Kinetics of Carboxylic Acids Formation During Polypropylene Thermooxidation in Water Saturated with Pressurized Oxygen
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
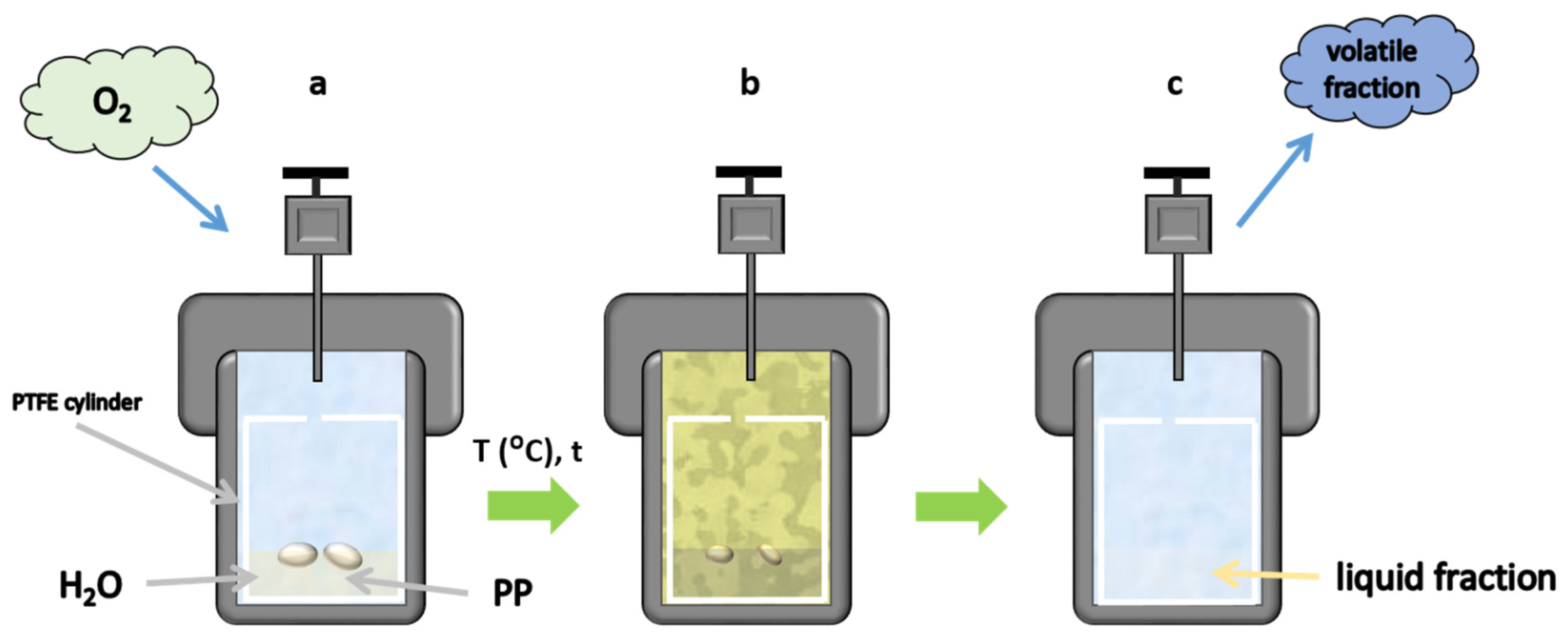
2.2. Chemical Processing of Polypropylene
2.3. Characterization of Liquid Products
2.4. Characterization of Solid Products
2.5. Gas Phase Characterization
3. Results and Discussion
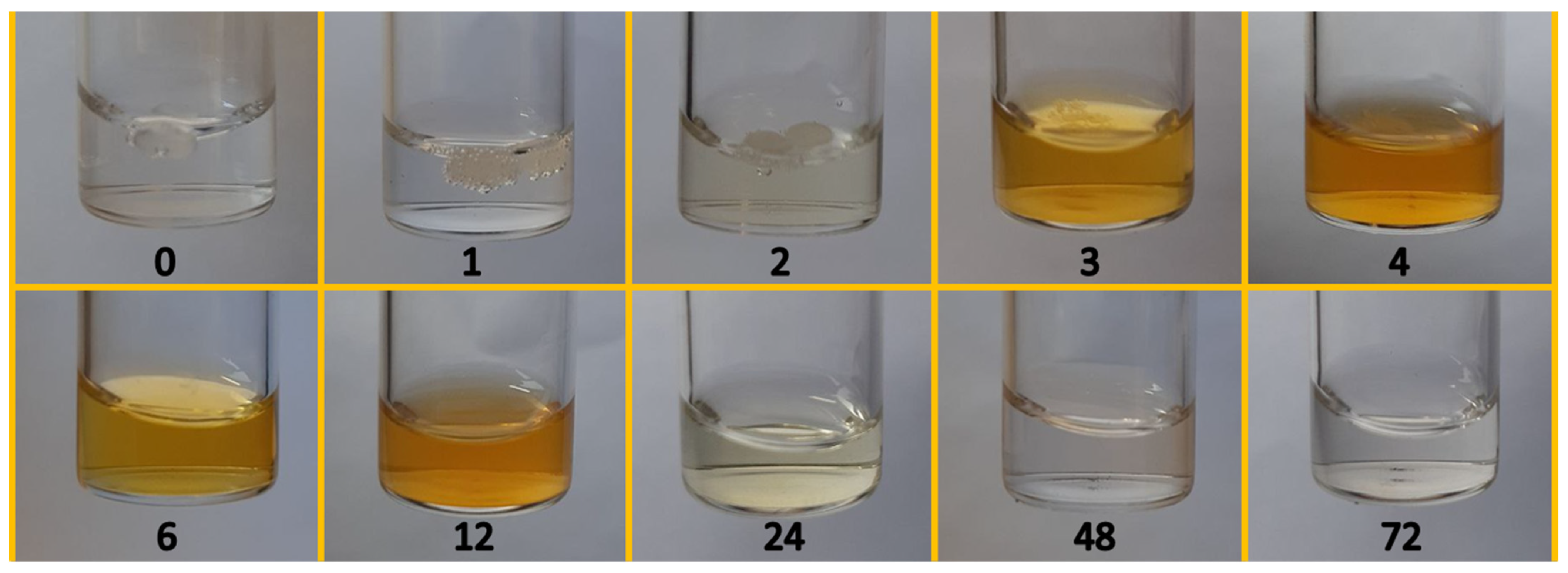
3.1. Overview of the Products of the TOD Process in H2O/O2 and D2O/O2 Mixture
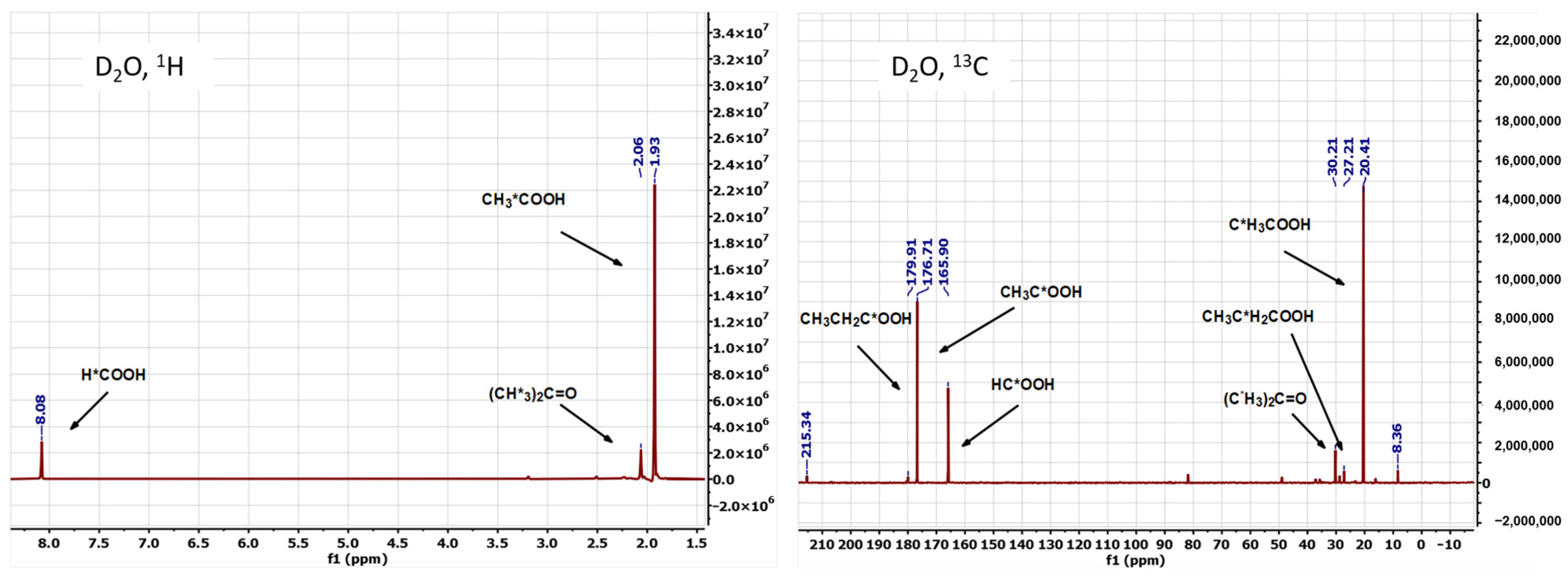
3.2. The Composition of the Liquid Products Obtained in the Water-Assisted TOD Process of PP
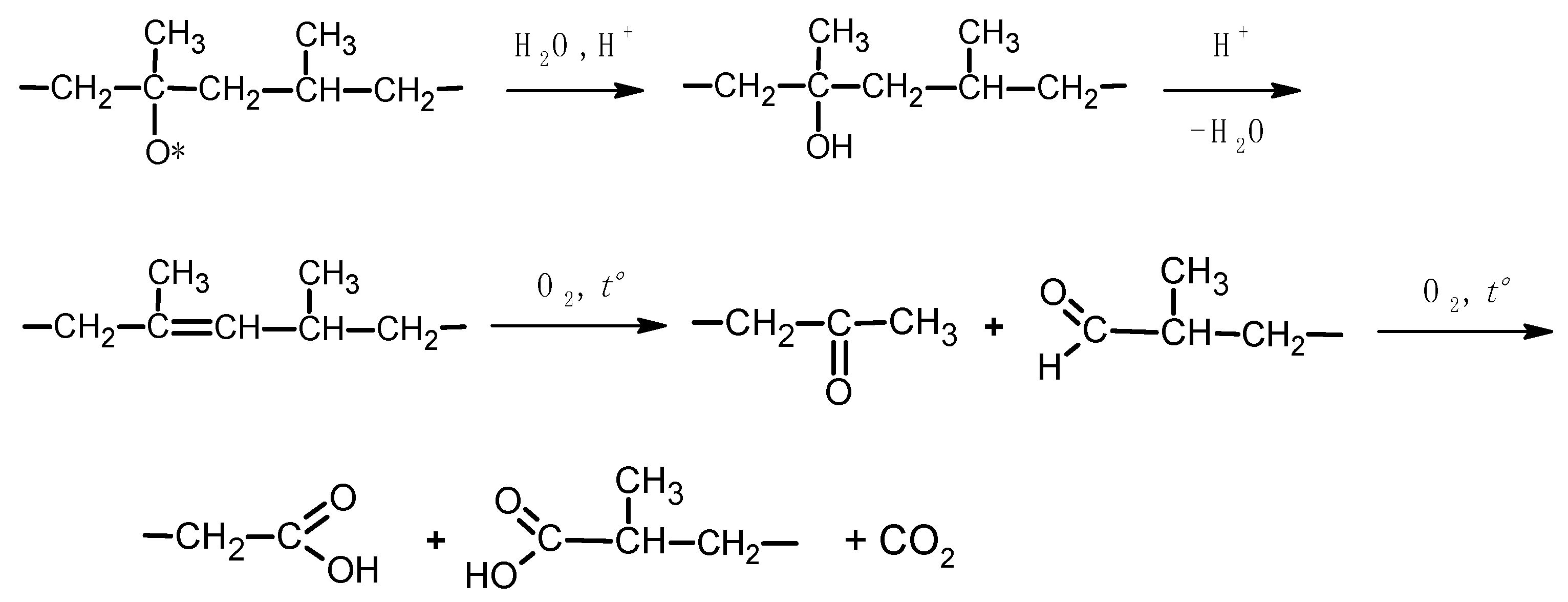
3.3. Possible Mechanisms of Water-Assisted TOD of PP
3.4. The Kinetics of the Monocarboxylic Acids Formation in the Water-Assisted TOD Process of PP
3.5. The Composition of the Solid Products Obtained in the Water-Assisted TOD Process of PP
3.6. The Composition of the Gas Phase Products Obtained in the Water-Assisted TOD Process of PP and Carbon Balance
3.7. The Role of Water in the Studied TOD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DSC | Differential scanning calorimetry |
| FTIR | Fourier IR spectroscopy |
| GC-MS | Gas chromatography–mass-spectrometry |
| GPC | Gel permeation chromatography |
| NMR | Nuclear magnetic resonance |
| OD | Oxidative decomposition |
| PE | Polyethylene |
| PP | Polypropylene |
| PTFE | Polytetrafluoroethylene |
| TGA | Thermogravimetric analysis |
| TOD | Thermal oxidative decomposition |
| UV | ultraviolet |
References
- Bushra, R.; Khayal, A.; Ahmad, M.; Song, J.; Jin, Y.; Xiao, H. Catalytic Conversion of Polymer Waste into High-Value Products for Advancing Circular Economy and Eco-Sustainability. J. Anal. Appl. Pyrolysis 2025, 189, 107052. [Google Scholar] [CrossRef]
- Alaghemandi, M. Sustainable Solutions Through Innovative Plastic Waste Recycling Technologies. Sustainability 2024, 16, 10401. [Google Scholar] [CrossRef]
- Martínez-Narro, G.; Hassan, S.; Phan, A.N. Chemical Recycling of Plastic Waste for Sustainable Polymer Manufacturing—A Critical Review. J. Environ. Chem. Eng. 2024, 12, 112323. [Google Scholar] [CrossRef]
- Ignatyev, I.A.; Thielemans, W.; Vander Beke, B. Recycling of Polymers: A Review. ChemSusChem 2014, 7, 1579–1593. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, e2000415. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; García, J.M. Chemical Recycling of Waste Plastics for New Materials Production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.H. Advancements and Future Directions in Waste Plastics Recycling: From Mechanical Methods to Innovative Chemical Processes. Chem. Eng. J. 2024, 493, 152727. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and Chemical Recycling of Solid Plastic Waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to Fuel: A Review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Davidson, M.G.; Furlong, R.A.; McManus, M.C. Developments in the Life Cycle Assessment of Chemical Recycling of Plastic Waste—A Review. J. Clean. Prod. 2021, 293, 126163. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J. Pyrolysis of Plastic Waste: Opportunities and Challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804. [Google Scholar] [CrossRef]
- Arabiourrutia, M.; Elordi, G.; Lopez, G.; Borsella, E.; Bilbao, J.; Olazar, M. Characterization of the Waxes Obtained by the Pyrolysis of Polyolefin Plastics in a Conical Spouted Bed Reactor. J. Anal. Appl. Pyrolysis 2012, 94, 230–237. [Google Scholar] [CrossRef]
- Jie, X.; Li, W.; Slocombe, D.; Gao, Y.; Banerjee, I.; Gonzalez-Cortes, S.; Yao, B.; AlMegren, H.; Alshihri, S.; Dilworth, J.; et al. Microwave-Initiated Catalytic Deconstruction of Plastic Waste into Hydrogen and High-Value Carbons. Nat. Catal. 2020, 3, 902–912. [Google Scholar] [CrossRef]
- Huo, E.; Lei, H.; Liu, C.; Zhang, Y.; Xin, L.; Zhao, Y.; Qian, M.; Zhang, Q.; Lin, X.; Wang, C.; et al. Jet Fuel and Hydrogen Produced from Waste Plastics Catalytic Pyrolysis with Activated Carbon and MgO. Sci. Total Environ. 2020, 727, 138411. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.T. Hydrogen and Carbon Nanotubes from Pyrolysis-Catalysis of Waste Plastics: A Review. Waste Biomass Valorization 2021, 12, 1–28. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Escola, J.M. Fuels from Waste Plastics by Thermal and Catalytic Processes: A Review. Ind. Eng. Chem. Res. 2008, 47, 7982–7992. [Google Scholar] [CrossRef]
- Fivga, A.; Dimitriou, I. Pyrolysis of Plastic Waste for Production of Heavy Fuel Substitute: A Techno-Economic Assessment. Energy 2018, 149, 865–874. [Google Scholar] [CrossRef]
- Irgolič, M.; Čolnik, M.; Kotnik, P.; Čuček, L.; Škerget, M. Hydrothermal Recycling of Polyolefins as Potential Alternative Method for Fuel Production. J. Clean. Prod. 2024, 463, 142718. [Google Scholar] [CrossRef]
- Watanabe, M.; Hirakoso, H.; Sawamoto, S.; Adschiri, T.; Arai, K. Polyethylene Conversion in Supercritical Water. J. Supercrit. Fluids 1998, 13, 247–252. [Google Scholar] [CrossRef]
- Bai, B.; Wang, W.; Jin, H. Experimental Study on Gasification Performance of Polypropylene (PP) Plastics in Supercritical Water. Energy 2020, 191, 116527. [Google Scholar] [CrossRef]
- Darzi, R.; Dubowski, Y.; Posmanik, R. Hydrothermal Processing of Polyethylene-Terephthalate and Nylon-6 Mixture as a Plastic Waste Upcycling Treatment: A Comprehensive Multi-Phase Analysis. Waste Manag. 2022, 143, 223–231. [Google Scholar] [CrossRef]
- Fakirov, S. Condensation Polymers: Their Chemical Peculiarities Offer Great Opportunities. Prog. Polym. Sci. 2019, 89, 1–18. [Google Scholar] [CrossRef]
- dos Passos, J.S. Synergistic Hydrothermal Liquefaction of Waste Materials. Ph.D. Thesis, Aarhus University, Aarhus, Denmark, January 2022. ISBN 9788775075270. [Google Scholar] [CrossRef]
- Inderthal, H.; Tai, S.L.; Harrison, S.T.L. Non-Hydrolyzable Plastics—An Interdisciplinary Look at Plastic Bio-Oxidation. Trends Biotechnol. 2021, 39, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Laredo, G.C.; Reza, J.; Meneses Ruiz, E. Hydrothermal Liquefaction Processes for Plastics Recycling: A Review. Clean. Chem. Eng. 2023, 5, 100094. [Google Scholar] [CrossRef]
- Chen, W.-T.; Jin, K.; Linda Wang, N.-H. Use of Supercritical Water for the Liquefaction of Polypropylene into Oil. ACS Sustain. Chem. Eng. 2019, 7, 3749–3758. [Google Scholar] [CrossRef]
- Jin, K.; Vozka, P.; Kilaz, G.; Chen, W.-T.; Wang, N.-H.L. Conversion of Polyethylene Waste into Clean Fuels and Waxes via Hydrothermal Processing (HTP). Fuel 2020, 273, 117726. [Google Scholar] [CrossRef]
- Oh, S.; Stache, E.E. Recent Advances in Oxidative Degradation of Plastics. Chem. Soc. Rev. 2024, 53, 7309–7327. [Google Scholar] [CrossRef]
- Iring, M.; Kelen, T.; Tudos, F.; Fuzes, L. Study of Thermal Oxidation of Polyolefins. J. Appl. Polym. Sci. 1976, 63, 55–63. [Google Scholar]
- Reich, L.; Jadrnicek, B.R.; Stivala, S.S. Kinetics of Thermal Oxidation of Polyolefins—A Review. Polym. Eng. Sci. 1971, 11, 265–273. [Google Scholar] [CrossRef]
- Richaud, E.; Farcas, F.; Bartoloméo, P.; Fayolle, B.; Audouin, L.; Verdu, J. Effect of Oxygen Pressure on the Oxidation Kinetics of Unstabilised Polypropylene. Polym. Degrad. Stab. 2006, 91, 398–405. [Google Scholar] [CrossRef]
- Hoff, A.; Jacobsson, S. Thermal Oxidation of Polypropylene in the Temperature Range of 120–280 °C. J. Appl. Polym. Sci. 1984, 29, 465–480. [Google Scholar] [CrossRef]
- François-Heude, A.; Richaud, E.; Leprovost, J.; Heninger, M.; Mestdagh, H.; Desnoux, E.; Colin, X. Real-Time Quantitative Analysis of Volatile Products Generated during Solid-State Polypropylene Thermal Oxidation. Polym. Test. 2013, 32, 907–917. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.H. Complete Degradation of Polyolefin Plastic Wastes to High-Value Products. ChemSusChem 2024, 17, 202301449. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Michalkiewicz, B.; Chen, X.; Mijowska, E.; Liu, J.; Jiang, Z.; Wen, X.; Tang, T. Sustainable Conversion of Mixed Plastics into Porous Carbon Nanosheets with High Performances in Uptake of Carbon Dioxide and Storage of Hydrogen. ACS Sustain. Chem. Eng. 2014, 2, 2837–2844. [Google Scholar] [CrossRef]
- Che, Y.; Wu, Y.; Niu, Z. Oxidative Degradation and Upcycling of Polyethylene Wastes. Sci. China Chem. 2025, 68, 430–443. [Google Scholar] [CrossRef]
- Kanbur, U.; Zang, G.; Paterson, A.L.; Chatterjee, P.; Hackler, R.A.; Delferro, M.; Slowing, I.I.; Perras, F.A.; Sun, P.; Sadow, A.D. Catalytic Carbon-Carbon Bond Cleavage and Carbon-Element Bond Formation Give New Life for Polyolefins as Biodegradable Surfactants. Chem 2021, 7, 1347–1362. [Google Scholar] [CrossRef]
- Xu, Z.; Munyaneza, N.E.; Zhang, Q.; Sun, M.; Posada, C.; Venturo, P.; Rorrer, N.A.; Miscall, J.; Sumpter, B.G.; Liu, G. Chemical Upcycling of Polyethylene, Polypropylene, and Mixtures to High-Value Surfactants. Science 2023, 381, 666–671. [Google Scholar] [CrossRef]
- Zhang, Q.; He, J.; Wei, X.; Shen, C.; Ye, P.; An, W.; Liu, X.; Li, H.; Xu, S.; Su, Z.; et al. Oxidative Upcycling of Polyethylene to Long Chain Diacid over Co-MCM-41 Catalyst. Angew. Chem. 2024, 136, e202407510. [Google Scholar] [CrossRef]
- Yeung, C.W.S.; Periayah, M.H.; Teo, J.Y.Q.; Goh, E.T.L.; Chee, P.L.; Loh, W.W.; Loh, X.J.; Lakshminarayanan, R.; Lim, J.Y.C. Transforming Polyethylene into Water-Soluble Antifungal Polymers. Macromolecules 2023, 56, 815–823. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, X.; Shen, C.; Ye, P.; An, W.; Liu, X.; Xu, S.; Wang, Y.-Z. Solvent-Regulated Tandem Oxidative Degradation of Polyethylene to Multifunctional Chemicals. ACS Sustain. Chem. Eng. 2024, 12, 5788–5798. [Google Scholar] [CrossRef]
- Zefirov, V.V.; Elmanovich, I.V.; Stakhanov, A.I.; Pavlov, A.A.; Stakhanova, S.V.; Kharitonova, E.P.; Gallyamov, M.O. A New Look at the Chemical Recycling of Polypropylene: Thermal Oxidative Destruction in Aqueous Oxygen-Enriched Medium. Polymers 2022, 14, 744. [Google Scholar] [CrossRef]
- Hermann, C.; Morrill, T.; Shriner, R.; Fuson, R. The Systematic Identification of Organic Compounds A Comprehensive Introduction to the Identification of Unknown Organic Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Bhargava, S.K.; Tardio, J.; Prasad, J.; Föger, K.; Akolekar, D.B.; Grocott, S.C. Wet Oxidation and Catalytic Wet Oxidation. Ind. Eng. Chem. Res. 2006, 45, 1221–1258. [Google Scholar] [CrossRef]
- Debellefontaine, H.; Chakchouk, M.; Foussard, J.N.; Tissot, D.; Striolo, P. Treatment of Organic Aqueous Wastes: Wet Air Oxidation and Wet Peroxide Oxidation®. Environ. Pollut. 1996, 92, 155–164. [Google Scholar] [CrossRef]
- Yadav, L.D.S. Organic Spectroscopy; Springer: Dordrecht, The Netherlands, 2005; ISBN 978-94-017-2508-8. [Google Scholar]
- Iring, M.; Tudos, F. Thermal Oxidation of Polyethylene and Polypropylene: Effects of Chemical Structure and Reaction Conditions on the Oxidation Process. Prog. Polym. Sci. 1990, 15, 217–262. [Google Scholar] [CrossRef]
- Dogan, F. Polypropylene; BoD—Books on Demand: Norderstedt, Germany, 2012. [Google Scholar]
- Mill, T.; Richardson, H.; Mayo, F.R. Aging and Degradation of Polyolefins. IV. Thermal and Photodecompositions of Model Peroxides. J. Polym. Sci. Polym. Chem. Ed. 1973, 11, 2899–2907. [Google Scholar] [CrossRef]
- Commereuc, S.; Vaillant, D.; Philippart, J.L.; Lacoste, J.; Lemaire, J.; Carlsson, D.J. Photo and Thermal Decomposition of IPP Hydroperoxides. Polym. Degrad. Stab. 1997, 57, 175–182. [Google Scholar] [CrossRef]
- Vaillant, D.; Lacoste, J.; Dauphin, G. The Oxidation Mechanism of Polypropylene: Contribution of 13C-NMR Spectroscopy. Polym. Degrad. Stab. 1994, 45, 355–360. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Q.; Yang, J.; Liu, W.; Wang, A. Recovering Valuable Chemicals from Polypropylene Waste via a Mild Catalyst-Free Hydrothermal Process. Environ. Sci. Technol. 2024, 58, 16611–16620. [Google Scholar] [CrossRef]
- Jin, F.; Zhou, Z.; Enomoto, H.; Moriya, T.; Higashijima, H. Conversion Mechanism of Cellulosic Biomass to Lactic Acid in Subcritical Water and Acid–Base Catalytic Effect of Subcritical Water. Chem. Lett. 2004, 33, 126–127. [Google Scholar] [CrossRef]
- Mangi, M.A.; Li, B.; Duan, P.; Cao, C. Preparation of Acetic Acid from Crop Residues via Hydrolysis Coupled with Oxidation in Subcritical Water. Ind. Crops Prod. 2025, 232, 121208. [Google Scholar] [CrossRef]
- Pigaleva, M.A.; Elmanovich, I.V.; Kononevich, Y.N.; Gallyamov, M.O.; Muzafarov, A.M. A Biphase H2O/CO2 System as a Versatile Reaction Medium for Organic Synthesis. RSC Adv. 2015, 5, 103573–103608. [Google Scholar] [CrossRef]
- Galkin, A.; Lunin, V. Water in Sub-and Supercritical States Is a Universal Medium for Chemical Reactions. Russ. Chem. Rev. 2005, 74, 21–35. [Google Scholar] [CrossRef]
- Avola, S.; Guillot, M.; da Silva-Perez, D.; Pellet-Rostaing, S.; Kunz, W.; Goettmann, F. Organic Chemistry under Hydrothermal Conditions. Pure Appl. Chem. 2012, 85, 89–103. [Google Scholar] [CrossRef]
- Partenheimer, W. Valuable Oxygenates by Aerobic Oxidation of Polymers Using Metal/Bromide Homogeneous Catalysts. Catal. Today 2003, 81, 117–135. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Romero, A.; Querol, J. Modeling of the Thermal N-Octane Oxidation in the Liquid Phase. Ind. Eng. Chem. Res. 1989, 28, 43–48. [Google Scholar] [CrossRef]
- Olivier, K.L.; Schaeffer, W.D. Butane Oxidation. U.S. Patent 3293292, 20 December 1966. [Google Scholar]
- Partenheimer, W. Methodology and Scope of Metal/Bromide Autoxidation of Hydrocarbons. Catal. Today 1995, 23, 69–158. [Google Scholar] [CrossRef]
- Calvo, L.; Vallejo, D. Formation of Organic Acids during the Hydrolysis and Oxidation of Several Wastes in Sub- and Supercritical Water. Ind. Eng. Chem. Res. 2002, 41, 6503–6509. [Google Scholar] [CrossRef]
- Čolnik, M.; Kotnik, P.; Knez, Ž.; Škerget, M. Hydrothermal Decomposition of Polyethylene Waste to Hydrocarbons Rich Oil. J. Supercrit. Fluids 2021, 169, 105136. [Google Scholar] [CrossRef]
- Djandja, O.S.; Liew, R.K.; Liu, C.; Liang, J.; Yuan, H.; He, W.; Feng, Y.; Lougou, B.G.; Duan, P.-G.; Lu, X.; et al. Catalytic Hydrothermal Carbonization of Wet Organic Solid Waste: A Review. Sci. Total Environ. 2023, 873, 162119. [Google Scholar] [CrossRef]
- Meyer, J.C.; Marrone, P.A.; Tester, J.W. Acetic Acid Oxidation and Hydrolysis in Supercritical Water. AIChE J. 1995, 41, 2108–2121. [Google Scholar] [CrossRef]
- Gallezot, P.; Chaumet, S.; Perrard, A.; Isnard, P. Catalytic Wet Air Oxidation of Acetic Acid on Carbon-Supported Ruthenium Catalysts. J. Catal. 1997, 168, 104–109. [Google Scholar] [CrossRef]
- Emanuel, N.; Buchachenko, A. Himicheskaya Fizika Molekulyarnogo Razrusheniya i Stabilizaciya Polimerov; Nauka: Moscow, Russia, 1988. [Google Scholar]
- Shlyapnikov, Y.; Kiryushkin, S.; Mar’in, A. Antioxidative Stabilization of Polymers; CRC Press: London, UK, 1996. [Google Scholar] [CrossRef]
- Zlatkevich, L. On the Kinetic Order of Decomposition of Polymeric Hydroperoxides. Polym. Degrad. Stab. 2004, 83, 369–371. [Google Scholar] [CrossRef]
- Shlyapnikov, Y.A.; Bogaevskaya, T.A.; Kiryushkin, S.G.; Monakhova, T.V. Specific Features of Formation and Properties of Hydroperoxides of Polyolefins. Eur. Polym. J. 1979, 15, 737–742. [Google Scholar] [CrossRef]
- Gugumus, F. Thermooxidative Degradation of Polyolefins in the Solid State: Part 1. Experimental Kinetics of Functional Group Formation. Polym. Degrad. Stab. 1996, 52, 131–144. [Google Scholar] [CrossRef]
- Gugumus, F. Thermooxidative Degradation of Polyolefins in the Solid State—6. Kinetics of Thermal Oxidation of Polypropylene. Polym. Degrad. Stab. 1998, 62, 235–243. [Google Scholar] [CrossRef]
- Snegirev, A.Y.; Talalov, V.A.; Stepanov, V.V.; Korobeinichev, O.P.; Gerasimov, I.E.; Shmakov, A.G. Autocatalysis in Thermal Decomposition of Polymers. Polym. Degrad. Stab. 2017, 137, 151–161. [Google Scholar] [CrossRef]
- Gryn’ova, G.; Hodgson, J.L.; Coote, M.L. Revising the Mechanism of Polymer Autooxidation. Org. Biomol. Chem. 2011, 9, 480–490. [Google Scholar] [CrossRef]
- Zlatkevich, L. Chemiluminescence in Autocatalytic Thermal Oxidation of Polymers. J. Polym. Sci. Polym. Lett. Ed. 1983, 21, 571–574. [Google Scholar] [CrossRef]
- Al-Malaika, S.; Axtell, F.; Rothon, R.; Gilbert, M. Additives for Plastics. In Brydson’s Plastics Materials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 127–168. [Google Scholar]
- Broska, R.; Rychly, J.; Csomorova, K. Carboxylic Acid Assisted Oxidation of Polypropylene Studied by Chemiluminescence. Polym. Degrad. Stab. 1999, 63, 231–236. [Google Scholar] [CrossRef]
- Bjerre, A.B.; Soerensen, E. Thermal Decomposition of Dilute Aqueous Formic Acid Solutions. Ind. Eng. Chem. Res. 1992, 31, 1574–1577. [Google Scholar] [CrossRef]
- Lacoste, J.; Carlsson, D.J. Gamma-, Photo-, and Thermally-initiated Oxidation of Linear Low Density Polyethylene: A Quantitative Comparison of Oxidation Products. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 493–500. [Google Scholar] [CrossRef]
- El-Hendawy, A.-N.A. Variation in the FTIR Spectra of a Biomass under Impregnation, Carbonization and Oxidation Conditions. J. Anal. Appl. Pyrolysis 2006, 75, 159–166. [Google Scholar] [CrossRef]
- Venegas-Sanchez, J.A.; Tagaya, M.; Kobayashi, T. Effect of Ultrasound on the Aqueous Viscosity of Several Water-Soluble Polymers. Polym. J. 2013, 45, 1224–1233. [Google Scholar] [CrossRef]
- Mori, Y.; Honda, T.; Lu, R.; Hayakawa, N.; Miyakoshi, T. Ultraviolet Degradation of Poly(Vinyl Alcohol) Used in Restoration of Historical and Cultural Properties. Polym. Degrad. Stab. 2015, 114, 30–36. [Google Scholar] [CrossRef]
- Chow, C.-F.; Wong, W.-L.; Chan, C.-W.; Chan, C.-S. Converting Inert Plastic Waste into Energetic Materials: A Study on the Light-Accelerated Decomposition of Plastic Waste with the Fenton Reaction. Waste Manag. 2018, 75, 174–180. [Google Scholar] [CrossRef]
- Kommula, B.; Chakraborty, S.; Banoo, M.; Roy, R.S.; Sil, S.; Swarnkar, A.; Rawat, B.; Kailasam, K.; Gautam, U.K. Waste Polyethylene-Derived Carbon Dots: Administration of Metal-Free Oxidizing Agents for Tunable Properties and Photocatalytic Hyperactivity. ACS Appl. Mater. Interfaces 2024, 16, 39470–39481. [Google Scholar] [CrossRef]
- Wexler, A.S. Infrared Determination of Structural Units in Organic Compounds by Integrated Intensity Measurements: Alkanes, Alkenes and Monosubstituted Alkyl Benzenes. Spectrochim. Acta 1965, 21, 1725–1742. [Google Scholar] [CrossRef]
- Purohit, V.; Orzel, R.A. Polypropylene: A Literature Review of the Thermal Decomposition Products and Toxicity. J. Am. Coll. Toxicol. 1988, 7, 221–242. [Google Scholar] [CrossRef]
- Odunlami, O.A.; Vershima, D.A.; Oladimeji, T.E.; Nkongho, S.; Ogunlade, S.K.; Fakinle, B.S. Advanced Techniques for the Capturing and Separation of CO2—A Review. Results Eng. 2022, 15, 100512. [Google Scholar] [CrossRef]
- Ingale, M.N.; Joshi, J.B.; Mahajani, V.V.; Gada, M.K. Waste Treatment of an Aqueous Waste Stream from a Cyclohexane Oxidation Unit. Process Saf. Environ. Prot. 1996, 74, 265–272. [Google Scholar] [CrossRef]
- Robert, R.; Barbati, S.; Ricq, N.; Ambrosio, M. Intermediates in Wet Oxidation of Cellulose: Identification of Hydroxyl Radical and Characterization of Hydrogen Peroxide. Water Res. 2002, 36, 4821–4829. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, D.; Gao, Y. Performance of High Temperature Phase-Stable High Entropy Oxide (MgCuMnCoFe)Ox in Catalytic Wet Air Oxidation of Chloroquine Phosphate. J. Mater. Sci. 2022, 57, 9104–9117. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nyui, M.; Kamibayashi, M.; Ozawa, T.; Nakanishi, I.; Anzai, K. Temperature-Dependent Free Radical Reaction in Water. J. Clin. Biochem. Nutr. 2011, 50, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Bruskov, V.I.; Masalimov, Z.K.; Chernikov, A.V. Heat-Induced Generation of Reactive Oxygen Species during Reduction of Dissolved Air Oxygen. Dokl. Biol. Sci. 2001, 381, 586–588. [Google Scholar] [CrossRef] [PubMed]
- Bruskov, V.I.; Masalimov, Z.K.; Chernikov, A.V. Heat-Induced Generation of Reactive Oxygen Species in Water. Dokl. Biochem. Biophys. 2002, 384, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Bartels, D.M. Temperature Dependence of Oxygen Diffusion in H2O and D2O. J. Phys. Chem. 1996, 100, 5597–5602. [Google Scholar] [CrossRef]

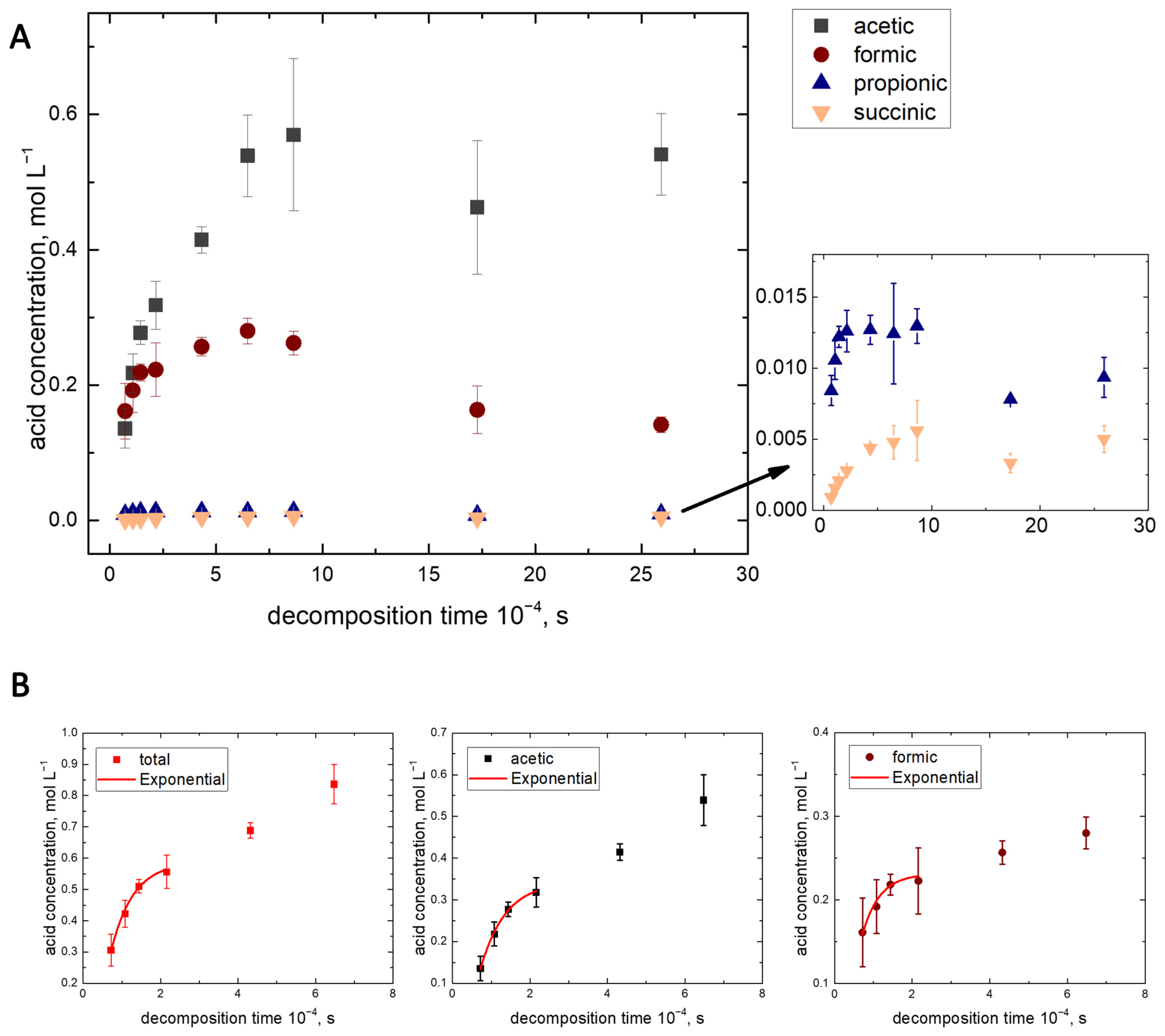
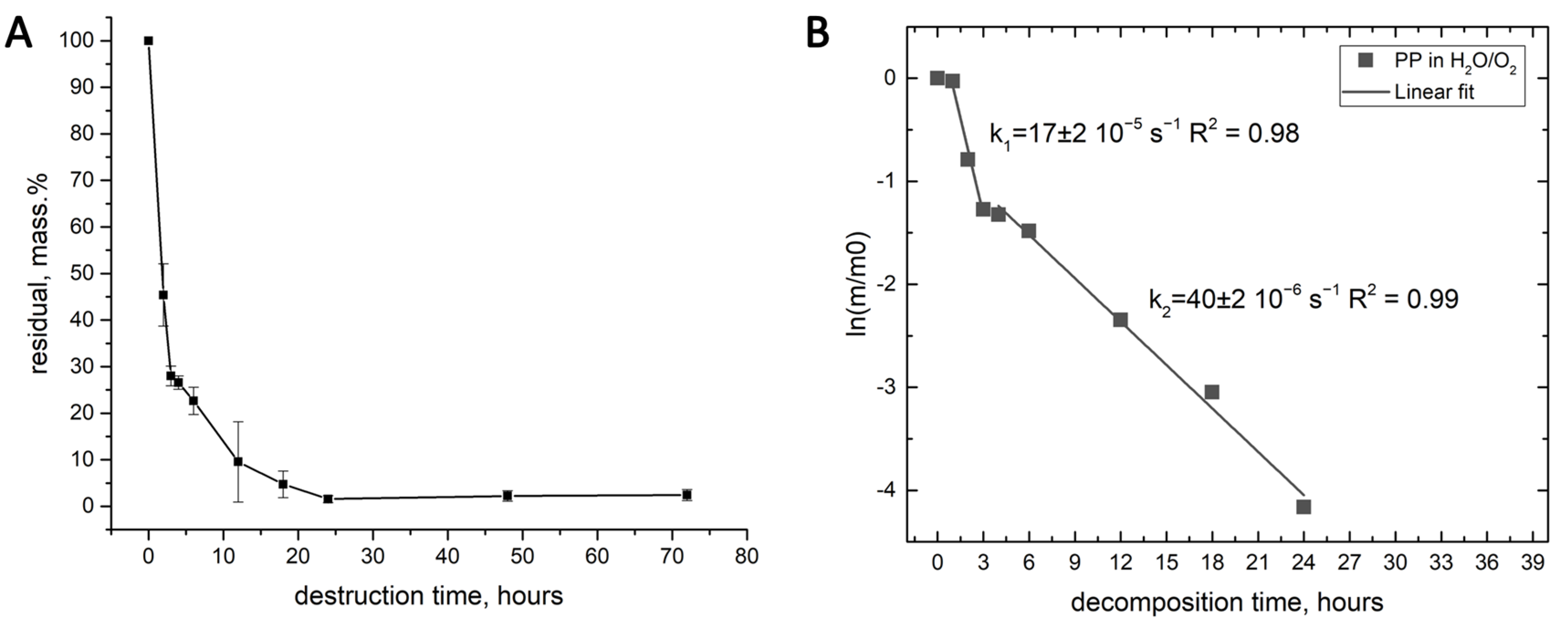

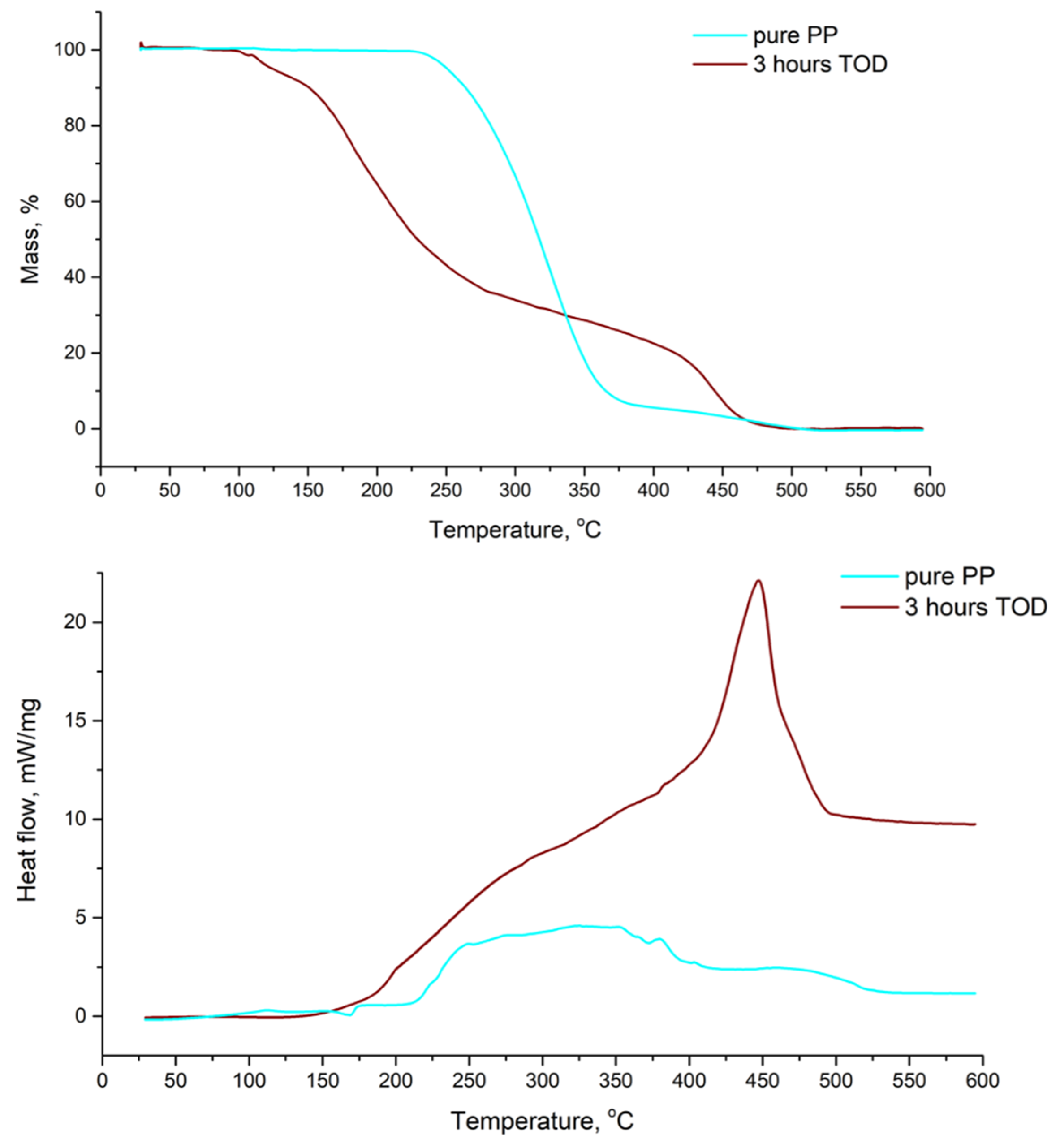
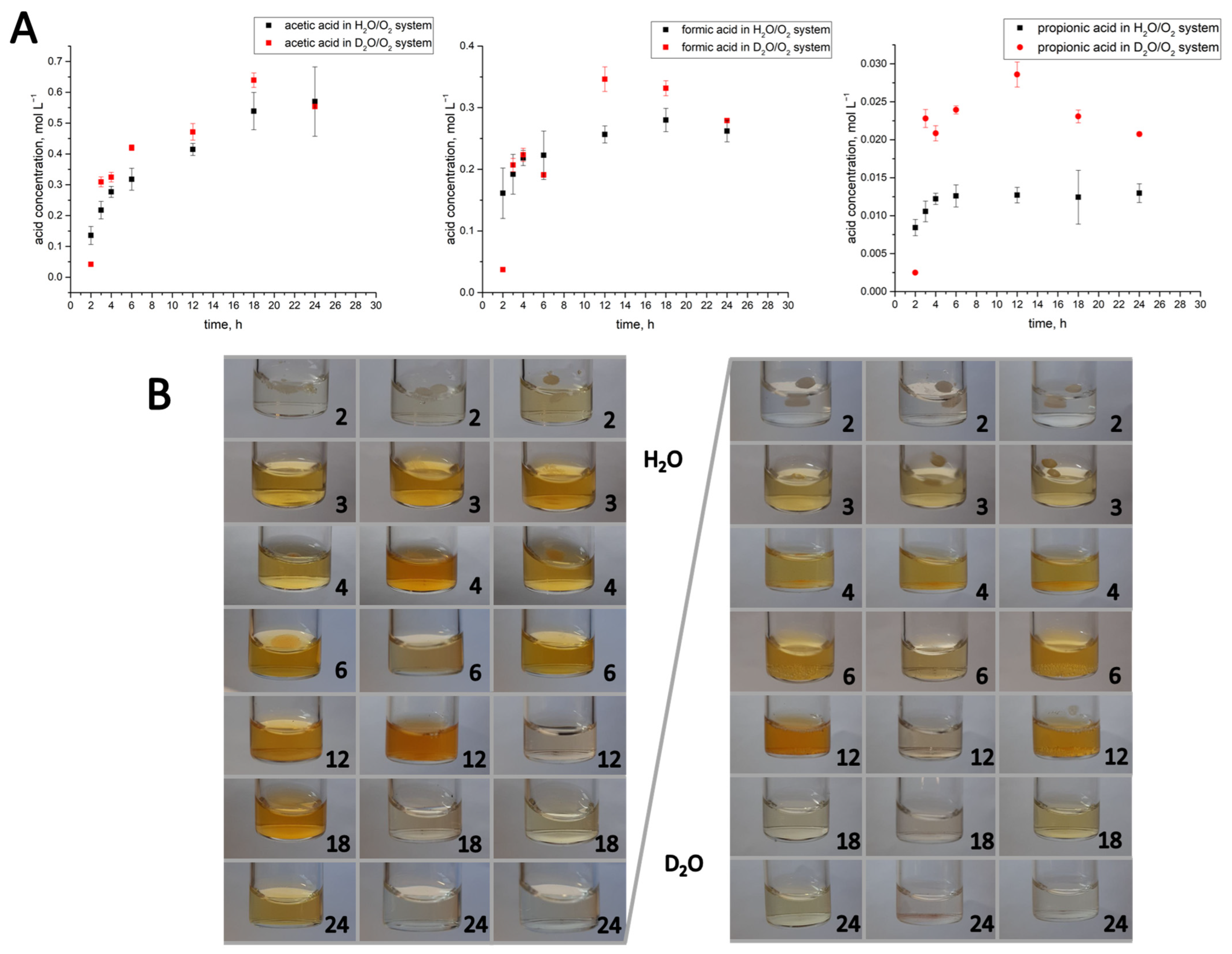
| Product | First-Order Kinetics of the TOD Process at t = 2–6 h and the Values of |
|---|---|
| acetic + formic + propionic + succinic acids | = 1.7 ± 0.5 × 10−4 s−1; R2 = 0.97 |
| acetic acid | = 1.5 ± 0.3 × 10−4 s−1; R2 = 0.99 |
| formic acid | = 2.3 ± 1.3 × 10−4 s−1; R2 = 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zefirov, V.V.; Kazaryan, P.S.; Stakhanov, A.I.; Stakhanova, S.V.; Ilyin, M.M.; Godovikov, I.A.; Shmakova, E.V.; Terentyev, A.G.; Dudkin, A.V.; Kharitonova, E.P.; et al. Kinetics of Carboxylic Acids Formation During Polypropylene Thermooxidation in Water Saturated with Pressurized Oxygen. Polymers 2025, 17, 2696. https://doi.org/10.3390/polym17192696
Zefirov VV, Kazaryan PS, Stakhanov AI, Stakhanova SV, Ilyin MM, Godovikov IA, Shmakova EV, Terentyev AG, Dudkin AV, Kharitonova EP, et al. Kinetics of Carboxylic Acids Formation During Polypropylene Thermooxidation in Water Saturated with Pressurized Oxygen. Polymers. 2025; 17(19):2696. https://doi.org/10.3390/polym17192696
Chicago/Turabian StyleZefirov, Vadim V., Polina S. Kazaryan, Andrey I. Stakhanov, Svetlana V. Stakhanova, Mikhail M. Ilyin, Ivan A. Godovikov, Elizaveta V. Shmakova, Andrey G. Terentyev, Alexander V. Dudkin, Elena P. Kharitonova, and et al. 2025. "Kinetics of Carboxylic Acids Formation During Polypropylene Thermooxidation in Water Saturated with Pressurized Oxygen" Polymers 17, no. 19: 2696. https://doi.org/10.3390/polym17192696
APA StyleZefirov, V. V., Kazaryan, P. S., Stakhanov, A. I., Stakhanova, S. V., Ilyin, M. M., Godovikov, I. A., Shmakova, E. V., Terentyev, A. G., Dudkin, A. V., Kharitonova, E. P., Gallyamov, M. O., & Khokhlov, A. R. (2025). Kinetics of Carboxylic Acids Formation During Polypropylene Thermooxidation in Water Saturated with Pressurized Oxygen. Polymers, 17(19), 2696. https://doi.org/10.3390/polym17192696







