Development of Low-Smoke Epoxy Resin Carbon Fiber Prepreg
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Synthesis of Modified Resins
2.2.1. Synthesis of PPPS-Modified E51
2.2.2. Synthesis of PPPS-Modified EOCN
2.3. Sample Preparation
2.3.1. Preparation of Resin Castings
2.3.2. Cone-and-Plate Viscometry
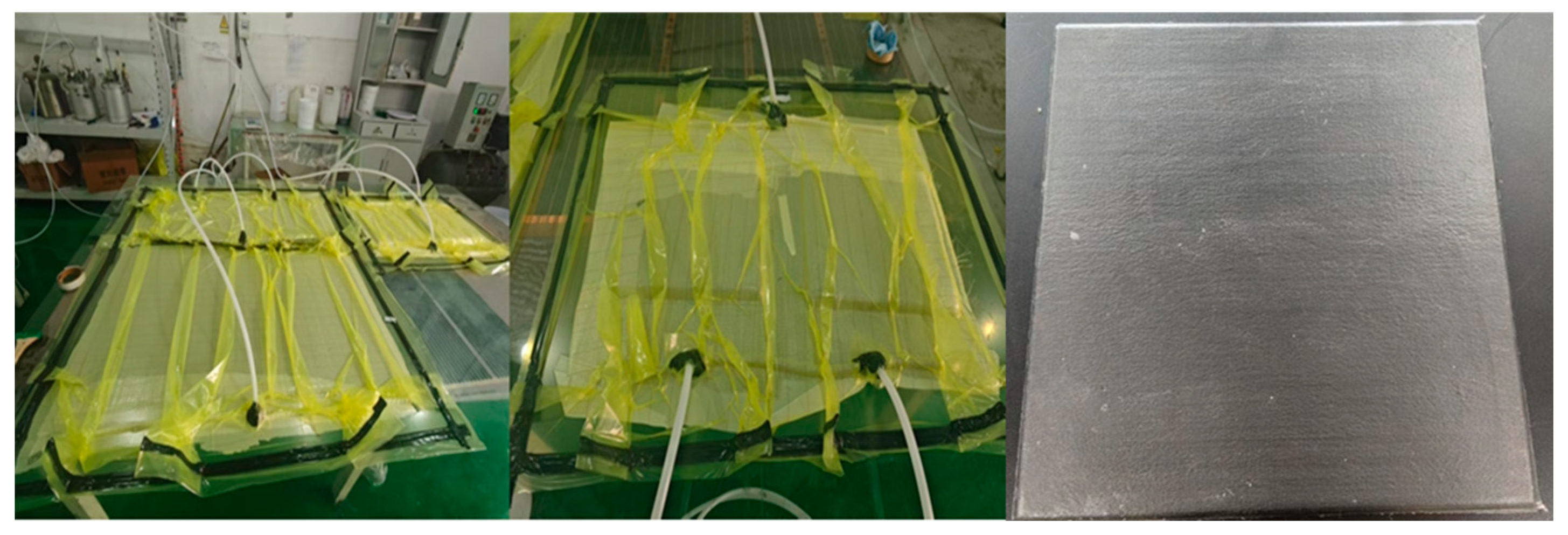
2.3.3. Preparation of Prepreg
2.3.4. Preparation of Composite Material
2.4. Characterization
- (1)
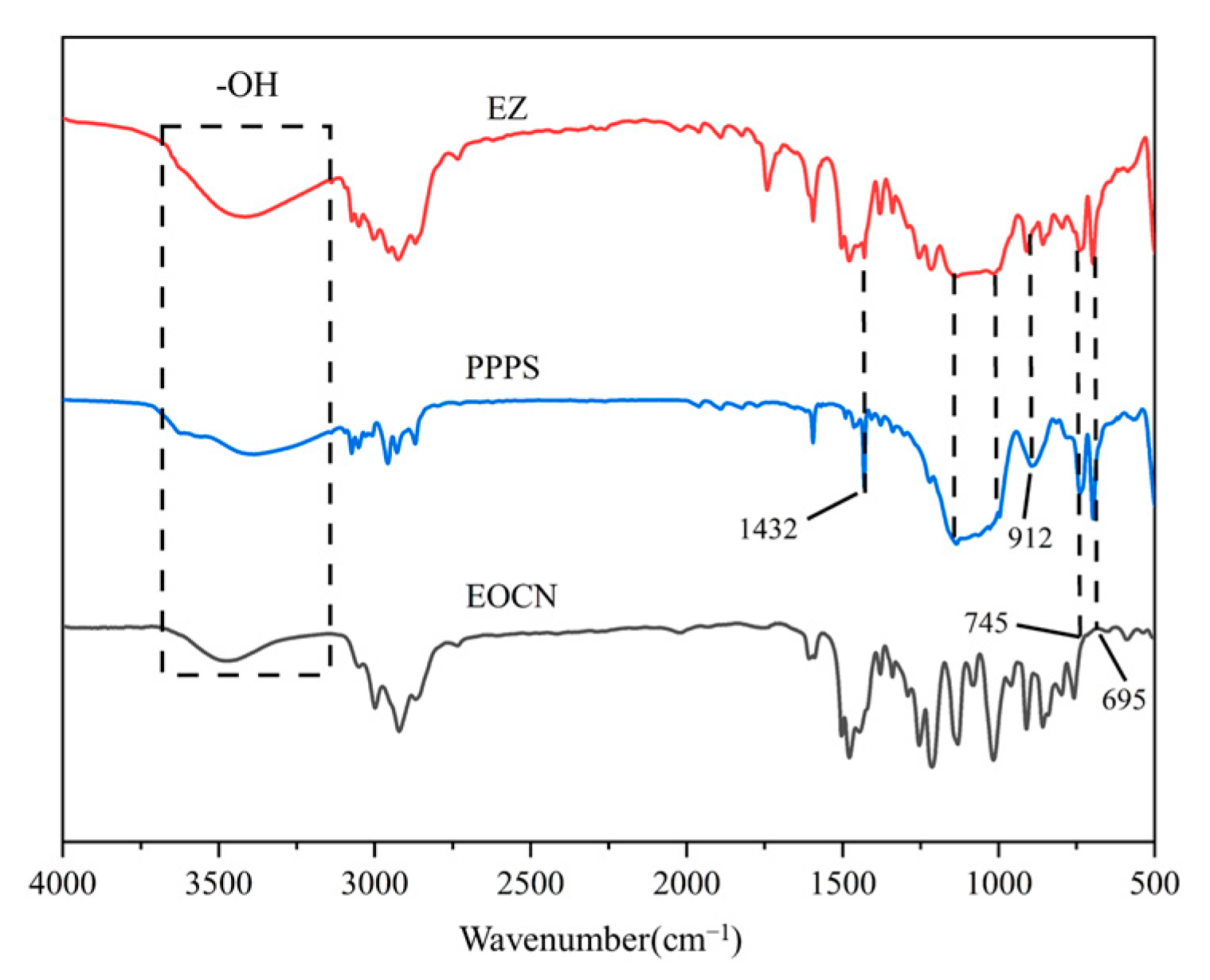
- Infrared testing: Infrared spectroscopy of EOCN, PPPS, and EZ was carried out by KBr compression method using a Nicolet 6700 FTIR spectrometer (Waltham, MA, USA) with a scanning wave number range of 4000–5000 cm−1;
- (2)
- Thermogravimetric analysis was performed using a STA449F3 simultaneous thermal analyzer (Selb, Germany). The resin sample was heated from 40 to 1000 °C at a constant heating rate of 10 °C/min under a dynamic air atmosphere.
- (3)
- Cone Calorimetry Test: According to ISO 5660 standard, FTT 0007 Cone Calorimeter is used to conduct cone calorimetry test on resin and composite materials.
- (4)
- The microscopic morphology of char residues of the resin and the composites was observed, and spot elemental analysis was performed using an FEI Scios 2 scanning electron microscope (SEM, Hillsboro, OR, USA) equipped with energy-dispersive X-ray spectroscopy (EDS, Hillsboro, OR, USA) at an accelerating voltage of 20 kV. Prior to analysis, the sample surfaces were sputter-coated with platinum to enhance conductivity.
- (5)
- Smoke Density Testing: In accordance with ISO 5659-2 [15], a single-chamber smoke density test was performed on the composite material to determine the specific optical density of smoke (abbreviated as smoke density). This test was performed to assess compliance with the smoke toxicity requirements of the International Maritime Organization’s FTP Code.
3. Results and Discussion
3.1. Structure and Properties of the Matrix Resin
3.1.1. Structural Characterization
3.1.2. Heat Resistance Analysis
3.1.3. Cone Calorimetric Analysis
3.1.4. Char Residue Analysis of Resin Matrix Combustion
3.1.5. Rheological Analysis
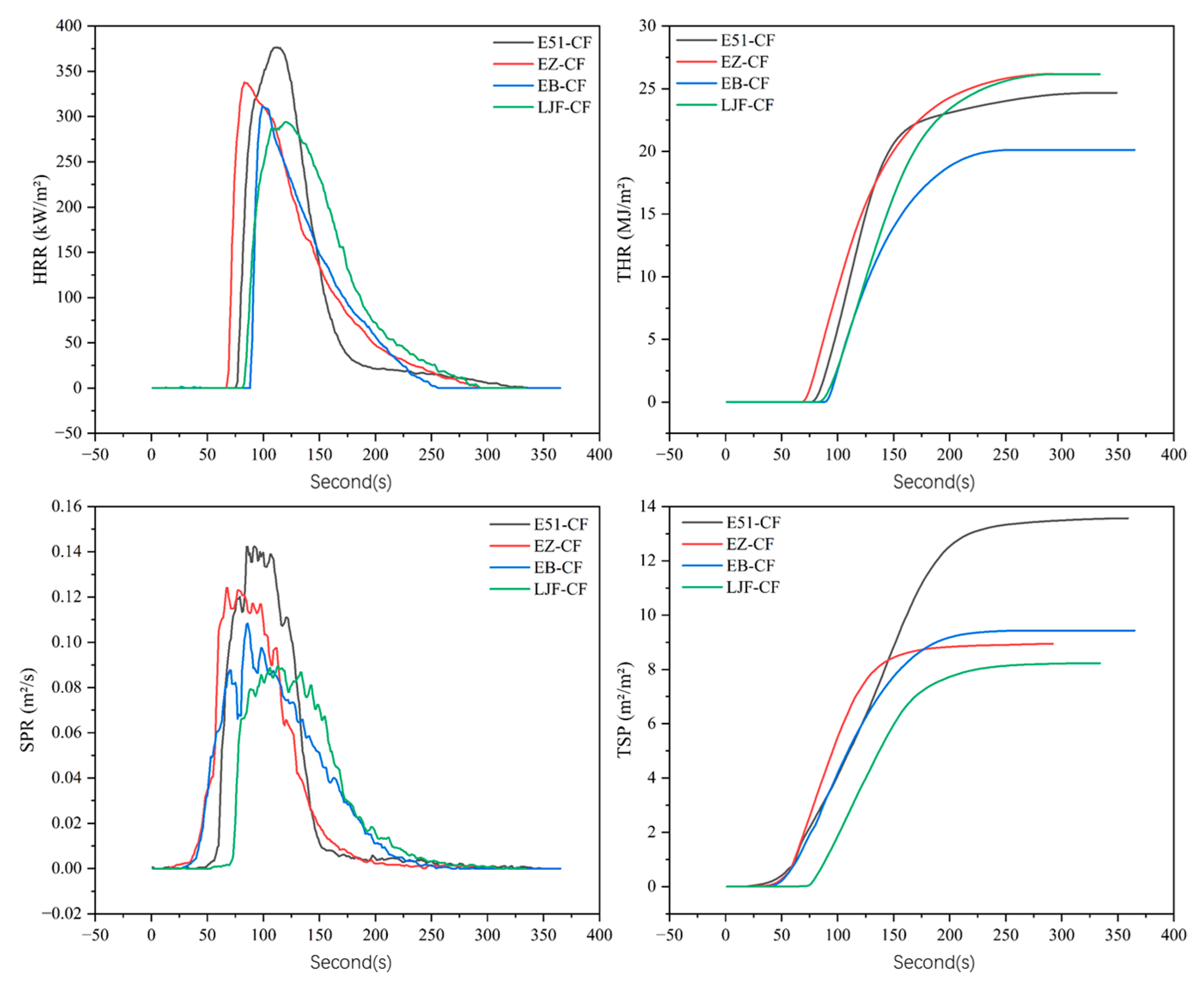
3.2. Smoke Production Analysis of Composite Materials
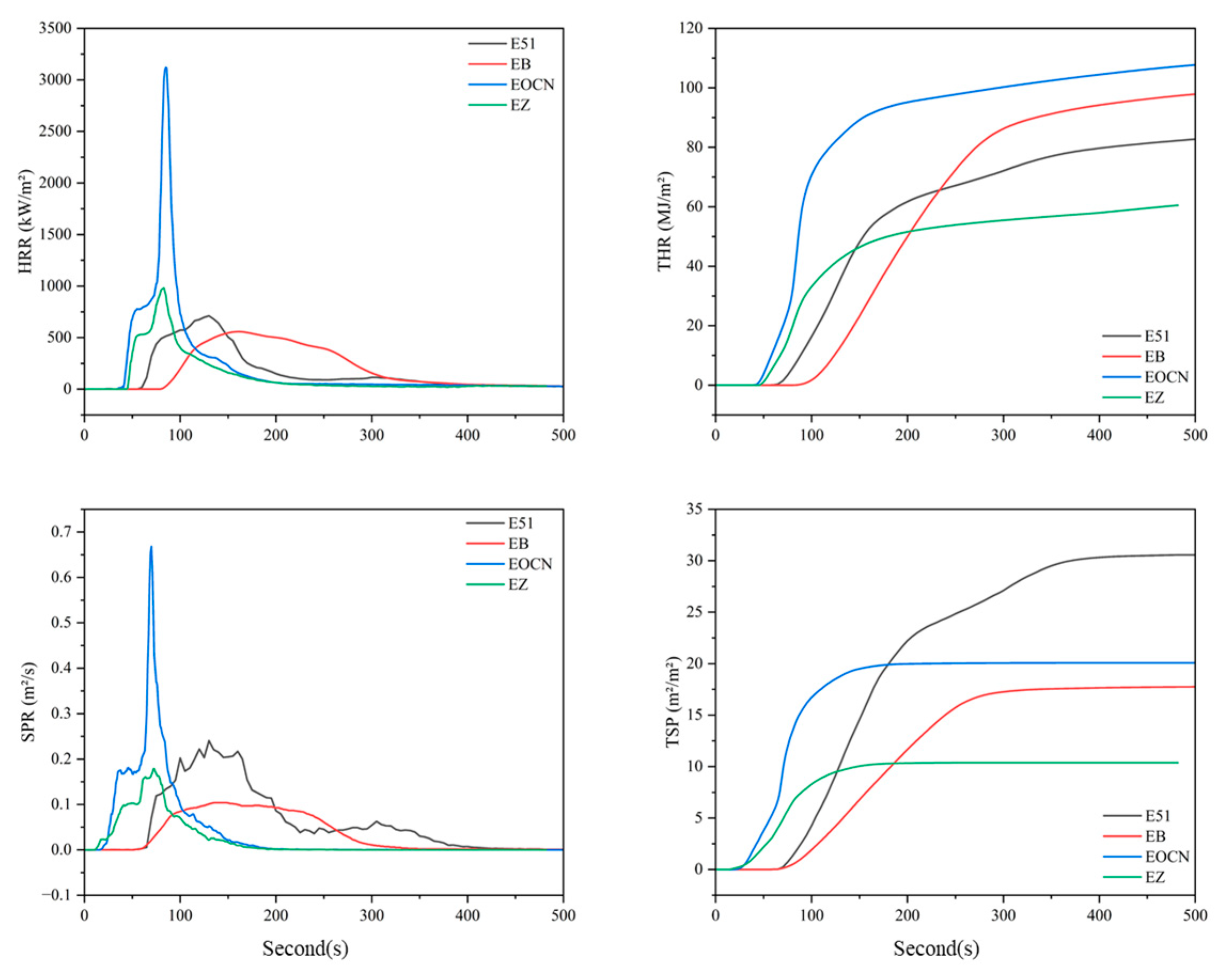
3.2.1. Cone Calorimetry Analysis
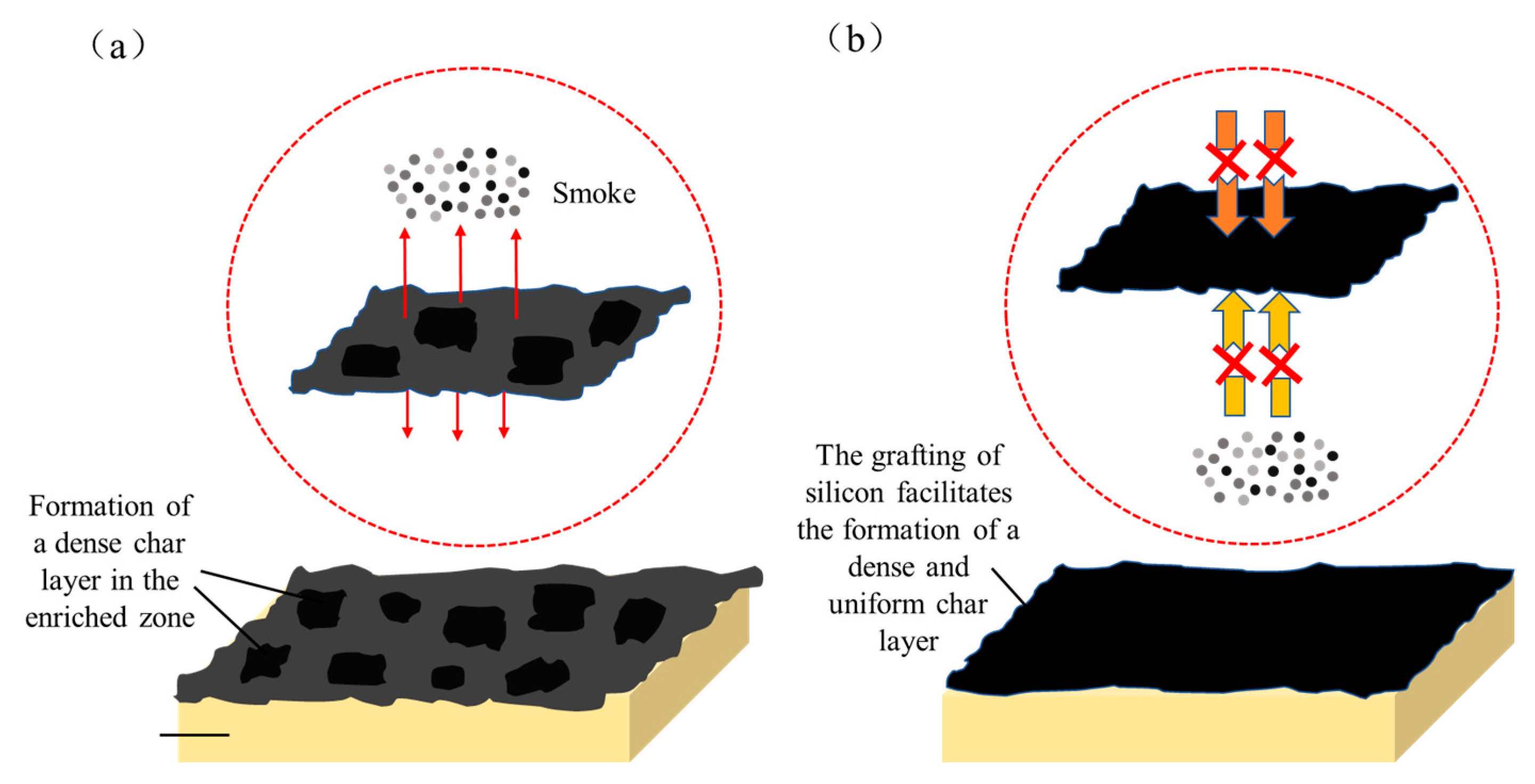
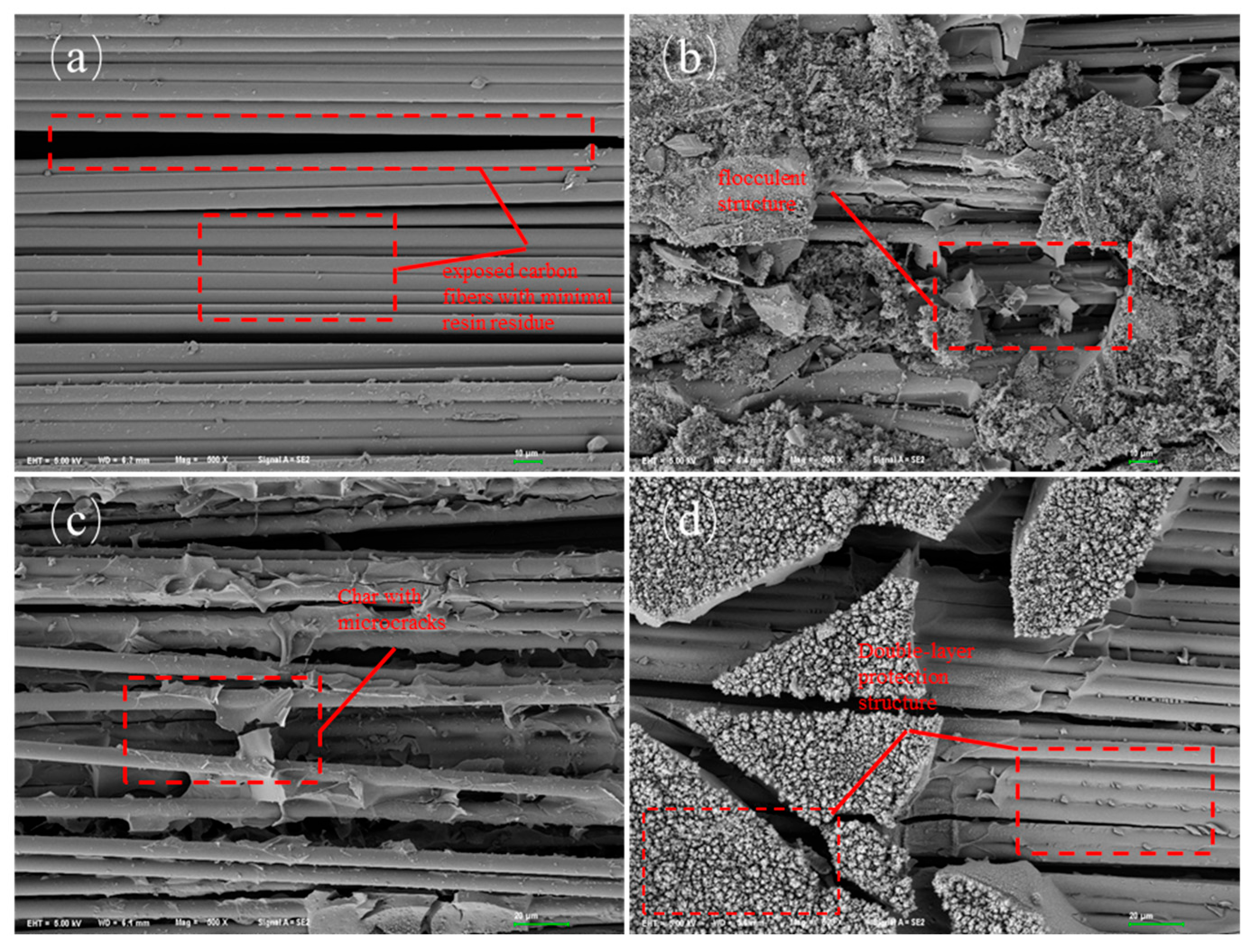
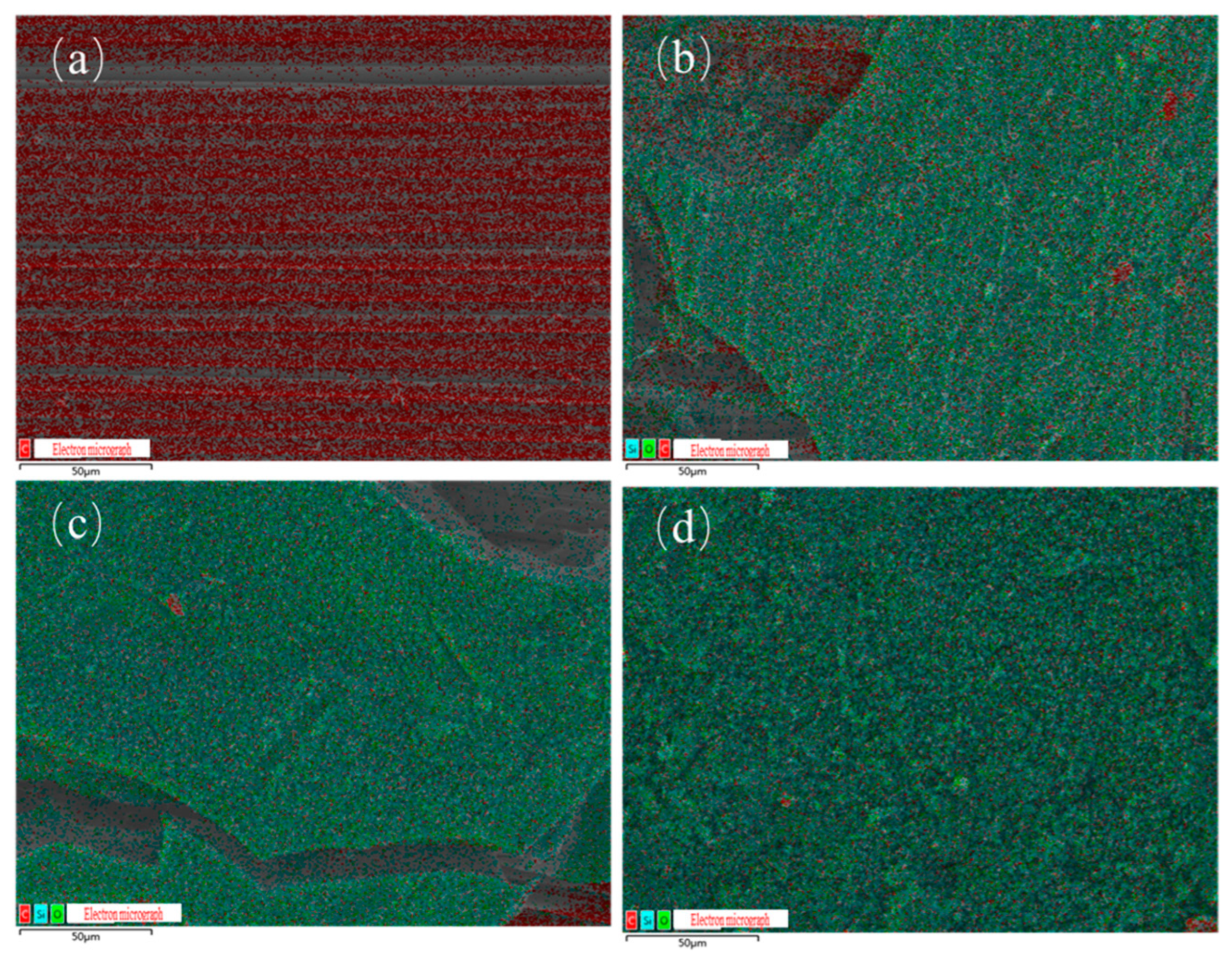
3.2.2. Char Residue Analysis of Composite Materials
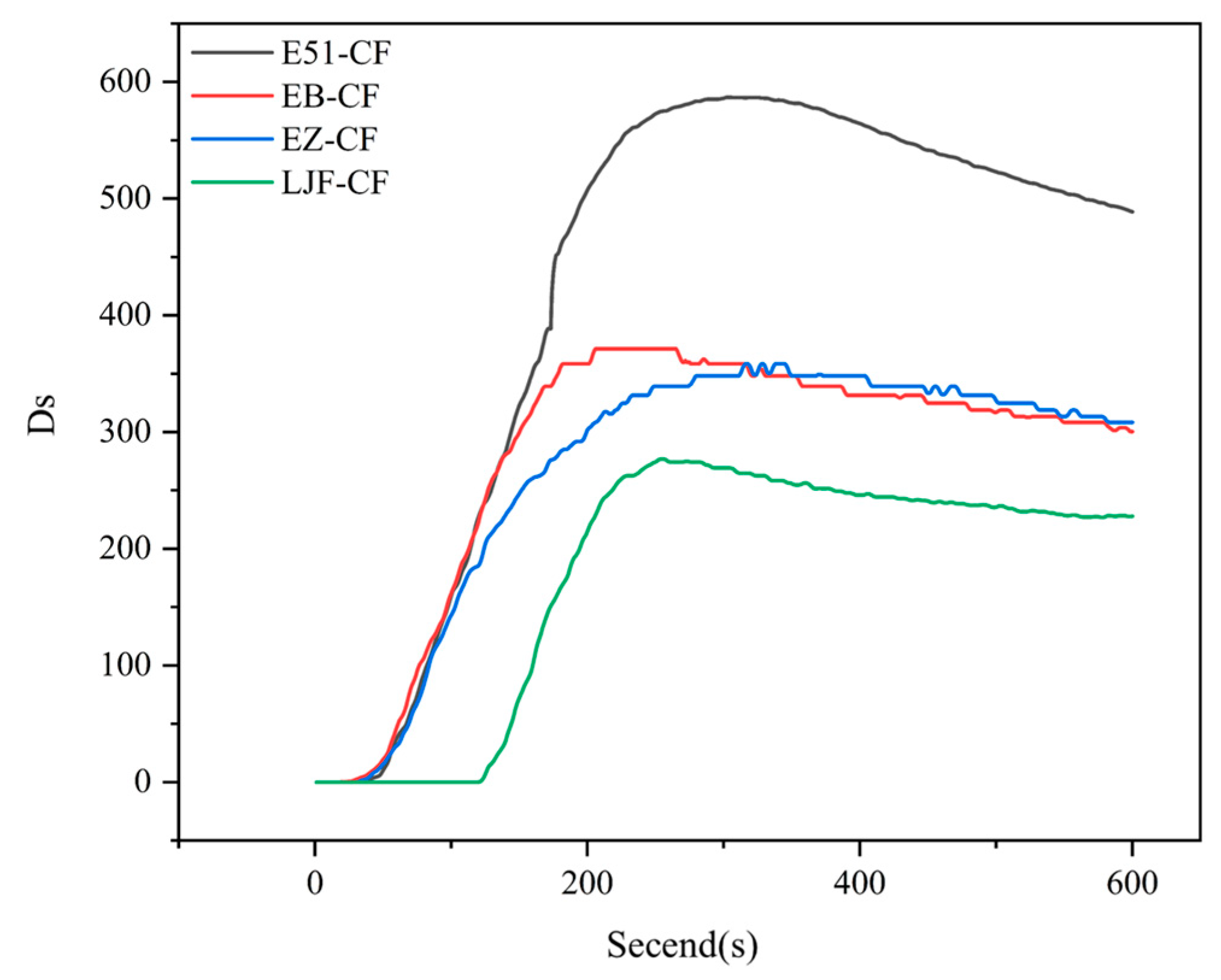
3.2.3. Smoke Density Test
4. Conclusions
- PPPS was chemically grafted into the molecular structures of E51 and EOCN resins, significantly improving their thermal stability and char residue yield. During combustion, silicon is uniformly distributed within the char residue as silicon oxides, forming a dense and homogeneous char layer that suppresses resin combustion, smoke transport, and reduces heat release and smoke generation. Compared to unmodified resins, the TSP of EB and EZ decreased by 54.8% and 48.3%, respectively. Compared with additive modification, the main reason for the significant smoke suppression effect is that the distribution of silicon elements introduced by grafting is more uniform, resulting in a more uniform distribution of silicon oxides after the resin pyrolysis.
- The significant reduction in TSP of modified resin-based composites is primarily attributed to the structural characteristics of the silicon-containing char residue. Microstructural analysis reveals that the silicon-rich char residue generated from the pyrolysis of modified resins uniformly accumulates on the fiber surface, forming a continuous protective layer. Notably, the char residue of LJF-CF exhibits a dual-layer architecture: a dense inner layer acts as an effective smoke barrier, drastically reducing the diffusion of smoke particles; a thickened outer layer effectively insulated heat, and delays the pyrolysis kinetics of the underlying resin matrix. This “dual-layer protection” mechanism achieves smoke suppression through a synergistic effect.
- LJF-CF composites meet the smoke density requirements for ship deck materials under the FTP Code. The LJF-CF composite material is a unidirectional carbon fiber laminatd unidirectionally structure, which only modify the resin matrix without adding any flame retardant/smoke suppressant. It is expected that through further smoke suppression measures, LJF-CF composites can be applied to the interior structural components of ships and other composite material fields with higher requirements for smoke toxicity.
- Although chemical modification achieves superior smoke suppression and flame retardancy, it is more complex and costly than additive blending. This study successfully tuned the hybrid resin’s viscosity via formulation optimization to meet the prepreg processing window (10–20 Pa·s), acknowledging that not all EB/EZ resin ratios yield suitable rheology. Future work will focus on industrial scale-up, cost–benefit analysis, and assessing long-term durability in marine environments (aging, water/salt spray resistance). Additionally, mechanical property evaluation (tensile strength, flexural modulus, fracture toughness) will be expanded to validate structural performance.
- The low-smoke prepreg developed in this study enriches the manufacturing techniques for low-smoke composites and significantly broadens their application scope.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, H.N.; Wang, X.L.; Xue, D.S. Application and Prospect of Advanced Composites in Ship Field. Synth. Fiber China. 2023, 52, 52–55. [Google Scholar]
- Li, J.T.; Luo, K.; Cao, M.F. Composite material and its latest application in ship. Ship Boat. 2013, 24, 10–16. [Google Scholar]
- Huang, Z.Q.; Wang, D.; Zhu, Y.L.; Zeng, W.R.; Hu, Y. The influence of mesoporous silica modified with phosphorus and nitrogen-containing hyperbranched molecules on thermal stability, combustion behavior, and toxic volatiles of epoxy resin. Polym. Adv. Technol. 2018, 29, 372–383. [Google Scholar] [CrossRef]
- Qiu, Y.; Qian, L.J.; Chen, Y.J.; Hao, J.W. Improving the fracture toughness and flame retardant properties of epoxy thermosets by phosphaphenanthrene/siloxane cluster-like molecules with multiple reactive groups. Compos. Part B-Eng. 2019, 178, 12. [Google Scholar] [CrossRef]
- Song, Y.C.; Chen, G.W.; Yao, H.L.; Zhao, D.N. Progress in Mechanism and Application of Silicon Containing Flame Retardants. Silicone Mater. 2018, 32, 496–500. [Google Scholar]
- Hu, Y.W.; Xu, Z.; Liu, S.P.; Xiong, Y.; Wu, X.Y.; Fan, Q.C.; Ding, Y.G.; Xu, L.L. Progress in synthesis and application of new phosphorus flame retardants. J. Wuhan Inst. Technol. 2024, 46, 473–481. [Google Scholar]
- You, G.Y.; Feng, B.; Liu, X.F.; Fan, F.F.; Ling, S.M. Synthesis of a Phosphorus/Nitrogen/Sulphur containing synergistic flame retardant and its flame retardancy on epoxy resin. Chem. Res. Appl. 2019, 31, 1898–1906. [Google Scholar]
- Liu, H.Y.; Zheng, P.L.; Li, J.W.; Sun, J.C.; Wang, R.; Zhao, H.H.; Wu, J.; Zheng, Y.; Liu, Q.Y. In-situ formation of macromolecule P/N/Si flame retardant for epoxy resin with low smoke toxicity and excellent flame-retardancy. J. Appl. Polym. Sci. 2023, 140, 13. [Google Scholar] [CrossRef]
- Chai, G.Q.; Zhu, G.Q.; Gao, S.; Zhou, J.J.; Gao, Y.J.; Wang, Y. On improving flame retardant and smoke suppression efficiency of epoxy resin doped with aluminum tri-hydroxide. Adv. Compos. Lett. 2019, 28, 12. [Google Scholar] [CrossRef]
- Xu, W.Z.; Zhang, B.L.; Wang, X.L.; Wang, G.S. The flame retardancy and smoke suppression effect of a hybrid containing dihydrogen phosphate anion modified reduced graphene oxide/layered double hydroxide on epoxy resin. RSC Adv. 2017, 7, 19662–19673. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.Y.; Feng, X.M.; Yu, B.; Lu, H.D.; Song, L.; Hu, Y. The effect of metal oxide decorated graphene hybrids on the improved thermal stability and the reduced smoke toxicity in epoxy resins. Chem. Eng. J. 2014, 250, 214–221. [Google Scholar] [CrossRef]
- Dong, Y.Y.; Gui, Z.; Hu, Y.; Wu, Y.; Jiang, S.H. The influence of titanate nanotube on the improved thermal properties and the smoke suppression in poly(methyl methacrylate). J. Hazard. Mater. 2012, 209, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.F.; Liu, J.B.; Ji, Y.D. Synthesis and Flame Retardancy of Silicon and Phosphorus Modified Epoxy Resin. Polym. Mater. Sci. Eng. 2023, 39, 40–50. [Google Scholar]
- ISO 5660-1:2015; Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method). International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 5659-2:2020; Plastics—Smoke Generation—Part 2: Determination of Optical Density by a Single-Chamber Test. International Organization for Standardization: Geneva, Switzerland, 2020.
- Mustata, F.; Bicu, I. Synthesis and properties of epoxy resins obtained from aniline/o-cresol/formaldehyde resins. J. Polym. Eng. 2004, 24, 391–408. [Google Scholar] [CrossRef]
- Wang, C.Z.; Li, S.X.; Yuan, Y.; Ji, Y.D.; Cao, D.F. Phenyl propyl polysiloxane modified epoxy resin I: The content, phase structures and macroproperties. Polymer 2024, 308, 13. [Google Scholar] [CrossRef]
- Ma, S.Q.; Liu, W.Q.; Wang, Z.F.; Hu, C.H.; Tang, C.Y. Simultaneously Increasing Impact Resistance and Thermal Properties of Epoxy Resins Modified by Polyether-Grafted-Epoxide Polysiloxane. Polym.-Plast. Technol. Eng. 2010, 49, 467–473. [Google Scholar] [CrossRef]
- Clarson, S.J.; Semlyen, J.A. Studies of cyclic and linear poly(dimethyl-siloxanes): 21. High temperature thermal behaviour. Polymer 1986, 27, 91–95. [Google Scholar] [CrossRef]
- Wang, Q.G.; Zhang, J.; Zhang, F. The Principle and Application of the Cone Calorimeter. Mod. Sci. Instrum. 2003, 6, 36–39. [Google Scholar]
- Jang, B.N.; Wilkie, C.A. The effects of triphenylphosphate and recorcinolbis(diphenylphosphate) on the thermal degradation of polycarbonate in air. Thermochim. Acta. 2005, 433, 1–12. [Google Scholar] [CrossRef]
- Wang, X.L.; Liu, M. Preparation of moderate-temperature curing epoxy resins used as hot-melt prepreg. Energy Chem. Ind. 2016, 37, 28–31. [Google Scholar]






| Sample | T5%/°C | Tmax1/°C | Tmax2/°C | MLRmax (%/°C) | R800/% |
|---|---|---|---|---|---|
| EOCN | 318.2 | 407.1 | 552.5 | 7.8 | 0 |
| EZ | 342.8 | 390.1 | 617.5 | 6.8 | 20 |
| Sample | E51 | EB | EOCN | EZ |
|---|---|---|---|---|
| PHRR (kW/m2) | 710.9 | 672.5 | 3121.4 | 981.87 |
| THR (MJ/m2) | 80.8 | 83.3 | 85.46 | 55.56 |
| PSPR (m2/s) | 0.24 | 0.10 | 0.67 | 0.18 |
| TSP (m2/m2) | 30.5 | 13.8 | 20.08 | 10.39 |
| Sample | Percentage of Residual Elements | |||
|---|---|---|---|---|
| C/wt% | O/wt% | Si/wt% | P/wt% | |
| EOCN | 74.7 | 25.0 | 0.2 | 0.1 |
| EZ | 56.6 | 22.3 | 21.0 | 0.1 |
| Sample | E51-CF | EB-CF | EZ-CF | LJF-CF |
|---|---|---|---|---|
| PHRR (kW/m2) | 376.6 | 311.7 | 337.1 | 294.0 |
| THR (MJ/m2) | 24.67 | 20.12 | 26.19 | 26.16 |
| PSPR (m2/s) | 0.14 | 0.11 | 0.12 | 0.09 |
| TSP (m2/m2) | 13.5 | 9.4 | 9.0 | 8.2 |
| Sample | Percentage of Residual Carbon Elements (%) | ||
|---|---|---|---|
| C | O | Si | |
| E51-CF | 100.00 | 0 | 0 |
| EB-CF | 50.06 | 37.94 | 12.00 |
| EZ-CF | 61.85 | 31.27 | 6.88 |
| LJF-CF | 59.08 | 30.84 | 10.08 |
| Sample | Ds max |
|---|---|
| E51-CF | 586.9 |
| EB-CF | 371.3 |
| EZ-CF | 358.5 |
| LJF-CF | 276.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wu, L.; Xu, Y.; Cao, D.; Ji, Y. Development of Low-Smoke Epoxy Resin Carbon Fiber Prepreg. Polymers 2025, 17, 2710. https://doi.org/10.3390/polym17192710
Zhao Y, Wu L, Xu Y, Cao D, Ji Y. Development of Low-Smoke Epoxy Resin Carbon Fiber Prepreg. Polymers. 2025; 17(19):2710. https://doi.org/10.3390/polym17192710
Chicago/Turabian StyleZhao, Yu, Lili Wu, Yujiao Xu, Dongfeng Cao, and Yundong Ji. 2025. "Development of Low-Smoke Epoxy Resin Carbon Fiber Prepreg" Polymers 17, no. 19: 2710. https://doi.org/10.3390/polym17192710
APA StyleZhao, Y., Wu, L., Xu, Y., Cao, D., & Ji, Y. (2025). Development of Low-Smoke Epoxy Resin Carbon Fiber Prepreg. Polymers, 17(19), 2710. https://doi.org/10.3390/polym17192710






