Cyclopentadienyl–Silsesquioxane Titanium Complexes in the Polymerizations of Styrene and L-Lactide
Abstract
1. Introduction
2. Results and Discussion
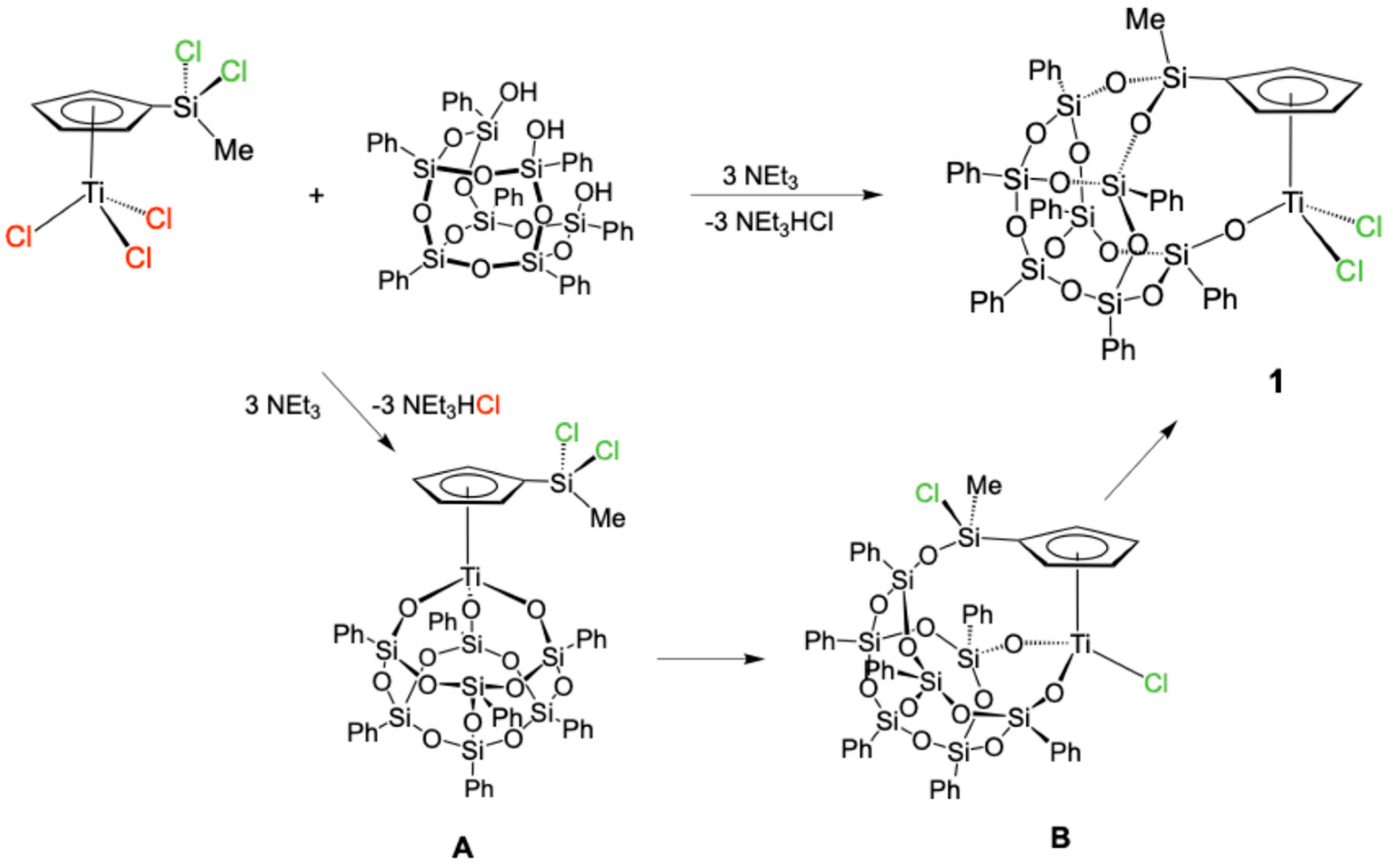
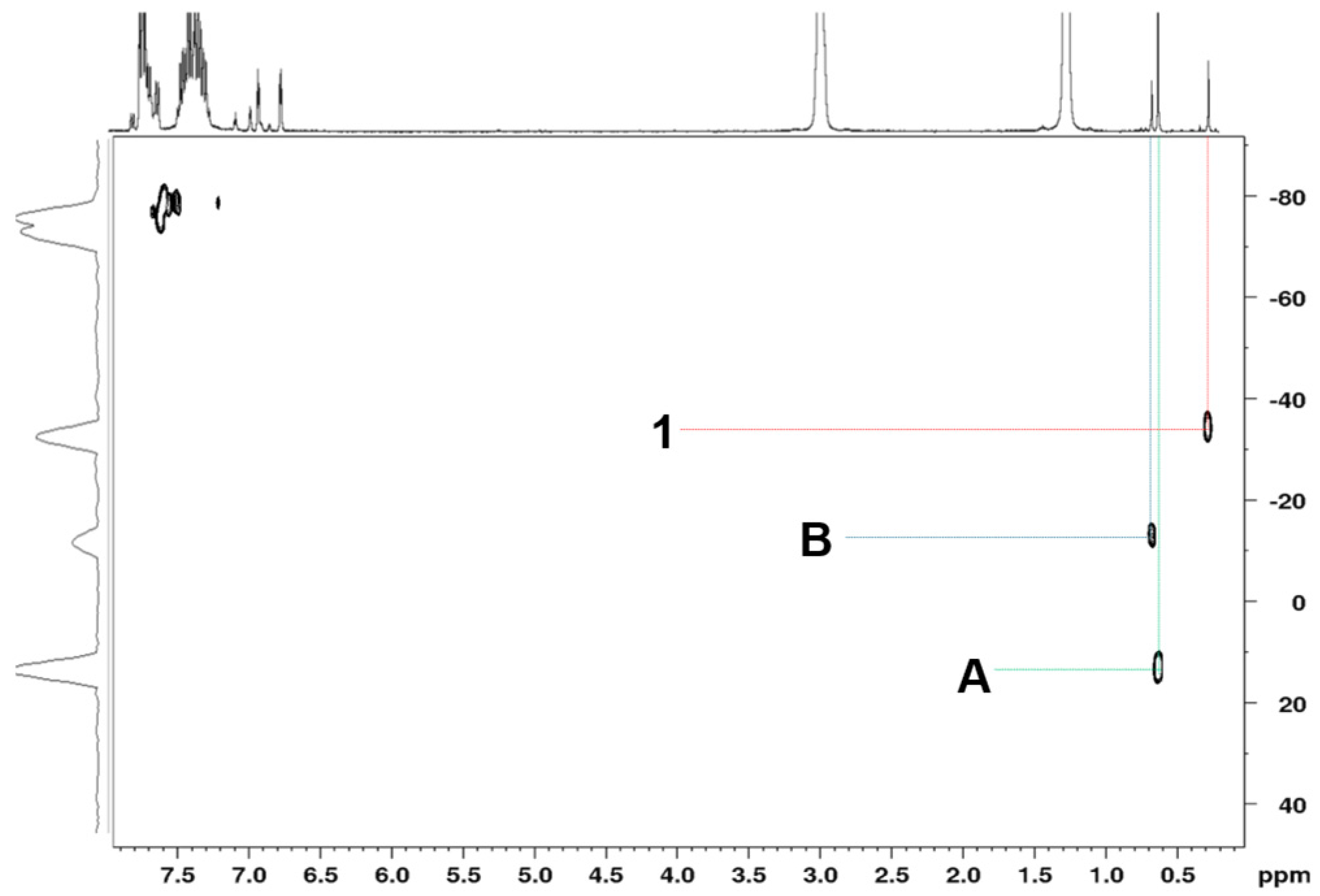
2.1. Synthesis of the Complex [Ti(η5-C5H4SiMeO2Ph7Si7O10-κO)Cl2] (1)
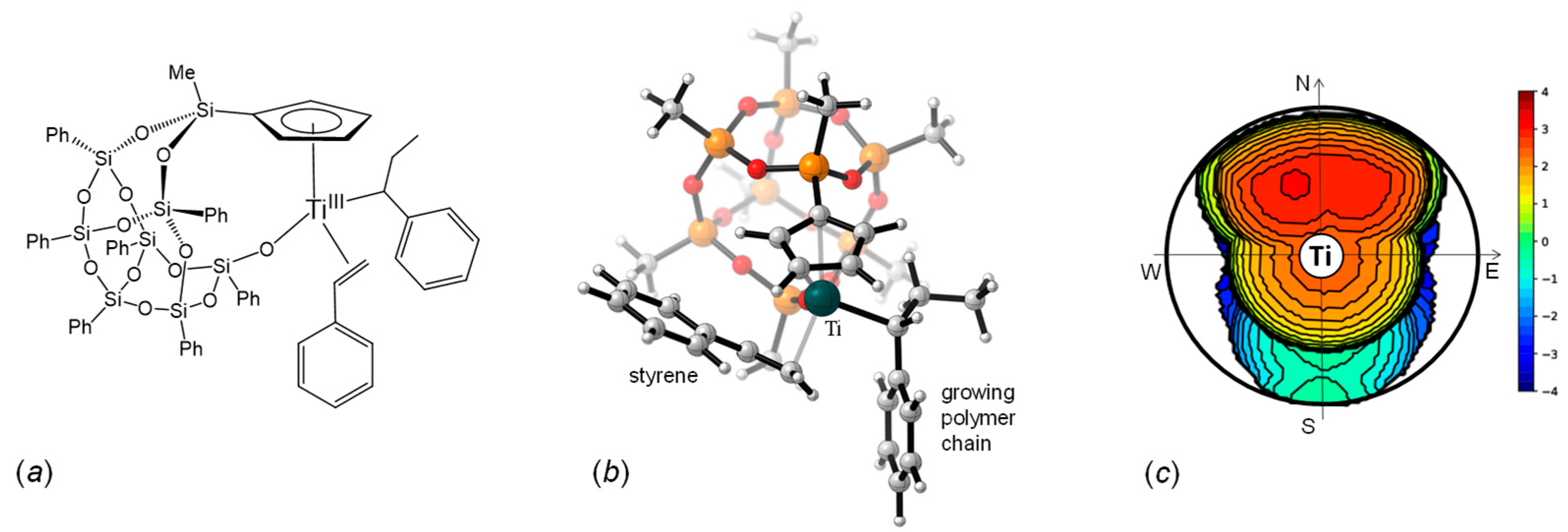
2.2. Styrene Polymerization
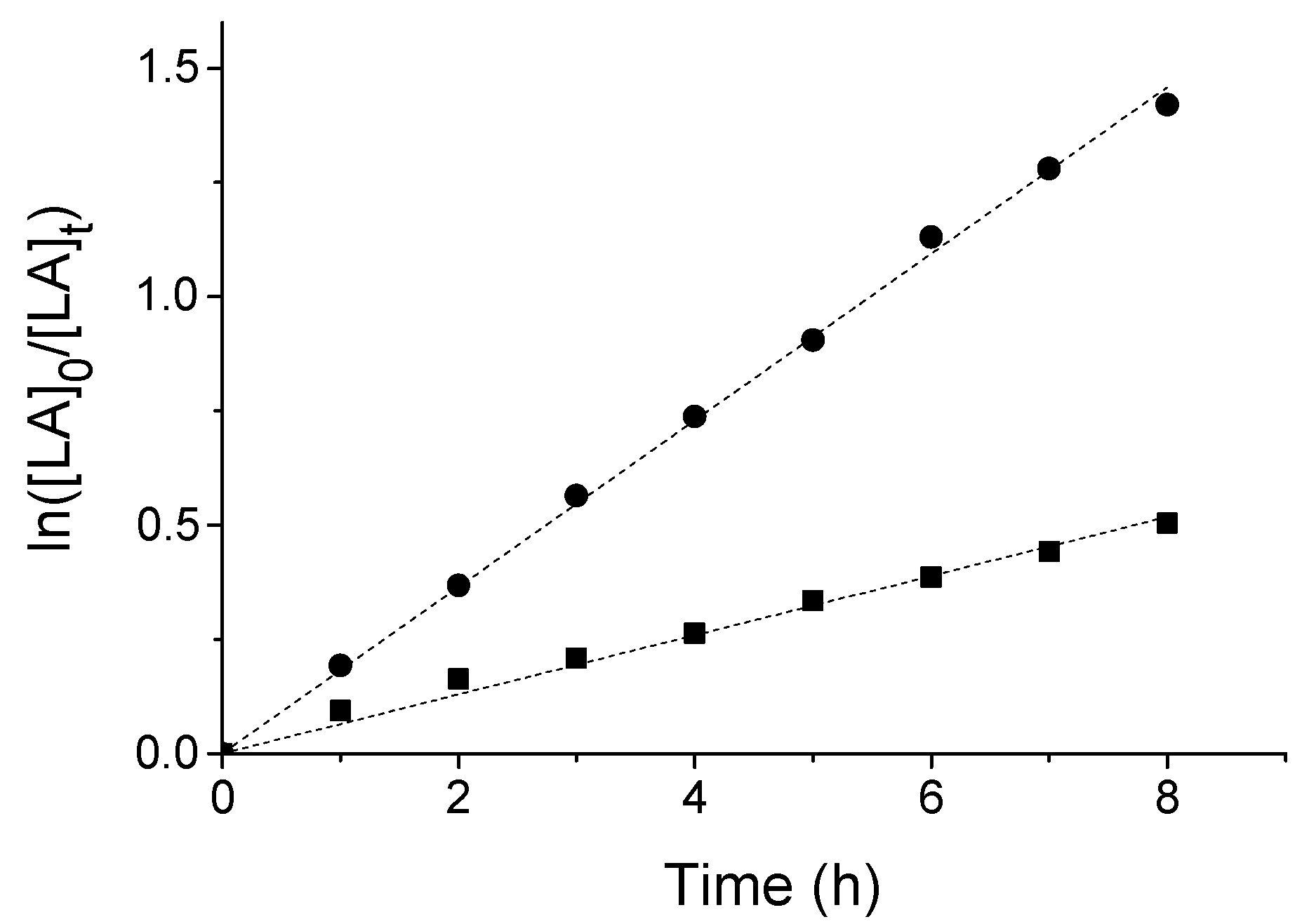
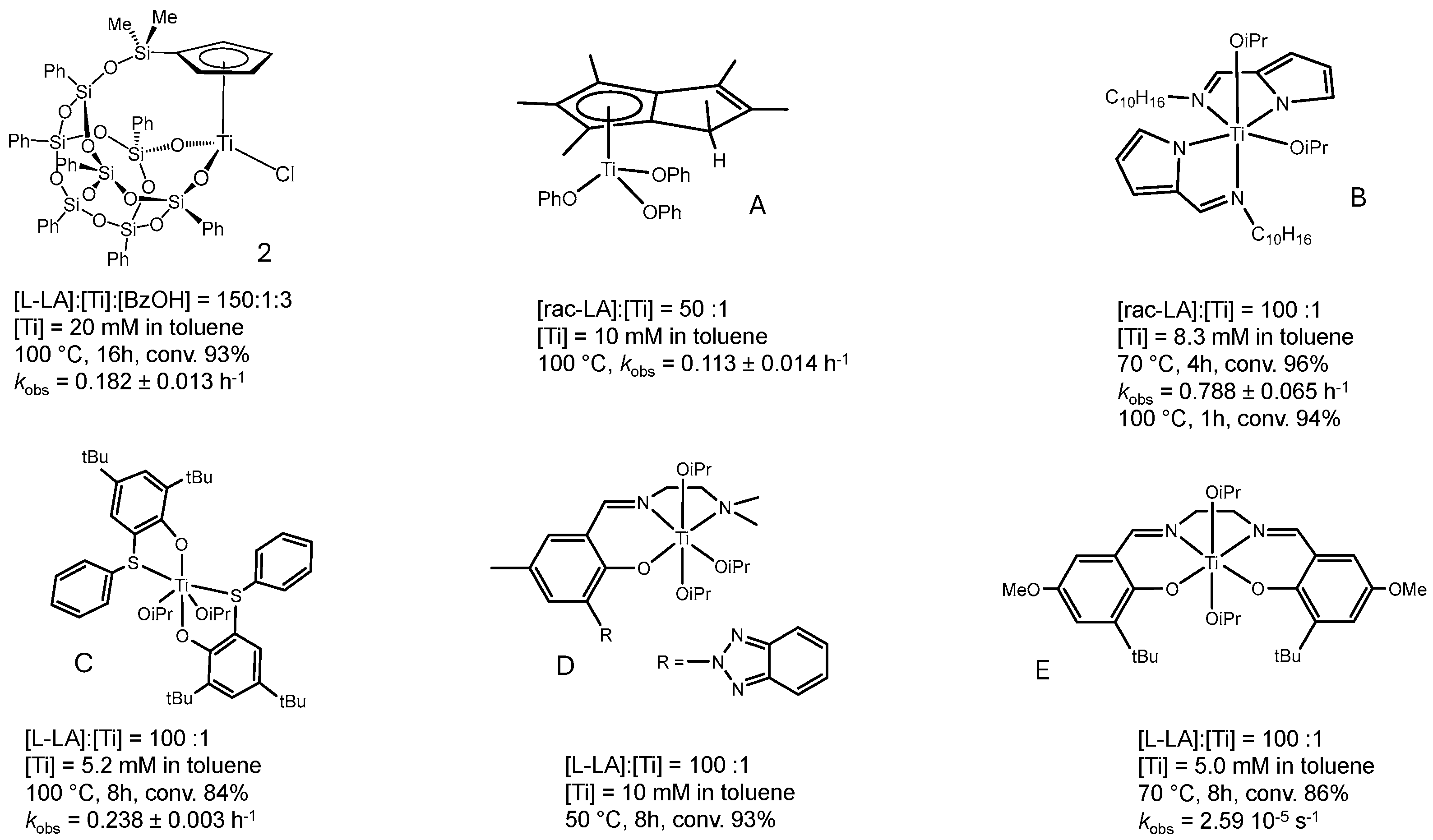
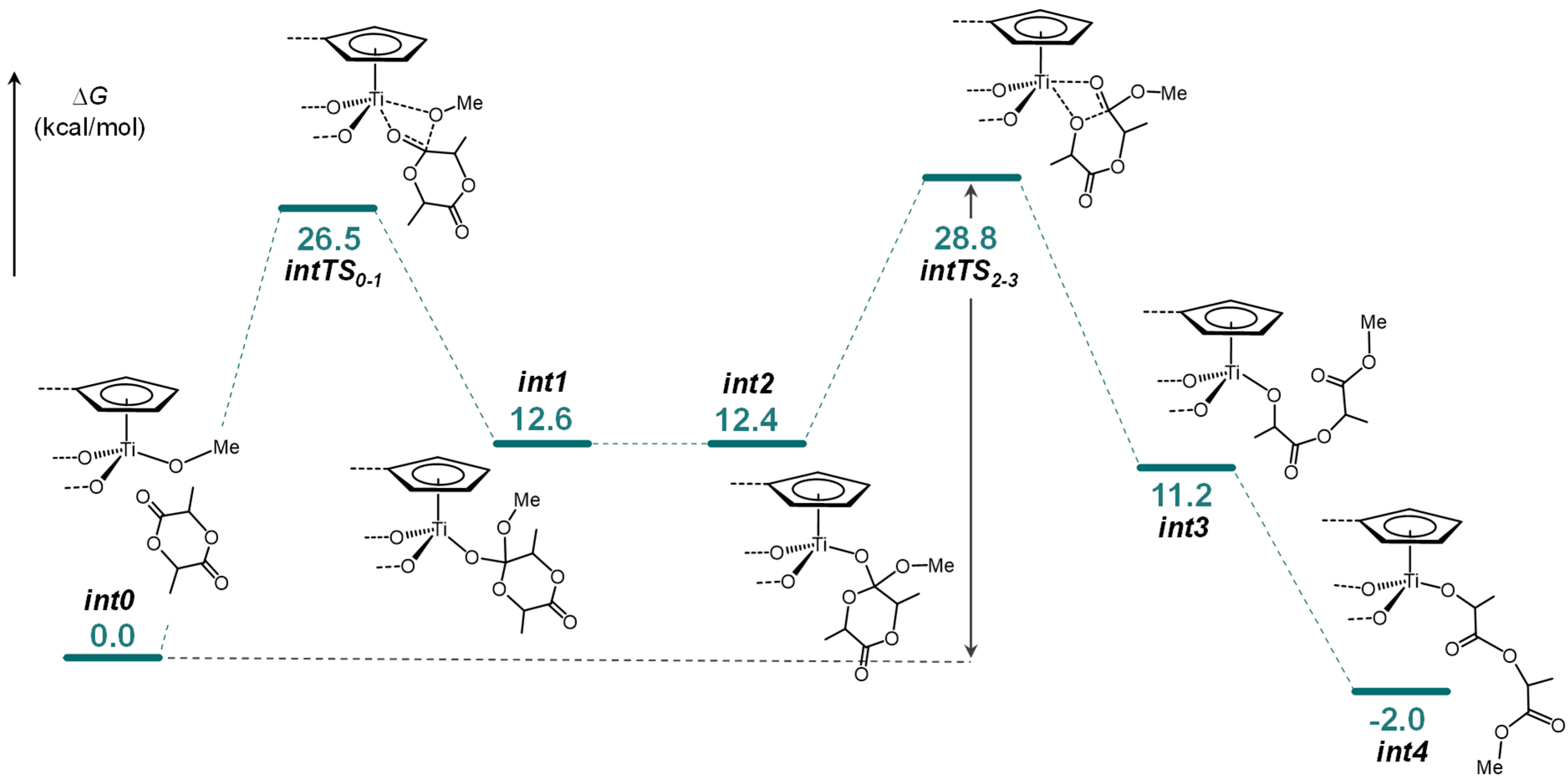
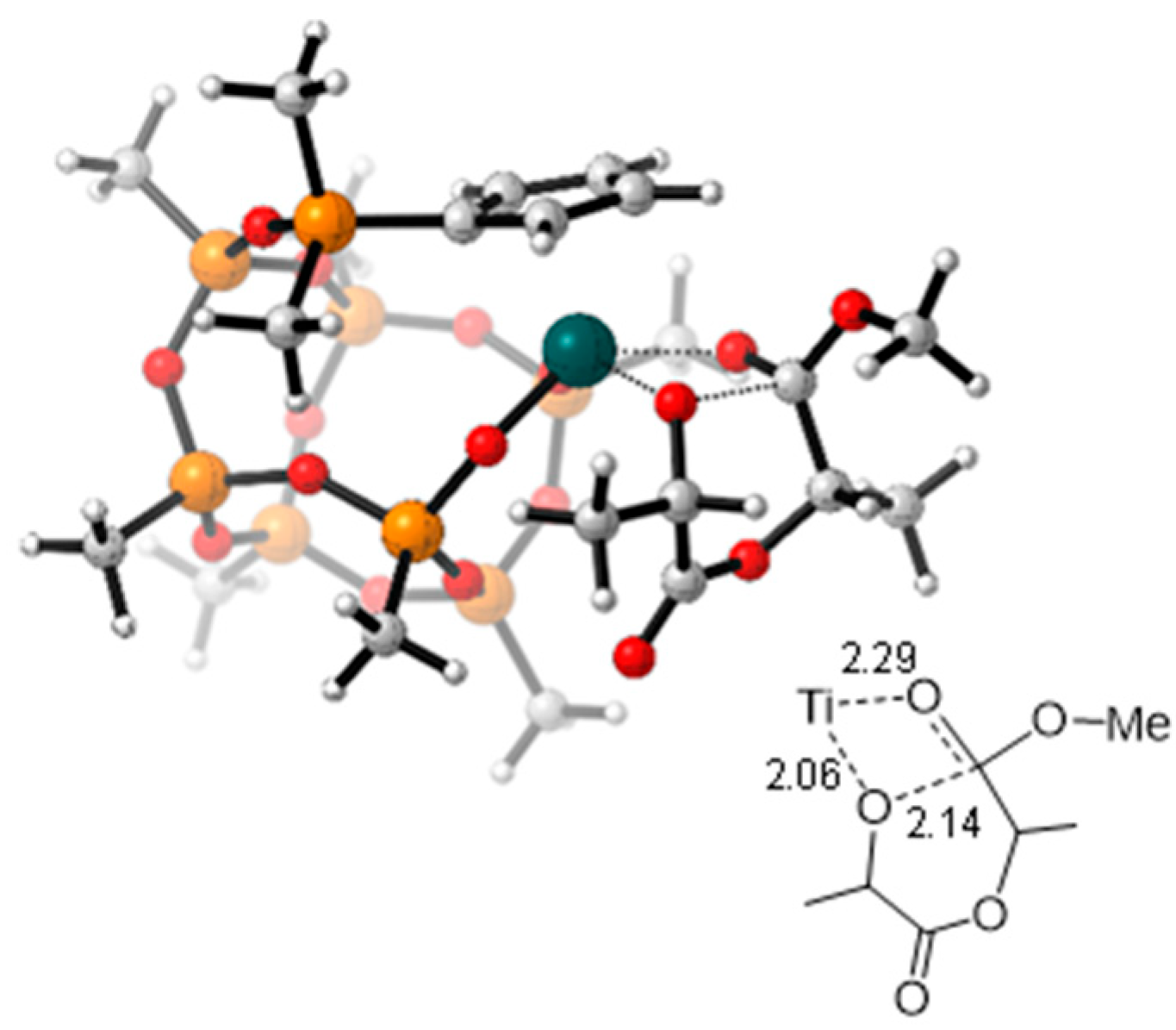
2.3. Lactide Polymerization
3. Conclusions
4. Experimental Section
4.1. Synthesis of [Ti{η5-C5H4SiMeO2Ph7Si7O10-κO}Cl2] (1)
4.2. Typical Procedure for Styrene Polymerization
4.3. Typical Procedure for L-Lactide Polymerization
4.4. Typical Procedure for L-Lactide Polymerization Without Solvent
4.5. Computational Details
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brintzinger, H.H.; Fischer, D.; Mülhaupt, R.; Rieger, B.; Waymouth, R.M. Stereospecific olefin polymerization with chiral metallocene catalysts. Angew. Chem. Int. Ed. Engl. 1995, 107, 1255–1283. [Google Scholar]
- Britovsek, G.J.P.; Gibson, V.C.; Wass, D.F. The Search for New-Generation Olefin Polymerization Catalysts: Life beyond Metallocenes. Angew. Chem. Int. Ed. Engl. 1999, 111, 448–468. [Google Scholar] [CrossRef]
- Gibson, V.C.; Spitzmesser, S.K. Advances in non-metallocene olefin polymerization catalysis. Chem. Rev. 2003, 103, 283–316. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-metal catalysts for ethylene homo- and copolymerization. Chem. Rev. 2000, 100, 1169–1204. [Google Scholar] [CrossRef]
- Baier, M.C.; Zuideveld, M.A.; Mecking, S. Post-Metallocenes in the Industrial Production of Polyolefins. Angew. Chem. Int. Ed. 2014, 53, 9722–9744. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.; Drzeżdżon, J.; Jacewicz, D. The greener side of polymers in the light of d-block metal complexes as precatalysts. Coord. Chem. Rev. 2023, 484, 215122. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Shi, C.; Quinn, E.C.; Diment, W.T.; Chen, E.Y.X. Recyclable and (Bio)degradable Polyesters in a Circular Plastics Economy. Chem. Rev. 2024, 124, 4393–4478, Erratum in Chem. Rev. 2024, 124, 11637. [Google Scholar] [CrossRef] [PubMed]
- Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F. Selectivity in Propene Polymerization with Metallocene Catalysts. Chem. Rev. 2000, 100, 1253–1346. [Google Scholar] [CrossRef] [PubMed]
- Braunschweig, H.; Breitling, F.M. Constrained geometry complexes—Synthesis and applications. Coord. Chem. Rev. 2006, 250, 2691–2720. [Google Scholar] [CrossRef]
- Massimiliano, D.; Marks, T.J. Multinuclear olefin polymerization catalysts. Chem. Rev. 2011, 111, 2450–2485. [Google Scholar] [CrossRef] [PubMed]
- Sita, L. Heterogeneous Ziegler-Natta to Homogeneous Single-Center Group 4 Organometallic Catalysts: A Primer on the Coordination Polymerization of Olefins. In Synthesis of Polymers; Schluter, D.A., Hawker, C., Sakamoto, J., Eds.; Wiley-VCH: Weinheim, Germany, 2012; pp. 25–66. [Google Scholar]
- Pinkas, J.; Lamač, M. Selective Transformations Mediated by Group 4 Metal Cyclopentadienyl Complexes. In Metallocenes in Regio- and Stereoselective Synthesis; Hapke, M., Kotora, M., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 117–160. [Google Scholar]
- Wang, W. Recent Advances in the Titanium-Based Catalysts for Ring-Opening Polymerization. ACS Omega 2024, 9, 29983–29993. [Google Scholar] [CrossRef]
- Le Roux, E. Recent advances on tailor-made titanium catalysts for biopolymer Synthesis. Coord. Chem. Rev. 2016, 306, 65–85. [Google Scholar] [CrossRef]
- Sauer, A.; Kol, M.; Kapelski, A.; Okuda, J. Structurally well-defined group 4 metal complexes initiators for the ring-opening polymerization of lactide monomers. Dalton Trans. 2013, 42, 9007–9023. [Google Scholar] [CrossRef]
- Impemba, S.; Milione, S. Group IV complexes with nitrogen based ligands in the ring opening polymerization of cyclic esters. Inorg. Chim. Acta 2024, 568, 122067. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, Y.; Rodriguez-Delgado, A.; Chen, E.Y.-X. Neutral metallocene ester enolate and non-metallocene alkoxy complexes of zirconium for catalytic ring-opening polymerization of cyclic esters. Organometallics 2008, 27, 5632–5640. [Google Scholar] [CrossRef]
- Petzetakis, N.; Pitsikalis, M.; Hadjichristidis, N. Titanium-mediated [CpTiCl2(OEt)] ring-opening polymerization of lactides: A novel route to well-defined polylactide-based complex macromolecular architectures. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1092–1103. [Google Scholar] [CrossRef]
- Saridis, E.; Maroulas, S.-D.; Pitsikalis, M. Ring-opening polymerization of L-lactide using half-titanocene complexes of the ATiCl2Nu type: Synthesis, characterization, and thermal properties. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1162–1174. [Google Scholar] [CrossRef]
- Patias, G.; Choinopoulos, I.; Koinis, S.; Pitsikalis, M. Employing (half-)titanocene complexes as initiators for the synthesis of end-functionalized polylactides by coordination polymerization. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2192–2202. [Google Scholar] [CrossRef]
- Białek, M.; Czaja, K. Application of Silsesquioxanes in the Preparation of Polyolefin-Based Materials. Materials 2023, 16, 1876. [Google Scholar] [CrossRef]
- Calabrese, C.; Aprile, C.; Gruttadauria, M.; Giacalone, F. POSS nanostructures in catalysis. Catal. Sci. Technol. 2020, 10, 7415–7447. [Google Scholar] [CrossRef]
- Quadrelli, E.A.; Basset, J.-M. On silsesquioxanes’ accuracy as molecular models for silica-grafted complexes in heterogeneous catalysis. Coord. Chem. Rev. 2010, 254, 707–728. [Google Scholar] [CrossRef]
- Severn, J.R.; Chadwick, J.C.; Duchateau, R.; Friederichs, N. “Bound but Not Gagged” Immobilizing Single-Site α-Olefin Polymerization Catalysts. Chem. Rev. 2005, 105, 4073–4147. [Google Scholar] [CrossRef] [PubMed]
- Duchateau, R. Incompletely Condensed Silsesquioxanes: Versatile Tools in Developing Silica-Supported Olefin Polymerization Catalysts. Chem. Rev. 2002, 102, 3525–3542. [Google Scholar] [CrossRef] [PubMed]
- Marciniec, B.; Maciejewski, H. Transition metal-siloxide complexes; synthesis, structure and application to catalysis. Coord. Chem. Rev. 2001, 223, 301–335. [Google Scholar] [CrossRef]
- Duchateau, R.; Abbenhuis, H.C.L.; Thiele, S.K.-H.; van Santen, R.A.; van Tol, M.F.H. Half-Sandwich Titanium Complexes Stabilized by a Novel Silsesquioxane Ligand: Soluble Model Systems for Silica-Grafted Olefin Polymerization Catalysts. Organometallics 1998, 17, 5222–5224. [Google Scholar] [CrossRef]
- Varga, V.; Pinkas, J.; Císařová, I.; Horáček, M.; Mach, K. Pentamethylcyclopentadienylmethyltitanium Silsesquioxanes and Their Zwitterionic Complexes with Tris(pentafluorophenyl)borane. Organometallics 2009, 28, 6944–6956. [Google Scholar] [CrossRef]
- Duchateau, R.; Abbenhuis, H.C.L.; van Santen, R.A.; Meetsma, A.; Thiele, S.K.-H.; van Tol, M.F.H. Ethylene polymerization with dimeric zirconium and hafnium silsesquioxane complexes. Organometallics 1998, 17, 5663–5673. [Google Scholar] [CrossRef]
- Duchateau, R.; Cremer, U.; Harmsen, R.J.; Mohamud, S.I.; Abbenhuis, H.C.L.; van Santen, R.A.; Meetsma, A.; Thiele, S.K.-H.; van Tol, M.F.H.; Kranenburg, M. Half-sandwich group 4 metal siloxy and silsesquioxane complexes: Soluble model systems for silica-grafted olefin polymerization catalysts. Organometallics 1999, 18, 5447–5459. [Google Scholar] [CrossRef]
- Kim, Y.; Han, Y.; Lee, M.H.; Yoon, S.W.; Choi, K.H.; Song, B.G.; Do, Y. New Half-Metallocene Catalysts Generating Polyethylene with Bimodal Molecular Weight Distribution and Syndiotactic Polystyrene. Macromol. Rapid Commun. 2001, 22, 573–578. [Google Scholar] [CrossRef]
- Severn, J.R.; Duchateau, R.; van Santen, R.A.; Ellis, D.D.; Spek, A.L. Homogeneous models for chemically tethered silica-supported olefin polymerization catalysts. Organometallics 2002, 21, 4–6. [Google Scholar] [CrossRef][Green Version]
- Mehta, A.; Tembe, G.; Bialek, M.; Parikh, P.; Mehta, G. Synthesis, characterization and ethylene polymerization by metallasilsesquioxane. Polym. Adv. Technol. 2013, 24, 441–445. [Google Scholar] [CrossRef]
- Byun, D.J.; Fudo, A.; Tanaka, A.; Fujiki, M.; Nomura, K. Effect of Cyclopentadienyl and Anionic Ancillary Ligand in Syndiospecific Styrene Polymerization Catalyzed by Nonbridged Half-Titanocenes Containing Aryloxo, Amide, and Anilide Ligands: Cocatalyst Systems. Macromolecules 2004, 37, 5520–5530. [Google Scholar] [CrossRef]
- Nomura, K.; Liub, J. Half-titanocenes for precise olefin polymerisation: Effects of ligand substituents and some mechanistic aspects. Dalton Trans. 2011, 40, 7666–7682. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, Q.; Liu, H.; Zhang, T.; Yang, X.; Zhu, F. Syndiospecific polymerization of styrene with half-titanocene catalysts containing fluorinated phenoxy ligands. RSC Adv. 2025, 15, 10711–10716. [Google Scholar] [CrossRef]
- Le Roux, E. Sustainable Catalysis: With Non-Endangered Metals, Part 1; North, M., North, M., Eds.; The Royal Society of Chemistry: London, UK, 2015; Chapter 6; pp. 116–139. [Google Scholar]
- Ventura, M.; Mosquera, M.E.G.; Cuenca, T.; Royo, B.; Jiménez, G. Cyclopentadienyl-Silsesquioxane Titanium Complexes: Highly Active Catalysts for Epoxidation of Alkenes with Aqueous Hydrogen Peroxide. Inorg. Chem. 2012, 51, 6345–6349. [Google Scholar] [CrossRef]
- Mateos, M.; Vinueza, J.; Mosquera, M.E.G.; Tabernero, V.; Cuenca, T.; Jiménez, G. Cyclopentadienyl-silsesquioxane titanium compounds as suitable candidates for immobilization on silica-based supports. Inorg. Chim. Acta 2020, 501, 119275. [Google Scholar] [CrossRef]
- Vinueza-Vaca, J.; Franco-Mateo, E.; Sessini, V.; Mosquera, M.E.G.; Souza-Egipsy, V.; Ramos, J.; Vega, J.F.; Jiménez, G.; Tabernero, V. Titanium-catalyzed synthesis of polymyrcene and polyanethol and application as sustainable additives for poly(lactic acid). Polymer 2024, 290, 126494. [Google Scholar] [CrossRef]
- Postigo, L.; Ventura, M.; Cuenca, T.; Jiménez, G.; Royo, B. Selective sulfoxidation with hydrogen peroxide catalysed by a titanium catalyst. Catal. Sci. Technol. 2015, 5, 320–324. [Google Scholar] [CrossRef]
- Napoli, M.; Grisi, F.; Longo, P. Half-Titanocene-Based Catalysts in the Syndiospecific Polymerization of Styrenes: Possible Oxidation States of the Titanium Species and Geometries of the Active Sites. Macromolecules 2009, 42, 2516–2522. [Google Scholar] [CrossRef]
- Xu, J.; Ouyang, J.; Fan, Z.; Chen, D.; Feng, L.; Yang, Y. Stereochemical Assignment of 13C NMR Spectra of Predominantly Syndiotactic Polystyrene. Polym. J. 1998, 30, 720–722. [Google Scholar] [CrossRef][Green Version]
- Ewart, S.W.; Baird, M.C. Olefin Polymerization by Pentamethylcyclopentadienyl Trimethyltitanium, Cp*TiMe3. Top. Catal. 1999, 7, 1–8. [Google Scholar] [CrossRef]
- Williams, E.F.; Murray, M.C.; Baird, M.C. Oxidation State(s) of the Active Titanium Species during Polymerization of Styrene to Syndiotactic Polystyrene Catalyzed by Cp*TiMe3/B(C6F5)3, Cp*TiMe3/[Ph3C][B(C6F5)], and Cp*TiCl2,3/MAO. Macromolecules 2000, 33, 261–268. [Google Scholar] [CrossRef]
- Grassi, A.; Zambelli, A.; Laschi, F. Reductive Decomposition of Cationic Half-Titanocene (IV) Complexes, Precursors of the Active Species in Syndiospecific Styrene Polymerization. Organometallics 1996, 15, 480–482. [Google Scholar] [CrossRef]
- Grassi, A.; Lamberti, C.; Zambelli, C.; Mingozzi, I. Syndiospecific Styrene Polymerization Promoted by Half-Titanocene Catalysts: A Kinetic Investigation Providing a Closer Insight to the Active Species. Macromolecules 1997, 30, 1884–1889. [Google Scholar] [CrossRef]
- Grassi, A.; Saccheo, S.; Zambelli, A.; Laschi, F. Reactivity of the [(η5-C5Me5)TiCH3][RB(C6F5)3] Complexes Identified as Active Species in Syndiospecific Styrene Polymerization. Macromolecules 1998, 31, 5588–5591. [Google Scholar] [CrossRef]
- Salvadori, E.; Chiesa, M.; Buonerba, A.; Grassi, A. Structure and dynamics of catalytically competent but labile paramagnetic metal-hydrides: The Ti(III)-H in homogeneous olefin polymerization. Chem. Sci. 2020, 11, 12436–12445. [Google Scholar] [CrossRef] [PubMed]
- Mahanthappa, M.M.; Waymouth, R.M. Titanium-mediated syndiospecific styrene polymerizations: Role of oxidation state. J. Am. Chem. Soc. 2001, 123, 12093–12094. [Google Scholar] [CrossRef]
- Zhang, H.; Nomura, K. Living copolymerization of ethylene with styrene catalyzed by (cyclopentadienyl)(ketimide)titanium(IV) complex—MAO catalyst system: Effect of anionic ancillary donor ligand. Macromolecules 2006, 39, 5266–5274. [Google Scholar] [CrossRef]
- Nomura, K.; Izawa, I.; Yi, J.; Nakatani, N.; Aoki, H.; Harakawa, H.; Ina, T.; Mitsudome, T.; Tomotsu, N.; Yamazoe, S. Solution XAS Analysis for Exploring Active Species in Syndiospecific Styrene Polymerization and 1-Hexene Polymerization Using Half Titanocene-MAO Catalysts: Significant Changes in the Oxidation State in the Presence of Styrene. Organometallics 2019, 38, 4497–4507. [Google Scholar] [CrossRef]
- Yi, J.; Nakatani, N.; Nomura, K. Solution XANES and EXAFS Analysis of Active Species of Titanium, Vanadium Complex Catalysts in Ethylene Polymerisation/Dimerisation and Syndiospecific Styrene Polymerisation. Dalton Trans. 2020, 49, 8008–8028. [Google Scholar] [CrossRef]
- Yi, J.; Nakatani, N.; Tomotsu, N.; Nomura, K.; Hada, M. Theoretical Studies of Reaction Mechanisms for Half-Titanocene Catalyzed Styrene Polymerization, Ethylene Polymerization, and Styrene-Ethylene Copolymerization: Roles of the Neutral Ti(III) and the Cationic Ti(IV) Species. Organometallics 2021, 40, 643–653. [Google Scholar] [CrossRef]
- Falivene, L.; Cao, Z.; Petta, A.; Serra, L.; Poater, A.; Oliva, R.; Scarano, V.; Cavallo, L. Towards the online computer-aided design of catalytic pockets. Nat. Chem. 2019, 11, 872–879. [Google Scholar] [CrossRef]
- Buchard, A.; Chuck, C.J.; Davidson, M.G.; Gobius Du Sart, G.; Jones, M.D.; McCormick, S.N.; Russell, A.D. A Highly Active and Selective Zirconium-Based Catalyst System for the Industrial Production of Poly(lactic acid). ACS Catal. 2023, 13, 2681–2695. [Google Scholar] [CrossRef]
- Hador, R.; Shuster, M.; Lipstman, S.; Kol, M. Fast-Tracking the l-Lactide Polymerization Activity of Group 4 Metal Complexes of Amine Tris(phenolate) Ligands. ACS Catal. 2022, 12, 4872–4879. [Google Scholar] [CrossRef]
- Turner, Z.R.; Buffet, J.-C.; O’Hare, D. Chiral Group 4 Cyclopentadienyl Complexes and Their Use in Polymerization of Lactide Monomers. Organometallics 2014, 33, 3891–3903. [Google Scholar] [CrossRef]
- Upitak, K.; Wattanathana, W.; Nanok, T.; Chuawong, P.; Hormnirun, P. Titanium complexes of pyrrolylaldiminate ligands and their exploitation for the ring-opening polymerization of cyclic esters. Dalton Trans. 2021, 50, 10964–10981. [Google Scholar] [CrossRef]
- Della Monica, F.; Luciano, E.; Roviello, G.; Grassi, A.; Milione, S.; Capacchione, C. Group 4 Metal Complexes Bearing Thioetherphenolate Ligands. Coordination Chemistry and Ring-Opening Polymerization Catalysis. Macromolecules 2014, 47, 2830–2841. [Google Scholar] [CrossRef]
- Liu, D.-C.; Li, C.-Y.; Lin, P.-H.; Chen, J.-D.; Tsai, C.-Y.; Lin, C.-H.; Ko, B.-T. Titanium complexes bearing benzotriazole iminophenolate ligands as efficient catalysts for ring-opening polymerization of cyclic esters. Inorg. Chem. Commun. 2018, 90, 1–7. [Google Scholar] [CrossRef]
- Gregson, C.K.A.; Blackmore, I.J.; Gibson, V.C.; Long, N.J.; Marshall, E.L.; White, A.J.P. Titanium–salen complexes as initiators for the ring opening polymerisation of rac-lactide. Dalton Trans. 2006, 3134–3140. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. (Eds.) Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824, Erratum in Phys. Rev. B 1986, 34, 7406. [Google Scholar] [CrossRef] [PubMed]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]



| Entry (a) | Catalyst | Al/Ti | Temper. (°C) | Time (h) | Yield. (g) | TOF (b) (h−1) | Mn(expt) (c) | Đ (c) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 500 | 60 | 3 | 0.089 | 28 | 6.6 | 1.7 |
| 2 | 1 | 500 | 60 | 6 | 0.130 | 21 | 6.7 | 1.7 |
| 3 | 1 | 500 | 60 | 18 | 0.172 | 9 | 8.2 | 3.2 |
| 4 | 1 | 500 | 80 | 18 | 0.252 | 13 | 6.1 | 1.6 |
| 5 | 1 | 250 | 80 | 18 | 0.241 | 6 | 6.4 | 1.4 |
| 6 | 1 | 1000 | 80 | 18 | 0.252 | 27 | 5.2 | 1.5 |
| Entry (a) | LA/2/BzOH | Temp. | Time (h) | Conv. (b) (%) | Mn(th) (c) | Mn(expt) (d) | Đ (d) |
|---|---|---|---|---|---|---|---|
| 1 | 150/1/0 | 100 | 16 | 23 | - | - | - |
| 2 | 150/1/1 | 100 | 16 | 71 | 15.4 | 12.3 | 1.24 |
| 3 | 150/1/1 | 100 | 20 | 87 | 18.8 | 12.5 | 1.32 |
| 4 | 150/1/2 | 100 | 16 | 90 | 9.7 | 7.7 | 1.24 |
| 5 | 150/1/2 | 100 | 20 | 95 | 10.3 | 8.9 | 1.23 |
| 6 | 150/1/3 | 100 | 16 | 93 | 6.7 | 5.3 | 1.19 |
| 7 (e) | 1500/1/3 | 175 | 1 | 81 | 58.3 | 10.2 | 2.22 |
| 8 (e) | 3000/1/6 | 175 | 1 | 83 | 59.8 | 11.6 | 2.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinueza-Vaca, J.; Anwar, S.; Impemba, S.; Grimaldi, I.; Jiménez, G.; Capacchione, C.; Tabernero, V.; Milione, S. Cyclopentadienyl–Silsesquioxane Titanium Complexes in the Polymerizations of Styrene and L-Lactide. Polymers 2025, 17, 2715. https://doi.org/10.3390/polym17192715
Vinueza-Vaca J, Anwar S, Impemba S, Grimaldi I, Jiménez G, Capacchione C, Tabernero V, Milione S. Cyclopentadienyl–Silsesquioxane Titanium Complexes in the Polymerizations of Styrene and L-Lactide. Polymers. 2025; 17(19):2715. https://doi.org/10.3390/polym17192715
Chicago/Turabian StyleVinueza-Vaca, Joan, Shoaib Anwar, Salvatore Impemba, Ilaria Grimaldi, Gerardo Jiménez, Carmine Capacchione, Vanessa Tabernero, and Stefano Milione. 2025. "Cyclopentadienyl–Silsesquioxane Titanium Complexes in the Polymerizations of Styrene and L-Lactide" Polymers 17, no. 19: 2715. https://doi.org/10.3390/polym17192715
APA StyleVinueza-Vaca, J., Anwar, S., Impemba, S., Grimaldi, I., Jiménez, G., Capacchione, C., Tabernero, V., & Milione, S. (2025). Cyclopentadienyl–Silsesquioxane Titanium Complexes in the Polymerizations of Styrene and L-Lactide. Polymers, 17(19), 2715. https://doi.org/10.3390/polym17192715









