Microbial Adhesion and Cytotoxicity of Heat-Polymerized and 3D-Printed Denture Base Materials when Modified with Dimethylaminohexadecyl Methacrylate and/or 2-Methacryloyloxyethyl Phosphorylcholine as Antimicrobial and Protein-Repellent Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Incorporation of DMAHDM and MPC
2.2. Preparation of the Testing Groups
2.2.1. Heat-Polymerized (HP) Denture Base Material
- (1)
- ProBase Hot control; “Control HP” (n = 10);
- (2)
- ProBase Hot + 1.5% MPC; “1.5% MPC HP” (n = 10);
- (3)
- ProBase Hot + 3% MPC; “3% MPC HP” (n = 10);
- (4)
- ProBase Hot + 1.5% DMAHDM; “1.5% DMAHDM HP” (n = 10);
- (5)
- ProBase Hot + 3% DMAHDM; “3% DMAHDM HP” (n = 10);
- (6)
- ProBase Hot + 3% MPC + 1.5% DMAHDM; “3% MPC + 1.5% DMAHDM HP” (n = 10).
2.2.2. Three-Dimensionally Printed (3DP) Denture Base Material
- (1)
- NextDent Denture 3D + control; “Control 3DP” (n = 10);
- (2)
- NextDent Denture 3D + 1.5% MPC; “1.5% MPC 3DP” (n = 10);
- (3)
- NextDent Denture 3D + 3% MPC; “3% MPC 3DP” (n = 10);
- (4)
- NextDent Denture 3D + 1.5% DMAHDM; “1.5% DMAHDM 3DP” (n = 10);
- (5)
- NextDent Denture 3D + 3% DMAHDM; “3% DMAHDM 3DP” (n = 10);
- (6)
- NextDent Denture 3D + 3% MPC + 1.5% DMAHDM; “3% MPC + 1.5% DMAHDM 3DP” (n = 10).
2.3. Sample Size
2.4. Randomization and Blinding
2.5. Candida albicans CFU Counts
2.6. Cytotoxicity MTT Test
2.7. Statistical Analysis
3. Results
3.1. Candida albicans CFU Counts
3.2. Cytotoxicity MTT Test
4. Discussion
5. Conclusions
- The incorporation of DMAHDM and/or MPC into 3DP is a novel approach filling a gap in the current knowledge.
- The incorporation of DMAHDM and/or MPC into HP and 3DP denture base resin materials significantly reduces microbial adhesion.
- The incorporation of DMAHDM and/or MPC into HP and 3DP denture base resin materials maintains acceptable cytotoxicity levels.
- The incorporation of DMAHDM and/or MPC into HP and 3DP denture base resin materials offers a promising strategy for improving the antimicrobial properties of the modified denture materials.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Revised American Dental Association Specification No. 12 for Denture Base Polymers—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/1089122/ (accessed on 10 February 2022).
- Sivakumar, I.; Arunachalam, K.S.; Sajjan, S.; Ramaraju, A.V.; Rao, B.; Kamaraj, B. Incorporation of antimicrobial macromolecules in acrylic denture base resins: A research composition and update. J. Prosthodont. 2014, 23, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Senpuku, H.; Sogame, A.; Inoshita, E.; Tsuha, Y.; Miyazaki, H.; Hanada, N. Systemic diseases in association with microbial species in oral biofilm from elderly requiring care. Gerontology 2003, 49, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Sumi, Y.; Miura, H.; Sunakawa, M.; Michiwaki, Y.; Sakagami, N. Colonization of denture plaque by respiratory pathogens in dependent elderly. Gerodontology 2002, 19, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Sumi, Y.; Kagami, H.; Ohtsuka, Y.; Kakinoki, Y.; Haruguchi, Y.; Miyamoto, H. High correlation between the bacterial species in denture plaque and pharyngeal microflora. Gerodontology 2003, 20, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef]
- Moura, J.S.; da Silva, W.J.; Pereira, T.; Del Bel Cury, A.A.; Rodrigues Garcia, R.C.M. Influence of acrylic resin polymerization methods and saliva on the adherence of four Candida species. J. Prosthet. Dent. 2006, 96, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Sesma, N.; Laganá, D.C.; Morimoto, S.; Gil, C. Effect of denture surface glazing on denture plaque formation. Braz. Dent. J. 2005, 16, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Inokoshi, M.; Kanazawa, M.; Minakuchi, S. Evaluation of a complete denture trial method applying rapid prototyping. Dent. Mater. J. 2012, 31, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Kattadiyil, M.T.; AlHelal, A. An update on computer-engineered complete dentures: A systematic review on clinical outcomes. J. Prosthet. Dent. 2017, 117, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Altarawneh, S.; Bencharit, S.; Mendoza, L.; Curran, A.; Barrow, D.; Barros, S.; Preisser, J.; Loewy, Z.G.; Gendreau, L.; Offenbacher, S. Clinical and histological findings of denture stomatitis as related to intraoral colonization patterns of Candida albicans, salivary flow, and dry mouth. J. Prosthodont. 2013, 22, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Martori, E.; Ayuso-Montero, R.; Martinez-Gomis, J.; Viñas, M.; Peraire, M. Risk factors for denture-related oral mucosal lesions in a geriatric population. J. Prosthet. Dent. 2014, 111, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.T.; Douglas, W.H. Micro-colonization of the denture-fitting surface by Candida albicans. J. Dent. 1973, 1, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Girard, B.; Landry, R.G.; Giasson, L. [Denture stomatitis: Etiology and clinical considerations]. J. Can. Dent. Assoc. 1996, 62, 808–812. [Google Scholar] [PubMed]
- Bachmann, S.P.; Ramage, G.; VandeWalle, K.; Patterson, T.F.; Wickes, B.L.; López-Ribot, J.L. Antifungal combinations against Candida albicans biofilms in vitro. Antimicrob. Agents Chemother. 2003, 47, 3657–3659. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Mukherjee, P.; Leidich, S.; Faddoul, F.; Hoyer, L.; Douglas, L.; Ghannoum, M. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J. Dent. Res. 2001, 80, 903–908. [Google Scholar] [CrossRef]
- Kuhn, D.M.; George, T.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Antifungal susceptibility of Candida biofilms: Unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 2002, 46, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Lamfon, H.; Porter, S.R.; McCullough, M.; Pratten, J. Susceptibility of Candida albicans biofilms grown in a constant depth film fermentor to chlorhexidine, fluconazole, and miconazole: A longitudinal study. J. Antimicrob. Chemother. 2004, 53, 383–385. [Google Scholar] [CrossRef]
- Imazato, S.; Torii, M.; Tsuchitani, Y.; McCabe, J.F.; Russell, R.R. Incorporation of bacterial inhibitor into resin composite. J. Dent. Res. 1994, 73, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Söderling, E.; Österblad, M.; Vallittu, P.K.; Lassila, L.V.J. Synthesis of methacrylate monomers with antibacterial effects against S. mutans. Molecules 2011, 16, 9755–9763. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Xu, H.H.K. Effects of quaternary ammonium chain length on antibacterial bonding agents. J. Dent. Res. 2013, 92, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Weir, M.D.; Zhang, K.; Deng, D.; Cheng, L.; Xu, H.H.K. Synthesis of new antibacterial quaternary ammonium monomer for incorporation into CaP nanocomposite. Dent. Mater. 2013, 29, 859–870. [Google Scholar] [CrossRef]
- Takahashi, N.; Iwasa, F.; Inoue, Y.; Morisaki, H.; Ishihara, K.; Baba, K. Evaluation of the durability and antiadhesive action of 2-methacryloyloxyethyl phosphorylcholine grafting on an acrylic resin denture base material. J. Prosthet. Dent. 2014, 112, 194–203. [Google Scholar] [CrossRef] [PubMed]
- al-Qarni, F.D.; Tay, F.; Weir, M.D.; Melo, M.A.; Sun, J.; Oates, T.W.; Xie, X.; Xu, H.H. Protein-repelling adhesive resin containing calcium phosphate nanoparticles with repeated ion-recharge and re-releases. J. Dent. 2018, 78, 91–99. [Google Scholar] [CrossRef]
- Ikeya, K.; Iwasa, F.; Inoue, Y.; Fukunishi, M.; Takahashi, N.; Ishihara, K.; Baba, K. Inhibition of denture plaque deposition on complete dentures by 2-methacryloyloxyethyl phosphorylcholine polymer coating: A clinical study. J. Prosthet. Dent. 2018, 119, 67–74. [Google Scholar] [CrossRef]
- Zhang, N.; Melo, M.A.S.; Bai, Y.; Xu, H.H.K. Novel protein-repellent dental adhesive containing 2-methacryloyloxyethyl phosphorylcholine. J. Dent. 2014, 42, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Fukumoto, K.; Iwasaki, Y.; Nakabayashi, N. Modification of polysulfone with phospholipid polymer for improvement of blood compatibility. Part 2. Protein adsorption and platelet adhesion. Biomaterials 1999, 20, 1553–1559. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Aiba, Y.; Morimoto, N.; Nakabayashi, N.; Ishihara, K. Semi-interpenetrating polymer networks composed of biocompatible phospholipid polymer and segmented polyurethane. J. Biomed. Mater. Res. 2000, 52, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Cumming, Z.L.; Goreish, H.H.; Kirkwood, L.C.; Tolhurst, L.A.; Stratford, P.W. Crosslinkable coatings from phosphorylcholine-based polymers. Biomaterials 2001, 22, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Bajunaid, S.O.; Baras, B.H.; Balhaddad, A.A.; Weir, M.D.; Xu, H.H.K. Antibiofilm and protein-repellent polymethylmethacrylate denture base acrylic resin for treatment of denture stomatitis. Materials 2021, 14, 1067. [Google Scholar] [CrossRef] [PubMed]
- Bajunaid, S.O.; Baras, B.H.; Weir, M.D.; Xu, H.H.K. Denture acrylic resin material with antibacterial and protein-repellent properties for the prevention of denture stomatitis. Polymers 2022, 14, 230. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.Y.; Lee, C.H.; Lee, C.J. Antifungal and physical characteristics of modified denture base acrylic incorporated with silver nanoparticles. Gerodontology 2012, 29, e413–e419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Melo, M.A.S.; Weir, M.D.; Reynolds, M.A.; Bai, Y.; Xu, H.H.K. Do dental resin composites accumulate more oral biofilms and plaque than amalgam and glass ionomer materials? Materials 2016, 9, 888. [Google Scholar] [CrossRef]
- AlAzzam, N.F.; Bajunaid, S.O.; Mitwalli, H.A.; Baras, B.H.; Weir, M.D.; Xu, H.H.K. The effect of incorporating dimethylaminohexadecyl methacrylate and/or 2-methacryloyloxyethyl phosphorylcholine on flexural strength and surface hardness of heat-polymerized and 3D-printed denture base materials. Materials 2024, 17, 4625. [Google Scholar] [CrossRef] [PubMed]
- ISO 20795-1:2013; Dentistry—Base Polymers—Part 1: Denture Base Polymers. International Organization for Standardization: Geneva, Switzerland, 2013. Available online: https://www.iso.org/standard/62277.html (accessed on 26 August 2024).
- Urbaniak, G.C.; Plous, S. Research Randomizer. Published online 22 June 2013. Available online: http://www.randomizer.org/ (accessed on 10 February 2022).
- Zarnowski, R.; Sanchez, H.; Andes, D.R. Large-Scale Production and Isolation of Candida Biofilm Extracellular Matrix. Nat. Protoc. 2016, 11, 2320–2327. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/03/64/36406.html (accessed on 17 February 2022).
- Al-Dulaijan, Y.A.; Balhaddad, A.A. Prospects on tuning bioactive and antimicrobial denture base resin materials: A narrative review. Polymers 2023, 15, 54. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT assay: Utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Malinowski, P.; Skała, K.; Jabłońska-Trypuć, A.; Koronkiewicz, A.; Wołejko, E.; Wydro, U.; Świderski, G.; Lewandowski, W. Comparison of the usefulness of MTT and CellTiter-Glo tests applied for cytotoxicity evaluation of compounds from the group of polyphenols. Environ. Sci. Proc. 2022, 18, 9. [Google Scholar] [CrossRef]
- Tolosa, L.; Donato, M.T.; Gómez-Lechón, M.J. General cytotoxicity assessment by means of the MTT assay. In Protocols in In Vitro Hepatocyte Research; Vinken, M., Rogiers, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 333–348. [Google Scholar] [CrossRef]
- Cao, L.; Xie, X.; Wang, B.; Weir, M.D.; Oates, T.W.; Xu, H.H.; Zhang, N.; Bai, Y. Protein-repellent and antibacterial effects of a novel polymethyl methacrylate resin. J. Dent. 2018, 79, 39–45. [Google Scholar] [CrossRef]
- Bajunaid, S.O. How effective are antimicrobial agents on preventing the adhesion of Candida albicans to denture base acrylic resin materials? A systematic review. Polymers 2022, 14, 908. [Google Scholar] [CrossRef]
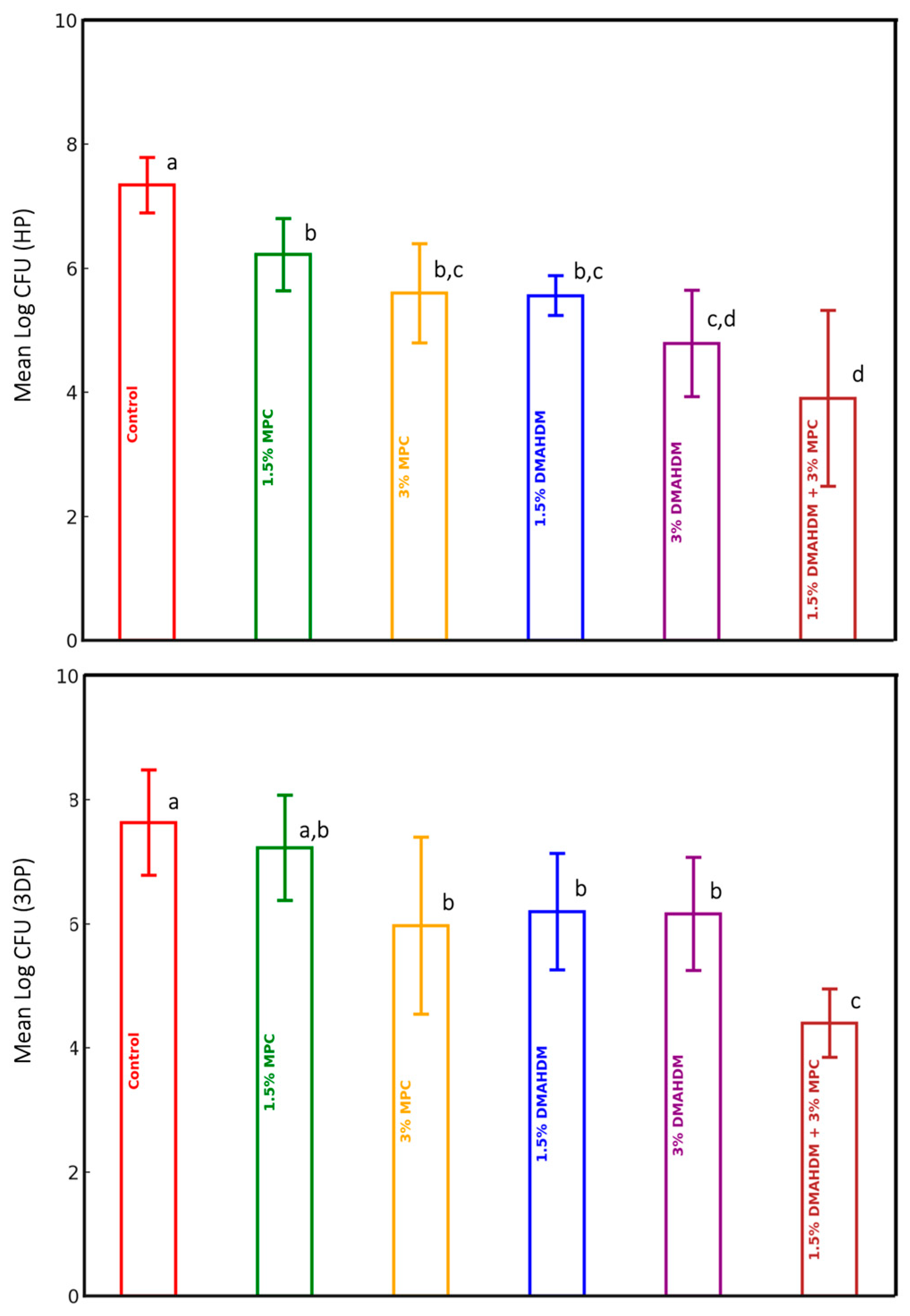

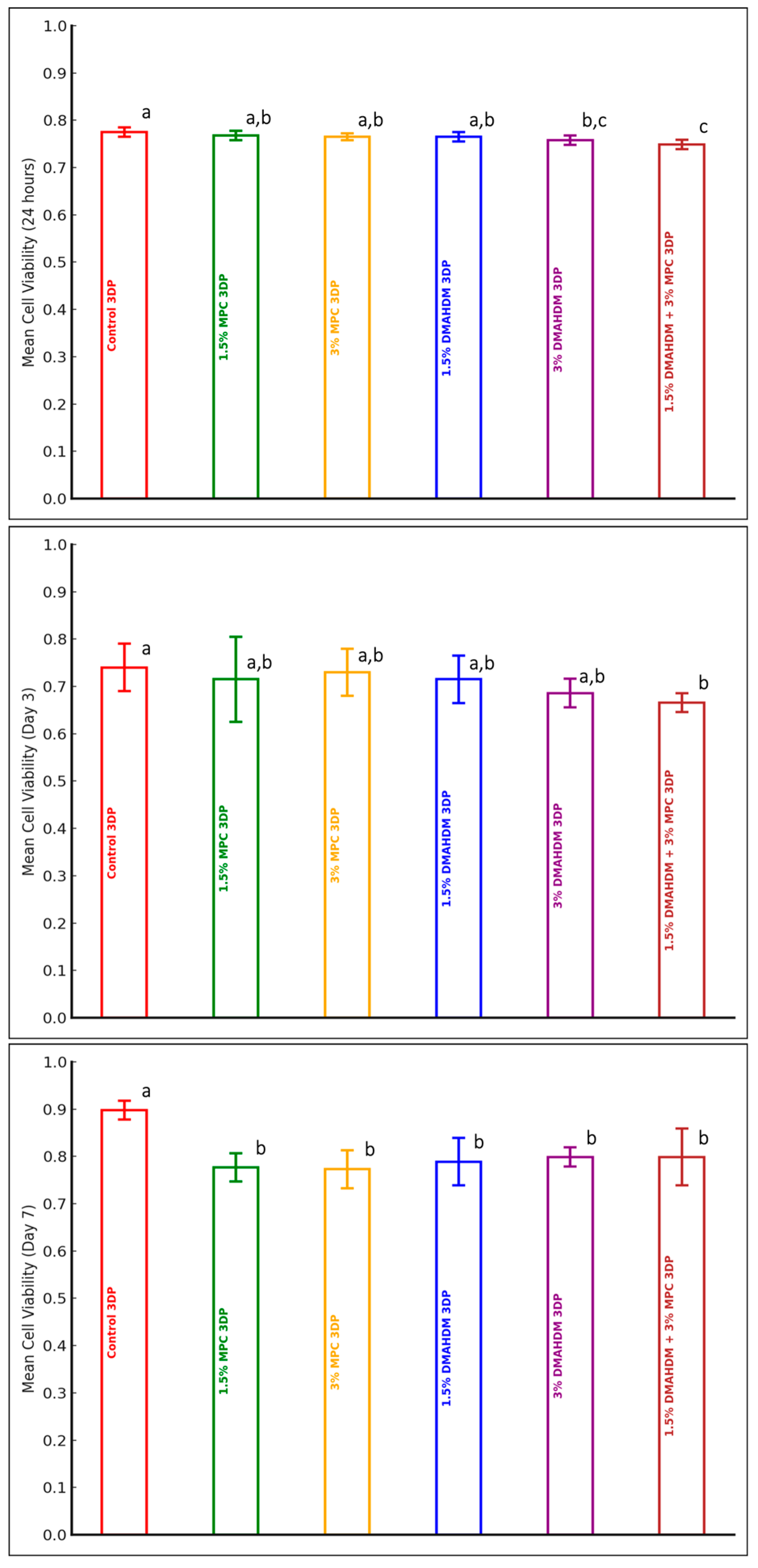
| Groups | Material | |||||
|---|---|---|---|---|---|---|
| HP | 3DP | |||||
| Mean Log CFU (SD) | F-Value | p-Value | Mean Log CFU (SD) | F-Value | p-Value | |
| Control (n = 10) | 7.34 (0.45) | 20.693 | <0.0001 | 7.63 (0.85) | 13.873 | <0.0001 |
| 1.5% MPC (n = 10) | 6.22 (0.58) | 7.23 (0.85) | ||||
| 3% MPC (n = 10) | 5.60 (0.80) | 5.97 (1.43) | ||||
| 1.5% DMAHDM (n = 10) | 5.56 (0.32) | 6.20 (0.94) | ||||
| 3% DMAHDM (n = 10) | 4.79 (0.86) | 6.16 (0.91) | ||||
| 1.5% DMAHDM + 3% MPC (n = 10) | 3.90 (1.42) | 4.40 (0.55) | ||||
| Groups | Time Points | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 h | Day 3 | Day 7 | |||||||
| Mean Cell Viability (SD) | F-Value | p-Value | Mean Cell Viability (SD) | F-Value | p-Value | Mean Cell Viability (SD) | F-Value | p-Value | |
| Control HP (n = 10) | 0.79 (0.07) | 4.830 | 0.001 | 0.77 (0.02) | 1.955 | 0.102 | 0.76 (0.07) | 0.430 | 0.826 |
| 1.5% MPC HP (n = 10) | 0.80 (0.05) | 0.76 (0.01) | 0.77 (0.05) | ||||||
| 3% MPC HP (n = 10) | 0.75 (0.03) | 0.76 (0.01) | 0.76 (0.06) | ||||||
| 1.5% DMAHDM HP (n = 10) | 0.74 (0.01) | 0.76 (0.01) | 0.79 (0.05) | ||||||
| 3% DMAHDM HP (n = 10) | 0.74 (0.01) | 0.75 (0.01) | 0.76 (0.06) | ||||||
| 1.5% DMAHDM + 3% MPC HP (n = 10) | 0.74 (0.01) | 0.77 (0.02) | 0.79 (0.08) | ||||||
| Groups | Time Points | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 h | Day 3 | Day 7 | |||||||
| Mean Cell Viability (SD) | F-Value | p-Value | Mean Cell Viability (SD) | F-Value | p-Value | Mean Cell Viability (SD) | F-Value | p-Value | |
| Control 3DP (n = 10) | 0.775 (0.01) | 7.29 | < 0.0001 | 0.740 (0.05) | 2.49 | 0.043 | 0.898 (0.02) | 10.37 | < 0.0001 |
| 1.5% MPC 3DP (n = 10) | 0.768 (0.01) | 0.715 (0.09) | 0.777 (0.03) | ||||||
| 3% MPC 3DP (n = 10) | 0.765 (0.007) | 0.730 (0.05) | 0.773 (0.04) | ||||||
| 1.5% DMAHDM 3DP (n = 10) | 0.765 (0.01) | 0.715 (0.05) | 0.789 (0.05) | ||||||
| 3% DMAHDM 3DP (n = 10) | 0.758 (0.01) | 0.686 (0.03) | 0.799 (0.02) | ||||||
| 1.5% DMAHDM + 3% MPC 3DP (n = 10) | 0.749 (0.01) | 0.666 (0.02) | 0.799 (0.06) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlAzzam, N.F.; Bajunaid, S.O.; Baras, B.H.; Mitwalli, H.A.; Weir, M.D.; Xu, H.H.K. Microbial Adhesion and Cytotoxicity of Heat-Polymerized and 3D-Printed Denture Base Materials when Modified with Dimethylaminohexadecyl Methacrylate and/or 2-Methacryloyloxyethyl Phosphorylcholine as Antimicrobial and Protein-Repellent Materials. Polymers 2025, 17, 228. https://doi.org/10.3390/polym17020228
AlAzzam NF, Bajunaid SO, Baras BH, Mitwalli HA, Weir MD, Xu HHK. Microbial Adhesion and Cytotoxicity of Heat-Polymerized and 3D-Printed Denture Base Materials when Modified with Dimethylaminohexadecyl Methacrylate and/or 2-Methacryloyloxyethyl Phosphorylcholine as Antimicrobial and Protein-Repellent Materials. Polymers. 2025; 17(2):228. https://doi.org/10.3390/polym17020228
Chicago/Turabian StyleAlAzzam, Njood F., Salwa O. Bajunaid, Bashayer H. Baras, Heba A. Mitwalli, Michael D. Weir, and Hockin H. K. Xu. 2025. "Microbial Adhesion and Cytotoxicity of Heat-Polymerized and 3D-Printed Denture Base Materials when Modified with Dimethylaminohexadecyl Methacrylate and/or 2-Methacryloyloxyethyl Phosphorylcholine as Antimicrobial and Protein-Repellent Materials" Polymers 17, no. 2: 228. https://doi.org/10.3390/polym17020228
APA StyleAlAzzam, N. F., Bajunaid, S. O., Baras, B. H., Mitwalli, H. A., Weir, M. D., & Xu, H. H. K. (2025). Microbial Adhesion and Cytotoxicity of Heat-Polymerized and 3D-Printed Denture Base Materials when Modified with Dimethylaminohexadecyl Methacrylate and/or 2-Methacryloyloxyethyl Phosphorylcholine as Antimicrobial and Protein-Repellent Materials. Polymers, 17(2), 228. https://doi.org/10.3390/polym17020228







