Morphological 3D Analysis of PLGA/Chitosan Blend Polymer Scaffolds and Their Impregnation with Olive Pruning Residues via Supercritical CO2
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Enhanced Solvent Extraction from Olive Pruning Waste
2.3. Foaming and Impregnation Supercritical Process
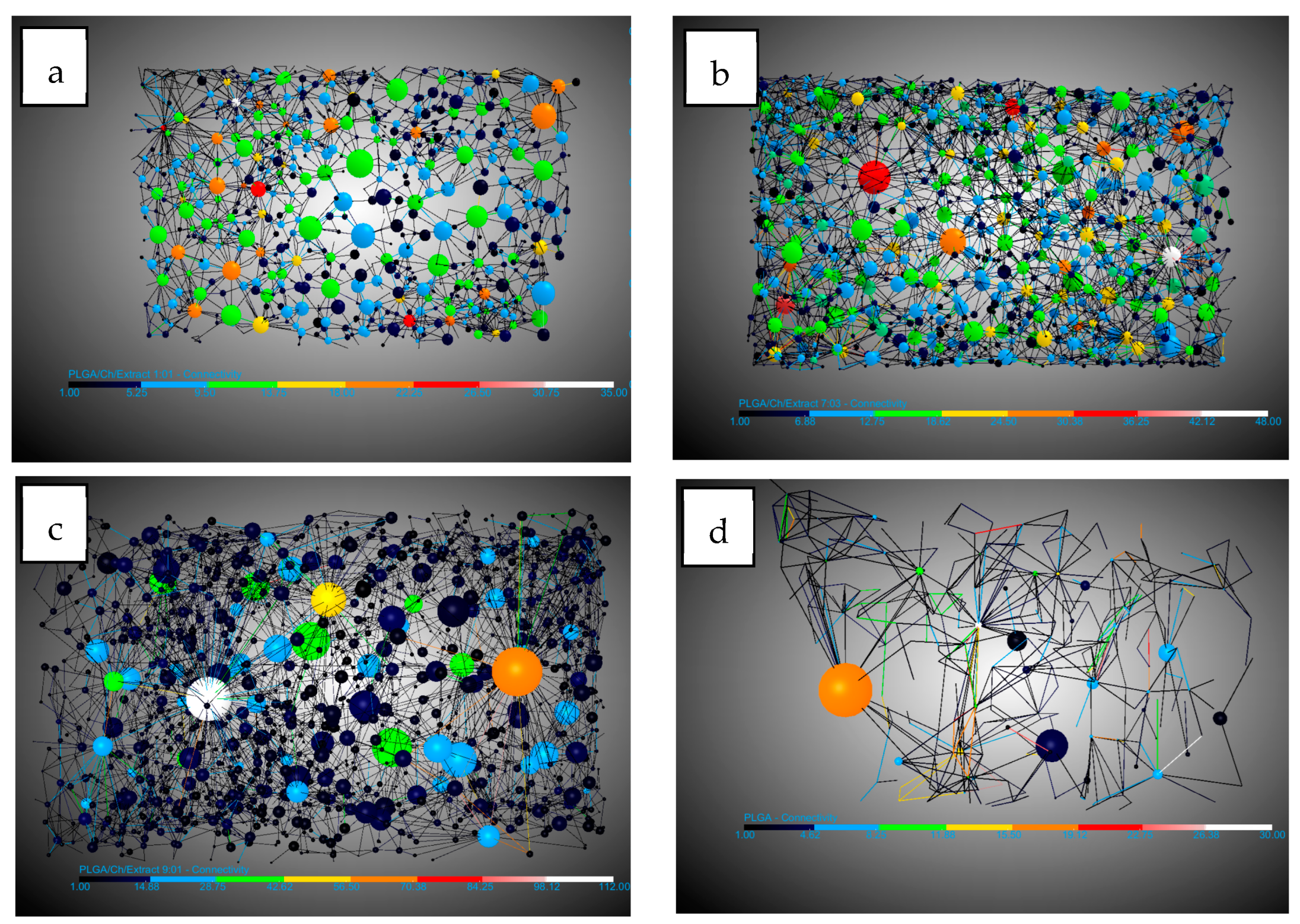
2.4. Tomography Analysis: Porosity, Connectivity, and Expansion Degree
2.5. Scanning Electron Microscopy
2.6. Total Loaded Impregnation
2.7. Antioxidant Capacity of Polymeric Scaffolds
3. Results and Discussion
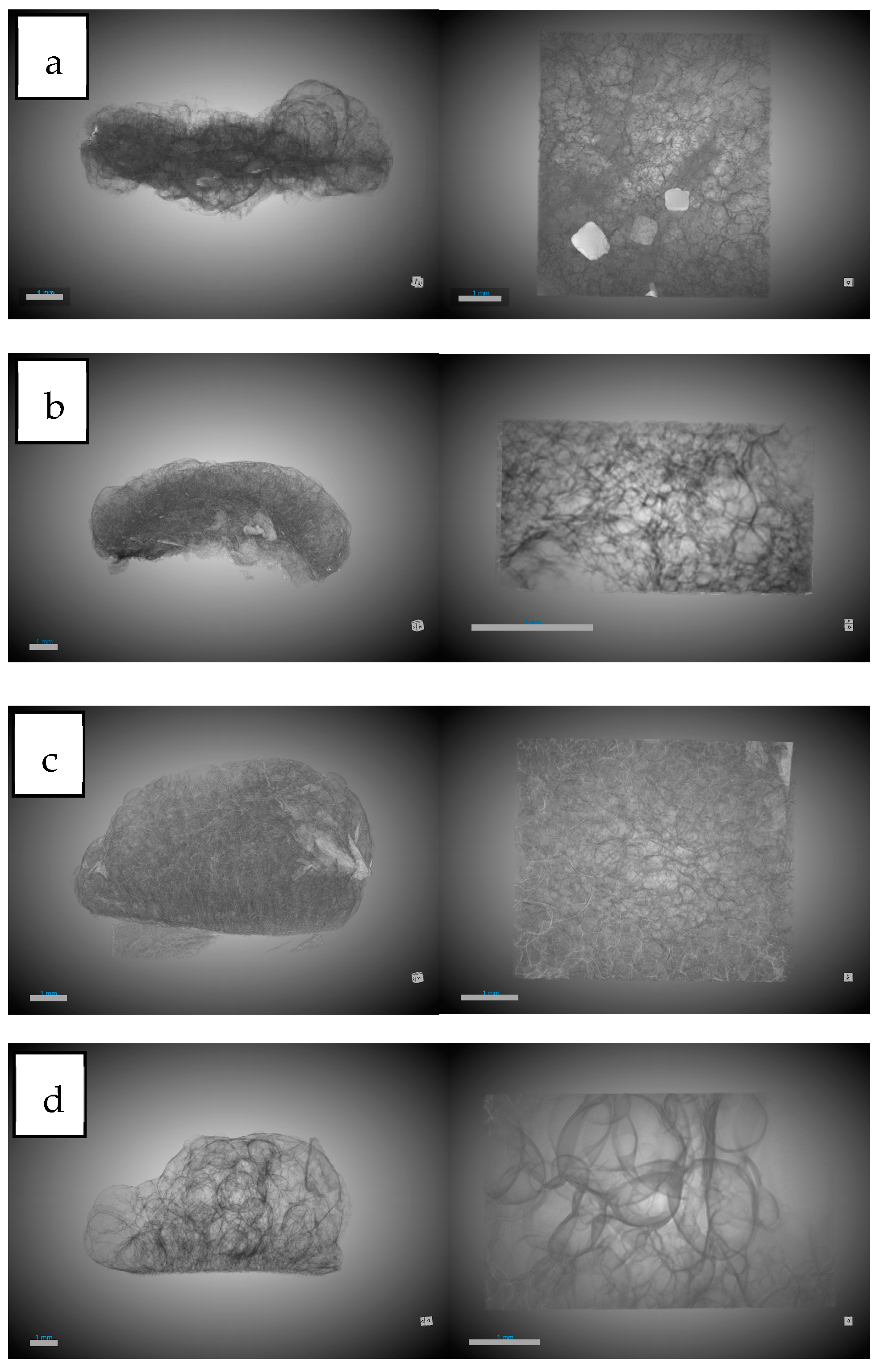
3.1. PLGA/Chitosan Morphological Analysis
3.1.1. Influence of Chitosan on Scaffold Formation
3.1.2. Influence of Olive Pruning Extract on Scaffold Formation
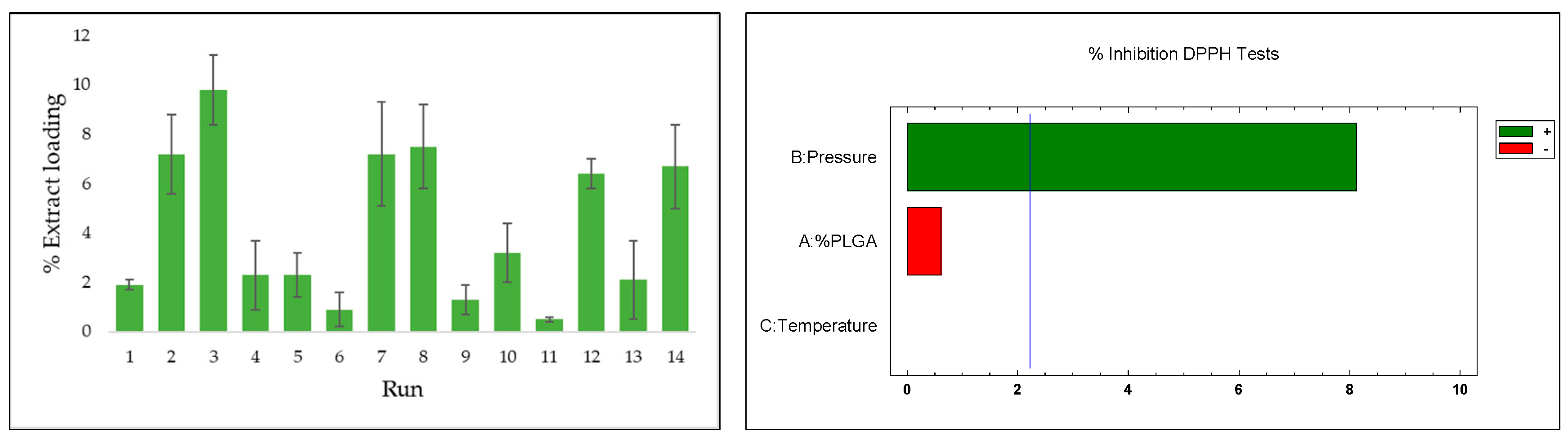
3.2. Extract Loading (%)
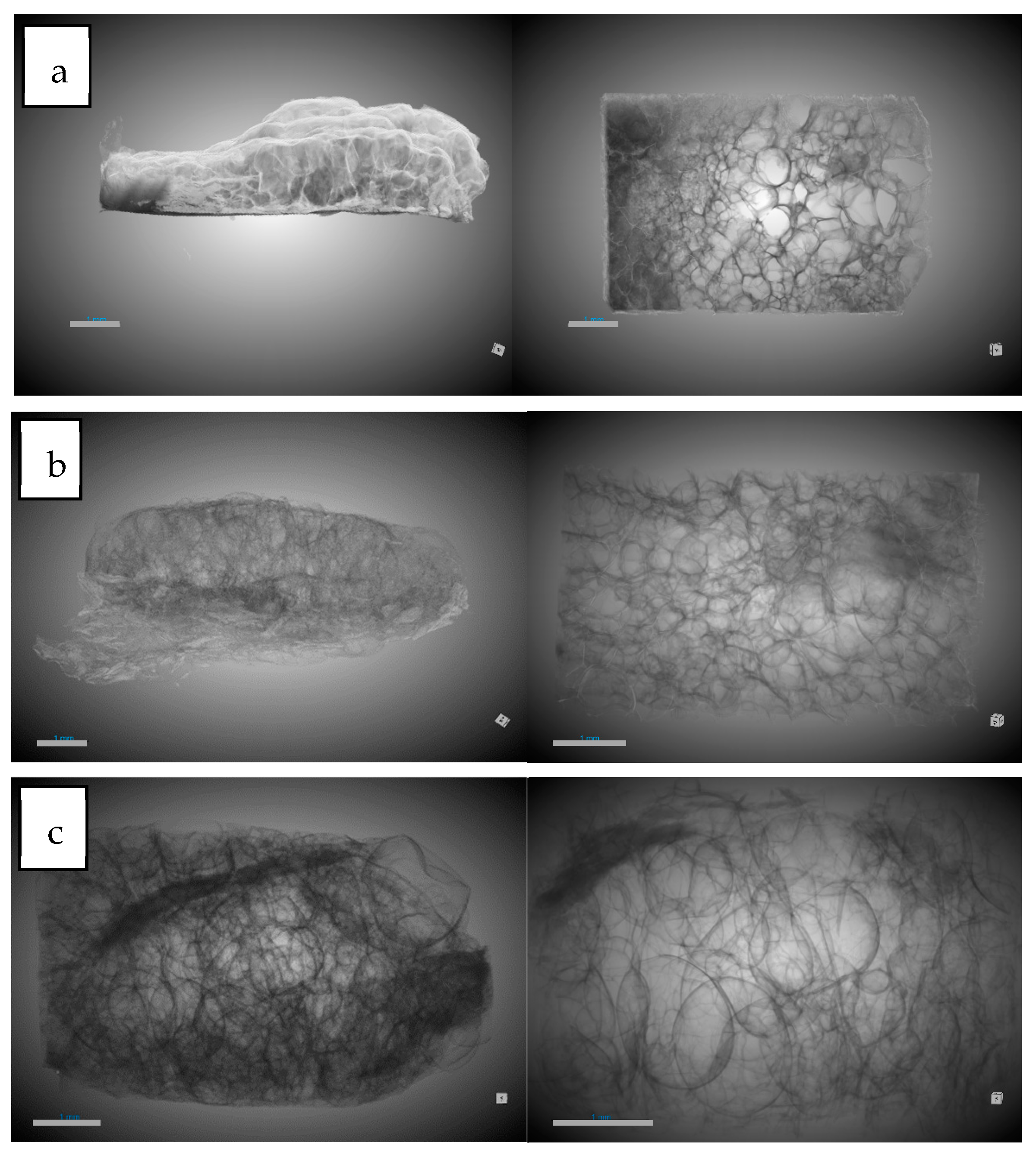
3.3. Scanning Electron Microscopy
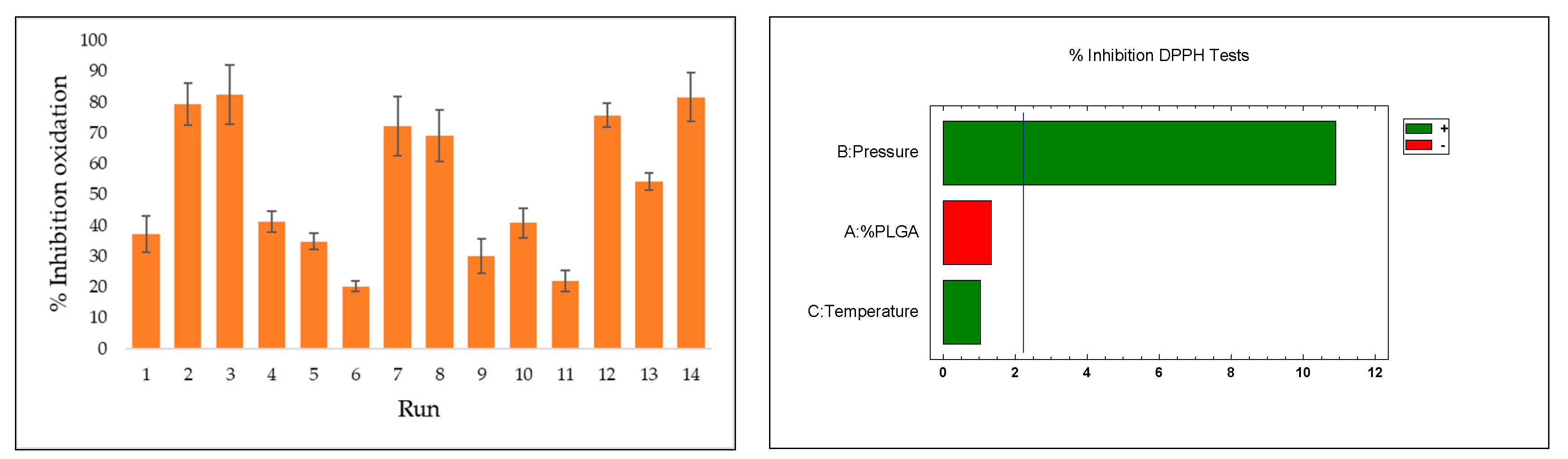
3.4. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramos-Román, M.J.; Jiménez-Moreno, G.; Anderson, R.S.; García-Alix, A.; Camuera, J.; Mesa-Fernández, J.M.; Manzano, S. Climate controlled historic olive tree occurrences and olive oil production in southern Spain. Glob. Planet. Chang. 2019, 182, 102996. [Google Scholar] [CrossRef]
- Ribas, J.C.R.; Lazzari, A.; Gonzalez, L.B.F.; da Silva, C.M.; Adamuchio, L.G.; Cuquel, F.L.; Sakurada, R.; Pintro, P.T.M. Bioactive compounds and antioxidant activity of leaves from olive trees grown in Paraná, Brazil|Compostos bioativos e atividade antioxidante de folhas de oliveiras cultivadas no Paraná, Brasil. Pesqui. Agropecu. Bras. 2023, 58, e03025. [Google Scholar] [CrossRef]
- Chinnarasu, C.; Montes, A.; Pereyra, C.; Casas, L.; Fernández-Ponce, M.T.; Mantell, C.; Pattabhi, S.; Martínez de la Ossa, E. Preparation of polyphenol fine particles potent antioxidants by a supercritical antisolvent process using different extracts of Olea europaea leaves. Korean J. Chem. Eng. 2016, 33, 594–602. [Google Scholar] [CrossRef]
- Rubel, S.A.; Yu, Z.N.; Murshed, H.M.; Islam, S.M.A.; Sultana, D.; Rahman, S.M.E.; Wang, J. Addition of olive (olea europaea) leaf extract as a source of natural antioxidant in mutton meatball stored at refrigeration temperature. J. Food Sci. Technol. 2021, 58, 4002–4010. [Google Scholar] [CrossRef]
- Khemakhem, I.; Abdelhedi, O.; Trigui, I.; Ayadi, M.A.; Bouaziz, M. Structural, antioxidant and antibacterial activities of polysaccharides extracted from olive leaves. Int. J. Biol. Macromol. 2018, 106, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Matthäus, B. A review: Benefit and bioactive properties of olive (Olea europaea L.) leaves. Eur. Food Res. Technol. 2017, 243, 89–99. [Google Scholar] [CrossRef]
- Debs, E.; Abi-Khattar, A.-M.; Rajha, H.N.; Abdel-Massih, R.M.; Assaf, J.-C.; Koubaa, M.; Maroun, R.G.; Louka, N. Valorization of Olive Leaves through Polyphenol Recovery Using Innovative Pretreatments and Extraction Techniques: An Updated Review. Separations 2023, 10, 587. [Google Scholar] [CrossRef]
- Dauber, C.; Parente, E.; Zucca, M.P.; Gámbaro, A.; Vieitez, I. Olea europea and By-Products: Extraction Methods and Cosmetic Applications. Cosmetics 2023, 10, 112. [Google Scholar] [CrossRef]
- Usman, I.; Hussain, M.; Imran, A.; Afzaal, M.; Saeed, F.; Javed, M.; Afzal, A.; Ashfaq, I.; Al Jbawi, E.; Saewan, S.A. Traditional and innovative approaches for the extraction of bioactive compounds. Int. J. Food Prop. 2022, 25, 1215–1233. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Seabra, I.J.; Braga, M.E.M.; Batista, M.T.; De Sousa, H.C. Effect of solvent (CO2/ethanol/H2O) on the fractionated enhanced solvent extraction of anthocyanins from elderberry pomace. J. Supercrit. Fluids 2010, 54, 145–152. [Google Scholar] [CrossRef]
- Carvalho, P.I.N.; Osorio-Tobón, J.F.; Rostagno, M.A.; Petenate, A.J.; Meireles, M.A.A. Techno-economic evaluation of the extraction of turmeric (Curcuma longa L.) oil and ar-turmerone using supercritical carbon dioxide. J. Supercrit. Fluids 2015, 105, 44–54. [Google Scholar] [CrossRef]
- Bendif, H.; Miara, M.D.; Kalboussi, Z.; Grauzdytė, D.; Povilaitis, D.; Venskutonis, P.R.; Maggi, F. Supercritical CO2 extraction of Rosmarinus eriocalyx growing in Algeria: Chemical composition and antioxidant activity of extracts and their solid plant materials. Ind. Crop. Prod. 2018, 111, 768–774. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. Supercritical CO2 extraction of Eucalyptus leaves oil and comparison with Soxhlet extraction and hydro-distillation methods. Sep. Purif. Technol. 2014, 133, 443–451. [Google Scholar] [CrossRef]
- Rovetto, L.J.; Aieta, N.V. Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L. J. Supercrit. Fluids 2017, 129, 16–27. [Google Scholar] [CrossRef]
- Difonzo, G.; Aresta, A.; Cotugno, P.; Ragni, R.; Squeo, G.; Summo, C.; Massari, F.; Pasqualone, A.; Faccia, M.; Zambonin, C.; et al. Supercritical CO2 extraction of phytocompounds from olive pomace subjected to different drying methods. Molecules 2021, 26, 598. [Google Scholar] [CrossRef] [PubMed]
- Dauber, C.; Carreras, T.; González, L.; Gámbaro, A.; Valdés, A.; Ibañez, E.; Vieitez, I. Characterization and incorporation of extracts from olive leaves obtained through maceration and supercritical extraction in Canola oil: Oxidative stability evaluation. LWT 2022, 160, 113274. [Google Scholar] [CrossRef]
- Dali, I.; Aydi, A.; Stamenic, M.; Kolsi, L.; Ghachem, K.; Zizovic, I.; Manef, A.; Delgado, D.R. Extraction of lyophilized olive mill wastewater using supercritical CO2 processes. Alex. Eng. J. 2022, 61, 237–246. [Google Scholar] [CrossRef]
- Marsudi, M.A.; Ariski, R.T.; Wibowo, A.; Cooper, G.; Barlian, A.; Rachmantyo, R.; Bartolo, P.J.D.S. Conductive polymeric-based electroactive scaffolds for tissue engineering applications: Current progress and challenges from biomaterials and manufacturing perspectives. Int. J. Mol. Sci. 2021, 22, 11543. [Google Scholar] [CrossRef]
- Tullii, G.; Giona, F.; Lodola, F.; Bonfadini, S.; Bossio, C.; Varo, S.; Desii, A.; Criante, L.; Sala, C.; Pasini, M.; et al. High-Aspect-Ratio Semiconducting Polymer Pillars for 3D Cell Cultures. ACS Appl. Mater. Interfaces 2019, 11, 28125–28137. [Google Scholar] [CrossRef]
- Valor, D.; Montes, A.; Monteiro, M.; García-Casas, I.; Pereyra, C.; de la Ossa, E.M. Determining the optimal conditions for the production by supercritical CO2 of biodegradable plga foams for the controlled release of rutin as a medical treatment. Polymers 2021, 13, 1645. [Google Scholar] [CrossRef]
- Gracia, E.; Mancini, A.; Colapietro, A.; Mateo, C.; Gracia, I.; Festuccia, C.; Carmona, M. Impregnation of Curcumin into a Biodegradable (Poly-lactic-co-glycolic acid, PLGA) Support, to Transfer Its Well Known In Vitro Effect to an In Vivo Prostate Cancer Model. Nutrients 2019, 11, 2312. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Sun, J.; Jiao, Z. Controllable fabrication of multi-modal porous PLGA scaffolds with different sizes of SPIONs using supercritical CO2 foaming. J. Appl. Polym. Sci. 2022, 139, 52287. [Google Scholar] [CrossRef]
- Lee, L.Y.; Ranganath, S.H.; Fu, Y.; Zheng, J.L.; Lee, H.S.; Wang, C.H.; Smith, K.A. Paclitaxel release from micro-porous PLGA disks. Chem. Eng. Sci. 2009, 64, 4341–4349. [Google Scholar] [CrossRef]
- Álvarez, I.; Gutiérrez, C.; Rodríguez, J.F.; de Lucas, A.; García, M.T. Production of biodegradable PLGA foams processed with high pressure CO2. J. Supercrit. Fluids 2020, 164, 104886. [Google Scholar] [CrossRef]
- Santos-Rosales, V.; Magariños, B.; Starbird, R.; Suárez-González, J.; Fariña, J.B.; Alvarez-Lorenzo, C.; García-González, C.A. Supercritical CO2 technology for one-pot foaming and sterilization of polymeric scaffolds for bone regeneration. Int. J. Pharm. 2021, 605, 120801. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, J.; Li, Z.; Jin, Z.; Hou, J.; Wang, X.; Li, Q. Solid-State Supercritical CO2 Foaming of PCL/PLGA Blends: Cell Opening and Compression Behavior. J. Polym. Environ. 2020, 28, 1880–1892. [Google Scholar] [CrossRef]
- Valor, D.; García-Casas, I.; Montes, A.; Danese, E.; Pereyra, C.; de la Ossa, E.M. Supercritical Impregnation of Mangifera indica Leaves Extracts into Porous Conductive PLGA-PEDOT Scaffolds. Polymers 2024, 16, 133. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wang, L.; Li, Q.; Chen, X.; Liu, S.; Zhou, Y. Poly(L-lactide)-grafted bioglass/poly(lactide-co-glycolide) scaffolds with supercritical CO2 foaming reprocessing for bone tissue engineering. Chem. Res. Chin. Univ. 2017, 33, 499–506. [Google Scholar] [CrossRef]
- Hassan, M.; Sulaiman, M.; Yuvaraju, P.D.; Galiwango, E.; Rehman, I.U.; Al-Marzouqi, A.H.; Khaleel, A.; Mohsin, S. Biomimetic PLGA/Strontium-Zinc Nano Hydroxyapatite Composite Scaffolds for Bone Regeneration. J. Funct. Biomater. 2022, 13, 13. [Google Scholar] [CrossRef]
- Chamorro, E.; Tenorio, M.J.; Calvo, L.; Torralvo, M.J.; Sáez-Puche, R.; Cabañas, A. One-step sustainable preparation of superparamagnetic iron oxide nanoparticles supported on mesoporous SiO2. J. Supercrit. Fluids 2020, 159, 104775. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Zhou, J.; Sun, J.; Jiao, Z. Multi-modal cell structure formation of poly (lactic-co-glycolic acid)/superparamagnetic iron oxide nanoparticles composite scaffolds by supercritical CO2 varying-temperature foaming. Polym. Adv. Technol. 2022, 33, 1906–1915. [Google Scholar] [CrossRef]
- Annu; Bhat, Z.I.; Imtiyaz, K.; Rizvi, M.M.A.; Ikram, S.; Shin, D.K. Comparative Study of ZnO-and-TiO2-Nanoparticles-Functionalized Polyvinyl Alcohol/Chitosan Bionanocomposites for Multifunctional Biomedical Applications. Polymers 2023, 15, 3477. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Lee, L.Y.; Tong, H.; Wang, C.-H. PLGA/chitosan composites from a combination of spray drying and supercritical fluid foaming techniques: New carriers for DNA delivery. J. Control. Release 2008, 129, 207–214. [Google Scholar] [CrossRef]
- Braga, M.E.M.; Pato, M.T.V.; Silva, H.S.R.C.; Ferreira, E.I.; Gil, M.H.; Duarte, C.M.M.; de Sousa, H.C. Supercritical solvent impregnation of ophthalmic drugs on chitosan derivatives. J. Supercrit. Fluids 2008, 44, 245–257. [Google Scholar] [CrossRef]
- Champeau, M.; Thomassin, J.-M.; Tassaing, T.; Jérôme, C. Drug loading of polymer implants by supercritical CO2 assisted impregnation: A review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Song, C.; Yang, S.; Yu, W.; Zhang, W.; Zhang, G.; Xi, Z.; Lu, E. Supercritical CO2 foamed composite scaffolds incorporating bioactive lipids promote vascularized bone regeneration via Hif-1α upregulation and enhanced type H vessel formation. Acta Biomater. 2019, 94, 253–267. [Google Scholar] [CrossRef]
- Xin, X.; Guan, Y.-X.; Yao, S.-J. Sustained release of dexamethasone from drug-loading PLGA scaffolds with specific pore structure fabricated by supercritical CO2 foaming. J. Appl. Polym. Sci. 2018, 135, 46207. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández-Ponce, M.T.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Supercritical impregnation of olive leaf extract to obtain bioactive films effective in cherry tomato preservation. Food Packag. Shelf Life 2019, 21, 100338. [Google Scholar] [CrossRef]
- Gostick, J.; Aghighi, M.; Hinebaugh, J.; Tranter, T.; Hoeh, M.A.; Day, H.; Spellacy, B.; Sharqawy, M.H.; Bazylak, A.; Burns, A.; et al. OpenPNM: A Pore Network Modeling Package. Comput. Sci. Eng. 2016, 18, 60–74. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent, Methods in Enzymology. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.I.; No, H.K.; Meyers, S.P. Physicochemical Characteristics and Functional Properties of Various Commercial Chitin and Chitosan Products. J. Agric. Food Chem. 1998, 46, 3839–3843. [Google Scholar] [CrossRef]
- Torres, A.; Romero, J.; Macan, A.; Guarda, A.; Galotto, M.J. Near critical and supercritical impregnation and kinetic release of thymol in LLDPE films used for food packaging. J. Supercrit. Fluids 2014, 85, 41–48. [Google Scholar] [CrossRef]
- Goñi, M.L.; Gañán, N.A.; Strumia, M.C.; Martini, R.E. Eugenol-loaded LLDPE films with antioxidant activity by supercritical carbon dioxide impregnation. J. Supercrit. Fluids 2016, 111, 28–35. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández Ponce, M.T.; Mantell Serrano, C.; Martínez de la Ossa-Fernández, E.J. Characterization of olive leaf extract polyphenols loaded by supercritical solvent impregnation into PET/PP food packaging films. J. Supercrit. Fluids 2018, 140, 196–206. [Google Scholar] [CrossRef]
- Milovanovic, S.; Markovic, D.; Mrakovic, A.; Kuska, R.; Zizovic, I.; Frerich, S.; Ivanovic, J. Supercritical CO2—Assisted production of PLA and PLGA foams for controlled thymol release. Mater. Sci. Eng. C 2019, 99, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Runge, T.; Wang, L.; Li, R.; Feng, J.; Shu, X.L.; Shi, Q.S. Hydrogen bonding impact on chitosan plasticization. Carbohydr. Polym. 2018, 200, 115–121. [Google Scholar] [CrossRef]
- Valor, D.; Montes, A.; García-Casas, I.; Pereyra, C.; Martínez de la Ossa, E.J. Supercritical solvent impregnation of alginate wound dressings with mango leaves extract. J. Supercrit. Fluids 2021, 178, 105357. [Google Scholar] [CrossRef]
- Rosales, J.M.; Cejudo, C.; Verano, L.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J. Supercritical impregnation of pla filaments with mango leaf extract to manufacture functionalized biomedical devices by 3d printing. Polymers 2021, 13, 2125. [Google Scholar] [CrossRef] [PubMed]











| Fixed Parameters | Variable | ||
|---|---|---|---|
| Pressure | 10 MPa | PLGA/Ch Ratio | 1:01, 7:03, 9:01 |
| Temperature | 308 K | Olive Pruning Extract | 15 mL |
| CO2 Flow Rate | 20 mg/min | ||
| Runs | P (MPa) | T (K) | Ratio |
|---|---|---|---|
| 1 | 10 | 308 | 1:1 |
| 2 | 30 | 328 | 7:3 |
| 3 | 30 | 308 | 1:1 |
| 4 | 10 | 328 | 9:1 |
| 5 | 10 | 328 | 7:3 |
| 6 | 10 | 308 | 7:3 |
| 7 | 30 | 328 | 9:1 |
| 8 | 30 | 308 | 9:1 |
| 9 | 10 | 328 | 1:1 |
| 10 | 20 | 318 | 7:3 |
| 11 | 10 | 308 | 9:1 |
| 12 | 30 | 308 | 7:3 |
| 13 | 20 | 318 | 7:3 |
| 14 | 30 | 328 | 1:1 |
| Experiments | Ratio (PLGA/Ch) | Expansion Factor (Ƹ) | Porosity (%) | Pore Diameter and SD (mm) | Number of Pores/mm3 | Connections per Pore and SD | Number of Throats/mm3 |
|---|---|---|---|---|---|---|---|
| PLGA/Ch | 1:01 | 76.50 | 64.06 | 0.11 ± 0.08 | 34.87 | 8.02 ± 4.30 | 133.04 |
| PLGA/Ch | 7:03 | 84.99 | 76.84 | 0.11 ± 0.08 | 46.32 | 8.41 ± 4.02 | 196.07 |
| PLGA/Ch | 9:01 | 89.67 | 88.55 | 0.15 ± 0.11 | 50.04 | 7.72 ± 2.95 | 193.35 |
| PLGA | – | 91.11 | 85.55 | 0.19 ± 0.14 | 8.37 | 3.26 ± 1.52 | 13.25 |
| Experiments | Ratio (PLGA/Ch) | Expansion Factor (Ƹ) | Porosity (%) | Pore Diameter (mm) | Number of Pores/mm3 | Connection per Pore | Number of Throats/mm3 |
|---|---|---|---|---|---|---|---|
| PLGA/Ch | 1:01 | 53.96 | 76.39 | 0.13 ±0.09 | 36.54 | 4.95 ± 2.24 | 90.86 |
| PLGA/Ch | 7:03 | 82.08 | 82.38 | 0.13 ± 0.09 | 47.41 | 6.90 ± 3.10 | 162.84 |
| PLGA/Ch | 9:01 | 88.54 | 87.35 | 0.14 ± 0.11 | 28.08 | 5.38 ± 3.38 | 73.27 |
| PLGA | – | 91.11 | 85.55 | 0.19 ± 0.14 | 8.37 | 3.26 ± 1.52 | 13.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Casas, I.; Valor, D.; Elayoubi, H.; Montes, A.; Pereyra, C. Morphological 3D Analysis of PLGA/Chitosan Blend Polymer Scaffolds and Their Impregnation with Olive Pruning Residues via Supercritical CO2. Polymers 2024, 16, 1451. https://doi.org/10.3390/polym16111451
García-Casas I, Valor D, Elayoubi H, Montes A, Pereyra C. Morphological 3D Analysis of PLGA/Chitosan Blend Polymer Scaffolds and Their Impregnation with Olive Pruning Residues via Supercritical CO2. Polymers. 2024; 16(11):1451. https://doi.org/10.3390/polym16111451
Chicago/Turabian StyleGarcía-Casas, Ignacio, Diego Valor, Hafsa Elayoubi, Antonio Montes, and Clara Pereyra. 2024. "Morphological 3D Analysis of PLGA/Chitosan Blend Polymer Scaffolds and Their Impregnation with Olive Pruning Residues via Supercritical CO2" Polymers 16, no. 11: 1451. https://doi.org/10.3390/polym16111451
APA StyleGarcía-Casas, I., Valor, D., Elayoubi, H., Montes, A., & Pereyra, C. (2024). Morphological 3D Analysis of PLGA/Chitosan Blend Polymer Scaffolds and Their Impregnation with Olive Pruning Residues via Supercritical CO2. Polymers, 16(11), 1451. https://doi.org/10.3390/polym16111451








