Advancements in Oral Delivery Systems for Probiotics Based on Polysaccharides
Abstract
1. Introduction
2. Polysaccharides for Probiotic Encapsulation
2.1. Cellulose
2.2. Chitosan
2.3. Alginate
2.4. Starch
2.5. Gum Arabic
3. Preparation Technique for Probiotic ODSs
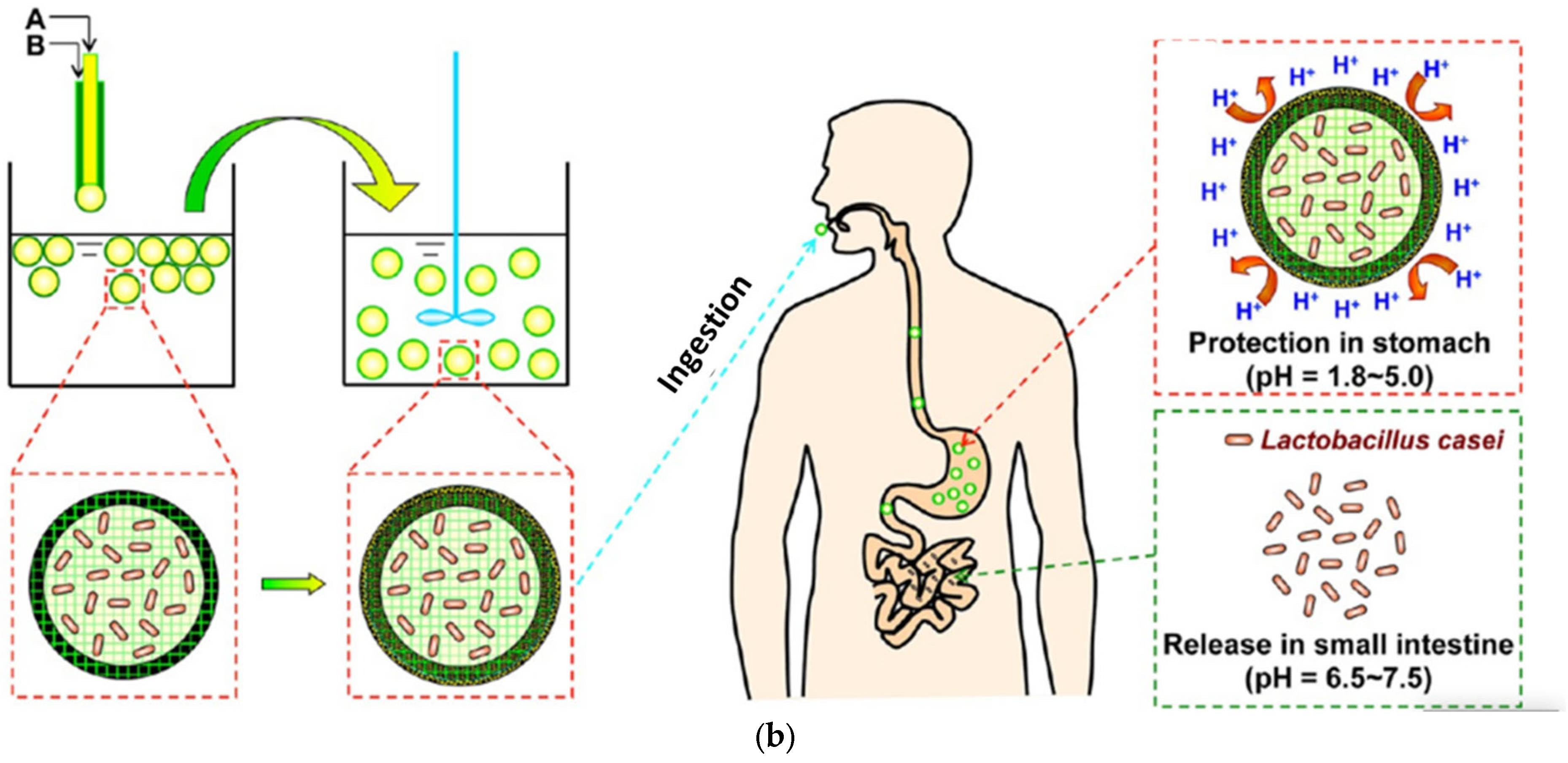
3.1. Extrusion
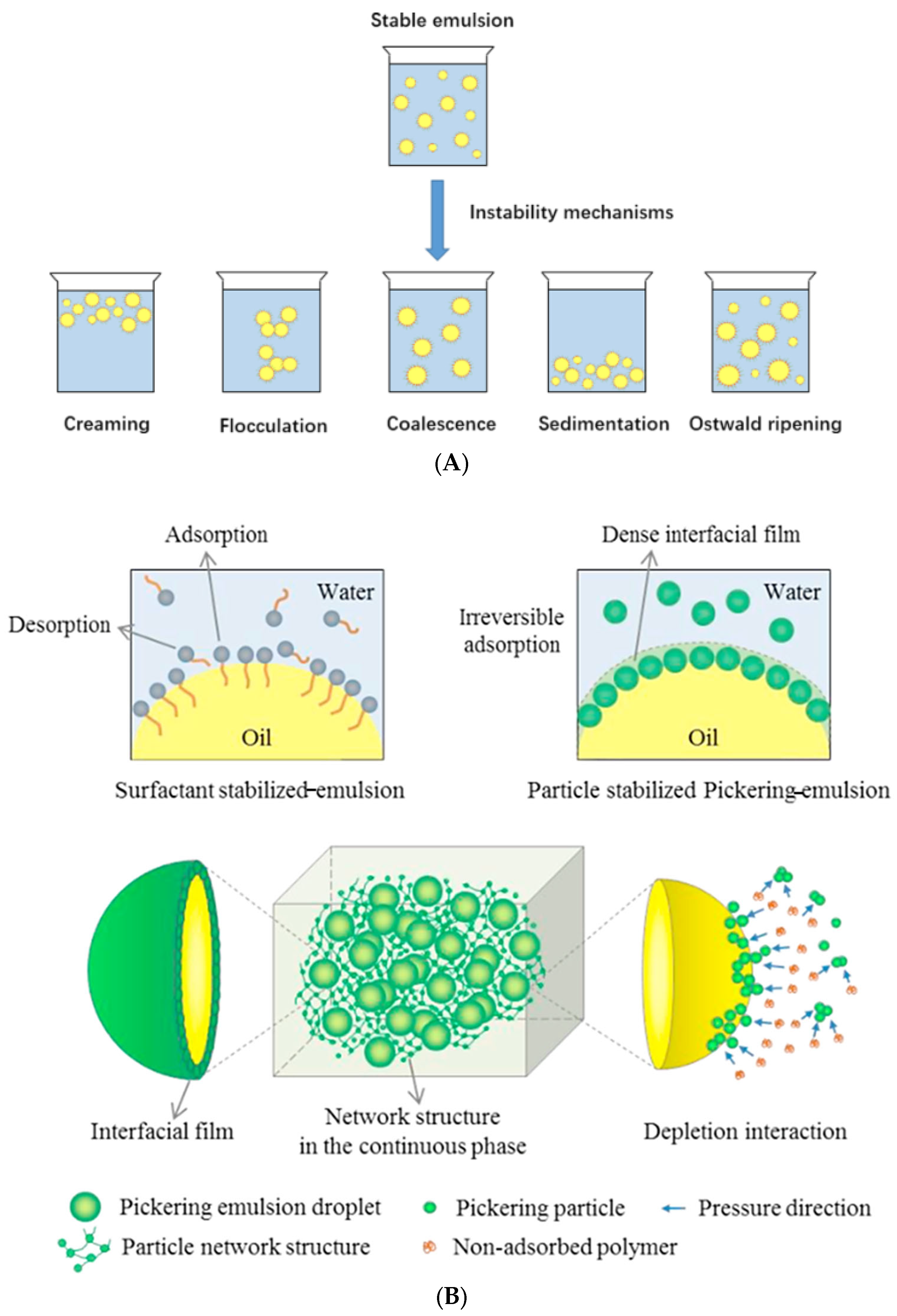
3.2. Emulsion
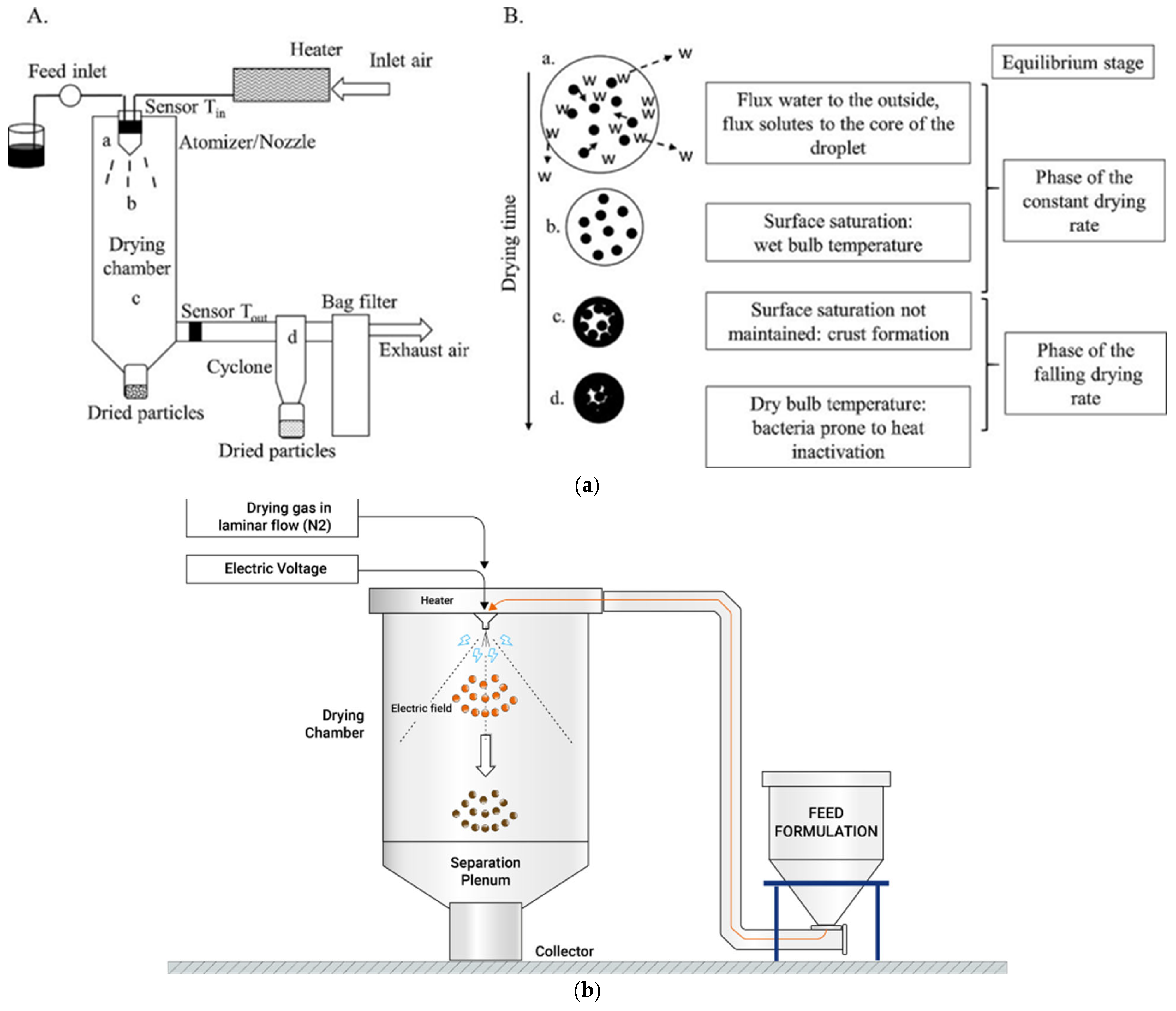
3.3. Spray Drying
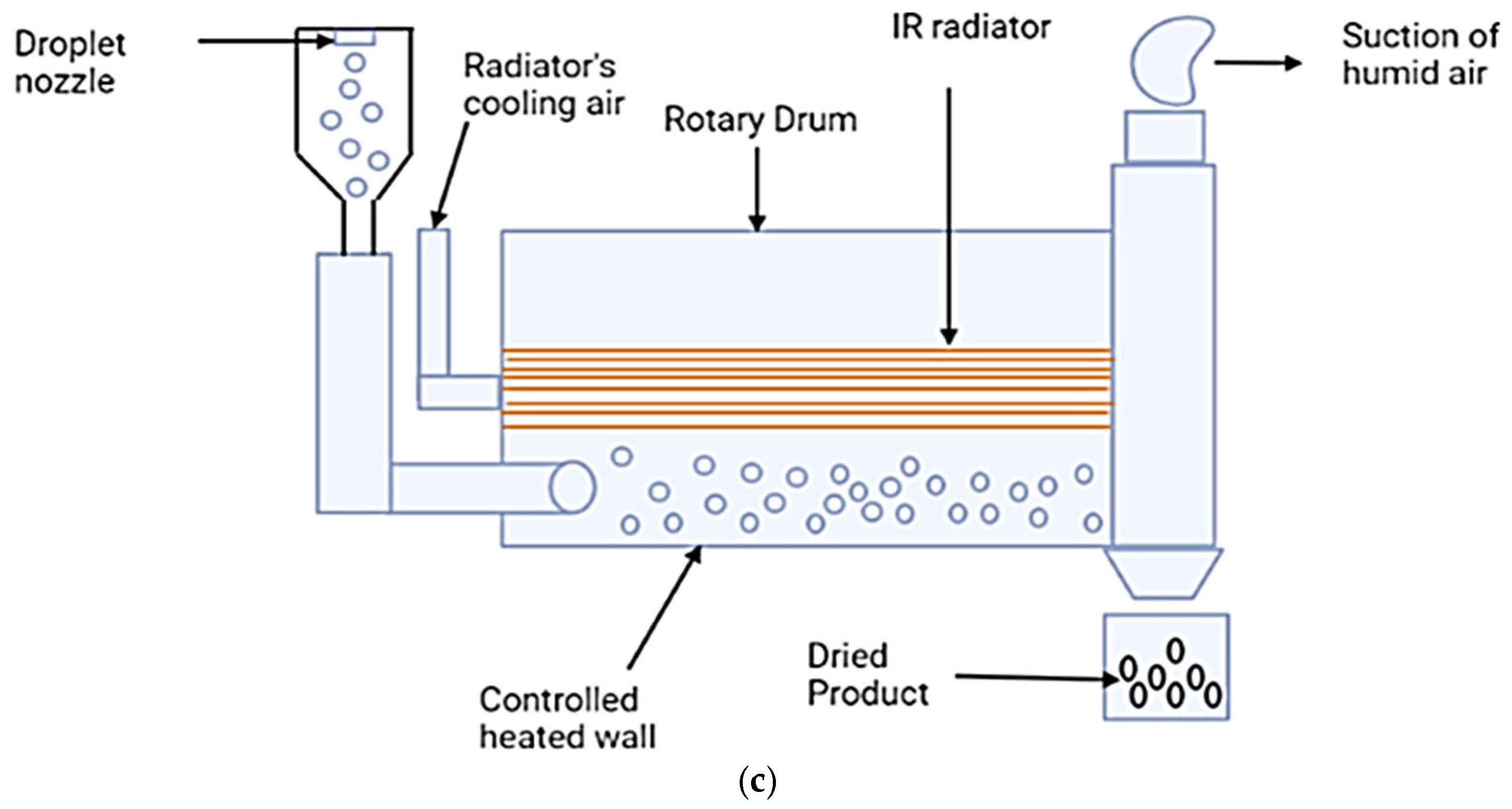
3.4. Innovative Technologies
4. Challenges and Future Prospects for Probiotic ODSs
4.1. Probiotic Viability Retention During the Dehydration Process
4.2. Core-Shell Probiotic Microparticle Based on a Polyelectrolyte Complex
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Maftei, N.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The potential impact of probiotics on human health: An update on their health-promoting properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Dudun, A.A.; Chesnokova, D.V.; Voinova, V.V.; Bonartsev, A.P.; Bonartseva, G.A. Changes in the gut microbiota composition during implantation of composite scaffolds based on poly(3-hydroxybutyrate) and alginate on the large-intestine wall. Polymers 2023, 15, 3649. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Arraño, V.; Martín-Peláez, S. Effects of probiotics and synbiotics on weight loss in subjects with overweight or obesity: A systematic review. Nutrients 2021, 13, 3627. [Google Scholar] [CrossRef]
- Huang, Y.P.; Shi, J.Y.; Luo, X.T.; Luo, S.C.; Cheung PC, K.; Corke, H.; Yang, Q.Q.; Zhang, B.B. How do probiotics alleviate constipation? A narrative review of mechanisms. Crit. Rev. Biotechnol. 2024, 45, 80–96. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Maleki, D.P.; Badehnoosh, B.; Asemi, Z.; Hallajzadeh, J.; Mansournia, M.A.; Yousefi, B.; Moazzami, B.; Chaichian, S. Anti-tumor activities of probiotics in cervical cancer. J. Ovarian Res. 2020, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Embong, H.; Othman, F.; Ghazi, H.F.; Maruthey, N.; Bahari, H. Probiotics for alzheimer’s disease: A systematic review. Nutrients 2021, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Oak, S.J.; Jha, R. The effects of probiotics in lactose intolerance: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1675–1683. [Google Scholar] [CrossRef]
- Qu, Q.Y.; He, P.P.; Zhang, Y.Q.; Yang, S.J.; Zeng, P.B. The intervention of probiotics on type 2 diabetes mellitus in animal models. Mol. Nutr. Food Res. 2024, 68, e2200815. [Google Scholar] [CrossRef] [PubMed]
- Umeano, L.; Iftikhar, S.; Alhaddad, S.F.; Paulsingh, C.N.; Riaz, M.F.; Garg, G.; Mohammed, L. Effectiveness of probiotic use in alleviating symptoms of irritable bowel syndrome: A systematic review. Cureus J. Med. Sci. 2024, 16, e58306. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.J.; Wang, J.Y. Children with atopic dermatitis show clinical improvement afterlactobacillus exposure. Clin. Exp. Allergy 2015, 45, 779–787. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.Q.; Wu, C.X. Modulation of gut microbiota and immune system by probiotics, pre-biotics, and post-biotics. Front. Nutr. 2021, 8, 634897. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Yu, Z.; Zhang, Z.; Liang, X.; Gong, P.M.; Yi, H.X.; Yang, L.Q.; Liu, T.J.; Shi, H.P.; Zhang, L.W. Bifidobacterium animalis f1-7 in combination with konjac glucomannan improves constipation in mice via humoral transport. Food Funct. 2021, 12, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Riezzo, G.; Chimienti, G.; Orlando, A.; D’Attoma, B.; Clemente, C.; Russo, F. Effects of long-term administration of lactobacillus reuteri Dsm-17938 on circulating levels of 5-Ht and BDNF in adults with functional constipation. Benef. Microbes 2019, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Meng, X.Y.; Wang, Y.; Zhang, J.; Zhao, Y.; Zheng, Z.C.; Zhang, T. Effects of probiotics on gastric cancer-related inflammation: A systematic review and meta-analysis. J. Food Biochem. 2022, 46, e14034. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Zhong, Q.X. Drying of probiotics to enhance the viability during preparation, storage, food application, and digestion: A review. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13287. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, I.; Kwiecień, M. Application of polysaccharide-based hydrogels as probiotic delivery systems. Gels 2018, 4, 47. [Google Scholar] [CrossRef]
- Han, C.T.; Fang, L.; Song, S.X.; Min, W.L. Polysaccharides-based delivery system for efficient encapsulation and controlled release of food-derived active peptides. Carbohydr. Polym. 2022, 291, 119580. [Google Scholar] [CrossRef]
- Wang, D.D.; Wang, W.B.; Wang, P.; Wang, C.; Niu, J.T.; Liu, Y.; Chen, Y.Z. Research progress of colon-targeted oral hydrogel system based on natural polysaccharides. Int. J. Pharm. 2023, 643, 123222. [Google Scholar] [CrossRef]
- Asgari, S.; Pourjavadi, A.; Licht, T.R.; Boisen, A.; Ajalloueian, F. Polymeric carriers for enhanced delivery of probiotics. Adv. Drug Deliv. Rev. 2020, 161–162, 1–21. [Google Scholar] [CrossRef]
- Villena MJ, M.; Lara-Villoslada, F.; Martínez MA, R.; Hernández ME, M. Development of gastro-resistant tablets for the protection and intestinal delivery of Lactobacillus fermentum cect 5716. Int. J. Pharm. 2015, 487, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; De Souza, C.; Ramachandran, M.; Wang, S.; Yi, H.; Ma, Z.; Zhang, L.; Lin, K. Precise oral delivery systems for probiotics: A review. J. Control. Release 2022, 352, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Arca, H.C.; Mosquera-Giraldo, L.I.; Bi, V.; Xu, D.Q.; Taylor, L.S.; Edgar, K.J. Pharmaceutical applications of cellulose ethers and cellulose ether esters. Biomacromolecules 2018, 19, 2351–2376. [Google Scholar] [CrossRef] [PubMed]
- Savitskaya, I.; Zhantlessova, S.; Kistaubayeva, A.; Ignatova, L.; Sinyavskiy, Y.; Shokatayeva, D.; Kushugulova, A.; Digel, I. Prebiotic cellulose—Pullulan matrix as a “vehicle” for probiotic biofilm delivery to the host large intestine. Polymers 2024, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, A.B.; Dufresne, A.; Boufi, S. Cellulose nanocrystal as ecofriendly stabilizer for emulsion polymerization and its application for waterborne adhesive. Carbohydr. Polym. 2020, 229, 115504. [Google Scholar] [CrossRef]
- Çanga, E.M.; Dudak, F.C. Improved digestive stability of probiotics encapsulated within poly(vinyl alcohol)/cellulose acetate hybrid fibers. Carbohydr. Polym. 2021, 264, 117990. [Google Scholar] [CrossRef]
- Dusso, D.; Salomon, C.J. Solving the delivery of lactococcus lactis: Improved survival and storage stability through the bioencapsulation with different carriers. J. Food Sci. 2023, 88, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Salmons, B.; Gunzburg, W.H. Release characteristics of cellulose sulphate capsules and production of cytokines from encapsulated cells. Int. J. Pharm. 2018, 548, 15–22. [Google Scholar] [CrossRef]
- Gunzburg, W.H.; Aung, M.M.; Toa, P.; Ng, S.; Read, E.; Tan, W.J.; Brandtner, E.M.; Dangerfield, J.; Salmons, B. Efficient protection of microorganisms for delivery to the intestinal tract by cellulose sulphate encapsulation. Microb. Cell Factories 2020, 19, 216. [Google Scholar] [CrossRef]
- Lupașcu, R.E.; Ghica, M.V.; Dinu-Pîrvu, C.; Popa, L.; Velescu, B.S.; Arsene, A.L. An overview regarding microbial aspects of production and applications of bacterial cellulose. Materials 2022, 15, 676. [Google Scholar] [CrossRef]
- Płoska, J.; Garbowska, M.; Pluta, A.; Stasiak-Różańska, L. Bacterial cellulose-innovative biopolymer and possibilities of its applications in dairy industry. Int. Dairy J. 2023, 140, 105586. [Google Scholar] [CrossRef]
- Fijałkowski, K.; Peitler, D.; Rakoczy, R.; Żywicka, A. Survival of probiotic lactic acid bacteria immobilized in different forms of bacterial cellulose in simulated gastric juices and bile salt solution. LWT—Food Sci. Technol. 2016, 68, 322–328. [Google Scholar] [CrossRef]
- Chitprasert, P.; Sudsai, P.; Rodklongtan, A. Aluminum carboxymethyl cellulose-rice bran microcapsules: Enhancing survival of Lactobacillus reuteri KUB-AC5. Carbohydr. Polym. 2012, 90, 78–86. [Google Scholar] [CrossRef]
- Li, W.; Luo, X.G.; Song, R.; Zhu, Y.; Li, B.; Liu, S.L. Porous cellulose microgel particle: A fascinating host for the encapsulation, protection, and delivery of Lactobacillus plantarum. J. Agric. Food Chem. 2016, 64, 3430–3436. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Xie, Y.X.; Li, Y.; Li, B.; Zhang, Y.Y.; Liu, S.L. Water-in-water emulsion stabilized by cellulose nanocrystals and their high enrichment effect on probiotic bacteria. J. Colloid Interface Sci. 2023, 633, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. The role of chitosan on oral delivery of peptide-loaded nanoparticle formulation. J. Drug Target. 2018, 26, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.Q.; Wang, T.; Sun, M.J.; Chen, X.G.; Liu, Y. Advances and applications of chitosan-based nanomaterials as oral delivery carriers: A review. Int. J. Biol. Macromol. 2020, 154, 433–445. [Google Scholar] [CrossRef]
- Collado-González, M.; González Espinosa, Y.; Goycoolea, F.M. Interaction between chitosan and mucin: Fundamentals and applications. Biomimetics 2019, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Osuna, I.; Vauthier, C.; Farabollini, A.; Palmieri, G.F.; Ponchel, G. Mucoadhesion mechanism of chitosan and thiolated chitosan-poly(isobutyl cyanoacrylate) core-shell nanoparticles. Biomaterials 2007, 28, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ma, Z.; De Souza, C.; Wang, S.L.; Qiao, F.Z.; Yi, H.X.; Gong, P.M.; Zhang, Z.; Liu, T.J.; Zhang, L.W.; et al. Microfluidic fabrication of encapsulated probiotic microspheres using cysteine-modified chitosan with dual functions of bacterial adhesion and intestinal mucosal adhesion. Food Hydrocoll. 2024, 149, 109602. [Google Scholar] [CrossRef]
- Tu, J.; Xu, Y.L.; Xu, J.Q.; Ling, Y.; Cai, Y.Q. Chitosan nanoparticles reduce LPS-induced inflammatory reaction via inhibition of NF-κB pathway in Caco-2 cells. Int. J. Biol. Macromol. 2016, 86, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Letocha, A.; Miastkowska, M.; Sikora, E. Preparation and characteristics of alginate microparticles for food, pharmaceutical and cosmetic applications. Polymers 2022, 14, 3834. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles-a review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Peng, P.; Feng, T.; Yang, X.; Nie, C.F.; Yu, L.F.; Ding, R.; Zhou, Q.; Jiang, X.Q.; Li, P. Gastrointestinal microenvironment responsive nanoencapsulation of probiotics and drugs for synergistic therapy of intestinal diseases. ACS Nano. 2023, 17, 14718–14730. [Google Scholar] [CrossRef]
- Mirmazloum, I.; Ladanyi, M.; Omran, M.; Papp, V.; Ronkainen, V.P.; Ponya, Z.; Papp, I.; Nemedi, E.; Kiss, A. Co-encapsulation of probiotic lactobacillus acidophilus and reishi medicinal mushroom (Ganoderma lingzhi) extract in moist calcium alginate beads. Int. J. Biol. Macromol. 2021, 192, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Zhu, Y.N.; Song, J.Y.; Yang, J.; Pan, C.; Xu, T.; Zhang, L. Novel balanced charged alginate/pei polyelectrolyte hydrogel that resists foreign-body reaction. ACS Appl. Mater. Interfaces 2018, 10, 6879–6886. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.Q.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y.P. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.; Afzaal, M.; Saeed, F.; Ahmad, A.; Imran, A.; Akram, N.; Asghar, A.; Shah, Y.A.; Ateeq, H.; Khan, M.R.; et al. Effect of starch-based nanoparticles on the viability and stability of probiotics under adverse conditions. Int. J. Food Prop. 2023, 26, 1841–1854. [Google Scholar] [CrossRef]
- Cortés, R.N.F.; Martínez, M.G.; Guzmán, I.V.; Llano SL, A.; Grosso CR, F.; Bustos, F.M. Evaluation of modified amaranth starch as shell material for encapsulation of probiotics. Cereal Chem. 2014, 91, 300–308. [Google Scholar] [CrossRef]
- Ashwar, B.A.; Gani, A.; Gani, A.; Shah, A.; Masoodi, F.A. Production of rs4 from rice starch and its utilization as an encapsulating agent for targeted delivery of probiotics. Food Chem. 2018, 239, 287–294. [Google Scholar] [CrossRef]
- Ma, J.G.; Li, T.Z.; Wang, Q.Y.; Xu, C.; Yu, W.; Yu, H.L.; Wang, W.; Feng, Z.B.; Chen, L.J.; Hou, J.C.; et al. Enhanced viability of probiotics encapsulated within synthetic/natural biopolymers by the addition of gum arabic via electrohydrodynamic processing. Food Chem. 2023, 413, 135680. [Google Scholar] [CrossRef] [PubMed]
- Arslan-Tontul, S.; Erbas, M. Single and double layered microencapsulation of probiotics by spray drying and spray chilling. Lwt-Food Sci. Technol. 2017, 81, 160–169. [Google Scholar] [CrossRef]
- Reyes, V.; Chotiko, A.; Chouljenko, A.; Campbell, V.; Liu, C.; Theegala, C.; Sathivel, S. Influence of wall material on production of spray dried lactobacillus plantarum nrrl b-4496 and its viability at different storage conditions. Dry. Technol. 2018, 36, 1738–1748. [Google Scholar] [CrossRef]
- Azeem, M.; Saeed, F.; Afzaal, M.; Ateeq, H.; Ahmad, A.; Liaqat, A.; Busquets, R.; Lorenzo, J.M.; Asif Shah, M. Encapsulation of probiotics in solid lipid micro particle for improved viability and stability under stressed conditions. Int. J. Food Prop. 2023, 26, 1612–1623. [Google Scholar] [CrossRef]
- Fareed, F.; Saeed, F.; Afzaal, M.; Imran, A.; Ahmad, A.; Mahmood, K.; Shah, Y.A.; Hussain, M.; Ateeq, H. Fabrication of electrospun gum arabic–polyvinyl alcohol blend nanofibers for improved viability of the probiotic. J. Food Sci. Technol. 2022, 59, 4812–4821. [Google Scholar] [CrossRef] [PubMed]
- How, Y.H.; Lai, K.W.; Pui, L.P.; In LL, A. Co-extrusion and extrusion microencapsulation: Effect on microencapsulation efficiency, survivability through gastrointestinal digestion and storage. J. Food Process Eng. 2022, 45, e13985. [Google Scholar] [CrossRef]
- Tan, L.L.; Mahotra, M.; Chan, S.Y.; Loo SC, J. In situ alginate crosslinking during spray-drying of lactobacilli probiotics promotes gastrointestinal-targeted delivery. Carbohydr. Polym. 2022, 286, 119279. [Google Scholar] [CrossRef]
- Yang, X.Y.; Nie, W.M.; Wang, C.; Fang, Z.L.; Shang, L.R. Microfluidic-based multifunctional microspheres for enhanced oral co-delivery of probiotics and postbiotics. Biomaterials 2024, 308, 122564. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.B.; Chen, J.; Li, S.; Zhang, J.; Zhu, C.E.; Ran, H.; Luo, M.; Pan, X.; Hu, H.; Wu, C. Preparation of acid-resistant microcapsules with shell-matrix structure to enhance stability of Streptococcus thermophilus iffi 6038. J. Food Sci. 2017, 82, 1978–1984. [Google Scholar] [CrossRef] [PubMed]
- Bennacef, C.; Desobry, S.; Probst, L.; Desobry-Banon, S. Alginate based core-shell capsules production through coextrusion methods: Recent applications. Foods 2023, 12, 1788. [Google Scholar] [CrossRef]
- Mei, L.; He, F.; Zhou, R.Q.; Wu, C.D.; Liang, R.; Xie, R.; Ju, X.J.; Wang, W.; Chu, L.Y. Novel intestinal-targeted Ca-alginate-based carrier for pH-responsive protection and release of lactic acid bacteria. Acs Appl. Mater. Interfaces 2014, 6, 5962–5970. [Google Scholar] [CrossRef]
- Farahmand, A.; Ghorani, B.; Emadzadeh, B.; Sarabi-Jamab, M.; Emadzadeh, M.; Modiri, A.; Tucker, N. Millifluidic-assisted ionic gelation technique for encapsulation of probiotics in double-layered polysaccharide structure. Food Res. Int. 2022, 160, 111699. [Google Scholar] [CrossRef]
- Li, Y.; Pei, Y.Q.; Shan, Z.Y.; Jiang, Y.M.; Cui, S.W.; He, Z.Y.; Zhang, Y.; Wang, H. A pH-sensitive w/o/w emulsion-bound carboxymethyl chitosan-alginate hydrogel bead system through the maillard reaction for probiotics intestine-targeted delivery. Food Hydrocoll. 2024, 153, 109956. [Google Scholar] [CrossRef]
- Sun, C.Y.; Wang, S.N.; Huang, X.Y.; Zhang, G.C.; Zhou, D.Y.; Wang, P.; Liu, H. Enhancing probiotic viability: Impact of soy hull polysaccharide concentration on stabilized high-internal-phase emulsions encapsulated with Lactobacillus plantarum and their release during gastrointestinal digestive. Food Hydrocoll. 2024, 152, 109959. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, C.M.; Tian, B.; Xu, J.L.; Wang, X.Y.; Dai, H.X.; Wang, H.; Xu, F.; Wang, C. 3D printed spiral tube-like cellulose scaffold for oral delivery of probiotics. Sci. Adv. 2024, 10, eadp3654. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Feng, J.R.; Sun, P.L.; Xiang, N.; Lu, B.Y.; Qiu, D. Recent advances in improving stability of food emulsion by plant polysaccharides. Food Res. Int. 2020, 137, 109376. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.Z.; Zhao, S.L.; Guan, X.; Mcclements, D.J.; Liu, X.B.; Liu, F.G.; Ngai, T. Polysaccharide-based pickering emulsions: Formation, stabilization and applications. Food Hydrocoll. 2021, 119, 106812. [Google Scholar] [CrossRef]
- Xie, Y.X.; Liu, C.; Zhang, J.; Li, Y.; Li, B.; Liu, S.L. Crosslinking alginate at water-in-water pickering emulsions interface to control the interface structure and enhance the stress resistance of the encapsulated probiotics. J. Colloid Interface Sci. 2024, 655, 653–663. [Google Scholar] [CrossRef]
- Ali, U.; Saeed, M.; Ahmad, Z.; Waseem, M.; Shah, F.; Khan, M.A.; Tariq, M.R.; Hafeez, H.; Kiran, W.; Nayik, G.A.; et al. Effect of high-speed homogenisation on the morphological characteristics and survivability of lactobacillus rhamnosus gg-coated microbeads. Int. J. Food Sci. Technol. 2024, 59, 2771–2779. [Google Scholar] [CrossRef]
- He, X.; Qin, Y.Y.; Liu, H.Y.; Cheng, K.; Yang, W.S.; Qin, X.S. Dual-responsive “egg-box” shaped microgel beads based on w1/o/w2 double emulsions for colon-targeted delivery of synbiotics. Foods 2024, 13, 2163. [Google Scholar] [CrossRef]
- Ding, X.Q.; Xu, Y.B.; Wang, Y.Y.; Xie, L.Y.; Liang, S.; Li, D.L.; Wang, Y.X.; Wang, J.S.; Zhan, X.A. Carboxymethyl konjac glucomannan-chitosan complex nanogels stabilized double emulsions incorporated into alginate hydrogel beads for the encapsulation, protection and delivery of probiotics. Carbohydr. Polym. 2022, 289, 119438. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, W.S.; Qin, X.S. Ultrasound-assisted multilayer pickering emulsion fabricated by wpi-egcg covalent conjugates for encapsulating probiotics in colon-targeted release. Ultrason. Sonochemistry 2023, 97, 106450. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.Y.; Guo, S.Y.; Cheung, P.C.; Hileuskaya, K.; You, L.J. Protection and delivery of probiotics by degraded fucoidan-chitosan nanogel-based w1 /o/w2 double emulsion incorporated in self-assembled hydrogel particles. Food Hydrocoll. 2024, 153, 109999. [Google Scholar] [CrossRef]
- Ruan, M.J.; Xie, Y.X.; Zhou, C.Y.; Li, Y.; Li, B.; Zhang, Y.Y.; Liu, S.L. Biomacromolecule based water-in-water pickering emulsion: A fascinating artificial cell-like compartment for the encapsulation of Lactobacillus plantarum. Food Biosci. 2023, 55, 102916. [Google Scholar] [CrossRef]
- Broeckx, G.; Vandenheuvel, D.; Claes IJ, J.; Lebeer, S.; Kiekens, F. Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. Int. J. Pharm. 2016, 505, 303–318. [Google Scholar] [CrossRef]
- Dantas, A.; Piella-Rifà, M.; Costa, D.P.; Felipe, X.; Gou, P. Innovations in spray drying technology for liquid food processing: Design, mechanisms, and potential for application. Appl. Food Res. 2024, 4, 100382. [Google Scholar] [CrossRef]
- Chhabra, N.; Arora, M.; Garg, D.; Samota, M.K. Spray freeze drying-a synergistic drying technology and its applications in the food industry to preserve bioactive compounds. Food Control 2024, 155, 110099. [Google Scholar] [CrossRef]
- Feng, K.; Huangfu, L.; Liu, C.; Bonfili, L.; Xiang, Q.; Wu, H.; Bai, Y. Electrospinning and electrospraying: Emerging techniques for probiotic stabilization and application. Polymers 2023, 15, 2402. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Maudhuit, A.; Gaiani, C.; Desobry, S. Encapsulation of bioactive compounds using competitive emerging techniques: Electrospraying, nano spray drying, and electrostatic spray drying. J. Food Eng. 2023, 339, 111260. [Google Scholar] [CrossRef]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R. Double layer co-encapsulation of probiotics and prebiotics by electro-hydrodynamic atomization. Food Sci. Technol. 2019, 110, 102–109. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, V.K.; Singh, R.; Dar, A.H. Spray-freeze-drying as emerging and substantial quality enhancement technique in food industry. Food Sci. Biotechnol. 2024, 33, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Semyonov, D.; Ramon, O.; Kaplun, Z.; Levin-Brener, L.; Gurevich, N.; Shimoni, E. Microencapsulation of lactobacillus paracasei by spray freeze drying. Food Res. Int. 2010, 43, 193–202. [Google Scholar] [CrossRef]
- Koster, K.L.; Lei, Y.P.; Anderson, M.; Martin, S.; Bryant, G. Effects of vitrified and nonvitrified sugars on phosphatidylcholine fluid-to-gel phase transitions. Biophys. J. 2000, 78, 1932–1946. [Google Scholar] [CrossRef]
- Pan, C.; Li, J.; Hou, W.; Lin, S.; Wang, L.; Pang, Y.; Wang, Y.; Liu, J. Polymerization-mediated multifunctionalization of living cells for enhanced cell-based therapy. Adv. Mater. 2021, 33, 2007379. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.; Castro, H.; Kirby, R. Evidence of membrane lipid oxidation of spray-dried lactobacillus bulgaricus during storage. Lett. Appl. Microbiol. 1996, 22, 34–38. [Google Scholar] [CrossRef]
- Santivarangkna, C.; Kulozik, U.; Foerst, P. Inactivation mechanisms of lactic acid starter cultures preserved by drying processes. J. Appl. Microbiol. 2008, 105, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cui, S.W.; Chen, M.S.; Li, Y.; Liang, R.; Xu, F.F.; Zhong, F. Protective approaches and mechanisms of microencapsulation to the survival of probiotic bacteria during processing, storage and gastrointestinal digestion: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2863–2878. [Google Scholar] [CrossRef]
- Bommasamudram, J.; Muthu, A.; Devappa, S. Studies on different polysaccharides used as carrier material for spray-drying of synbiotics and its viability under different storage conditions. Bioact. Carbohydr. Diet. Fibre 2024, 31, 100421. [Google Scholar] [CrossRef]
- Tao, T.; Ding, Z.; Hou, D.P.; Prakash, S.; Zhao, Y.N.; Fan, Z.P.; Zhang, D.M.; Wang, Z.P.; Liu, M.; Han, J. Influence of polysaccharide as co-encapsulant on powder characteristics, survival and viability of microencapsulated Lactobacillus paracasei Lpc-37 by spray drying. J. Food Eng. 2019, 252, 10–17. [Google Scholar] [CrossRef]
- Cazorla-Luna, R.; Martin-Illana, A.; Notario-Perez, F.; Ruiz-Caro, R.; Veiga, M.D. Naturally occurring polyelectrolytes and their use for the development of complex-based mucoadhesive drug delivery systems: An overview. Polymers 2021, 13, 2241. [Google Scholar] [CrossRef]
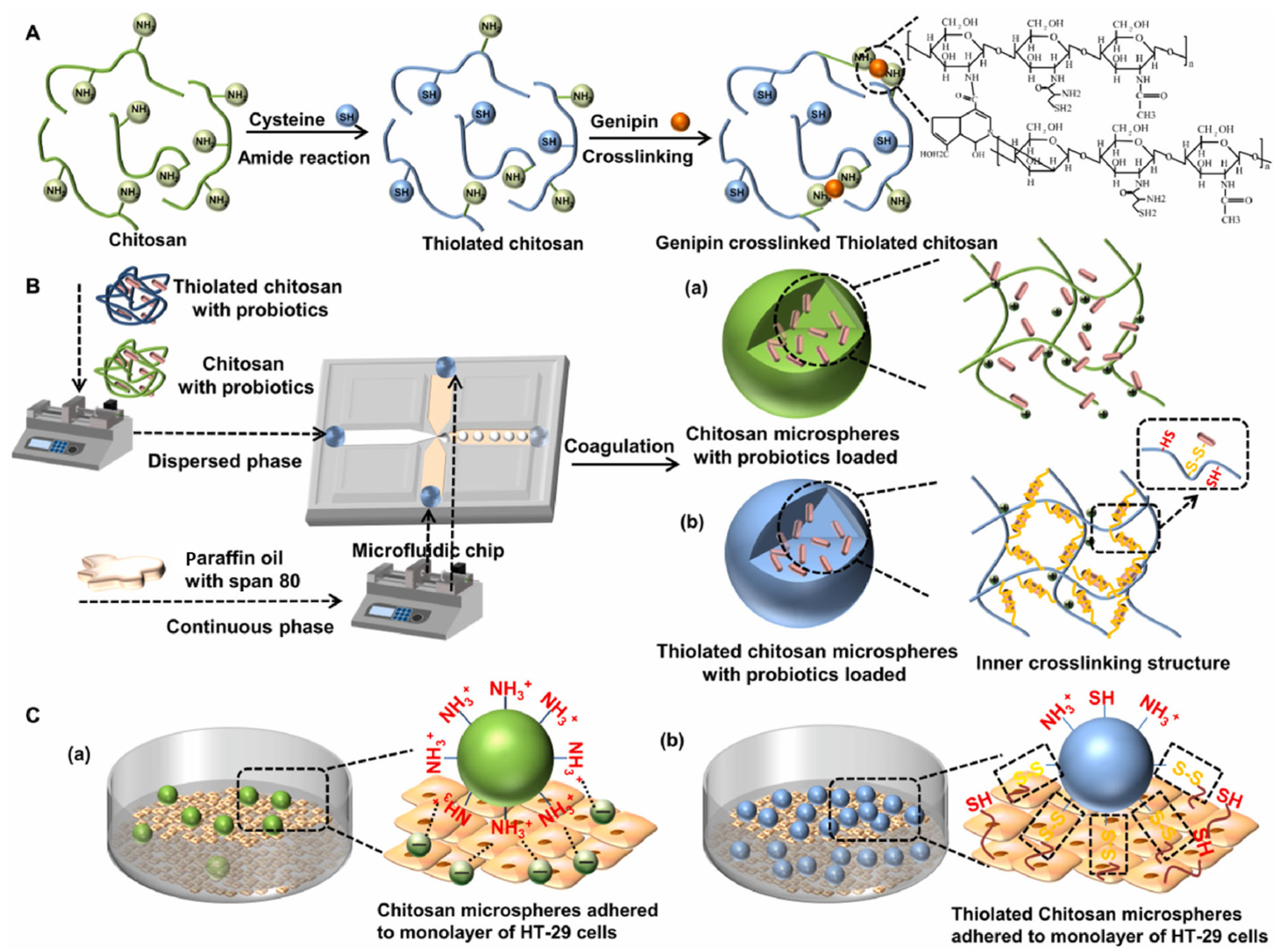
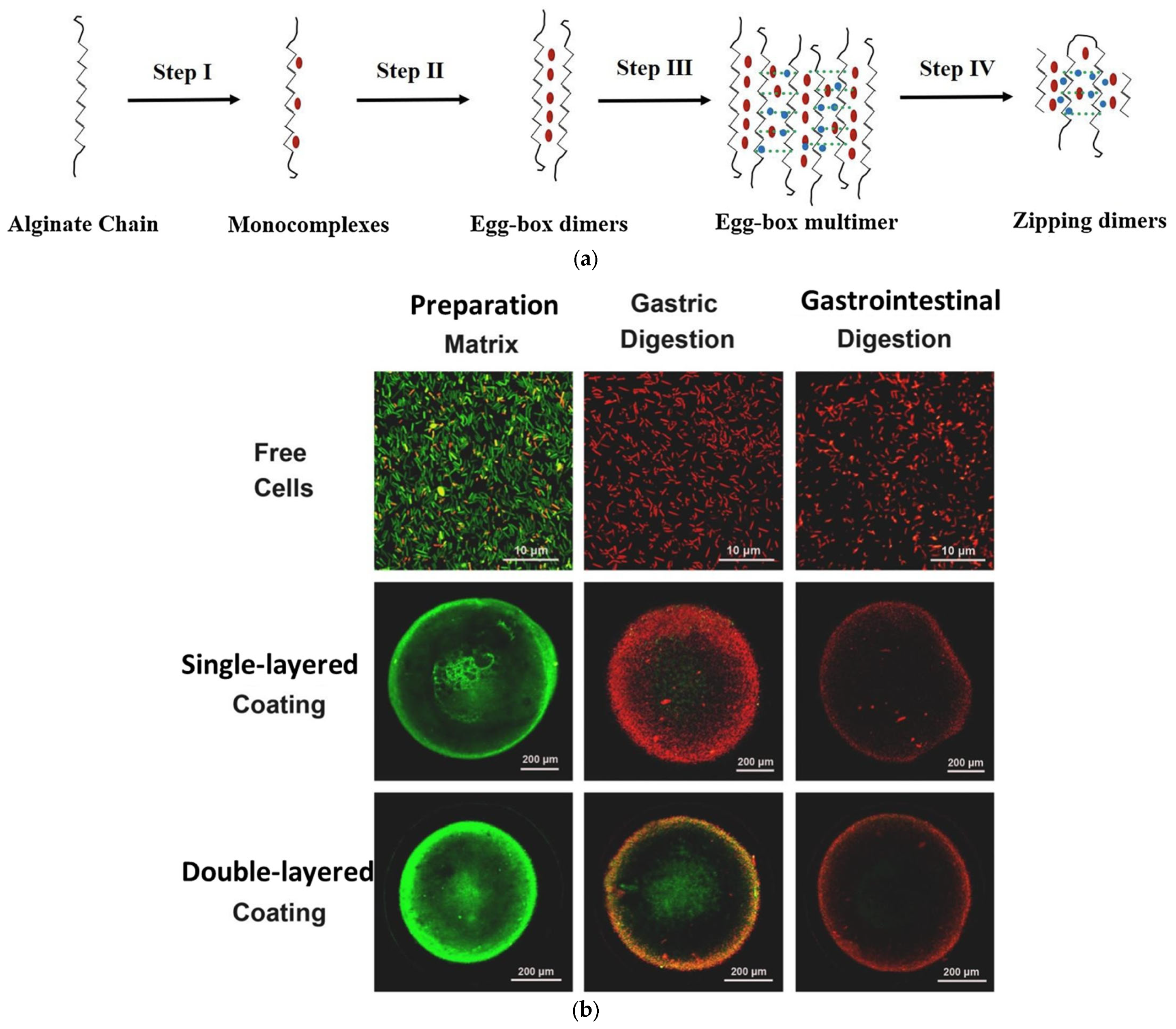
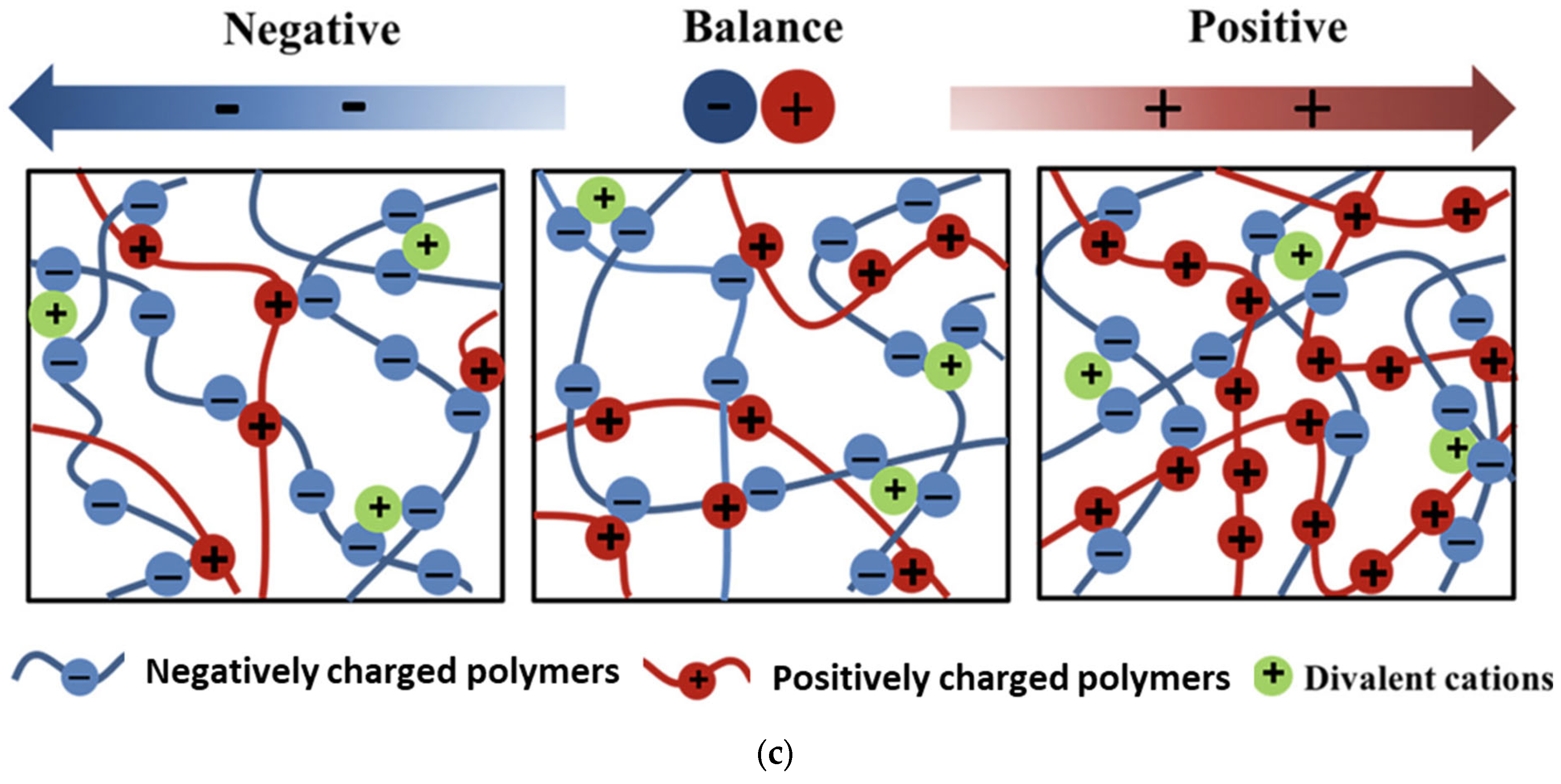
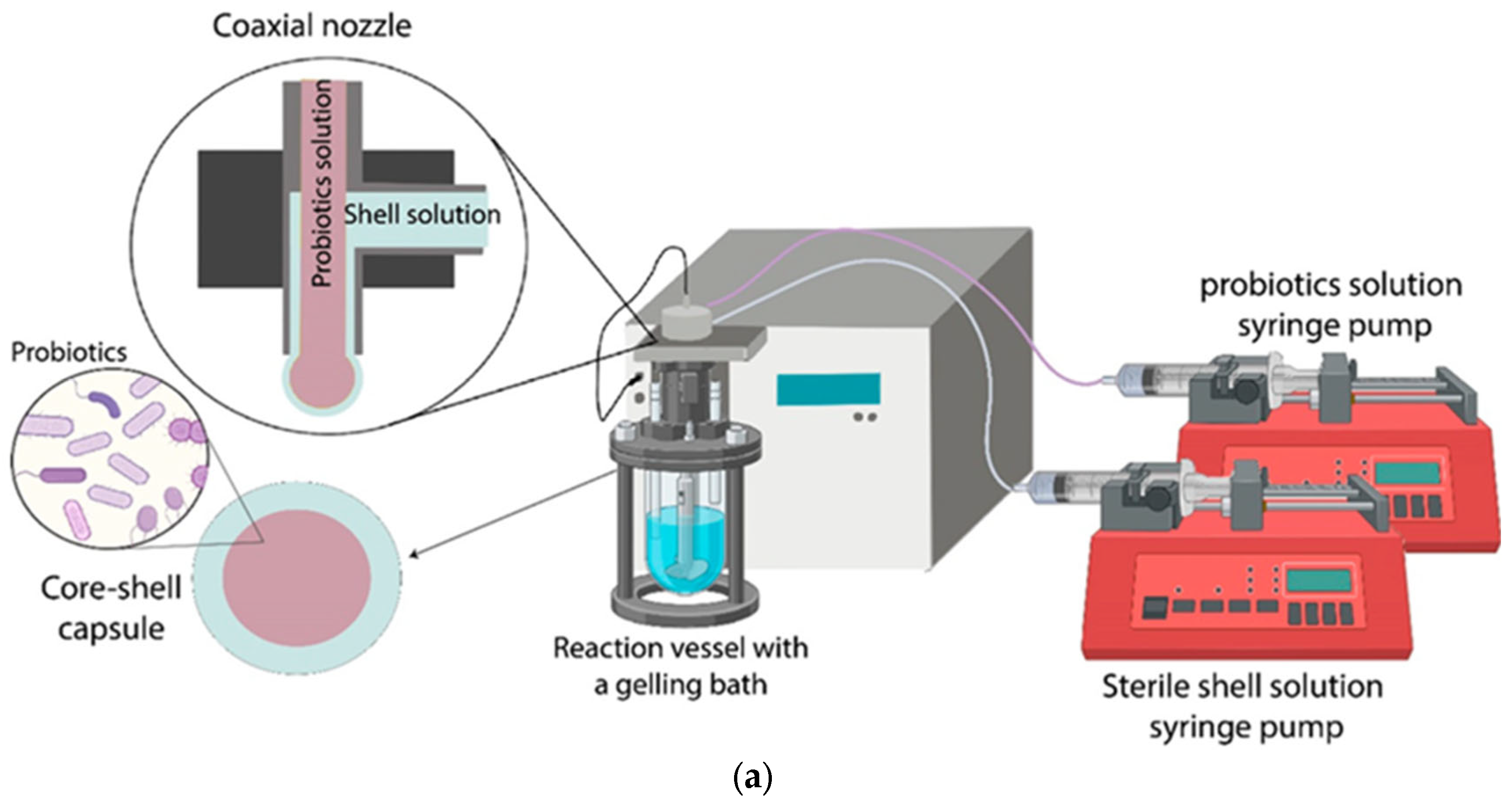
| Cellulose/ Cellulose Derivate | Probiotics | Encapsulation System | Characterization | Superiority | Reference |
|---|---|---|---|---|---|
| CMC-Na | Lactobacillus reuteri KUB-AC5 |  Microcapsule Microcapsule | 159.92 ± 3.96 μm; Nearly spherical microcapsules |
| [33] |
| Cellulose acetate | Escherichia coli Nissle 1917 |  Hybrid fiber Hybrid fiber | 754 ± 497 nm; Uniform fibers |
| [26] |
| Cellulose | Lactobacillus plantarum CICC 6240 |  Microgel Microgel | 500 μm; Core-shell spherical microgels |
| [34] |
| Nanocrystal cellulose | Lactobacillus helveticus CICC 22536 |  w/w emulsion w/w emulsion | 5–6 μm |
| [35]) |
| Cellulose sulphate | Lactobacillus acidophilus DMS 20079, Lactobacillus johnsonii DMS 10533, Lactobacillus casei DSM 20011 and Bifidobacterium longum subsp. infantis (B. infantis) DMS 20088 |  Porous capsule Porous capsule | 0.7 mm |
| [29] |
| Polysaccharide | Probiotic | Emulsion Type | Stability Mechanism andAdvantages |
|---|---|---|---|
| Soy hull polysaccharide | Lactobacillus plantarum | o/w |
|
| Apple pectin/alginate | Lactobacillus reuteri | w/o/w |
|
| κCarrageenan/carboxymethyl chitosan/alginate | Lactobacillus rhamnosus | w/o/w |
|
| Alginate/carboxymethyl konjac glucomannan/chitosan | Lactobacillus reuteri | w/o/w |
|
| Chitosan/alginate | Lactobacillus plantarum | w/o/w |
|
| Fucoidan/chitosan | Lactobacillus bulgaricus | w/o/w |
|
| Hydroxypropyl methylcellulose (HPMC)/dextran | Lactobacillus plantarum | w/w Pickering emulsion |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-D.; Zhang, W.; Liang, T.-X. Advancements in Oral Delivery Systems for Probiotics Based on Polysaccharides. Polymers 2025, 17, 144. https://doi.org/10.3390/polym17020144
Wang Z-D, Zhang W, Liang T-X. Advancements in Oral Delivery Systems for Probiotics Based on Polysaccharides. Polymers. 2025; 17(2):144. https://doi.org/10.3390/polym17020144
Chicago/Turabian StyleWang, Zi-Dan, Wei Zhang, and Tian-Xin Liang. 2025. "Advancements in Oral Delivery Systems for Probiotics Based on Polysaccharides" Polymers 17, no. 2: 144. https://doi.org/10.3390/polym17020144
APA StyleWang, Z.-D., Zhang, W., & Liang, T.-X. (2025). Advancements in Oral Delivery Systems for Probiotics Based on Polysaccharides. Polymers, 17(2), 144. https://doi.org/10.3390/polym17020144







