Establishment of Optimal Drug Delivery System and Evaluation of Utilization of Hydrogel Contact Lens According to the Addition Method of Tretinoin and Bovine Serum Albumin
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Nanoparticle Manufacturing
2.3. Polymerization
2.4. Measuring Device and Methods
2.4.1. Evaluation of Optical and Physical Properties of Contact Lens
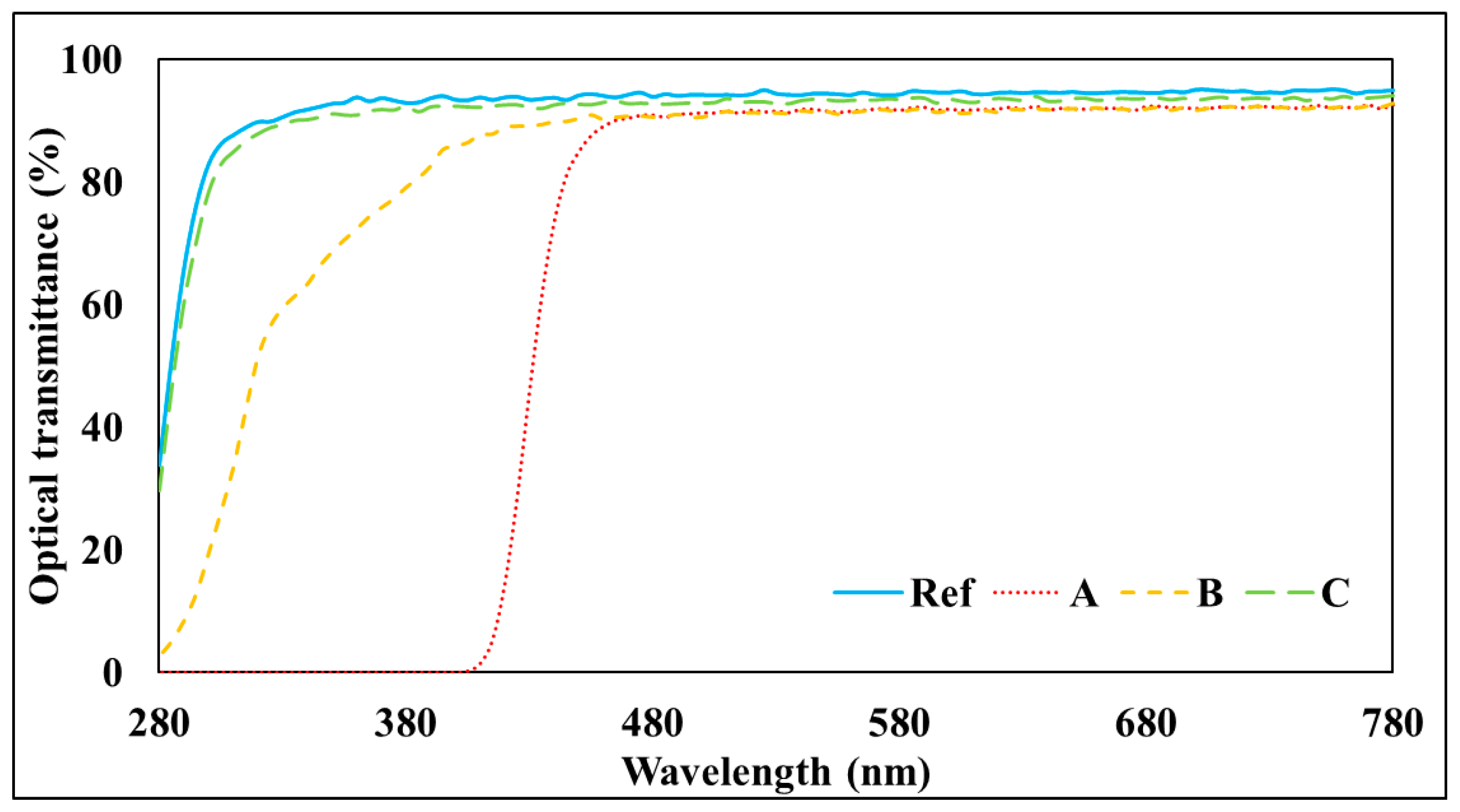
2.4.2. Spectral Transmittance
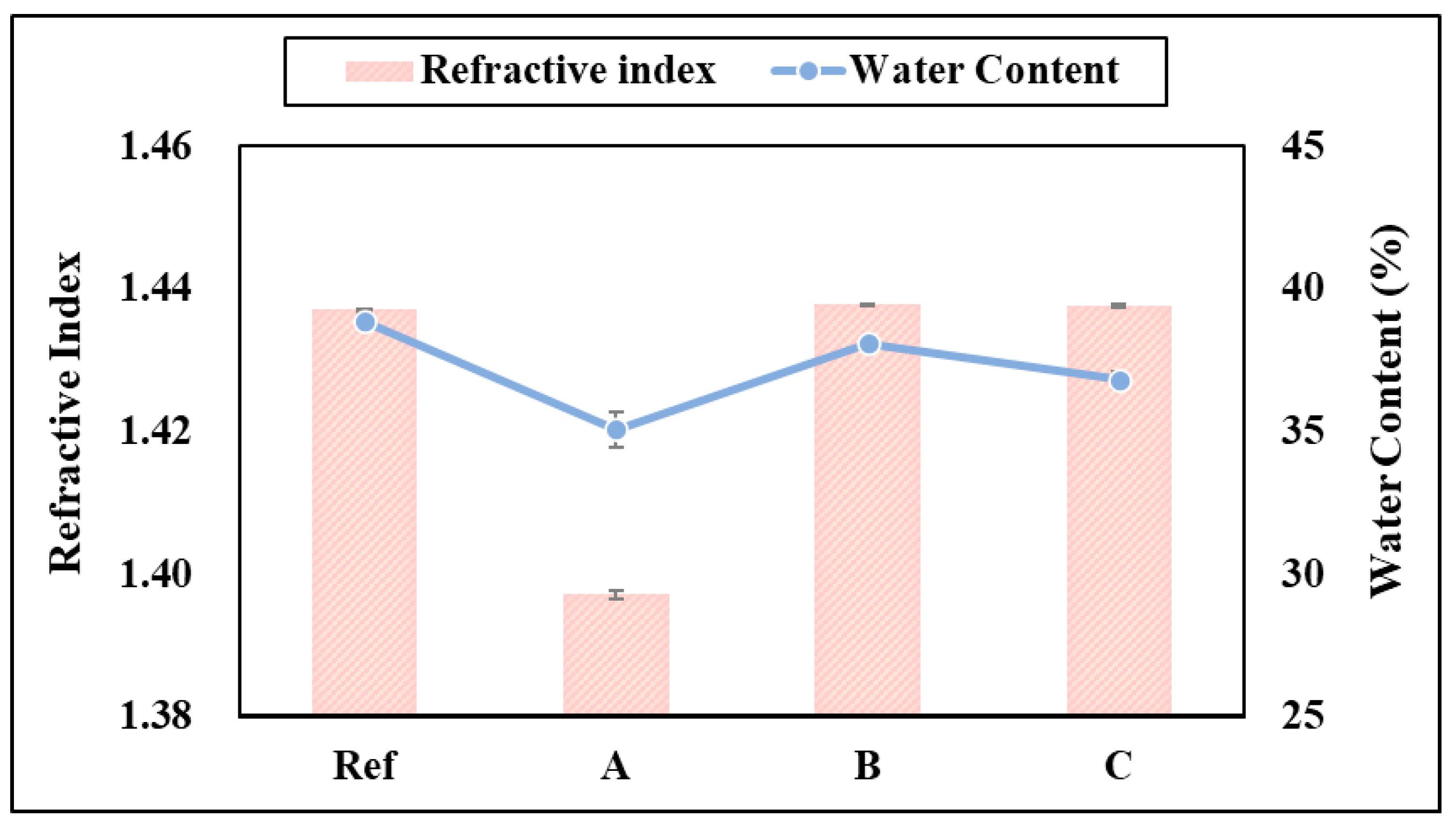
2.4.3. Refractive Index
2.4.4. Water Content
- * : Water content;
- ** : water-contained sample;
- *** : dried sample.
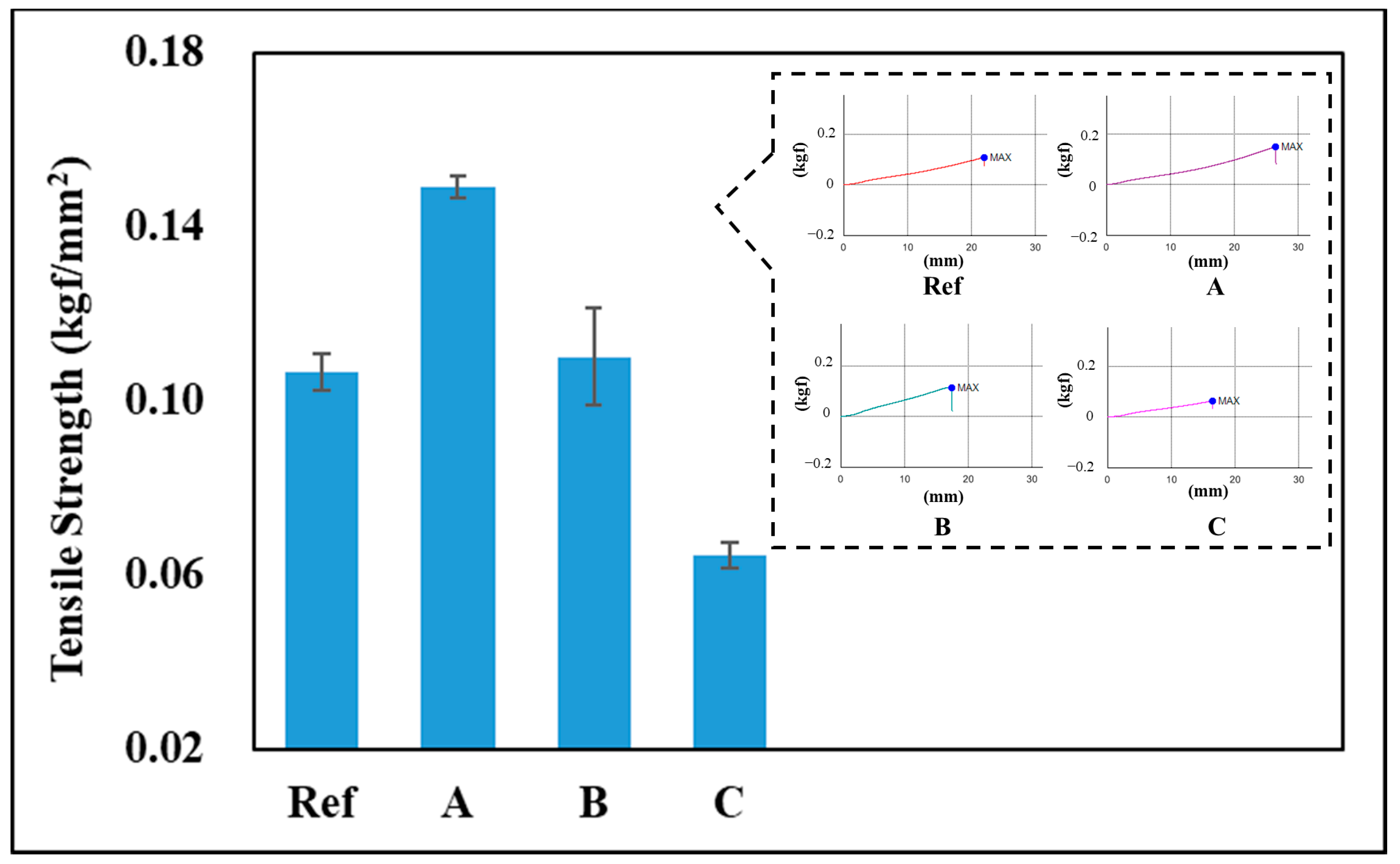
2.4.5. Tensile Strength
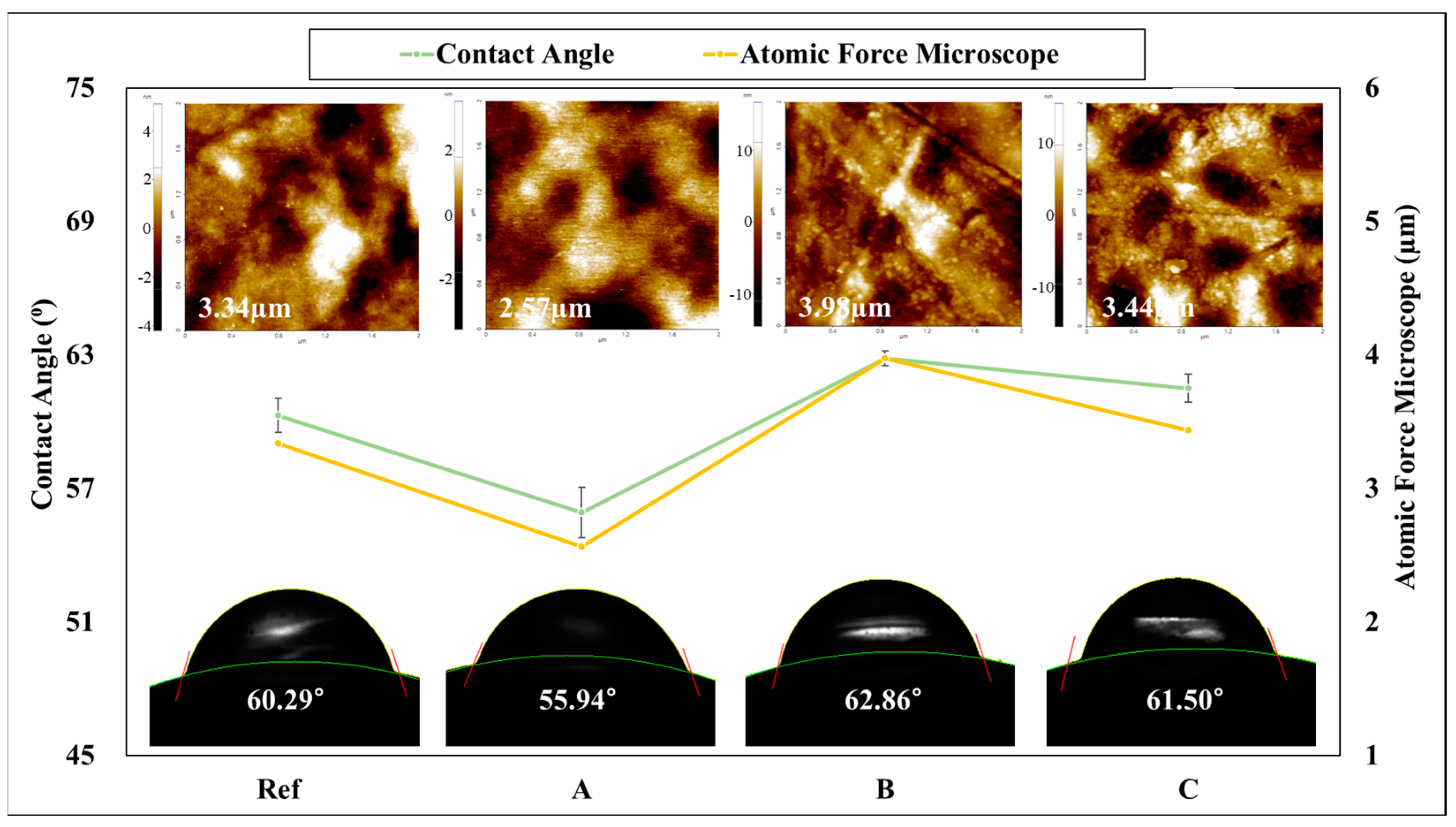
2.4.6. Contact Angle
2.4.7. Atomic Force Microscope (AFM)
2.4.8. Drug Concentration
- * : drug elution concentration;
- ** : molar absorptivity path length;
- *** A: absorbance.
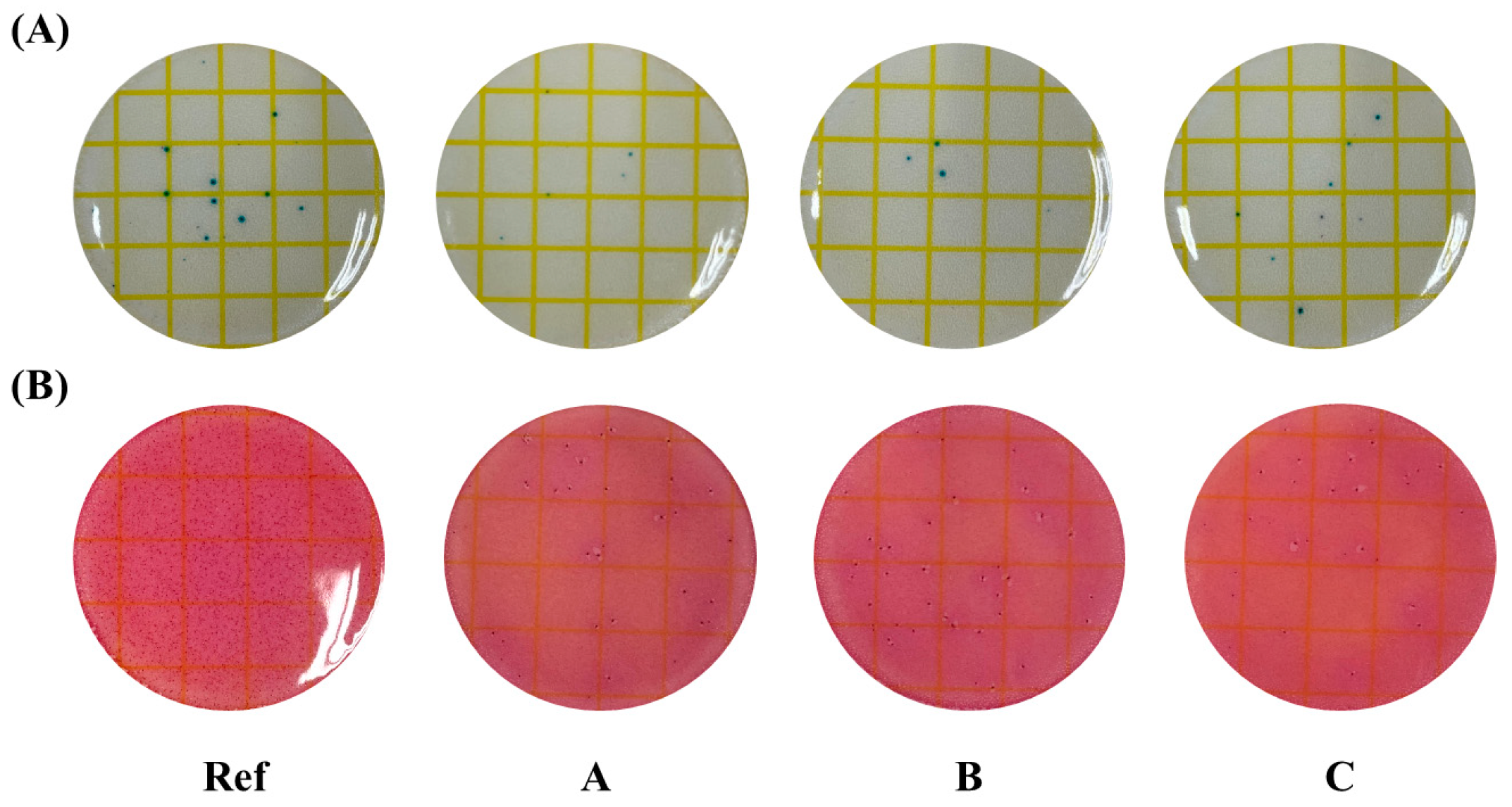
2.4.9. Antimicrobial Property
3. Results and Discussion
3.1. Spectral Transmittance
3.2. Refractive Index and Water Content
3.3. Contact Angle and AFM
3.4. Tensile Strength
3.5. Drug Delivery
3.6. Antibacterial Properties
3.7. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chitte, S.; Gadhari, V. Application of Hydrogel-Polymer in Various Drug Deliveries: An Overview. Int. J. Pharm. Res. Hum. J. 2021, 22, 361–384. [Google Scholar]
- Shailesh, K.S.; Archana, D.; Divya, S.J. Hydrogel: Preparation, Characterization and Applications. Pharma Innov. J. 2017, 6, 25–32. [Google Scholar]
- Neha, K.; Neeraj, K.; Kumar, M.R. Hydrogels for Pharmaceutical and Biomedical Applications. Crit. Rev. Ther. Drug Carr. Syst. 2005, 22, 107–149. [Google Scholar]
- An, H.G.; Lee, H.M. Antioxidant Properties and Characterization of Contact Lenses Containing Tannic Acid and Seaweed Polysaccharides. Polymer 2022, 46, 775–782. [Google Scholar]
- Wang, K.; Hao, Y.; Wang, Y.; Chen, H.; Mao, L.; Deng, Y.; Chen, J.; Yuan, S.; Zhang, T.; Ren, J.; et al. Functional Hydrogels and Their Application in Drug Delivery, Biosensors, and Tissue Engineering. Int. J. Polym. Sci. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Song, S.C.; Jo, J.K.; Jeon, C.S. Drug Delivery Technology Using Hydrogel. NICE 2010, 28, 171–177. [Google Scholar]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A. Pre-clinical Immunotoxicity Studies of Nanotechnology-Formulated Drugs: Challenges, Considerations and Strategy. J. Control. Release 2015, 220, 571–583. [Google Scholar] [CrossRef]
- Kwak, K.M.; Kim, J.U.; Kim, M.I. Recent Advances in Research on Nanotechnology-based Drug Delivery System. Korean Soc. Biotechnol. Bioeng. J. 2023, 38, 203–211. [Google Scholar] [CrossRef]
- Gugleva, V.; Velichka, A. Recent Progress of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as ocular drug delivery platforms. Pharmaceuticals 2023, 16, 474. [Google Scholar] [CrossRef]
- Hong, S.Y.; Park, H.H. Drug Delivery System Using Protein Nanoparticles. Korean Soc. Biotechnol. Bioeng. J. 2020, 35, 10–22. [Google Scholar]
- Raghu, S.; Krunal, P.; Sunita, P. Bovine Serum Albumin Nanoparticles for the Efficient Delivery of Berberine: Preparation, Characterization and In vitro biological studies. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125501. [Google Scholar]
- Wang, L.; Hess, A.; Chang, T.Z.; Wang, Y.C.; Champion, J.A.; Compans, R.W.; Wang, B.Z. Nanoclusters self-assem bled from conformation-stabilized influenza M2e as broadly cross protective influenza vaccines. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 473–482. [Google Scholar] [CrossRef]
- Kim, Y.J. Ophthalmic Drug Delivery System Using Contact Lens. Ann. Optom. Contact Lens 2023, 22, 48–51. [Google Scholar] [CrossRef]
- Korea Pharmaceutial Information Center. Drug Encylopedia. Available online: https://www.health.kr/Menu.PharmReview/_uploadfiles/%EC%A7%80%EC%9A%A9%EC%84%B1%20%EB%B9%84%ED%83%80%EB%AF%BC.pdf (accessed on 24 October 2024).
- Kryczyk-Poprawa, A.; Kwiecień, A.; Opoka, W. Photostability of Topical Agents Applied to the Skin: A Review. Pharmaceutics 2020, 12, 10. [Google Scholar] [CrossRef]
- Tseng, S.C.G. Topical tretinoin treatment for severe dry-eye disorders. J. Am. Acad. Dermatol. 1986, 15, 860–866. [Google Scholar] [CrossRef]
- Tseng, S.C.G.; Maumenee, A.E.; Stark, W.J.; Maumenee, I.H.; Jensen, A.D.; Green, W.R.; Kenyon, K.R. Topical Retinoid Treatment for Various Dry-eye Disorders. Ophthalmology 1985, 92, 717–727. [Google Scholar] [CrossRef]
- Kim, H.J.; Lim, S.L. Adsorption of Antibiotics on Serum Albumin Nanoparticle. Clean Technol. 2021, 27, 55–60. [Google Scholar]
- Meng, F.; Liu, F.; Lan, M.; Zou, T.; Li, L.; Cai, T.; Cai, Y. Preparation and evaluation of folate-modified albumin baicalin-loaded nanoparticles for the targeted treatment of breast cancer. J. Drug Deliv. Sci. Technol. 2021, 65, 102603. [Google Scholar] [CrossRef]
- ISO 8599:1994; Optics and Optical Instruments—Contact Lenses—Determination of the Spectral and Luminous Transmittance. International Standard: Genève, Switzerland, 1994.
- ANSI Z80.20:2004; Ophthalmics—Contact Lenses—Standard Terminology, Tolerances, Measurements and Physicochemical Properties. American National Standards Institute: Merrifield, VA, USA, 2004.
- Huh, C.; Mason, S.G. Effects of surface roughness on wetting (theoretical). J. Colloid Interface Sci. 1977, 60, 11–38. [Google Scholar] [CrossRef]
- Sterner, O.; Aeschlimann, R.; Zurcher, S.; Scales, C.; Riederer, D.; Spencer, N.D.; Tosatti, S.G.P. Tribological Classification of Contact Lenses: From Coefficient of Friction to Sliding Work. Tribol. Lett. 2016, 63, 1–13. [Google Scholar] [CrossRef]
- Park, J.H. A study on Antibacterial and Antioxidant Activity of Contact Lens Made of Natural Functional Substances. Ph.D. Thesis, Department of Chemistry Graduate School of Chosun University, Gwangju, Korea, 2024. [Google Scholar]
- Kearns, V.R.; Williams, R.L. Drug Delivery Systems for the Eye. Expert Rev. Med. Devices 2009, 6, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Lee, S.K.; Hwang, S.H.; Kim, H. Photocatalytic deposition of silver nanoparticles onto organic/inorganic composite nanofibers. Macromol. Mater. Eng. 2006, 291, 1265–1270. [Google Scholar] [CrossRef]

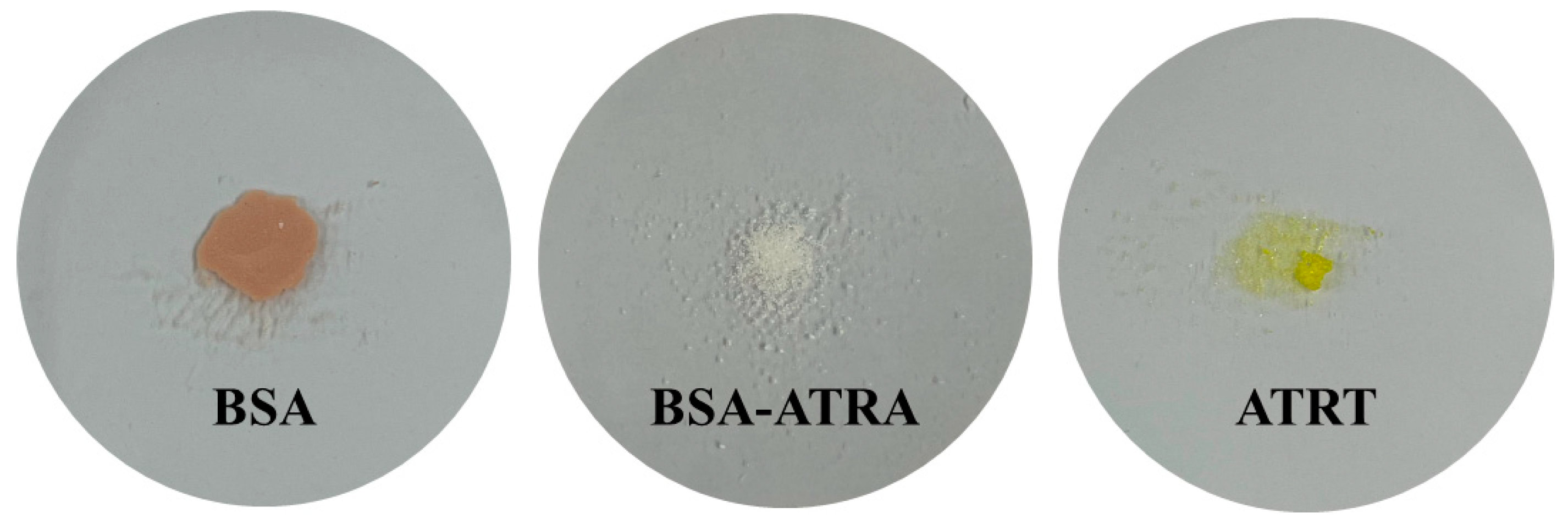


| Sample | HEMA | EGDMA | 2H2M | ATRA | BSA | BSA-ATRA |
|---|---|---|---|---|---|---|
| Ref | 98.52 | 0.99 | 0.49 | - | - | - |
| A | 97.54 | 0.98 | 0.49 | 0.99 | ||
| B | 97.06 | 0.97 | 0.49 | 0.49 | 0.99 | |
| C | 97.54 | 0.98 | 0.49 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-I.; Sung, A.-Y. Establishment of Optimal Drug Delivery System and Evaluation of Utilization of Hydrogel Contact Lens According to the Addition Method of Tretinoin and Bovine Serum Albumin. Polymers 2025, 17, 159. https://doi.org/10.3390/polym17020159
Park H-I, Sung A-Y. Establishment of Optimal Drug Delivery System and Evaluation of Utilization of Hydrogel Contact Lens According to the Addition Method of Tretinoin and Bovine Serum Albumin. Polymers. 2025; 17(2):159. https://doi.org/10.3390/polym17020159
Chicago/Turabian StylePark, Hye-In, and A-Young Sung. 2025. "Establishment of Optimal Drug Delivery System and Evaluation of Utilization of Hydrogel Contact Lens According to the Addition Method of Tretinoin and Bovine Serum Albumin" Polymers 17, no. 2: 159. https://doi.org/10.3390/polym17020159
APA StylePark, H.-I., & Sung, A.-Y. (2025). Establishment of Optimal Drug Delivery System and Evaluation of Utilization of Hydrogel Contact Lens According to the Addition Method of Tretinoin and Bovine Serum Albumin. Polymers, 17(2), 159. https://doi.org/10.3390/polym17020159






