Evolution of Microplastics Released from Tea Bags into Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Tea Bag Preparation for the Analysis
2.4. Evaluation of Cytotoxicity
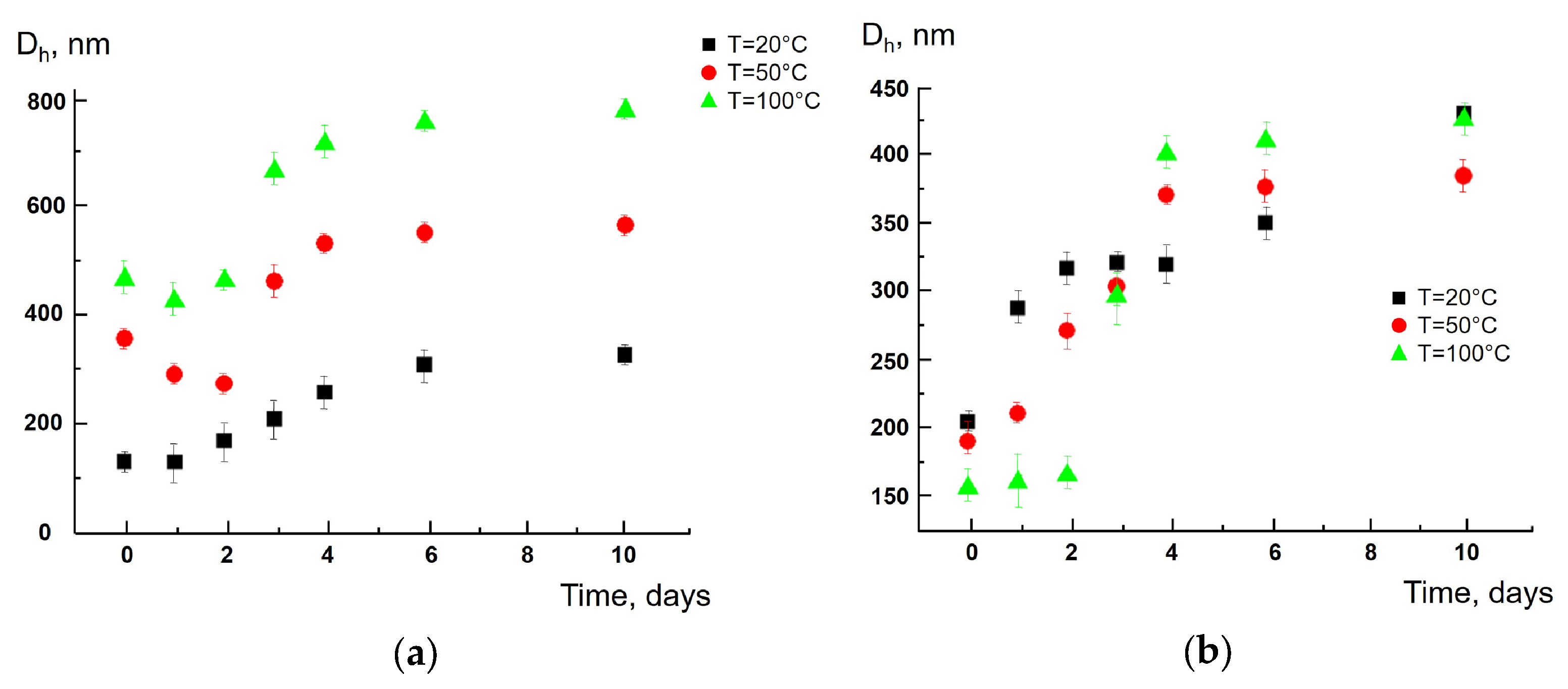
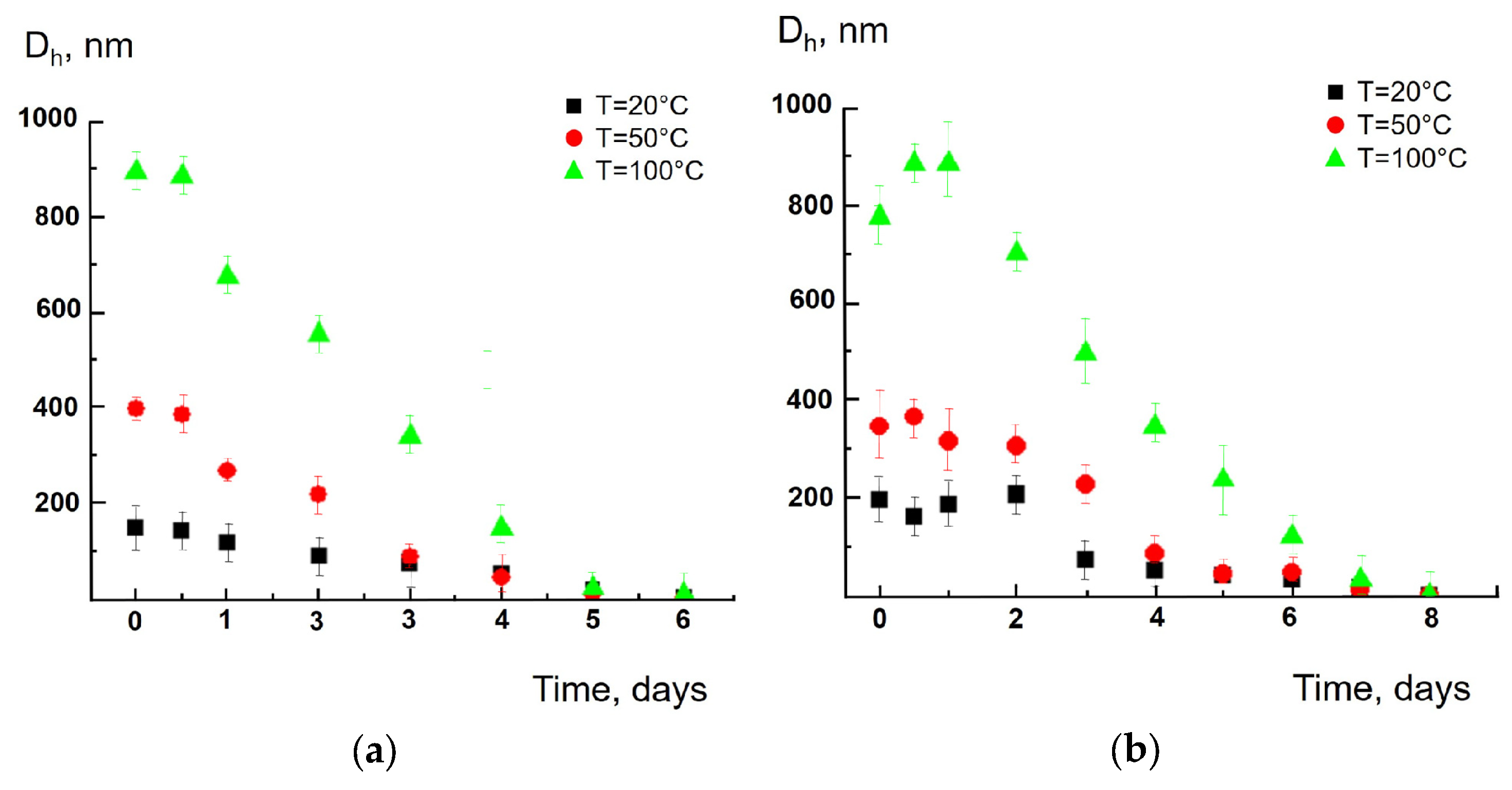
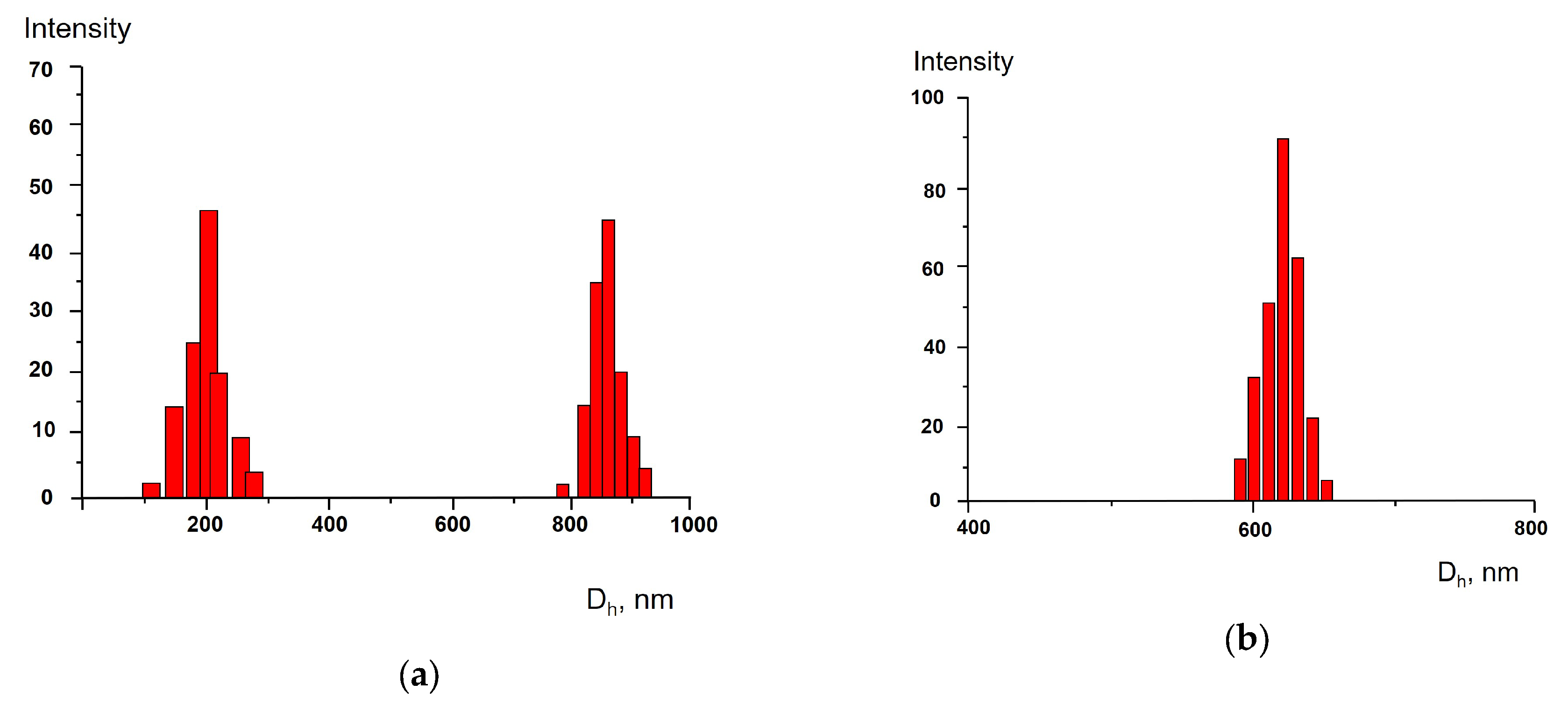
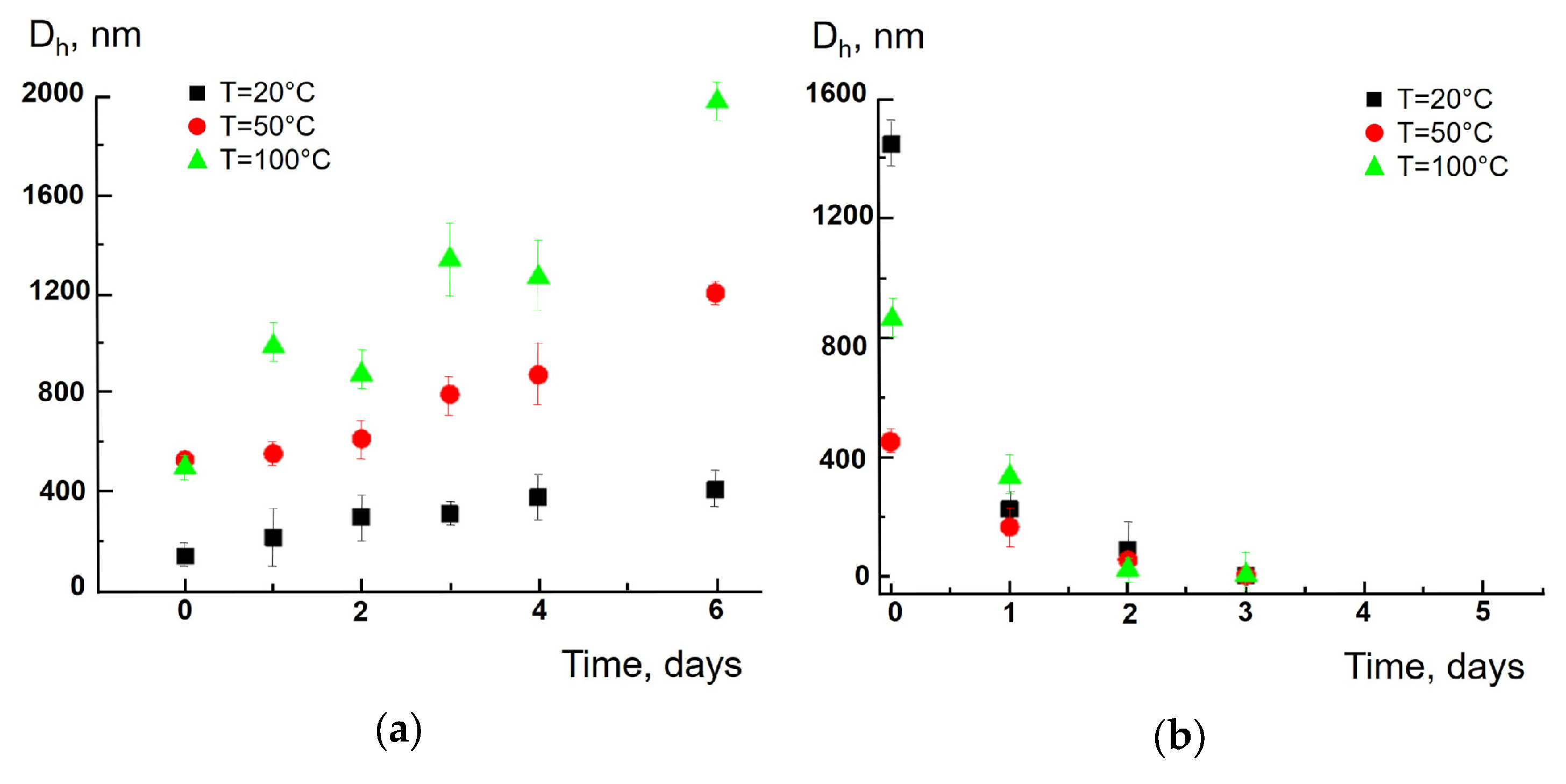
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- UNEA Resolution 5/14: End Plastic Pollution: Towards an International Legally Binding Instrument; United Nations Environment Programme (UNEP). 2022. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/39812/OEWG_PP_1_INF_1_UNEA%20resolution.pdf (accessed on 5 September 2025).
- The Lancet Planetary Health. Microplastics and human health—An urgent problem. Lancet Planet. Health 2017, 1, e254. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of nanoplastic in the environment and possible impact on human health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef]
- Vethaak, A.D.; Legler, J. Microplastics and human health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Anastopoulos, I.; Pashalidis, I.; Kayan, B.; Kalderis, D. Microplastics as carriers of hydrophilic pollutants in an aqueous environment. J. Mol. Liq. 2022, 350, 118182. [Google Scholar] [CrossRef]
- Fu, L.; Li, J.; Wang, G.; Luan, Y.; Dai, W. Adsorption behavior of organic pollutants on microplastics. Ecotoxicol. Environ. Saf. 2021, 217, 112207. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- SAPEA: Science Advice for Policy by European Academies. A Scientific Perspective on Microplastics in Nature and Society; SAPEA: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- De Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef]
- Gouin, T.; Ellis-Hutchings, R.; Pemberton, M.; Wilhelmus, B. Addressing the relevance of polystyrene nano- and microplastic particles used to support exposure, toxicity and risk assessment: Implications and recommendations. Part. FibreToxicol. 2024, 21, 39. [Google Scholar] [CrossRef]
- Lyulin, S.V.; Gurtovenko, A.A.; Saliu, F.; Galli, P.; Surroop, D.; Yaroslavov, A.A.; Radionova, S.G.; Kuznetsova, T.A.; Kenny, J.M. Microplastics in the environment: The role of polymer science. Sci. Total Environ. 2025, 998, 180267. [Google Scholar] [CrossRef]
- Dong, H.; Wang, X.; Niu, X.; Zeng, J.; Zhou, Y.; Suona, Z.; Yuan, Y.; Chen, X. Overview of analytical methods for the determination of microplastics: Current status and trends. Trends Anal. Chem. 2023, 167, 117261. [Google Scholar] [CrossRef]
- De-la-Torre, G.E.; Pizarro-Ortega, C.I.; Dioses-Salinas, D.C.; Ammendolia, J.; Okoffo, E.D. Investigating the current status of COVID-19 related plastics and their potential impact on human health. Curr. Opin. Toxicol. 2021, 27, 47–53. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human consumption of microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shi, Y.; Yang, L.; Xiao, L.; Kehoe, D.K.; Gun’ko, Y.K.; Boland, J.J.; Wang, J.J. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat. Food 2020, 1, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Hale, R.C.; Seeley, M.E.; King, A.E.; Yu, L.H. Analytical Chemistry of Plastic Debris: Sampling, Methods, and Instrumentation. In Microplastic in the Environment: Pattern and Process; Bank, M.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 17–69. [Google Scholar] [CrossRef]
- Zangmeister, C.D.; Radney, J.G.; Benkstein, K.D.; Kalanyan, B. Common single-use consumer plastic products release trillions of sub-100 nm nanoparticles per liter into water during normal use. Environ. Sci. Technol. 2022, 56, 5448–5455. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Cheng, Q.; Ding, T.; Qian, Y.; Zhang, Y.; Sun, H.; Peng, C.; Naz, I.; Li, J.; Liu, J. Micro- and nanoplastics in the environment: Occurrence, detection, characterization and toxicity—A critical review. J. Clean. Prod. 2021, 313, 127863. [Google Scholar] [CrossRef]
- Prata, J.C.; Paço, A.; Reis, V.; da Costa, J.P.; Fernandes, A.S.; da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Micro(nano)plastics—Analytical challenges towards risk evaluation. Trends Anal. Chem. 2019, 111, 173–184. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of various microplastics in human stool: A prospective case series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.B.; He, H.R.; Zhang, J.F.; Ma, G.S. You are what you eat: Microplastics in the feces of young men living in Beijing. Sci. Total Environ. 2021, 767, 144345. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Trasande, L.; Kannan, K. Occurrence of polyethylene terephthalate and polycarbonate microplastics in infant and adult feces. Environ. Sci. Technol. Lett. 2021, 8, 989–994. [Google Scholar] [CrossRef]
- Gigault, J.; El Hadri, H.; Nguyen, B.; Grassl, B.; Rowenczyk, L.; Tufenkji, N.; Feng, S.; Wiesner, M. Nanoplastics are neither microplastics nor engineered nanoparticles. Nat. Nanotechnol. 2021, 16, 501–507. [Google Scholar] [CrossRef]
- Sripada, K.; Wierzbicka, A.; Abass, K.; Grimalt, J.O.; Erbe, A.; Röllin, H.B.; Weihe, P.; Díaz, G.J.; Singh, R.R.; Visnes, T.; et al. A children’s health perspective on nano- and microplastics. Environ. Health Perspect. 2022, 130, 015001. [Google Scholar] [CrossRef]
- Anjum, F.; Azam, A.; Faseeh, H.; Bano, R.; Latif, M.; Fahid, A. Effects of microplastics on living organisms and their trophic transfer: An ecotoxicological review. Futur. Biotechnol. 2023, 3, 2–11. [Google Scholar] [CrossRef]
- Volkova, A.; Molodina, L.; Golikova, E.; Ermakova, L.; Bogdanova, N. Aggregation stability of a positively charged γ-Al2O3 sol prepared from an air-dry nanopowder. Colloid J. 2014, 76, 395–407. [Google Scholar] [CrossRef]
- Rudenskaya, G.N.; Isaev, V.A.; Shmoylov, A.M.; Karabasova, M.A.; Shvets, S.V.; Miroshnikov, A.I.; Brusov, A.B. Preparation of proteolytic enzymes from Kamchatka crab Paralithodes camchatica hepatopancreas and their application. Appl. Biochem. Biotechnol. 2000, 88, 175–183. [Google Scholar] [CrossRef]
- Van Holde, K.E.; Johnson, W.C.; Ho, P.S. Principles of Physical Biochemistry; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2006; ISBN 0-13-720459-0. [Google Scholar]
- Pérez-Camargo, R.A.; Liu, G.M.; Wang, D.J.; Müller, A.J. Experimental and data fitting guidelines for the determination of polymer crystallization kinetics. Chin. J. Polym. Sci. 2022, 40, 658–691. [Google Scholar] [CrossRef]
- Fernandez, C.E.; Bermudez, M.; Alla, A.; Mancera, M.; García-Martín, M.G.; Benito, E.; Roffe, I.; Galbis, J.A.; Munoz-Guerra, S. Crystallization studies on linear aliphatic polyamides derived from naturally occurring carbohydrates. J. Appl. Polym. Sci. 2010, 116, 2515–2525. [Google Scholar] [CrossRef]
- Blanco, I.; Siracusa, V. The use of thermal techniques in the characterization of bio-sourced polymers. Materials 2021, 14, 1686. [Google Scholar] [CrossRef]
- Tagashira, K.; Maruyama, M.; Mizutani, Y.; Kajioka, H.; Sakai, K.; Okada, K.N.; Hikosaka, M. Melting behavior and structural and morphological changes of isotactic polypropylene from heat treatment. Polymer 2019, 51, 227–235. [Google Scholar] [CrossRef]
- Shen, D.; Xiao, R.; Gu, S.; Zhang, H. The overview of thermal decomposition of cellulose in lignocellulosic biomass. In Cellulose—Biomass Conversion; Van de Ven, T., Kadla, J., Eds.; InTech: Rijeka, Croatia, 2013; pp. 193–226. [Google Scholar] [CrossRef]
- Vasile, C.; Popescu, C.-M.; Popescu, M.-C.; Brebu, M.; Willfor, S. Thermal behavior/treatment of some vegetable residues. IV. Thermal decomposition of eucalyptus wood-cellulose. Cellulose Chem. Technol. 2011, 45, 29–42. [Google Scholar]
- Agarwal, V.; Dauenhauer, P.J.; Huber, G.W.; Auerbach, S.M. Ab initio dynamics of cellulose pyrolysis: Nascent decomposition pathways at 327 and 600 °C. J. Am. Chem. Soc. 2012, 134, 14958–14972. [Google Scholar] [CrossRef]
- Xu, J.L.; Lin, X.; Hugelier, S.; Herrero-Langreo, A.; Gowen, A.A. Spectral imaging for characterization and detection of plastic substances in branded teabags. J. Hazard. Mater. 2021, 418, 126328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Hamidian, A.H.; Tubić, A.; Zhang, Y.; Fang, J.K.H.; Wu, C.; Lam, P.K.S. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef] [PubMed]
- Gałko, G.; Sajdak, M. Trends for the thermal degradation of polymeric materials: Analysis of available techniques, issues, and opportunities. Appl. Sci. 2022, 12, 9138. [Google Scholar] [CrossRef]
- Marqués-Calvo, M.; Regaño, C.; Nyugen, J.; Aidanpa, L.; Muñoz-Guerra, S. Hydrolytic and fungal degradation of polyamides derived from tartaric acid and hexamethylenediamine. Polymer 2000, 41, 2765–2772. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of nanoparticle tracking analysis (NTA) by nanosight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Träuble, H. Membrane Electrostatics. In Structure of Biological Membranes; Abrahamsson, S., Pascher, I., Eds.; Nobel Foundation Symposia; Springer: New York, NY, USA, 1977; pp. 509–550. [Google Scholar] [CrossRef]
- Panova, T.V.; Sybachin, A.V.; Zhao, Y.; Zhu, X. Interaction of liposomes with silica nanocapsules: From lipid bilayer coating to multi-liposomal composites. Mendeleev Commun. 2021, 31, 830–832. [Google Scholar] [CrossRef]
- Efimova, A.A.; Popov, A.S.; Kazantsev, A.V.; Semenyuk, P.I.; Le-Deygen, I.M.; Lukashev, N.V.; Yaroslavov, A.A. pH-sensitive liposomes with embedded 3-(isobutylamino)cholan-24-oic acid: What is the possible mechanism of fast cargo release? Membranes 2023, 13, 407. [Google Scholar] [CrossRef] [PubMed]
- Yaroslavov, A.A.; Efimova, A.A.; Krasnikov, E.A.; Trosheva, K.S.; Popov, A.S.; Melik-Nubarov, N.S.; Krivtsov, G.G. Chitosan-based multi-liposomal complexes: Synthesis, biodegradability and cytotoxicity. Int. J. Biol. Macromol. 2021, 177, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Sybachin, A.V.; Efimova, A.A.; Litmanovich, E.A.; Menger, F.M.; Yaroslavov, A.A. Complexation of polycations to anionic liposomes: Composition and structure of the interfacial complexes. Langmuir 2007, 23, 10034–10039. [Google Scholar] [CrossRef] [PubMed]
- Spiridonov, V.; Orlova, M.; Ivanov, I.; Panova, I.; Orlov, A.; Antonova, Y.; Yaroslavov, A. Synthesis of microgels based on carboxymethylcellulose cross-linked with zinc(II) ions and heterocyclic effectors of NO-synthase. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124104. [Google Scholar] [CrossRef]
- Grozdova, I.; Melik-Nubarov, N.; Efimova, A.; Ezhov, A.; Krivtsov, G.; Litmanovich, E.; Yaroslavov, A. Intracellular delivery of drugs by chitosan-based multi-liposomal complexes. Colloids Surf. B Biointerfaces 2020, 193, 111062. [Google Scholar] [CrossRef] [PubMed]
| Tea Bag Sample | Experiment | Control | Polymer | ||
|---|---|---|---|---|---|
| DSC, °Tmelt | TG,°Tdecomp | DSC, °Tmelt | TG, °Tdecomp | ||
| I | 220 | 408 | 225 | 400 | Polyamide (nylon) [33] |
| II | 210 | 390 | 219 | 394 | Polyamide (nylon) [33] |
| III | 168 | 220 | 175 | 290 | Polypropylene [34,35] |
| IV | 170 | 270 | 175 | 290 | Polypropylene [34,35] |
| V | - | 310 | - | 350 | Cellulose [36,37] |
| VI | - | 343 | - | 350 | Cellulose [36,37] |
| VII | - | 340 | - | 350 | Cellulose [36,37] |
| VIII | 130 admixture | 290 | - | 300 | Cellulose [36,38] |
| Sample | Ultimate Size, nm | ||
|---|---|---|---|
| °C | |||
| 20 | 50 | 100 | |
| I | 320 | 580 | 760 |
| II | 330 | 570 | 780 |
| III | 590 | 630 | 610 |
| IV | 350 | 570 | 680 |
| V | 0 | 0 | 0 |
| VI | 0 | 0 | 0 |
| VII | 0 | 0 | 0 |
| VIII | 0 | 0 | 0 |
| Sample | Viability of Caco-2 Cells, r.u. |
|---|---|
| I | 0.98 ± 0.03 |
| II | 1.04 ± 0.03 |
| III | 1.06 ± 0.04 |
| IV | 1.02 ± 0.04 |
| V | 0.81 ± 0.05 |
| VI | 0.98 ± 0.03 |
| VII | 1.06 ± 0.01 |
| VIII | 0.96 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaroslavov, A.A.; Efimova, A.A.; Grokhovskaya, T.E.; Badikova, A.G.; Spiridonov, V.V.; Pozdyshev, D.V.; Lyulin, S.V.; Kenny, J.M. Evolution of Microplastics Released from Tea Bags into Water. Polymers 2025, 17, 2700. https://doi.org/10.3390/polym17192700
Yaroslavov AA, Efimova AA, Grokhovskaya TE, Badikova AG, Spiridonov VV, Pozdyshev DV, Lyulin SV, Kenny JM. Evolution of Microplastics Released from Tea Bags into Water. Polymers. 2025; 17(19):2700. https://doi.org/10.3390/polym17192700
Chicago/Turabian StyleYaroslavov, Alexander A., Anna A. Efimova, Tatyana E. Grokhovskaya, Anastasiia G. Badikova, Vasily V. Spiridonov, Denis V. Pozdyshev, Sergey V. Lyulin, and Jose M. Kenny. 2025. "Evolution of Microplastics Released from Tea Bags into Water" Polymers 17, no. 19: 2700. https://doi.org/10.3390/polym17192700
APA StyleYaroslavov, A. A., Efimova, A. A., Grokhovskaya, T. E., Badikova, A. G., Spiridonov, V. V., Pozdyshev, D. V., Lyulin, S. V., & Kenny, J. M. (2025). Evolution of Microplastics Released from Tea Bags into Water. Polymers, 17(19), 2700. https://doi.org/10.3390/polym17192700









