Effect of Hydroxyvalerate Molar Percentage on Physicochemical and Degradation Properties of Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Fibrous Membranes and Potential Application for Air Filtration
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrospinning Process
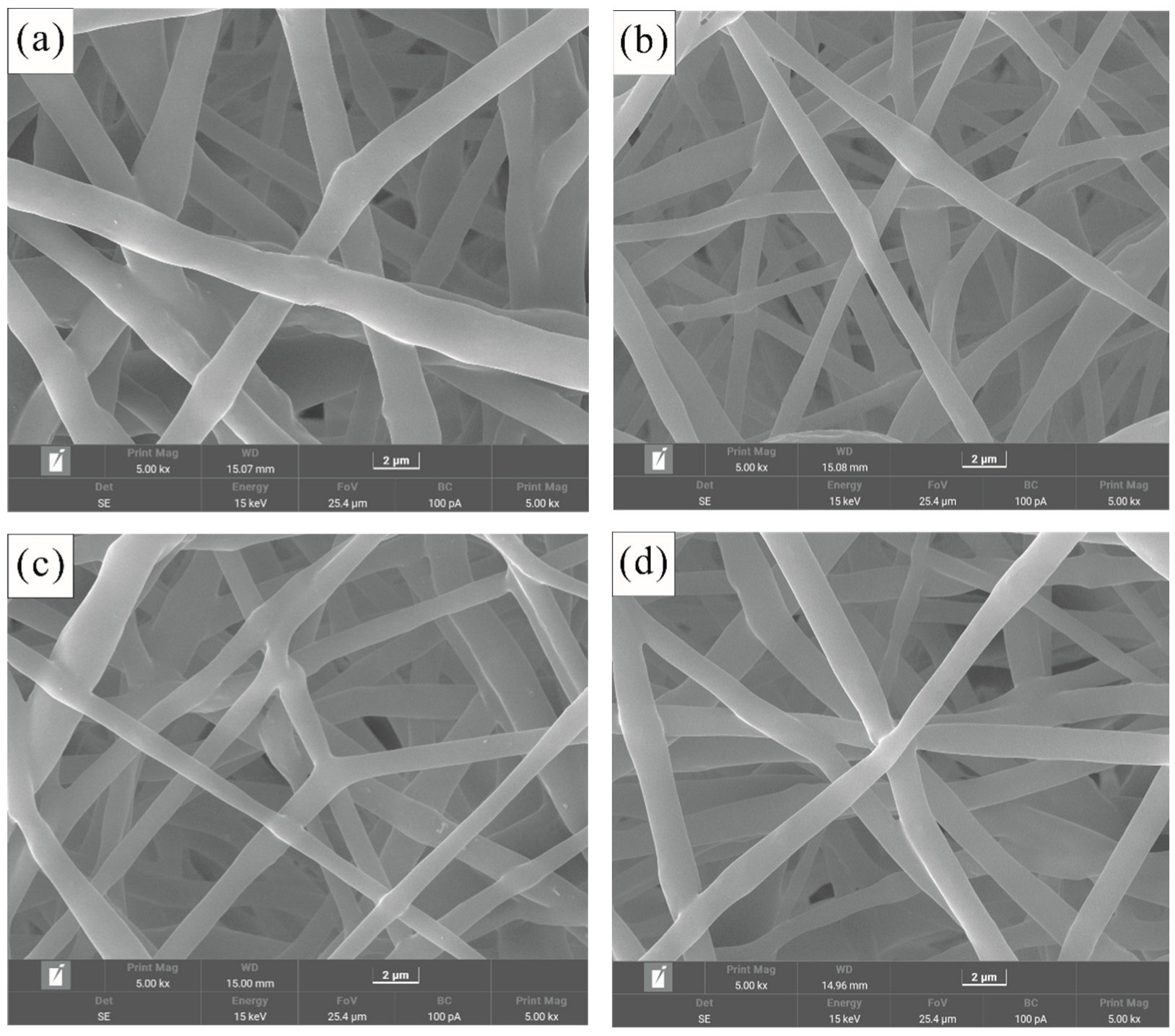
2.2. Fiber Morphology
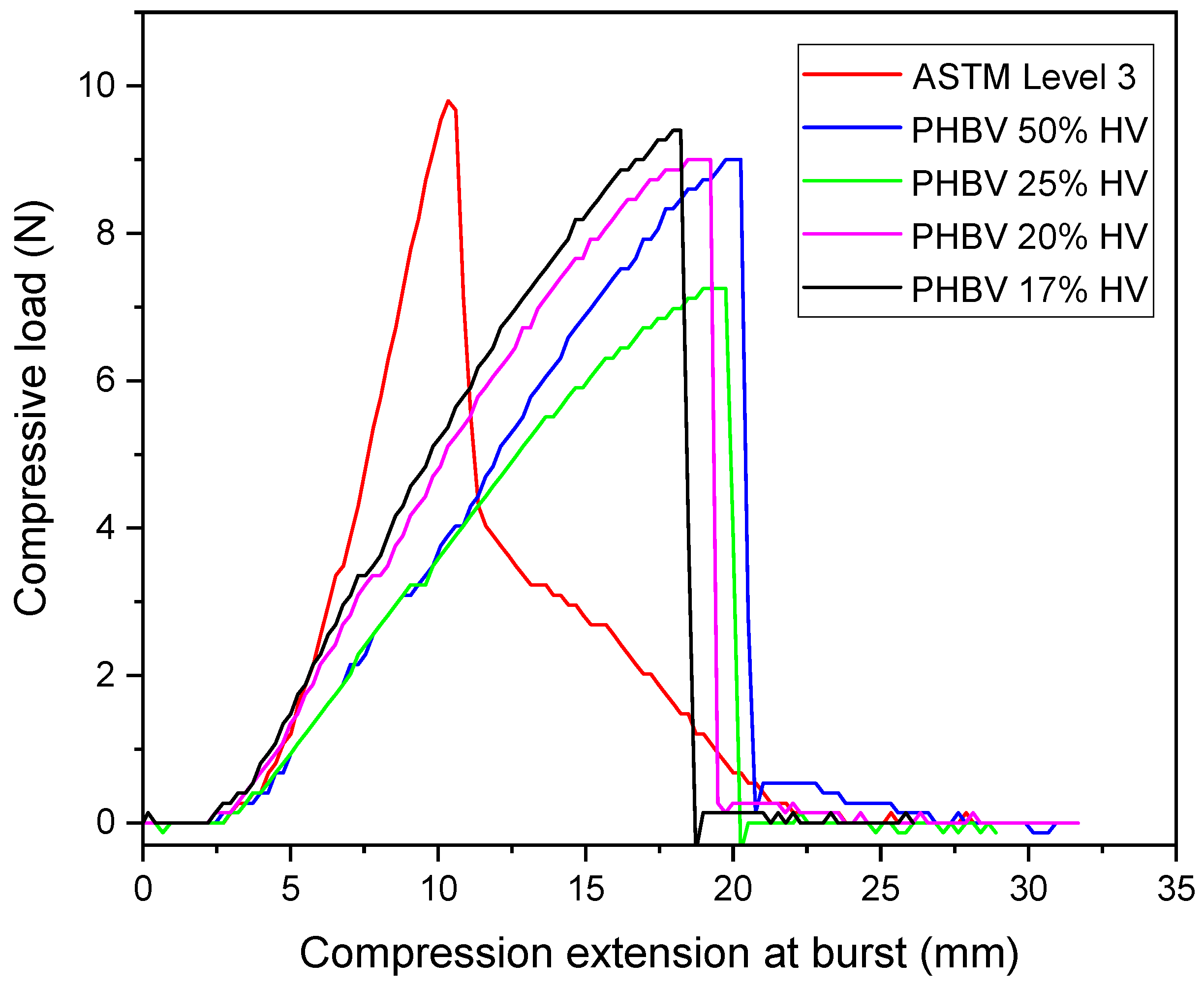
2.3. Ball Burst Test
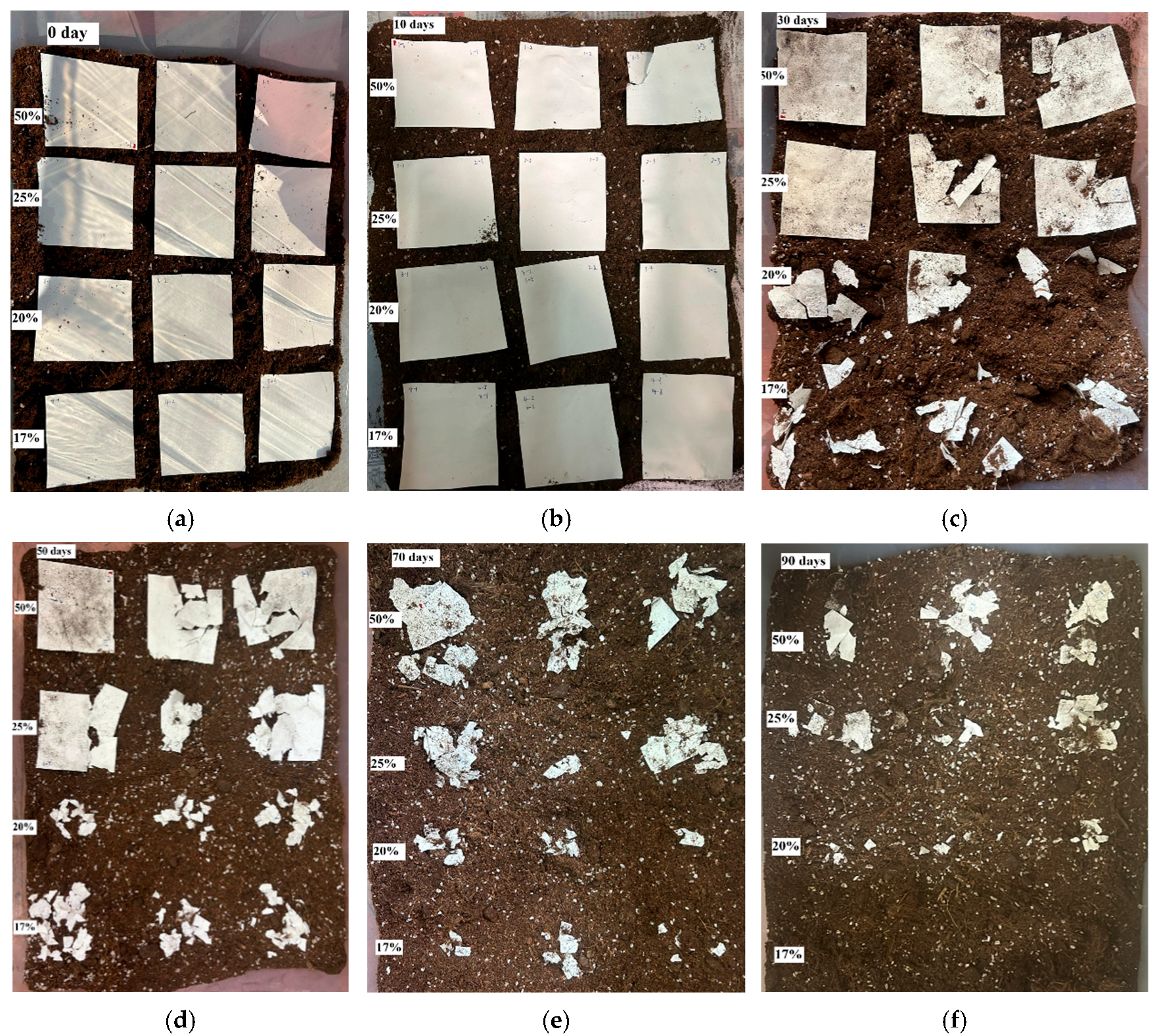
2.4. Soil Burial Degradation Test
2.5. Thermal Analysis
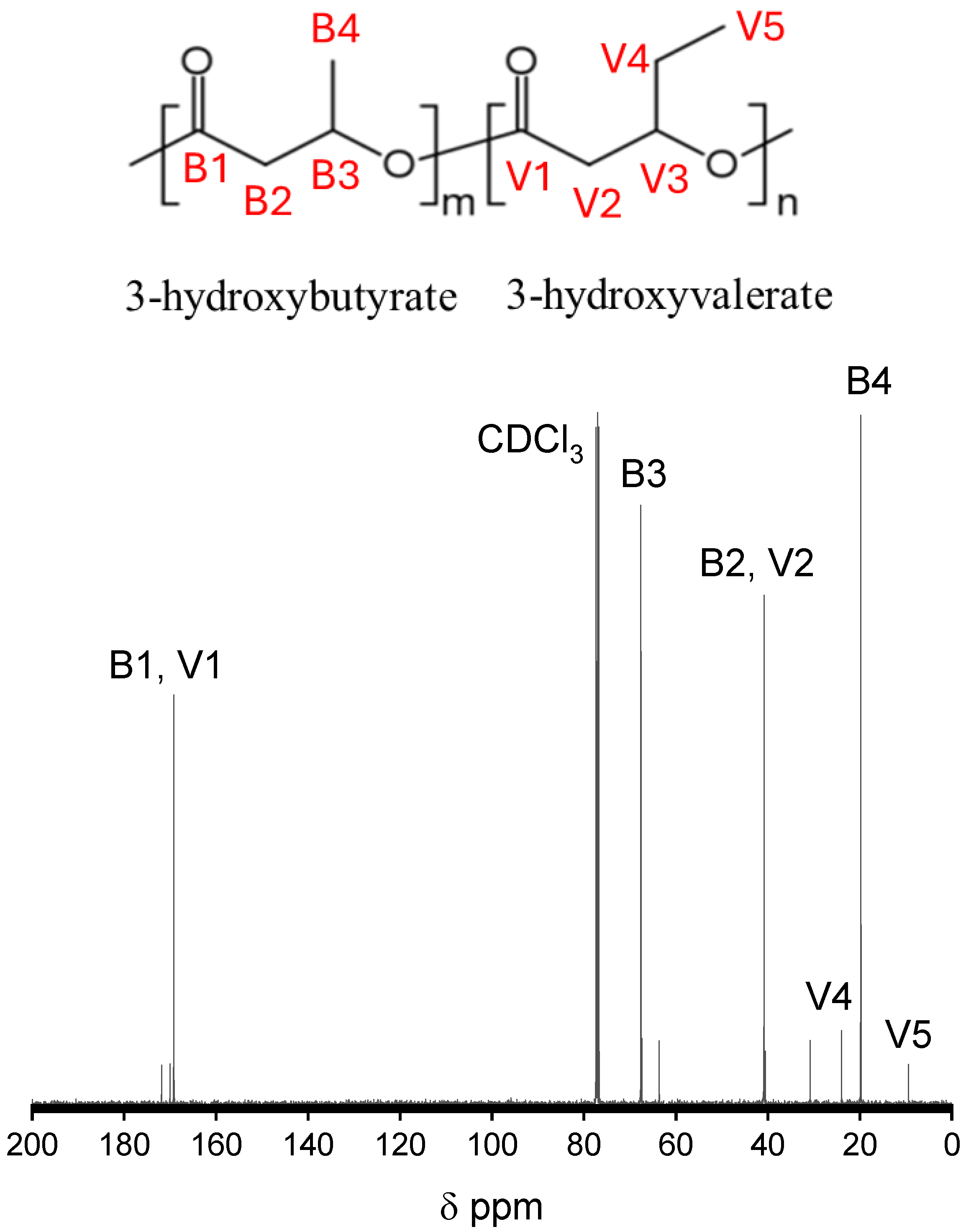
2.6. Qualitative 13C Nuclear Magnetic Resonances
2.7. Filtration Measurement
2.8. Brunauer–Emmett–Teller (BET) Surface Area Analysis
3. Results
3.1. Qualitative 13C Nuclear Magnetic Resonances
3.2. Surface Morphology of Fibrous Membrane
3.3. Thermal Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)
3.4. Ball Burst Strength (BBS)
3.5. Filtration Efficiency
3.6. Specific Surface Area and Pore Size Distribution
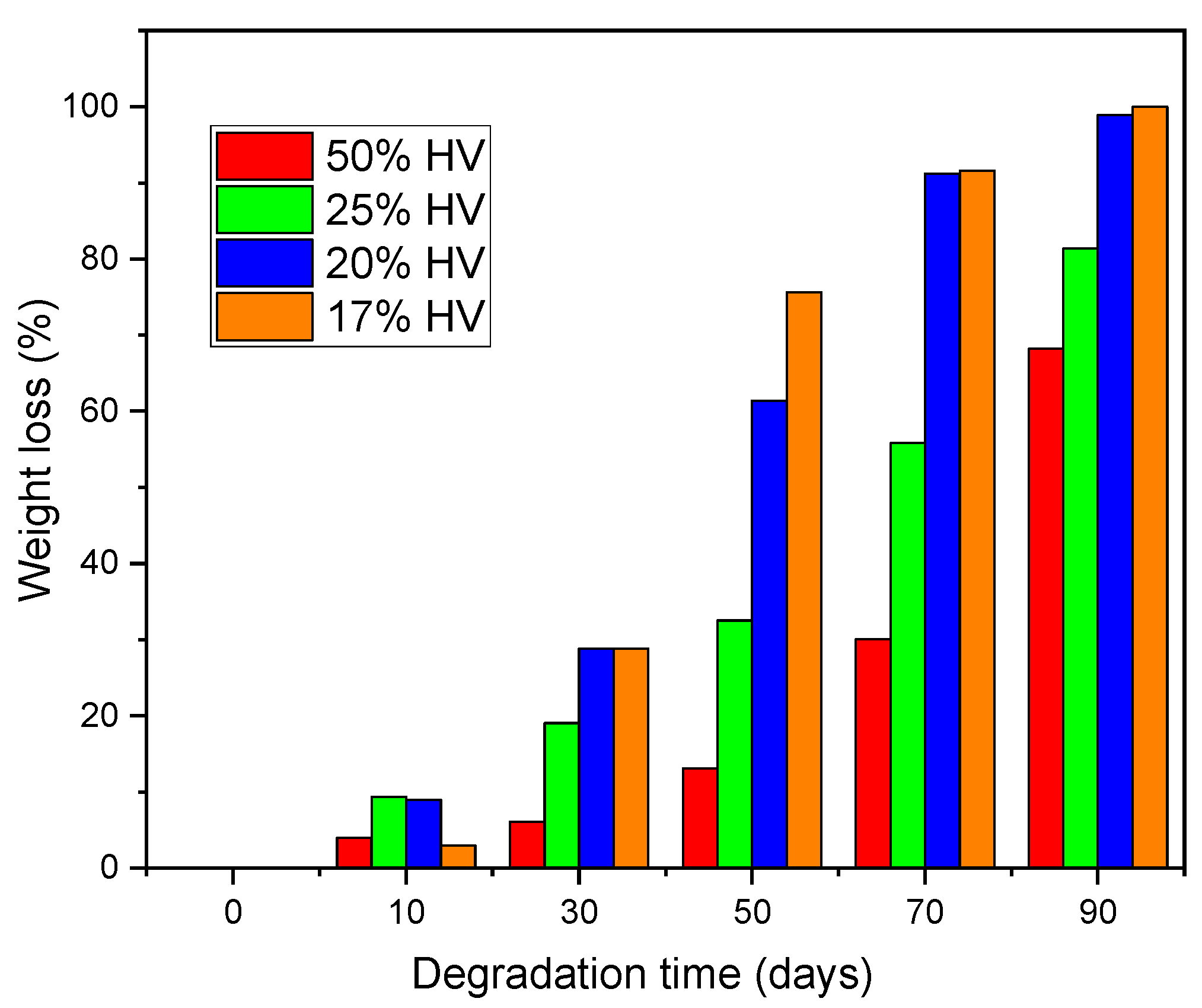
3.7. Degradation of Electrospun Fibrous Membrane in Soil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Z.; Wang, W.; Qi, D.; Luo, Y.; Liu, Y.; Xu, Y.; Cui, F.; Wang, C.; Chen, X. Calcinable Polymer Membrane with Revivability for Efficient Oily-Water Remediation. Adv. Mater. 2018, 30, 1801870. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- McCann, J.T.; Marquez, M.; Xia, Y. Highly Porous Fibers by Electrospinning into a Cryogenic Liquid. J. Am. Chem. Soc. 2006, 128, 1436–1437. [Google Scholar] [CrossRef]
- Tian, X.; Bai, H.; Zheng, Y.; Jiang, L. Bio-inspired Heterostructured Bead-on-String Fibers That Respond to Environmental Wetting. Adv. Funct. Mater. 2011, 21, 1398–1402. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, C.; Hsu, P.C.; Zhang, C.; Liu, N.; Zhang, J.; Lee, H.R.; Lu, Y.; Qiu, Y.; Chu, S.; et al. Nanofiber Air Filters with High-Temperature Stability for Efficient PM2.5 Removal from the Pollution Sources. Nano Lett. 2016, 16, 3642–3649. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Hou, H.; Zhao, Y.; Zhu, Z.; Fong, H. Electrospun polyimide nanofibers and their applications. Prog. Polym. Sci. 2016, 61, 67–103. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Wang, Y.; Zhang, Q.; Ma, W.; Huang, C. Electrospun nanofiber membranes for wastewater treatment applications. Sep. Purif. Technol. 2020, 250, 117116. [Google Scholar] [CrossRef]
- Zhu, X.; Feng, S.; Zhao, S.; Zhang, F.; Xu, C.; Hu, M.; Zhong, Z.; Xing, W. Perfluorinated superhydrophobic and oleophobic SiO2@PTFE nanofiber membrane with hierarchical nanostructures for oily fume purification. J. Membr. Sci. 2020, 594, 117473. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef]
- Thavasi, V.; Singh, G.; Ramakrishna, S. Electrospun nanofibers in energy and environmental applications. Energy Environ. Sci. 2008, 1, 205–221. [Google Scholar] [CrossRef]
- Thuvander, J.; Jönsson, A.S. Techno-economic impact of air sparging prior to purification of alkaline extracted wheat bran hemicelluloses by membrane filtration. Sep. Purif. Technol. 2020, 253, 117498. [Google Scholar] [CrossRef]
- Chen, G.-Q. Polyhydroxyalkanoates (PHAs): Separation, Purification and Manufacturing Methods. In Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology; Flickinger, M.C., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 1–23. [Google Scholar]
- Wei, L.; Liang, S.; Coats, E.R.; McDonald, A.G. Valorization of residual bacterial biomass waste after polyhydroxyalkanoate isolation by hydrothermal treatment. Bioresour. Technol. 2015, 198, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Coats, E.R.; McDonald, A.G. Multivariate near infrared spectroscopy for predicting polyhydroxybutyrate biosynthesis by mixed microbial consortia cultured on crude glycerol. Biomass-Bioenergy 2015, 81, 490–495. [Google Scholar] [CrossRef]
- Kim, M.; Cho, K.-S.; Ryu, H.W.; Lee, E.G.; Chang, Y.K. Recovery of poly(3-hydroxybutyrate) from high cell density culture of Ralstonia eutropha by direct addition of sodium dodecyl sulfate. Biotechnol. Lett. 2003, 25, 55–59. [Google Scholar] [CrossRef]
- Kulkarni, S.O.; Kanekar, P.P.; Jog, J.P.; Patil, P.A.; Nilegaonkar, S.S.; Sarnaik, S.S.; Kshirsagar, P.R. Characterisation of copolymer, poly (hydroxybutyrate-co-hydroxyvalerate) (PHB-co-PHV) produced by Halomonas campisalis (MCM B-1027), its biodegradability and potential application. Bioresour. Technol. 2011, 102, 6625–6628. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Cai, J.; Liu, Z.; Zheng, Y.; Wang, H.; Li, Q.; He, N. Biosynthesis and Thermal Properties of PHBV Produced from Levulinic Acid by Ralstonia eutropha. PLoS ONE 2013, 8, e60318. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Guho, N.M.; Coats, E.R.; McDonald, A.G. Characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) biosynthesized by mixed microbial consortia fed fermented dairy manure. J. Appl. Polym. Sci. 2014, 131, 5516–5528. [Google Scholar] [CrossRef]
- Modi, S.; Koelling, K.; Vodovotz, Y. Assessment of PHB with varying hydroxyvalerate content for potential packaging applications. Eur. Polym. J. 2011, 47, 179–186. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, J.-C.; Ma, Y.-M.; Chen, G.-Q. Engineering biosynthesis of polyhydroxyalkanoates (PHA) for diversity and cost reduction. Metab. Eng. 2019, 58, 82–93. [Google Scholar] [CrossRef]
- Lammi, S.; Gastaldi, E.; Gaubiac, F.; Angellier-Coussy, H. How olive pomace can be valorized as fillers to tune the biodegradation of PHBV based composites. Polym. Degrad. Stabil. 2019, 166, 325–333. [Google Scholar] [CrossRef]
- Vermeer, C.M.; Nielsen, M.; Eckhardt, V.; Hortensius, M.; Tamis, J.; Picken, S.J.; Meesters, G.M.H.; Kleerebezem, R. Systematic solvent screening and selection for polyhydroxyalkanoates (PHBV) recovery from biomass. J. Environ. Chem. Eng. 2022, 10, 108573. [Google Scholar] [CrossRef]
- Pal, A.K.; Wu, F.; Misra, M.; Mohanty, A.K. Reactive extrusion of sustainable PHBV/PBAT-based nanocomposite films with organically modified nanoclay for packaging applications: Compression moulding vs. cast film extrusion. Compos. B Eng. 2020, 198, 108141. [Google Scholar] [CrossRef]
- Mehrpouya, M.; Vahabi, H.; Barletta, M.; Laheurte, P.; Langlois, V. Additive manufacturing of polyhydroxyalkanoates (PHAs) biopolymers: Materials, printing techniques, and applications. Mater. Sci. Eng. C 2021, 127, 112216. [Google Scholar] [CrossRef] [PubMed]
- Bonnenfant, C.; Gontard, N.; Aouf, C. Biobased and biodegradable polymers in a circular economy context: Understanding quercetin and gallic acid impacts on PHBV thermal properties. Polym. Degrad. Stabil. 2022, 201, 109975. [Google Scholar] [CrossRef]
- Salomez, M.; George, M.; Fabre, P.; Touchaleaume, F.; Cesar, G.; Lajarrige, A.; Gastaldi, E. A comparative study of degradation mechanisms of PHBV and PBSA under laboratory-scale composting conditions. Polym. Degrad. Stabil. 2019, 167, 102–113. [Google Scholar] [CrossRef]
- Weng, Y.X.; Wang, Y.; Wang, X.L.; Wang, Y.Z. Biodegradation behavior of PHBV films in a pilot-scale composting condition. Polym. Test. 2010, 29, 579–587. [Google Scholar] [CrossRef]
- Kumar, R.; Sadeghi, K.; Jang, J.; Seo, J. Mechanical, Chemical, and Bio-Recycling of Biodegradable Plastics: A Review. Sci. Total Environ. 2023, 882, 163446. [Google Scholar] [CrossRef]
- Bellache, R.; Hammiche, D.; Bettache, A.; Boukerrou, A. Enzymatic degradation of prickly pear seed (PPS)/Polyhydroxy(butyrate-co-valerate) (PHBV) biocomposite. Mater. Today Proc. 2022, 53, 113–116. [Google Scholar] [CrossRef]
- Numata, K.; Abe, H.; Doi, Y. Enzymatic processes for biodegradation of poly(hydroxyalkanoate)s crystals. Can. J. Chem. 2008, 86, 471–483. [Google Scholar] [CrossRef]
- Sang, B.I.; Hori, K.; Tanji, Y.; Unno, H. Fungal contribution to in situ biodegradation of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) film in soil. Appl. Microbiol. Biotechnol. 2002, 58, 241–247. [Google Scholar] [CrossRef]
- Rutkowska, M.; Krasowska, K.; Heimowska, A.; Adamus, G.; Sobota, M.; Musioł, M.; Janeczek, H.; Sikorska, W.; Krzan, A.; Žagar, E.; et al. Environmental degradation of blends of atactic poly[(R,S)-3-hydroxybutyrate] with natural PHBV in baltic Sea Water and compost with activated sludge. J. Polym. Environ. 2008, 16, 183–191. [Google Scholar] [CrossRef]
- Luzier, W.D. Materials derived from biomass/biodegradable materials. Proc. Natl. Acad. Sci. USA 1992, 89, 839–842. [Google Scholar] [CrossRef]
- Ashori, A.; Jonoobi, M.; Ayrilmis, N.; Shahreki, A.; Fashapoyeh, M.A. Preparation and characterization of polyhydroxybutyrate-co-valerate (PHBV) as green composites using nano reinforcements. Int. J. Biol. Macromol. 2019, 136, 1119–1124. [Google Scholar] [CrossRef]
- Montanheiro, T.L.d.A.; Montagna, L.S.; Viorica Patrulea, V.; Jordan, O.; Borchard, G.; Ribas, R.G.; Campos, T.M.B.; Thim, G.P.; Lemes, A.P. Enhanced water uptake of PHBV scaffolds with functionalized cellulose nanocrystals. Polym. Test. 2019, 79, 106079. [Google Scholar] [CrossRef]
- ASTM F2100-21; Standard Specification for Performance of Materials Used in Medical Face Masks. ASTM: West Conshohocken, PA, USA, 2023.
- ASTM D3787-07; Standard Test Method for Bursting Strength of Textiles-Constant-Rate-of-Traverse (CRT) Ball Burst Test. ASTM: West Conshohocken, PA, USA, 2011.
- Ahmed, T.; Shahid, M.; Azeem, F.; Rasul, I.; Shah, A.A.; Noman, M.; Hameed, A.; Manzoor, N.; Manzoor, I.; Muhammad, S. Biodegradation of plastics: Current scenario and future prospects for environmental safety. Environ. Sci. Pollut. Res. Int. 2018, 25, 7287–7298. [Google Scholar] [CrossRef] [PubMed]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Folino, A.; Karageorgiou, A.; Calabrò, P.S.; Komilis, D. Biodegradation of wasted bioplastics in natural and industrial environments: A review. Sustainability 2020, 12, 6030. [Google Scholar] [CrossRef]
- Berthet, M.A.; Gontard, N.; Angellier-Coussy, H. Impact of fiber moisture content on the structure/mechanical properties relationships of PHBV/wheat straw fibers biocomposites. Compos. Sci. Technol. 2015, 117, 386–391. [Google Scholar] [CrossRef]
- Kamiya, N.; Yamamoto, Y.; Inoue, Y.; Chujo, R.; Doi, Y. Microstructure of bacterially synthesized poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Macromolecules 1989, 22, 1676–1682. [Google Scholar] [CrossRef]
- Žagar, E.; Kržan, A.; Adamus, G.; Kowalczuk, M. Sequence Distribution in Microbial Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Co-polyesters Determined by NMR and MS. Biomacromolecules 2006, 7, 2210–2216. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Gapes, D.J.; Newman, R.H.; Dare, P.H. Physico-Chemical Properties of Polyhydroxyalkanoate Produced by Mixed-Culture Nitrogen-Fixing Bacteria. Appl. Microbiol. Biotechnol. 2009, 82, 545–555. [Google Scholar] [CrossRef]
- Ivanova, G.; Serafim, L.S.; Lemos, P.C.; Ramos, A.M.; Reis, M.A.M.; Cabrita, E.J. Influence of Feeding Strategies of Mixed Microbial Cultures on the Chemical Composition and Microstructure of Copolyesters P(3HB-Co-3HV) Analyzed by NMR and Statistical Analysis. Magn. Reson. Chem. 2009, 47, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Irorere, V.U.; Bagheriasl, S.; Blevins, M.; Kwiecień, I.; Stamboulis, A.; Radecka, I. Electrospun Fibres of Polyhydroxybutyrate Synthesized by Ralstonia Eutropha from Different Carbon Sources. Int. J. Polym. Sci. 2014, 2014, 705359. [Google Scholar] [CrossRef]
- Dai, Y.; Lambert, L.; Yuan, Z.; Keller, J. Characterisation of Polyhydroxyalkanoate Copolymers with Controllable Four-Monomer Composition. J. Biotechnol. 2008, 134, 137–145. [Google Scholar] [CrossRef]
- Doi, Y.; Kunioka, M.; Nakamura, Y.; Soga, K. Nuclear Magnetic Resonance Studies on Poly (b-hydroxybutyrate) and a Copolyester of P-Hydroxybutyrate and P-Hydroxyvalerate Isolated from Alcaligenes Eutrophus H16. Macromolecules 1986, 19, 2860–2864. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Cabedo, L.; Lagaron, J.M.; Torres-Giner, S. Development of Electrospun Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Monolayers Containing Eugenol and Their Application in Multilayer Antimicrobial Food Packaging. Front. Nutr. 2020, 7, 140. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Bernardos, A.; Martínez-Máñez, R.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Electrospun Antimicrobial Films of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Containing Eugenol Essential Oil Encapsulated in Mesoporous Silica Nanoparticles. Nanomaterials 2019, 9, 227. [Google Scholar] [CrossRef]
- Guho, N.M.; Pokhrel, D.; Abbasi, M.; McDonald, A.G.; Alfaro, M.; Brinkman, C.K.; Coats, E.R. Pilot-scale production of poly3-hydroxybutyrate-co-3-hydroxyvalerate from fermented dairy manure: Process performance, polymer characterization, and scale-up implications. Bioresour. Technol. Rep. 2020, 12, 100588. [Google Scholar]
- Abbasi, M.; Coats, E.R.; McDonald, A.G. Green solvent extraction and properties characterization of Poly(3-hydroxybutyrate-co3-hydroxyvalerate) biosynthesized by mixed microbial consortia fed fermented dairy manure. Bioresour. Technol. Rep. 2022, 18, 101065. [Google Scholar]
- Souza Junior, O.F.; Staffa, L.H.; Costa, L.C.; Chinelatto, M.A. Thermal and Rheological Behavior of Binary Blends of Poly(hydroxybutyrate-co-hydroxyvalerate) and Poly(ethylene-co-vinyl acetate) with Different Vinyl Acetate Content. Macromol. Symp. 2019, 383, 1800020. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Diao, M.; Wang, W.; Hua, W.; Wu, S.; Chen, P.; Ruan, R.; Cheng, Y. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Production by Rhodospirillum rubrum Using a Two-Step Culture Strategy. J. Chem. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Zhuikov, V.A.; Zhuikova, Y.V.; Makhina, T.K.; Myshkina, V.L.; Rusakov, A.; Useinov, A.; Voinova, V.V.; Bonartseva, G.A.; Berlin, A.A.; Bonartsev, A.P.; et al. Comparative Structure-Property Characterization of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)s Films under Hydrolytic and Enzymatic Degradation: Finding a Transition Point in 3-Hydroxyvalerate Content. Polymers 2020, 12, 728. [Google Scholar] [CrossRef] [PubMed]
- Thiré, R.M.D.S.M.; Arruda, L.C.; Barreto, L.S. Morphology and thermal properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/attapulgite nanocomposites. Mater. Res. 2011, 14, 340–344. [Google Scholar] [CrossRef]
- Vahabi, H.; Michely, L.; Moradkhani, G.; Akbari, V.; Cochez, M.; Vagner, C.; Renard, E.; Saeb, M.R.; Langlois, V. Thermal Stability and Flammability Behavior of Poly(3-hydroxybutyrate) (PHB) Based Composites. Materials 2019, 12, 2239. [Google Scholar] [CrossRef]
- García, A.; Pérez, D.; Castro, M.; Urtuvia, V.; Castillo, T.; Díaz-Barrera, A.; Espín, G.; Peña, C. Production and recovery of poly-3-hydroxybutyrate [P(3HB)] of ultra-high molecular weight using fed-batch cultures of Azotobacter vinelandii OPNA strain. J. Chem. Technol. Biotechnol. 2019, 94, 1853–1860. [Google Scholar] [CrossRef]
- Koller, M.; Bona, R.; Chiellini, E.; Braunegg, G. Extraction of short-chain-length poly-[(R)-hydroxyalkanoates] (scl-PHA) by the “anti-solvent” acetone under elevated temperature and pressure. Biotechnol. Lett. 2013, 35, 1023–1028. [Google Scholar] [CrossRef]
- Chen, Y.; Chou, I.-N.; Tsai, Y.-H.; Wu, H.-S. Thermal degradation of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in drying treatment. J. Appl. Polym. Sci. 2013, 130, 3659–3667. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Zhu, M.-F.; Wu, W.-H.; Qin, Z.-Y. Reducing the formation of six-membered ring ester during thermal degradation of biodegradable PHBV to enhance its thermal stability. Polym. Degrad. Stab. 2009, 94, 18–24. [Google Scholar] [CrossRef]
- Samorì, C.; Basaglia, M.; Casella, S.; Favaro, L.; Galletti, P.; Giorgini, L.; Marchi, D.; Mazzocchetti, L.; Torri, C.; Tagliavini, E. Dimethyl carbonate and switchable anionic surfactants: Two effective tools for the extraction of polyhydroxyalkanoates from microbial biomass. Green Chem. 2014, 17, 1047–1056. [Google Scholar] [CrossRef]
- Deeken, C.R.; Abdo, M.S.; Frisella, M.M.; Matthews, B.D. Physicomechanical evaluation of polypropylene, poly ester, and poly tetrafluoroethylene meshes for inguinal hernia repair. J. Am. Coll. Surg. 2011, 212, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Pölöskei, K.; Csézi, G.; Hajba, S.; Tábi, T. Investigation of the thermoformability of various D-Lactide content poly(lactic acid) films by ball burst test. Polym. Eng. Sci. 2020, 60, 1266–1277. [Google Scholar] [CrossRef]
- GB 19083-2010; Technical Requirements for Protective Face Mask for Medical Use. Standardization Administration of China: Beijing, China, 2010.
- Li, Y.; Yin, X.; Yu, J.; Ding, B. Electrospun nanofibers for high-performance air filtration. Compos. Commun. 2019, 15, 6–19. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.-G.; Wang, K.; Arias, J.L.; Scherf, U.; El-Hammadi, M.M.; Zhou, Y.; et al. Electro-spun nanofiber membranes for air filtration: A review. Nanomaterial 2022, 12, 1077. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, T.; Cui, J.; Keshari Samal, S.; Xiong, R.; Huang, C. Bio-based electrospun nanofiber as building blocks for a novel eco-friendly air filtration membrane: A review. Sep. Purif. Technol. 2021, 277, 119623. [Google Scholar] [CrossRef]
- Qin, X.-H.; Wang, S.-Y. Filtration properties of electrospinning nanofibers. J. Appl. Polym. Sci. 2006, 102, 1285–1290. [Google Scholar] [CrossRef]
- Lei, L.; Shang, L.; Li, Y.; Yang, C. Three-layer composite filter media containing electrospun polyimide nanofibers for the removal of fine particles. Fibers Polym. 2017, 18, 749–757. [Google Scholar] [CrossRef]
- Bidabadi, M.; Fereidooni, J.; Tavakoli, R.; Mafi, M. Premixed filtration combustion of micron and sub-micronparticles in inert porous media: A theoretical analysis. Korean J. Chem. Eng. 2011, 28, 461–469. [Google Scholar] [CrossRef]
- Imaizumi, S.; Matsumoto, H.; Suzuki, K.; Minagawa, M.; Kimura, M.; Tanioka, A. Phenolic resin-based carbon thin fibers prepared by electrospinning: Additive effects of poly(vinyl butyral) and electrolytes. Polymer J. 2009, 41, 1124–1128. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Li, X.H.; Wang, C.H.; Fu, S.W.; Liu, Y.C.; Shao, C.L. Polyacrylonitrile and carbon nanofibers with controllable nanoporous structures by electrospinning. Macromol. Mater. Eng. 2009, 294, 673–678. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Piorkowska, E.; Kulpinski, P.; Pracella, M. Mechanical and thermal properties of PLA composites with cellulose nanofibers and standard size fibers. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1509–1514. [Google Scholar] [CrossRef]
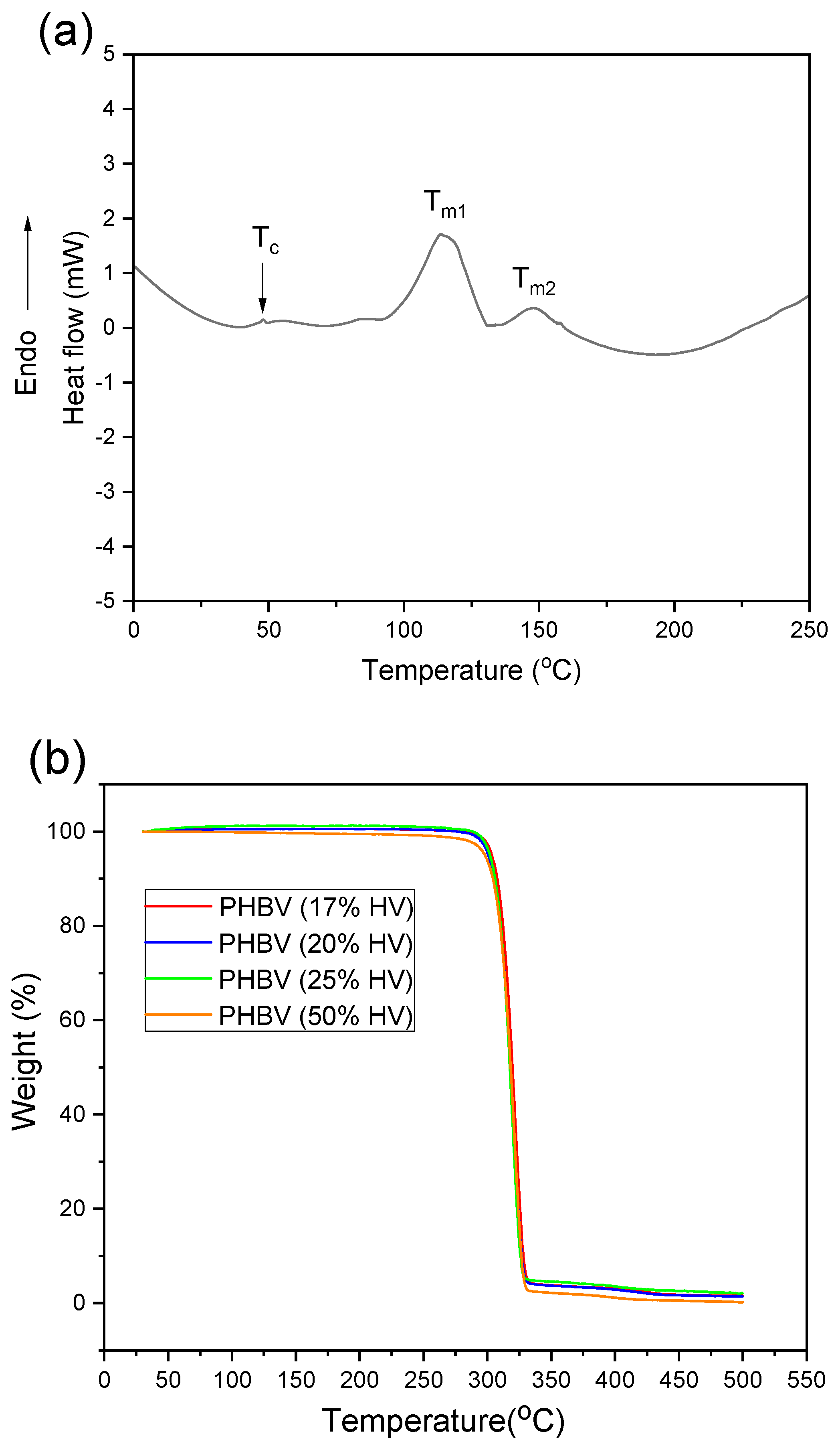
| PHBV (%HV) | Tm1 (°C) | Tm2 (°C) | Tc (°C) | Xc (%) |
|---|---|---|---|---|
| 50% | 120.52 | 141.74 | 54.56 | 23.37 |
| 25% | 121.02 | 143.86 | 58.39 | 25.96 |
| 20% | 121.07 | 146.84 | 63.53 | 33.62 |
| 17% | 121.18 | 147.31 | 78.55 | 50.71 |
| PHBV (%HV) | Td (°C) | Tonset (°C) | Toffset (°C) |
|---|---|---|---|
| 50% | 287.86 | 266.72 | 298.72 |
| 25% | 296.07 | 276.76 | 303.93 |
| 20% | 296.11 | 274.54 | 301.62 |
| 17% | 296.85 | 274.94 | 302.48 |
| Maximum Compressive Load (N) | Compression Elongation at Burst (mm) | |
|---|---|---|
| ASTM F2100 level 3 mask | 9.71 | 11.00 |
| PHBV 50% HV | 8.86 | 20.05 |
| PHBV 25% HV | 7.39 | 19.32 |
| PHBV 20% HV | 9.27 | 19.13 |
| PHBV 17% HV | 9.34 | 18.21 |
| Particle Diameter (μm) | ASTM F2100 Level 3 | PHBV (50% HV) | PHBV (25% HV) | PHBV (20% HV) | PHBV (17% HV) |
|---|---|---|---|---|---|
| 10 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 |
| 5.0 | 100.000 | 100.000 | 100.000 | 100.000 | 100.000 |
| 2.5 | 99.999 | 99.999 | 99.999 | 99.999 | 99.999 |
| 1.0 | 99.999 | 99.999 | 99.999 | 99.999 | 99.999 |
| 0.5 | 100.000 | 96.321 | 96.688 | 96.4848 | 97.378 |
| 0.3 | 89.588 | 95.691 | 96.422 | 96.193 | 97.367 |
| PFE(%) | 98.231 | 98.668 | 98.851 | 98.753 | 99.124 |
| Electrospun Membrane with HV mol.% | BET Surface Area (m2/g) | Average Pore Diameter (nm) |
|---|---|---|
| untreated | 0.927 | 10.251 |
| One heating-cooling cycle | 2.343 | 7.124 |
| Three heating-cooling cycles | 2.692 | 6.745 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Lee, C.-H.; Wang, Y.; Kan, C.-W.; Lu, X.-Y. Effect of Hydroxyvalerate Molar Percentage on Physicochemical and Degradation Properties of Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Fibrous Membranes and Potential Application for Air Filtration. Polymers 2025, 17, 2719. https://doi.org/10.3390/polym17202719
Liu Y, Lee C-H, Wang Y, Kan C-W, Lu X-Y. Effect of Hydroxyvalerate Molar Percentage on Physicochemical and Degradation Properties of Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Fibrous Membranes and Potential Application for Air Filtration. Polymers. 2025; 17(20):2719. https://doi.org/10.3390/polym17202719
Chicago/Turabian StyleLiu, Yaohui, Cheng-Hao Lee, Yanming Wang, Chi-Wai Kan, and Xiao-Ying Lu. 2025. "Effect of Hydroxyvalerate Molar Percentage on Physicochemical and Degradation Properties of Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Fibrous Membranes and Potential Application for Air Filtration" Polymers 17, no. 20: 2719. https://doi.org/10.3390/polym17202719
APA StyleLiu, Y., Lee, C.-H., Wang, Y., Kan, C.-W., & Lu, X.-Y. (2025). Effect of Hydroxyvalerate Molar Percentage on Physicochemical and Degradation Properties of Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Fibrous Membranes and Potential Application for Air Filtration. Polymers, 17(20), 2719. https://doi.org/10.3390/polym17202719







