Recent Progress on the Characterization of Polymer Crystallization by Atomic Force Microscopy
Abstract
1. Introduction
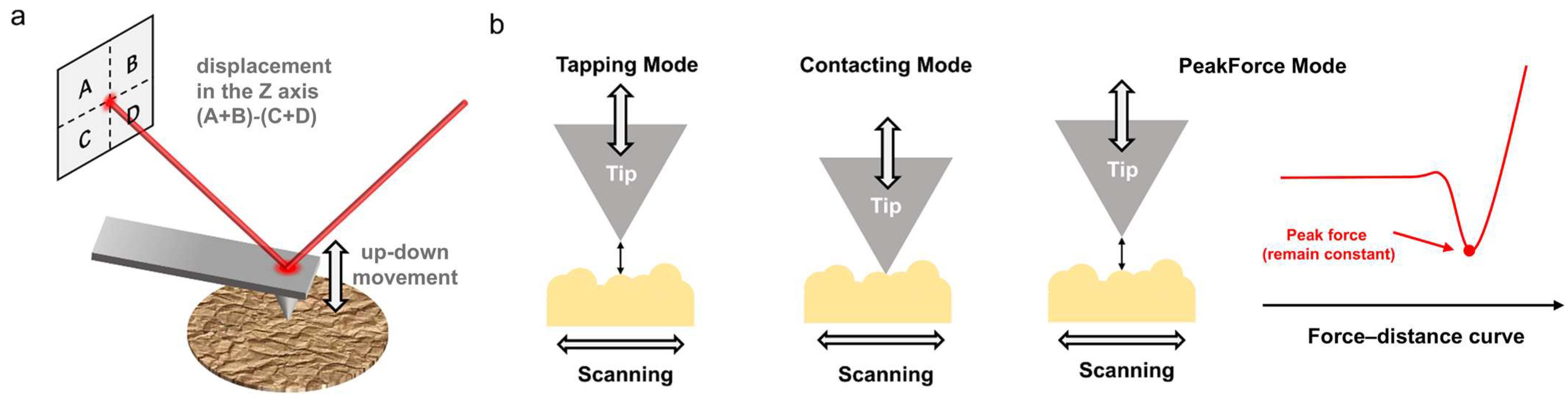
2. Basic Principles of AFM Technology
- Tapping mode: The probe periodically approaches the sample surface at its resonant frequency. As it nears the sample, the probe undergoes compressed oscillation due to the interaction forces, causing changes in amplitude. These changes are captured by a four-quadrant detector through the reflected laser and converted into surface topography information via post-processing. Since the probe does not contact with the sample surface, there is no risk of damage to the sample, making it suitable for studying soft or fragile samples, such as thin film surfaces and molecular-scale images. This is particularly important for in situ observation of the crystallization process in polymer films and for characterizing the structure of polymer crystals at the molecular scale. By changing the setpoint and drive amplitude of the probe, it is possible to select imaging based on intermolecular attractive forces or repulsive forces [23].
- Contact mode: As the probe glides across the surface while maintaining contact between the tip and the sample, changes in the sample’s topography cause vertical deflection of the cantilever. This results in the position of the reflected laser changing. By measuring the positional shifts in the reflected laser corresponding to each scanning point through the four-quadrant detector, information about the sample’s surface topography is obtained. This mode offers high sensitivity and resolution but can easily damage soft samples. Many AFM functionalization tests are based on contact mode, such as lateral force microscopy (LFM), piezo force microscopy (PFM), etc. [24]. These are widely used for testing the physical properties of polymer crystals, which help researchers to establish relationships between structure and properties.
- PeakForce mode: The principle behind PeakForce mode is relatively complex, so only a brief introduction is provided here. Further details can be found in Ref. [25]. The peak force between the probe and sample is maintained constant (by moving the scanning tube). When scanning along the X and Y axes, the detector’s movement in the Z direction is recorded to obtain the surface topography image of the sample and nanomechanical information. Unlike conventional tapping mode, this approach employs a cantilever oscillating at a non-resonant frequency. Under these conditions, each interaction between the tip and the sample can be measured, yielding a continuous force–distance curve. This mode is crucial for measuring the mechanical and electronical properties of polymer crystals under pressure [26].
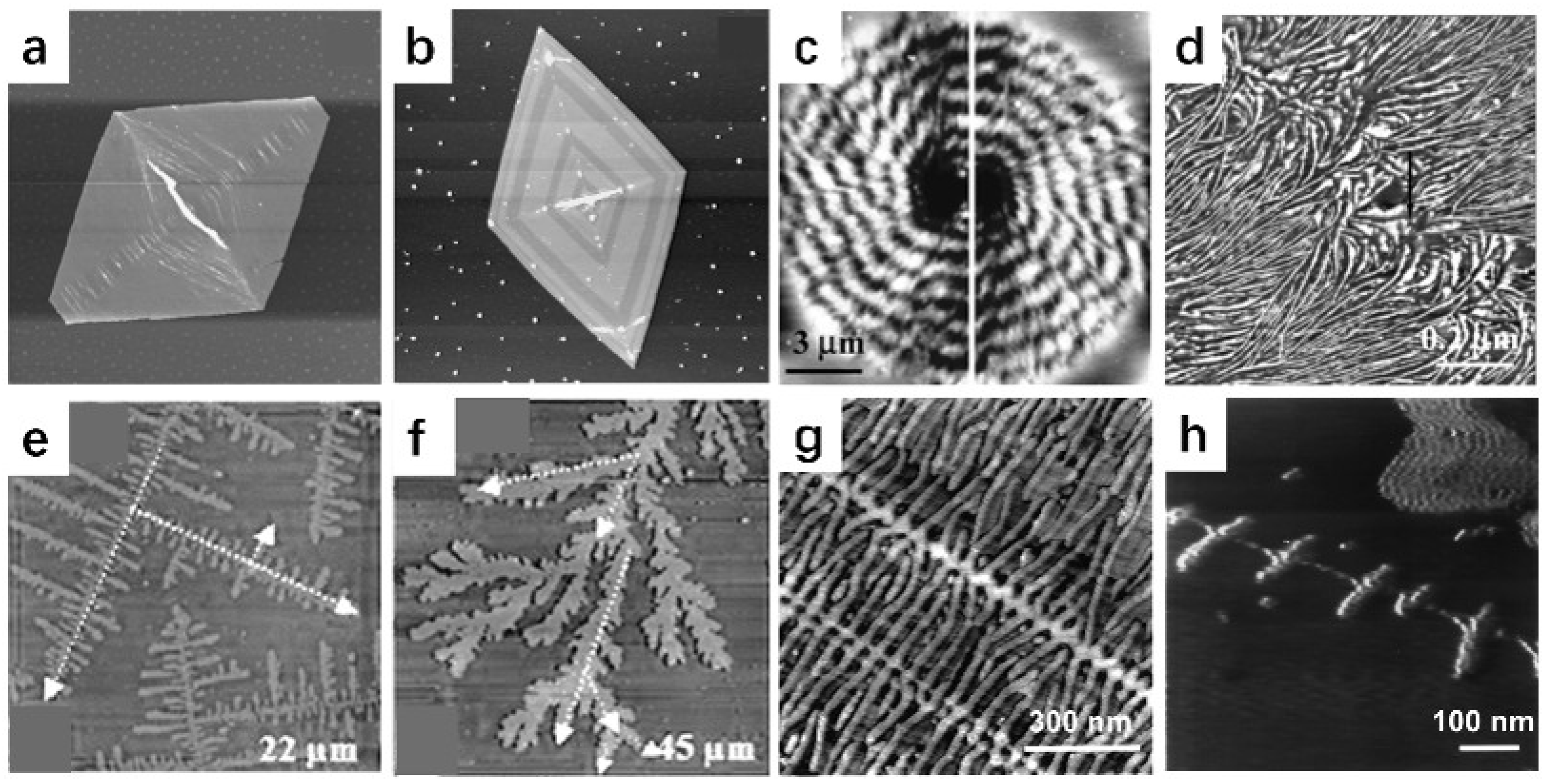
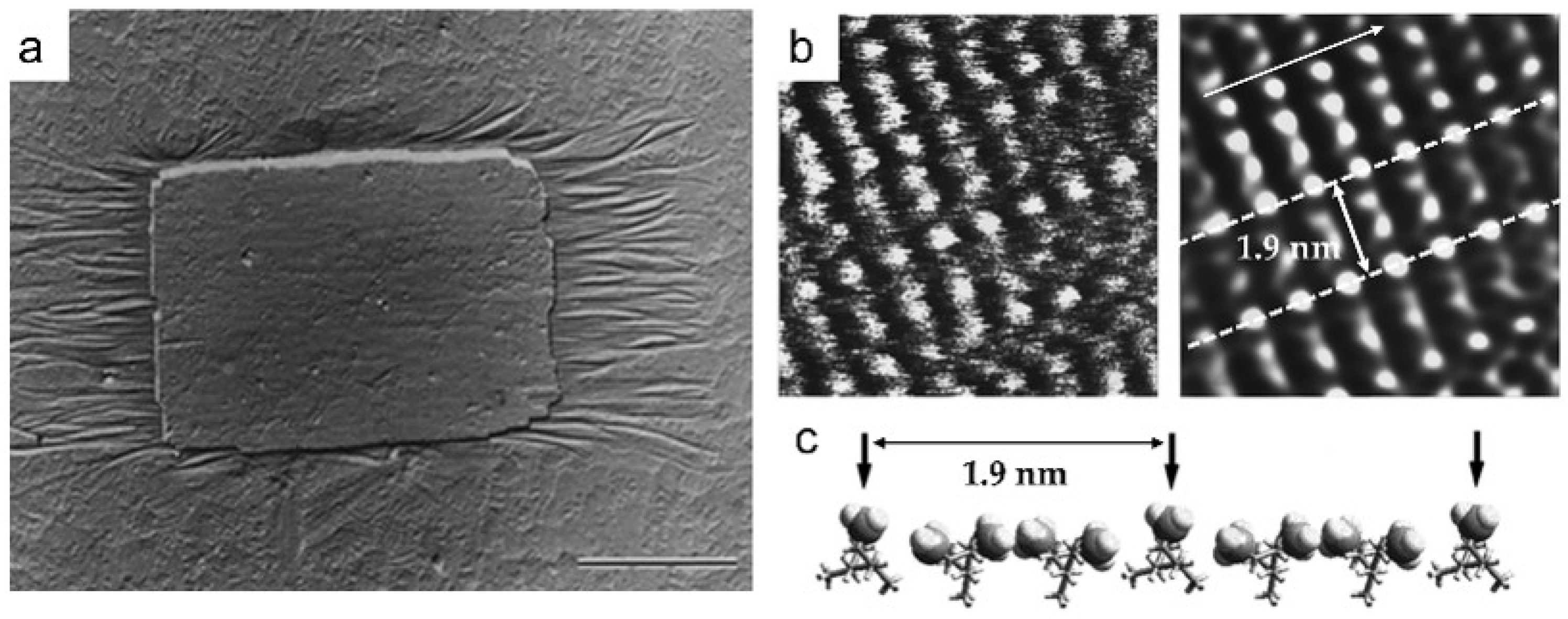
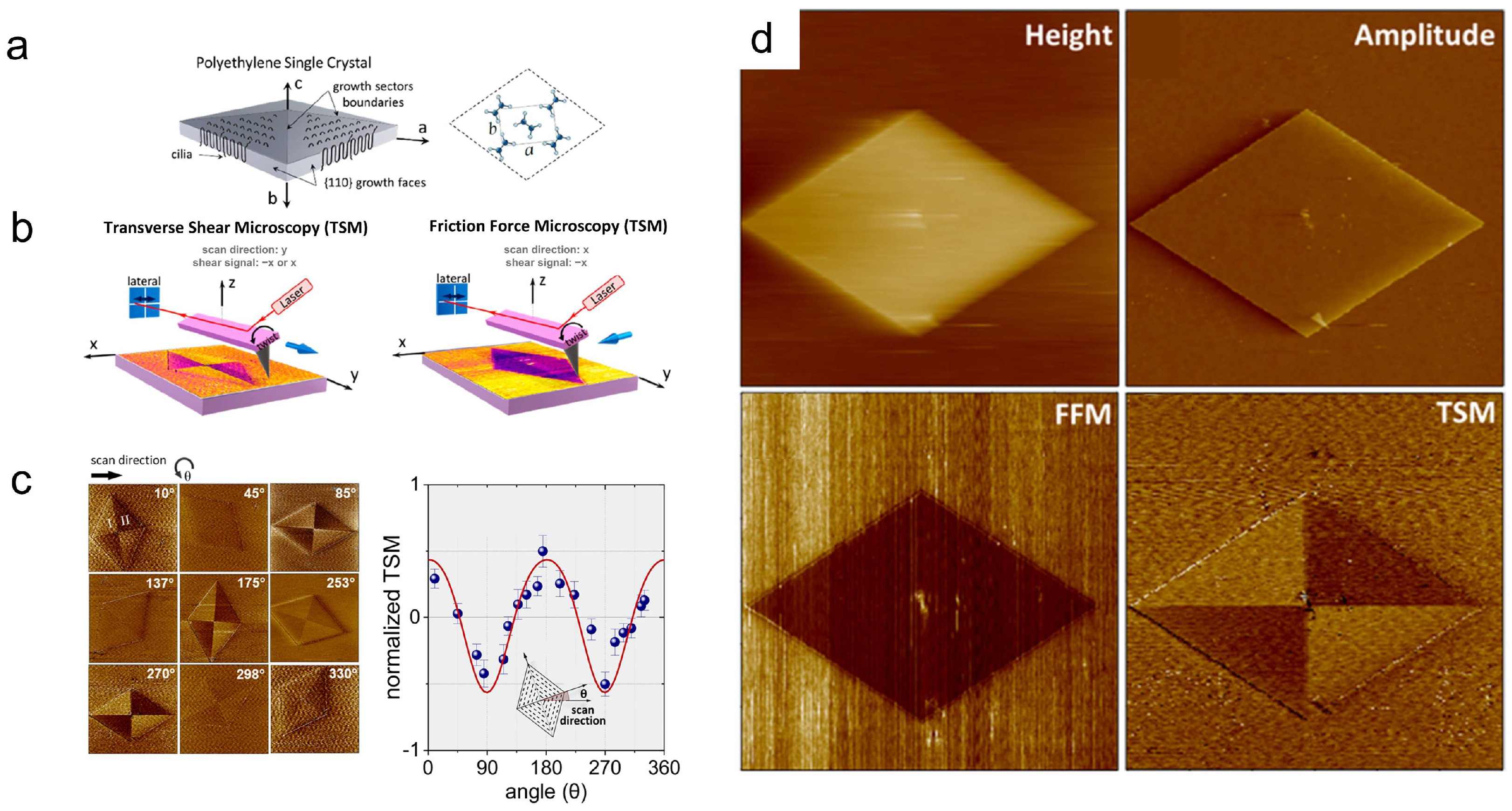
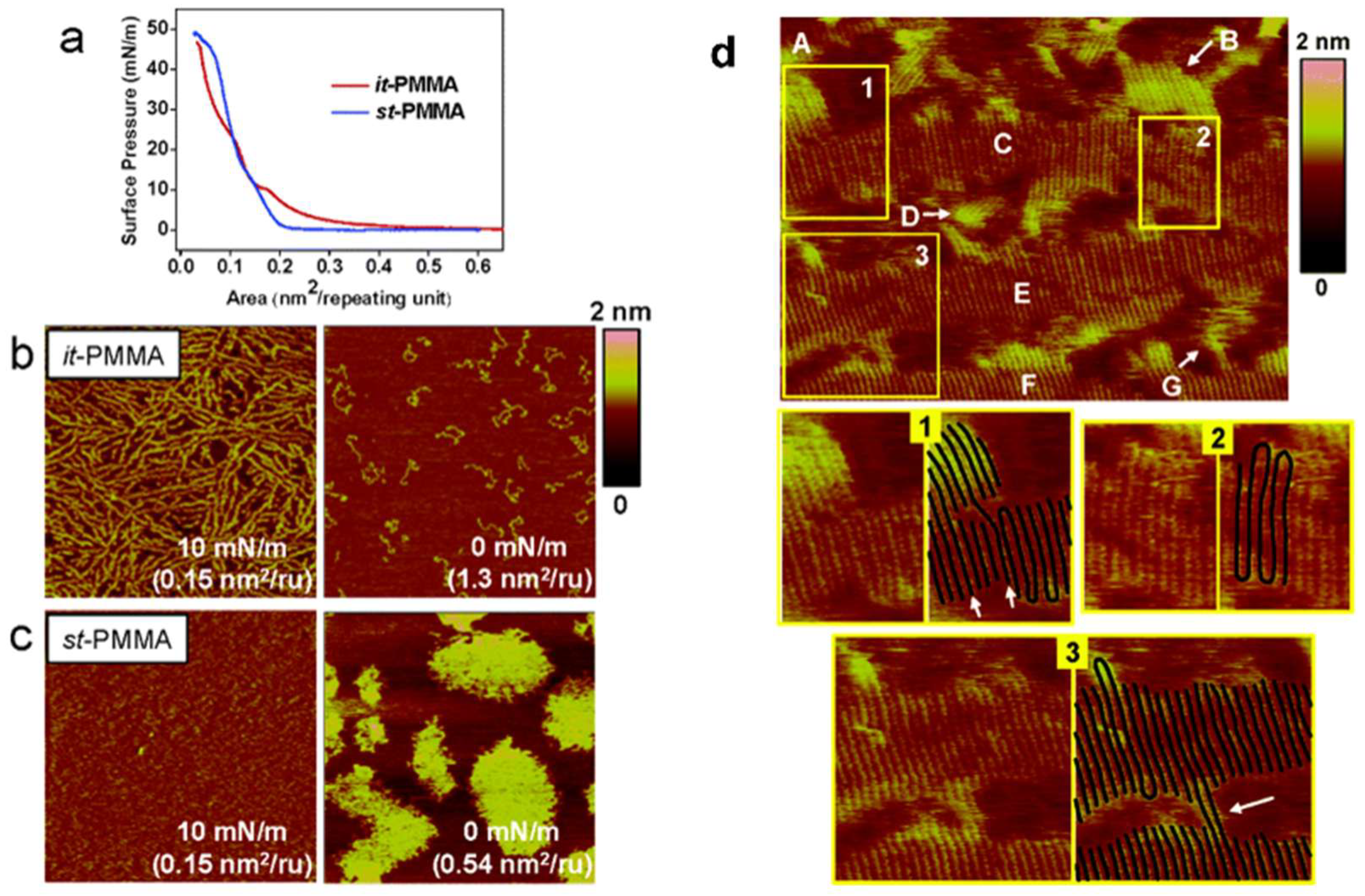
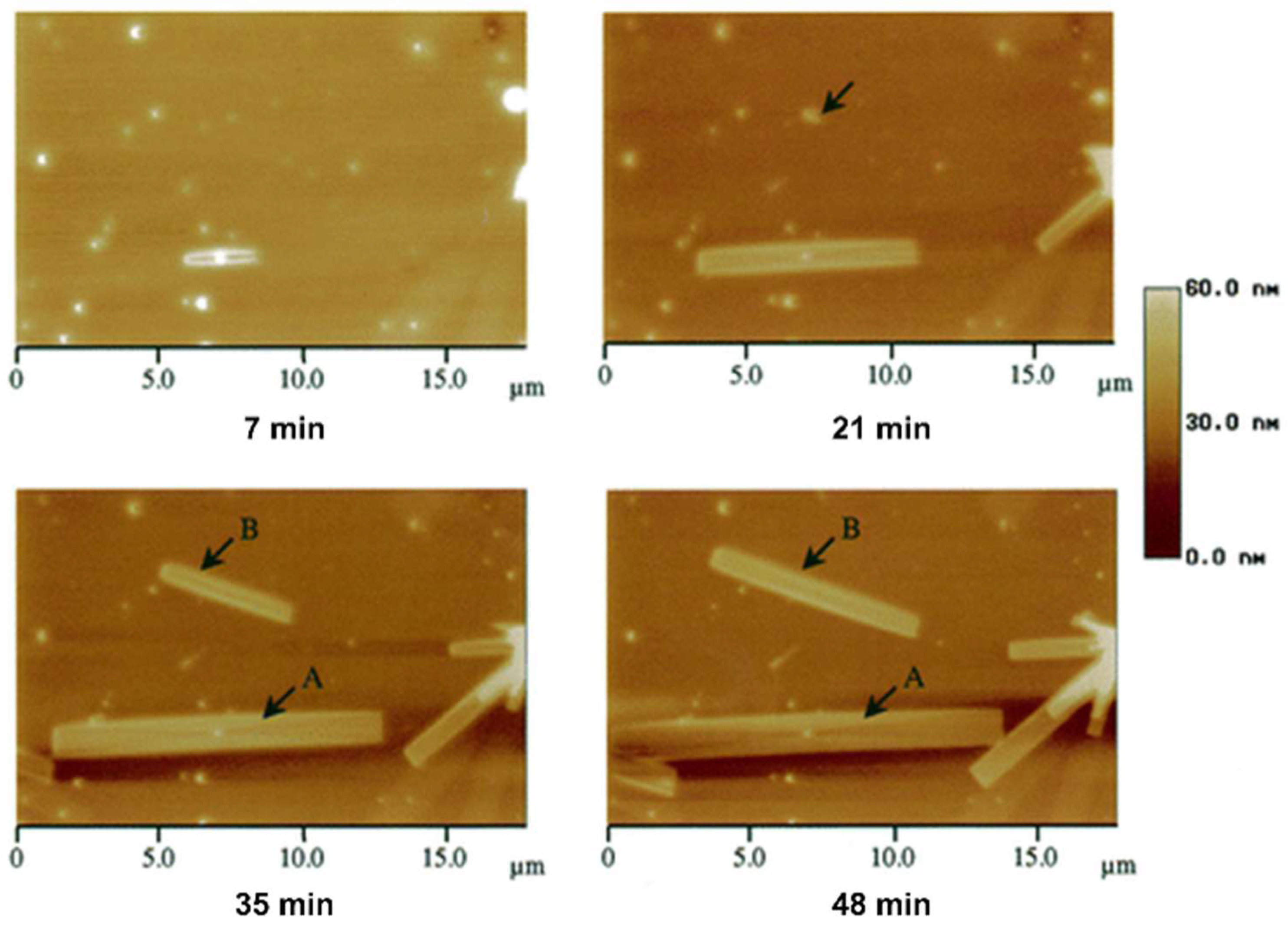
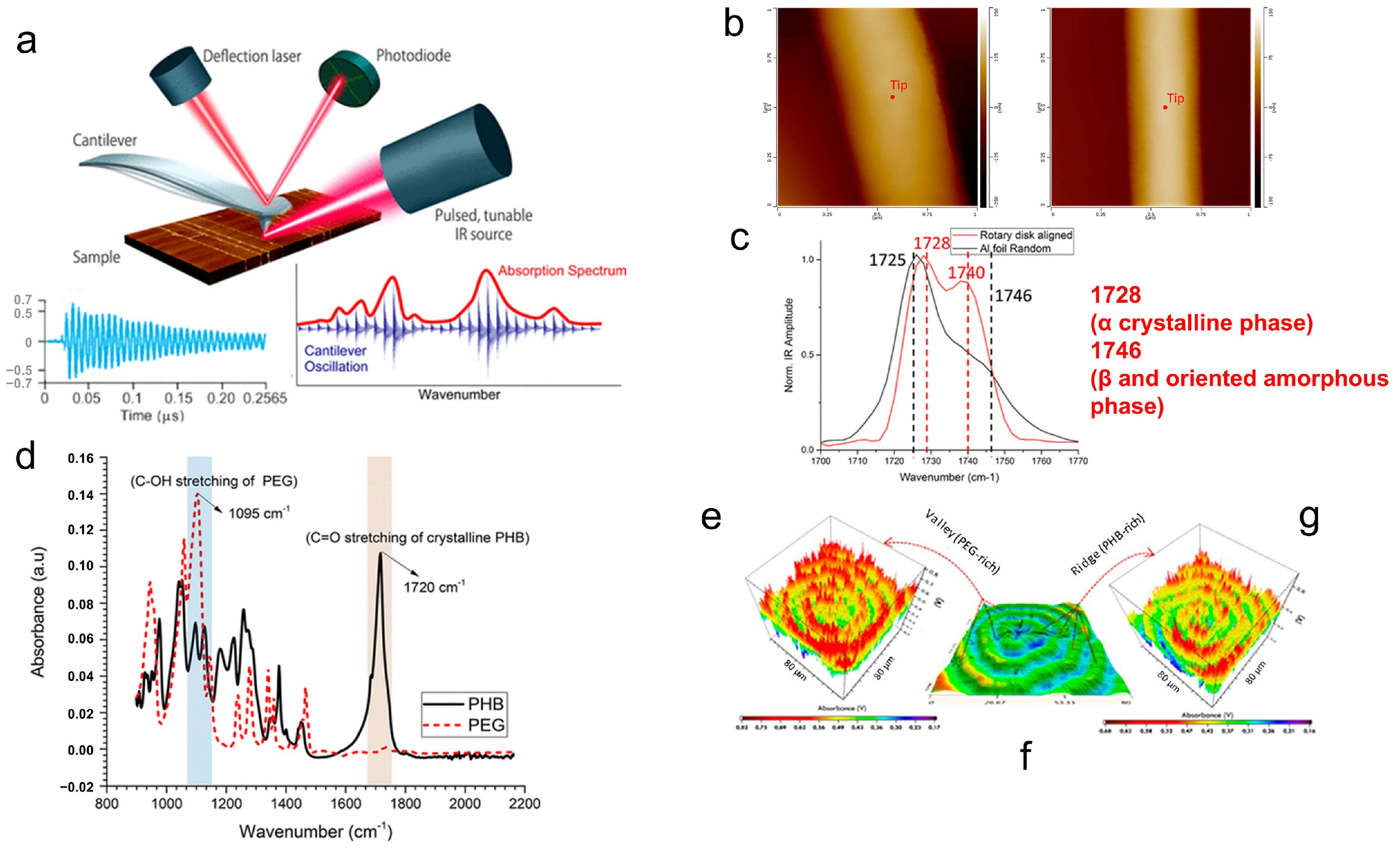
3. Characterization of Polymer Crystal Structures
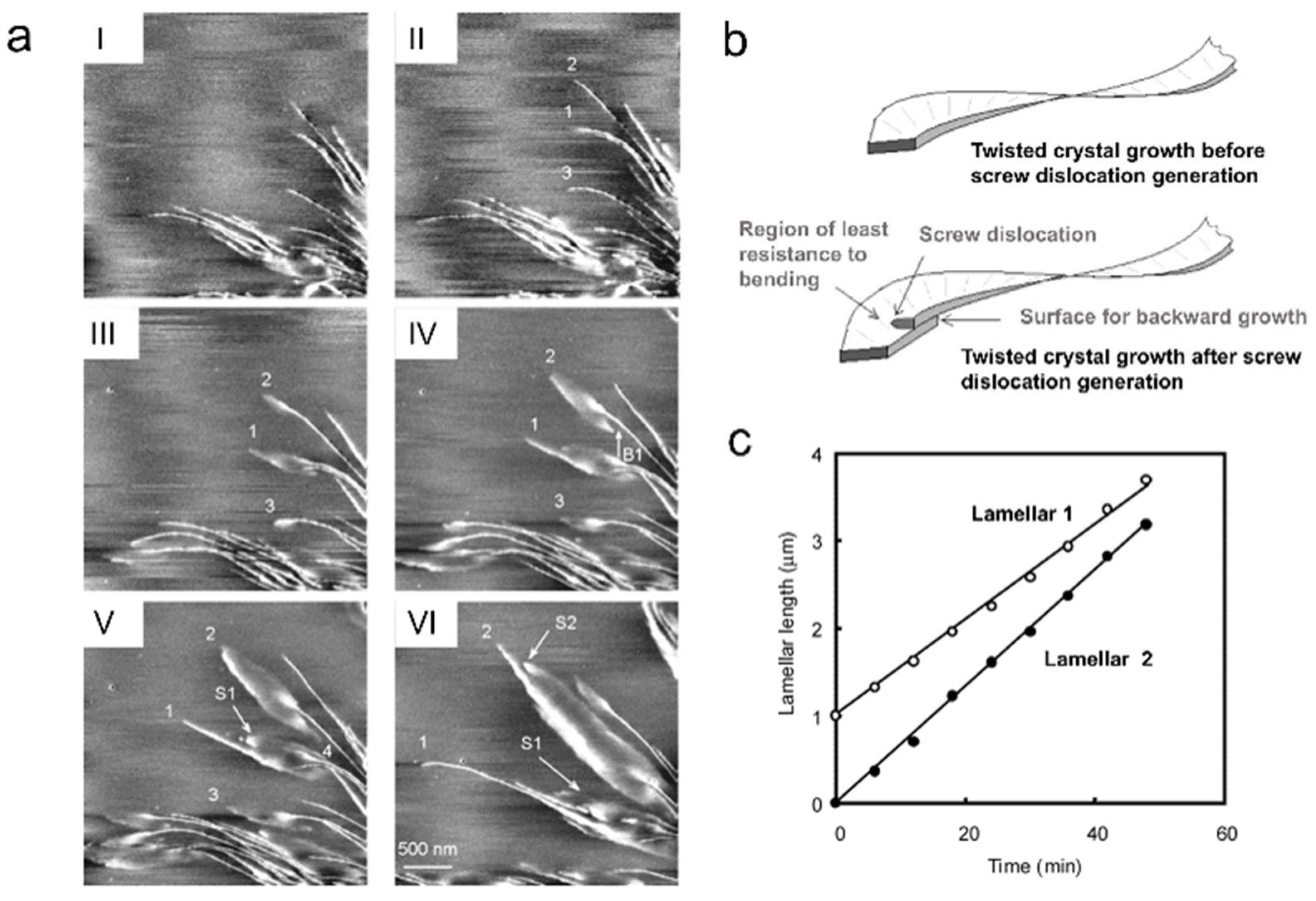
4. Characterization of Polymer Crystallization Kinetics
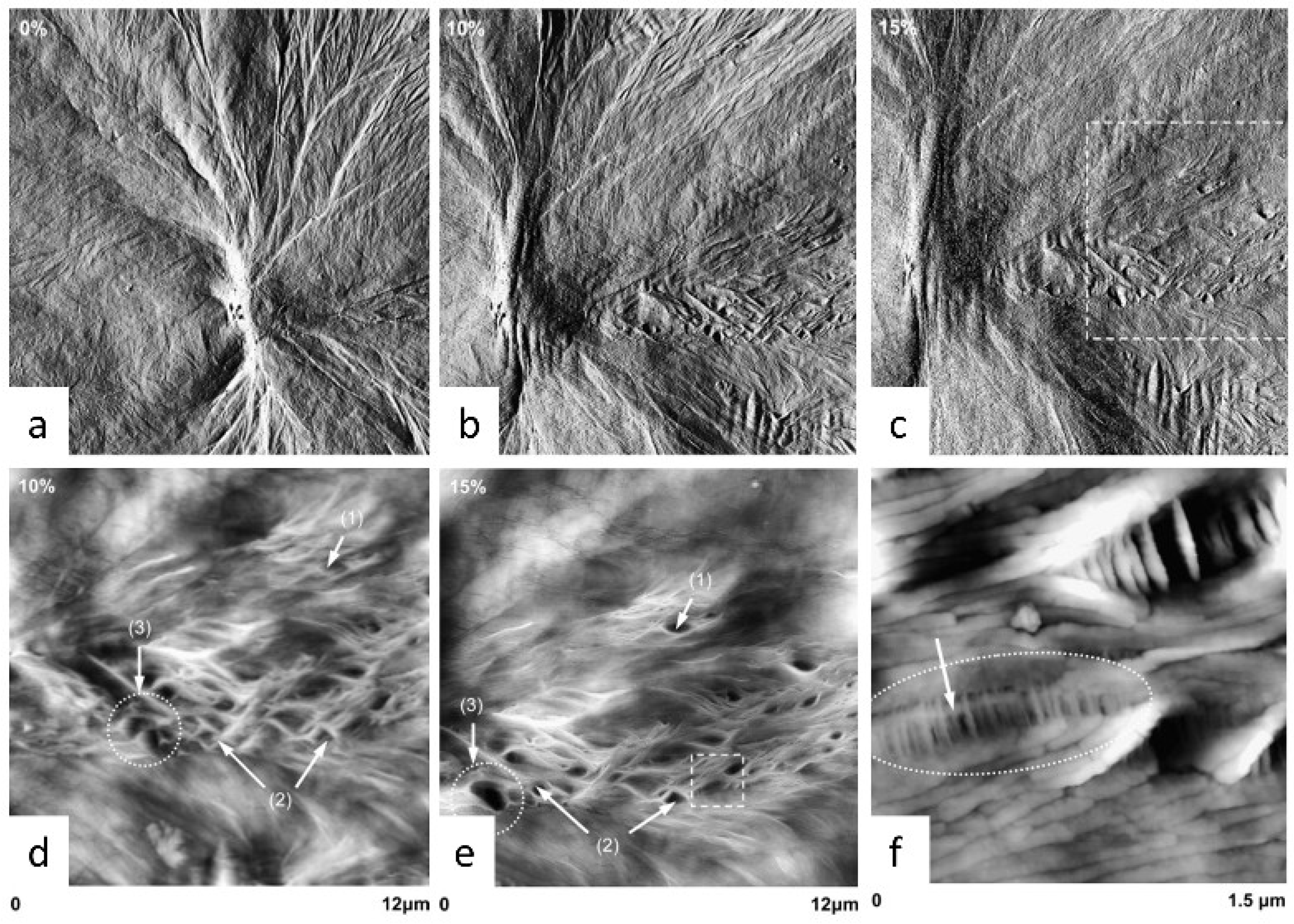
5. Characterization of the Structure–Property Relationship
6. Application of the Latest AFM Technology
7. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lotz, B.; Miyoshi, T.; Cheng, S.Z.D. 50th Anniversary Perspective: Polymer Crystals and Crystallization: Personal Journeys in a Challenging Research Field. Macromolecules 2017, 50, 5995–6025. [Google Scholar] [CrossRef]
- Hobbs, J.K.; Farrance, O.E.; Kailas, L. How Atomic Force Microscopy Has Contributed to Our Understanding of Polymer Crystallization. Polymer 2009, 50, 4281–4292. [Google Scholar] [CrossRef]
- Chen, H.-L.; Li, L.-J.; Lin, T.-L. Formation of Segregation Morphology in Crystalline/Amorphous Polymer Blends: Molecular Weight Effect. Macromolecules 1998, 31, 2255–2264. [Google Scholar] [CrossRef]
- Lynd, N.A.; Meuler, A.J.; Hillmyer, M.A. Polydispersity and Block Copolymer Self-Assembly. Prog. Polym. Sci. 2008, 33, 875–893. [Google Scholar] [CrossRef]
- Nichetti, D.; Manas-Zloczower, I. Influence of Molecular Parameters on Material Processability in Extrusion Processes. Polym. Eng. Sci. 1999, 39, 887–895. [Google Scholar] [CrossRef]
- Collis, M.W.; Mackley, M.R. The Melt Processing of Monodisperse and Polydisperse Polystyrene Melts within a Slit Entry and Exit Flow. J. Non-Newton. Fluid Mech. 2005, 128, 29–41. [Google Scholar] [CrossRef]
- Matsumoto, A. Polymer Structure Control Based on Crystal Engineering for Materials Design. Polym. J. 2003, 35, 93–121. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed]
- Pourrahimi, A.M.; Olsson, R.T.; Hedenqvist, M.S. The Role of Interfaces in Polyethylene/Metal-Oxide Nanocomposites for Ultrahigh-Voltage Insulating Materials. Adv. Mater. 2018, 30, 1703624. [Google Scholar] [CrossRef]
- Kasirajan, S.; Ngouajio, M. Polyethylene and Biodegradable Mulches for Agricultural Applications: A Review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Brown, A. X-Ray Diffraction Studies of the Stretching and Relaxing of Polyethylene. J. Appl. Phys. 1949, 20, 552–558. [Google Scholar] [CrossRef]
- Strain, H.H.; Dore, W.H. Polymerization of Dihydroxyacetone. J. Am. Chem. Soc. 1934, 56, 2649–2650. [Google Scholar] [CrossRef]
- Fielding-Russell, G.S.; Pillai, P.S. A Study of the Crystallization of Polyethylene Terephthalate Using Differential Scanning Calorimetry and X-ray Techniques. Makromol. Chem. 1970, 135, 263–274. [Google Scholar] [CrossRef]
- Gray, A.P. Polymer Crystallinity Determinations by DSC. Thermochim. Acta 1970, 1, 563–579. [Google Scholar] [CrossRef]
- Booth, A.; Hay, J.N. The Use of Differential Scanning Calorimetry to Study Polymer Crystallization Kinetics. Polymer 1969, 10, 95–104. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Ghassemi, P.; Carriere, P.; Nanda, S.; Assadi, A.A.; Nguyen, D.D. Recent Applications of Advanced Atomic Force Microscopy in Polymer Science: A Review. Polymers 2020, 12, 1142. [Google Scholar] [CrossRef]
- Kajiyama, T.; Tanaka, K.; Ge, S.-R.; Takahara, A. Morphology and Mechanical Properties of Polymer Surfaces via Scanning Force Microscopy. Prog. Surf. Sci. 1996, 52, 1–52. [Google Scholar] [CrossRef]
- Wu, X.; Shi, S.; Yu, Z.; Russell, T.P.; Wang, D. AFM Nanomechanical Mapping and Nanothermal Analysis Reveal Enhanced Crystallization at the Surface of a Semicrystalline Polymer. Polymer 2018, 146, 188–195. [Google Scholar] [CrossRef]
- Reisecker, V.; Kuhness, D.; Haberfehlner, G.; Brugger-Hatzl, M.; Winkler, R.; Weitzer, A.; Loibner, D.; Dienstleder, M.; Kothleitner, G.; Plank, H. Spectral Tuning of Plasmonic Activity in 3D Nanostructures via High-Precision Nano-Printing. Adv. Funct. Mater. 2024, 34, 2310110. [Google Scholar] [CrossRef]
- Mathurin, J.; Deniset-Besseau, A.; Bazin, D.; Dartois, E.; Wagner, M.; Dazzi, A. Photothermal AFM-IR Spectroscopy and Imaging: Status, Challenges, and Trends. J. Appl. Phys. 2022, 131, 010901. [Google Scholar] [CrossRef]
- Chang, J.; Tavakol, M.; Besnard, C.; Korsunsky, A.M.; Tan, J.-C. Biomimetic Enamel-like Crystals: A Versatile Platform for Unraveling the Basic Mechanisms of Demineralization and Remineralization. ACS Appl. Mater. Interfaces 2025, 17, 50505–50518. [Google Scholar] [CrossRef]
- Radmacher, M.; Cleveland, J.P.; Fritz, M.; Hansma, H.G.; Hansma, P.K. Mapping Interaction Forces with the Atomic Force Microscope. Biophys. J. 1994, 66, 2159–2165. [Google Scholar] [CrossRef]
- Hansma, P.K.; Cleveland, J.P.; Radmacher, M.; Walters, D.A.; Hillner, P.E.; Bezanilla, M.; Fritz, M.; Vie, D.; Hansma, H.G.; Prater, C.B.; et al. Tapping Mode Atomic Force Microscopy in Liquids. Appl. Phys. Lett. 1994, 64, 1738–1740. [Google Scholar] [CrossRef]
- Giessibl, F.J. Advances in Atomic Force Microscopy. Rev. Mod. Phys. 2003, 75, 949. [Google Scholar] [CrossRef]
- Chang, X.; Hallais, S.; Roux, S.; Danas, K. Model Reduction Techniques for Quantitative Nano-Mechanical AFM Mode. Meas. Sci. Technol. 2021, 32, 075406. [Google Scholar] [CrossRef]
- Chang, X.; Hallais, S.; Danas, K.; Roux, S. PeakForce AFM Analysis Enhanced with Model Reduction Techniques. Sensors 2023, 23, 4730. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, M.R. Non-Contact Atomic Force Microscope: Modeling and Simulation Using van Der Pol Averaging Method. Izv. Vyss. Uchebnykh Zaved. Appl. Nonlinear Dyn. 2021, 29, 345–355. [Google Scholar]
- Yamada, H.; Fukuma, T.; Umeda, K.; Kobayashi, K.; Matsushige, K. Local Structures and Electrical Properties of Organic Molecular Films Investigated by Non-Contact Atomic Force Microscopy. Appl. Surf. Sci. 2002, 188, 391–398. [Google Scholar] [CrossRef]
- McDonough, M.; Perov, P.; Johnson, W.; Radojev, S. Data Processing & Analysis for Atomic Force Microscopy (AFM). Bachelor’s Thesis, Suffolk University, Boston, MA, USA, 2020. [Google Scholar]
- Jagtap, R. Overview Literature on Atomic Force Microscopy (AFM): Basics and Its Important Applications for Polymer Characterization. Indian J. Eng. Mater. Sci. 2006, 13, 368–384. [Google Scholar]
- Magonov, S.N.; Elings, V.; Whangbo, M.-H. Phase Imaging and Stiffness in Tapping-Mode Atomic Force Microscopy. Surf. Sci. 1997, 375, L385–L391. [Google Scholar] [CrossRef]
- Maddali, H.; House, K.L.; Emge, T.J.; O’Carroll, D.M. Identification of the Local Electrical Properties of Crystalline and Amorphous Domains in Electrochemically Doped Conjugated Polymer Thin Films. RSC Adv. 2020, 10, 21454–21463. [Google Scholar] [CrossRef]
- Ando, T. High-Speed AFM Imaging. Curr. Opin. Struct. Biol. 2014, 28, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, B. Recent Trends in AFM Imaging Speed and Improvement Methods. Curr. Nanosci. 2022, 18, 277–290. [Google Scholar] [CrossRef]
- Wei, M.; Zhang, Y.; Wang, Y.; Liu, X.; Li, X.; Zheng, X. Employing Atomic Force Microscopy (AFM) for Microscale Investigation of Interfaces and Interactions in Membrane Fouling Processes: New Perspectives and Prospects. Membranes 2024, 14, 35. [Google Scholar] [CrossRef]
- Tian, M.; Dosière, M.; Hocquet, S.; Lemstra, P.J.; Loos, J. Novel Aspects Related to Nucleation and Growth of Solution Grown Polyethylene Single Crystals. Macromolecules 2004, 37, 1333–1341. [Google Scholar] [CrossRef]
- Keller, A. A Note on Single Crystals in Polymers: Evidence for a Folded Chain Configuration. Philos. Mag. 1957, 2, 1171–1175. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.-H.; Zhang, Z.-M.; Zhou, J.-J.; Jiang, Y.; Yan, S.; Li, L.; Wu, Q.; Chen, G.-Q.; Schultz, J.M. Direct AFM Observation of Crystal Twisting and Organization in Banded Spherulites of Chiral Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate). Macromolecules 2004, 37, 4118–4123. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, W.; Zhang, G.; He, B. Crystal Pattern Formation and Transitions of PEO Monolayers on Solid Substrates from Nonequilibrium to near Equilibrium. Macromolecules 2006, 39, 324–329. [Google Scholar] [CrossRef]
- Hobbs, J.K.; Humphris, A.D.L.; Miles, M.J. In-Situ Atomic Force Microscopy of Polyethylene Crystallization. 1. Crystallization from an Oriented Backbone. Macromolecules 2001, 34, 5508–5519. [Google Scholar] [CrossRef]
- Blackadder, D.A.; Schleinitz, H.M. Effect of Ultrasonic Radiation on the Crystallization of Polyethylene from Dilute Solution. Nature 1963, 200, 778–779. [Google Scholar] [CrossRef]
- McMaster, T.J.; Carr, H.J.; Miles, M.J.; Cairns, P.; Morris, V.J. Scanning Tunneling Microscopy of Poly(γ-Benzyl L-Glutamate). Macromolecules 1991, 24, 1428–1430. [Google Scholar] [CrossRef]
- Yang, R.; Naoi, K.; Evans, D.F.; Smyrl, W.H.; Hendrickson, W.A. Scanning Tunneling Microscope Study of Electropolymerized Polypyrrole with Polymeric Anion. Langmuir 1991, 7, 556–558. [Google Scholar] [CrossRef]
- Jeon, D.; Kim, J.; Gallagher, M.C.; Willis, R.F. Scanning Tunneling Spectroscopic Evidence for Granular Metallic Conductivity in Conducting Polymeric Polyaniline. Science 1992, 256, 1662–1664. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, R.H.; McGonigal, G.C.; Schneider, R.; Thomson, D.J. Mechanisms for the Deposition of Nanometer-Sized Structures from Organic Fluids Using the Scanning Tunneling Microscope. J. Vac. Sci. Technol. A Vac. Surf. Film. 1990, 8, 667–671. [Google Scholar] [CrossRef]
- Rabe, J.P.; Buchholz, S. Commensurability and Mobility in Two-Dimensional Molecular Patterns on Graphite. Science 1991, 253, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.J.; Jandt, K.D.; McMaster, T.J.; Williamson, R.L. Atomic Force Microscopy of Polymer Single Crystals and Melt-Drawn Films. Colloids Surf. A Physicochem. Eng. Asp. 1994, 87, 235–243. [Google Scholar] [CrossRef]
- Jones, A.T.; Aizlewood, J.M.; Beckett, D.R. Crystalline Forms of Isotactic Polypropylene. Makromol. Chem. 1964, 75, 134–158. [Google Scholar] [CrossRef]
- Lotz, B.; Wittmann, J.C.; Lovinger, A.J. Structure and Morphology of Poly(Propylenes): A Molecular Analysis. Polymer 1996, 37, 4979–4992. [Google Scholar] [CrossRef]
- Lotz, B. Molecular Aspects of Structure and Morphology of Isotactic Polypropylene. J. Macromol. Sci. Part B 2002, 41, 685–709. [Google Scholar] [CrossRef]
- Keith, H.D.; Padden, F.J. The Optical Behavior of Spherulites in Crystalline Polymers. Part II. The Growth and Structure of the Spherulites. J. Polym. Sci. 1959, 39, 123–138. [Google Scholar] [CrossRef]
- Lotz, B.; Wittmann, J.-C.; Stocker, W.; Magonov, S.N.; Cantow, H.-J. Atomic Force Microscopy on Epitaxially Crystallized Isotactic Polypropylene. Polym. Bull. 1991, 26, 209–214. [Google Scholar] [CrossRef]
- Meille, S.V.; Ferro, D.R.; Brueckner, S.; Lovinger, A.J.; Padden, F.J. Structure of β-Isotactic Polypropylene: A Long-Standing Structural Puzzle. Macromolecules 1994, 27, 2615–2622. [Google Scholar] [CrossRef]
- Stocker, W.; Schumacher, M.; Graff, S.; Thierry, A.; Wittmann, J.-C.; Lotz, B. Epitaxial Crystallization and AFM Investigation of a Frustrated Polymer Structure: Isotactic Poly(Propylene), β Phase. Macromolecules 1998, 31, 807–814. [Google Scholar] [CrossRef]
- Sheng, Q.; Peng, B.; Ji, C.; Li, H. Enhancing the Uniformity of Organic Field-Effect Transistors by a Single-Crystalline Layer-Controlled Active Channel. Adv. Mater. 2023, 35, 2304736. [Google Scholar] [CrossRef]
- Tian, X.; Yao, J.; Zhang, L.; Han, B.; Shi, J.; Su, J.; Liu, J.; Li, C.; Liu, X.; Zhai, T.; et al. Few-Layered Organic Single-Crystalline Heterojunctions for High-Performance Phototransistors. Nano Res. 2022, 15, 2667–2673. [Google Scholar] [CrossRef]
- Hansma, H.; Motamedi, F.; Smith, P.; Hansma, P.; Wittman, J.C. Molecular Resolution of Thin, Highly Oriented Poly (Tetrafluoroethylene) Films with the Atomic Force Microscope. Polymer 1992, 33, 647–649. [Google Scholar] [CrossRef]
- Mullin, N.; Hobbs, J.K. Direct Imaging of Polyethylene Films at Single-Chain Resolution with Torsional Tapping Atomic Force Microscopy. Phys. Rev. Lett. 2011, 107, 197801. [Google Scholar] [CrossRef] [PubMed]
- Snetivy, D.; Vancso, G.J.; Rutledge, G.C. Atomic Force Microscopy of Polymer Crystals. 6. Molecular Imaging and Study of Polymorphism in Poly(p-Phenyleneterephthalamide) Fibers. Macromolecules 1992, 25, 7037–7042. [Google Scholar] [CrossRef]
- Snétivy, D.; Yang, H.; Glomm, B.; Julius Vancso, G. Short-Range Order in Extended-Chain Crystals of Polyoxymethylene from a True Molecular Perspective: An Atomic Force Microscopy Study. J. Mater. Chem. 1994, 4, 55–59. [Google Scholar] [CrossRef]
- Snétivy, D.; Vancso, G.J. AFM Studies of Macromolecules in Extended-Chain Crystals of Polymers. Colloids Surf. A Physicochem. Eng. Asp. 1994, 87, 257–262. [Google Scholar] [CrossRef]
- Stocker, W.; Bar, G.; Kunz, M.; Möller, M.; Magonov, S.N.; Cantow, H.-J. Atomic Force Microscopy on Polymers and Polymer Related Compounds. Polym. Bull. 1991, 26, 215–222. [Google Scholar] [CrossRef]
- Davé, R.S.; Farmer, B.L. Energetics of Chain Folding in Polyethylene Crystals. Polymer 1988, 29, 1544–1554. [Google Scholar] [CrossRef]
- Passaglia, E.; Khoury, F. Crystal Growth Kinetics and the Lateral Habits of Polyethylene Crystals. Polymer 1984, 25, 631–644. [Google Scholar] [CrossRef]
- Toda, A. Growth Mode and Curved Lateral Habits of Polyethylene Single Crystals. Faraday Discuss. 1993, 95, 129–143. [Google Scholar] [CrossRef]
- Wittmann, J.C.; Lotz, B. Polymer Decoration: The Orientation of Polymer Folds as Revealed by the Crystallization of Polymer Vapors. J. Polym. Sci. Polym. Phys. Ed. 1985, 23, 205–226. [Google Scholar] [CrossRef]
- Hamidinejad, M.; Arif, T.; Wang, G.; Rezaei, S.; Serles, P.; Taylor, H.K.; Park, C.B.; Filleter, T. Sectorization of Macromolecular Single Crystals Unveiled by Probing Shear Anisotropy. ACS Macro Lett. 2022, 11, 53–59. [Google Scholar] [CrossRef]
- Kumaki, J. Observation of Polymer Chain Structures in Two-Dimensional Films by Atomic Force Microscopy. Polym. J. 2016, 48, 3–14. [Google Scholar] [CrossRef]
- Kumaki, J.; Kawauchi, T.; Yashima, E. Two-Dimensional Folded Chain Crystals of a Synthetic Polymer in a Langmuir−Blodgett Film. J. Am. Chem. Soc. 2005, 127, 5788–5789. [Google Scholar] [CrossRef]
- Mullin, N.; Vasilev, C.; Tucker, J.D.; Hunter, C.N.; Weber, C.H.M.; Hobbs, J.K. “Torsional Tapping” Atomic Force Microscopy Using T-Shaped Cantilevers. Appl. Phys. Lett. 2009, 94, 173109. [Google Scholar] [CrossRef]
- Huang, L.; Su, C. A Torsional Resonance Mode AFM for In-Plane Tip Surface Interactions. Ultramicroscopy 2004, 100, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Mullin, N.; Hobbs, J. Torsional Resonance Atomic Force Microscopy in Water. Appl. Phys. Lett. 2008, 92, 053103. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, S.Z.D.; Putthanarat, S.; Eby, R.K.; Reneker, D.H.; Lotz, B.; Magonov, S.; Hsieh, E.T.; Geerts, R.G.; Palackal, S.J.; et al. Crystallization, Melting and Morphology of Syndiotactic Polypropylene Fractions. 4. In Situ Lamellar Single Crystal Growth and Melting in Different Sectors. Macromolecules 2000, 33, 6861–6868. [Google Scholar] [CrossRef]
- Point, J.J.; Colet, M.C.; Dosiere, M. Experimental Criterion for the Crystallization Regime in Polymer Crystals Grown from Dilute Solution: Possible Limitation Due to Fractionation. J. Polym. Sci. Part B Polym. Phys. 1986, 24, 357–388. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, X.; He, Y.; Guo, B.; Xu, J. In Situ Monitoring and Tuning Multilayer Stacking of Polymer Lamellar Crystals in Solution with Aggregation-Induced Emission. Nano Lett. 2024, 24, 4717–4724. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, J.K.; McMaster, T.J.; Miles, M.J.; Barham, P.J. Direct Observations of the Growth of Spherulites of Poly(Hydroxybutyrate-Co-Valerate) Using Atomic Force Microscopy. Polymer 1998, 39, 2437–2446. [Google Scholar] [CrossRef]
- Li, L.; Chan, C.-M.; Yeung, K.L.; Li, J.-X.; Ng, K.-M.; Lei, Y. Direct Observation of Growth of Lamellae and Spherulites of a Semicrystalline Polymer by AFM. Macromolecules 2001, 34, 316–325. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Goswami, D.K.; Okasinski, J.S.; Salaita, K.; Sun, P.; Bedzyk, M.J.; Mirkin, C.A. The Controlled Evolution of a Polymer Single Crystal. Science 2005, 307, 1763–1766. [Google Scholar] [CrossRef]
- Humphris, A.D.L.; Miles, M.J.; Hobbs, J.K. A Mechanical Microscope: High-Speed Atomic Force Microscopy. Appl. Phys. Lett. 2005, 86, 034106. [Google Scholar] [CrossRef]
- Howard-Knight, J.P.; Hobbs, J.K. Video Rate Atomic Force Microscopy Using Low Stiffness, Low Resonant Frequency Cantilevers. Appl. Phys. Lett. 2008, 93, 104101. [Google Scholar] [CrossRef]
- Hobbs, J.K.; Vasilev, C.; Humphris, A.D.L. Real Time Observation of Crystallization in Polyethylene Oxide with Video Rate Atomic Force Microscopy. Polymer 2005, 46, 10226–10236. [Google Scholar] [CrossRef]
- Statton, W.O.; Geil, P.H. Recrystallization of Polyethylene during Annealing. J. Appl. Polym. Sci. 1960, 3, 357–361. [Google Scholar] [CrossRef]
- Organ, S.J.; Hobbs, J.K.; Miles, M.J. Reorganization and Melting of Polyethylene Single Crystals: Complementary TEM, DSC, and Real-Time AFM Studies. Macromolecules 2004, 37, 4562–4572. [Google Scholar] [CrossRef]
- Roe, R.-J.; Bair, H.E. Thermodynamic Study of Fold Surfaces of Polyethylene Single Crystals. Macromolecules 1970, 3, 454–458. [Google Scholar] [CrossRef]
- Hocquet, S.; Dosière, M.; Thierry, A.; Lotz, B.; Koch, M.H.J.; Dubreuil, N.; Ivanov, D.A. Morphology and Melting of Truncated Single Crystals of Linear Polyethylene. Macromolecules 2003, 36, 8376–8384. [Google Scholar] [CrossRef]
- Magonov, S.N.; Yerina, N.A.; Ungar, G.; Reneker, D.H.; Ivanov, D.A. Chain Unfolding in Single Crystals of Ultralong Alkane C390H782 and Polyethylene: An Atomic Force Microscopy Study. Macromolecules 2003, 36, 5637–5649. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, J.; Baier, M.C.; Mecking, S.; Reiter, R.; Mülhaupt, R.; Reiter, G. Molecular-Weight-Dependent Changes in Morphology of Solution-Grown Polyethylene Single Crystals. Macromol. Rapid Commun. 2015, 36, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Organ, S.J.; Keller, A. Solution Crystallization of Polyethylene at High Temperatures. J. Mater. Sci. 1985, 20, 1586–1601. [Google Scholar] [CrossRef]
- Nakamura, J.; Kawaguchi, A. In Situ Observations of Annealing Behavior of Polyethylene Single Crystals on Various Substrates by AFM. Macromolecules 2004, 37, 3725–3734. [Google Scholar] [CrossRef]
- Thomas, C.; Ferreiro, V.; Coulon, G.; Seguela, R. In Situ AFM Investigation of Crazing in Polybutene Spherulites under Tensile Drawing. Polymer 2007, 48, 6041–6048. [Google Scholar] [CrossRef]
- Plummer, C.J.G.; Kausch, H.-H. Deformation and Entanglement in Semicrystalline Polymers. J. Macromol. Sci. Part B 1996, 35, 637–657. [Google Scholar] [CrossRef]
- Thomas, C.; Seguela, R.; Detrez, F.; Miri, V.; Vanmansart, C. Plastic Deformation of Spherulitic Semi-Crystalline Polymers: An in Situ AFM Study of Polybutene under Tensile Drawing. Polymer 2009, 50, 3714–3723. [Google Scholar] [CrossRef]
- Gregorio, R.; Borges, D.S. Effect of Crystallization Rate on the Formation of the Polymorphs of Solution Cast Poly(Vinylidene Fluoride). Polymer 2008, 49, 4009–4016. [Google Scholar] [CrossRef]
- Sencadas, V.; Branciforti, M.C.; Gregorio, R., Jr.; Lanceros-Méndez, S. Molecular Orientation and Degree of Crystallinity of Piezoelectric Poly(Vinylidene Fluoride) Films Exclusively in the β Phase. Ferroelectrics 2008, 370, 29–35. [Google Scholar] [CrossRef]
- Su, Y.P.; Sim, L.N.; Li, X.; Coster, H.G.L.; Chong, T.H. Anti-Fouling Piezoelectric PVDF Membrane: Effect of Morphology on Dielectric and Piezoelectric Properties. J. Membr. Sci. 2021, 620, 118818. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Zeng, F. Ferroelectric Performance, Surface and Mechanical Properties of Poled PVDF Sheet with Metal Coating: Assessing through PFM, FTIR, and Nanoindentation and Nanoscratch. Ferroelectrics 2017, 515, 134–142. [Google Scholar] [CrossRef]
- Fortunato, M.; Bidsorkhi, H.C.; De Bellis, G.; Sarto, F.; Sarto, M.S. Piezoelectric Response of Graphene-Filled PVDF Nanocomposites through Piezoresponse Force Microscopy (PFM). In Proceedings of the 2017 IEEE 17th International Conference on Nanotechnology (IEEE-NANO), Pittsburgh, PA, USA, 25–28 July 2017; pp. 125–129. [Google Scholar]
- Wu, L.; Jin, Z.; Liu, Y.; Ning, H.; Liu, X.; Alamusi; Hu, N. Recent Advances in the Preparation of PVDF-Based Piezoelectric Materials. Nanotechnol. Rev. 2022, 11, 1386–1407. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Zhu, Z.; Rui, G.; Du, B.; Chen, D.; Huang, Y.-F.; Zhu, L. Recent Progress on Structure Manipulation of Poly(Vinylidene Fluoride)-Based Ferroelectric Polymers for Enhanced Piezoelectricity and Applications. Adv. Funct. Mater. 2023, 33, 2301302. [Google Scholar] [CrossRef]
- Bolsée, J.-C.; Oosterbaan, W.D.; Lutsen, L.; Vanderzande, D.; Manca, J. CAFM on Conjugated Polymer Nanofibers: Capable of Assessing One Fiber Mobility. Org. Electron. 2011, 12, 2084–2089. [Google Scholar] [CrossRef]
- Lee, J.S.; Prabu, A.A.; Kim, K.J. Annealing Effect upon Chain Orientation, Crystalline Morphology, and Polarizability of Ultra-Thin P(VDF-TrFE) Film for Nonvolatile Polymer Memory Device. Polymer 2010, 51, 6319–6333. [Google Scholar] [CrossRef]
- Liscio, A.; Palermo, V.; Samorì, P. Probing Local Surface Potential of Quasi-One-Dimensional Systems: A KPFM Study of P3HT Nanofibers. Adv. Funct. Mater. 2008, 18, 907–914. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, W. Feeling Inter- or Intramolecular Interactions with the Polymer Chain as Probe: Recent Progress in SMFS Studies on Macromolecular Interactions. ChemPhysChem 2012, 13, 2238–2256. [Google Scholar] [CrossRef]
- Hughes, M.L.; Dougan, L. The Physics of Pulling Polyproteins: A Review of Single Molecule Force Spectroscopy Using the AFM to Study Protein Unfolding. Rep. Prog. Phys. 2016, 79, 076601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, P.; Lu, S.; Deng, Y.; Ma, Z.; Song, Y.; Zhang, W. Dynamic Distribution of αc-Relaxation in Polyethylene Single Crystals Investigated by Single-Molecule Force Spectroscopy. Macromolecules 2023, 56, 4621–4628. [Google Scholar] [CrossRef]
- Liu, K.; Song, Y.; Feng, W.; Liu, N.; Zhang, W.; Zhang, X. Extracting a Single Polyethylene Oxide Chain from a Single Crystal by a Combination of Atomic Force Microscopy Imaging and Single-Molecule Force Spectroscopy: Toward the Investigation of Molecular Interactions in Their Condensed States. J. Am. Chem. Soc. 2011, 133, 3226–3229. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Feng, W.; Zhang, W. Investigation on the Folding Mode of a Polymer Chain in a Spiral Crystal by Single Molecule Force Spectroscopy. Chin. J. Polym. Sci. 2014, 32, 1149–1157. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, P.; Zhang, X.; Jiang, K.; Song, Y.; Zhang, W. Quantifying the Chain Folding in Polymer Single Crystals by Single-Molecule Force Spectroscopy. ACS Macro Lett. 2019, 8, 1194–1199. [Google Scholar] [CrossRef]
- Oesterhelt, F.; Rief, M.; Gaub, H.E. Single Molecule Force Spectroscopy by AFM Indicates Helical Structure of Poly(Ethylene-Glycol) in Water. New J. Phys. 1999, 1, 6. [Google Scholar] [CrossRef]
- Song, Y.; Ma, Z.; Yang, P.; Zhang, X.; Lyu, X.; Jiang, K.; Zhang, W. Single-Molecule Force Spectroscopy Study on Force-Induced Melting in Polymer Single Crystals: The Chain Conformation Matters. Macromolecules 2019, 52, 1327–1333. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Lu, S.; Song, Y.; Zhang, W. Direct Detection of the Chain Trajectory and Long-Range Correlation in Crystalline–Amorphous Polymer Networks by Single-Molecule Force Spectroscopy. Macromolecules 2025, 58, 6170–6177. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, W. Hybrid AFM for Nanoscale Physicochemical Characterization: Recent Development and Emerging Applications. Small 2017, 13, 1603525. [Google Scholar] [CrossRef]
- Lasch, P.; Naumann, D. Spatial Resolution in Infrared Microspectroscopic Imaging of Tissues. Biochim. Biophys. Acta (BBA) Biomembr. 2006, 1758, 814–829. [Google Scholar] [CrossRef]
- Dazzi, A.; Prazeres, R.; Glotin, F.; Ortega, J.M. Local Infrared Microspectroscopy with Subwavelength Spatial Resolution with an Atomic Force Microscope Tip Used as a Photothermal Sensor. Opt. Lett. 2005, 30, 2388–2390. [Google Scholar] [CrossRef] [PubMed]
- Dazzi, A.; Prater, C.B. AFM-IR: Technology and Applications in Nanoscale Infrared Spectroscopy and Chemical Imaging. Chem. Rev. 2017, 117, 5146–5173. [Google Scholar] [CrossRef]
- Gong, L.; Chase, D.B.; Noda, I.; Liu, J.; Martin, D.C.; Ni, C.; Rabolt, J.F. Discovery of β-Form Crystal Structure in Electrospun Poly[(R)-3-Hydroxybutyrate-Co-(R)-3-Hydroxyhexanoate] (PHBHx) Nanofibers: From Fiber Mats to Single Fibers. Macromolecules 2015, 48, 6197–6205. [Google Scholar] [CrossRef]
- Tri, P.N.; Prud’homme, R.E. Nanoscale Lamellar Assembly and Segregation Mechanism of Poly(3-Hydroxybutyrate)/Poly(Ethylene Glycol) Blends. Macromolecules 2018, 51, 181–188. [Google Scholar] [CrossRef]
- Mikhalchan, A.; Banas, A.M.; Banas, K.; Borkowska, A.M.; Nowakowski, M.; Breese, M.B.H.; Kwiatek, W.M.; Paluszkiewicz, C.; Tay, T.E. Revealing Chemical Heterogeneity of CNT Fiber Nanocomposites via Nanoscale Chemical Imaging. Chem. Mater. 2018, 30, 1856–1864. [Google Scholar] [CrossRef]
- Purohit, H.S.; Taylor, L.S. Miscibility of Itraconazole–Hydroxypropyl Methylcellulose Blends: Insights with High Resolution Analytical Methodologies. Mol. Pharm. 2015, 12, 4542–4553. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.-T.; Xu, F.; Yang, J.; Liu, J.; Cai, W.; Zhu, G. Resistive Switching and Nanoscale Chemical Mapping of Phase Separation in PVDF/PMMA/F8T2 Ternary Thin Films. Polymer 2018, 153, 498–506. [Google Scholar] [CrossRef]
- Frogley, M.D.; Lekkas, I.; Kelley, C.S.; Cinque, G. Performances for Broadband Synchrotron Photothermal Infrared Nano-Spectroscopy at Diamond Light Source. Infrared Phys. Technol. 2020, 105, 103238. [Google Scholar] [CrossRef]
- Anderson, N.; Hartschuh, A.; Novotny, L. Near-Field Raman Microscopy. Mater. Today 2005, 8, 50–54. [Google Scholar] [CrossRef]
- Stöckle, R.M.; Suh, Y.D.; Deckert, V.; Zenobi, R. Nanoscale Chemical Analysis by Tip-Enhanced Raman Spectroscopy. Chem. Phys. Lett. 2000, 318, 131–136. [Google Scholar] [CrossRef]



| Characterization Methods | Sample Requirements | Crystallographic Information | Advantages | Limitations |
|---|---|---|---|---|
| X-Ray Diffraction (XRD) | Available in bulk, powder, and film | Crystallinity, crystal form, orientation, etc. | Non-destructive, highly automated, high sensitivity | Low spatial resolution |
| Differential Scanning Calorimetry (DSC) | Available in bulk, powder, and film | Crystallinity, crystallization (melting), enthalpy, etc. | Highly automated, capable of quantitative analysis | Low spatial resolution, destructive |
| Optical Microscopy (OM) | Morphology | Simple operation, wide range of applications, non-destructive | Resolution limited to the micrometer level | |
| Scanning Electron Microscopy (SEM) | Must be conductive (non-conductive samples require gold or carbon spraying to enhance conductivity) | Morphology | High spatial resolution (around 1 nm), large depth of field, minimal electron damage | Vacuum test environment, low resolution in the vertical direction |
| Transmission Electron Microscopy (TEM) | Small (less than 2 mm in width) and thin (less than 500 nm in thickness), generally requires special processing (deposited on copper mesh, ultrathin sectioning, or surface replica) | Morphology, crystal structure | Extremely high spatial resolution (better than 0.2 nm) | Vacuum test environment, high electron damage, difficulties in sample preparation |
| Atomic Force Microscopy (AFM) | Surface roughness less than 1 μm | Morphology, physical properties (modulus, electrical conductivity, piezoelectricity, etc.) | Almost non-destructive, high spatial resolution (vertical: better than 0.1 nm; horizontal: depends on the probe diameter, typically around 1 nm), can be tested in gas, liquid, and vacuum environments | Slow scanning speed, size limitation, operational experience required |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Chen, M.; Li, H. Recent Progress on the Characterization of Polymer Crystallization by Atomic Force Microscopy. Polymers 2025, 17, 2692. https://doi.org/10.3390/polym17192692
Chen S, Chen M, Li H. Recent Progress on the Characterization of Polymer Crystallization by Atomic Force Microscopy. Polymers. 2025; 17(19):2692. https://doi.org/10.3390/polym17192692
Chicago/Turabian StyleChen, Shen, Min Chen, and Hanying Li. 2025. "Recent Progress on the Characterization of Polymer Crystallization by Atomic Force Microscopy" Polymers 17, no. 19: 2692. https://doi.org/10.3390/polym17192692
APA StyleChen, S., Chen, M., & Li, H. (2025). Recent Progress on the Characterization of Polymer Crystallization by Atomic Force Microscopy. Polymers, 17(19), 2692. https://doi.org/10.3390/polym17192692






