Plant-Based Scaffolds for Tissue Engineering: A Review
Abstract
1. Introduction
Why Plants?
2. Methodology
2.1. Protocol
2.2. Eligibility Criteria
2.3. Information Sources and Search
2.4. Selection of Sources of Evidence and Data Charting
3. Results
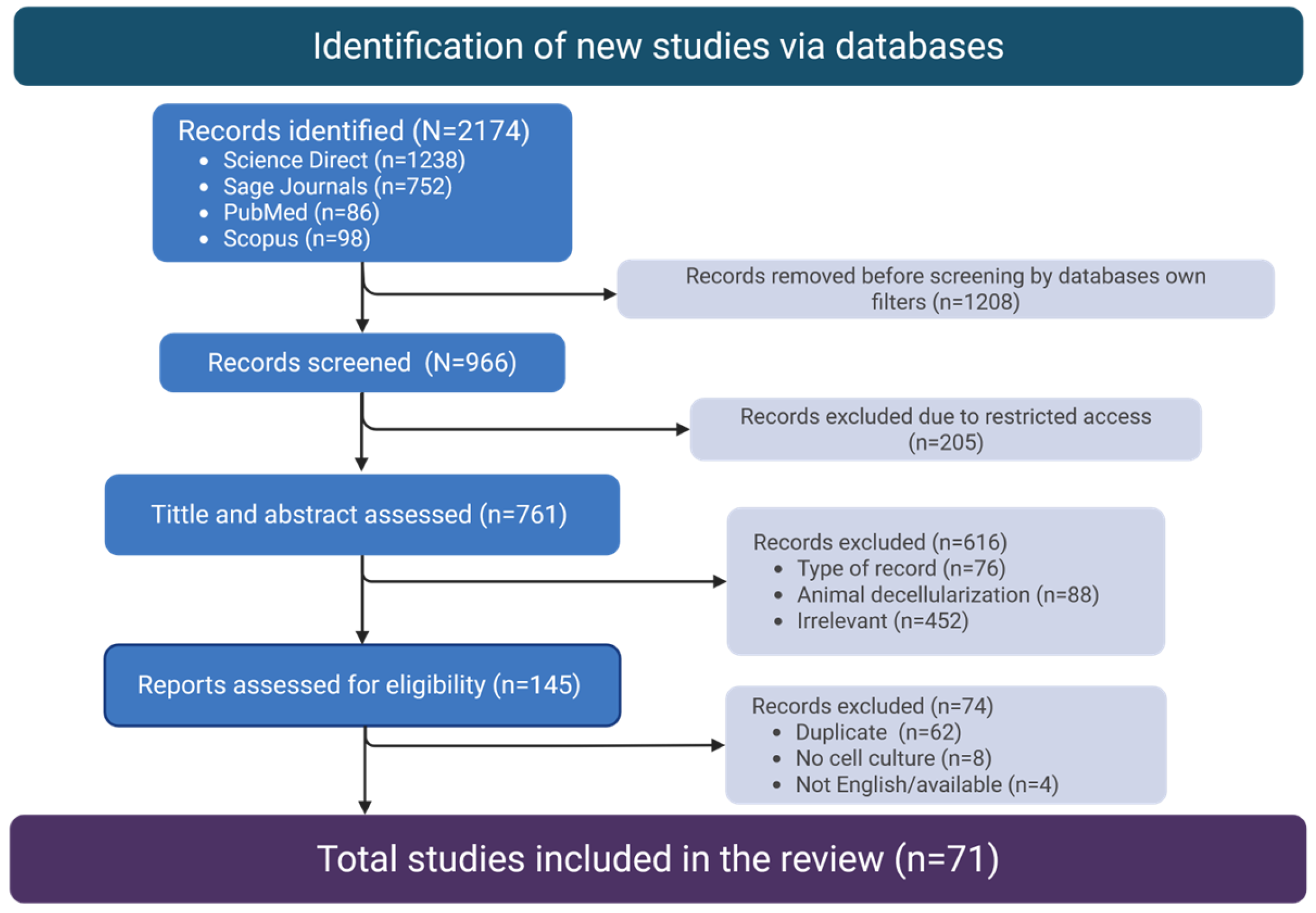
3.1. Article Selection
3.2. Characteristics of Sources of Evidence
3.3. Synthesis of Results
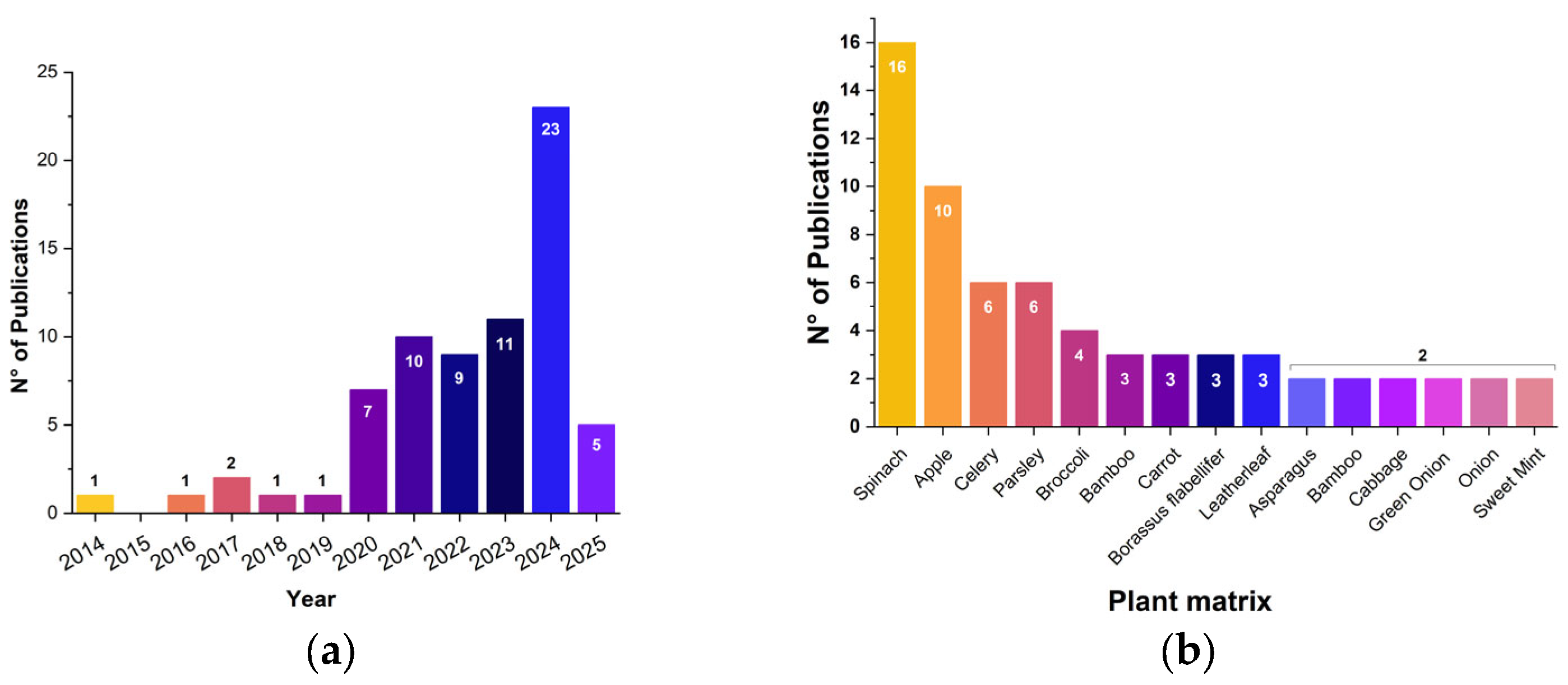
3.3.1. Selection of the Plant Matrix
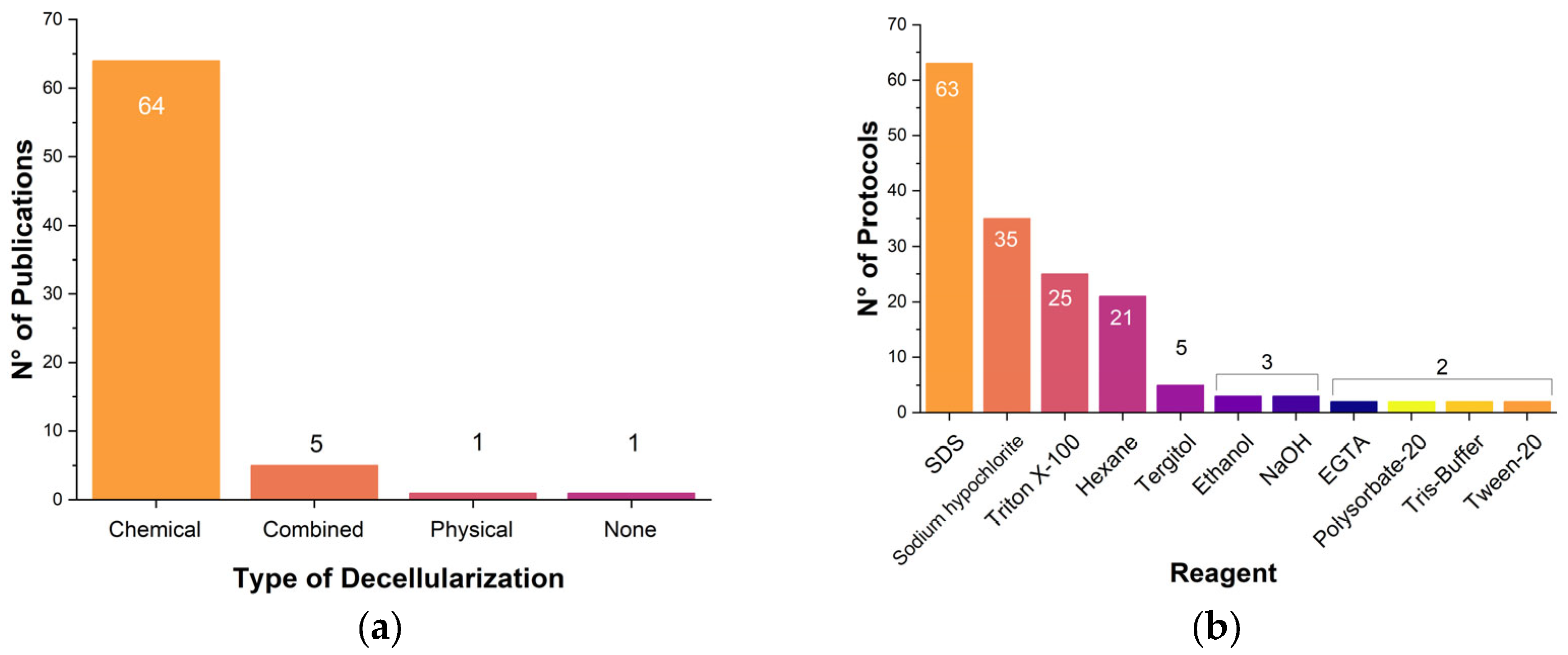
3.3.2. Decellularization Protocol
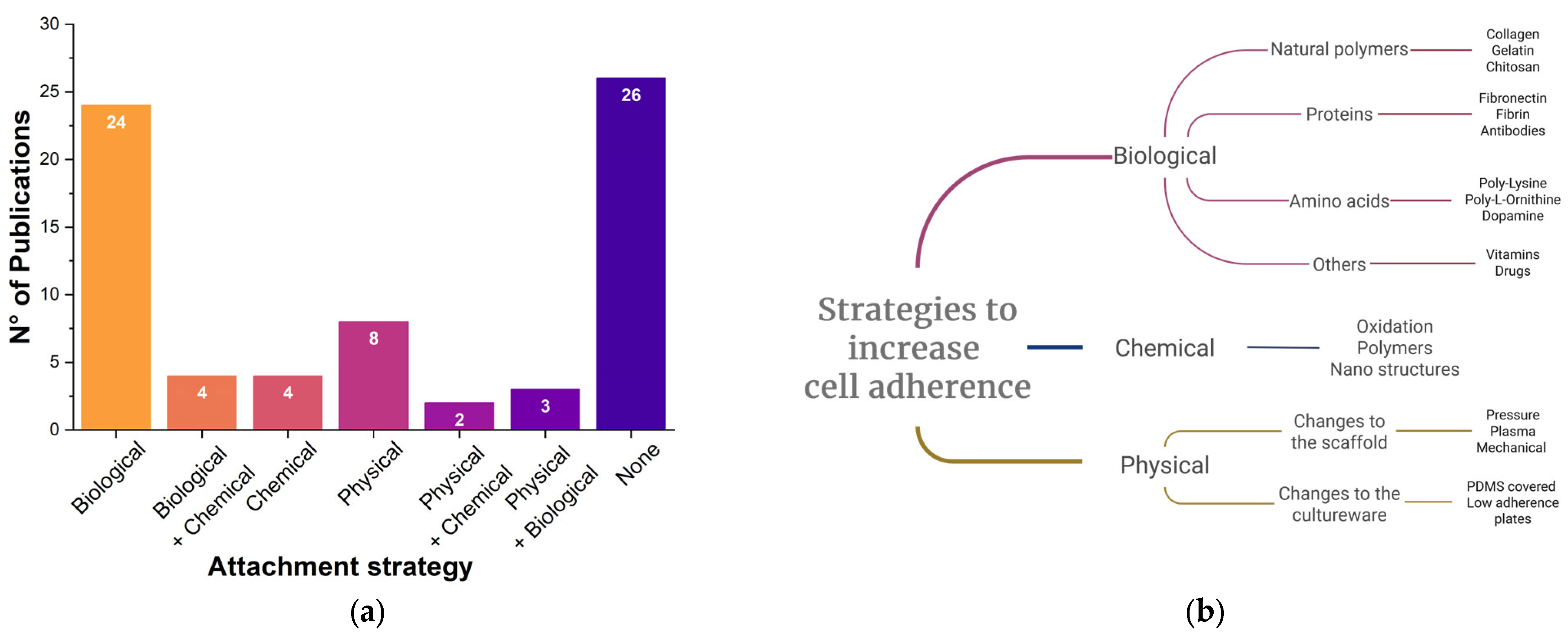
3.3.3. Cell Adhesion Strategy
3.3.4. Additional Tests
| Plant Matrix | Mechanical Test | Ref. | ||
|---|---|---|---|---|
| Type | Parameters | Main Results | ||
| Spinach Parsley Artemisia annua Peanut hairy leaves | Tensile | Native and decellularized spinach leaves were uniaxially stretched at a constant strain of 10 mm/min until failure. | Decellularized leaves displayed significantly lower ultimate tensile strength (p = 0.00925) and strain at failure (p = 0.000287) than native samples. Maximum tangent modulus for decellularized spinach leaves was 0.3 MPa. | [31] |
| Ficus hispida Paquira aquatica Garcinia sp. | Tensile | Maximum tangent modulus (MTM), strain at failure (SAF), and ultimate tensile strength (UTS) for F. hispida and Pachira aquatica samples. | P. aquatica samples prepared using both decellularization protocols displayed similar mechanical properties across all three of the parameters measured. The F. hispida samples showed a similar trend in all the cases except the UTS testing. Samples prepared using the SDS had higher average UTS results than those prepared with bleach. | [32] |
| Apple Carrot Celery | Hysteresis compression and tensile | Apple: hysteresis compression cycle. Each test consisted of a load phase at a rate of 2.5% min−1 down to −30% strain. Carrot: compression load applied at a rate of 5%/min up to 60% strain. Celery: tensile load applied at a rate of 20% strain. | After the different tests, these are the elastic moduli of the different matrices: E apple = 4 kPa, E carrot = 43 kPa, E celery = 590 kPa. | [35] |
| Spinach | Tensile | Tensile test at a loading velocity of 5 mm/min. | The peak of stress–strain curve was 1.4 MPa and its elongation at break 4.57%. | [39] |
| Bambusa vulgaris | Compression | Uniaxial compression down to 80% compression at a crosshead speed of 5 mm/min. | The decellularized bamboo had a compressive strength of 1.52 ± 0.346 MPa, whereas the values of the ones oxidized (ODP 0.01, ODP 0.1 and ODP 0.5) were 1.36 ± 0.47 MPa, 1.078 ± 0.2 MPa and 0.6 ± 0.045 MPa, respectively. Thus, the strength was decreased, in correlation with the oxidation process (DP > ODP0.01 > ODP0.1 > ODP0.5). | [41] |
| Chive Spinach | Tensile | Uniaxial tensile loading until failure. | It was not possible to apply traverse tensile loading on the chive for mechanical testing. Only tensile loading in the longitudinal direction along the cellulose fibers. When possible, results show stress at break was 0.7 ± 0.2 MPa, and the strain at break was 20 ± 13% compared with 13 ± 3% in native tissue. | [45] |
| Borassus flabellifer | Compression | Hydrated scaffold samples compressed at a rate of 1 mm/min. | The stress–strain curve is typical for soft polymers with the stress plateau between 60 and 70% of strain. The compressive strength was found to be higher in cellulose–chitosan group. | [47] |
| Onion | Tensile | Tensile test with a loading speed of 5 mm/min. | The results demonstrated that scaffold revealed a high tensile strength (8.197 MPa), and an elongation at break (3.23%) close to the normal bone tissue. | [48] |
| Cabbage | Tensile | Cut samples subjected to a loading velocity of 50 mm/min. | Peak of stress-strain curve was 4.32 MPa and its elongation at break was 18.54%. | [49] |
| Alstroemeria flower | Compression | Each stem was placed between the grips of the machine and compressed in the axial direction at the rate of 1 mm/min. | The decellularized scaffold showed an approximately 40% increase in length, while this factor was about 80% for the decellularized and chitosan ones. In terms of the Young’s modulus, the decellularized scaffolds had (0.15 MPa) versus (0.8 MPa) for the ones coated with chitosan. | [52] |
| Fennel Wild Fennel Dill | Compression | The test was performed by applying a hysteresis compression cycle on the decellularized samples with GelMA and GelMA scaffolds as controls. A preload force (F = 0.001 N) was applied, followed by a loading phase at a rate of 2.5%/min, down to −30% strain, and an unloading phase at a rate of 5%/min. | A decrease in mechanical characteristics was found for all the samples containing plant structures compared to GelMA samples. GelMA-Fennel (4.27 +/− 1.16 kPa), GelMA-Wild Fennel (5.68 +/− 0.76 kPa), GelMA-Dill (3.55 +/− 0.34 kPa) versus GelMA (9.32 +/− 1.85 kPa). | [53] |
| Spinach Sweet Mint Tomato leaves | Tensile | PDMS, decellularized spinach and porcine lung tissue were evaluated. The samples’ stretching properties were analyzed in a custom build device. Additionally, the stretched and non-stretched spinach leaves were subjected to a constant strain of 5 mm/min until failure. | The local longitudinal strain values of the PDMS were between 10.85–12.71%) while the decellularized leaf and lung scaffolds showed greater variation, ranging from 7.76–15.88% and 10.67–19.67%, respectively. | [54] |
| Apple | Compression | Cyclic hydrostatic pressure (HP) stimulation. | Data showed no significant changes between samples incubated in osteogenic media with applied HP (16.1 ± 2.1 kPa) and without applied HP (17.2 ± 3.2 kPa) after 1 week and 2 weeks (13.9 ± 0.8 kPa and 18.7 ± 0.7 kPa, respectively). | [55] |
| Celery | Tensile | A universal mechanical testing machine was used to measure the Young’s modulus (no more details indicated in the experimental section). | The Young’s modulus values of decellularized celery with nanoamyloids and decellularized celery with nanohydroxyapatite and were significantly higher than that of decellularized celery. | [56] |
| Borassus flabellifer | Compression | Samples under hydrated conditions were evaluated at a rate of 1 mm/min. | All scaffold groups (cellulose, cellulose-chitosan, cellulose-chitosan-PRP and cellulose-PRP) exhibited linear elastic behavior at lower stress values. Up to 80% strain, all the scaffold groups exhibited an extended linear elastic region without plateau or final densification region. The compressive strength was higher among the chitosan coated samples than in the uncoated decellularized ones. The compressive moduli were found to be in the following decreasing order: Cellulose-Chitosan-PRP, Cellulose-Chitosan, Cellulose-PRP and Cellulose. The presence of PRP did not modify the mechanical behavior. | [57] |
| Borassus flabellifer | Compression | Compressive mechanical properties of the scaffolds were estimated under hydrated conditions with a speed of 1 mm/min. | The compressive moduli were found to be 0.59 ± 0.19 kPa for oxidized scaffolds, 5.73 ± 1.76 kPa oxidized + APTES, and 8.33 ± 1.52 kPa for OCS and OTS. Surface modification by APTES and OTS has a significant effect in enhancing the compressive mechanical behavior of OCS scaffolds. | [58] |
| Asparagus | Compression | Each scaffold was compressed mechanically to a maximum 30% strain, at a compression speed of 50 µm/s. | The Young’s modulus of the scaffold in culture media at 37 °C is 128 ± 20 kPa when measured parallel to the long axis. | [61] |
| Leatherleaf Spinach Parsley | Tensile | Samples (from the different tissues leatherleaf, spinach, and parsley, and the different decellularization protocols, as well as cross-linked gelatin) were pulled uniaxially at 0.08 mm/s until failure. | Elastic modulus was 3.8 ± 0.2, 4.0 ± 1.4, 3.9 ± 0.3, 2.7 ± 0.9, and 2.8 ± 0.2 MPa for intact, SDS-, SDS/EGTA-, Tergitol/EGTA-, and Tergitol/SDS-decellularized leatherleaf, respectively. Elastic modulus of spinach was 1.15 ± 0.22, 1.62 ± 0.58, 0.54 ± 0.77, 4.05 ± 1.33, and 2.34 ± 1.09 MPa for intact, SDS-, SDS/EGTA-, Tergitol/EGTA-, and Tergitol/SDS-decellularization, respectively. Parsley was 4.47 ± 0.16, 3.59 ± 1.47, 2.28 ± 0.62, 1.21 ± 0.17, and 0.21 ± 0.29 MPa, respectively. Elastic modulus of cross-linked gelatin was 0.3 ± 0.1 MPa, and failure strain was 0.1 ± 0.02. Maximum modulus of 3D grafts constructed from SDS-decellularized leatherleaf and gelatin was 1.3 ± 0.1 MPa. Maximum tensile stress at failure was 5.5 ± 1.1 MPa, and failure strain was 4.1 ± 0.7. | [63] |
| Corn Husk Jackfruit rind | Compression and tensile | Fresh and decellularized jackfruit and corn husk samples were pulled to failure. Jackfruit samples were axially affixed into the machine and pulled at a constant 10 mm/min. Corn husk samples were pulled to failure both parallel to leaf venation and orthogonal to it. | Following decellularization, corn husk scaffold stiffnesses decreased from 56.67 ± 16.71 MPa to 12.95 ± 2.43 MPa in fiber-aligned direction, while jackfruit decreased from 7.54 ± 2.42 MPa to 2.47 ± 1.47 MPa. | [65] |
| Apple | Compression (hardness and stiffness) | To measure hardness, samples were compressed to 50% of the initial thickness. Maximum force (N) of compression was measured. To measure stiffness, Young’s modulus (MPa) was calculated based on the force displacement curve. | The hardness of the decellularized apple tissues was reduced 1.5 times from the native ones (33,42 ± 7.62 N) vs. (51.99 ± 7.33 N). The Young’s Modulus on the other hand was reduced from a native one of (0.3 ± 0.09 MPa) to (0.1 ± 0.01 MPa) in decellularized samples. | [66] |
| Olive Leaves | Tensile | A uniaxial tensile test at constant force at a speed of 10 mm/min was applied, and the sheets were pulled to the rupture point. | The elasticity modulus of acellular samples was significantly reduced compared to normal leaf samples. The no decellularized sample showed a modulus of elasticity of about 20.45 MPa and a maximum tensile stress of 2.50 MPa. While the decellularized samples showed an elasticity modulus of 4.99 MPa and a maximum tensile stress of 0.60 MPa. | [67] |
| Nopal | Tensile | Native and decellularized nopal samples. A cross speed of 1 mm/min applied until failure occurred. | Native tissue showed a tensile strength of 12.5 +/− 1 MPa and decellularized tissue of 11.8 +/− 0.5 MPa. No significant difference between the two groups. | [70] |
| Watermelon rind | Tensile | Tensile testing at the strain rate of 10 mm/min. | The sample possess an elastic modulus of 1.335 MPa and 0.595 MPa in the dry and wet states, respectively. The stiffness of the scaffold declines upon soaking in water. | [72] |
| Parsley | Tensile and suture retention | For the tensile tests, wet and dry decellularized parsley stems were cut and attached to the tensile grips 10 mm away from both ends. The tensile tests were performed at a rate of 6 mm/min until failure occurred. Suture retention tests were performed according to BS EN ISO 7198:2017. The tensile tests were performed at a rate of 6 mm/min until failure. | The test results demonstrated that the elastic modulus, UTS, and suture retention strength of the decellularized parsley stems under wet conditions were 5.182 ± 0.856 MPa, 0.471 ± 0.044 MPa, and 0.066 ± 0.029 MPa (0.242 ± 0.067 N), respectively. The percentage elongation of the samples at UTS was 12.3 ± 2.4%. Under dry conditions, the samples were more brittle, giving a significantly higher elastic modulus (32.148 ± 1.649 MPa). The tensile strength of the dry samples was also significantly higher, with a UTS of 1.301 ± 0.051 MPa. The elongation of the dry test samples at UTS was 8.1 ± 0.6%, slightly lower than that observed under wet conditions. The mechanical properties of the developed TEVG were similar to artery-like behavior with a strain of 13% and a UTS of 0.14 MPa. | [74,105] |
| Spinach | Tensile and suture retention | The samples were attached to the tensile grips from both ends, and the tensile test was performed at a rate of 6 mm/min until failure. For suture retention: wet samples were sutured 2 mm from the top end and attached to the tensile grip. The bottom end of the samples was fixed to the lower grip 10 mm from the end, and suture retention tests were performed at a rate of 0.1 mm/s until the load bearing was reduced to 10% of the maximum force. | The tensile test results showed that the average elastic moduli of the longitudinal and transverse samples were significantly different from each other. The elastic moduli of the longitudinal and transverse testing samples were 0.843 ± 0.096 MPa and 0.250 ± 0.032 MPa, respectively. The UTSs of the longitudinal and transverse test samples were 0.145 ± 0.007 MPa and 0.101 ± 0.006 MPa, respectively. The suture retention strengths of the longitudinal and transverse samples were 0.090 ± 0.017 MPa and 0.073 ± 0.002 MPa, respectively. | [76] |
| Spinach | Tensile | Samples were stretched in quasi-static mode. All static tests aiming to identify tensile moduli, strength, and ultimate strain were performed with a strain rate of 5%/min and a preload force of 0.01 N. | The tensile modulus of the decellularized scaffolds was 2.2 ± 0.9 MPa, 4-fold lower than that of native primary veins at 10.1 ± 2.4 MPa. Likewise, the decellularized primary vein demonstrated a 3-fold lower ultimate strain and 8-fold lower ultimate tensile strength than the native tissue. | [77] |
| Celery | Compression | Unconfined compression tests, with a bath chamber. Hydrated samples were evaluated at a displacement rate of 0.01 mm/s up to 50% deformation. | Celery scaffolds after 24 h decellularization had a Young’s Modulus of 46.76 +/− 8.43 kPa in slices cut transversely and 42.51 +/− 7.78 kPa in slices cut longitudinally. | [78] |
| Leatherleaf viburnum | Suture retention | Suture retention tests were performed on plant-based grafts and rat aorta to compare maximum load of 8–0 and 10–0 Prolene sutures. The graft or aorta was clamped to one end of the Testing machine, and the suture holder clamped to the other. Tension was applied to sutures at a rate of 1 mm/s until a tear in the graft or aorta was observed. | Retention force of 8–0 and 10–0 Prolene sutures was 0.74 ± 0.13 and 0.65 ± 0.28 N for plant-based grafts and 1.01 ± 0.17 and 0.32 ± 0.10 N for rat aorta, respectively. | [79] |
| Pumpkin | Tensile | The mechanical properties of the scaffolds were measured with a strain rate of 5 mm/min. | The coated MgO2-Pumpkin scaffold exhibits higher (~21%) tensile strength (1.71 MPa) compared to the uncoated version (1.42 MPa). The compressive strength of both coated (0.5721 MPa) and uncoated (0.5633 MPa) pumpkin showed no difference. | [82] |
| Apple | Compression | Custom-built uniaxial compression apparatus, at a constant rate of 3 mm/min. | No significant difference was observed in the modulus between the blank scaffolds (31.6 kPa ± 4.8 kPa) and the cell-seeded scaffolds cultured in non-differentiation medium (24.1 kPa ± 8.8 kPa; p = 0.88). In contrast, a significant difference was noted between the modulus of the blank scaffolds (31.6 kPa ± 4.8 kPa) and that of the cell-seeded scaffolds cultured in differentiation medium (192.0 kPa ± 16.6 kPa; p < 0.001). | [86] |
| Lisianthus sp. | Tensile | Uniaxial tensile strength at the 1 mm/min rate | Elastic modulus of 31 MPa | [89] |
| Bougainvillea sp. | Tensile | Native and decellularized samples with a load of 500 N and stretching speed of 1 mm min−1 at room temperature. | The bracts exhibited tensile strength values (around 1.5 MPa) with no significant difference between native and decellularized. | [90] |
| Apple | Compression | The coated and uncoated scaffolds were subjected to a strain of 30% at a strain rate of 2 mm/min. | The Young’s modulus for uncoated and coated scaffolds were calculated as 10.13 kPa and 6.78 kPa, respectively. | [91] |
| Taraxacum Ruderalia | Hydrostatic conductivity | The hydrostatic conductivity at the average pressure of lymphatic collectors and density of lymph fluid were measured. The risk of kinking was determined by curving the conduit around a plastic template of predefined decreasing diameter ranging from 50 to 5 mm. As comparative controls for the hydrostatic conductivity and kink resistance test, fresh arteries, veins, and lymphatic collectors were harvested from swine extremities and decellularized using the same method. | The size of the tubes generated ranged between 1 mm and 1 cm, making them candidates for anastomosis of lymphatic collectors, small and large blood vessels. These observations were quantified by the kinking resistance test, where the cellulose tube proved to be statistically equally resistant to kinking as decellularized veins, lymphatic collectors, and arteries. The hydraulic conductivity in the physiologic range of a lymphangion (65 cm H2O) and fluid of normal lymph viscosity (0.0018 Pa) was found to be statistically equivalent (α 0.05) to the conductivity of decellularized lymphatic and vascular vessels of the swine.- | [92] |
| Pomelo | Tensile, shear and compression | The mechanical properties were evaluated using an electromechanical universal tester. For tensile tests, samples were stretched at 2 mm/min until failure. For shear tests, samples were adhered between two pork skin slices and pulled apart at 2 mm/min. For compression tests, samples were compressed at 2 mm/min to 90% strain. | The compressive stress of the decellularized pomelo with MOF and Gel (4.53 ± 0.14 MPa) was much greater than decellularized pomelo (3.59 ± 0.09 MPa) and MOF and Gel (0.28± 0.03 MPa) at 90% strain. The tensile stress of decellularized pomelo with MOF and Gel before fracture could reach 337.52 ± 7.54 kPa, much higher than decellularized pomelo (299.03 ± 10.23 kPa). | [93] |
| Leatherleaf viburnum | Tensile | Samples were subjected to uniaxial tensile testing at a rate of 0.08 mm/s until failure. | Trypsin/Tergitol-, Trypsin/Tergitol/EGTA, and SDS/Tergitol-treated samples exhibited the highest tensile strength and elastic modulus, followed by SDS-treated samples with 6 h of clearing (1.2–2.6 N/mm2). Extended clearing times (>12 h) weakened scaffold structure, reducing both tensile strength and elasticity. | [94] |
| Walnut leaves | Tensile | Native and decellularized samples were evaluated in wet conditions at a displacement rate of 0.1 mm/s and with a preload of 0.1 N. | For native samples, the Young’s modulus was 5.16 ± 0.89 MPa, for the decellularized ones it was 4.17 ± 0.91 MPa. Statistical analyses indicated no significant differences between native and decellularized walnut leaves scaffolds in terms of mechanical properties. | [95] |
| Water spinach Green onion Water horsetail | Tensile | A tensile test was performed to determine Young’s modulus, tensile strength, and maximum elongation of the plant scaffolds. | Native and decellularized water horsetail showed the highest Young’s module of 343.7 ± 15.6 and 73.9 ± 15.69 MPa respectively, compared to native and decellularized water spinach (21.54 ± 1.18 and 10.35 ± 2.33 MPa) and green onion (19.49 ± 1.38 MPa and 8.42 ± 2.30 MPa) respectively. Water spinach is the most promising graft candidate in suturability tests besides presenting the highest elongation before rupture in tensile test, with a maximum value of 7.31 ± 0.64% after decellularization. In comparison, GO and WH showed similar maximum elongations of 2.80 ± 1.13% and 2.37 ± 0.59%. | [97] |
4. Discussion
4.1. Cellular Adhesion, Growth, and Differentiation In Vitro
4.2. In Vivo Results
4.3. Other Emerging Biotechnological Applications
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline Phosphatase |
| APTES | Amino (NH2)-Terminated 3-Aminopropyltriethoxysilane |
| RGP peptide | Arginine–Glycine–Aspartate Peptide sequence commonly found in ECM Adhesion Proteins |
| AFM | Atomic Force Microscopy |
| BET | Brunauer–Emmett–Teller |
| CaCl2 | Calcium Chloride |
| ciPTEC | Conditionally Immortalized Proximal Tubule Epithelial Cell |
| dASC | Dog-Adipose-Derived Stromal Cell |
| EGTA | Ethylene Glycol-Bis (Β-Aminoethyl Ether)-N, N,N′,N′-Tetraacetic Acid |
| ECM | Extracellular Matrix |
| FTIR | Fourier-Transform Infrared Analysis |
| GO | Graphene Oxide |
| THP-1 | Human Acute Monocytic Leukemia |
| A549 | Human Adenocarcinoma Alveolar Basal Epithelial Cell |
| hASC | Human-Adipose-Derived Stem Cell |
| hBM-MSC | Human-Bone-Marrow-Derived Mesenchymal Stem Cell |
| MCF-7 | Human Breast Adenocarcinoma Cell |
| MDA-MB321 | Human Breast Cancer Cell Line Expressing GFP |
| hDP-SC | Human-Dental-Pulp-Derived Stem Cell |
| hDF | Human Dermal Fibroblast |
| HDLECs | Human Dermal Lymphatic endothelial cells |
| hESC | Human Embryonic Stem Cell |
| hEC | Human Endothelial Cell |
| HeLa | Human Epithelial Cells Derived from Cervical Cancer |
| hFFs | Human Foreskin Fibroblasts |
| HepG2 | Human Hepatocarcinoma |
| BJ/5Ta | Human-Immortalized Dermal Fibroblast |
| HaCaTs | Human-Immortalized Keratinocytes |
| hiPS-CMs | Human-Induced Pluripotent-Stem-Cell-Derived Cardiomyocytes |
| hiPSC | Human-Induced Pluripotent Stem Cell |
| Lin Sca-1pos cMSCs | Human Lin Sca-1pos Cardiac Mesenchymal Cells (CMSC) |
| BEAS-2B | Human Lung Epithelial Cell Line |
| hMSC | Human Mesenchymal Stem Cell |
| MG63 | Human Osteosarcoma Cell Line |
| hPS-CMs | Human Pluripotent Stem-Cell-Derived Cardiomyocytes |
| PC3 | Human Prostate Cancer Cell |
| hRBC | Human Red Blood Cell |
| hSMC | Human Skeletal Muscle Cell |
| HUVEC | Human Umbilical Vein Epithelial Cell |
| HCl | Hydrochloric Acid |
| IVC | Inferior Vena Cava |
| A549 | Lung Adenocarcinoma Cell Line |
| MOFs | Metal-Organic Frameworks |
| OTS | Methyl (CH3)-Terminated Octadecyltrichlorosilane |
| C619 | Mouse-Derived Epithelial Cell Line from Skin BALB/C |
| 3T3-L1 | Mouse Embryonic Pre-Adipocytes |
| MC-3T3-E1 | Mouse Embryonic Pre-Osteoblasts |
| NIH-3T3 | Mouse Fibroblast |
| L929 | Mouse Fibroblast Cell Line |
| C2C12 | Mouse Muscle Myoblast |
| MC-3T3 | Mouse Pre-Osteoblastic Cell Line |
| NHDF | Normal Human Dermal Fibroblast |
| PRP | Platelet-Rich Plasma |
| PLGA | Polylactic-Co-Glycolic Acid |
| PB-SC | Primary Bovine Satellite Cell |
| PC-SC | Primary Canine Satellite Cell |
| QM7 | Quail Muscle Clone 7 Cell Line |
| rASC | Rat-Adipose-Derived Stem Cell |
| rAEC | Rat Aortic Endothelial Cell |
| rBMSC | Rat Bone Marrow Stromal Cell |
| PC12 | Rat Cell Line derived from a pheochromocytoma of the adrenal medulla, which has an embryonic origin in the neural crest |
| rEC | Rat Aortic Endothelial Cell |
| rH-NSC | Rat Hippocampal Neural Stem Cell |
| rASCs | Rat Mesenchymal Stem Cells from Adipose Tissue |
| rVSMC | Rat Vascular Smooth Muscle Cell |
| SEM | Scanning Electron Microscopy |
| SK-MEL-28 | Skin Melanoma Cell |
| NaOH | Sodium Hydroxide |
| TGA | Thermogravimetric Analysis |
| TCP | Tissue Culture Plate |
| XRD | X-Ray Diffraction |
References
- Global Observatory on Donation and Transplantation (GODT). Organ Donation and Transplantation Activities 2022 Report; Global Observatory on Donation and Transplantation Activities: Madrid, Spain, 2022. [Google Scholar]
- Sánchez-Ibañez, J.; Humphreys, C.; Lomero, M.; Escoto, M.; Weiss, M.J.; Wilson, M.; López-Fraga, M. Tissue and Cell Donation: Recommendations From an International Consensus Forum. Transplant. Direct 2023, 9, e1466. [Google Scholar] [CrossRef]
- Peneda Pacheco, D.; Suárez Vargas, N.; Visentin, S.; Petrini, P. From Tissue Engineering to Engineering Tissues: The Role and Application of in Vitro Models. Biomater. Sci. 2021, 9, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Elkasabgy, N.A. Biomaterials for Tissue Engineering Applications and Current Updates in the Field: A Comprehensive Review. AAPS PharmSciTech 2022, 23, 267. [Google Scholar] [CrossRef]
- Pang, K.; Du, L.; Wu, X. A Rabbit Anterior Cornea Replacement Derived from Acellular Porcine Cornea Matrix, Epithelial Cells and Keratocytes. Biomaterials 2010, 31, 7257–7265. [Google Scholar] [CrossRef]
- Demitri, C.; Giuri, A.; De Benedictis, V.M.; Raucci, M.G.; Giugliano, D.; Sannino, A.; Ambrosio, L. Microwave-Induced Porosity and Bioactivation of Chitosan-PEGDA Scaffolds: Morphology, Mechanical Properties and Osteogenic Differentiation: Bioactivation of Chitosan-PEGDA Scaffolds and Osteogenic Differentiation. J. Tissue Eng. Regen. Med. 2017, 11, 86–98. [Google Scholar] [CrossRef]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A Review of Water Interactions, Applications in Composites, and Water Treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef]
- Tayeb, A.; Amini, E.; Ghasemi, S.; Tajvidi, M. Cellulose Nanomaterials—Binding Properties and Applications: A Review. Molecules 2018, 23, 2684. [Google Scholar] [CrossRef] [PubMed]
- Kunjalukkal Padmanabhan, S.; Lamanna, L.; Friuli, M.; Sannino, A.; Demitri, C.; Licciulli, A. Carboxymethylcellulose-Based Hydrogel Obtained from Bacterial Cellulose. Molecules 2023, 28, 829. [Google Scholar] [CrossRef]
- Madaghiele, M.; Demitri, C.; Surano, I.; Silvestri, A.; Vitale, M.; Panteca, E.; Zohar, Y.; Rescigno, M.; Sannino, A. Biomimetic Cellulose-Based Superabsorbent Hydrogels for Treating Obesity. Sci. Rep. 2021, 11, 21394. [Google Scholar] [CrossRef] [PubMed]
- Villani, S.; Kunjalukkal Padmanabhan, S.; Stoppa, M.; Nisi, R.; Calcagnile, M.; Alifano, P.; Demitri, C.; Licciulli, A. Neem-Hypericum-Bacterial Cellulose Wound Care Paste Characterized in Vitro and in Galleria Mellonella in Vivo Model. Carbohydr. Polym. Technol. Appl. 2024, 7, 100431. [Google Scholar] [CrossRef]
- Courtenay, J.; Sharma, R.; Scott, J. Recent Advances in Modified Cellulose for Tissue Culture Applications. Molecules 2018, 23, 654. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Alexander, A.; Shukla, R.; Jain, S.; Bisht, A.; Kumari, K.; Verma, K.; Sharma, S. Tissue Regeneration Properties of Hydrogels Derived from Biological Macromolecules: A Review. Int. J. Biol. Macromol. 2024, 271, 132280. [Google Scholar] [CrossRef]
- Modulevsky, D.J.; Lefebvre, C.; Haase, K.; Al-Rekabi, Z.; Pelling, A.E. Apple Derived Cellulose Scaffolds for 3D Mammalian Cell Culture. PLoS ONE 2014, 9, e97835. [Google Scholar] [CrossRef]
- Bilirgen, A.C.; Toker, M.; Odabas, S.; Yetisen, A.K.; Garipcan, B.; Tasoglu, S. Plant-Based Scaffolds in Tissue Engineering. ACS Biomater. Sci. Eng. 2021, 7, 926–938. [Google Scholar] [CrossRef]
- Joshi, S.; Pendyala, G.; Shah, P.; Mopagar, V.; Padmawar, N.; Padubidri, M. Scaffolds— The Ground for Regeneration: A Narrative Review. J. Int. Soc. Prev. Community Dent. 2020, 10, 692. [Google Scholar] [CrossRef]
- Rabbani, M.; Salehani, A.A.; Farnaghi, M.; Moshtaghi, M. Plant Decellularization by Chemical and Physical Methods for Regenerative Medicine: A Review Article. J. Med. Signals Sens. 2024, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Tardy, B.L.; Mattos, B.D.; Otoni, C.G.; Beaumont, M.; Majoinen, J.; Kämäräinen, T.; Rojas, O.J. Deconstruction and Reassembly of Renewable Polymers and Biocolloids into Next Generation Structured Materials. Chem. Rev. 2021, 121, 14088–14188. [Google Scholar] [CrossRef] [PubMed]
- Jayanth, N.; Venkata Roshan, M.; Sakthi Balaji, S.; Durga Karthik, P.; Barathwaj, A.; Rishiyadhav, G. Additive Manufacturing of Biomaterials: A Review. Mater. Today Proc. 2024, 113, 287–298. [Google Scholar] [CrossRef]
- Francis, A.P.; Augustus, A.R.; Chandramohan, S.; Bhat, S.A.; Priya, V.V.; Rajagopalan, R. A Review on Biomaterials-Based Scaffold: An Emerging Tool for Bone Tissue Engineering. Mater. Today Commun. 2023, 34, 105124. [Google Scholar] [CrossRef]
- Predeina, A.L.; Dukhinova, M.S.; Vinogradov, V.V. Bioreactivity of Decellularized Animal, Plant, and Fungal Scaffolds: Perspectives for Medical Applications. J. Mater. Chem. B 2020, 8, 10010–10022. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F. Xenogeneic Extracellular Matrix as a Scaffold for Tissue Reconstruction. Transpl. Immunol. 2004, 12, 367–377. [Google Scholar] [CrossRef]
- Halper, J.; Kjaer, M. Basic Components of Connective Tissues and Extracellular Matrix: Elastin, Fibrillin, Fibulins, Fibrinogen, Fibronectin, Laminin, Tenascins and Thrombospondins. In Progress in Heritable Soft Connective Tissue Diseases; Halper, J., Ed.; Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2014; Volume 802, pp. 31–47. ISBN 978-94-007-7892-4. [Google Scholar]
- Capuana, E.; Lopresti, F.; Carfì Pavia, F.; Brucato, V.; La Carrubba, V. Solution-Based Processing for Scaffold Fabrication in Tissue Engineering Applications: A Brief Review. Polymers 2021, 13, 2041. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, M.; Mujumdar, A.S. Progress of Plant-derived Non-starch Polysaccharides and Their Challenges and Applications in Future Foods. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13361. [Google Scholar] [CrossRef]
- Chen, L.; Guttieres, D.; Koenigsberg, A.; Barone, P.W.; Sinskey, A.J.; Springs, S.L. Large-Scale Cultured Meat Production: Trends, Challenges and Promising Biomanufacturing Technologies. Biomaterials 2022, 280, 121274. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Modulevsky, D.J.; Cuerrier, C.M.; Pelling, A.E. Biocompatibility of Subcutaneously Implanted Plant-Derived Cellulose Biomaterials. PLoS ONE 2016, 11, e0157894. [Google Scholar] [CrossRef] [PubMed]
- Fontana, G.; Gershlak, J.; Adamski, M.; Lee, J.; Matsumoto, S.; Le, H.D.; Binder, B.; Wirth, J.; Gaudette, G.; Murphy, W.L. Biofunctionalized Plants as Diverse Biomaterials for Human Cell Culture. Adv. Healthc. Mater. 2017, 6, 1601225. [Google Scholar] [CrossRef] [PubMed]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.K.; Dolivo, D.M.; Yang, T.; Dominko, T.; et al. Crossing Kingdoms: Using Decellularized Plants as Perfusable Tissue Engineering Scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef]
- Adamski, M.; Fontana, G.; Gershlak, J.R.; Gaudette, G.R.; Le, H.D.; Murphy, W.L. Two Methods for Decellularization of Plant Tissues for Tissue Engineering Applications. J. Vis. Exp. 2018, 57586. [Google Scholar] [CrossRef]
- Lee, J.; Jung, H.; Park, N.; Park, S.-H.; Ju, J.H. Induced Osteogenesis in Plants Decellularized Scaffolds. Sci. Rep. 2019, 9, 20194. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-W.; Shiwarski, D.J.; Ball, R.L.; Whitehead, K.A.; Feinberg, A.W. Engineering Aligned Skeletal Muscle Tissue Using Decellularized Plant-Derived Scaffolds. ACS Biomater. Sci. Eng. 2020, 6, 3046–3054. [Google Scholar] [CrossRef]
- Contessi Negrini, N.; Toffoletto, N.; Farè, S.; Altomare, L. Plant Tissues as 3D Natural Scaffolds for Adipose, Bone and Tendon Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 723. [Google Scholar] [CrossRef]
- Lacombe, J.; Harris, A.F.; Zenhausern, R.; Karsunsky, S.; Zenhausern, F. Plant-Based Scaffolds Modify Cellular Response to Drug and Radiation Exposure Compared to Standard Cell Culture Models. Front. Bioeng. Biotechnol. 2020, 8, 932. [Google Scholar] [CrossRef]
- Phan, N.V.; Wright, T.; Rahman, M.M.; Xu, J.; Coburn, J.M. In Vitro Biocompatibility of Decellularized Cultured Plant Cell-Derived Matrices. ACS Biomater. Sci. Eng. 2020, 6, 822–832. [Google Scholar] [CrossRef]
- Robbins, E.R.; Pins, G.D.; Laflamme, M.A.; Gaudette, G.R. Creation of a Contractile Biomaterial from a Decellularized Spinach Leaf without ECM Protein Coating: An in Vitro Study. J. Biomed. Mater. Res. A 2020, 108, 2123–2132. [Google Scholar] [CrossRef]
- Salehi, A.; Mobarhan, M.A.; Mohammadi, J.; Shahsavarani, H.; Shokrgozar, M.A.; Alipour, A. Efficient Mineralization and Osteogenic Gene Overexpression of Mesenchymal Stem Cells on Decellularized Spinach Leaf Scaffold. Gene 2020, 757, 144852. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dominko, T.; Weathers, P.J. Using Decellularized Grafted Leaves as Tissue Engineering Scaffolds for Mammalian Cells. Vitro Cell. Dev. Biol.-Plant 2020, 56, 765–774. [Google Scholar] [CrossRef]
- Aswathy, S.H.; Mohan, C.C.; Unnikrishnan, P.S.; Krishnan, A.G.; Nair, M.B. Decellularization and Oxidation Process of Bamboo Stem Enhance Biodegradation and Osteogenic Differentiation. Mater. Sci. Eng. C 2021, 119, 111500. [Google Scholar] [CrossRef]
- Bai, H.; Xie, B.; Wang, Z.; Li, M.; Sun, P.; Wei, S.; Wang, W.; Wu, H.; Bai, L.; Li, J. Application of the Tissue-Engineered Plant Scaffold as a Vascular Patch. ACS Omega 2021, 6, 11595–11601. [Google Scholar] [CrossRef]
- Cancelliere, R.; Zurlo, F.; Micheli, L.; Melino, S. Vegetable Waste Scaffolds for 3D-Stem Cell Proliferating Systems and Low Cost Biosensors. Talanta 2021, 223, 121671. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.F.; Lacombe, J.; Zenhausern, F. The Emerging Role of Decellularized Plant-Based Scaffolds as a New Biomaterial. Int. J. Mol. Sci. 2021, 22, 12347. [Google Scholar] [CrossRef]
- Jansen, K.; Evangelopoulou, M.; Pou Casellas, C.; Abrishamcar, S.; Jansen, J.; Vermonden, T.; Masereeuw, R. Spinach and Chive for Kidney Tubule Engineering: The Limitations of Decellularized Plant Scaffolds and Vasculature. AAPS J. 2021, 23, 11. [Google Scholar] [CrossRef]
- Jones, J.D.; Rebello, A.S.; Gaudette, G.R. Decellularized Spinach: An Edible Scaffold for Laboratory-Grown Meat. Food Biosci. 2021, 41, 100986. [Google Scholar] [CrossRef]
- Mahendiran, B.; Muthusamy, S.; Selvakumar, R.; Rajeswaran, N.; Sampath, S.; Jaisankar, S.N.; Krishnakumar, G.S. Decellularized Natural 3D Cellulose Scaffold Derived from Borassus flabellifer (Linn.) as Extracellular Matrix for Tissue Engineering Applications. Carbohydr. Polym. 2021, 272, 118494. [Google Scholar] [CrossRef]
- Salehi, A.; Mobarhan, M.A.; Mohammadi, J.; Shahsavarani, H.; Shokrgozar, M.A.; Alipour, A. Natural Cellulose-Based Scaffold for Improvement of Stem Cell Osteogenic Differentiation. J. Drug Deliv. Sci. Technol. 2021, 63, 102453. [Google Scholar] [CrossRef]
- Salehi, A.; Mobarhan, M.A.; Mohammadi, J.; Shahsavarani, H.; Shokrgozar, M.A.; Alipour, A. Cabbage-derived Three-dimensional Cellulose Scaffold-induced Osteogenic Differentiation of Stem Cells. J. Cell. Physiol. 2021, 236, 5306–5316. [Google Scholar] [CrossRef]
- Walawalkar, S.; Almelkar, S. Fabricating a Pre-Vascularized Large-Sized Metabolically-Supportive Scaffold Using Brassica Oleracea Leaf. J. Biomater. Appl. 2021, 36, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Bai, X.; Sun, P.; Zhang, L.; Wei, S.; Bai, H. A Novel Plant Leaf Patch Absorbed With IL-33 Antibody Decreases Venous Neointimal Hyperplasia. Front. Bioeng. Biotechnol. 2021, 9, 742285. [Google Scholar] [CrossRef]
- Esmaeili, J.; Jadbabaee, S.; Far, F.M.; Lukolayeh, M.E.; Kırboğa, K.K.; Rezaei, F.S.; Barati, A. Decellularized Alstroemeria Flower Stem Modified with Chitosan for Tissue Engineering Purposes: A Cellulose/Chitosan Scaffold. Int. J. Biol. Macromol. 2022, 204, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Grilli, F.; Pitton, M.; Altomare, L.; Farè, S. Decellularized Fennel and Dill Leaves as Possible 3D Channel Network in GelMA for the Development of an in Vitro Adipose Tissue Model. Front. Bioeng. Biotechnol. 2022, 10, 984805. [Google Scholar] [CrossRef]
- Harris, A.F.; Lacombe, J.; Sanchez-Ballester, N.M.; Victor, S.; Curran, K.A.J.; Nordquist, A.R.; Thomas, B.; Gu, J.; Veuthey, J.-L.; Soulairol, I.; et al. Decellularized Spinach Biomaterials Support Physiologically Relevant Mechanical Cyclic Strain and Prompt a Stretch-Induced Cellular Response. ACS Appl. Bio Mater. 2022, 5, 5682–5692. [Google Scholar] [CrossRef]
- Leblanc Latour, M.; Pelling, A.E. Mechanosensitive Osteogenesis on Native Cellulose Scaffolds for Bone Tissue Engineering. J. Biomech. 2022, 135, 111030. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Y.; Zhang, H.; Wang, X.; Chen, T.; Wu, Y.; Xu, X.; Yang, S.; Ji, P.; Song, J. Natural Plant Tissue with Bioinspired Nano Amyloid and Hydroxyapatite as Green Scaffolds for Bone Regeneration. Adv. Healthc. Mater. 2022, 11, 2102807. [Google Scholar] [CrossRef]
- Mahendiran, B.; Muthusamy, S.; Sampath, S.; Jaisankar, S.N.; Selvakumar, R.; Krishnakumar, G.S. In Vitro and in Vivo Biocompatibility of Decellularized Cellulose Scaffolds Functionalized with Chitosan and Platelet Rich Plasma for Tissue Engineering Applications. Int. J. Biol. Macromol. 2022, 217, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Mahendiran, B.; Muthusamy, S.; Janani, G.; Mandal, B.B.; Rajendran, S.; Krishnakumar, G.S. Surface Modification of Decellularized Natural Cellulose Scaffolds with Organosilanes for Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2022, 8, 2000–2015. [Google Scholar] [CrossRef] [PubMed]
- Thyden, R.; Perreault, L.R.; Jones, J.D.; Notman, H.; Varieur, B.M.; Patmanidis, A.A.; Dominko, T.; Gaudette, G.R. An Edible, Decellularized Plant Derived Cell Carrier for Lab Grown Meat. Appl. Sci. 2022, 12, 5155. [Google Scholar] [CrossRef]
- Ahmadian, M.; Hosseini, S.; Alipour, A.; Jahanfar, M.; Farrokhi, N.; Homaeigohar, S.; Shahsavarani, H. In Vitro Modeling of Hepatocellular Carcinoma Niche on Decellularized Tomato Thorny Leaves: A Novel Natural Three-Dimensional (3D) Scaffold for Liver Cancer Therapeutics. Front. Bioeng. Biotechnol. 2023, 11, 1189726. [Google Scholar] [CrossRef]
- Couvrette, L.J.; Walker, K.L.A.; Bui, T.V.; Pelling, A.E. Plant Cellulose as a Substrate for 3D Neural Stem Cell Culture. Bioengineering 2023, 10, 1309. [Google Scholar] [CrossRef]
- Galefi, A.; Nourany, M.; Hosseini, S.; Alipour, A.; Azari, S.; Jahanfar, M.; Farrokhi, N.; Homaeigohar, S.; Shahsavarani, H. Enhanced Osteogenesis on Proantocyanidin-Loaded Date Palm Endocarp Cellulosic Matrices: A Novel Sustainable Approach for Guided Bone Regeneration. Int. J. Biol. Macromol. 2023, 242, 124857. [Google Scholar] [CrossRef] [PubMed]
- Gorbenko, N.; Rinaldi, G.; Sanchez, A.; Merna, N. Small-Caliber Vascular Grafts Engineered from Decellularized Leaves and Cross-Linked Gelatin. Tissue Eng. Part A 2023, 29, 397–409. [Google Scholar] [CrossRef]
- Jones, J.D.; Thyden, R.; Perreault, L.R.; Varieur, B.M.; Patmanidis, A.A.; Daley, L.; Gaudette, G.R.; Dominko, T. Decellularization: Leveraging a Tissue Engineering Technique for Food Production. ACS Biomater. Sci. Eng. 2023, 9, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Perreault, L.R.; Thyden, R.; Kloster, J.; Jones, J.D.; Nunes, J.; Patmanidis, A.A.; Reddig, D.; Dominko, T.; Gaudette, G.R. Repurposing Agricultural Waste as Low-Cost Cultured Meat Scaffolds. Front. Food Sci. Technol. 2023, 3, 1208298. [Google Scholar] [CrossRef]
- Rai, R.; Nitin, N. Apple-Derived 3D Scaffold for Improving Gastrointestinal Viability and in-Situ Growth of Probiotics. Food Res. Int. 2023, 168, 112758. [Google Scholar] [CrossRef] [PubMed]
- Ahangar Salehani, A.; Rabbani, M.; Biazar, E.; Heidari Keshel, S.; Pourjabbar, B. The Effect of Chemical Detergents on the Decellularization Process of Olive Leaves for Tissue Engineering Applications. Eng. Rep. 2023, 5, e12560. [Google Scholar] [CrossRef]
- Xia, N.; Zhu, Y.; Liu, R.; Chen, W.; Zhao, Y.; Sun, L. Decellularized Lotus Petioles Integrated Microfluidic Chips for Neural Cell Alignment Monitoring. Compos. Part B Eng. 2023, 255, 110621. [Google Scholar] [CrossRef]
- Yun, J.; Robertson, S.; Kim, C.; Suzuki, M.; Murphy, W.L.; Gopalan, P. Aligned Skeletal Muscle Assembly on a Biofunctionalized Plant Leaf Scaffold. Acta Biomater. 2023, 171, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Zamudio-Ceja, R.B.; Garcia-Contreras, R.; Chavez-Granados, P.A.; Aranda-Herrera, B.; Alvarado-Garnica, H.; Jurado, C.A.; Fischer, N.G. Decellularized Scaffolds of Nopal (Opuntia Ficus-Indica) for Bioengineering in Regenerative Dentistry. J. Funct. Biomater. 2023, 14, 252. [Google Scholar] [CrossRef]
- Ahmadian, M.; Hosseini, S.; Alipour, A.; Kazemi, J.; Farrokhi, N.; Jahanfar, M.; Homaeigohar, S.; Shahsavarani, H. Enhanced Liver Cancer Cellular Response to a Drug on a 3D Nanostructured Matrix of Decellularized Eggplant Leaves. Mater. Today Commun. 2024, 39, 109318. [Google Scholar] [CrossRef]
- Banaeyan, R.; Nourany, M.; Hosseini, S.; Galefi, A.; Alipour, A.; Jahanfar, M.; Wang, P.Y.; Homaeigohar, S.; Shahsavarani, H. Polydopamine-based Surface Functionalization of Watermelon Rind as a 3D Nanofibrous Cellulose Scaffold for Osteogenesis. Cellulose 2024, 31, 443–461. [Google Scholar] [CrossRef]
- Berry-Kilgour, C.; Oey, I.; Cabral, J.; Dowd, G.; Wise, L. Decellularized Green and Brown Macroalgae as Cellulose Matrices for Tissue Engineering. J. Funct. Biomater. 2024, 15, 390. [Google Scholar] [CrossRef]
- Cevik, M.; Dikici, S. Development of Tissue-Engineered Vascular Grafts from Decellularized Parsley Stems. Soft Matter 2024, 20, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xiong, W.; Guo, Y.; Jin, X.; Wang, L.; Ge, C.; Tan, W.; Zhou, Y. Three-dimensional Pore Structure of the Decellularized Parsley Scaffold Regulates Myogenic Differentiation for Cell Cultured Meat. J. Food Sci. 2024, 89, 5646–5658. [Google Scholar] [CrossRef] [PubMed]
- Dikici, S. Enhancing Wound Regeneration Potential of Fibroblasts Using Ascorbic Acid-Loaded Decellularized Baby Spinach Leaves. Polym. Bull. 2024, 81, 9995–10016. [Google Scholar] [CrossRef]
- Filiz, Y.; Arslan, Y.; Duran, E.; Saglam-Metiner, P.; Horozoglu, S.; Paradiso, A.; Martinez, D.C.; Sabour-Takanlou, M.; Heljak, M.; Jaroszewicz, J.; et al. Decellularized Plant-Derived Vasculature-on-a-Chip Interacting with Breast Cancer Spheroids to Evaluate a Dual-Drug Therapy. Appl. Mater. Today 2024, 36, 102015. [Google Scholar] [CrossRef]
- Fiorelli, E.; Scioli, M.G.; Terriaca, S.; Ul Haq, A.; Storti, G.; Madaghiele, M.; Palumbo, V.; Pashaj, E.; De Matteis, F.; Ribuffo, D.; et al. Comparison of Bioengineered Scaffolds for the Induction of Osteochondrogenic Differentiation of Human Adipose-Derived Stem Cells. Bioengineering 2024, 11, 920. [Google Scholar] [CrossRef] [PubMed]
- Gorbenko, N.; Vaccaro, J.C.; Fagan, R.; Cerro, R.A.; Khorrami, J.M.; Galindo, L.; Merna, N. Perfusion Bioreactor Conditioning of Small-Diameter Plant-Based Vascular Grafts. Tissue Eng. Regen. Med. 2024, 21, 1189–1201. [Google Scholar] [CrossRef]
- Hasanzadeh, A.; Alipour, A.; Ghasemi, S.; Hosseini, S.; Farrokhi, N.; Wang, P.-Y.; Zarrabi, A.; Mohammadi, J.; Shahsavarani, H. Proanthocyanidin-Imbued Cellulosic 3-Dimensional Intrinsic Aligned Nanostructures: A Novel Approach for Dental and Bone Regeneration Using Dental Pulp Derived Stem Cells. J. Sci. Adv. Mater. Devices 2024, 9, 100820. [Google Scholar] [CrossRef]
- Hong, T.K.; Do, J.T. Generation of Chicken Contractile Skeletal Muscle Structure Using Decellularized Plant Scaffolds. ACS Biomater. Sci. Eng. 2024, 10, 3500–3512. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Galefi, A.; Hosseini, S.; Shaabani, A.; Farrokhi, N.; Jahanfar, M.; Nourany, M.; Homaeigohar, S.; Alipour, A.; Shahsavarani, H. Magnesium Oxide Nanoparticle Reinforced Pumpkin-Derived Nanostructured Cellulose Scaffold for Enhanced Bone Regeneration. Int. J. Biol. Macromol. 2024, 281, 136303. [Google Scholar] [CrossRef]
- Hu, J.; He, S.; Zhang, D.; Wang, Z.; Zhao, S.; Shi, X.; Li, W.; Guo, Q.; Guan, W.; Yan, L. Constructing Liver-like Tissue in Situ Based on Plant-Derived Cellulose Scaffolds Alleviating Acute Liver Injury. Mater. Des. 2024, 240, 112856. [Google Scholar] [CrossRef]
- Ksouri, R.; Aksel, H.; Saghrouchni, H.; Saygideger, Y. Biocompatible and Safe Decellularized Spinach With Antibacterial and Wound Healing Activity. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35489. [Google Scholar] [CrossRef]
- Singh, P.K.; Gangwar, A.K.; Khangembam, S.D.; Verma, R.K.; Yadav, V.K.; Singh, Y.; Shekher, C.; Goyal, R.P.; Kumar, N. Development of Cellulose Scaffolds Derived from Ficus Benjamina Leaves for Tissue Engineering Applications. Trends Biomater. Artif. Organs 2024, 38, 85. [Google Scholar]
- Leblanc Latour, M.; Tarar, M.; Hickey, R.J.; Cuerrier, C.M.; Catelas, I.; Pelling, A.E. Decellularized Apple-Derived Scaffolds for Bone Tissue Engineering In Vitro and In Vivo. J. Vis. Exp. 2024, e65226. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Ruan, H.; Ye, C.; Jiang, W.; Wang, X.; Chen, S.; Chen, Z.; Li, D. Plant-Derived Leaf Vein Scaffolds for the Sustainable Production of Dog Cell-Cultured Meat. Food Chem. X 2024, 23, 101603. [Google Scholar] [CrossRef]
- Raundal, K.; Kharat, A.; Sanap, A.; Kheur, S.; Potdar, P.; Sakhare, S.; Bhonde, R. Decellularized Leaf-Based Biomaterial Supports Osteogenic Differentiation of Dental Pulp Mesenchymal Stem Cells. Vitro Cell. Dev. Biol. - Anim. 2024, 60, 926–934. [Google Scholar] [CrossRef]
- Zaman, M.S.; Khosravieh, Z.F.; Ahssan, M.; Salehiamin, M.; Ghoraishizadeh, S.; Darvishnia, F.; Rahmani, E.; Esmaeili, J. Fabrication of a Conduit for Future Peripheral Nerve Regeneration Using Decellularized Plant Tissue Modified with Polyaniline/Graphene Oxide Nanosheet. Mater. Today Commun. 2024, 39, 109204. [Google Scholar] [CrossRef]
- Singh, P.; Maparu, A.K.; Mishra, M.; Rai, B.; Sivakumar, S. In Vitro Evaluation of Decellularized Floral Scaffold with Surface Nanotopography for Skin Tissue Engineering. Mater. Today Commun. 2024, 41, 111056. [Google Scholar] [CrossRef]
- Sood, A.; Singhmar, R.; Son, Y.; Jo, C.-H.; Choi, S.; Kumar, A.; Soo Han, S. Tuning the Efficacy of Decellularized Apple by Coating with Alginate/Gelatin to Behave as a Bioscaffold for Cultured Meat Production. Food Res. Int. 2024, 177, 113907. [Google Scholar] [CrossRef] [PubMed]
- Will, P.A.; Taqatqeh, F.; Fricke, F.; Berner, J.E.; Lindenblatt, N.; Kneser, U.; Hirche, C. Tissue-Engineered Cellulose Tubes for Microvascular and Lymphatic Reconstruction: A Translational and Feasibility Study. J. Plast. Reconstr. Aesthet. Surg. 2024, 97, 200–211. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Z.; Tan, J.; Pan, J.; Chen, S.; Wan, W. Copper Ion/Gallic Acid MOFs-Laden Adhesive Pomelo Peel Sponge Effectively Treats Biofilm-Infected Skin Wounds and Improves Healing Quality. Bioact. Mater. 2024, 32, 260–276. [Google Scholar] [CrossRef]
- Imeidopf, G.; Khaimov, D.; John, S.; Merna, N. Optimization and Standardization of Plant-Derived Vascular Scaffolds. Int. J. Mol. Sci. 2025, 26, 2752. [Google Scholar] [CrossRef]
- Kian, M.; Hashemi, S.S.; Derakhshanfar, A.; Darya, G.-H.; Shahhossein, Z.; Saharkhiz, M.J. Decellularized Persian Walnut Leaf (Juglans Regia) as a Potential Wound Dressing Scaffold: An Experimental Study. Front. Bioeng. Biotechnol. 2025, 13, 1524956. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Lee, K.H.; Cheong, D.Y.; Lee, S.W.; Park, I.; Lee, G. Perfusable Cellulose Channels from Decellularized Leaf Scaffolds for Modeling Vascular Amyloidosis. Int. J. Biol. Macromol. 2025, 308, 142509. [Google Scholar] [CrossRef]
- Salehi, A.; Ernez, M.; Salido, G.L.; Cattaneo, G. Toward “Green” Vessels: Characterization of Microstructure, Mechanics, and Endothelial Cell Interaction on Three Macro-Tubular Plants for Vascular Tissue Engineering Applications. Adv. Mater. Technol. 2025, 10, 2401129. [Google Scholar] [CrossRef]
- Yang, D.H.; Lee, I.-H.; Kim, W.-J. Evaluation of Various Mushroom-Based Scaffolds for Application to Cellular Agriculture. Food Chem. 2025, 488, 144827. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Xie, B.; Li, M.; Sun, P.; Wei, S.; Zhang, L.; Zhang, C. Biodegraded PCl and Gelatin Fabricated Vascular Patch in Rat Aortic and Inferior Vena Cava Angioplasty. Microvasc. Res. 2022, 141, 104314. [Google Scholar] [CrossRef] [PubMed]
- Halle, W. Toxizitätsprüfungen in Zellkulturen für eine Vorhersage der akuten Toxizität (LD50) zur Einsparung von Tierversuchen. 1998, Volume 1. Available online: http://hdl.handle.net/2128/11850 (accessed on 29 August 2025).
- Harris, A.F.; Lacombe, J.; Liyanage, S.; Han, M.Y.; Wallace, E.; Karsunky, S.; Abidi, N.; Zenhausern, F. Supercritical Carbon Dioxide Decellularization of Plant Material to Generate 3D Biocompatible Scaffolds. Sci. Rep. 2021, 11, 3643. [Google Scholar] [CrossRef]
- Brandl, F.P.; Seitz, A.K.; Teßmar, J.K.V.; Blunk, T.; Göpferich, A.M. Enzymatically Degradable Poly(Ethylene Glycol) Based Hydrogels for Adipose Tissue Engineering. Biomaterials 2010, 31, 3957–3966. [Google Scholar] [CrossRef]
- Arnold, N.; Scott, J.; Bush, T.R. A Review of the Characterizations of Soft Tissues Used in Human Body Modeling: Scope, Limitations, and the Path Forward. J. Tissue Viability 2023, 32, 286–304. [Google Scholar] [CrossRef]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef] [PubMed]
- BS EN ISO 7198:2017; Cardiovascular Implants and Extracorporeal Systems. Vascular Prostheses. Tubular Vascular Grafts and Vascular Patches. British Standard: London, UK, 2017.


| Database | Search Handle | Used Website Filters |
|---|---|---|
| PubMed | << (((decellularized) AND (scaffold)) AND ((plant) OR (vegetable))) >> | None |
| Science Direct | << decellularized AND scaffold AND (plant OR vegetable)>> | Article type > Research article |
| Scopus | <<(TITLE-ABS-KEY (decellularized) AND TITLE-ABS-KEY (scaffold) AND TITLE-ABS-KEY (plant) OR TITLE-ABS-KEY (vegetable) AND NOT TITLE-ABS-KEY (review))>> | None |
| Sage Journals | <<‘plant’ OR ‘vegetable’ AND ‘decellularized’ AND ‘scaffold’>> | Article type > Research article |
| Author, Publication Year | Plant Matrix | Decellularization Protocol | Cell Line(s) Used/ Animal Model | Cell Interactions/ In Vivo Study | Summary of Findings | Ref. |
|---|---|---|---|---|---|---|
| Modulevsky, 2014 | Apple | Chemical: SDS | NIH3T3, C2C12, and HeLa | Cellular adhesion, proliferation, and invasion | Apple scaffolds were functionalized with either Type I collagen, glutaraldehyde +NaBH4, or control (PBS). After 4 weeks of culture, all three cell lines showed similar proliferation in the biological and chemical-modified scaffolds. Particularly, NIH3T3 and HeLa cells also proliferated on the unmodified scaffolds. | [15] |
| Modulevsky, 2016 | Apple | Chemical: SDS > PBS | Wild-type and C57BL/10ScSnJ mice | Subcutaneous implantation | Scaffold biocompatibility and cell infiltration were examined with H&E staining of fixed cellulose scaffolds following their implantation. The results demonstrate that by 8 weeks post implantation, the host accepted the cellulose scaffold. | [29] |
| Fontana, 2017 | Bamboo, Parsley, Vanilla, Anthurium magnificum, Anthurium waroquaenum, Calathea zebrina, Laelia ancepts, Schoenoplectus tabernaemontani, and Solenostemon scutellarioides | Chemical: SDS > Triton X-100 + 10% Sodium Hypochlorite > n-Hexane | hDF and MSC | Adhesion | Decellularized plants coated with the RGD–dopamine conjugate (RGDOPA) supported the adhesion of hDF and MSCs, while noncoated plants did not support cell attachment on parsley stems. Decellularized plants were also functionalized via biomineralization; these plants also supported the attachment of hDF. | [30] |
| Gershlak, 2017 | Spinach, Parsley, Artemisia annua, and Peanut Hairy Leaves | Chemical: [n-Hexane > PBS] > Triton X-100 + Sodium hypochlorite | HUVEC and hPS-CM | Cytocompatibility, adhesion, proliferation, and contraction | HUVECs were seeded on the inside to mimic the endothelium and hPS-Cm on the outside. After 3 days, the hPS-CM formed clusters; after 5 days, they spontaneously contracted. There was no difference between clusters grown on the decellularized scaffolds and on TCP. | [31] |
| Adamski, 2018 | Ficus hispida, Paquira aquatica, and Garcinia sp. | Chemical: (a) SDS > Nonionic surfactant + Sodium hypochlorite > TRIS-HCl [10 mM, pH 8.5] (b) Sodium hypochlorite + Sodium Bicarbonate + Temperature | hDF and MSC | Cell viability and cell adhesion | The hDFs had higher metabolic activities on bleached scaffolds than on SDS-treated ones. RGD–Dopamine-coated F. hispida leaves showed the adhesion and growth of MSCs. | [32] |
| Lee, 2019 | Apple, Broccoli, Sweet Pepper, Carrot, Jujube, and Persimmon | Chemical: SDS > Ethanol | hiPSC Sprague–Dawley rats | Cell attachment and proliferation, osteogenic differentiation. Rat calvarial defects | Apple, carrot, and persimmon scaffolds were evaluated for cell viability, all with satisfactory results. Apple scaffolds were used for osteogenic differentiation. Elevated levels of mRNA expressed in a time-dependent manner were recorded by osteogenic markers, such as osteocalcin (OCN), and type I collagen (COL-1) was constant. | [33] |
| Cheng, 2020 | Carrot, Broccoli, Cucumber, Potato, Apple, Asparagus, Green Onion, Leek, and Celery | Chemical: SDS | C2C12 and hSMC | Cell attachment, proliferation, and alignment | The outer white section of the green onion had a microstructure that guided C2C12 cell differentiation into aligned myotubes. Quantitative analysis of both cell lines’ alignments revealed an almost complete anisotropic organization compared to that of 2D isotropic controls. | [34] |
| Contessi-Negrini, 2020 | Apple, Carrot, and Celery | Chemical: SDS > CaCl2 | 3T3-L1 preadipocytes, MC3T3-E1 pre-osteoblasts, and L929 fibroblasts | Adhesion, proliferation, and differentiation (adipogenic, osteogenic, and guided anisotropy) | Polylysine-coated samples. No scaffold showed cytotoxic effects. In terms of adipogenic differentiation, higher metabolic activity values were detected in TCP wells compared to the apple scaffolds. There was no difference between the MC3T3-E1 cells grown on the carrot scaffolds and the ones on TCP. For the L929 cells and celery, there was a higher metabolic activity in TCP wells, but the percentage increase in metabolic activity at t = 14 days compared to that at t = 1 day was significantly higher on celery-derived scaffolds. Celery scaffolds also offered the possibility to align the cells. | [35] |
| Lacombe, 2020 | Spinach, Solanum lycopersicum, Echinodorus grisebachii), A. Borealis, and Luckly Bamboo | Chemical: n-Hexanes > SDS > Triton X-100 + Sodium hypochlorite | SK-MEL-28 PC3 | Tissue/disease model | The mechano-regulation of both cell lines on decellularized spinach-leaf scaffolds was decreased compared to cells deposited on standard rigid cell culture substrates. | [36] |
| Phan, 2020 | Tobacco BY-2 and Rice (Cells) and Tobacco Hairy Roots (Tissue) | Physical + Enzymatic: (Lyophilization > DNase) | hFF and THP-1 | Cellular attachment and macrophage response test | When exposed to decellularized BY-2 cell-derived matrices, monolayer cultures of hFFs maintained or increased metabolic activity. Furthermore, hFFs were able to attach, spread, and proliferate when cultured with the decellularized BY-2 cell-derived matrices in the aggregate mode. Directly treating THP-1-derived macrophages with the BY-2 cell-derived matrices for 48 h resulted in increased TNF-α secretion as compared to that in the untreated control group, indicating the possible presence of endotoxin remnants from the decellularization. | [37] |
| Robbins, 2020 | Spinach | Chemical: [n-Hexane > PBS] > SDS > Triton X-100 + Sodium hypochlorite > TRIS Buffer | hiPS-CM | Cell viability, attachment, proliferation, and contraction | Similar cell viabilities between the fibronectin-coated, collagen IV, and uncoated-spinach decellularized scaffolds. No differences in contraction were found between coated leaves, (TCPs), noncoated leaves, or noncoated TCP at most points (on all but day 14). | [38] |
| Salehi, 2020 | Spinach | Chemical: n-Hexane > SDS > Triton X-100 + Sodium hypochlorite > Hexane > Sodium hypochlorite | BM-MSC | Biocompatibility, osteoinductivity, and osteogenic differentiation | The staining results showed that the cells spread on the surface of the scaffold and did not aggregate. Additionally, ALP activity and calcium content measurements in BM-MSCs cultured on the spinach leaf decellularized scaffold on day 18 were significantly higher than those of BM-MSCs cluttered on the TCP. The expression levels of Runx2, osteocalcin, and Col-I genes in the BM-MSCs cultured on the decellularized scaffold at days 9 and 18 were significantly higher than those of the BM-MSCs cultured on the TCP. | [39] |
| Wang, 2020 | Aptenia cordifolia | Chemical: n-Hexane > SDS > TritonX-100 + Sodium hypochlorite | MDA-MB23-expressing GFP | Graft tissue | Specially grown and modified Aptenia cordifolia plants were decellularized to create a grafted scaffold with double-ended vascularity. Characteristic spindle-shaped green fluorescent cells were readily observed on both the TCP and on the decellularized grafted scaffolds. | [40] |
| Aswathy, 2021 | Bambusa vulgaris | Chemical: (a) SDS + Sodium hypochlorite (b) Triton X-100 + Sodium hypochlorite (c) SDS + Triton X-100 (d) Sodium hypochlorite | rA-MSC Wistar rats | Cytocompatibility, cell viability, and osteogenic differentiation Subcutaneous implantation | Oxidized bamboo scaffolds had better MSC adhesion, viability, and osteogenic differentiation than non-oxidized ones. The animal test showed the scaffolds were able to induce angiogenesis and were biocompatible and biodegradable. | [41] |
| Bai, 2021 | Onion Leaf and Onion Skin | Chemical: SDS > PBS > Sodium hypochlorite | Male Sprague–Dawley rat | Inferior vena cava patch venoplasty model | The onion leaf was decellularized, and the scaffold was loaded with polylactic-co-glycolic acid (PLGA)-based rapamycin nanoparticles. Both leaf- and onion-cellulose-laced scaffolds showed decreased neointimal thickness, with the leaf scaffolds also showing fewer CD68+ cells and PCNA+ cells. | [42] |
| Cancelliere, 2021 | Broccoli | Chemical: SDS | NHDF human Lin− Sca-1pos cardiac mesenchymal cells (cMSC) | Biosensor and in vitro toxicity model of cellular attachment and growth | Microstructured scaffolds from stalks of broccoli, named BrcS, were either functionalized for the production of enzymatic 3D biosensors to monitor glucose uptake over time or preconditioned to be used as 3D scaffolds for cMSC cultures. After the preconditioning of the broccoli scaffold with the cell culture medium, it was able to support the cell attachment and growth of cMSCs. | [43] |
| Harris, 2021 | Spinach, Sweet Mints, Celery, and Parsley | Physical vs. Chemical: (a) ssCO2 (b) SDS | hDF | Cell viability, attachment, and proliferation | The hDFs were seeded on and attached to scCO2-decellularized scaffolds, showing viable cells at 14 days and able to respond to a drug stimulus. As per the authors’ recollection, further analyses are needed to evaluate the influence of the ssCO2 decellularization on seeded cells. | [44] |
| Jansen, 2021 | Chive and Spinach | Chemical: n-Hexane > SDS | Tetramethylrhodamine-isothiocyanate (TRITC)-labeled (ciPTEC) | Differentiation into possible vascular grafts | L-Dopa coated samples allowed renal cells to grow on the lumens of chive and spinach petioles, but they did not reach the spinach leaf vasculature through petiole injection. Although recellularization was performed successfully, both spinach and chive tissues quickly disintegrated in the culture. Unsuitable for kidney tubule tissue engineering. | [45] |
| Jones, 2021 | Spinach | Chemical: n-Hexane > SDS > Triton X-100 + Sodium hypochlorite | BP-SC | Lab-grown meat | Cells grown on the decellularized scaffolds showed comparable cell viability compared to cells grown on TCP. Moreover, there was evidence of increased cell differentiation over time. | [46] |
| Mahendiran, 2021 | Borassus flabellifer | Chemical: SDS | L929 | Cytocompatibility, cell viability, and attachment | Significant increase in cell population on day 5 on the chitosan-decellularized samples compared to the uncoated scaffolds. | [47] |
| Salehi, 2021 | Onion | Chemical: n-Hexane > SDS > Triton X-100 + Sodium hypochlorite > Hexane | BM-MSC | Osteogenic differentiation | ALP activity, calcium deposition, and expressions of bone-related genes, such as Runx2, ALP, osteocalcin, and Collagen type-I (col-I), were higher on onion-decellularized scaffolds than on TCP. | [48] |
| Salehi, 2021 | Cabbage | Chemical: n-Hexane > PBS > SDS > Triton X-100 + Sodium hypochlorite > H2O > Hexane | BM-MSC | Osteogenic differentiation | BM-MSCs’ ALP activity and mineralization were increased significantly for cells cultured on the decellularized cabbage leaves compared to those of the cells cultured on TCP. The same applied to the genes: Runx2, ALP, collagen-1 (Col-I), and osteocalcin. | [49] |
| Walawalkar, 2021 | Brassica oleracea | Chemical: SDS > Triton X-100 | HUVECs | Cell attachment and proliferation | The fibrin-coated cabbage leaf scaffold showed no cytotoxicity and was able to maintain the metabolic activities and identity. | [50] |
| Xie, 2021 | Epipremnum aureum | None | A human spiral saphenous vein graft (SVG) implanted in the popliteal vein was harvested from a patient with trauma. Male Sprague–Dawley rats | Drug delivery of IL-33 | Plant leaves absorbed with rhodamine, distilled water (control), rapamycin, IL-33, and IL-33 antibodies were implanted into the rat IVC. There was a large number of IL-33 (p = 0.006) and IL-1β (p = 0.012) positive cells in the human SVG neointima compared to those in the human great saphenous vein. | [51] |
| Esmaeili, 2022 | Alstroemeria Flower | Chemical + Physical: SDS > NaOCl + NaOH at 60 °C | MC3T3 | Cytotoxicity, cell attachment, and differentiation | Both chitosan-coated and uncoated scaffolds were cytocompatible. Chitosan-coated plant-based scaffolds had increase roughness, potential swelling, degradation, diffusion, mechanical behavior, and a porous structure when compared to uncoated scaffolds. Chitosan-coated samples also showed good attachment, proliferation, and migration. | [52] |
| Grilli, 2022 | Fennel, Wild Fennel, and Dill | Chemical: SDS + CaCl2 | 3T3-L1 preadipocyte murine cell | Direct cytocompatibility and adipogenic differentiation | No significant differences in cytocompatibility among the three matrices. Adipogenic differentiation studied on dill samples showed greater metabolic activity. | [53] |
| Harris, 2022 | Spinach, Sweet Mint, and Tomato Leaves | Chemical: n-Hexane > SDS > Sodium hypochlorite | A549 | Disease model/cancer model | A549 lung cells seeded on stretched scaffolds displayed modified cellular morphologies, like that of cells under strain constraints. Custom-built machine could help to mimic breathing motions. Also, cells seeded on the scaffold could sense the mechanical strain, as demonstrated by a nuclear reorientation perpendicular to the strain direction, a nuclear location of YAP, and increased expression of YAP target genes, a high cytoplasmic calcium level, and elevated expression levels of collagen genes (COL1A1, COL3A1, COL4A1, and COL6A), with increased collagen secretion. | [54] |
| Leblanc-Latour, 2022 | Apple | Chemical: SDS > CaCl2 > Ethanol | MC3T3-E1 Subclone 4 | Osteogenic differentiation | The application of hydrostatic pressure significantly increased the density of cells after 1 week compared to static condition. Same for ALP activity, Alizarin Red. | [55] |
| Li, 2022 | Celery | Chemical + Enzymatic: (SDS > Triton X-100 + Sodium hypochlorite > Lysozyme > CaCl2) | MC3T3-E1, HUVEC, and C2C12 rBM-MSC Mouse RBC Male rats | Biocompatibility, orientation, and arrangement study on RBC adhesion, and osteogenesis in vitro. Evaluation of osteogenesis in vivo | Since day 1, both nanoamyloid-loaded scaffolds and nanohydroxyapatite loaded scaffolds showed greater live cell numbers than decellularized celery, but with no difference among the former two groups. HUVEC and C2C12 myoblasts on celery scaffolds can grow and form a two-layer tube-like structure along plant stems and a membrane-like structure on leaves. Nanohydroxyapatite crystals deposited on amyloid further prompted the osteogenic differentiation of preosteoblasts. Adhesion of rBM-MSC was increased by the presence of nanoamyloids. Compared to decellularized tissue, there were greater expressions of Osx, Alpl, and Runx-2 in MC3T3E1 cells seeded on scaffolds with nanoamyloids and nanohydroxyapatite. In vivo experiments proved successful trabeculae regeneration on the scaffold, with nanohydroxyapatite scaffolds showing infiltration of ingrown cells and the newly formed collagen matrix. | [56] |
| Mahendiran, 2022 | Borassus flabellifer | Chemical: SDS | MG63 Wistar rats | Cytotoxicity, cell viability, and biocompatibility in vitro and in vivo (subcutaneous implantation). Drug delivery of growth factors from PRP | All the scaffolds (Cellulose, Cellulose–PRP, Cellulose–Chitosan, and Cellulose–Chitosan–PRP) showed no evidence of cytotoxicity. The addition of PRP proved to aid cell viability. The best performance in terms of cell viability was the from the Cellulose–Chitosan–PRP scaffold, followed by the cellulose–PRP, the cellulose–chitosan, and, finally, the cellulose one. The scaffold groups Cellulose–PRP and Cellulose–Chitosan–PRP were able to release PDGF-BB. | [57] |
| Mahendiran, 2022 | Borassus flabellifer | Chemical: SDS | hRBC MG63 Raw 264.7 Wistar rats | Hemolytic assay Osteogenic differentiation In vitro inflammatory response In vivo studies | All three scaffolds (oxidized, oxidized + APTEs, and oxidized + OTS) showed to be non-hemolytic and hemocompatible. The oxidized, APTES, and OTS-treated scaffolds showed good cellular adhesion, proliferation, and differentiation of osteoblasts. Animal models demonstrated angiogenesis, degradation, and compatibility with native collagen matrix. | [58] |
| Thyden, 2022 | Broccoli | Chemical: SDS + Tween-20 + Sodium hypochlorite | PB-SC | Lab-grown meat | Cell adhesion was observed, and cell death was limited to 2.55 ± 1.09%. An average cell death of 2.55 +/− 1.09% was observed across all the replicates. There was no significant difference between the different animals of origin. There was an average of 2818 +/− 1062 cells/mm2 for all the samples. | [59] |
| Ahmadian, 2023 | Tomato Leaves | Chemical: SDS > Sodium hypochlorite > n-Hexane | hFF HepG2 | Cytotoxicity and cell viability. Tissue/Disease model | Over a 7-day culture, the viability of hFF cells seeded on the decellularized tomato leaves increased. Moreover, HepG2 cells formed colonies on both the decellularized scaffolds and the TCP controls. Vitality staining showed that even if HepG2 cells grew faster on TCP, there were also more dead cells when compared to the decellularized scaffold. HepG2 seeded on these scaffolds improved the cells’ response to the drug and increased cell survival in comparison to TCP. | [60] |
| Couvrette, 2023 | Asparagus | Chemical: SDS > CaCl2 > Ethanol | Adult rat hippocampal neural stem cells | Attachment, proliferation, and differentiation | NSCs differentiated on the poly-L-ornithine-coated asparagus scaffold showed significant increases in their expressions of neuron-specific beta-III tubulin and glial fibrillary acidic protein compared to TCP, indicating that the scaffold may enhance the differentiation of NSCs toward astrocytic and neuronal lineages. | [61] |
| Galefi, 2023 | Phoenix dactyliferous | Chemical: SDS | MG63 | Cell viability and cell adhesion, osteogenic differentiation | The cells were evaluated on decellularized date scaffolds and decellularized date scaffolds treated with grape seed proanthocyanin extract. Cells cultivated on the later retained a differentiated phenotype, as evidenced by the presence of pseudopodia; moreover, the cell density on this scaffold was greater than that on the non-treated ones. In terms of osteogenic differentiation, there was a greater deposition of calcium on the scaffolds treated at days 7 and 14 of culturing than the non-treated ones, yet this difference disappeared after 21 days of culturing. Higher ALP activity was found on the treated scaffolds. The same applies to the expressions of Col1A and OCN genes. | [62] |
| Gorbenko, 2023 | Leatherleaf, Spinach, and Parsley | Chemical: (a) SDS > clearing solution (b) SDS > EGTA > clearing solution (c) Tergitol > EGTA > clearing solution (d) Tergitol > SDS > clearing solution | rEC | Adhesion, proliferation, and alignment. | Decellularized leatherleaf, spinach, and parsley were wrapped once around an acrylic rod to form a 3D straw-like structure and then coated with fibronectin and cross-linked gelatin. The rate of proliferation was lower on leatherleaf 2D scaffolds when compared with ECs cultured on TCP. ECs were successfully seeded on 3D grafts made from SDS- and SDS/EGTA-decellularized leatherleaf | [63] |
| Jones, 2023 | Spinach | Chemical: (a) n-Hexane > SDS > Tx100 + Sodium hypochlorite (b) n-Hexane > SDS > PS20 + Sodium hypochlorite (c) PS20 + Sodium hypochlorite | PB-SC | Cultured meat | The cells seeded on non-decellularized scaffolds did not attach, whereas as the cells seeded on the decellularized scaffolds did adhere. The implementation of PS20 as a secondary decellularization agent did not appear to affect cell viability. | [64] |
| Perreault, 2023 | Corn Husk and Jackfruit Rind | Chemical: SDS + Polysorbate-20 + Sodium hypochlorite | PB-SC QM7 | Cultured meat | QM7 cultured on corn husk scaffolds yielded increased protein, but PBSCs seeded on corn husks did not yield protein content higher than controls (QM7 on corn husk: 16.28 ± 3.55, PBSCs on corn husks: 9.57 ± 1.56 ug/ul lysate/Gram, control: 6.35 ± 1.43 ug/ul lysate/Gram). | [65] |
| Rai, 2023 | Apple | Chemical: SDS | Infusion with Lactobacillus sp. cells | Drug delivery/ Probiotic delivery | Alginate-coated scaffolds aided in the survival of the Lactobacillus sp. after gastric- and intestinal-simulated transits. | [66] |
| Salehani, 2023 | Olive Leaves | Chemical: (a)n-Hexane > SDS > NaClO (b) n-Hexane > Triton-X 100 and NaClO | C619 | Cytotoxicity | Samples with higher SDS concentrations showed higher cytotoxic effects. | [67] |
| Xia, 2023 | Lotus Petioles | Chemical: SDS > Sodium hypochlorite | hMSC HUVEC hDF hESC | Cell attachment, proliferation, and alignment | Poly-L-lysine, PVG copolymer coating, and peptide (CGGGRGDSP-am (RGD) or CGGGK*(FITC)-am) immobilization on the lotus scaffold. All four cell lines showed polygonal morphologies after incubation for 1 h on to the RGD-decellularized scaffold and no attachment on the PVG- decellularized s ones. After 7 days of culturing, the hESC, hDF, and hMSC align along the plant’s topography. | [68] |
| Yun, 2023 | Sorghum Leaves | Chemical: NaOH > Sodium hypochlorite | hESC | Adhesion, growth, and alignment Myogenic differentiation and contraction | The leaf scaffold was biofunctionalized with poly (PEGMEMA-r-VDM-r-GMA) copolymer, which prevented non-specific protein adsorption, and was modified with cell adhesive RGD peptide to enable cell adhesion and growth in serum-free media. The hESC-derived myogenic progenitor cells cultured on the biofunctionalized leaf scaffold adopted a parallel orientation along the leaf’s topography and showed uniaxial contraction. | [69] |
| Zamudio-Ceja and Garcia-Contreras, 2023 | Nopal | Chemical: SDS > CaCl2 | hDPSC | Osteogenic differentiation | hDPSCs showed significant increases in cell viability of 95% and 106% at 168 h for native and decellularized scaffolds, respectively. Cells grown on TCP showed fusiform elongated morphology, and the ones on the decellularized scaffold showed a spherical shape. Also, the scaffold by itself did not induce the pro-inflammatory expressions of COX-1 or COX-2 but permitted physiological cell function under IL-1β stimulation. | [70] |
| Ahmadian, 2024 | Eggplant Leaves | Chemical: SDS > n-Hexane> | HepG2 cells | Tissue/disease model | Model for hepatocarcinoma tissue. The 3D model was assessed by seeding HepG2 cells on decellularized eggplant leaves to check the effect of prilocaine on cancer cells. Evidence suggests that HepG2 cells were able to thrive and proliferate effectively on the scaffold, and prilocaine demonstrated its efficacy in inhibiting the growth of cancer cells. The model successfully mimics the tissue and the drug interactions. | [71] |
| Banaeyan, 2024 | Watermelon Rind | Chemical: SDS | hFF hMSC | Cytotoxicity and metabolic activity, osteogenic differentiation | Polydopamine treated watermelon rind had a higher cell density and aided in the osteoinduction of the hMSCs evidenced by the mineralization, deposition of hydroxyapatite crystals and raised gene expression of COL1A1, BGLAP, ALP, RUNX2 and SPP1 after 21 days of culture. The coated scaffolds showed the greatest calcium deposits and ALP activity when compared to the TCP and uncoated scaffolds. | [72] |
| Berry-Kilgour, 2024 | Ulva lactuca, Ecklonia radiata, and Durvillaea poha | Chemical + Physical: (a) SDS > NaCO3 + NaClO + Temperature (b) SDS > Triton X-100 + NaClO | Immortalized dermal fibroblasts (BJ/5Ta) | Cytotoxicity and attachment | No matrix showed cytotoxic effects. When seeded on D. poha or U. lactuca scaffolds, fibroblasts were rounded with limited cellulose contact. By contrast, fibroblasts attached to the fibrous inner layer of the E. radiata scaffolds. | [73] |
| Cevik, 2024 | Parsley | Chemical: SDS > Triton X-100 > Sodium hypochlorite | L929 HUVEC | Cytotoxicity attachment, proliferation, and development of tissue-engineered vascular grafts | Parsley stems were used to produce a biocompatible scaffold for TEVG applications. Cytotoxicity and proliferation assays with L929 cells showed no difference when compared to the TCP control. No short-term and long-term cytotoxicity was found for the decellularized parsley stems. The scaffolds were suitable for the culture of human endothelial cells, where monolayer formation was observed over 7 days. | [74] |
| Chen, 2024 | Parsley | Chemical (a) SDS > Sodium hypochlorite (b) Triton X-100 > Sodium hypochlorite | C2C12 | Cultured meat | Plant based scaffolds were modified with type A gelatin and crosslinked with transglutaminase. After induced differentiation, the fibrous scaffolds were more inclined to form multinucleated myotubes with higher expression of myogenic genes and proteins, and the final cell-based meat contained higher total protein content than the honeycomb structure. | [75] |
| Dikici, 2024 | Spinach | Chemical: Acetic acid > SDS > Triton X-100 > Sodium hypochlorite | L929 | Drug delivery | Baby spinach leaf scaffolds were loaded with L-ascorbic and then released within the effective dose range. The spinach scaffolds releasing the ascorbic acid showed an increase in cells’ metabolic activity. | [76] |
| Filiz, 2024 | Spinach | Chemical: n-Hexane > SDS > Triton X-100 > Sodium chlorite | MCF-7 and HUVEC, and spheroids. HDFs embedded in GelMA to mimic breast tissue with tumor | Cancer/disease/tissue model | The endothelialization of decellularized spinach without any surface modification was done. The developed micro platform enabled the co-culture of breast cancer spheroids and plant-derived vasculature under perfusion flow, resulting in close-to-real breast cancer modeling. | [77] |
| Fiorelli, 2024 | Celery | Chemical: SDS | hASC | Cell viability and osteochondrogenic differentiation | 24h decellularized scaffolds were used. After 3 weeks the cells showed osteogenic differentiation. Comparison with GelMA scaffolds correlates. Higher content of GelMA, indicated greater stiffness, and more osteogenic differentiation. Lesser content, chondrogenic differentiation. | [78] |
| Gorbenko, 2024 | Leatherleaf Viburnum | Chemical: SDS > Triton X-100 and Sodium hypochlorite | rEC Vascular smooth muscle cells Rat citrated blood White cell assay | Cell adhesion, proliferation. In vitro thrombosis. Foreign body reactions in vitro. | Grafts coated with fibronectin were seeded with vascular smooth muscle cells and endothelial cells. Endothelial cell density after 24 h of exposure to fluid flow did not change significantly compared to static culture. Endothelial cell density significantly increased by 30% after 3 weeks of bioreactor treatment with fluid compared to 3 weeks of culture under static conditions. Endothelialization of leatherleaf scaffolds significantly reduces thrombus formation in vitro. The application of fluid flow and pressure can further reduce thrombosis. After 24 h in culture, the greatest white cell density was found in endothelial cell and vascular smooth muscle cell seeded leatherleaf, followed by TCP control and acellular decellularized scaffold. | [79] |
| Hasanzadeh, 2024 | Beaucarnea recurvata Leaves | Chemical: n-Hexane > SDS > Triton X-100 + Sodium hypochlorite > Hexane | hDP-SC | Cell attachment, proliferation, osteogenic differentiation | Grape seed proanthocyanidin coated plant-based scaffold had improved physicochemical properties, as well as biological ones such as cell proliferation, protein absorption, osteogenic differentiation. This last one was evident by an increase in ALP activity, and mineral deposition. | [80] |
| Hong, 2024 | Celery | Chemical: SDS > Ethanol | Chicken myoblast | Cultured meat | SDS decellularized celery scaffolds were nontoxic for the cells and supported proliferation and differentiation. After 2 weeks fully grown myoblasts completely covered the surface of the scaffold. | [81] |
| Hosseini, 2024 | Pumpkin | Chemical: NaClO + NaHCO3 | HFF MG63 Wistar rats hASC | Cytotoxicity, cell adhesion. In vivo biocompatibility and inflammation. In vitro osteoinductivity (osteogenic differentiation). | Both coated with magnesium oxide and uncoated scaffolds did not induce cytotoxicity. For cell adhesion and proliferation in the magnesium oxide coated scaffolds showed a higher percentage of live cells at all time points. In vivo, tests showed no adverse effects on the overall health of the animals. Moreover, the animals with coated scaffolds showed the highest wound closure percentage. | [82] |
| Hu, 2024 | Apple | Chemical: SDS | ASC BALB/c mice | Cell attachment and proliferation. Hepatocyte-like induction. Acute liver injury. | Apple-derived cellulose scaffolds served as successful platforms for the growth and attachment of ASC. Liver function recovered in ALI mice transplanted with the apple decellularized scaffold and the implants developed vasculature and bile duct structures. | [83] |
| Ksouri, 2024 | Spinach | Chemical: SDS > Tween-20 > SDS + Sodium hypochlorite | S. aureus NIH3T3 A549 BEAS-2B | Antimicrobial activity. Cell viability, wound healing ability. | Decellularized spinach scaffold showed antibacterial activity. The scaffolds showed no cytotoxicity. Decellularized constructs had the capability to boost the migration of cells, ideal for wound healing applications. | [84] |
| Singh, 2024 | Ficus benjamina | Chemical: n-Hexane > SDS > Tri-n-butyl phosphate > Sodium hypochlorite | Rabbit RBC New Zealand White rabbits | In vitro hemocompatibility test. In vivo biocompatibility analysis. | The decellularized scaffolds showed less hemolysis than the native F. benjamina. Histological analysis of the implanted scaffolds showed encapsulation of the native tissue indicating a moderate to severe immune response. On the other hand, the decellularized scaffolds showed epidermal cells and less inflammatory infiltrate. | [85] |
| Leblanc-Latour, 2024 | Apple | Chemical: SDS | MC3T3-E1 Sprague-Dawley rats | Cell adhesion, proliferation, osteogenic differentiation. Calvarial defect model | Seeded scaffolds showed mineralized deposits after 4 weeks in culture with differentiation medium. Visual assessment indicated the scaffolds seemed well integrated in the skull surrounding tissues. H&E staining revealed cellular infiltration within the scaffold pores and evidence of vascularization, as shown by the presence of blood vessels within the scaffolds. | [86] |
| Luo, 2024 | Natural Leaf Veins (NLVs) | Chemical + Physical: NaOH + Temperature > H2O2 | PC-SC dASC | Cultured meat | After adhesion, proliferation, and differentiation of the cell lines onto the natural leaf veins scaffolds, muscle and fat slices were produced. | [87] |
| Raundal, 2024 | Spinach | Chemical: Ethanol + NaOH + HCl | hDP-SC | Cell attachment, osteogenic differentiation | Cells were able to attach to the cell surface and penetrate it. On day 14, spinach scaffold had significantly higher osteonectin gene expression than the control. | [88] |
| Sadegh-Zaman, 2024 | Lisianthus sp. | Chemical: NaOH + NaOCl as Sodium hypochlorite | Schwann cells | Cell proliferation and migration for neural tissue engineering | To assess this potential, the Lisianthus flower stems were decellularized and then modified with polyaniline and graphene oxide nanosheets (0.05% (G0), 0.1% (G1), 0.2% (G2), and 5% w/v (G3)) and graphene oxide nanosheets. Modified and decellularized stems had no cytotoxicity to the Schwann cells | [89] |
| Singh, 2024 | Bougainvillea sp. | Chemical: TRIS-HCl (0.5 M, pH 8.0) > NaOH + SDS > Triton X-100 + Sodium hypochlorite | HaCaT NIH/3T3 | Cell proliferation | The bougainvillea scaffold did not show cytotoxicity. The actin morphology of NIH/3T3 cells on the floral scaffold exhibited formation of a well spread filamentous network. The increase in cellular density of HaCaT signifies the preference of the cellular attachment onto the floral scaffold. | [90] |
| Sood, 2024 | Apple | Chemical: n-Hexane > SDS > Triton x-100 + Sodium hypochlorite | PB-SC and in co-culture with NIH/3T3 fibroblasts | Cultured meat | Decellularized apple coated with a polymer mixture of gelatin/alginate. Coated scaffolds showed enhanced capability to adhere and proliferate the two cell lines on their surface, compared to uncoated apple scaffolds. Also coated ones were more easily digested in simulated gastric fluid with pepsin. Both coated and uncoated scaffolds behaved similarly when incubated with simulated gastric fluid and PBS. | [91] |
| Will, 2024 | Taraxacum Ruderalia | Chemical: SDS > CaCl2 | Human and murine skin fibroblasts and dermal lymphatic endothelial cells HDLEC | Biocompatibility, proliferative capacity, and ex-vivo endothelialization | No statistical difference in cell growth was found for HDLEC in-vitro on equivalent sheets of SDS-decellularized cellulose compared to a commercial neo-dermis. The tubes showed adequate biocompatibility, supported cell proliferation, and facilitated spontaneous ex-vivo endothelialization of lymphatic endothelial cells. In the ex vivo swine limb model, Lympho-Venous Anastomoses using the engineered cellulose tubes was successfully performed. | [92] |
| Yang, 2024 | Pomelo | Chemical: SDS > Triton X-100 + Sodium hypochlorite | L929, Raw264.7 HUVECs S. aureus + E. coli Sprague-Dawley rats | Cytotoxicity In vitro cell migration, angiogenesis test. In vitro antibacterial test In vivo antibacterial and wound healing in infected wounds. | Hybrid wound dressing, with the decellularized pomelo as the substrate material. The PVA-TSPBA hydrogel works as a coating material to enhance the pomelo’s adhesive and moisture retention ability. The gallic acid/Cu MOFs are antibacterial agents. In vitro, the DPP + MOF@Gel exhibits good biocompatibility. Moreover, the DPP + MOF@Gel can inhibit the viability of S. aureus and E. coli both in vitro and in vivo. | [93] |
| Imeidopf, 2025 | Leatherleaf viburnum | Chemical: (a) Trypsin > Tergitol (b) Trypsin > Tergitol > EGTA (c) SDS > Tergitol (d) SDS > clearing/Sodium hypochlorite | rEC | Adhesion and proliferation | Fibronectin coated decellularized scaffolds were seeded with rat aortic EC. Results showed that the best protocol in terms of cell proliferation and survival was SDs with clearance of less than 6 h. Scaffolds treated with Trypsin/Tergitol/EGTA showed the least survival rate. Additionally, for the SDS treated scaffolds, an increment in the clearance time was inversely proportional to the cell density. | [94] |
| Kian, 2025 | Walnut Leaves | Chemical: n-Hexane > SDS > TritonX-100 + sodium chlorite | hMSC Balb/c mice | Cell adhesion and proliferation. Wound closure analysis and histopathological analysis. | The decellularized walnut leaves scaffold is nontoxic and cytocompatible. SEM images show a mixture of circular and elongated morphologies on the surface of the scaffolds. Animal treated with the walnut decellularized scaffolds showed a higher wound closure percentage on days 3 and 14 when compared to controls. Same for histopathological scores and collagen deposition. Wounds treated with the walnut scaffold had better re-epithelialization, collagen deposition, and angiogenesis. | [95] |
| Lee, 2025 | Spinach | Chemical: Acetone > SDS > Triton x-100 > Sodium hypochlorite | None | None | The decellularized spinach scaffold effectively mimics the vasculature architecture. The scaffolds maintain an intact cellulose framework and vein system, as evidenced by the flow of fluorescent molecules and the reversible color changes of adsorbed colorimetric nanoparticles in response to pH variations. | [96] |
| Salehi, 2025 | Water Spinach, Green Onion, and Water Horsetail | Chemical: n-Hexane > SDS > Triton X-100 + Sodium hypochlorite | HUVEC | Cell adhesion, vitality, and proliferation | HUVECs on the luminal surfaces of decellularized scaffolds show higher expression of Ki-67 protein and a consistent increase in cell number on water spinach and green onion scaffolds compared to TCP. | [97] |
| Yang, 2025 | Shiitake Mushroom, Oyster Mushroom, King Oyster Mushroom, and Wood Ear Mushroom | Chemical: SDS | C2C12 | Cultured meat | Shiitake, Oyster, and Wood ear mushroom microcarriers showed 92.7%, 90%, and 62.7% and of initial attachment of animal cells, respectively. King Oyster mushrooms samples failed to clear the remnants of the SDS, so there was no cell survival. There was no significant difference in proliferation between the Shiitake mushroom microcarrier and the TCP control. | [98] |
| Chemical | Inhibitory Concentration 50× (mM) | Oral Rat or Mouse LD50 (mmol/kg) | Oral Rat or Mouse LD50 (mg/kg) |
|---|---|---|---|
| Acetone | 444 | 168 | 9759.1 |
| Ethanol | 379 | 304 | 14,008.3 |
| Sodium chloride | 75.9 | 51.3 | 2998 |
| Sodium dodecyl sulfate | 0.27 | 4.45 | 1288 |
| Triton X-100 | 0.055 | 2.78 | 1798.7 |
| Tween 80 | 0.49 | 19.1 | 25,021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Ovalle, M.I.; Demitri, C.; Madaghiele, M. Plant-Based Scaffolds for Tissue Engineering: A Review. Polymers 2025, 17, 2705. https://doi.org/10.3390/polym17192705
Vargas-Ovalle MI, Demitri C, Madaghiele M. Plant-Based Scaffolds for Tissue Engineering: A Review. Polymers. 2025; 17(19):2705. https://doi.org/10.3390/polym17192705
Chicago/Turabian StyleVargas-Ovalle, Maria Isabela, Christian Demitri, and Marta Madaghiele. 2025. "Plant-Based Scaffolds for Tissue Engineering: A Review" Polymers 17, no. 19: 2705. https://doi.org/10.3390/polym17192705
APA StyleVargas-Ovalle, M. I., Demitri, C., & Madaghiele, M. (2025). Plant-Based Scaffolds for Tissue Engineering: A Review. Polymers, 17(19), 2705. https://doi.org/10.3390/polym17192705








