Influence of Surface Energy and Phase Composition on Electroadhesive Interactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Electroadhesive Systems
2.2. Methods
- (1)
- Setting the gap between the electroadhesive and the substrate (polymer film) to 0.1 mm;
- (2)
- Setting the minimum peel speed to 0.000001 mm/h;
- (3)
- Setting the specified value of electrical voltage and minimum current (10 μA);
- (4)
- Applying electrical voltage and recording the maximum electroadhesive forces.
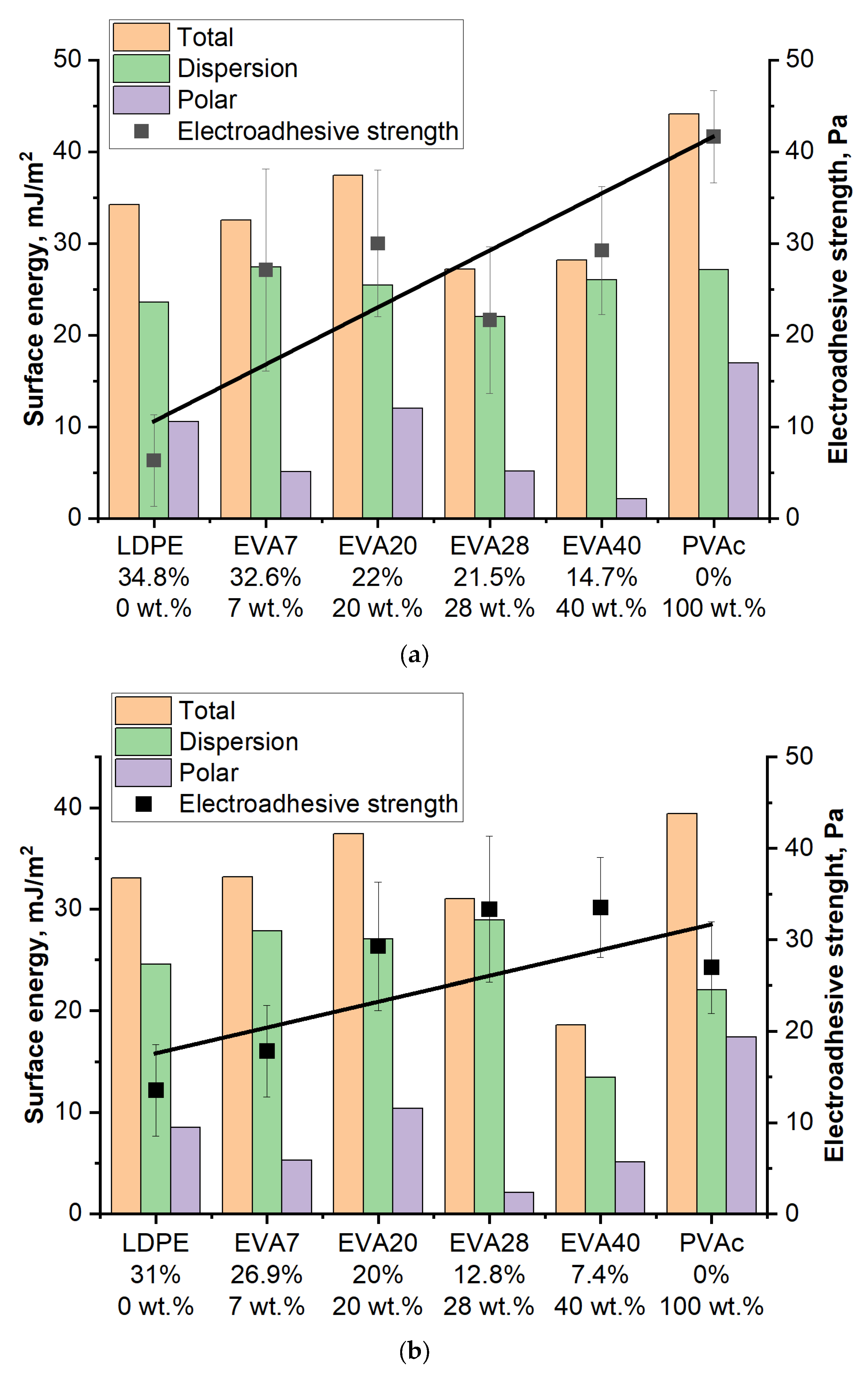
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DC source | Direct current source |
| LDPE | Low-density polyethylene |
| EVA | Ethylene-vinyl acetate copolymer |
| PVAc | Polyvinyl acetate |
| TP | Pressing temperature |
| P | Pressure |
| tP | Pressing time |
| Vp | Cooling rate after pressing |
| TA | Annealing temperature |
| tA | Annealing time |
| VA | Cooling rate after annealing |
| DSC | Differential scanning calorimetry |
| TEM | Transmission electron microscopy |
| α | Degree of crystallinity |
| ES | Electroadhesion strength |
| γtotal | Total surface energy |
| γD | Dispersion component of surface energy |
| γP | Polar component of surface energy |
| Vcooling | Cooling rate |
References
- Guo, J.; Leng, J.; Rossiter, J. Electroadhesion Technologies for Robotics: A Comprehensive Review. IEEE Trans. Robot. 2020, 36, 313. [Google Scholar] [CrossRef]
- AliAbbasi, E.; Muzammil, M.; Sirin, O.; Lefèvre, P.; Martinsen, Ø.G.; Basdogan, C. Effect of Finger Moisture on Tactile Perception of Electroadhesion. IEEE Trans. Haptics 2024, 17, 841. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sun, X.; Wang, D.; Liu, Y.; Zhang, Y. Effect of Electrostatic Tactile Feedback on Accuracy and Efficiency of Pan Gestures on Touch Screens. IEEE Trans. Haptics 2018, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Shultz, C.D.; Peshkin, M.A.; Colgate, J.E. Surface Haptics via Electroadhesion: Expanding Electrovibration with Johnsen and Rahbek. In Proceedings of the 2015 IEEE World Haptics Conference (WHC), Evanston, IL, USA, 22–25 June 2015; pp. 57–62. [Google Scholar]
- Jeong, Y.-J.; Hong, T.-H.; Lee, H.-J.; Kim, K. Optimal Design of the Electroadhesion Pad with a Dual-Insulating Layer for Climbing Robots. Actuators 2022, 11, 36. [Google Scholar] [CrossRef]
- Persson, B.N.J.; Guo, J. Electroadhesion for Soft Adhesive Pads and Robotics: Theory and Numerical Results. Soft Matter 2019, 15, 8032. [Google Scholar] [CrossRef]
- Ruffatto, D.; Shah, J.; Spenko, M. Optimization of Electrostatic Adhesives for Robotic Climbing and Manipulation. In Proceedings of the Volume 4: 36th Mechanisms and Robotics Conference; Parts A and B; American Society of Mechanical Engineers: Chicago, IL, USA, 2012; pp. 1143–1152. [Google Scholar] [CrossRef]
- Tang, H.; Beebe, D.J. A Microfabricated Electrostatic Haptic Display for Persons with Visual Impairments. IEEE Trans. Rehabil. Eng. 1998, 6, 241. [Google Scholar] [CrossRef]
- Ruffatto, D.; Parness, A.; Spenko, M. Improving Controllable Adhesion on Both Rough and Smooth Surfaces with a Hybrid Electrostatic/Gecko-like Adhesive. J. R. Soc. Interface 2014, 11, 20131089. [Google Scholar] [CrossRef]
- Guo, J.; Tailor, M.; Bamber, T.; Chamberlain, M.; Justham, L.; Jackson, M. Investigation of Relationship between Interfacial Electroadhesive Force and Surface Texture. J. Phys. D Appl. Phys. 2016, 49, 035303. [Google Scholar] [CrossRef]
- Guo, J.; Bamber, T.; Chamberlain, M.; Justham, L.; Jackson, M. Optimization and Experimental Verification of Coplanar Interdigital Electroadhesives. J. Phys. D Appl. Phys. 2016, 49, 415304. [Google Scholar] [CrossRef]
- Dadkhah, M.; Ruffatto, D.; Zhao, Z.; Spenko, M. Increasing Adhesion via a New Electrode Design and Improved Manufacturing in Electrostatic/Microstructured Adhesives. J. Electrost. 2018, 91, 48. [Google Scholar] [CrossRef]
- Fimbel, A.; Abensur, T.; Le, M.-Q.; Capsal, J.-F.; Cottinet, P.-J. Accurate Electroadhesion Force Measurements of Electrostrictive Polymers: The Case of High Performance Plasticized Terpolymers. Polymers 2021, 14, 24. [Google Scholar] [CrossRef]
- Choi, K.; Kim, Y.C.; Sun, H.; Kim, S.-H.; Yoo, J.W.; Park, I.-K.; Lee, P.-C.; Choi, H.J.; Choi, H.R.; Kim, T.; et al. Quantitative Electrode Design Modeling of an Electroadhesive Lifting Device Based on the Localized Charge Distribution and Interfacial Polarization of Different Objects. ACS Omega 2019, 4, 7994. [Google Scholar] [CrossRef]
- Nakamura, T.; Yamamoto, A. Modeling and Control of Electroadhesion Force in DC Voltage. Robomech J. 2017, 4, 18. [Google Scholar] [CrossRef]
- Park, S.; Chang, H.; Kim, J.; Gwak, Y.; Moon, J. Superior Electroadhesion Force with Permittivity-Engineered Bilayer Films Using Electrostatic Simulation and Machine Learning Approaches. Sci. Rep. 2024, 14, 17026. [Google Scholar] [CrossRef]
- Park, S.; Shintake, J.; Piskarev, Y.; Wei, Y.; Joshipura, I.; Frey, E.; Neumann, T.; Floreano, D.; Dickey, M.D. Stretchable and Soft Electroadhesion Using Liquid-Metal Subsurface Microelectrodes. Adv. Mater. Technol. 2021, 6, 2100263. [Google Scholar] [CrossRef]
- Ciavarella, M.; Papangelo, A. A Simplified Theory of Electroadhesion for Rough Interfaces. Front. Mech. Eng. 2020, 6, 27. [Google Scholar] [CrossRef]
- Gao, X.; Cao, C.; Guo, J.; Conn, A. Elastic Electroadhesion with Rapid Release by Integrated Resonant Vibration. Adv. Mater. Technol. 2019, 4, 1800378. [Google Scholar] [CrossRef]
- Xie, G.; Wang, W.; Zhao, X.; Wang, H. Low-Voltage Electroadhesive Pad with Thin Insulation Layer Fabricated by Parylene Deposition. In Proceedings of the 2019 IEEE 9th Annual International Conference on CYBER Technology in Automation, Control, and Intelligent Systems (CYBER), Suzhou, China, 29 July–2 August 2019; pp. 197–202. [Google Scholar]
- Guo, J.; Elgeneidy, K.; Xiang, C.; Lohse, N.; Justham, L.; Rossiter, J. Soft Pneumatic Grippers Embedded with Stretchable Electroadhesion. Smart Mater. Struct. 2018, 27, 055006. [Google Scholar] [CrossRef]
- Chen, R.; Liu, F.; Wang, H.; Zhu, X.; Tao, X.; Zhang, S.; Jiang, G. Theoretical and Experimental Analyses of the Dynamic Electroadhesion Force. Extreme Mech. Lett. 2022, 56, 101892. [Google Scholar] [CrossRef]
- Li, Q.; Le Duigou, A.; Guo, J.; Thakur, V.K.; Rossiter, J.; Liu, L.; Leng, J.; Scarpa, F. Biobased and Programmable Electroadhesive Metasurfaces. ACS Appl. Mater. Interfaces 2022, 14, 47198. [Google Scholar] [CrossRef]
- Rajagopalan, P.; Muthu, M.; Liu, Y.; Luo, J.; Wang, X.; Wan, C. Advancement of Electroadhesion Technology for Intelligent and Self-Reliant Robotic Applications. Adv. Intell. Syst. 2022, 4, 2200064. [Google Scholar] [CrossRef]
- Cao, C.; Gao, X.; Guo, J.; Conn, A. De-Electroadhesion of Flexible and Lightweight Materials: An Experimental Study. Appl. Sci. 2019, 9, 2796. [Google Scholar] [CrossRef]
- Xiang, C.; Guan, Y.; Zhu, H.; Lin, S.; Song, Y. All 3D Printed Ready-to-Use Flexible Electroadhesion Pads. Sens. Actuators A Phys. 2022, 344, 113747. [Google Scholar] [CrossRef]
- Plyusnina, O.; Nikulova, U.V.; Khasbiullin, R.R.; Shapagin, A.V. Regulation of the Phase Structure in the Crystallizing Curing System PCL–DGEBA. Polymers 2024, 16, 2695. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, A.D.; Nikulova, U.V.; Shapagin, A.V. Phase Equilibria and Interdiffusion in the Ternary System Epoxy Oligomer–Polysulfone–Alkyl Glycidyl Ether. Polymers 2023, 16, 130. [Google Scholar] [CrossRef]
- Sharov, I.; Stepanenko, V.Y.; Nikulova, U.V.; Shapagin, A.V. Influence of Polymer Nature on Electroadhesion. Polymers 2024, 16, 3344. [Google Scholar] [CrossRef]
- Psarras, G.C. Fundamentals of Dielectric Theories. In Dielectric Polymer Materials for High-Density Energy Storage; Elsevier: Amsterdam, The Netherlands, 2018; pp. 11–57. ISBN 978-0-12-813215-9. [Google Scholar]
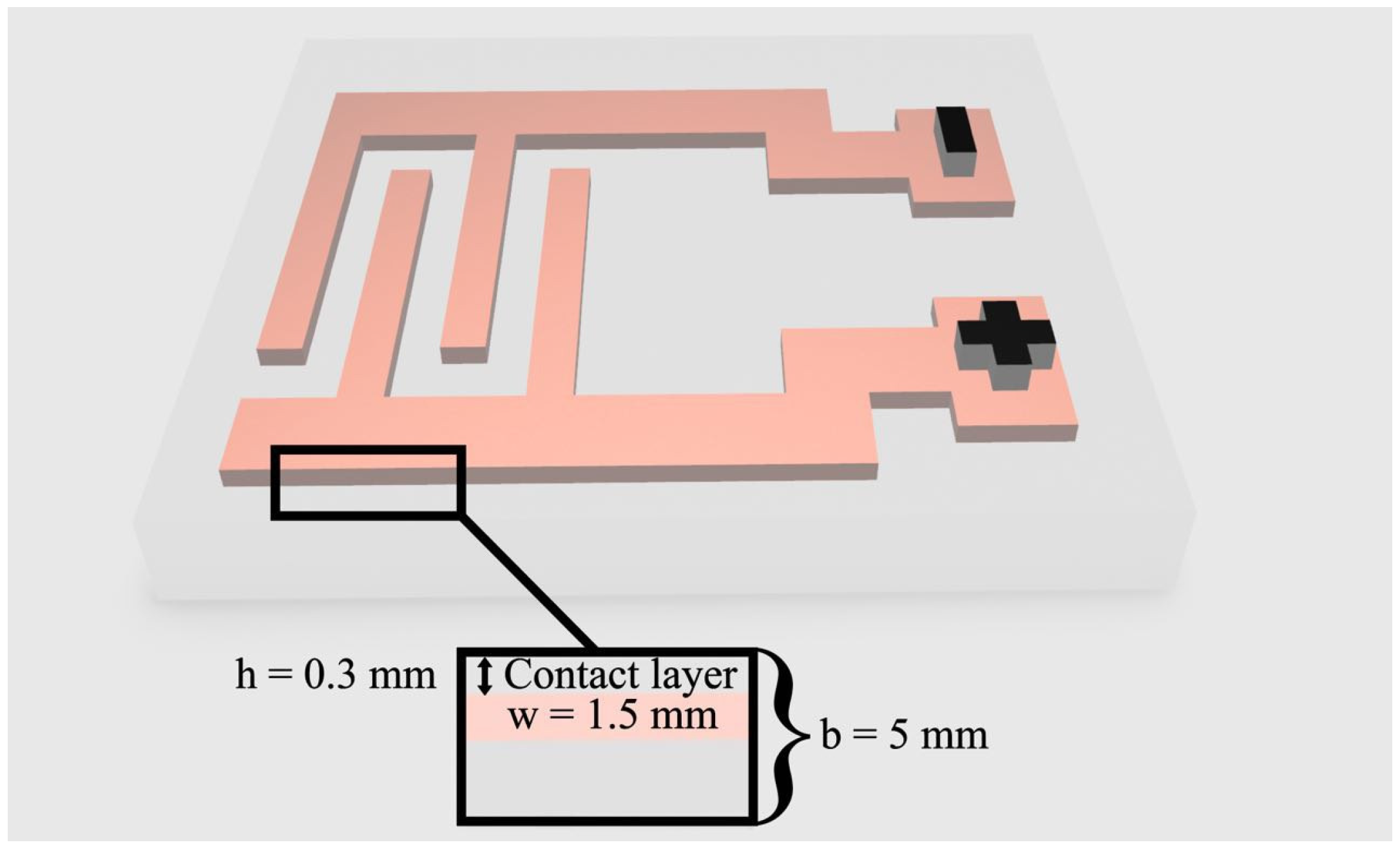
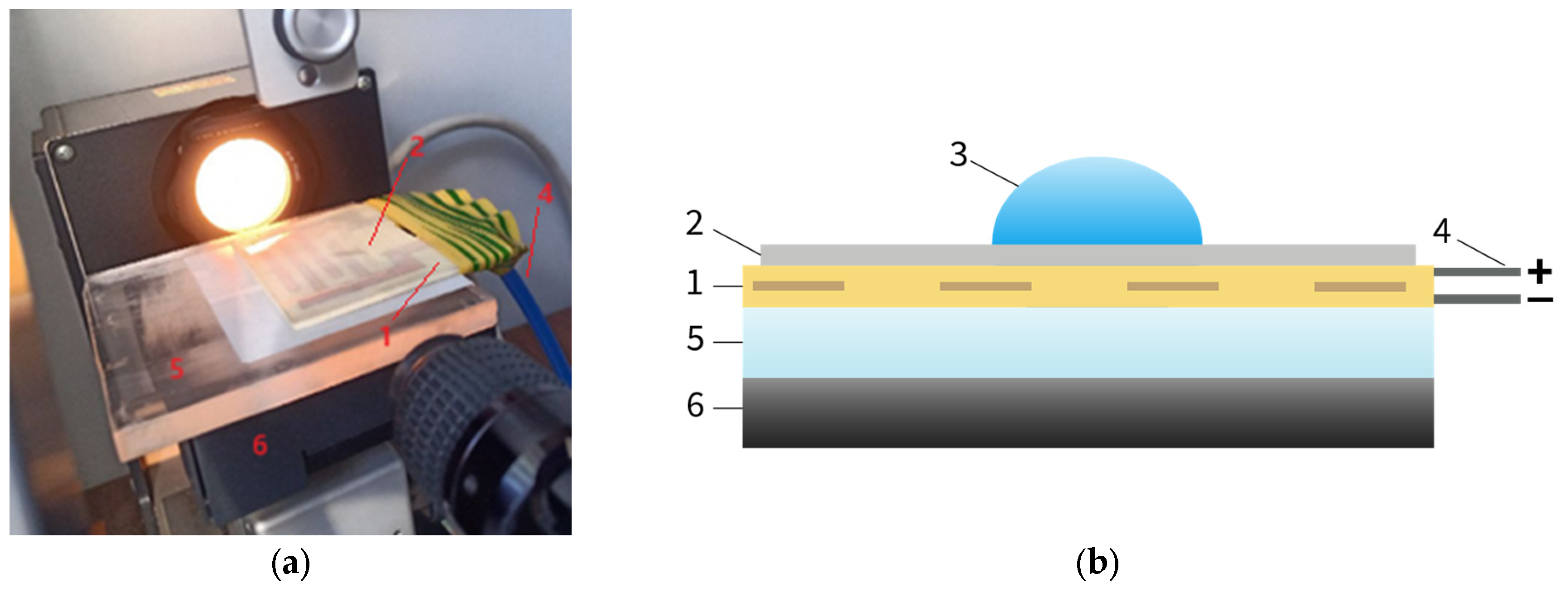
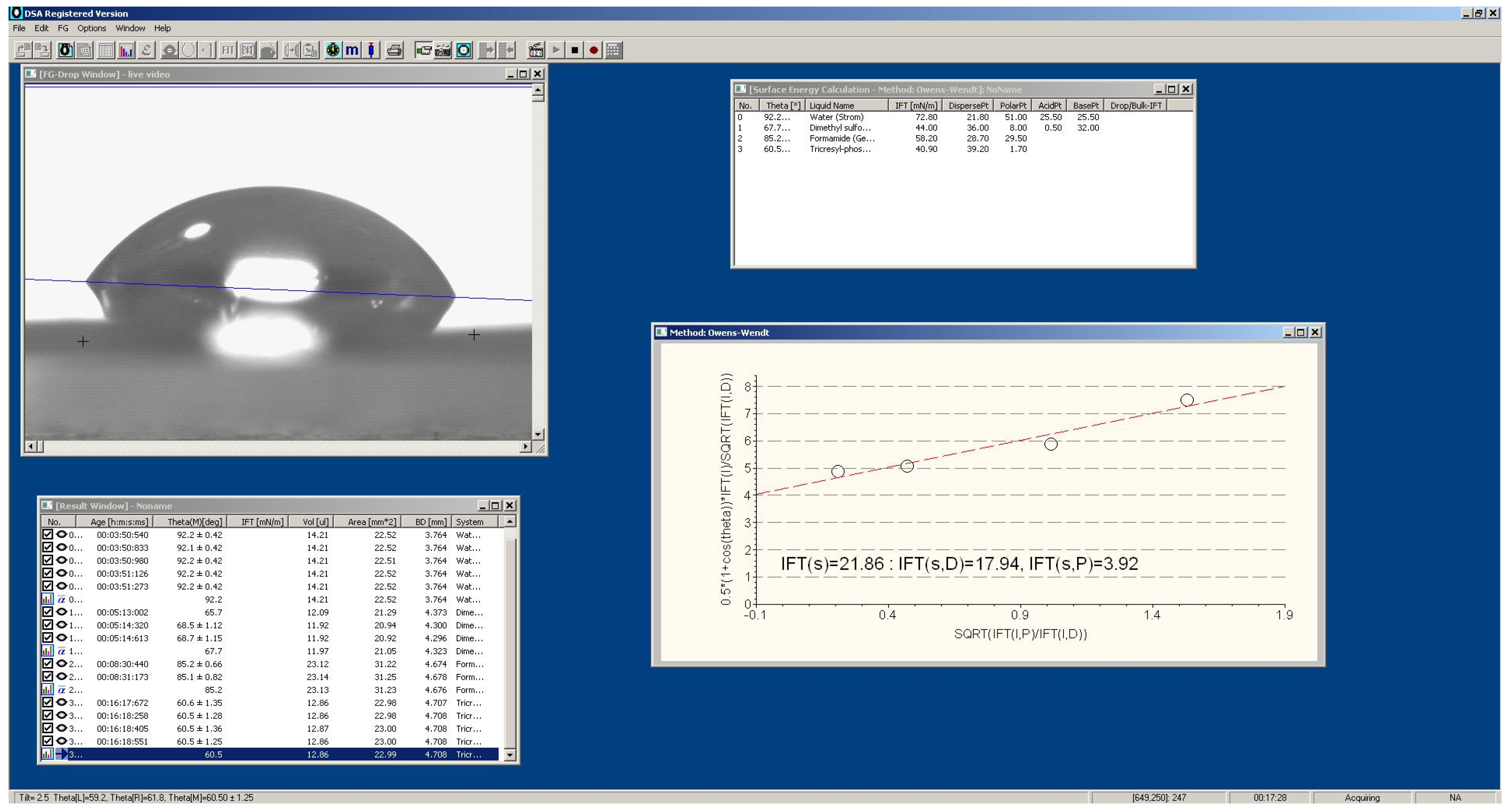
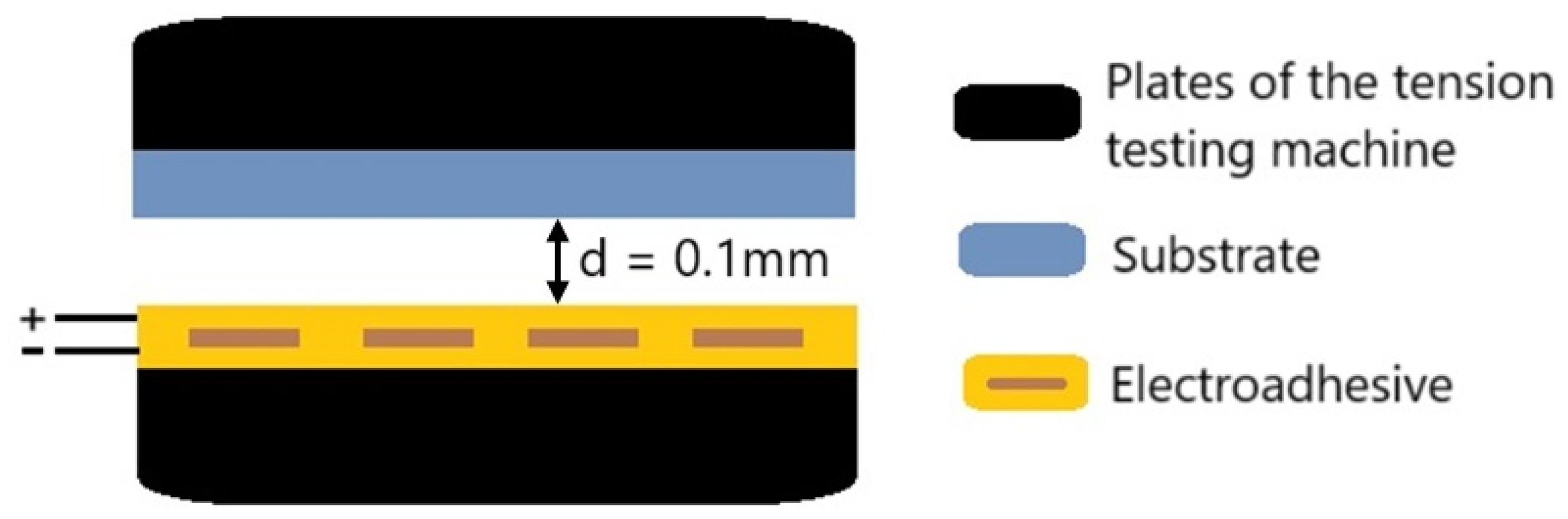
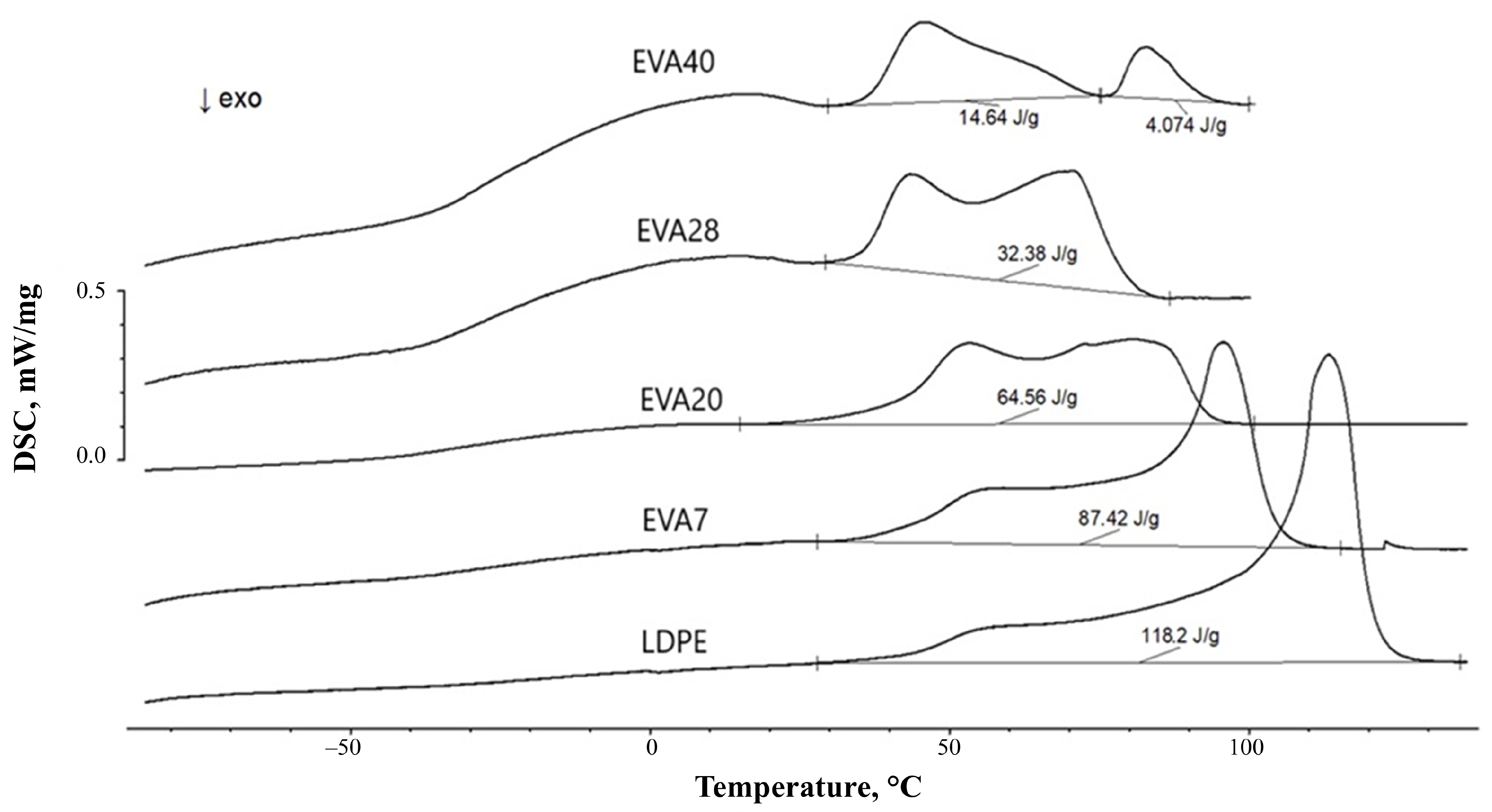
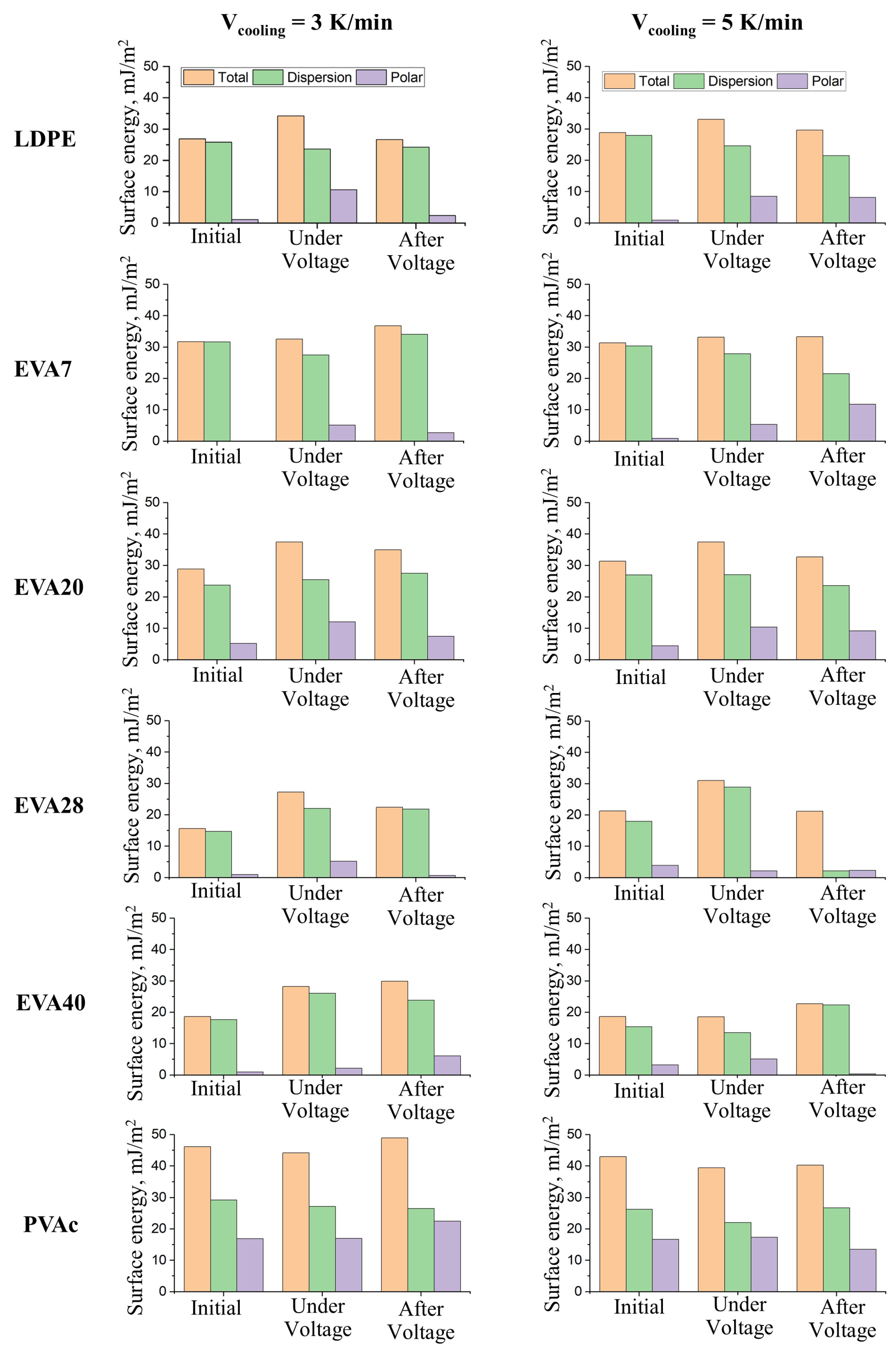
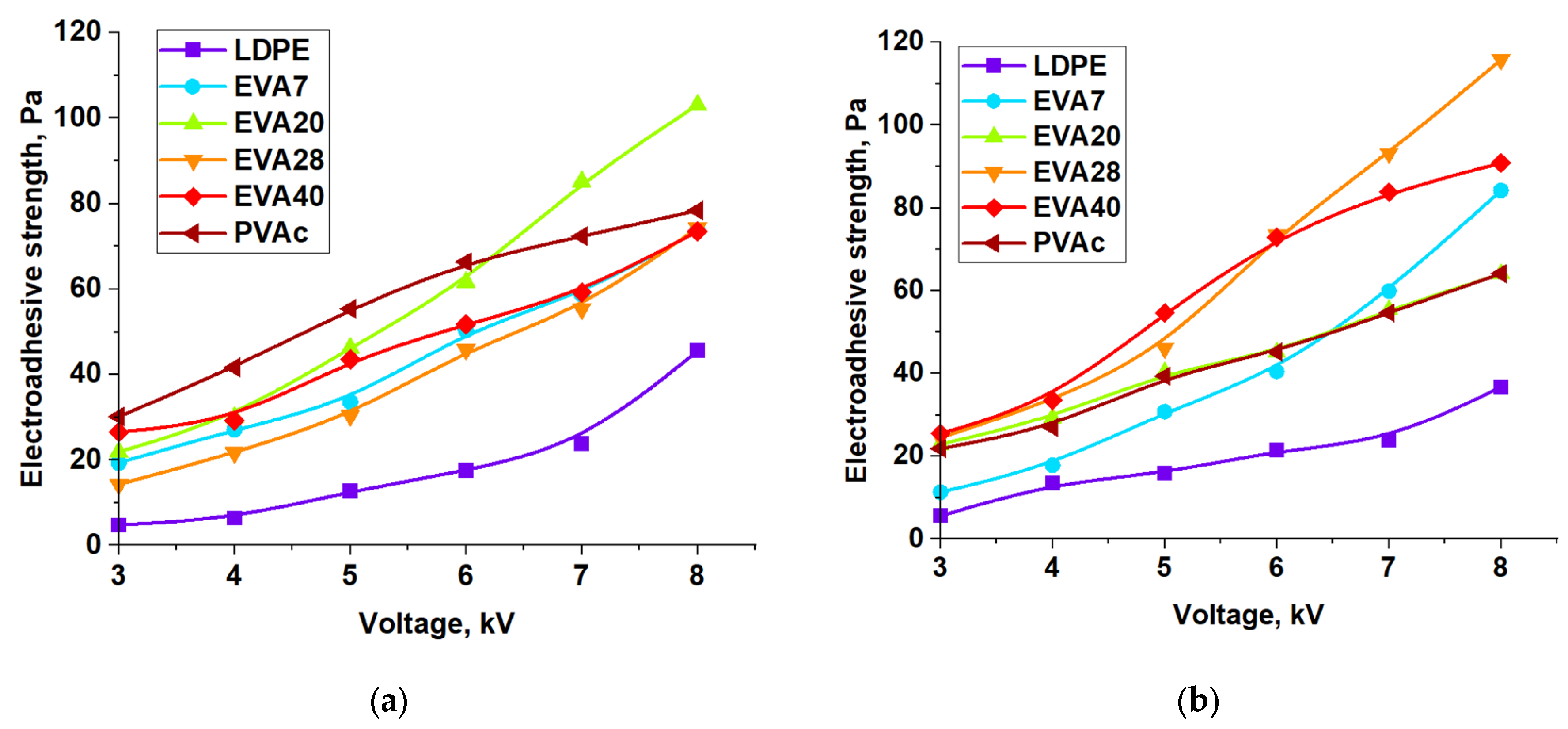
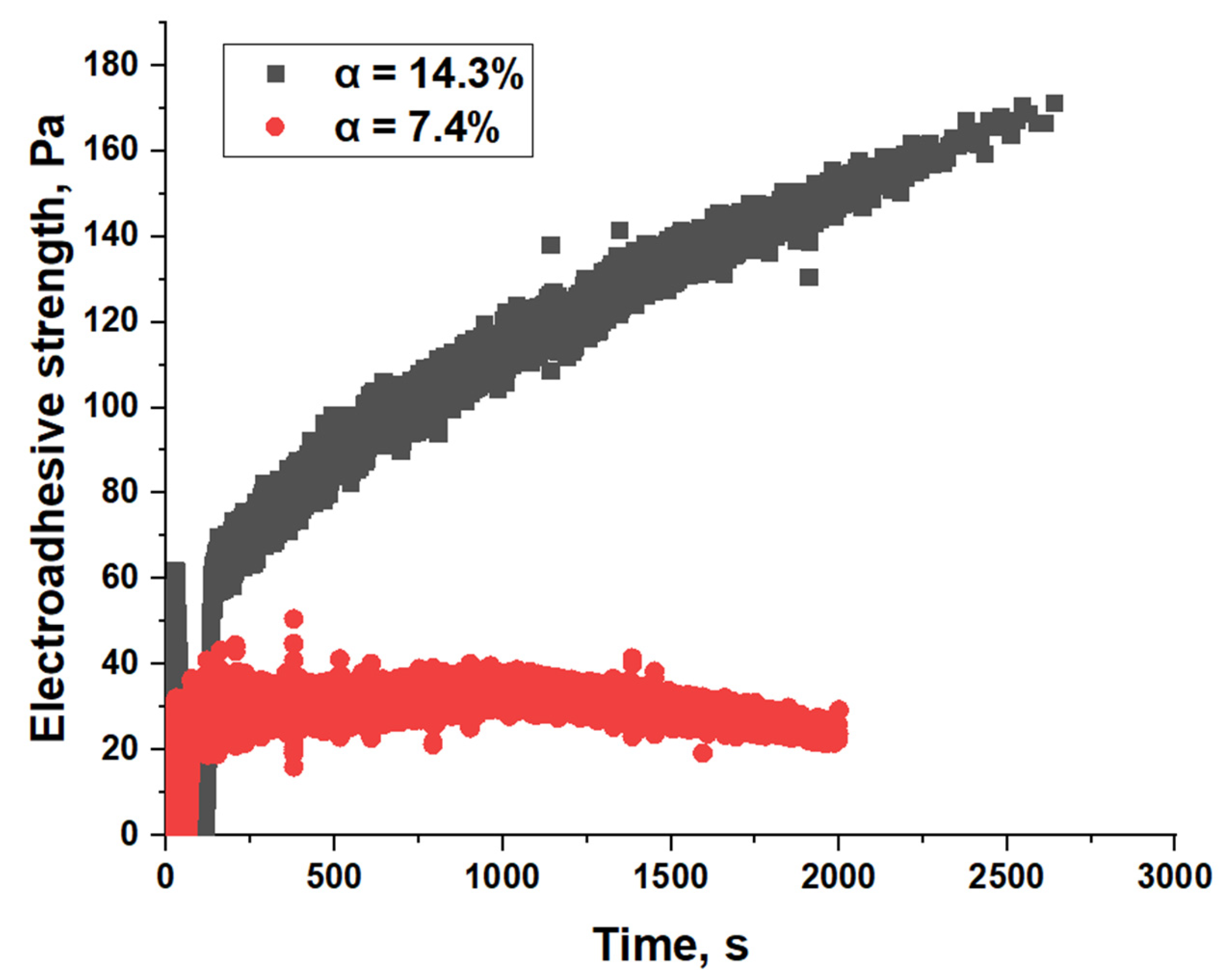
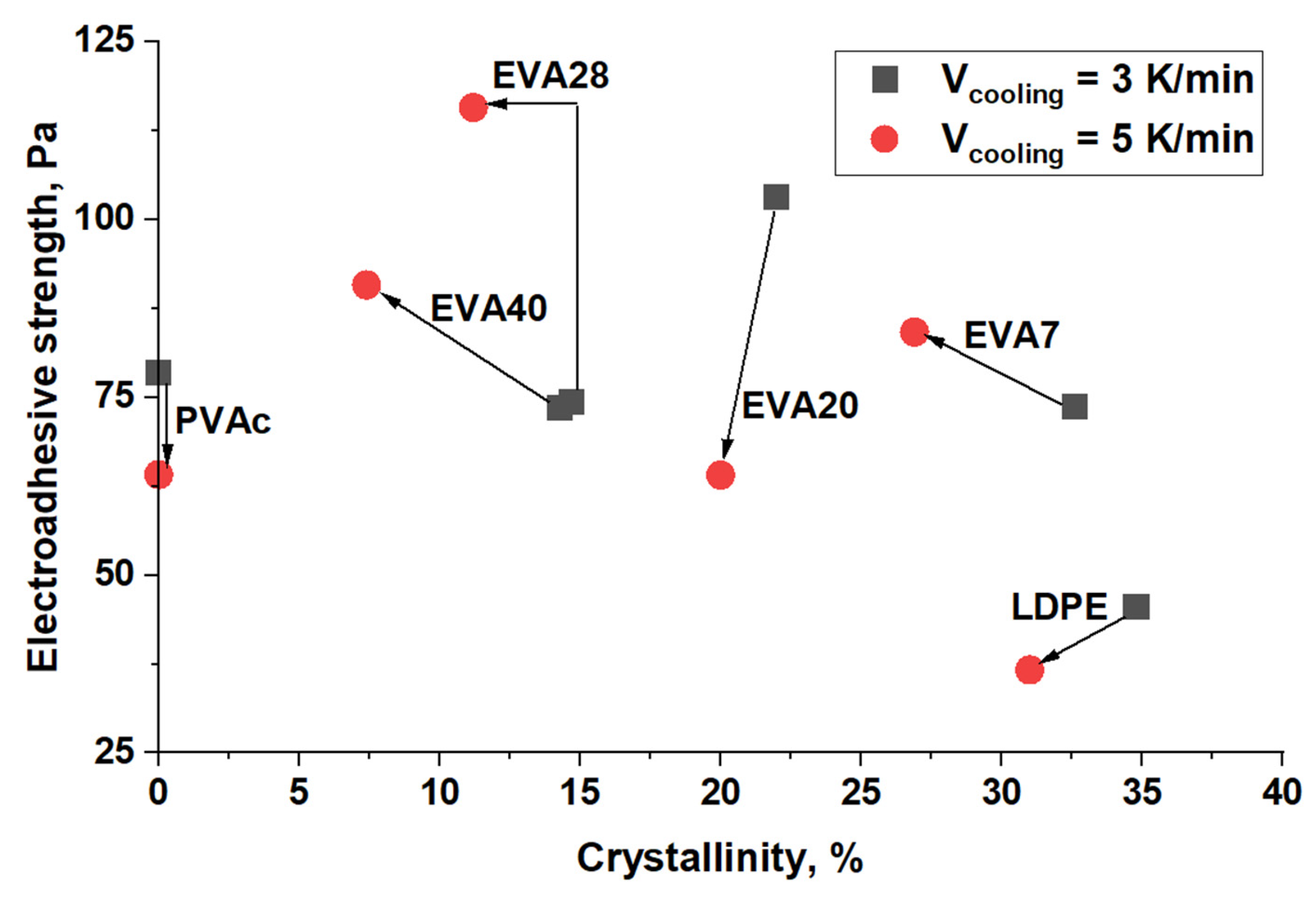
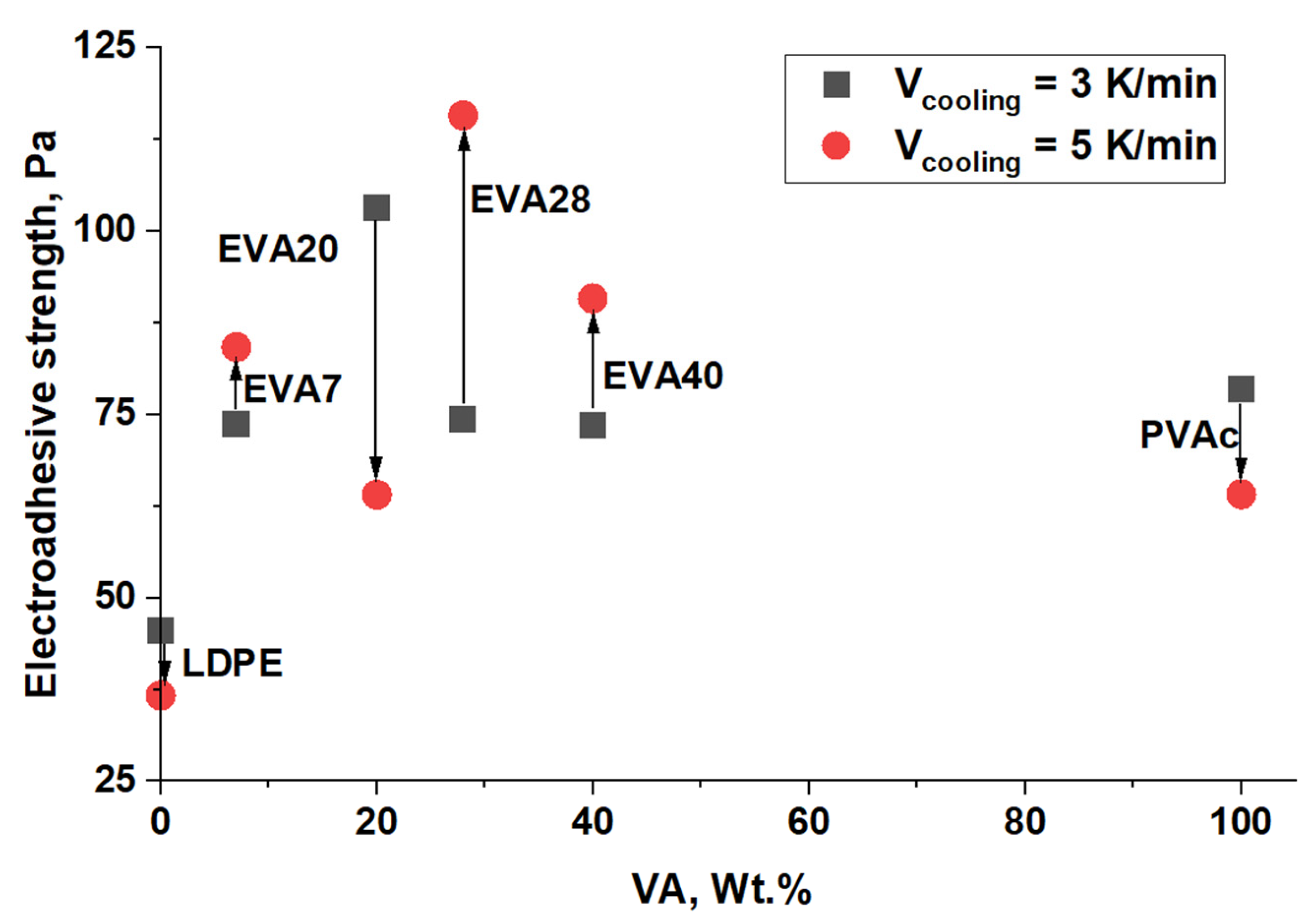
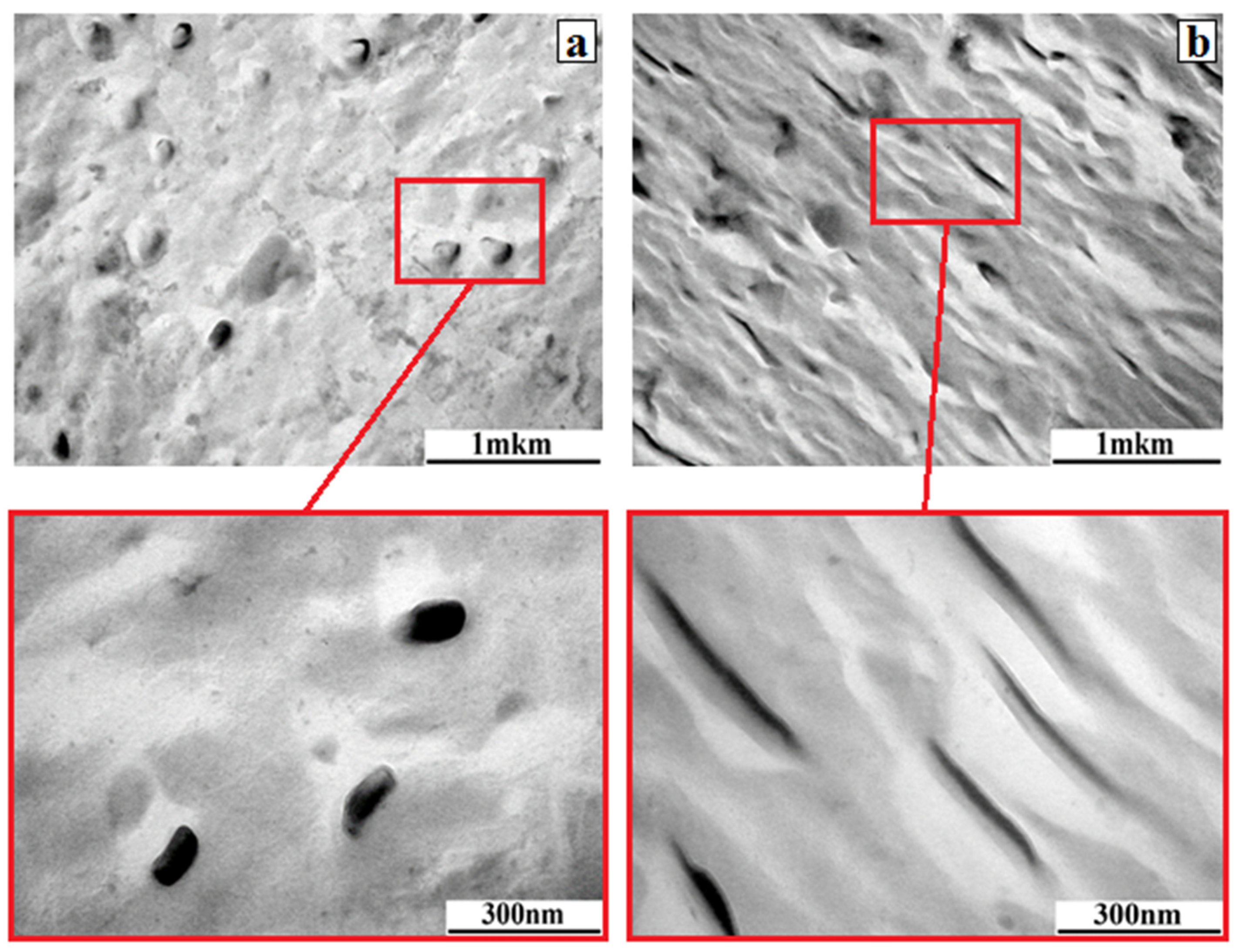
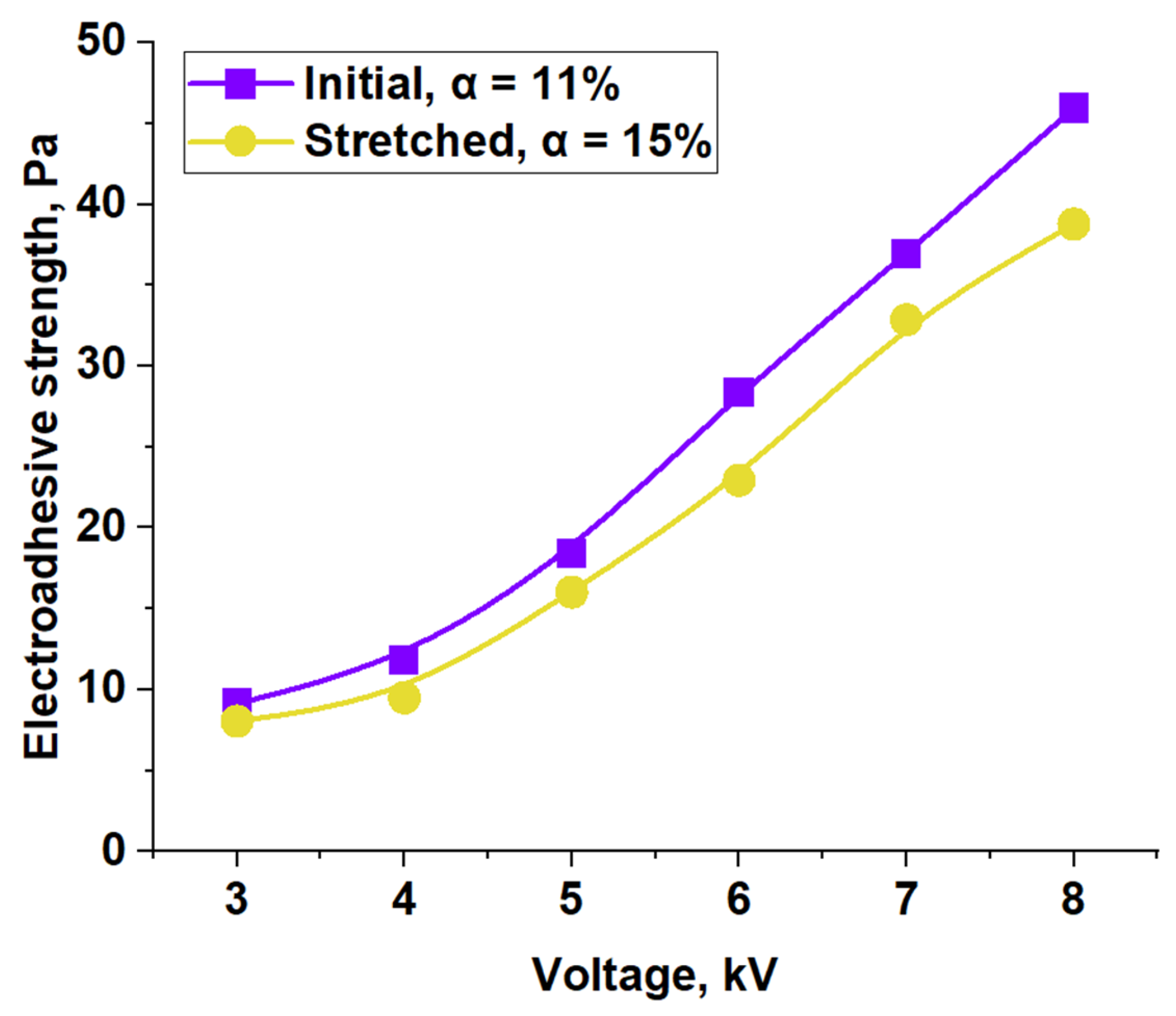
| Material | Pressing Temperature (TP), °C | Pressure (P), MPa | Pressing Time (tP), s | Cooling Rate After Pressing (VP), K/min | Annealing Temperature (TA), °C | Annealing Time (tA), min | Cooling Rate After Annealing (VA), K/min |
|---|---|---|---|---|---|---|---|
| LDPE | 160 | 10 | 5 | 3 | 150 | 60 | 5 |
| EVA7 | 120 | 10 | 5 | 3 | 110 | 60 | 5 |
| EVA20 | 110 | 10 | 5 | 3 | 100 | 60 | 5 |
| EVA28 | 95 | 10 | 5 | 3 | 85 | 60 | 5 |
| EVA40 | 85 | 10 | 5 | 3 | 75 | 60 | 5 |
| PVAc | 85 | 10 | 5 | 3 | 75 | 60 | 5 |
| Material | α, % (Vcooling = 3 K/min) | α, % (Vcooling = 5 K/min) |
|---|---|---|
| LDPE | 34 | 31 |
| EVA7 | 32.6 | 26.9 |
| EVA20 | 22 | 20 |
| EVA28 | 14.7 | 11.2 |
| EVA40 | 14.3 | 7.4 |
| PVAc | 0 | 0 |
| Material | ES, Pa (4 kV) | ES, Pa (8 kV) | Surface Energy, mJ/m2 (4 kV) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial State | Under Voltage | After Voltage | ||||||||||
| γtotal | γD | γP | γtotal | γD | γP | γtotal | γD | γP | ||||
| LDPE | Without annealing | 6.34 | 45.5 | 26.89 | 25.79 | 1.1 | 34.23 | 23.62 | 10.61 | 26.69 | 24.27 | 2.42 |
| Annealed | 13.51 | 36.69 | 28.81 | 27.92 | 0.88 | 33.08 | 24.57 | 8.51 | 29.69 | 21.53 | 8.16 | |
| EVA7 | Without annealing | 27.11 | 73.6 | 31.69 | 31.65 | 0.04 | 32.55 | 27.45 | 5.1 | 36.78 | 34.06 | 2.72 |
| Annealed | 17.81 | 84.21 | 31.29 | 30.39 | 0.89 | 33.17 | 27.86 | 5.31 | 33.32 | 21.53 | 11.79 | |
| EVA20 | Without annealing | 30.01 | 103.1 | 28.83 | 23.67 | 5.15 | 37.48 | 25.47 | 12.01 | 34.96 | 27.49 | 7.46 |
| Annealed | 29.27 | 64.08 | 31.35 | 26.93 | 4.42 | 37.43 | 27.03 | 10.4 | 32.67 | 23.52 | 9.15 | |
| EVA28 | Without annealing | 30.01 | 74.34 | 15.6 | 14.68 | 0.92 | 27.2 | 22.05 | 5.19 | 22.43 | 21.77 | 0.66 |
| Annealed | 33.33 | 115.8 | 21.86 | 17.94 | 3.92 | 31.04 | 28.93 | 2.11 | 21.17 | 18.85 | 2.32 | |
| EVA40 | Without annealing | 29.25 | 73.46 | 18.66 | 17.69 | 0.97 | 28.23 | 26.06 | 2.17 | 29.91 | 23.83 | 6.08 |
| Annealed | 33.52 | 90.81 | 18.63 | 15.41 | 3.21 | 18.6 | 13.46 | 5.14 | 22.69 | 22.33 | 0.36 | |
| PVAc | Without annealing | 41.66 | 78.39 | 46.15 | 29.24 | 16.91 | 44.13 | 27.14 | 16.99 | 48.96 | 26.49 | 22.47 |
| Annealed | 26.96 | 64.14 | 42.94 | 26.24 | 16.69 | 39.43 | 22.04 | 17.39 | 40.22 | 26.75 | 13.47 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharov, K.I.; Stepanenko, V.Y.; Khasbiullin, R.R.; Matveev, V.V.; Nikulova, U.V.; Shapagin, A.V. Influence of Surface Energy and Phase Composition on Electroadhesive Interactions. Polymers 2025, 17, 2739. https://doi.org/10.3390/polym17202739
Sharov KI, Stepanenko VY, Khasbiullin RR, Matveev VV, Nikulova UV, Shapagin AV. Influence of Surface Energy and Phase Composition on Electroadhesive Interactions. Polymers. 2025; 17(20):2739. https://doi.org/10.3390/polym17202739
Chicago/Turabian StyleSharov, Konstantin I., Valentina Yu. Stepanenko, Ramil R. Khasbiullin, Vladimir V. Matveev, Uliana V. Nikulova, and Aleksey V. Shapagin. 2025. "Influence of Surface Energy and Phase Composition on Electroadhesive Interactions" Polymers 17, no. 20: 2739. https://doi.org/10.3390/polym17202739
APA StyleSharov, K. I., Stepanenko, V. Y., Khasbiullin, R. R., Matveev, V. V., Nikulova, U. V., & Shapagin, A. V. (2025). Influence of Surface Energy and Phase Composition on Electroadhesive Interactions. Polymers, 17(20), 2739. https://doi.org/10.3390/polym17202739








