1H Time Domain Nuclear Magnetic Resonance and Oscillatory Rheology as a Tool for Uncovering the Impact of UV-C Radiation on Polypropylene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. UV-C Chamber Assembly
2.3. Physicochemical Analysis
3. Results
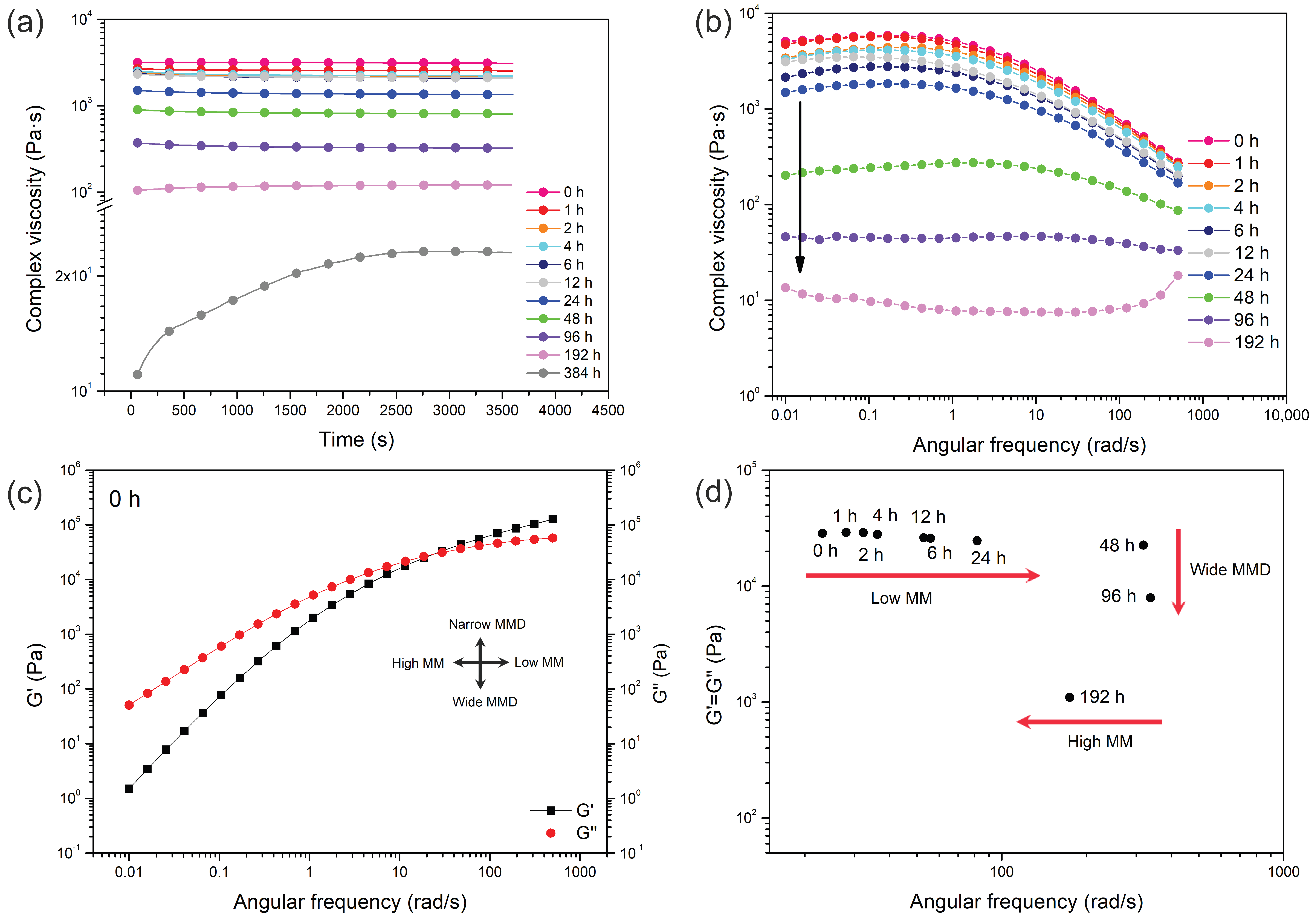
3.1. Rheological and 1H DQ-TDNMR Analyses for the Samples
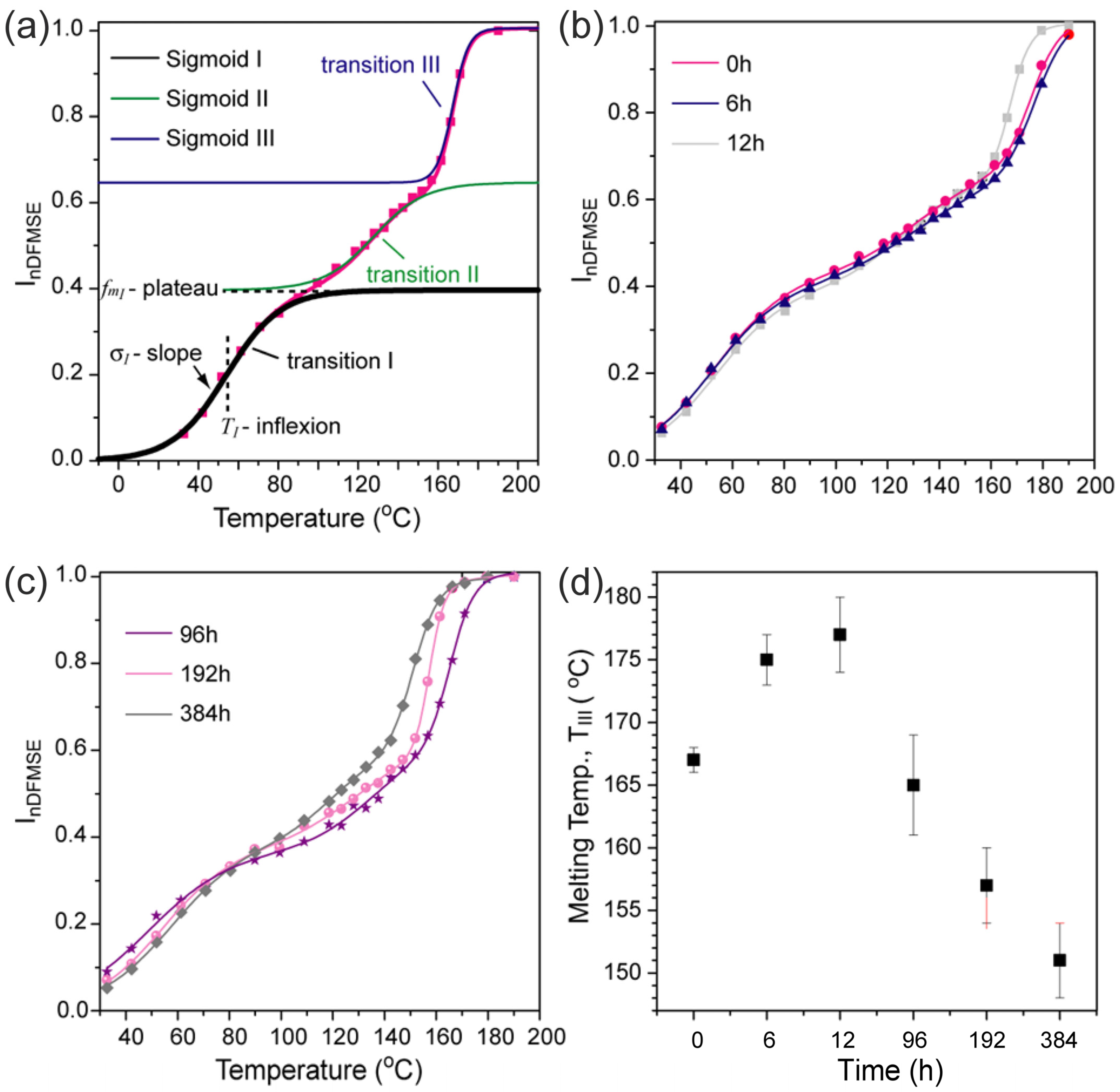
3.2. Effect of UV-C Light on the Molecular Mobility and Microstructure
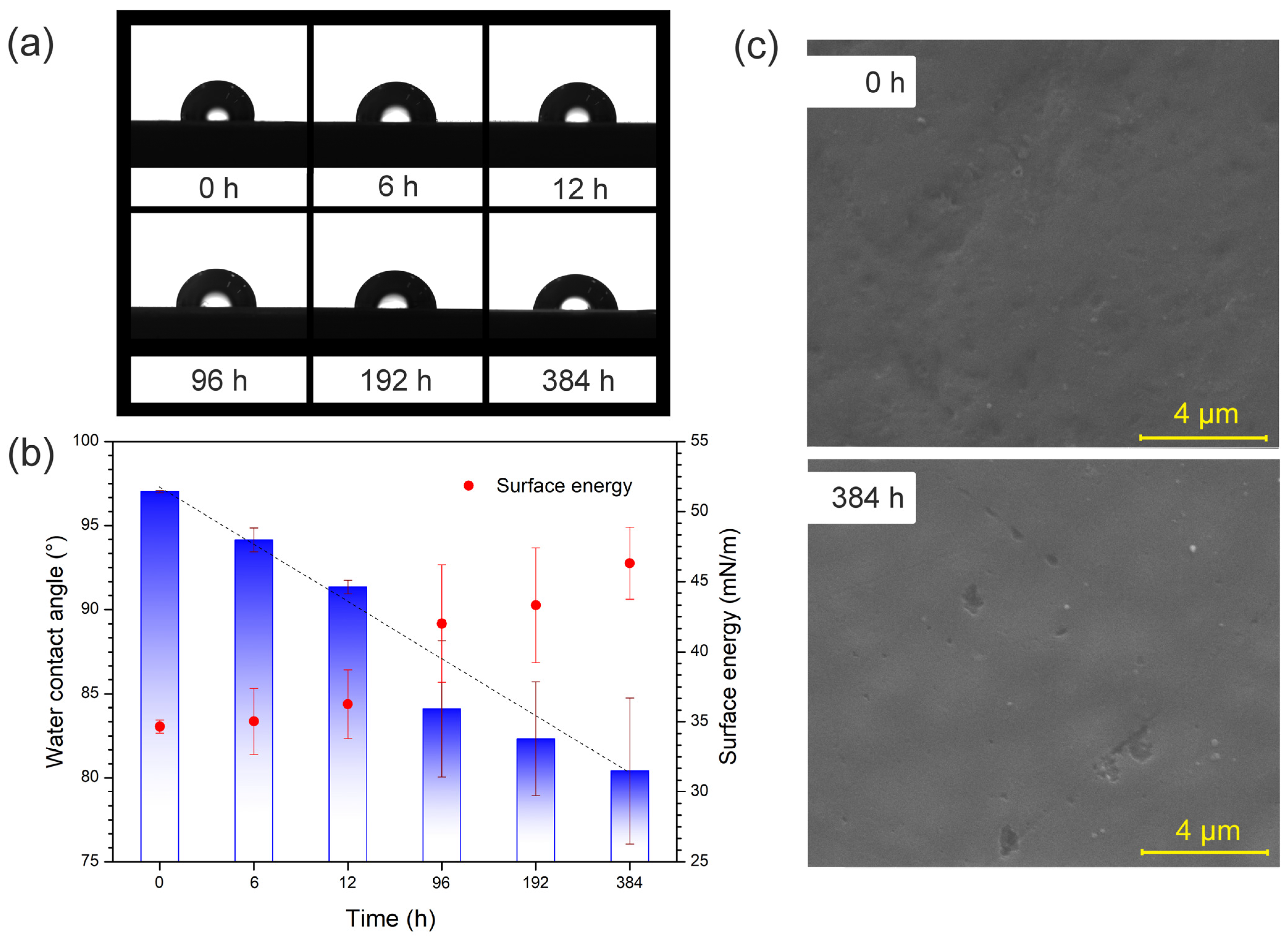
3.3. Chemical and Morphological Alterations for the Samples
4. Discussion
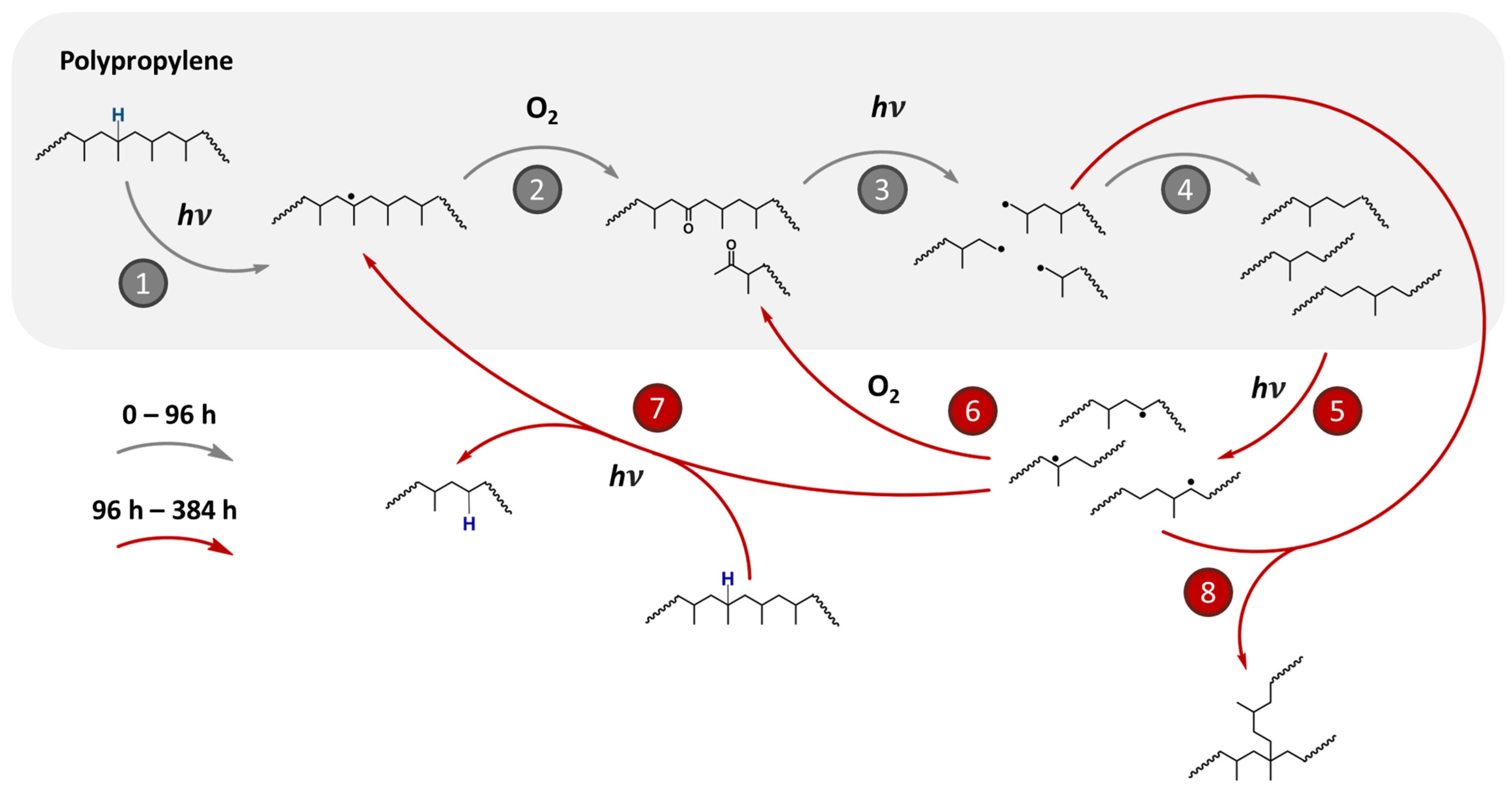
- The low molar mass chains formed in the previous stage (4) undergo homolytic bond cleavage upon photon energy absorption, leading to the formation of new radicals (stage 5), which are quickly attacked by molecular oxygen, resulting in the production of other oxidized species (stage 6). At this point, other polypropylene species contribute to the photocatalytic cycle, undergoing hydrogen abstraction by free radical molecules formed in stage (stage 7).
- With the increase in the number of reactive species, i.e., free radicals, from stages (5) and (3), a slight increase in molar mass is observed, between 192 h and 384 h, after the abrupt decrease, between 0 and 96 h. We propose that these species begin recombining, forming branched chains of high molar mass (stage 8). This observation is supported by the increase in complex viscosity (Figure 1) and 1H-TD-Low Field NMR (Figure 2), presented in the section Rheological and 1H DQ-TDNMR analyses for the samples, as well as the decrease in the crystallinity degree, and in the section Effect of UV-C light on the molecular mobility and microstructure, and also by the increase in the CH vibration shown in the Raman spectroscopy analysis in Figure S8 in the Supplementary section.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PP | Polypropylene |
| 1H TD-NMR | 1H Time Domain Nuclear Magnetic Resonance |
| FTIR-ATR | Attenuated Total Reflectance Fourier Transform Infrared spectroscopy |
| DSC | Differential scanning calorimetry |
| SEM | Scanning Electron Microscopy |
| 1H nuclei spins | |
| G′(ω) | Storage modulus |
| G″(ω) | Loss modulus |
| MI | Methyl index |
| CI | Carbonyl index |
| MM | Molar mass |
| MMD | Molar mass distribution |
| fd | Fraction of unconstrained molecular segments |
| T2 | Relaxation time NMR |
| DF-MSE | Dipolar Filtered Magic Sandwich Echo |
References
- Yun, J.; Yan, R.; Fan, X.; Gurtler, J.; Phillips, J. Fate of E. coli O157:H7, Salmonella Spp. and Potential Surrogate Bacteria on Apricot Fruit, Following Exposure to UV-C Light. Int. J. Food Microbiol. 2013, 166, 356–363. [Google Scholar] [CrossRef]
- Gayán, E.; Álvarez, I.; Condón, S. Inactivation of Bacterial Spores by UV-C Light. Innov. Food Sci. Emerg. Technol. 2013, 19, 140–145. [Google Scholar] [CrossRef]
- Woo, H.; Beck, S.; Boczek, L.; Carlson, K.; Brinkman, N.; Linden, K.; Lawal, O.; Hayes, S.; Ryu, H. Efficacy of Inactivation of Human Enteroviruses by Dual-Wavelength Germicidal Ultraviolet (UV-C) Light Emitting Diodes (LEDs). Water 2019, 11, 1131. [Google Scholar] [CrossRef]
- Welch, D.; Buonanno, M.; Grilj, V.; Shuryak, I.; Crickmore, C.; Bigelow, A.W.; Randers-Pehrson, G.; Johnson, G.W.; Brenner, D.J. Far-UVC Light: A New Tool to Control the Spread of Airborne-Mediated Microbial Diseases. Sci. Rep. 2018, 8, 2752. [Google Scholar] [CrossRef]
- Fino, V.R.; Kniel, K.E. UV Light Inactivation of Hepatitis A Virus, Aichi Virus, and Feline Calicivirus on Strawberries, Green Onions, and Lettuce. J. Food Prot. 2008, 71, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Ha, S. Do Ultraviolet-C Radiation on the Fresh Chicken Breast: Inactivation of Major Foodborne Viruses and Changes in Physicochemical and Sensory Qualities of Product. Food Bioprocess Technol. 2015, 8, 895–906. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA—J. Am. Med. Assoc. 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Zayat, M.; Garcia-Parejo, P.; Levy, D. Preventing UV-Light Damage of Light Sensitive Materials Using a Highly Protective UV-Absorbing Coating. Chem. Soc. Rev. 2007, 36, 1270–1281. [Google Scholar] [CrossRef]
- Mohr, L.C.; Capelezzo, A.P.; Baretta, C.R.D.M.; Martins, M.A.P.M.; Fiori, M.A.; Mello, J.M.M. Titanium Dioxide Nanoparticles Applied as Ultraviolet Radiation Blocker in the Polylactic Acid Bidegradable Polymer. Polym. Test. 2019, 77, 105867. [Google Scholar] [CrossRef]
- Zadbuke, N.; Shahi, S.; Gulecha, B.; Padalkar, A.; Thube, M. Recent Trends and Future of Pharmaceutical Packaging Technology. J. Pharm. Bioallied Sci. 2013, 5, 98–110. [Google Scholar] [CrossRef] [PubMed]
- McKeen, L.W. Plastics Used in Medical Devices; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780323221696. [Google Scholar]
- Assis, M.; Ribeiro, L.K.; Gonçalves, M.O.; Staffa, L.H.; Paiva, R.S.; Lima, L.R.; Coelho, D.; Almeida, L.F.; Moraes, L.N.; Rosa, I.L.V.; et al. Polypropylene Modified with Ag-Based Semiconductors as a Potential Material against SARS-CoV-2 and Other Pathogens. ACS Appl. Polym. Mater. 2022, 4, 7102–7114. [Google Scholar] [CrossRef] [PubMed]
- Paiva, R.; Wrona, M.; Nerín, C.; Cruz, S.A. Hydrogenated Amorphous Carbon Film Deposited by Plasma on Recycled Polypropylene as a Functional Barrier to Hazardous Migrants. Food Packag. Shelf Life 2022, 33, 100864. [Google Scholar] [CrossRef]
- Rabek, J.F. Polymer Photodegradation; Springer Netherlands: Dordrecht, The Netherlands, 1995; ISBN 978-94-010-4556-8. [Google Scholar]
- Wen, B.; Wang, F.; Xu, X.; Ding, Y.; Zhang, S.; Yang, M. The Effect of Encapsulation of Nano Zinc Oxide with Silica on the UV Resistance of Polypropylene. Polym. Plast. Technol. Eng. 2011, 50, 1375–1382. [Google Scholar] [CrossRef]
- Qi, L.; Ding, Y.F.; Dong, Q.X.; Wen, B.; Liu, P.; Wang, F.; Zhang, S.M.; Yang, M.S. UV Photodegradation of Polypropylene Thick Bars Containing Rutile-Type TiO2 Nanorods. Chinese J. Polym. Sci. (Engl. Ed.) 2014, 32, 834–843. [Google Scholar] [CrossRef]
- Basfar, A.A.; Idriss Ali, K.M.; Mofti, S.M. UV Stability and Radiation-Crosslinking of Linear Low Density Polyethylene and Low Density Polyethylene for Greenhouse Applications. Polym. Degrad. Stab. 2003, 82, 229–234. [Google Scholar] [CrossRef]
- Gijsman, P.; Meijers, G.; Vitarelli, G. Comparison of the UV-Degradation Chemistry of Polypropylene, Polyethylene, Polyamide 6 and Polybutylene Terephthalate. Polym. Degrad. Stab. 1999, 65, 433–441. [Google Scholar] [CrossRef]
- Zepp, R.; Ruggiero, E.; Acrey, B.; Acrey, B.; Davis, M.J.B.; Davis, M.J.B.; Han, C.; Han, C.; Han, C.; Hsieh, H.S.; et al. Fragmentation of Polymer Nanocomposites: Modulation by Dry and Wet Weathering, Fractionation, and Nanomaterial Filler. Environ. Sci. Nano 2020, 7, 1742–1758. [Google Scholar] [CrossRef] [PubMed]
- Barkoula, N.M.; Alcock, B.; Cabrera, N.O.; Peijs, T. Flame-Retardancy Properties of Intumescent Ammonium Poly(Phosphate) and Mineral Filler Magnesium Hydroxide in Combination with Graphene. Polym. Polym. Compos. 2008, 16, 101–113. [Google Scholar]
- Staffa, L.H.; Agnelli, J.A.M.; de Souza, M.L.; Bettini, S.H.P. Considerations about the Role of Compatibilizer in Coir Fiber Polypropylene Composites Containing Different Stabilization Systems When Submitted to Artificial Weathering. Cellulose 2020, 27, 9409–9422. [Google Scholar] [CrossRef]
- Goodwin, D.G.; Shen, S.-J.; Lyu, Y.; Lankone, R.; Barrios, A.C.; Kabir, S.; Perreault, F.; Wohlleben, W.; Nguyen, T.; Sung, L. Graphene/Polymer Nanocomposite Degradation by Ultraviolet Light: The Effects of Graphene Nanofillers and Their Potential for Release. Polym. Degrad. Stab. 2020, 182, 109365. [Google Scholar] [CrossRef]
- Butler, C.H.; Whitmore, P.M. Measurement of Peroxides in the Volatile Degradation Products of Polypropylene Photooxidation. Polym. Degrad. Stab. 2013, 98, 471–473. [Google Scholar] [CrossRef]
- Yousif, E.; Ahmed, D.S.; El-Hiti, G.A.; Alotaibi, M.H.; Hashim, H.; Hameed, A.S.; Ahmed, A. Fabrication of Novel Ball-like Polystyrene Films Containing Schiff Base Microspheres as Photostabilizers. Polymers 2018, 10, 1185. [Google Scholar] [CrossRef]
- de Freitas, A.d.S.M.; Rodrigues, J.S.; Botaro, V.R.; Lemes, A.P.; Cruz, S.A.; Waldman, W.R. Formation of Craze-like Pattern in Polypropylene UV-Induced Surface Cracking. J. Polym. Res. 2022, 29, 506. [Google Scholar] [CrossRef]
- Mylläri, V.; Ruoko, T.P.; Syrjälä, S. A Comparison of Rheology and FTIR in the Study of Polypropylene and Polystyrene Photodegradation. J. Appl. Polym. Sci. 2015, 132, 1–6. [Google Scholar] [CrossRef]
- Boronat, C.; Correcher, V.; García-Guinea, J.; Bravo-Yagüe, J.C. Ultraviolet C Radiation on Polypropylene: A Potential Way to Reduce Plastic Pollution. Polym. Degrad. Stab. 2024, 225, 110784. [Google Scholar] [CrossRef]
- FDA Classify Your Medical Device. Silver Spring, MD, USA, 2020. Available online: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/ClassifyYourDevice/ (accessed on 18 September 2025).
- Basaglia, M.V.; Ferreira Gimenez, J.C.; Petrucelli Homem, M.G.; Cruz, S.A.; Staffa, L.H.; Bettini, S.H.P. Improving UV-C Stability in Polypropylene through Synergistic Phenolic or Hydroxylamine-Based Additives with UV Absorbers. Express Polym. Lett. 2025, 19, 124–139. [Google Scholar] [CrossRef]
- Munaro, A.P.; da Cunha, G.P.; Filgueiras, J.G.; Pinto, J.M.; Munaro, M.; de Azevedo, E.R.; Akcelrud, L.C. Ageing and Structural Changes in PDMS Rubber Investigated by Time Domain NMR. Polym. Degrad. Stab. 2019, 166, 300–306. [Google Scholar] [CrossRef]
- Paul, J.; Hansen, E.W.; Roots, J. Probing the Molecular Dynamics in XLPE Aged at Different Temperatures by 1H NMR Relaxation Time Measurements. Polym. Degrad. Stab. 2012, 97, 2403–2411. [Google Scholar] [CrossRef]
- Perez, M.G.; Lima, A.P.; Moraes, T.B.; Chaves, E.G.; da Silva Ruiz, N.M.; dos Santos Teixeira, S.C.; de Angeli Honorato, H.H.; de Menezes, S.M.C.; DeAzevedo, E.R. 1H Time Domain NMR to Probe Microstructural and Mobility Changes in Polyamide 11 Exposed to H2S Scavengers. What Type of Information Can Be Assessed? Polym. Degrad. Stab. 2022, 202, 110001. [Google Scholar] [CrossRef]
- Numata, K.; Asano, A.; Nakazawa, Y. Solid-State and Time Domain NMR to Elucidate Degradation Behavior of Thermally Aged Poly (Urea-Urethane). Polym. Degrad. Stab. 2020, 172, 109052. [Google Scholar] [CrossRef]
- Baum, J.; Munowitz, M.; Garroway, A.N.; Pines, A. Multiple-Quantum Dynamics in Solid State NMR. J. Chem. Phys. 1985, 83, 2015–2025. [Google Scholar] [CrossRef]
- Saalwächter, K.; Herrero, B.; López-Manchado, M.A. Chain Order and Cross-Link Density of Elastomers As Investigated by Proton Multiple-Quantum NMR. Macromolecules 2005, 38, 9650–9660. [Google Scholar] [CrossRef]
- Chassé, W.; Lang, M.; Sommer, J.U.; Saalwächter, K. Cross-Link Density Estimation of PDMS Networks with Precise Consideration of Networks Defects. Macromolecules 2012, 45, 899–912, Erratum in Macromolecules 2015, 48, 1267–1268. [Google Scholar] [CrossRef]
- ASTM D1238-23a; Standard Test Method for Melt Flow Rates of Thermoplastics by Extrusion Plastometer 2023. ASTM International: West Conshohocken, PA, USA; pp. 1–19. Available online: https://knowledge.bsigroup.com/products/standard-test-method-for-melt-flow-rates-of-thermoplastics-by-extrusion-plastometer-2 (accessed on 18 September 2025).
- ASTM D638; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2014; pp. 1–16. Available online: https://www.zwickroell.com/zh/industries/plastics/thermoplastics-and-thermosetting-molding-materials/tensile-properties-astm-d638/ (accessed on 18 September 2025).
- ASTM D256-23; Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics. ASTM International: West Conshohocken, PA, USA, 2010; pp. 1–20. Available online: https://standards.iteh.ai/catalog/standards/astm/9f48a40f-3127-4a44-a35b-d4179fce7a31/astm-d256-23?srsltid=AfmBOor2hs17dPTAonppp43ZGlnMd4n9lUXL4w5sNX0rYMJ-emrtK1fL (accessed on 18 September 2025).
- ASTM D792-07; Standard Test Methods for Density and Specific Gravity (Relative Density) of Plastics. ASTM International: West Conshohocken, PA, USA, 2007; pp. 1–5. Available online: https://file.yizimg.com/175706/2012061422022437.pdf (accessed on 18 September 2025).
- Wu, S. Calculation of Interfacial Tension in Polymer Systems. J. Polym. Sci. Part C Polym. Symp. 1971, 34, 19–30. [Google Scholar] [CrossRef]
- Staffa, L.H.; Agnelli, J.A.M.; de Souza, M.L.; Bettini, S.H.P. Evaluation of Interactions between Compatibilizers and Photostabilizers in Coir Fiber Reinforced Polypropylene Composites. Polym. Eng. Sci. 2017, 57, 1179–1185. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Shentu, B.; Chen, S.; Chen, M. Effect of Titanium Dioxide on the UV-C Ageing Behavior of Silicone Rubber. J. Appl. Polym. Sci. 2018, 135, 46099. [Google Scholar] [CrossRef]
- Cruz, S.A.; Zanin, M. Evaluation and Identification of Degradative Processes in Post-Consumer Recycled High-Density Polyethylene. Polym. Degrad. Stab. 2003, 80, 31–37. [Google Scholar] [CrossRef]
- Filgueiras, J.G.; da Silva, U.B.; Paro, G.; D’Eurydice, M.N.; Cobo, M.F.; DeAzevedo, E.R. Dipolar Filtered Magic-Sandwich-Echoes as a Tool for Probing Molecular Motions Using Time Domain NMR. J. Magn. Reson. 2017, 285, 47–54. [Google Scholar] [CrossRef]
- De Azevedo, E.R.; Hu, W.-G.; Bonagamba, T.J.; Schmidt-Rohr, K. Centerband-Only Detection of Exchange: Efficient Analysis of Dynamics in Solids by NMR. J. Am. Chem. Soc. 1999, 121, 8411–8412. [Google Scholar] [CrossRef]
- Rutledge, G.C.; Suter, U.W. Helix Jump Mechanisms in Crystalline Isotactic Polypropylene. Macromolecules 1992, 25, 1546–1553. [Google Scholar] [CrossRef]
- Rabello, M.; White, J. Crystallization and Melting Behaviour of Photodegraded Polypropylene — I. Chemi-Crystallization. Polymer 1997, 38, 6379–6387. [Google Scholar] [CrossRef]
- Yakimets, I.; Lai, D.; Guigon, M. Effect of Photo-Oxidation Cracks on Behaviour of Thick Polypropylene Samples. Polym. Degrad. Stab. 2004, 86, 59–67. [Google Scholar] [CrossRef]
- Rouillon, C.; Bussiere, P.-O.; Desnoux, E.; Collin, S.; Vial, C.; Therias, S.; Gardette, J.-L. Is Carbonyl Index a Quantitative Probe to Monitor Polypropylene Photodegradation? Polym. Degrad. Stab. 2016, 128, 200–208. [Google Scholar] [CrossRef]
- Almond, J.; Sugumaar, P.; Wenzel, M.N.; Hill, G.; Wallis, C. Determination of the Carbonyl Index of Polyethylene and Polypropylene Using Specified Area under Band Methodology with ATR-FTIR Spectroscopy. e-Polymers 2020, 20, 369–381. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, L.; Sutton, D.; Wang, X.; Lin, T. Needleless Melt-Electrospinning of Polypropylene Nanofibres. J. Nanomater. 2012, 2012, 382639. [Google Scholar] [CrossRef]
- McDonald, M.P.; Ward, I.M. The Assignment of the Infra-Red Absorption Bands and the Measurement of Tacticity in Polypropylene. Polymer 1961, 2, 341–355. [Google Scholar] [CrossRef]
- Nishikida, K.; Coates, J. Infrared And Raman Analysis Of Polymers. In Handbook of Plastics Analysis; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Carlsson, D.J.; Clark, F.R.S.; Wiles, D.M. The Photo-Oxidation of Polypropylene Monofilaments. Text. Res. J. 1976, 46, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Grause, G.; Chien, M.F.; Inoue, C. Changes during the Weathering of Polyolefins. Polym. Degrad. Stab. 2020, 181, 109364. [Google Scholar] [CrossRef]
- Curcio, M.S.; Canela, M.C.; Waldman, W.R. Selective Surface Modification of TiO2-Coated Polypropylene by Photodegradation. Eur. Polym. J. 2018, 101, 177–182. [Google Scholar] [CrossRef]
- Fechine, G.J.M.; Demarquette, N.R. Cracking Formation on the Surface of Extruded Photodegraded Polypropylene Plates. Polym. Eng. Sci. 2008, 48, 365–372. [Google Scholar] [CrossRef]
- Allen, N.S.; Chirinos-Padron, A.; Henman, T.J. Photoinitiated Oxidation of Polypropylene: A Review. Prog. Org. Coatings 1985, 13, 97–122. [Google Scholar] [CrossRef]



| Sample | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| PP 0 h | 54 ± 3 | 128 ± 3 | 167 ± 1 | 14 ± 1 | 13 ± 1 | 3.9 ± 0.5 | 0.39 ± 0.02 | 0.25 ± 0.05 | 0.36 ± 0.02 |
| PP 6 h | 54 ± 2 | 131 ± 5 | 175 ± 1 | 14 ± 1 | 12 ± 2 | 5 ± 1 | 0.42 ± 0.02 | 0.23 ± 0.05 | 0.35 ± 0.03 |
| PP 12 h | 52 ± 2 | 131 ± 4 | 177 ± 2 | 13 ± 1 | 16 ± 2 | 6 ± 1 | 0.40 ± 0.02 | 0.25 ± 0.05 | 0.37 ± 0.03 |
| PP 96 h | 52 ± 4 | 133 ± 5 | 165 ± 2 | 16 ± 1 | 13 ± 2 | 4.5 ± 0.5 | 0.38 ± 0.02 | 0.25 ± 0.05 | 0.38 ± 0.03 |
| PP 192 h | 53 ± 2 | 130 ± 5 | 157 ± 2 | 14 ± 1 | 15 ± 2 | 3.0 ± 0.5 | 0.37 ± 0.02 | 0.26 ± 0.05 | 0.38 ± 0.03 |
| PP 384 h | 59 ± 2 | 128 ± 4 | 151 ± 2 | 14 ± 1 | 15 ± 2 | 3.0 ± 0.5 | 0.40 ± 0.02 | 0.26 ± 0.05 | 0.33 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gimenez, J.C.F.; Bonatti, S.H.F.; Basaglia, M.V.; Garcia, R.H.d.S.; dos Santos, A.; Staffa, L.H.; Samara, M.; Bettini, S.H.P.; de Azevedo, E.R.; Helal, E.; et al. 1H Time Domain Nuclear Magnetic Resonance and Oscillatory Rheology as a Tool for Uncovering the Impact of UV-C Radiation on Polypropylene. Polymers 2025, 17, 2727. https://doi.org/10.3390/polym17202727
Gimenez JCF, Bonatti SHF, Basaglia MV, Garcia RHdS, dos Santos A, Staffa LH, Samara M, Bettini SHP, de Azevedo ER, Helal E, et al. 1H Time Domain Nuclear Magnetic Resonance and Oscillatory Rheology as a Tool for Uncovering the Impact of UV-C Radiation on Polypropylene. Polymers. 2025; 17(20):2727. https://doi.org/10.3390/polym17202727
Chicago/Turabian StyleGimenez, Jessica Caroline Ferreira, Sophia Helena Felisbino Bonatti, Marcos Vinícius Basaglia, Rodrigo Henrique dos Santos Garcia, Alef dos Santos, Lucas Henrique Staffa, Mazen Samara, Silvia Helena Prado Bettini, Eduardo Ribeiro de Azevedo, Emna Helal, and et al. 2025. "1H Time Domain Nuclear Magnetic Resonance and Oscillatory Rheology as a Tool for Uncovering the Impact of UV-C Radiation on Polypropylene" Polymers 17, no. 20: 2727. https://doi.org/10.3390/polym17202727
APA StyleGimenez, J. C. F., Bonatti, S. H. F., Basaglia, M. V., Garcia, R. H. d. S., dos Santos, A., Staffa, L. H., Samara, M., Bettini, S. H. P., de Azevedo, E. R., Helal, E., Demarquette, N. R., Homem, M. G. P., & Cruz, S. A. (2025). 1H Time Domain Nuclear Magnetic Resonance and Oscillatory Rheology as a Tool for Uncovering the Impact of UV-C Radiation on Polypropylene. Polymers, 17(20), 2727. https://doi.org/10.3390/polym17202727











