Electrospinning Membrane with Polyacrylate Mixed Beta-Cyclodextrin: An Efficient Adsorbent for Cationic Dyes
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Polyacrylate (PA) Copolymer
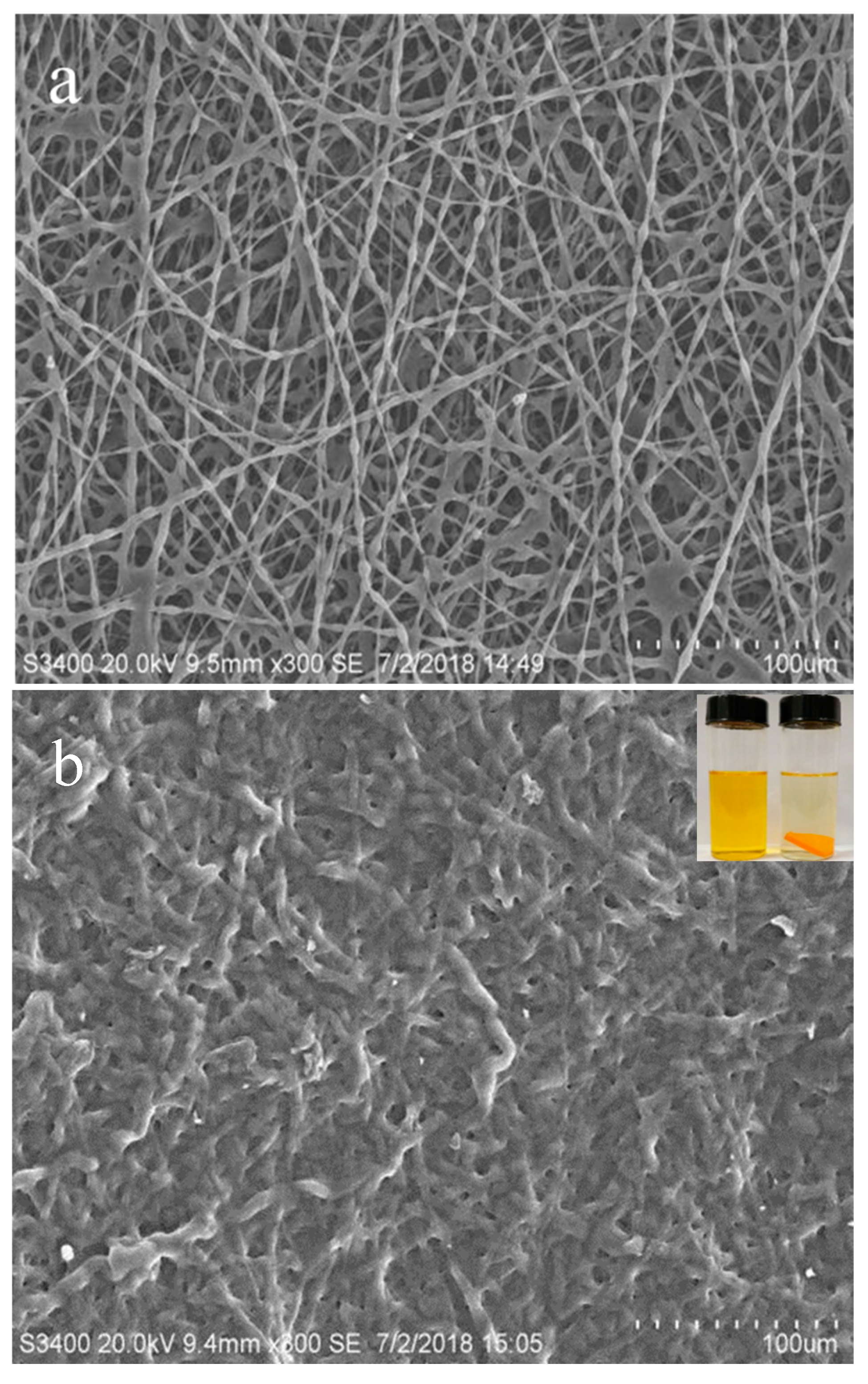
2.3. Preparation of Electrospun β-CD/PA Nanofiber Membrane
2.4. Characterization
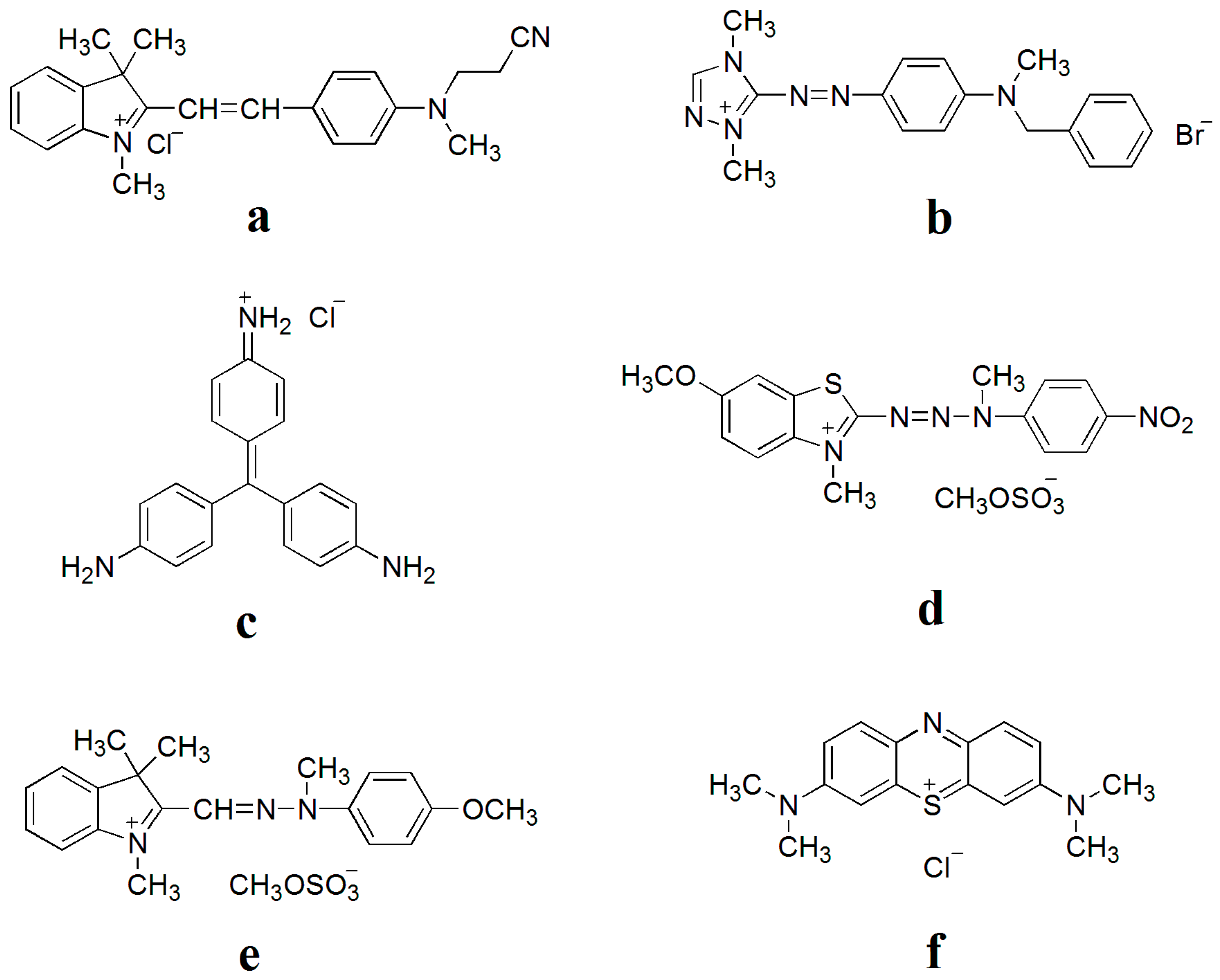
2.5. Batch Adsorption Experiments
3. Results and Discussion
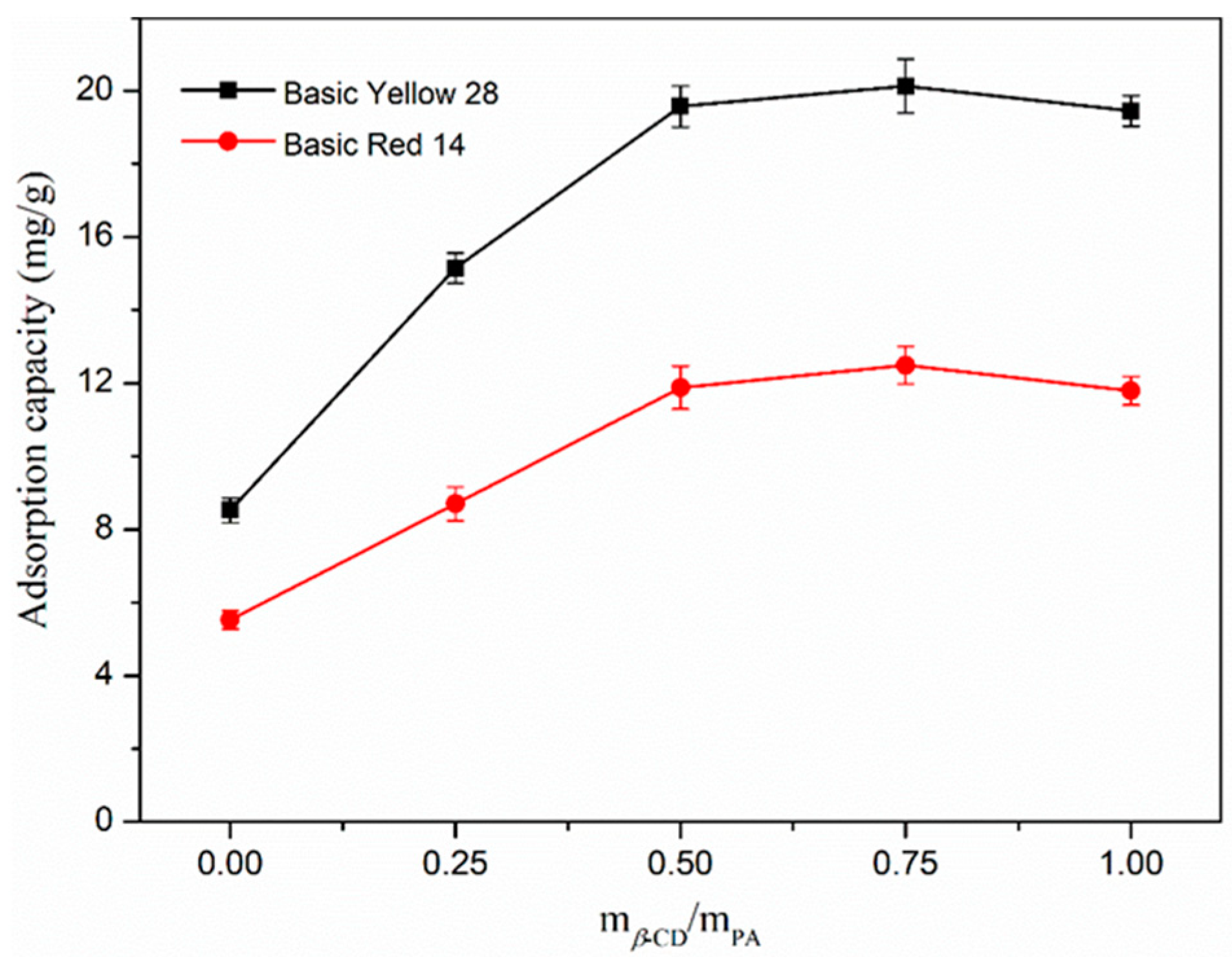
3.1. Determination of Optimum Adsorption Capacity of Nanofibers
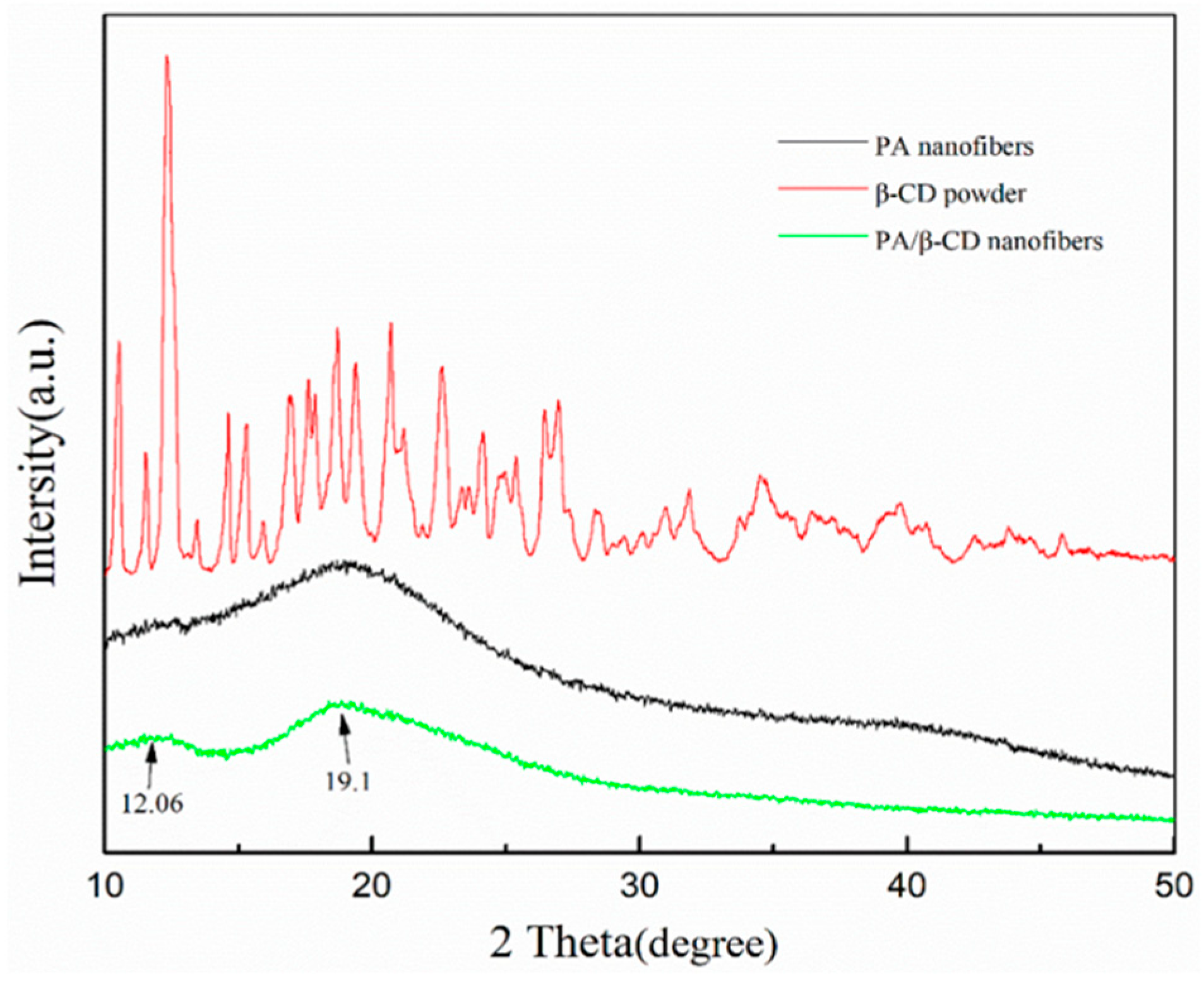
3.2. XRD Characterization of the Obtained Nanofibers
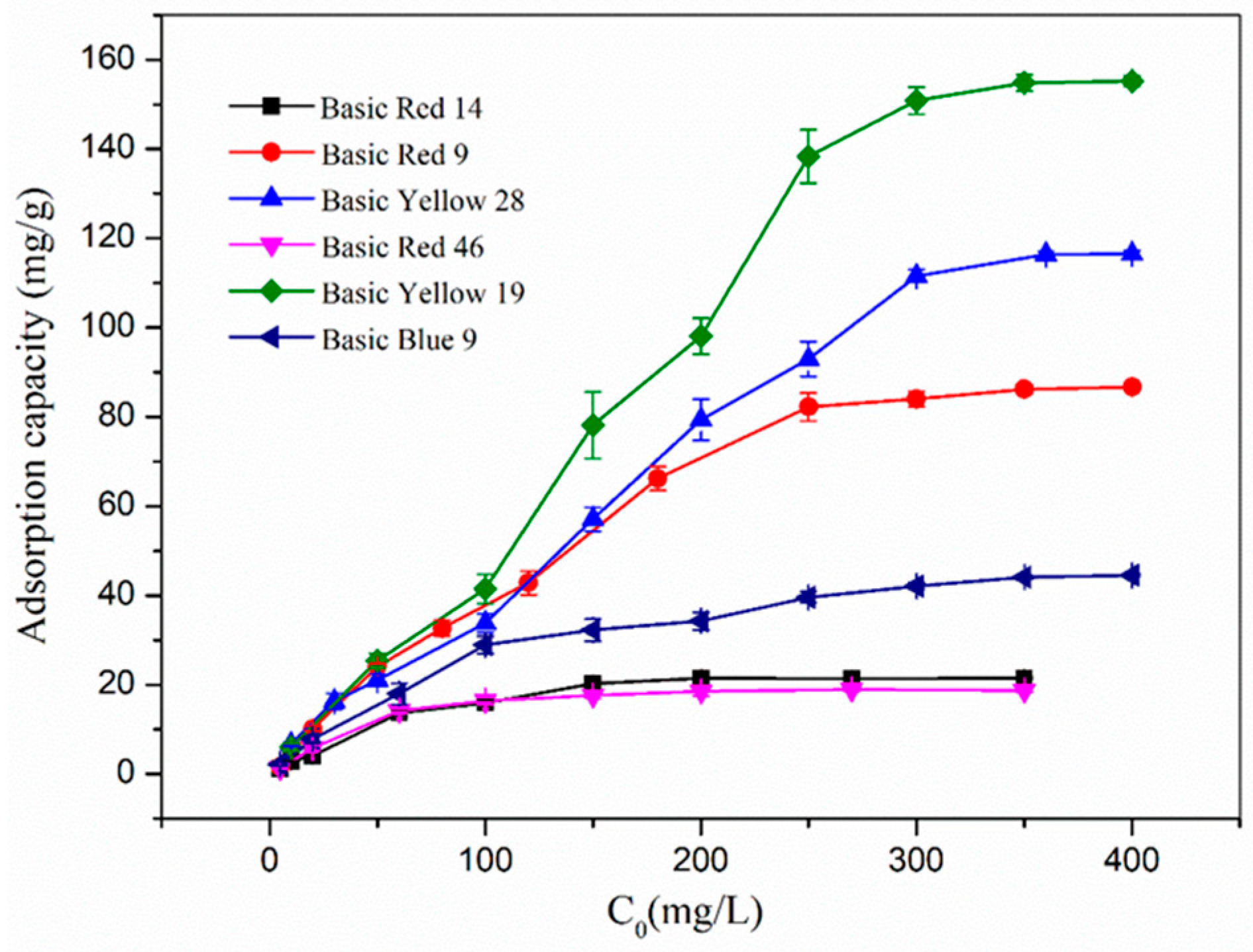
3.3. Effect of Initial Concentration of Cationic Dye Solution on the Adsorption of Nanofibers
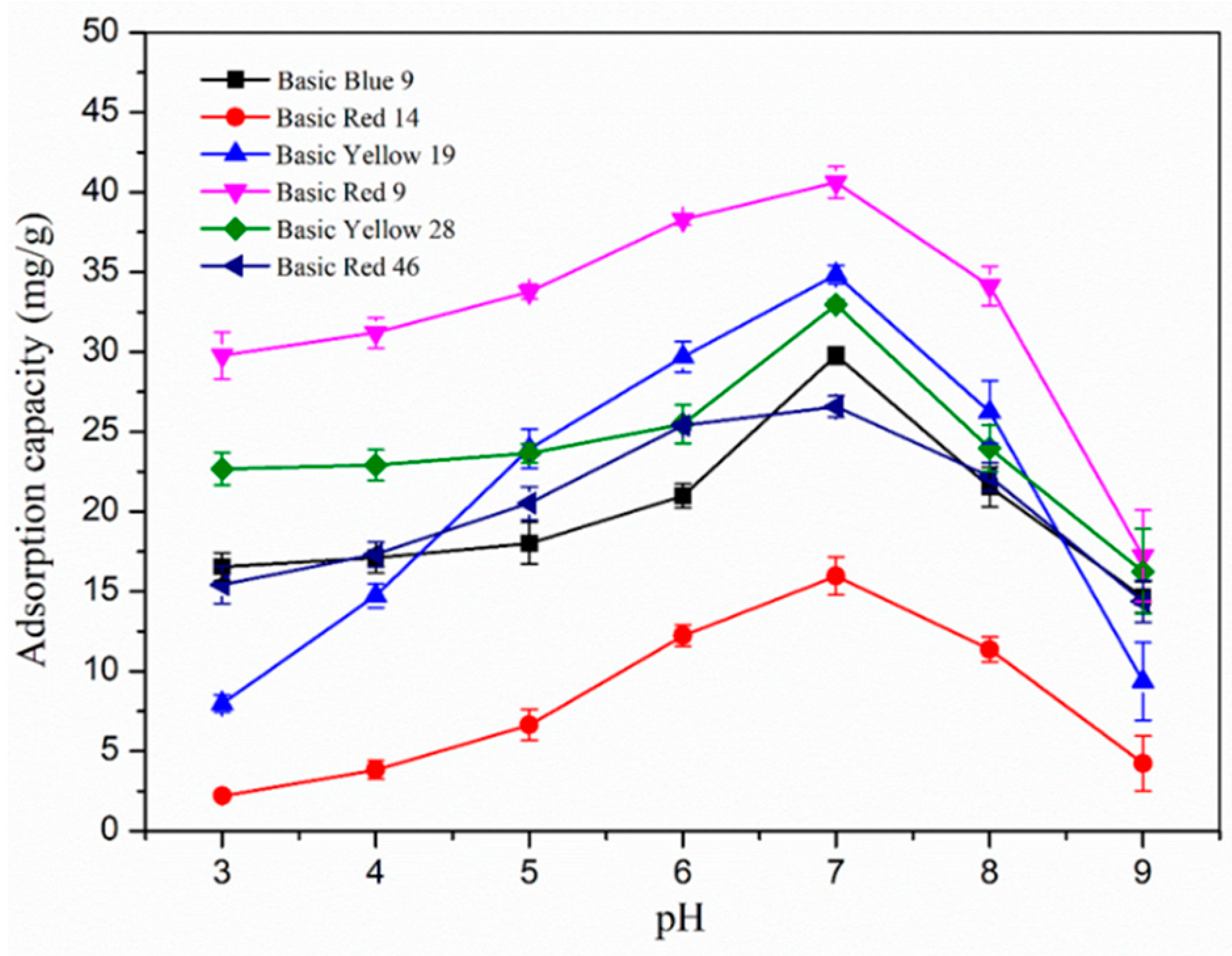
3.4. Effect of pH on the Adsorption of Nanofibers and the Adsorption Mechanism
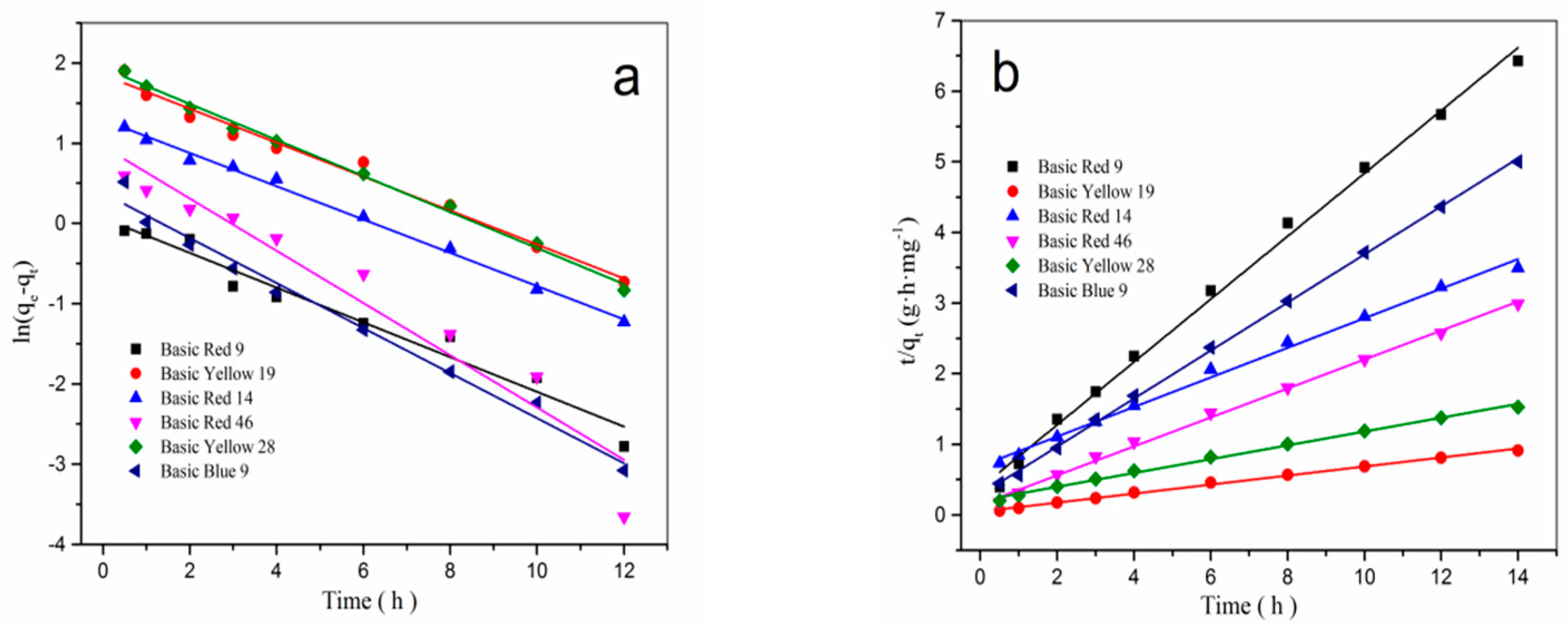
3.5. Adsorption Kinetics of β-CD/PA Nanofiber for Cationic Dyes
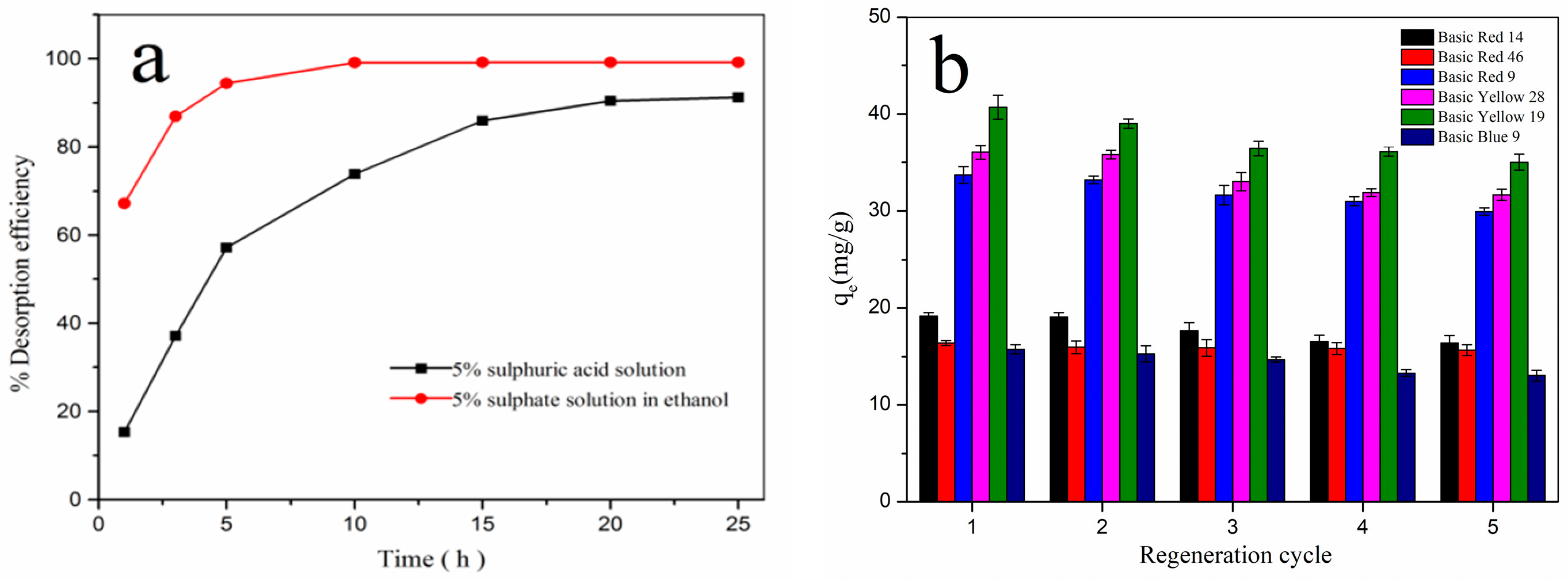
3.6. Desorption and Recycling Ability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Feng, J.; Yan, W. Synthesis of polypyrrole-modified TiO2 composite adsorbent and its adsorption performance on acid Red G. J. Appl. Polym. Sci. 2013, 128, 3231–3239. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Y.; Li, X.; Sun, B.; Jiang, Z.; Wang, C. Water-insoluble sericin/β-cyclodextrin/PVA composite electrospun nanofibers as effective adsorbents towards methylene blue. Colloids Surf. B Biointerfaces 2015, 136, 375–382. [Google Scholar] [CrossRef]
- McMullan, G.; Meehan, C.; Conneely, A.; Kirby, N.; Robinson, T.; Nigam, P.; Banat, I.M.; Marchant, R.; Smyth, W.F. Microbial decolourisation and degradation of textile dyes. Appl. Microbiol. Biot. 2001, 56, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Jah, A.H.; Khodadoust, S.; Sahraei, R.; Daneshfar, A.; Mihandoost, A.; Purkait, M.K. Cadmium telluride nanoparticles loaded on activated carbon as adsorbent for removal of sunset yellow. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 90, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, Y.; Luo, C.; Lu, F.; Qiu, H.; Sun, M. Synthesis and characterization of magnetic β-cyclodextrin–chitosan nanoparticles as nano-adsorbents for removal of methyl blue. Int. J. Biol. Macromol. 2012, 50, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Feng, N.; Wei, Y.; Zhang, G. An in situ gelatin-assisted hydrothermal synthesis of ZnO-reduced graphene oxide composites with enhanced photocatalytic performance under ultraviolet and visible light. RSC Adv. 2014, 4, 7933. [Google Scholar] [CrossRef]
- Ayekoe, C.Y.P.; Robert, D.; Lanciné, D.G. Combination of coagulation-flocculation and heterogeneous photocatalysis for improving the removal of humic substances in real treated water from Agbô River (Ivory-Coast). Catal. Today 2017, 281, 2–13. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, W.; Guan, K.; Peng, C.; Wu, J. Freeze-casting of alumina ultra-filtration membranes with good performance for anionic dye separation. Ceram. Int. 2018, 44, 11901–11904. [Google Scholar] [CrossRef]
- Hua, Y.; Xiao, J.; Zhang, Q.; Cui, C.; Wang, C. Facile synthesis of surface-functionalized magnetic nanocomposites for effectively selective adsorption of cationic dyes. Nanoscale Res. Lett. 2018, 13, 99. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohyd. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, Y.; Wang, T.; Wu, Y.; He, Y.; Yang, R.; Zheng, S. Regulation of the surface area and surface charge property of MOFs by multivariate strategy: Synthesis, characterization, selective dye adsorption and separation. Micropor. Mesopor. Mat. 2018, 272, 101–108. [Google Scholar] [CrossRef]
- Martín, D.M.; Faccini, M.; García, M.A.; Amantia, D. Highly efficient removal of heavy metal ions from polluted water using ion-selective polyacrylonitrile nanofibers. J. Environ. Chem. Eng. 2018, 6, 236–245. [Google Scholar] [CrossRef]
- Wu, J.; Wang, N.; Zhao, Y.; Jiang, L. Electrospinning of multilevel structured functional micro-/nanofibers and their applications. J. Mater. Chem. A 2013, 1, 7290. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospinning of nanofibers from non-polymeric systems: Polymer-free nanofibers from cyclodextrin derivatives. Nanoscale 2012, 4, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Zhu, M.; Yu, S.; Sui, G.; Yang, X. Studies on soy protein isolate/polyvinyl alcohol hybrid nanofiber membranes as multi-functional eco-friendly filtration materials. Mater. Sci. Eng. B 2016, 214, 1–10. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, M.; Diao, G. Electrospinning β-cyclodextrin/poly(vinyl alcohol) nanofibrous membrane for molecular capture. Carbohyd. Polym. 2011, 86, 1410–1416. [Google Scholar] [CrossRef]
- Uyar, T.; Nur, Y.; Hacaloglu, J.; Besenbacher, F. Electrospinning of functional poly(methyl methacrylate) nanofibers containing cyclodextrin-menthol inclusion complexes. Nanotechnology 2009, 20, 125703. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, C.; Fang, W.; Wang, J.; Zhang, W.; Jin, G.; Diao, G. Electrospinning of Calixarene-Functionalized Polyacrylonitrile Nanofiber Membranes and Application as an Adsorbent and Catalyst Support. Langmuir 2013, 29, 11858–11867. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, A.; Rafati, A.A. Preparation of silica mesoporous nanoparticles functionalized with β-cyclodextrin and its application for methylene blue removal. J. Mol. Liq. 2015, 209, 239–245. [Google Scholar] [CrossRef]
- Mallard, I.; Städe, L.W.; Ruellan, S.; Jacobsen, P.A.L.; Larsen, K.L.; Fourmentin, S. Synthesis, characterization and sorption capacities toward organic pollutants of new β-cyclodextrin modified zeolite derivatives. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 50–57. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Hazel, G.S.S.; Hidajat, K.; Uddin, M.S. Synthesis of carboxymethyl-β-cyclodextrin conjugated magnetic nano-adsorbent for removal of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2010, 367, 85–95. [Google Scholar] [CrossRef]
- Liu, X.; Yan, L.; Yin, W.; Zhou, L.; Tian, G.; Shi, J.; Yang, Z.; Xiao, D.; Gu, Z.; Zhao, Y. A magnetic graphene hybrid functionalized with beta-cyclodextrins for fast and efficient removal of A magnetic graphene hybrid functionalized with beta-cyclodextrins for fast and efficient removal of organic dyes. J. Mater. Chem. A 2014, 12296–12303. [Google Scholar] [CrossRef]
- Liu, M.; Xue, Y.; Cai, Y.L.; Zhu, Y.Q.; Li, L.F.; Zheng, C.L. Water resistance of β-cyclodextrin/polyacrylate nanofiber films and their supramolecular capturing performance for heavy metal ions. J. Text. Res. 2016, 37, 1–5. [Google Scholar]
- Wang, X.; Shen, Y.; Lai, X. Micromorphology and mechanism of polyurethane/polyacrylate membranes modified with epoxide group. Prog. Org. Coat. 2014, 77, 268–276. [Google Scholar] [CrossRef]
- Guo, Y.; Li, S.; Wang, G.; Ma, W.; Huang, Z. Waterborne polyurethane/poly(n-butyl acrylate-styrene) hybrid emulsions: Particle formation, film properties, and application. Prog. Org. Coat. 2012, 74, 248–256. [Google Scholar] [CrossRef]
- Sun, J.; Yuan, A.L.; Zheng, C.L. Dyes Chemistry; Chemical Industry Press: Beijing, China, 2022. [Google Scholar]
- Liu, L.; Xu, S.Q. The adsorption performance of β-cyclodextrin functionalized polyacrylonitrile (PAN) electrospun nanofibers. Shanghai Text. Sci. Technol. 2014, 42, 5–8. [Google Scholar]
- Zhao, L.; Yu, B.; Xue, F.; Xie, J.; Zhang, X.; Wu, R.; Wang, R.; Hu, Z.; Yang, S.; Luo, J. Facile hydrothermal preparation of recyclable S-doped graphene sponge for Cu2+ adsorption. J. Hazard. Mater. 2015, 286, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, L.; Xu, G.; Chen, C. Synthesis of β-cyclodextrin-calix[4]arene coupling product and its adsorption of basic fuchsin and methylene blue from water. Jincl. Phenom. Macro 2013, 75, 147–153. [Google Scholar] [CrossRef]


| Sample | PA/g | β-CD/g | DMF/mL | (mβ-CD/mPA)/% | Conductivity/μS·cm−1 | Viscosity/mPa·s | Fiber’s Diameter/μm |
|---|---|---|---|---|---|---|---|
| a | 2 | 0 | 16 | 0 | 138.9 | 143.3 | 25 |
| b | 2 | 0.5 | 16 | 25 | 125.1 | 154.1 | 12.2 |
| c | 2 | 1 | 16 | 50 | 106.3 | 168.5 | 11.3 |
| d | 2 | 1.5 | 16 | 75 | 94.3 | 187.5 | 10.8 |
| e | 2 | 2 | 16 | 100 | 72.7 | 235.0 | 11.8 |
| Cationic Dyes | Pseudo-First-Order Kinetic | Pseudo-Second-Order Kinetic | ||||
|---|---|---|---|---|---|---|
| qe | k1 | R2 | qe | k2 | R2 | |
| Basic Red 9 | 2.11 | 0.2165 | 0.958 | 2.18 | 0.4452 | 0.995 |
| Basic Yellow 19 | 14.78 | 0.2116 | 0.983 | 15.26 | 0.0639 | 0.997 |
| Basic Red 14 | 3.71 | 0.2073 | 0.995 | 4.01 | 0.2095 | 0.996 |
| Basic Red 46 | 4.66 | 0.3256 | 0.925 | 4.68 | 0.2050 | 0.995 |
| Basic Yellow 28 | 8.73 | 0.2245 | 0.995 | 9.16 | 0.0978 | 0.997 |
| Basic Blue 9 | 2.75 | 0.2807 | 0.983 | 2.80 | 0.3402 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, C.; Zhao, W.; Tu, X.; Zhou, S. Electrospinning Membrane with Polyacrylate Mixed Beta-Cyclodextrin: An Efficient Adsorbent for Cationic Dyes. Polymers 2025, 17, 243. https://doi.org/10.3390/polym17020243
Zheng C, Zhao W, Tu X, Zhou S. Electrospinning Membrane with Polyacrylate Mixed Beta-Cyclodextrin: An Efficient Adsorbent for Cationic Dyes. Polymers. 2025; 17(2):243. https://doi.org/10.3390/polym17020243
Chicago/Turabian StyleZheng, Chunling, Wei Zhao, Xiaoqian Tu, and Shaoqiang Zhou. 2025. "Electrospinning Membrane with Polyacrylate Mixed Beta-Cyclodextrin: An Efficient Adsorbent for Cationic Dyes" Polymers 17, no. 2: 243. https://doi.org/10.3390/polym17020243
APA StyleZheng, C., Zhao, W., Tu, X., & Zhou, S. (2025). Electrospinning Membrane with Polyacrylate Mixed Beta-Cyclodextrin: An Efficient Adsorbent for Cationic Dyes. Polymers, 17(2), 243. https://doi.org/10.3390/polym17020243






