Biocompatible Interpenetrating Network Hydrogels with Dually Cross-Linked Polyol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
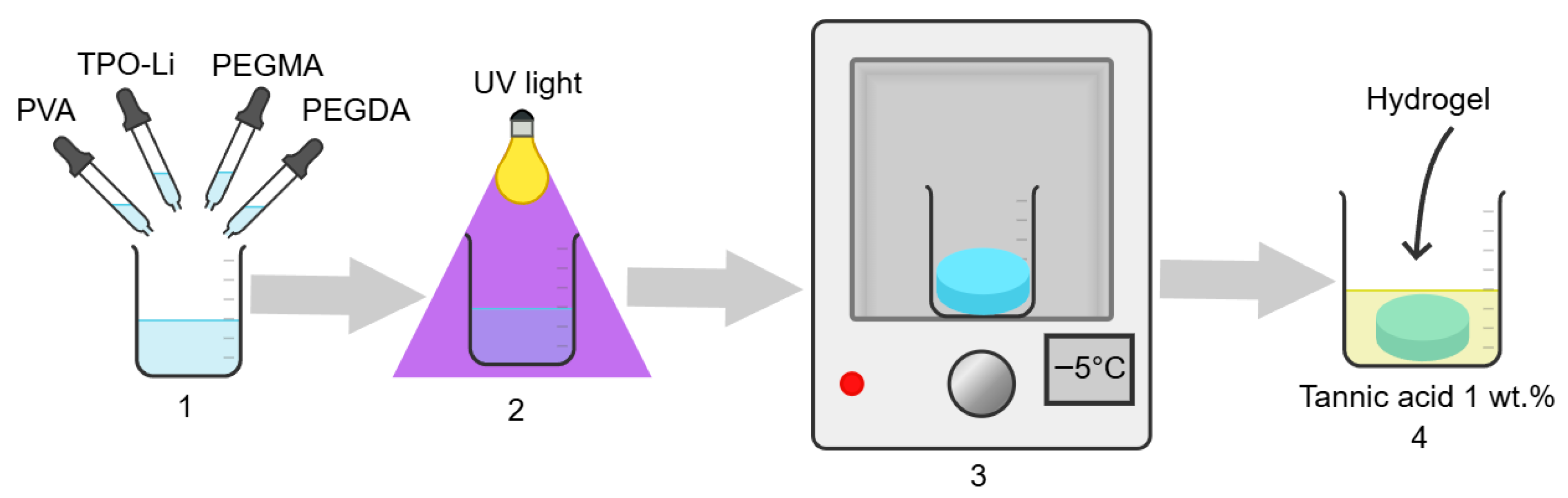
2.2. Sample Preparation
2.3. Rheology
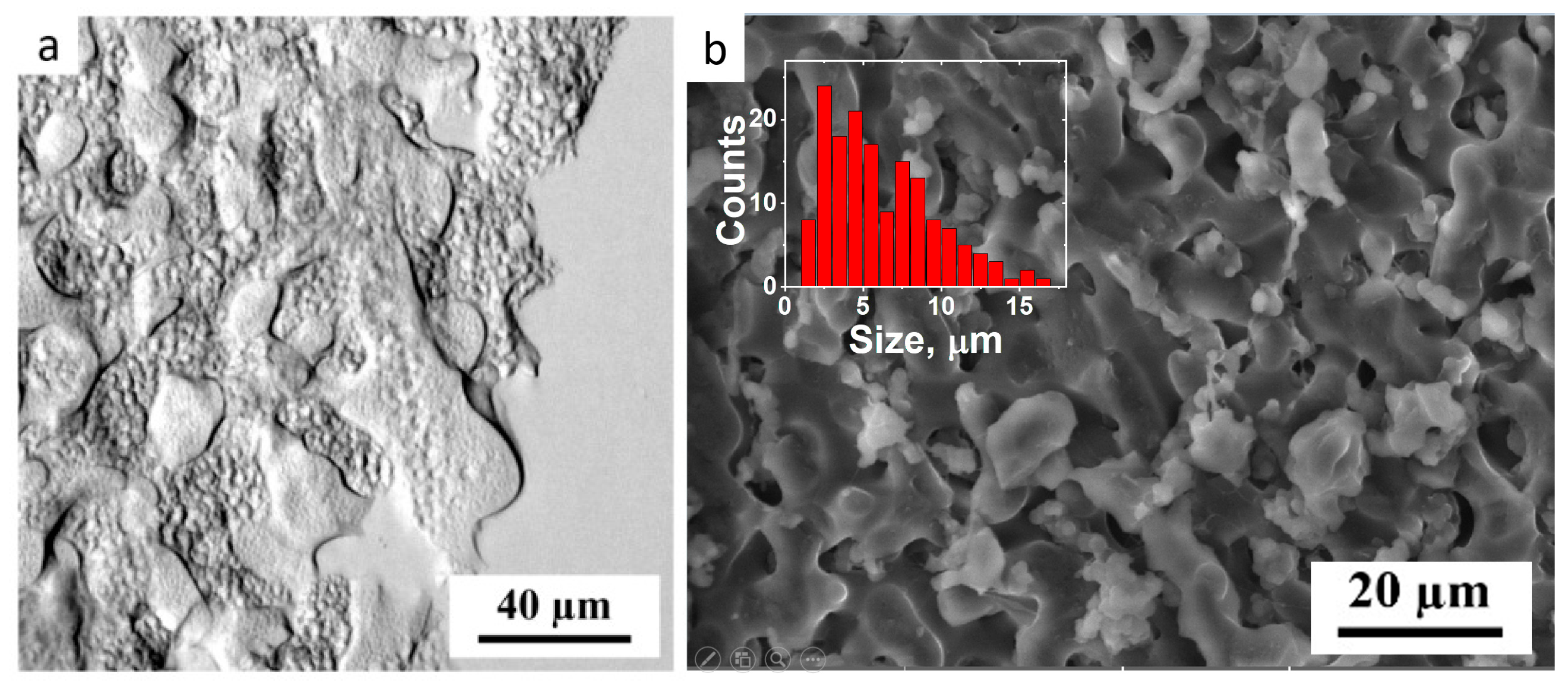
2.4. Light Microscopy
2.5. Cryogenic Scanning Electron Microscopy
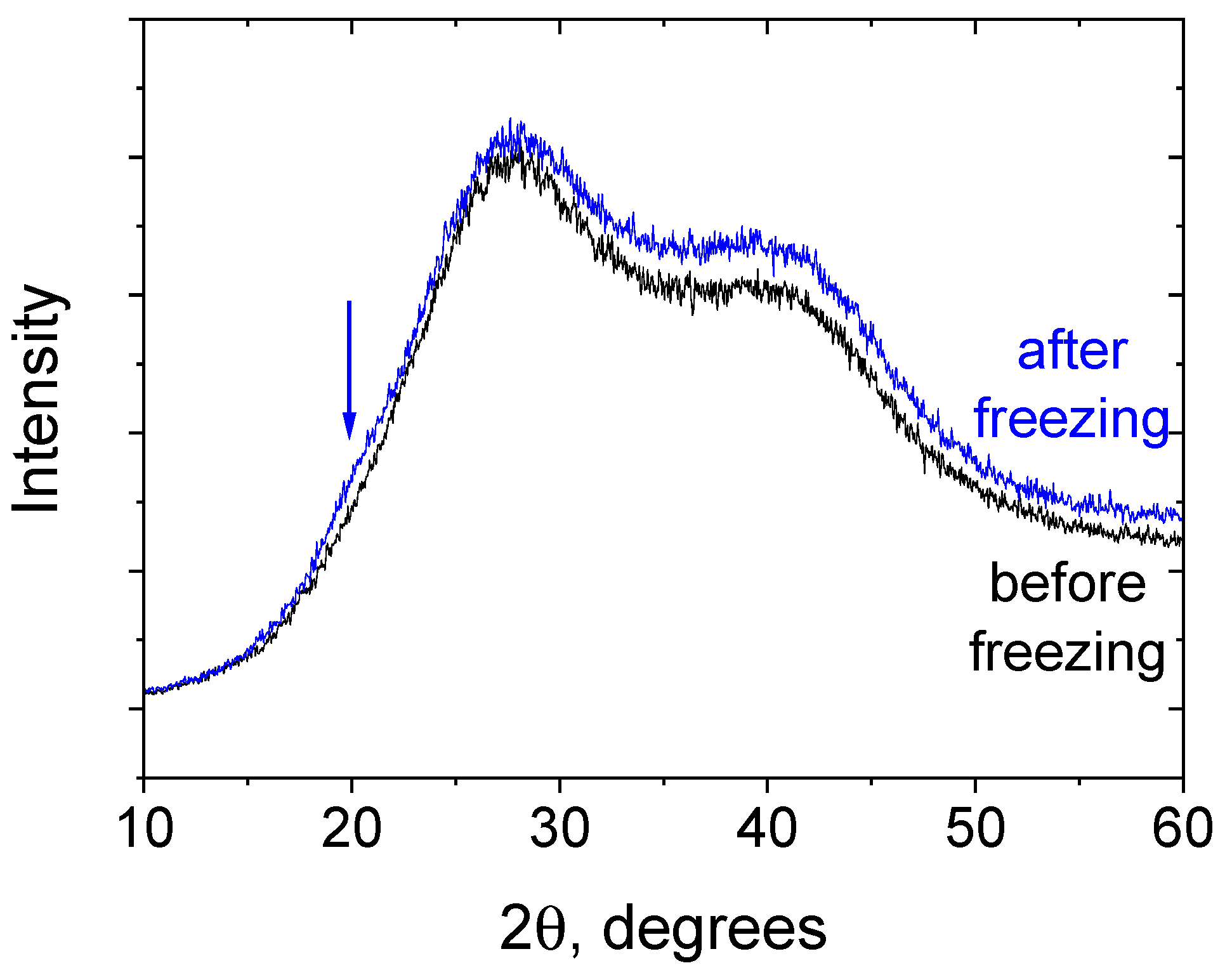
2.6. X-Ray Diffraction
2.7. UV-VIS Spectroscopy
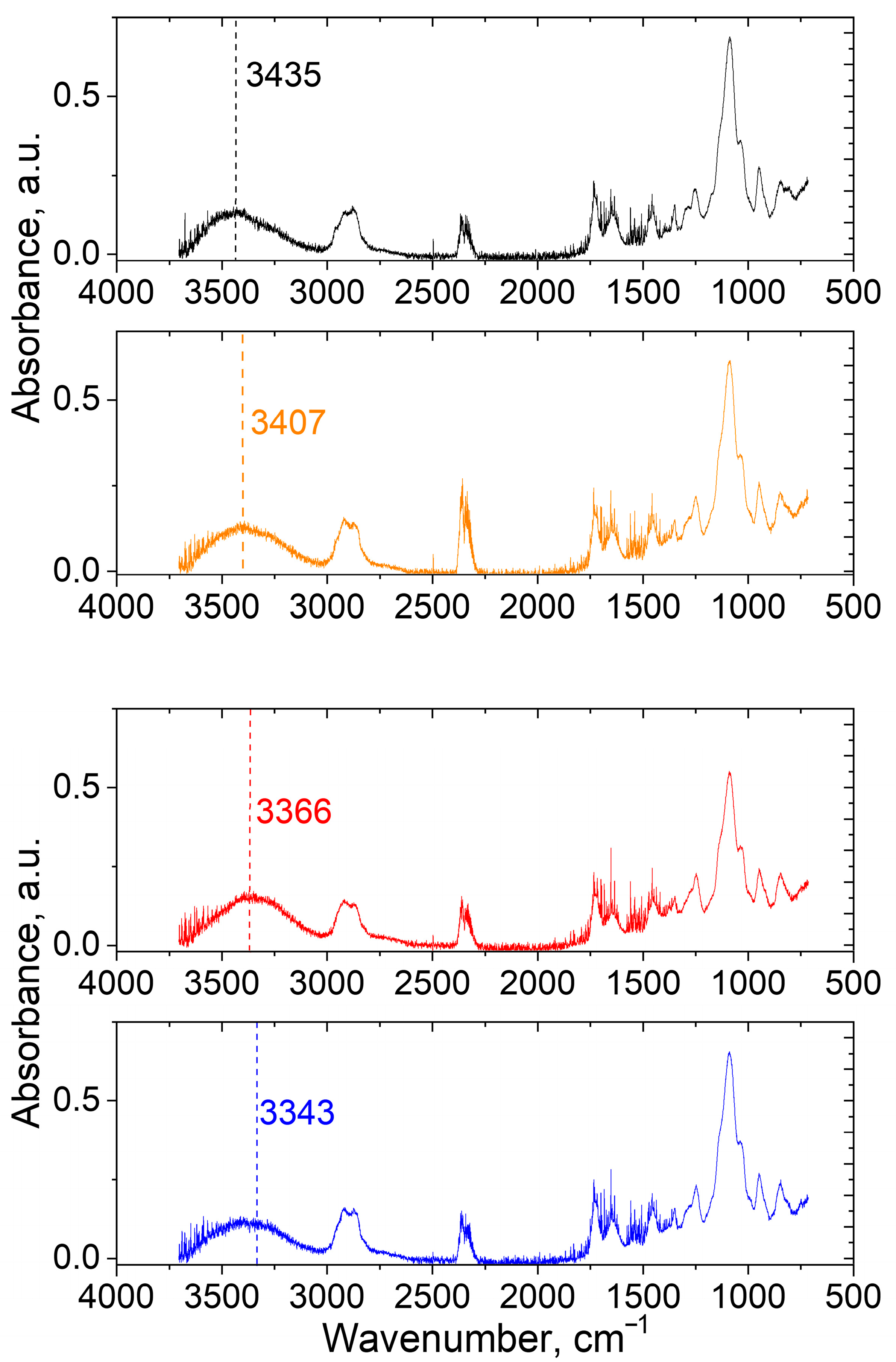
2.8. Fourier-Tranform Infrared Spectroscopy
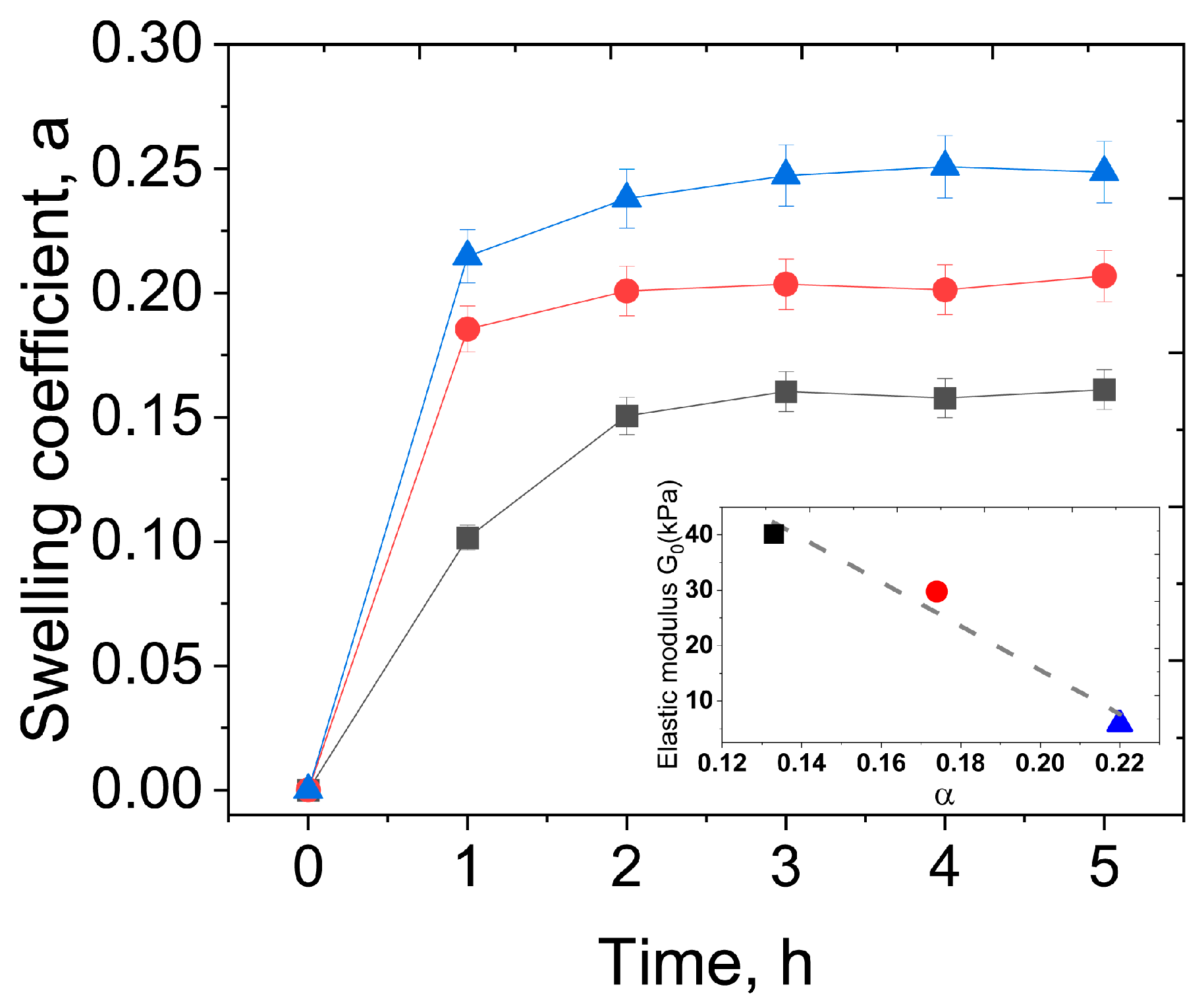
2.9. Swelling
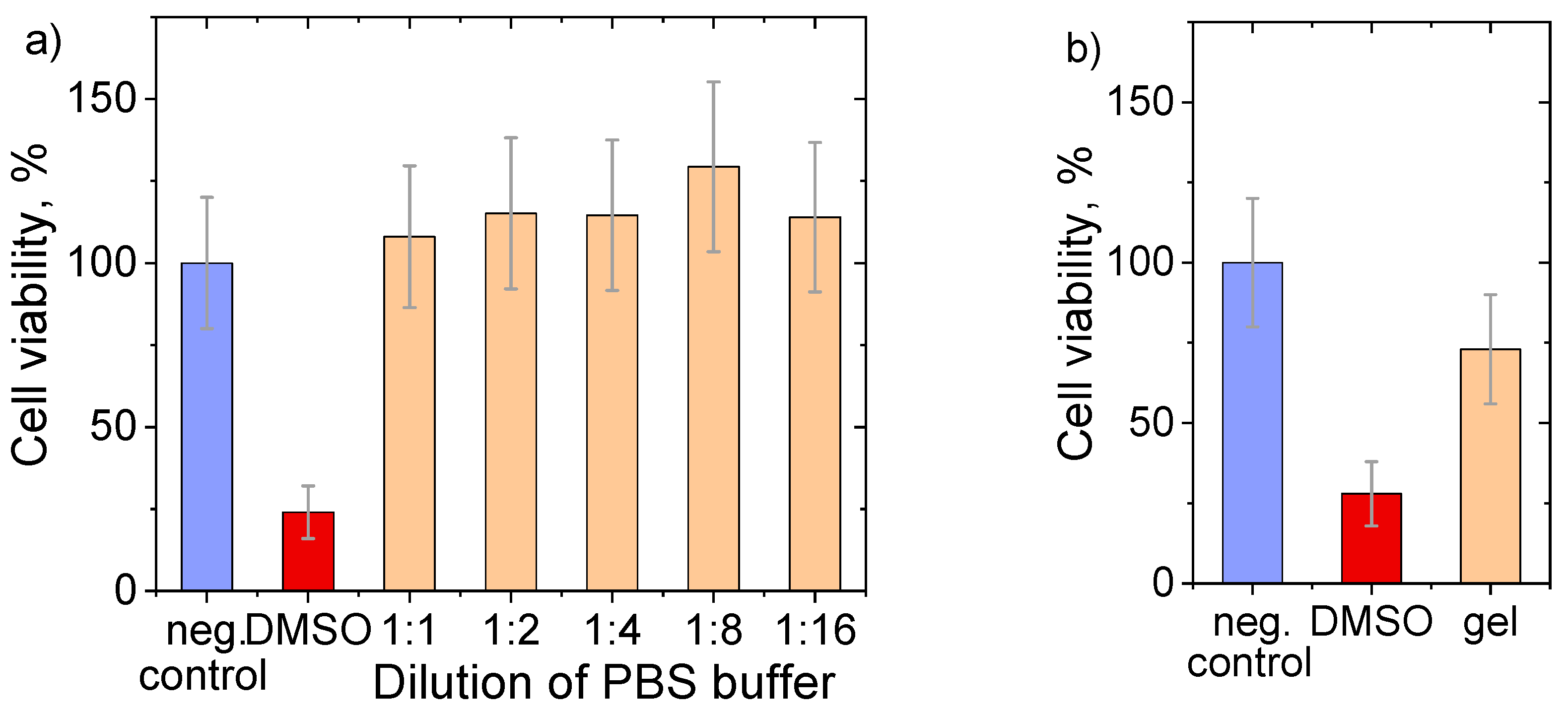
2.10. Biocompatibility Analysis
2.10.1. Hydrogel Stock Buffers
2.10.2. Hydrogels in Direct Contact with Cells
3. Results and Discussion
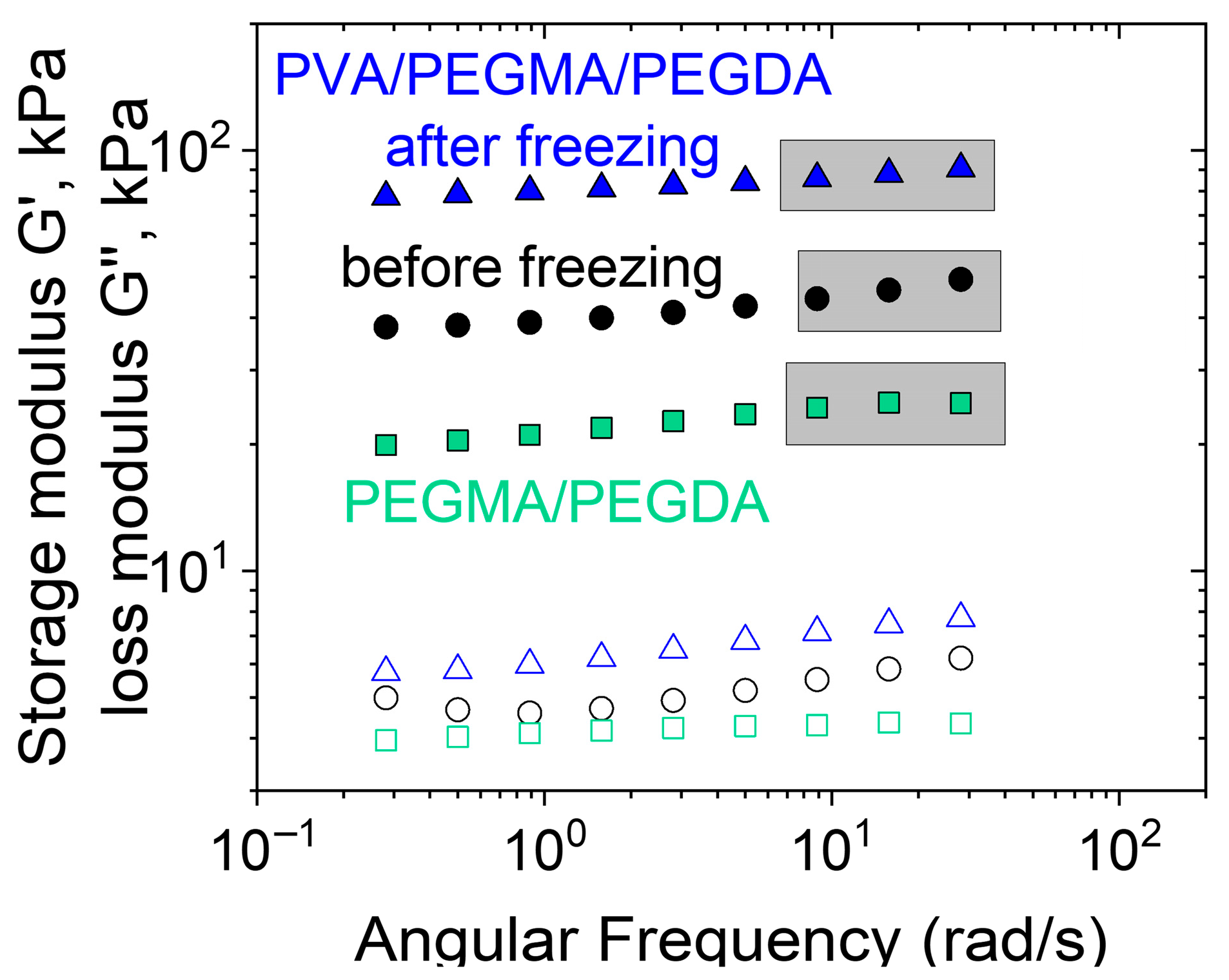
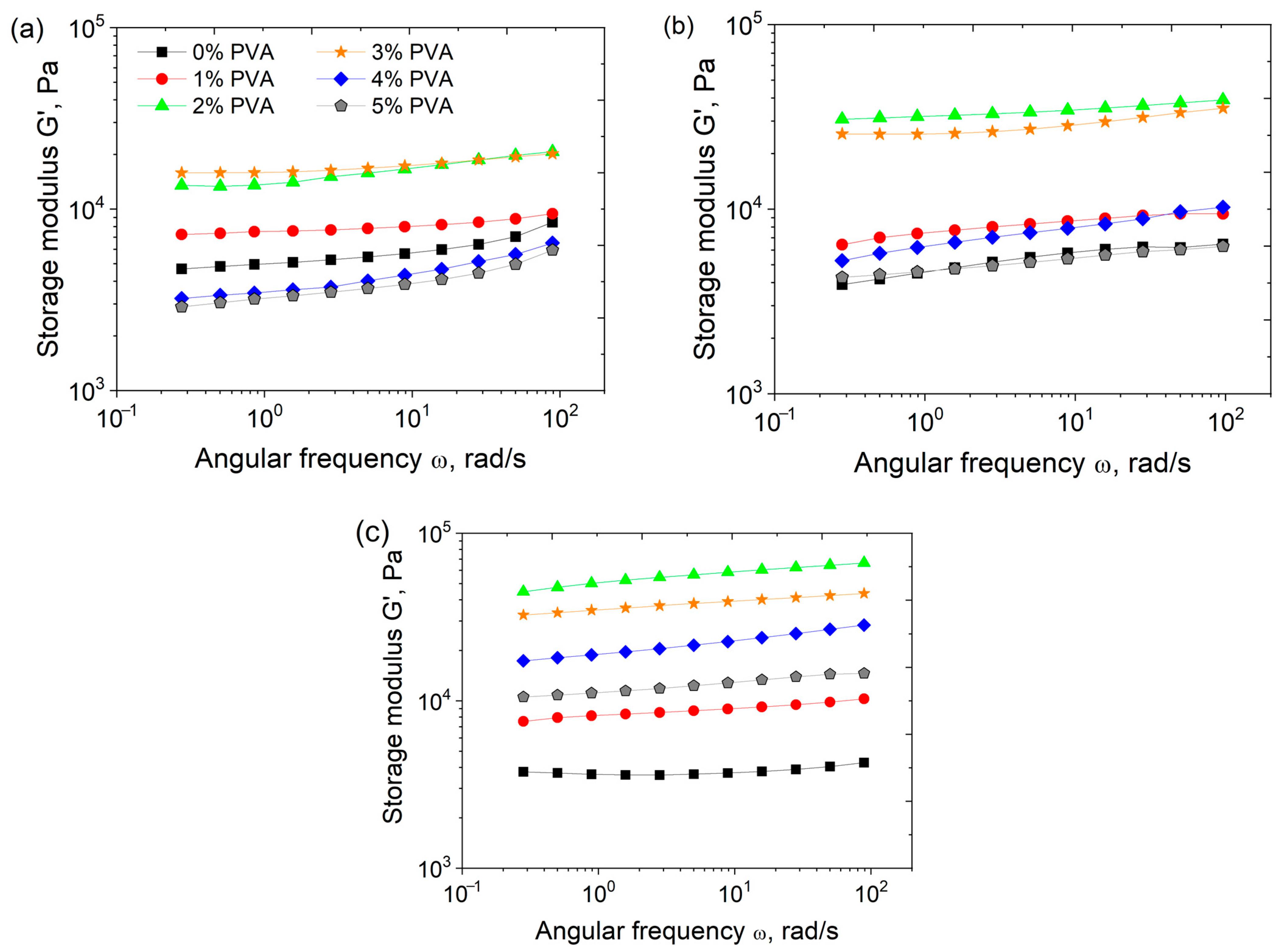
3.1. Rheological Properties and Microstructure
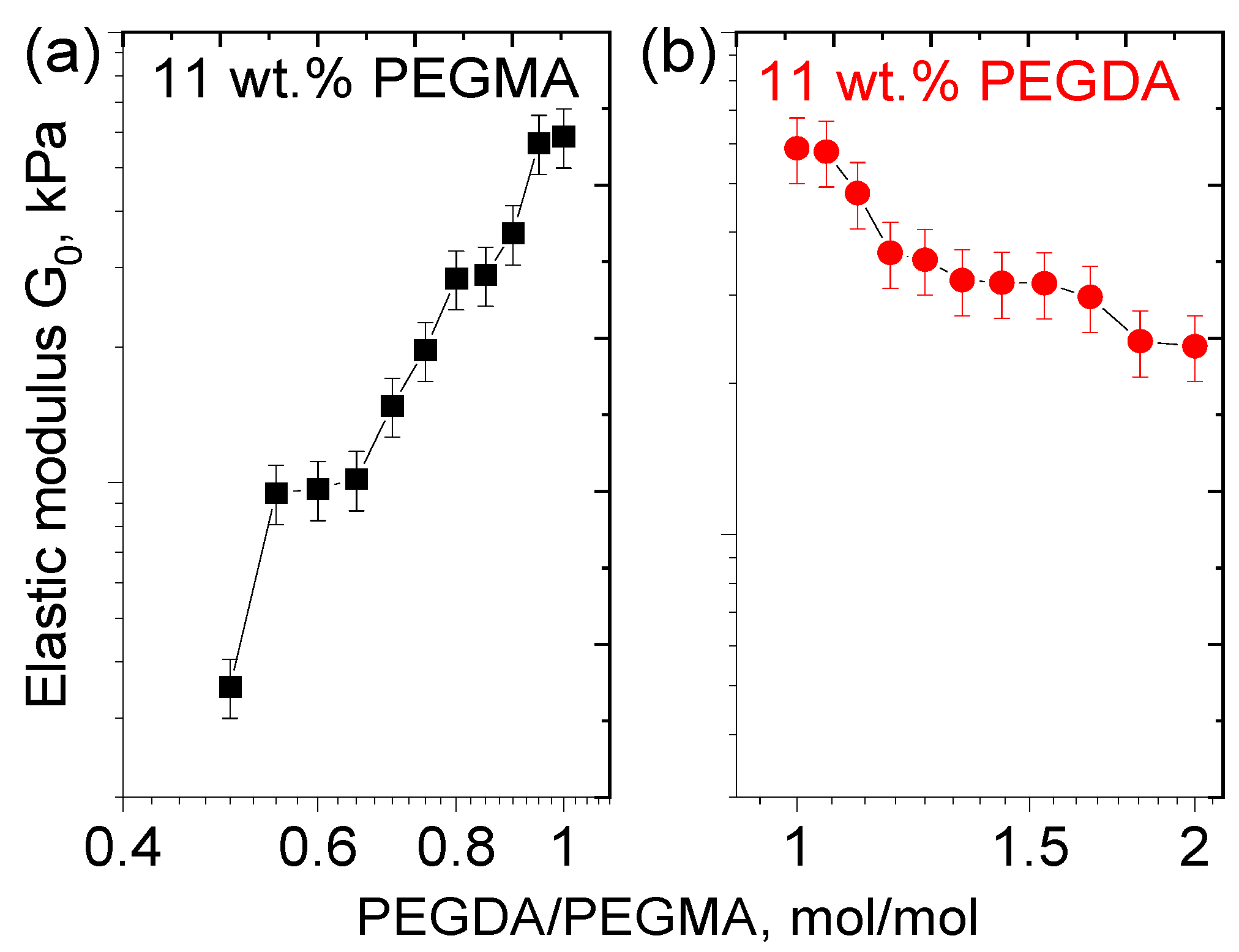
3.2. Effect of PEGMA and PEGDA Concentrations
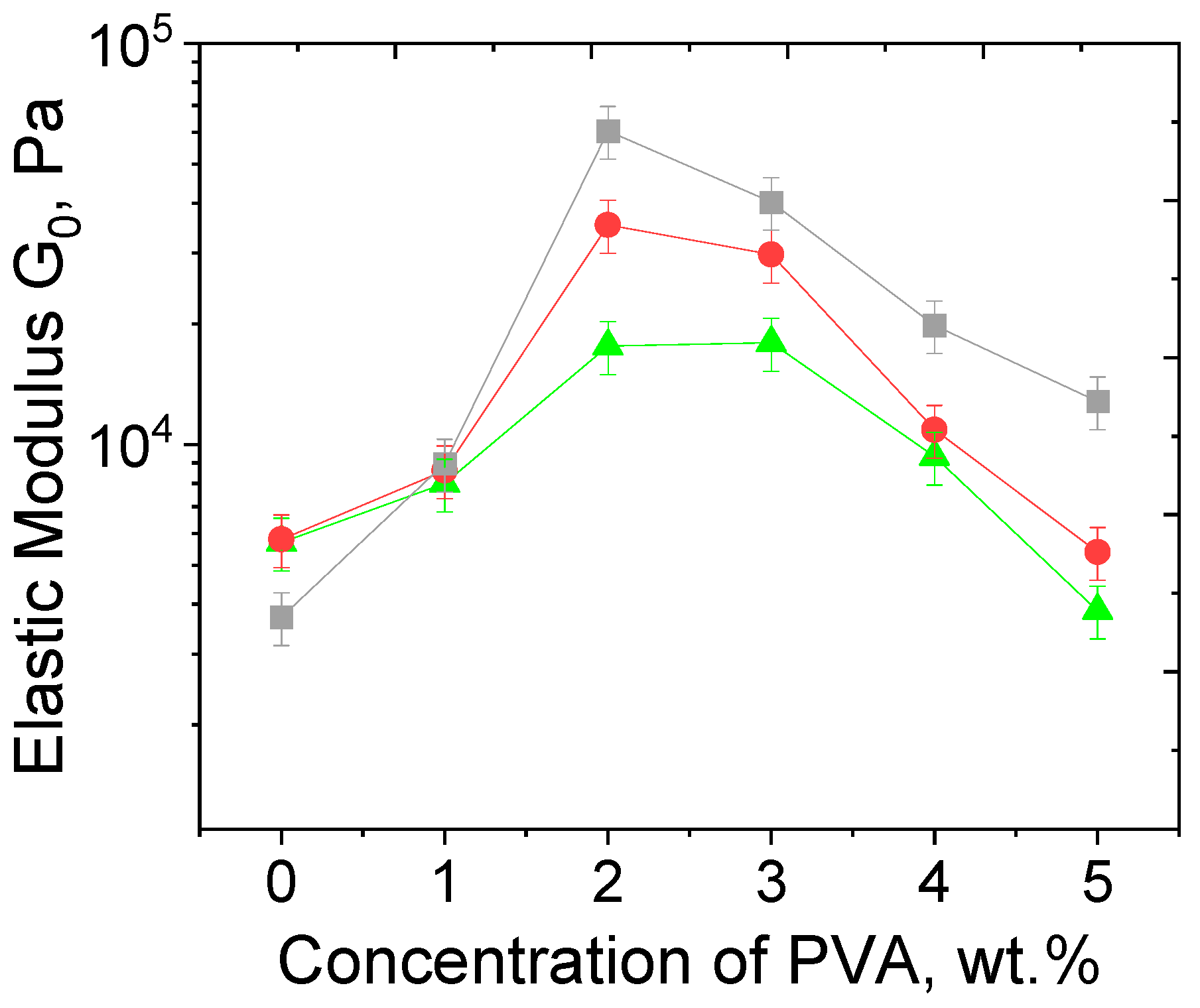
3.3. Effect of PVA Concentration and TA Addition
3.4. Biocompatibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PEGMA | poly(ethylene glycol) methacrylate |
| PEGDA | poly(ethylene glycol) diacrylate |
| PVA | poly(vinyl alcohol) |
| TA | tannic acid |
| TPO-Li | lithium 2,4,6-trimethylbenzoyl phosphinate |
| LM | light microscopy |
| cryo-VSEM | cryogenic low-vacuum scanning electron microscopy |
| cryo-SEM | cryogenic scanning electron microscopy |
| FTIR | Fourier-transform infrared spectroscopy |
References
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of Natural Hydrogels for Regenerative Medicine Applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Lu, F.; Chang, Q. The Diversified Hydrogels for Biomedical Applications and Their Imperative Roles in Tissue Regeneration. Biomater. Sci. 2023, 11, 2639–2660. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Stojanović, G.M.; Bin Abdullah, M.F.; Dolatshahi-Pirouz, A.; Marei, H.E.; Ashammakhi, N.; Hasan, A. Fundamental Properties of Smart Hydrogels for Tissue Engineering Applications: A Review. Int. J. Biol. Macromol. 2024, 254, 127882. [Google Scholar] [CrossRef]
- Li, Y.; Feng, G.; Liu, J.; Yang, T.; Hou, R.; Liu, J.; Wang, X. Progress in Glucose-Sensitive Hydrogels for Biomedical Applications. Macromol. Chem. Phys. 2023, 224, 2300257. [Google Scholar] [CrossRef]
- Hama, R.; Ulziibayar, A.; Reinhardt, J.W.; Watanabe, T.; Kelly, J.; Shinoka, T. Recent Developments in Biopolymer-Based Hydrogels for Tissue Engineering Applications. Biomolecules 2023, 13, 280. [Google Scholar] [CrossRef] [PubMed]
- Serafin, A.; Culebras, M.; Collins, M.N. Synthesis and Evaluation of Alginate, Gelatin, and Hyaluronic Acid Hybrid Hydrogels for Tissue Engineering Applications. Int. J. Biol. Macromol. 2023, 233, 123438. [Google Scholar] [CrossRef] [PubMed]
- Kirschning, A.; Dibbert, N.; Dräger, G. Chemical Functionalization of Polysaccharides—Towards Biocompatible Hydrogels for Biomedical Applications. Chem. A Eur. J. 2018, 24, 1231–1240. [Google Scholar] [CrossRef]
- Li, C.; Deng, R.; Yang, M.; Yuan, F.; Zhang, C.; Yu, J. Advanced Hydrogel Material for Meniscus Repair. Adv. Funct. Mater. 2024, 34, 2312276. [Google Scholar] [CrossRef]
- Eslahi, N.; Abdorahim, M.; Simchi, A. Smart Polymeric Hydrogels for Cartilage Tissue Engineering: A Review on the Chemistry and Biological Functions. Biomacromolecules 2016, 17, 3441–3463. [Google Scholar] [CrossRef]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic Hydrogels: Synthesis, Novel Trends, and Applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Y.; Zhao, S.; Li, D.; Tang, X.; Zhang, H.; Huang, J.; Guo, Z.; Liu, W. Mechanical Regulation of Polymer Gels. Chem. Rev. 2024, 124, 10435–10508. [Google Scholar] [CrossRef]
- Crosby, C.O.; Stern, B.; Kalkunte, N.; Pedahzur, S.; Ramesh, S.; Zoldan, J. Interpenetrating Polymer Network Hydrogels as Bioactive Scaffolds for Tissue Engineering. Rev. Chem. Eng. 2022, 38, 347–361. [Google Scholar] [CrossRef]
- Nonoyama, T.; Gong, J.P. Tough Double Network Hydrogel and Its Biomedical Applications. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 393–410. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Philippova, O.E. New Approaches to the Design of Double Polymer Networks: A Review. Polym. Sci. Ser. C 2022, 64, 26–39. [Google Scholar] [CrossRef]
- Panteli, P.A.; Patrickios, C.S. Multiply Interpenetrating Polymer Networks: Preparation, Mechanical Properties, and Applications. Gels 2019, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Wanasinghe, S.V.; De Alwis Watuthanthrige, N.; Konkolewicz, D. Interpenetrated Triple Network Polymers: Synergies of Three Different Dynamic Bonds. Polym. Chem. 2022, 13, 3705–3712. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and Applications of Interpenetrating Polymer Network Hydrogels. A Review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Nakajima, T.; Furukawa, H.; Tanaka, Y.; Kurokawa, T.; Osada, Y.; Gong, J.P. True Chemical Structure of Double Network Hydrogels. Macromolecules 2009, 42, 2184–2189. [Google Scholar] [CrossRef]
- Waters, D.J.; Engberg, K.; Parke-Houben, R.; Ta, C.N.; Jackson, A.J.; Toney, M.F.; Frank, C.W. Structure and Mechanism of Strength Enhancement in Interpenetrating Polymer Network Hydrogels. Macromolecules 2011, 44, 5776–5787. [Google Scholar] [CrossRef]
- Rodin, M.; Li, J.; Kuckling, D. Dually Cross-Linked Single Networks: Structures and Applications. Chem. Soc. Rev. 2021, 50, 8147–8177. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xiang, D.; Luo, Z.; Feng, L.; Li, J.; Lin, Y.; Luo, Z.; Li, M.; Xie, X.; Xiang, B. Lignin-Based Hyper-Cross-Linked Resin as an Adsorbent for Aniline from Aqueous Solution. Int. J. Biol. Macromol. 2025, 289, 138892. [Google Scholar] [CrossRef]
- Zhong, M.; Liu, Y.-T.; Liu, X.-Y.; Shi, F.-K.; Zhang, L.-Q.; Zhu, M.-F.; Xie, X.-M. Dually Cross-Linked Single Network Poly(Acrylic Acid) Hydrogels with Superior Mechanical Properties and Water Absorbency. Soft Matter 2016, 12, 5420–5428. [Google Scholar] [CrossRef]
- Stillman, Z.; Jarai, B.M.; Raman, N.; Patel, P.; Fromen, C.A. Degradation Profiles of Poly(Ethylene Glycol)Diacrylate (PEGDA)-Based Hydrogel Nanoparticles. Polym. Chem. 2020, 11, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Mayumi, K.; Ducouret, G.; Hébraud, P. Viscoelastic Properties of Poly(Vinyl Alcohol) Hydrogels Having Permanent and Transient Cross-Links Studied by Microrheology, Classical Rheometry, and Dynamic Light Scattering. Macromolecules 2013, 46, 4174–4183. [Google Scholar] [CrossRef]
- Mayumi, K.; Marcellan, A.; Ducouret, G.; Creton, C.; Narita, T. Stress–Strain Relationship of Highly Stretchable Dual Cross-Link Gels: Separability of Strain and Time Effect. ACS Macro Lett. 2013, 2, 1065–1068. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and Properties of Poly(Vinyl Alcohol) Hydrogels with High Strength and Toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, H.; Zhao, Y. Poly(Vinyl Alcohol) Hydrogel Can Autonomously Self-Heal. ACS Macro Lett. 2012, 1, 1233–1236. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Jiao, C.; Zhao, Y.; Zhang, J.; Wang, H. Self-Assembled Polyvinyl Alcohol–Tannic Acid Hydrogels with Diverse Microstructures and Good Mechanical Properties. ACS Omega 2018, 3, 11788–11795. [Google Scholar] [CrossRef]
- Lee, D.; Hwang, H.; Kim, J.-S.; Park, J.; Youn, D.; Kim, D.; Hahn, J.; Seo, M.; Lee, H. VATA: A Poly(Vinyl Alcohol)- and Tannic Acid-Based Nontoxic Underwater Adhesive. ACS Appl. Mater. Interfaces 2020, 12, 20933–20941. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Huang, M.; Sun, X.; Wei, N.; Shi, H.; Li, H.; Lin, M.; Sun, J. Super-Strong, Nonswellable, and Biocompatible Hydrogels Inspired by Human Tendons. ACS Appl. Mater. Interfaces 2022, 14, 2638–2649. [Google Scholar] [CrossRef]
- Pacelli, S.; Rampetsreiter, K.; Modaresi, S.; Subham, S.; Chakravarti, A.R.; Lohfeld, S.; Detamore, M.S.; Paul, A. Fabrication of a Double-Cross-Linked Interpenetrating Polymeric Network (IPN) Hydrogel Surface Modified with Polydopamine to Modulate the Osteogenic Differentiation of Adipose-Derived Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 24955–24962. [Google Scholar] [CrossRef]
- Pal, A.; Nayak, J.; Roy, B.; Maiti, S.; Nath Chowdhury, S.; Das, P.; Katheria, A.; Ray, S.K.; Chattopadhyay, S.; Das, N.C. Dual Crosslinked Interpenetrating Polymer Network-Based Porous Hydrogel Membrane for Solid-State Supercapacitor Applications. Polymer 2024, 308, 127408. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, W.; Zhang, Q. Constructing Phase Separation in Polymer Gels: Strategies, Functions and Applications. Prog. Polym. Sci. 2024, 154, 101847. [Google Scholar] [CrossRef]
- Burke, G.; Barron, V.; Geever, T.; Geever, L.; Devine, D.M.; Higginbotham, C.L. Evaluation of the Materials Properties, Stability and Cell Response of a Range of PEGDMA Hydrogels for Tissue Engineering Applications. J. Mech. Behav. Biomed. Mater. 2019, 99, 1–10. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-Based Hydrogels for Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Kuklin, A.I.; Torocheshnikov, V.N.; Orekhov, A.S.; Roland, S.; Miquelard-Garnier, G.; Matsarskaia, O.; Iliopoulos, I.; Philippova, O.E. Double Dynamic Hydrogels Formed by Wormlike Surfactant Micelles and Cross-Linked Polymer. J. Colloid. Interface Sci. 2022, 611, 46–60. [Google Scholar] [CrossRef]
- Strachota, B.; Strachota, A.; Horodecka, S.; Šlouf, M.; Dybal, J. Monolithic Nanocomposite Hydrogels with Fast Dual T- and PH- Stimuli Responsiveness Combined with High Mechanical Properties. J. Mater. Res. Technol. 2021, 15, 6079–6097. [Google Scholar] [CrossRef]
- Kaberova, Z.; Karpushkin, E.; Nevoralová, M.; Vetrík, M.; Šlouf, M.; Dušková-Smrčková, M. Microscopic Structure of Swollen Hydrogels by Scanning Electron and Light Microscopies: Artifacts and Reality. Polymers 2020, 12, 578. [Google Scholar] [CrossRef]
- Blinova, E.; Korel, A.; Zemlyakova, E.; Pestov, A.; Samokhin, A.; Zelikman, M.; Tkachenko, V.; Bets, V.; Arzhanova, E.; Litvinova, E. Cytotoxicity and Degradation Resistance of Cryo- and Hydrogels Based on Carboxyethylchitosan at Different pH Values. Gels 2024, 10, 272. [Google Scholar] [CrossRef]
- Gursel, I.; Balcik, C.; Arica, Y.; Akkus, O.; Akkas, N.; Hasirci, V. Synthesis and Mechanical Properties of Interpenetrating Networks of Polyhydroxybutyrate-Co-Hydroxyvalerate and Polyhydroxyethyl Methacrylate. Biomaterials 1998, 19, 1137–1143. [Google Scholar] [CrossRef]
- Kozhunova, E.Y.; Rudyak, V.Y.; Li, X.; Shibayama, M.; Peters, G.S.; Vyshivannaya, O.V.; Nasimova, I.R.; Chertovich, A.V. Microphase Separation of Stimuli-Responsive Interpenetrating Network Microgels Investigated by Scattering Methods. J. Colloid. Interface Sci. 2021, 597, 297–305. [Google Scholar] [CrossRef]
- Lewandowska, K.; Staszewska, D.U.; Bohdanecký, M. The Huggins Viscosity Coefficient of Aqueous Solution of Poly(Vinyl Alcohol). Eur. Polym. J. 2001, 37, 25–32. [Google Scholar] [CrossRef]
- Ricciardi, R.; Auriemma, F.; De Rosa, C.; Lauprêtre, F. X-Ray Diffraction Analysis of Poly(Vinyl Alcohol) Hydrogels, Obtained by Freezing and Thawing Techniques. Macromolecules 2004, 37, 1921–1927. [Google Scholar] [CrossRef]
- Adelnia, H.; Ensandoost, R.; Shebbrin Moonshi, S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/Thawed Polyvinyl Alcohol Hydrogels: Present, Past and Future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Muravlev, D.A.; Muravleva, A.K.; Matveev, V.V.; Chalykh, A.E.; Philippova, O.E. pH-Dependent Gelation of a Stiff Anionic Polysaccharide in the Presence of Metal Ions. Polymers 2020, 12, 868. [Google Scholar] [CrossRef] [PubMed]
- Beamish, J.A.; Zhu, J.; Kottke-Marchant, K.; Marchant, R.E. The Effects of Monoacrylated Poly(Ethylene Glycol) on the Properties of Poly(Ethylene Glycol) Diacrylate Hydrogels Used for Tissue Engineering. J. Biomed. Mater. Res. A 2010, 92A, 441–450. [Google Scholar] [CrossRef]
- Hakim, K.M.; Zhang, R.; Wilson, S.; Goel, S.; Impey, S.A.; Aria, A.I. Additive Manufacturing and Physicomechanical Characteristics of PEGDA Hydrogels: Recent Advances and Perspective for Tissue Engineering. Polymers 2023, 15, 2341. [Google Scholar] [CrossRef]
- Shutava, T.; Prouty, M.; Kommireddy, D.; Lvov, Y. pH Responsive Decomposable Layer-by-Layer Nanofilms and Capsules on the Basis of Tannic Acid. Macromolecules 2005, 38, 2850–2858. [Google Scholar] [CrossRef]
- Larin, D.E.; Shibaev, A.V.; Liu, C.-Y.; Emelyanenko, A.V. The effect of dynamic cross-links and mesogenic groups on the swelling and collapse of polymer gels. Giant 2024, 20, 100341. [Google Scholar] [CrossRef]
- Preobrazhenskiy, I.I.; Tikhonov, A.A.; Evdokimov, P.V.; Shibaev, A.V.; Putlyaev, V.I. DLP printing of hydrogel/calcium phosphate composites for the treatment of bone defects. Open Ceram. 2020, 6, 100115. [Google Scholar] [CrossRef]
- Fumio, U.; Hiroshi, Y.; Kumiko, N.; Sachihiko, N.; Kenji, S.; Yasunori, M. Swelling and mechanical properties of poly(vinyl alcohol) hydrogels. Int. J. Pharm. 1990, 58, 135–142. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Q. Understanding elasticity and swellability of polymer gels from a perspective of polymer/solvent interaction. Curr. Opinion. Colloid. Interface Sci. 2025, 75, 101872. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Doroganov, A.P.; Larin, D.E.; Smirnova, M.E.; Cherkaev, G.V.; Kabaeva, N.M.; Kitaeva, D.K.; Buyanovskaya, A.G.; Philippova, O.E. Hydrogels of Polysaccharide Carboxymethyl Hydroxypropyl Guar Crosslinked by Multivalent Metal Ions. Polym. Sci. Ser. A 2021, 63, 24–33. [Google Scholar] [CrossRef]
- Morozova, S.; Muthukumar, M. Elasticity at Swelling Equilibrium of Ultrasoft Polyelectrolyte Gels: Comparisons of Theory and Experiments. Macromolecules 2017, 50, 2456–2466. [Google Scholar] [CrossRef]
- Stricher, M.; Sarde, C.-O.; Guénin, E.; Egles, C.; Delbecq, F. Cellulosic/Polyvinyl Alcohol Composite Hydrogel: Synthesis, Characterization and Applications in Tissue Engineering. Polymers 2021, 13, 3598. [Google Scholar] [CrossRef] [PubMed]
- Shibaev, A.V.; Abrashitova, K.A.; Kuklin, A.I.; Orekhov, A.S.; Vasiliev, A.L.; Iliopoulos, I.; Philippova, O.E. Viscoelastic Synergy and Microstructure Formation in Aqueous Mixtures of Nonionic Hydrophilic Polymer and Charged Wormlike Surfactant Micelles. Macromolecules 2017, 50, 339–348. [Google Scholar] [CrossRef]
- Qian, K.; Ding, S.-P.; Ye, Z.; Xia, D.-L.; Du, B.-Y.; Xu, J.-T. Microphase separation behavior of polystyrene-block-poly(4-vinyl pyridine)/poly(acrylic acid) polyelectrolyte complexes in bulk. Polymer 2025, 325, 128292. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, H.; Poh, P.; Machens, H.-G.; Schilling, A. Hydrogels for Engineering of Perfusable Vascular Networks. Int. J. Mol. Sci. 2015, 16, 15997–16016. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuleuov, U.B.; Kwiatkowski, A.L.; Kazhmuratova, A.T.; Zhaparova, L.Z.; Nassikhatuly, Y.; Šlouf, M.; Shibaev, A.V.; Petrenko, V.I.; Lanceros-Méndez, S.; Tazhbayev, Y.M. Biocompatible Interpenetrating Network Hydrogels with Dually Cross-Linked Polyol. Polymers 2025, 17, 2737. https://doi.org/10.3390/polym17202737
Tuleuov UB, Kwiatkowski AL, Kazhmuratova AT, Zhaparova LZ, Nassikhatuly Y, Šlouf M, Shibaev AV, Petrenko VI, Lanceros-Méndez S, Tazhbayev YM. Biocompatible Interpenetrating Network Hydrogels with Dually Cross-Linked Polyol. Polymers. 2025; 17(20):2737. https://doi.org/10.3390/polym17202737
Chicago/Turabian StyleTuleuov, Ulygbek B., Alexander L. Kwiatkowski, Akerke T. Kazhmuratova, Lyazzat Zh. Zhaparova, Yermauyt Nassikhatuly, Miroslav Šlouf, Andrey V. Shibaev, Viktor I. Petrenko, Senentxu Lanceros-Méndez, and Yerkeblan M. Tazhbayev. 2025. "Biocompatible Interpenetrating Network Hydrogels with Dually Cross-Linked Polyol" Polymers 17, no. 20: 2737. https://doi.org/10.3390/polym17202737
APA StyleTuleuov, U. B., Kwiatkowski, A. L., Kazhmuratova, A. T., Zhaparova, L. Z., Nassikhatuly, Y., Šlouf, M., Shibaev, A. V., Petrenko, V. I., Lanceros-Méndez, S., & Tazhbayev, Y. M. (2025). Biocompatible Interpenetrating Network Hydrogels with Dually Cross-Linked Polyol. Polymers, 17(20), 2737. https://doi.org/10.3390/polym17202737







