4.2. Performance Evaluation of TASS-Based Fracturing Fluid
4.2.1. Thermal Rheology and Viscoelastic Performance
As shown in
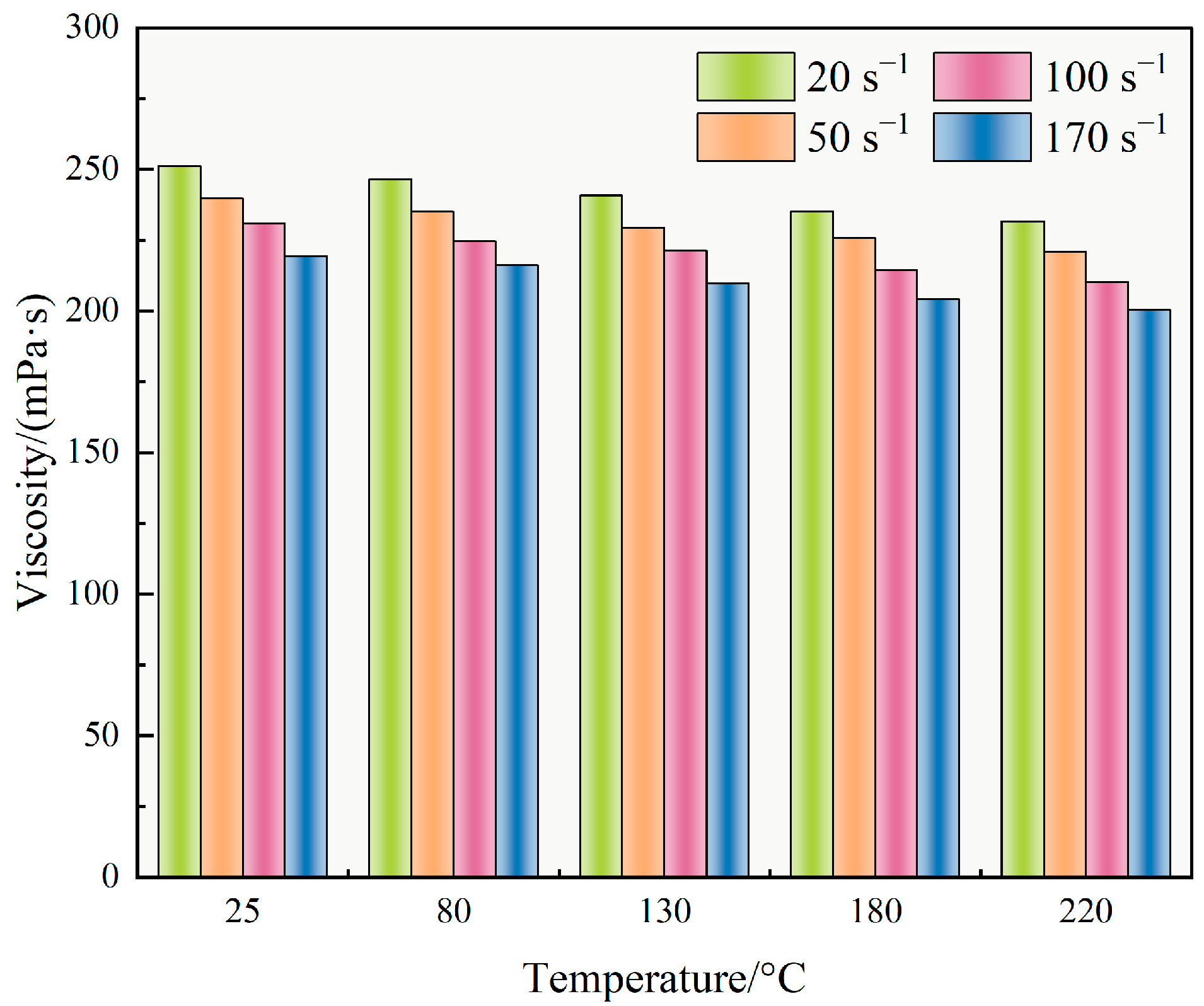
Figure 7a, the TASS fracturing fluid exhibited typical pseudoplastic behavior, with viscosity significantly decreasing as shear rate increased. At 25 °C, viscosity decreased from 5003 mPa·s at 0.1 s
−1 to 222 mPa·s at 1000 s
−1. With increasing temperature, this trend became more pronounced: viscosity at 1000 s
−1 reduced to 205 mPa·s at 100 °C, indicating weakened inter-chain interactions due to thermal agitation.
Power law fitting results (
Table 2) further confirmed this trend. The flow behavior index (n) increased slightly from 0.52 at 25 °C to 0.59 at 100 °C, reflecting a reduction in shear-thinning intensity. Simultaneously, the consistency index (K) decreased from 6.47 Pa·s
n to 4.31 Pa·s
n, implying lower viscous resistance at elevated temperatures. These changes suggest that the TASS molecular network partially relaxes under heat, as evidenced by the moderate decrease in storage modulus (G′) and the slight increase in the loss factor (tan δ = G″/G′) with rising temperature, yet retains adequate viscosity for proppant transport.
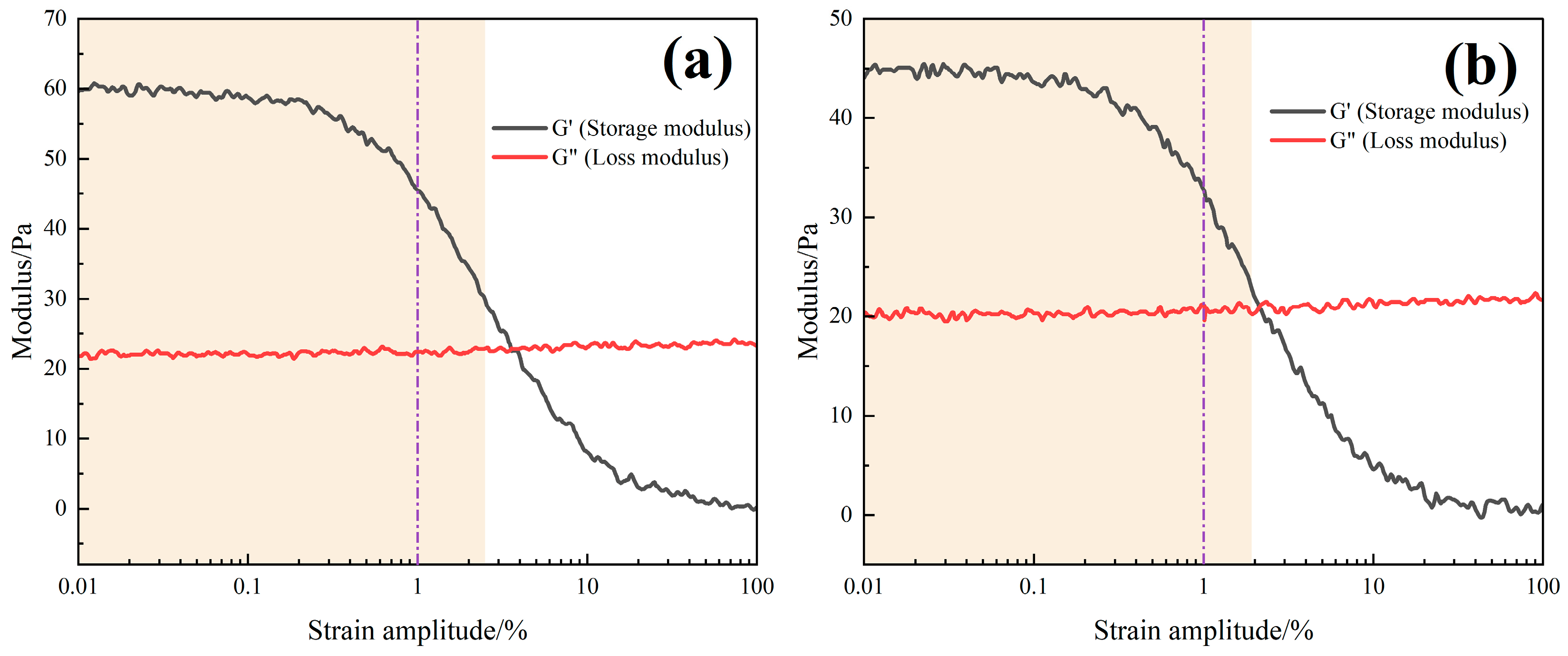
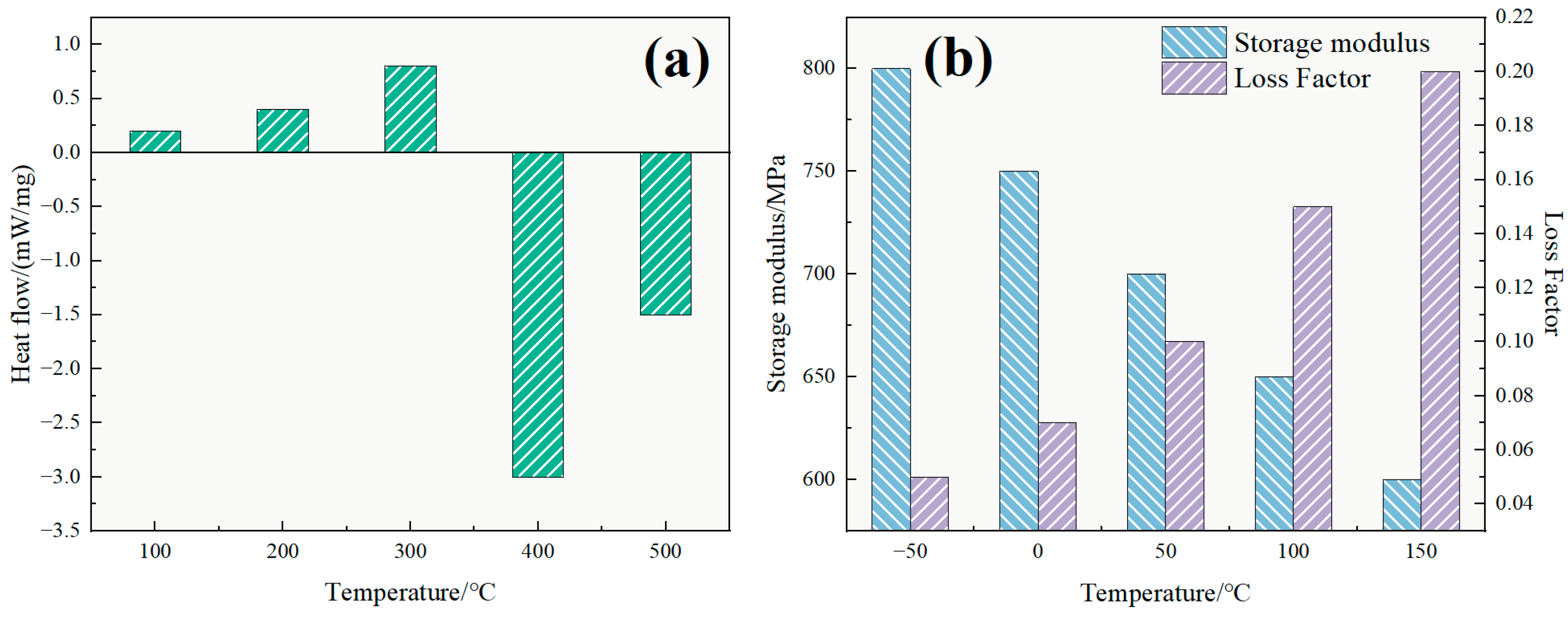
In
Figure 7b, the storage modulus (G′) consistently exceeded the loss modulus (G″) across all temperatures and frequencies, indicating that the system behaves as an elastic-dominant viscoelastic fluid. At 25 °C, G′ reached 203 Pa while G″ was 182 Pa, and at 100 °C, G′ remained at 262 Pa while G″ increased slightly to 241 Pa. The stable separation of moduli across the tested range confirms that the TASS system maintains its elastic structure under thermal stress.
This behavior is attributed to the formation of a 3D supramolecular network via hydrophobic associations and electrostatic interactions. The silane groups appear to enhance steric hindrance and thermal resistance, while the sulfonic acid groups may facilitate hydration and ion–dipole interactions. These synergistic mechanisms could help preserve viscoelasticity under thermal stress, making the TASS system robust against high-temperature degradation.
4.2.2. Interfacial Activity Under Salinity Influence
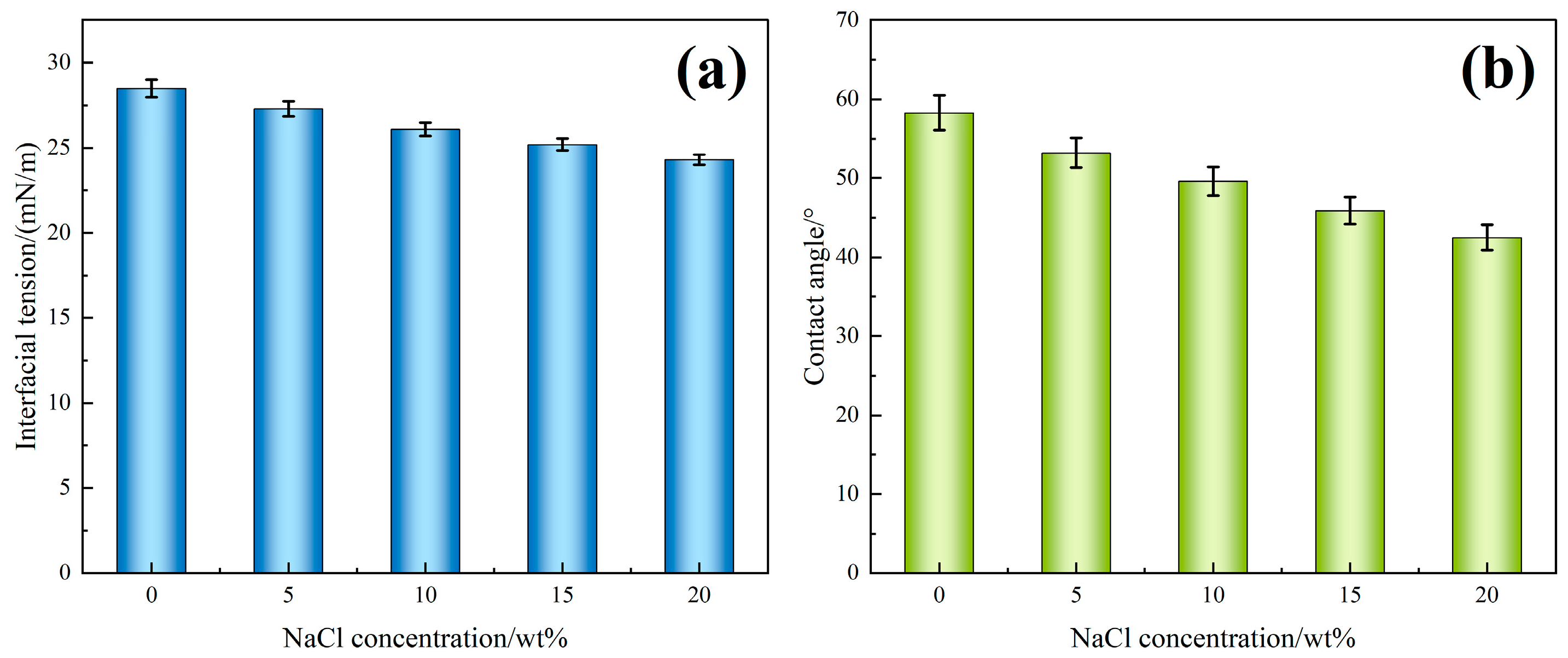
Figure 8a illustrates the variation in interfacial tension of the TASS solution with increasing NaCl concentration. As the NaCl content increases from 0 wt% to 20 wt%, the interfacial tension drops from 28.5 mN/m to 24.3 mN/m, representing a 14.7% reduction. This decline indicates that TASS molecules retain strong surface activity even in high-salinity environments. The amphoteric molecular structure—with both hydrophilic and hydrophobic moieties—facilitates adsorption at the oil–water interface. In addition, Na
+ ions compress the electrical double layer and reduce electrostatic repulsion, enabling tighter packing of surfactant molecules at the interface. Simultaneously, the salting-out effect promotes the migration of hydrophobic chains toward the interface, which further amplifies the reduction in interfacial tension. All measurements were repeated three times (
n = 3) under identical conditions, and the results are expressed as mean ± SD. Error bars in
Figure 8a represent 95% confidence intervals, confirming that the observed decrease in IFT is statistically significant and reproducible across the tested salinity range.
Figure 8b shows that the contact angle on sandstone surfaces decreases from 58.3° to 42.5° with increasing NaCl concentration. This trend confirms that TASS enhances surface wettability under saline conditions. Mechanistically, Na
+ and Cl
− ions interact with both mineral surfaces and the amphoteric head groups of TASS, forming hydration layers and hydrogen-bonding networks that increase rock surface hydrophilicity. Similarly, all contact angle tests were conducted in triplicate (
n = 3), and
Figure 8b shows mean ± SD values with 95% confidence intervals. The consistent monotonic decline in contact angle demonstrates the reproducibility of wettability enhancement under saline conditions. These synergistic mechanisms explain why higher NaCl concentrations improve both interfacial tension and wettability, thereby enhancing oil displacement efficiency in high-salinity reservoirs.
In summary, the TASS solution exhibits excellent interfacial activity under salinity influence, characterized by a significant decrease in IFT and enhanced rock wettability. These properties contribute to improved oil displacement efficiency in high-salinity reservoirs.
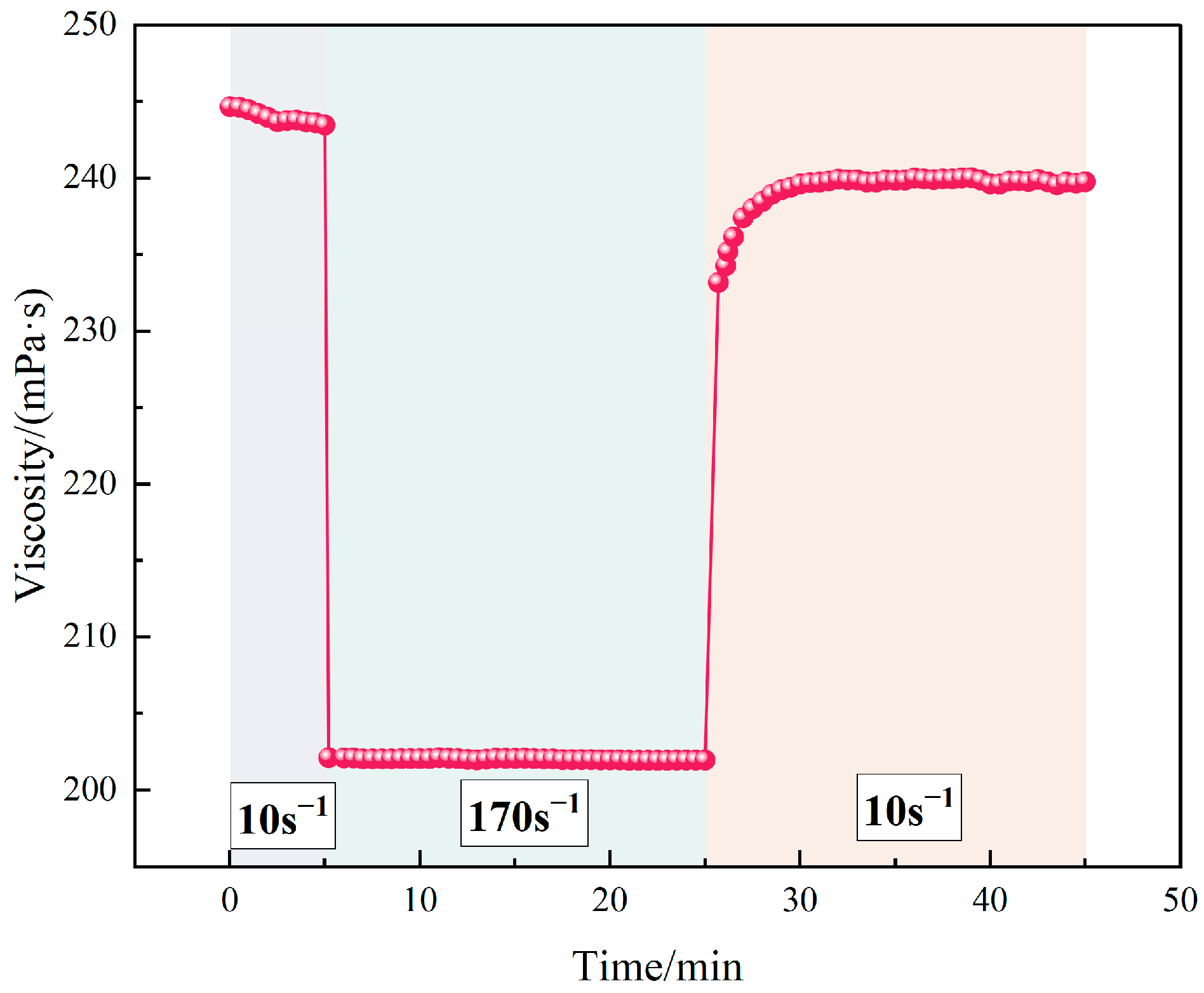
4.2.3. Resistance to Elevated Temperature and Shear
Figure 9 illustrates the thermal and shear resistance of the TASS-based fracturing fluid. As temperature increased from 25 °C to 220 °C, the viscosity gradually declined from 231.1 mPa·s to 210.2 mPa·s, corresponding to a minimal reduction of only 9.04%. This result highlights the system’s robust thermal tolerance, which is critical for ensuring proppant suspension and transport in ultra-deep formations.
The observed viscosity retention may be associated with the molecular architecture of TASS, wherein silane groups appear to provide steric rigidity and sulfonic acid moieties are likely to enhance ionic stability. These features promote intermolecular interactions and network stability under elevated thermal energy, thus mitigating thermal degradation.
Under varying shear rates at 130 °C, the viscosity decreased from 251.2 mPa·s at 20 s−1 to 209.8 mPa·s at 170 s−1, indicating a total reduction of 16.48%. This moderate shear-thinning behavior suggests pseudoplastic fluid characteristics, enabling energy-efficient pumping while maintaining sufficient viscosity for proppant transport. The shear resistance stems from reversible hydrophobic associations and steric hindrance among TASS chains, which facilitate transient network formation even under strong mechanical disturbance.
Overall, the TASS-based fluid demonstrated stable rheological performance across a wide temperature (25–220 °C) and shear rate (20–170 s−1) spectrum, with viscosity variations remaining below 17%. This rheological stability, driven by the rationally designed polymer backbone, makes the fluid system particularly suitable for applications in deep, high-temperature, and high-salinity reservoirs.
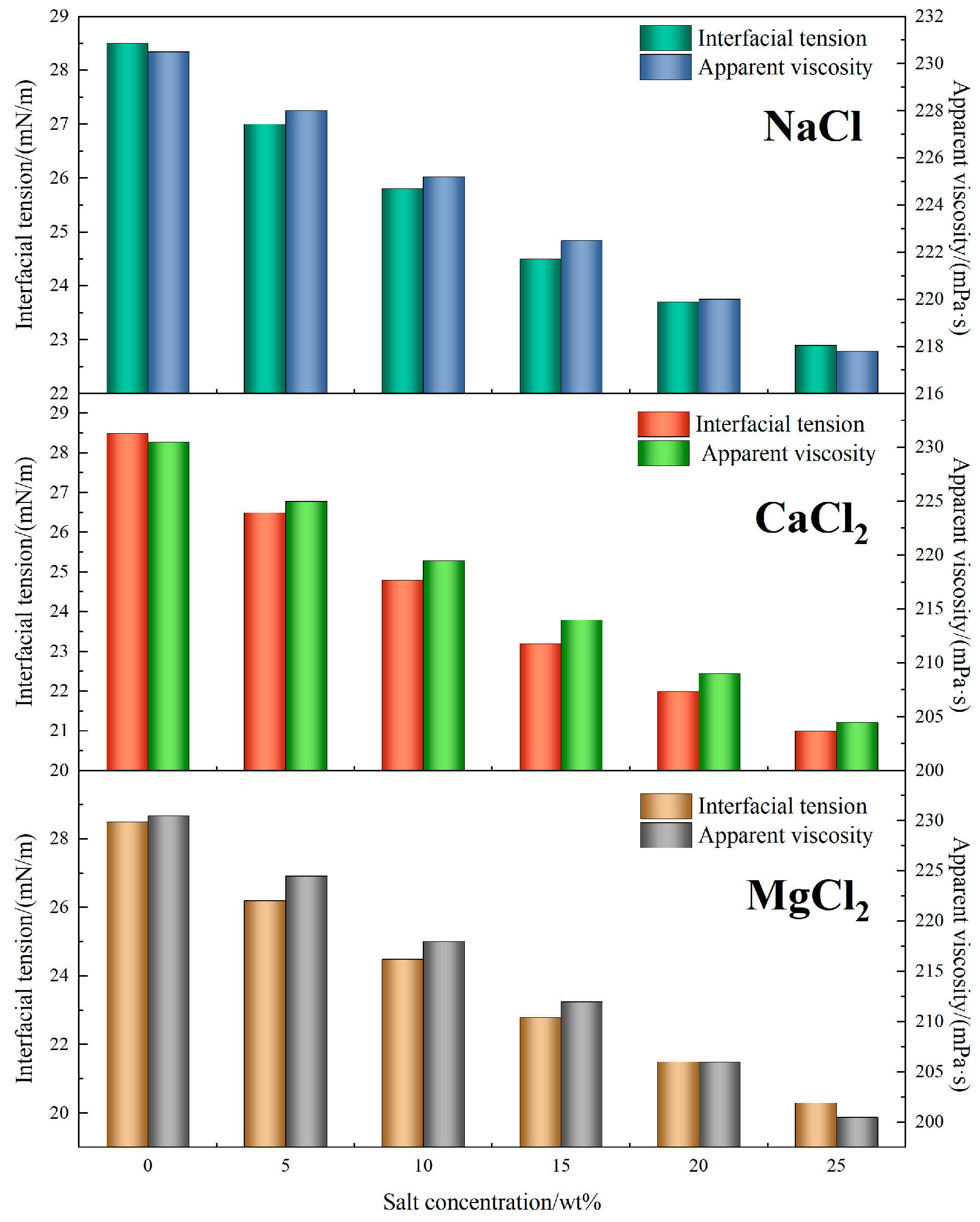
4.2.4. Interfacial and Rheological Stability Under Salinity
Figure 10 illustrates the influence of salt concentration on the interfacial tension and viscosity of TASS-based fracturing fluids. With increasing NaCl content from 0 to 25 wt%, the interfacial tension decreased from 28.5 mN/m to 22.9 mN/m (↓19.6%), indicating a strong salting-out effect. In comparison, solutions with divalent salts showed more pronounced reductions—particularly with MgCl
2, where interfacial tension dropped to 20.3 mN/m. This enhancement can be attributed to stronger electrostatic interactions between divalent cations and the amphoteric head groups of TASS, which promote tighter molecular packing and interfacial film stability.
In terms of viscosity, the TASS system maintained high rheological integrity across all salinity conditions. For NaCl, the viscosity declined modestly from 230.5 mPa·s to 217.8 mPa·s (↓5.5%) as salt concentration increased. In CaCl2 and MgCl2 environments, reductions were slightly greater, reaching 204.5 mPa·s and 200.5 mPa·s, respectively, at 25 wt%. Despite the decrease, viscosities remained above the threshold required for effective fracturing performance.
This performance stability is primarily related to the synergistic effect of sulfonic acid and zwitterionic groups within the TASS molecular framework. Sulfonic acid groups are expected to enable ion–dipole interactions that stabilize the network in monovalent salt solutions, while divalent cations further promote crosslinking, enhancing viscosity retention and interfacial adsorption. These features ensure that TASS not only withstands high-salinity degradation but also maintains effective flowback and proppant transport performance.
In conclusion, the TASS fracturing fluid demonstrated robust resistance to both monovalent and divalent salts, with less than 13% viscosity loss and significant interfacial tension reduction. These results affirm its suitability for fracturing operations in high-salinity, high-temperature reservoirs.
4.2.5. Stability and Reservoir Compatibility Under Extreme Conditions
As shown in
Table 3, the TASS-based fracturing fluid demonstrated remarkable stability during 72 h static aging under 150 °C and 20 wt% NaCl conditions. The viscosity slightly decreased from 200.5 mPa·s to 194.0 mPa·s (↓3.3%), and no phase separation was observed throughout the duration. This high thermal–saline stability can be attributed to the unique molecular design of TASS, where silane groups introduce steric hindrance that stabilizes the three-dimensional network, while sulfonic acid groups provide ionic interaction that mitigates chain degradation at elevated temperature.
Table 4 further confirms the compatibility of the TASS fluid with reservoir rocks. Across five core samples, post-treatment permeability decreased only marginally, with damage rates ranging from 4.1% to 4.4% and an average of 4.26%. This low formation damage was primarily due to TASS’s amphoteric structure, which minimizes electrostatic adsorption on mineral surfaces, and its low-residue gel-breaking formulation (0.1 wt% ammonium persulfate) [
27]. In addition, the partial inclusion of CMHPG (0.3–0.6 wt%) improved thermal viscosity retention without contributing to pore blockage, as gel-breaking by-products were water-soluble and easily removable.
Collectively, the TASS system achieves low reservoir damage (<5%) while maintaining viscosity stability, salt tolerance, and minimal residue, thereby surpassing conventional fracturing fluids in both performance and reservoir compatibility.
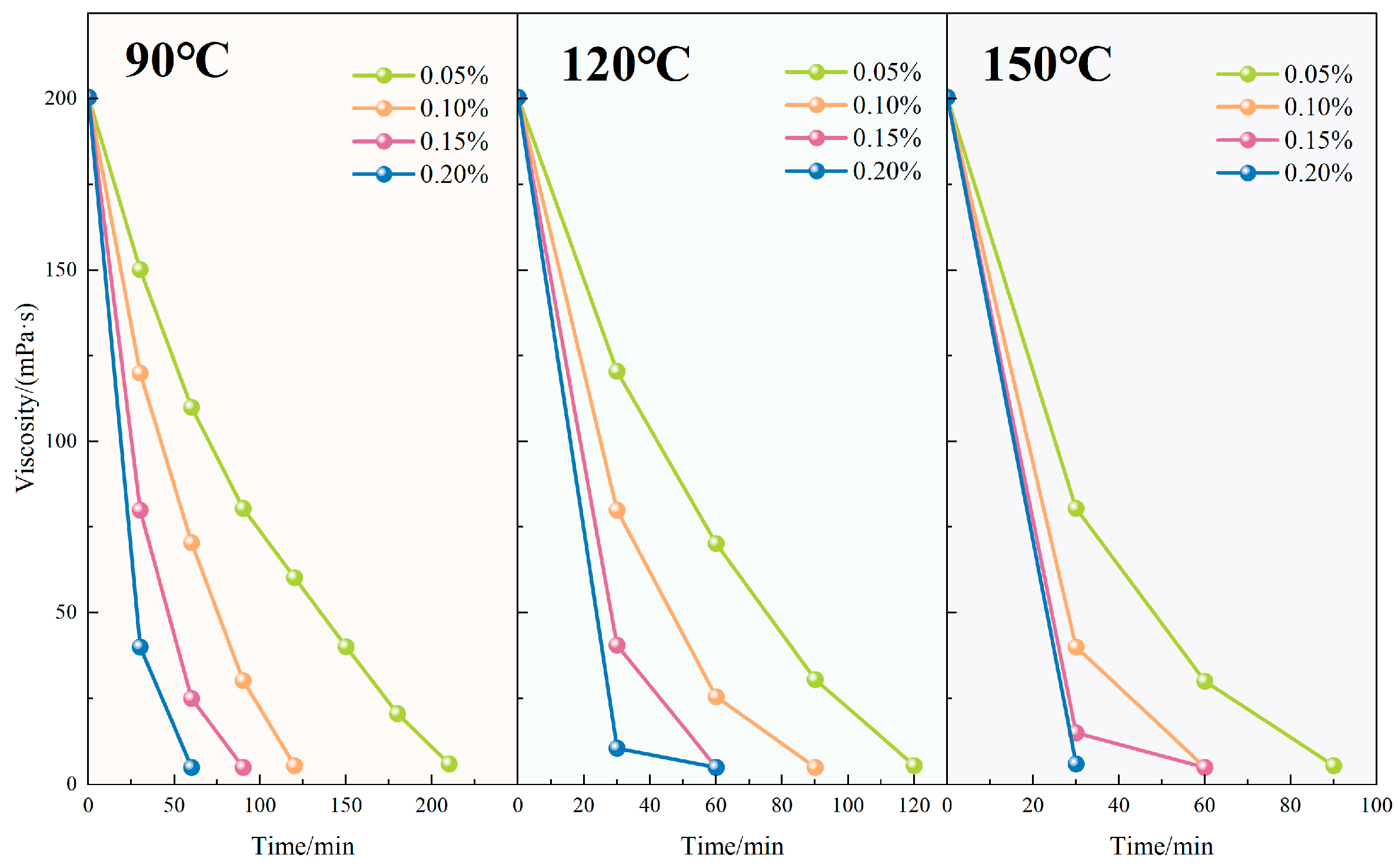
4.2.6. Gel-Breaking Performance and Residue Control
Figure 11 presents the viscosity evolution of the TASS fracturing fluid under different gel breaker concentrations and temperatures. At 120 °C, viscosity reduction occurred more rapidly with increased ammonium persulfate concentration. Specifically, with 0.05 wt% gel breaker, the viscosity declined from 200.5 mPa·s to 5 mPa·s over 180 min, while at 0.20 wt%, the same endpoint was achieved within just 60 min. This inverse relationship confirmed that higher gel breaker concentrations significantly enhanced the degradation kinetics of the polymer network.
In parallel, temperature also played a decisive role. Under a fixed gel breaker concentration of 0.10 wt%, raising the reaction temperature from 90 °C to 150 °C reduced the gel-breaking time from 120 to 60 min. This acceleration is attributed to the thermal activation of ammonium persulfate decomposition, which increases the generation of free radicals that cleave degradable linkages in the TASS molecular chains, thus expediting viscosity loss.
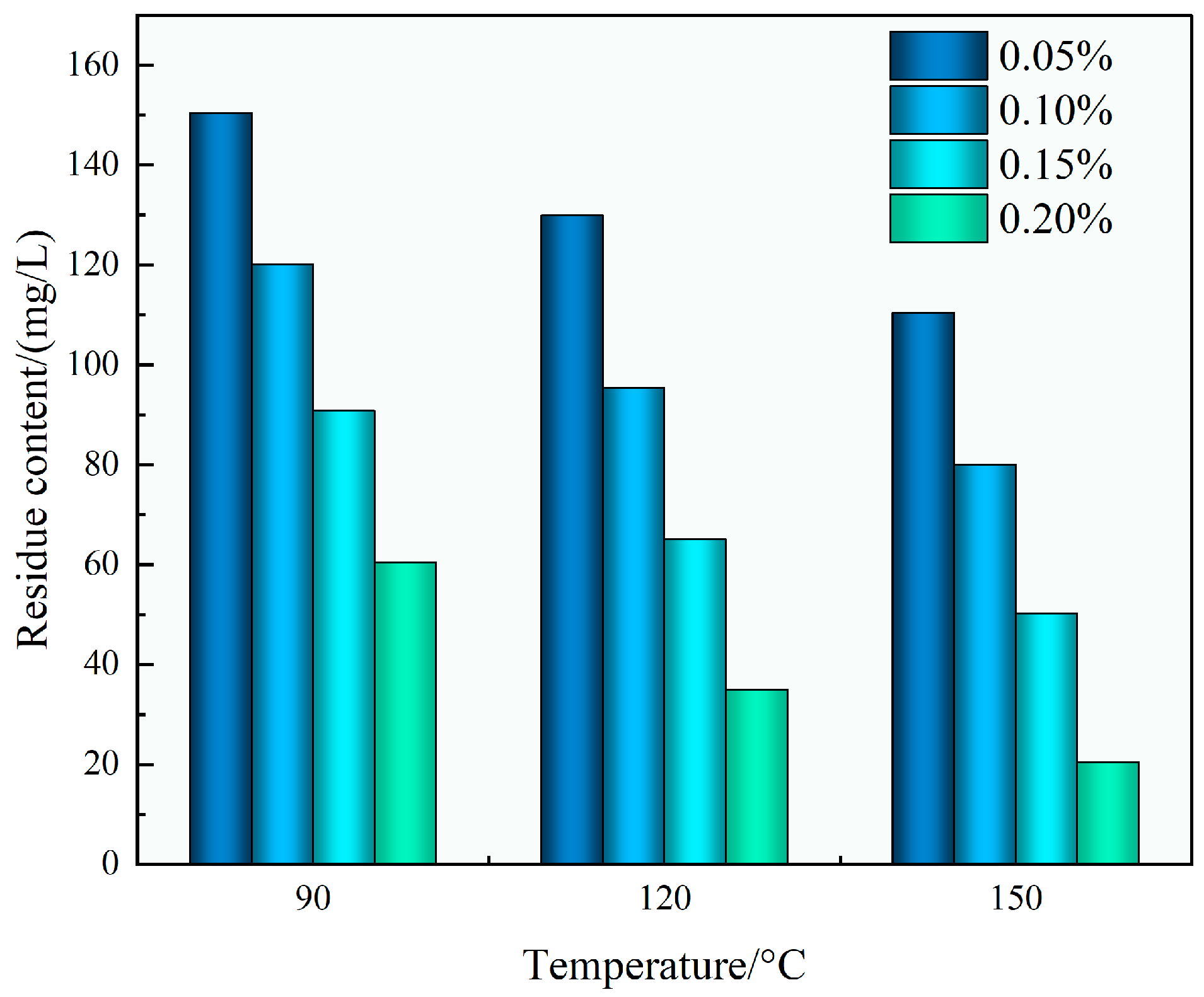
As shown in
Figure 12, the residual content after gel breaking decreased systematically with both rising gel breaker concentration and increasing temperature. The minimum residue content of 20.5 mg/L was obtained under the most favorable tested condition of 0.20 wt% gel breaker at 150 °C. Such low residue values indicate nearly complete gel degradation, which is crucial to avoid post-fracturing formation damage, particularly pore blockage and permeability loss.
The superior gel-breaking efficiency of the TASS system can be attributed the molecular structure design, which incorporates thermally cleavable and oxidant-sensitive bonds. These likely facilitate rapid breakdown when exposed to oxidative conditions and heat, resulting in swift viscosity decay and minimal residual matter. Combined with its amphoteric structure and low adsorption properties, this enables the fluid to minimize retention within the reservoir rock matrix.
In conclusion, the TASS fracturing fluid demonstrated rapid and thorough gel-breaking performance, achieving low viscosity (<5 mPa·s) and minimal residue (<25 mg/L) under most favorable tested conditions of gel breaker and temperature conditions. These properties ensure smooth flowback, reservoir protection, and operational efficiency, positioning the TASS system as a robust candidate for deployment in high-temperature and high-salinity oilfield environments.
4.2.7. Performance Benchmarking Against Commercial Systems
To contextualize the novelty of this work, we conducted a direct benchmark under 20 wt% NaCl between the TASS fracturing fluid and a commercial betaine surfactant system. The comparative results are shown in
Table 5. Both fluid systems were prepared at 0.5 wt% concentration, and the viscosity was measured across a temperature range from 120 °C to 220 °C at a fixed shear rate of 100 s
−1. The comparative results are summarized in
Table 5.
As shown in
Table 5, both systems exhibited viscosity degradation with rising temperature; however, the TASS-based fluid demonstrated significantly enhanced thermal stability throughout the test range. At 220 °C, the TASS system retained a viscosity of 210.5 mPa·s, whereas the commercial betaine surfactant system dropped sharply to 120.4 mPa·s, indicating a 45% lower viscosity than TASS at this condition. This substantial discrepancy underscores the robust high-temperature performance of the TASS formulation.
The enhanced thermal resistance of TASS is likely associated with its tailored molecular architecture. The incorporation of silane functional groups within the amphoteric backbone appears to increase molecular rigidity and steric hindrance, which may help delay thermal chain scission. Simultaneously, the presence of sulfonic acid groups introduces strong ionic interactions and hydration shell effects, which improve thermal and salt stability by preserving intermolecular associations under ionic and thermal stress. These synergistic structural features confer improved molecular cohesion, allowing TASS to maintain a stable three-dimensional network even under harsh thermal environments.
This comparative analysis not only confirms the superior rheological stability of the TASS fluid over traditional betaine-based formulations but also provides direct evidence of its novelty as a silane-grafted amphoteric design tailored for high-temperature and high-salinity reservoirs.
To place these results against industry baselines, we compiled a conservative benchmark (
Table 6) that anchors on our measured TASS values and contrasts them with literature-reported ranges for standard polymer-based fluids (guar/CMHPG, HPAM) at comparable high temperatures. (Note on comparability: literature ranges for CMHPG/HPAM commonly use fresh or low-salinity water; presenting TASS in 20 wt% NaCl makes the comparison conservative.)
In brief, standard polymer fluids generally operate near 120~150 °C at moderate shear; near 150 °C, reported apparent viscosities are often ≤100 mPa·s and show marked salinity sensitivity, while permeability-damage rates ~15~40% are frequently cited for crosslinked guar in core tests. By contrast, in this study, TASS sustained ~220 mPa·s at 160 °C and 170 s−1 in 20 wt% NaCl brine and kept core damage < 5% under reservoir-simulated conditions. Taken together, the quantitative benchmarks indicate that TASS not only outperforms a commercial surfactant system but also exceeds standard polymer fluids by a substantial margin under high-temperature/high-salinity conditions.
4.2.8. Field Application
To validate the field applicability and operational reliability of the TASS-based high-temperature and salt-resistant fracturing fluid, a pilot application was carried out in a vertical well located in a moderately tight sandstone reservoir. The target reservoir had a depth of approximately 4133 m, with a bottom-hole static temperature (BHST) of 126 °C and a brine salinity of about 20 wt% NaCl, representing a typical high-temperature and high-salinity environment. The well had undergone limited prior stimulation and exhibited only moderate initial productivity, providing a suitable baseline for performance benchmarking.
Prior to implementation, a series of laboratory qualification tests was conducted to ensure the fracturing fluid met industrial standards. As shown in
Table 7, the TASS system demonstrated excellent rheological, thermal, and filtration characteristics. The fluid viscosity at 25 °C (100 s
−1) reached 800 mPa·s, and remained above 220 mPa·s at 160 °C under high shear (170 s
−1), surpassing conventional thresholds for high-temperature fracturing fluids. Other critical parameters, such as pH (7.5) and viscoelastic modulus G′ (50 Pa at 1 Hz), also satisfied operational criteria, indicating sufficient elasticity and thermal robustness for field deployment.
Following the fracturing operation, the well demonstrated a marked improvement in productivity. As summarized in
Table 8, daily oil output increased from 10.2 tons to 18.5 tons, representing an 81% enhancement, while daily gas production rose from 0.6 × 10
4 m
3 to 1.4 × 10
4 m
3, an increase of 133%. Simultaneously, the water cut dropped from 35% to 22%, indicating effective flowback and reduced fluid retention in the formation.
The return fluid analysis further confirmed the in-field gel-breaking efficiency and environmental compatibility of the TASS system. As presented in
Table 9, the flowback rate reached 72%, and the residual content remained as low as 40 mg/L, demonstrating effective degradation of the polymer matrix. The pH of the return fluid remained near neutral (7.0), and the microbial count was below 10
3 CFU/mL, indicating low biological activity and minimal risk of microbial-induced damage.
Throughout the field operation, the fracturing fluid exhibited consistent rheological behavior with no observed phase separation or viscosity degradation, even at elevated downhole temperatures. The pressure profile during post-fracturing production was stable, and no signs of formation plugging, capillary locking, or excessive retention were detected—further attesting to the low-damage nature of the system. By providing detailed reservoir characteristics and baseline productivity data, the stimulation performance of the TASS system can now be more transparently interpreted and compared with other case studies.
The observed production improvements and favorable flowback parameters collectively demonstrate the technical feasibility and operational robustness of the TASS fracturing fluid under high-temperature and high-salinity conditions. These advantages are primarily attributed to its amphoteric molecular design, which integrates thermal-stable silane chains and ionic-tolerant sulfonic acid groups, ensuring both structural integrity and degradability during in situ application. Compared with conventional polymer–surfactant hybrids, the TASS system shows enhanced adaptability to thermally stressed reservoirs while minimizing permeability impairment.
Given the promising performance demonstrated in this field case, further applications in similar reservoir environments are warranted. Additionally, fine-tuning of additive ratios, gel breaker dosage, and fracturing schedules may further improve cost-efficiency and stimulation effectiveness in scaled operations.
4.2.9. Mechanism Analysis of Temperature and Salt Resistance
The superior thermal and salt resistance exhibited by the TASS-based fracturing fluid may be attributed to its molecular-level architecture, which integrates multiple functional moieties to achieve cooperative performance enhancements. The presence of sulfonic acid groups is expected to enable ion–dipole interactions with common cations (Na
+, Ca
2+, Mg
2+), forming stable hydration shells that likely help prevent molecular precipitation even under 25 wt% salinity, consistent with previous reports [
28]. Interestingly, although divalent ions typically exert stronger electrostatic shielding, NaCl exhibited a more pronounced effect in reducing oil–water interfacial tension. This deviation from classical electrostatic trends could be related to the greater interfacial mobility facilitated by monovalent Na
+, which may promote dynamic rearrangement of TASS molecules, in contrast to Ca
2+ and Mg
2+ which are suggested to form coordination complexes with sulfonate groups, restricting molecular orientation and reducing interfacial efficiency.
From a thermal perspective, the integration of silane functionalities within the TASS backbone appears to introduce structural rigidity and steric hindrance, limiting chain mobility and thereby enhancing resistance to thermal degradation at temperatures up to 220 °C. Furthermore, the amphoteric nature of TASS molecules may help balance electrostatic interactions, reducing intermolecular aggregation and improving colloidal stability in complex ionic environments.
Notably, the broad polydispersity index (PDI = 5.62) observed in TASS suggests a contribution to its unique viscoelastic behavior under shear stress. The coexistence of long-chain and short-chain polymer segments is likely to form a hierarchical network that can resist deformation and rapidly recover upon shear cessation. Long segments may provide entanglement and elastic memory, while shorter, more flexible chains facilitate reversible hydrophobic associations, thereby sustaining fluid viscosity during high-shear operations.
Collectively, these molecular design strategies—including ionic interaction control, thermal shielding mechanisms, and dynamic network reformation—offer a plausible mechanistic interpretation for the exceptional adaptability of TASS-based fracturing fluids in high-temperature and high-salinity reservoirs. However, these interpretations remain speculative and require further experimental validation in future work.
Unlike conventional betaine VES, whose activity typically degrades above ~150–180 °C, the silane-reinforced amphoteric architecture of TASS preserves network integrity and interfacial packing to 220 °C in high-salinity brines, explaining the superior viscosity retention observed in
Table 5.
In addition, the practical scalability and long-term reservoir compatibility of TASS were briefly considered. From a scalability standpoint, the synthesis route is based on free radical polymerization of widely available feedstocks (epoxy ethylene, glycine methyl ester, dodecanol, chlorosulfonated olefin), which aligns with existing surfactant production infrastructure and is cost-effective for industrial upscaling. The relatively low dosage required in fracturing operations (0.5 wt%) further enhances its economic feasibility. In terms of long-term reservoir compatibility, the amphoteric structure minimizes irreversible adsorption on rock surfaces, as reflected in the core-flow tests where permeability damage was consistently below 5%. The gel-breaking process generates low levels of water-soluble residues (<25 mg/L), reducing the risk of pore plugging. Furthermore, field trial flowback fluids exhibited near-neutral pH (~7.0) and low microbial activity, indicating stable environmental and reservoir compatibility over extended timescales. These considerations suggest that TASS not only performs effectively at the laboratory and pilot scales but also has promising potential for industrial deployment in complex reservoirs.