Diffusion Behavior of Polyethylene Furanoate (PEF) and Tritan as Sustainable Polyester Packaging Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Film Samples
- 14 µm biaxially oriented PEF film (provided by Avantium, Amsterdam, The Netherlands).
- 51 µm TritanTM film (provided by Eastman, Kingsport TN, USA).
2.2. Determination of Diffusion Coefficients
2.3. Diffusion Modelling
3. Results
4. Discussion
4.1. Diffusion Modelling Parameters
4.2. Literature Data for the Migration from PEF and Tritan
4.3. Prediction of Maximum Concentrations of Oligomers and Other NIAS
- Storage for 365 days at 25 °C (long-term storage conditions, e.g., for mineral water bottles as applied by EFSA for the evaluation of recyclate containing PET bottles [19]).
- Storage for 10 days at 60 °C as official testing conditions for long-term storage at room temperature according to Regulation 10/2011 [10].
- Heating to 70 °C for 2 h to simulate hot fill conditions.
- Heating to 100 °C for 30 min to simulate microwave treatment.
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Cort, S.; Godts, F.; Moreau, A. Packaging Materials: 1. Polyethylene Terephthalate for Food Packaging Applications; ILSI Report Series; ILSI Europe: Brussels, Belgium, 2017; ISBN 9789078637431. Available online: https://ilsi.eu/publication/packaging-materials-1-polyethylene-terephthalate-pet-for-food-packaging-applications-updated-version (accessed on 20 June 2025).
- The European Commission. Regulation No 2022/1616 of 15 September 2022 on recycled plastic materials and articles intended to come into contact with foods, and repealing Regulation (EC) No 282/2008. Off. J. Eur. Union 2022, L243/3–L243/46. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022R1616 (accessed on 20 June 2025).
- De Jong, E.; Visser, H.A.; Sousa Dias, A.; Harvey, C.; Gruter, G.-J.M. The road to bring FDCA and PEF to the market. Polymers 2022, 14, 943. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.; Gruter, G.-J.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5963–6098. [Google Scholar] [CrossRef]
- Gandini, A.; Silvestre, A.J.D.; Pascoal Neto, C.; Sousa, A.F.; Gomes, M. The furan counterpart of poly (ethylene terephthalate): An alternative material based on renewable resources. J. Polym. Sci. Part A Polym. Chem. 2009, 5, 295–298. [Google Scholar] [CrossRef]
- Burgess, S.K.; Kriegel, R.M.; Koros, W.J. Carbon dioxide sorption and transport in amorphous poly(ethylene furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Muburak, C.R.; Kriegel, R.M.; Koros, W.J. Chain mobility, thermal and mechanical properties of poly(ethylene furanoate) compared to poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- The European Commission. Regulation No. 2025/40 of the European Parliament and of the Council of 19 December 2024 on Packaging and Packaging Waste, Amending Regulation (EU) 2019/1020 and Directive (EU) 2019/904, and Repealing Directive 94/62/EC. Official Journal of the European Commission 2025. Available online: https://eur-lex.europa.eu/legal-content/ET/TXT/PDF/?uri=OJ:L_202500040 (accessed on 20 June 2025).
- The European Commission. Regulation No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC. Off. J. Eur. Union 2004, L338/4. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:338:0004:0017:en:PDF (accessed on 20 June 2025).
- The European Commission. Regulation No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come Into Contact with Food. Off. J. Eur. Union 2011, L12/1–L12/89. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R0010&from=EN (accessed on 20 June 2025).
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on the safety assessment of the substance, furan-2,5-dicarboxylic acid, CAS No 3238-40-2, for use in food contact materials. EFSA J. 2014, 12, 3866. [Google Scholar] [CrossRef]
- EFSA Scientific Committee. Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment. EFSA J. 2019, 17, 5708. [Google Scholar] [CrossRef]
- Kroes, R.; Kleiner, J.; Renwick, A. The threshold of toxicological concern concept in risk assessment. Toxicol. Sci. 2005, 86, 226–230. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on the safety assessment of the substance, 2,2,4,4-tetramethylcyclobutane-1,3-diol (TMCD), CAS No 3010-96-6, for use in food contact materials. EFSA J. 2013, 11, 3388. [Google Scholar] [CrossRef]
- Eckardt, M.; Schneider, J.; Simat, T.J. In vitro intestinal digestibility of cyclic aromatic polyester oligomers from polyethylene terephthalate (PET) and polybutylene terephthalate (PBT). Food Addit. Contam. Part A 2019, 12, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Schreier, V.N.; Odermatt, A.; Welle, F. Modeling the migration of polyethylene terephthalate oligomers for exposure risk assessment. Molecules 2023, 28, 173. [Google Scholar] [CrossRef]
- Juric, M.; Franz, R.; Welle, F. Determination of diffusion coefficient of bisphenol A (BPA) in polyethylene terephthalate (PET) to estimate migration of BPA from recycled PET into foods. Appl. Siences 2024, 14, 7704. [Google Scholar] [CrossRef]
- Barthelemy, E.; Spyropoulos, D.; Milana, M.-R.; Pfaff, K.; Gontard, N.; Lampi, E.; Castle, L. Safety evaluation of mechanical recycling processes used to produce polyethylene terephthalate (PET) intended for food contact applications. Food Addit. Contam. Part A 2014, 31, 490–497. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids. Scientific Guidance on the criteria for the evaluation and on the preparation of applications for the safety assessment of post-consumer mechanical PET recycling processes intended to be used for manufacture of materials and articles in contact with food. EFSA J. 2024, 22, e8879. [Google Scholar] [CrossRef]
- European Union. Practical Guidelines on the Application of Migration Modelling for the Estimation of Specific Migration; EU Report 27529 EN; Publications Office of the European Union: Luxembourg, 2015; ISBN 978-92-79-52790-6. Available online: https://op.europa.eu/de/publication-detail/-/publication/1b79bc61-97f6-11e5-983e-01aa75ed71a1 (accessed on 16 March 2024).
- Begley, T.; Castle, L.; Feigenbaum, A.; Franz, R.; Hinrichs, K.; Lickly, T.; Mercea, P.; Milana, M.; O’Brian, A.; Rebre, S.; et al. Evaluation of migration models that might be used in support of regulations for food-contact plastics. Food Addit. Contam. 2005, 22, 73–90. [Google Scholar] [CrossRef]
- Welle, F. A new method for the prediction of diffusion coefficients in poly(ethylene terephthalate). J. Appl. Polym. Sci. 2013, 129, 1845–1851. [Google Scholar] [CrossRef]
- Michaels, A.S.; Vieth, W.R.; Barrie, J.A. Diffusion of gases in polyethylene terephthalate. J. Appl. Phys. 1963, 34, 13–20. [Google Scholar] [CrossRef]
- Roduit, B.; Borgeat, C.H.; Cavin, S.; Fragniere, C.; Dudler, V. Application of finite element analysis (FEA) for the simulation of release of additives from multilayer polymeric packaging structures. Food Addit. Contam. Part A 2005, 22, 945–955. [Google Scholar] [CrossRef]
- Ewender, J.; Welle, F. Diffusion coefficients of n-alkanes and 1-alcohols in polyethylene naphthalate (PEN). Int. J. Polym. Sci. 2019, 2019, 2748649. [Google Scholar] [CrossRef]
- Molinspiration. Available online: https://www.molinspiration.com/ (accessed on 20 June 2025).
- Zheng, J.-M.; Qui, J.; Madeira, L.M.; Mendes, A. Polymer structure and compensation effect of the diffusion pre-exponential factor and activation energy of a permeating solute. J. Phys. Chem. B 2007, 111, 2828–2835. [Google Scholar] [CrossRef]
- Overington, A.R.; Wong, M.; Harrison, J.A.; Ferreira, L.B. Estimation of mass transfer rates through hydrophobic pervaporation membranes. Sep. Sci. Technol. 2009, 44, 787–816. [Google Scholar] [CrossRef]
- Hoppe, M.; De Voogt, P.; Franz, R. Oligomers in polyethylene furanoate—Identification and quantification approach via LC-UV LC-MS response ratio. Food Addit. Contam. Part A 2018, 35, 2244–2255. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.E.; Kendig, E.L.; Belcher, S.M. Assessment of bisphenol A released from reusable plastic, aluminium and stainless steel water bottles. Chemosphere 2011, 85, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Simoneau, C.; Van den Eede, L.; Valzacchi, S. Identification and quantification of the migration of chemicals from plastic baby bottles used as substitutes for polycarbonate. Food Addit. Contam. Part A 2012, 29, 469–480. [Google Scholar] [CrossRef]
- Guart, A.; Wagner, M.; Mezquida, A.; Lacorte, S.; Oehlmann, J.; Borrell, A. Migration of plasticisers from Tritan and polycarbonate bottles and toxicological evaluation. Food Chem. 2013, 141, 373–380. [Google Scholar] [CrossRef]
- Onghena, M.; van Hoeck, E.; Vervliet, P.; Scippo, M.L.; Simon, C.; van Loco, J.; Covaci, A. Development and application of a non-targeted extraction method for the analysis of mi-grating compounds from plastic baby bottles by GCMS. Food Addit. Contam. Part A 2014, 31, 2090–2102. [Google Scholar] [CrossRef]
- Kubicova, M.; Eckardt, M.; Simat, T.J. Migration of oligomers from TritanTM copolyester: Application of hydrolysis for overall oligomer determination. Food Addit. Contam. Part A 2023, 40, 1074–1095. [Google Scholar] [CrossRef]
- Brenz, F.; Linke, S.; Simat, T.J. Linear and cyclic oligomers in PET, glycol-modified PET and TritanTM used for food contact materials. Food Addit. Contam. Part A 2021, 38, 160–179. [Google Scholar] [CrossRef]
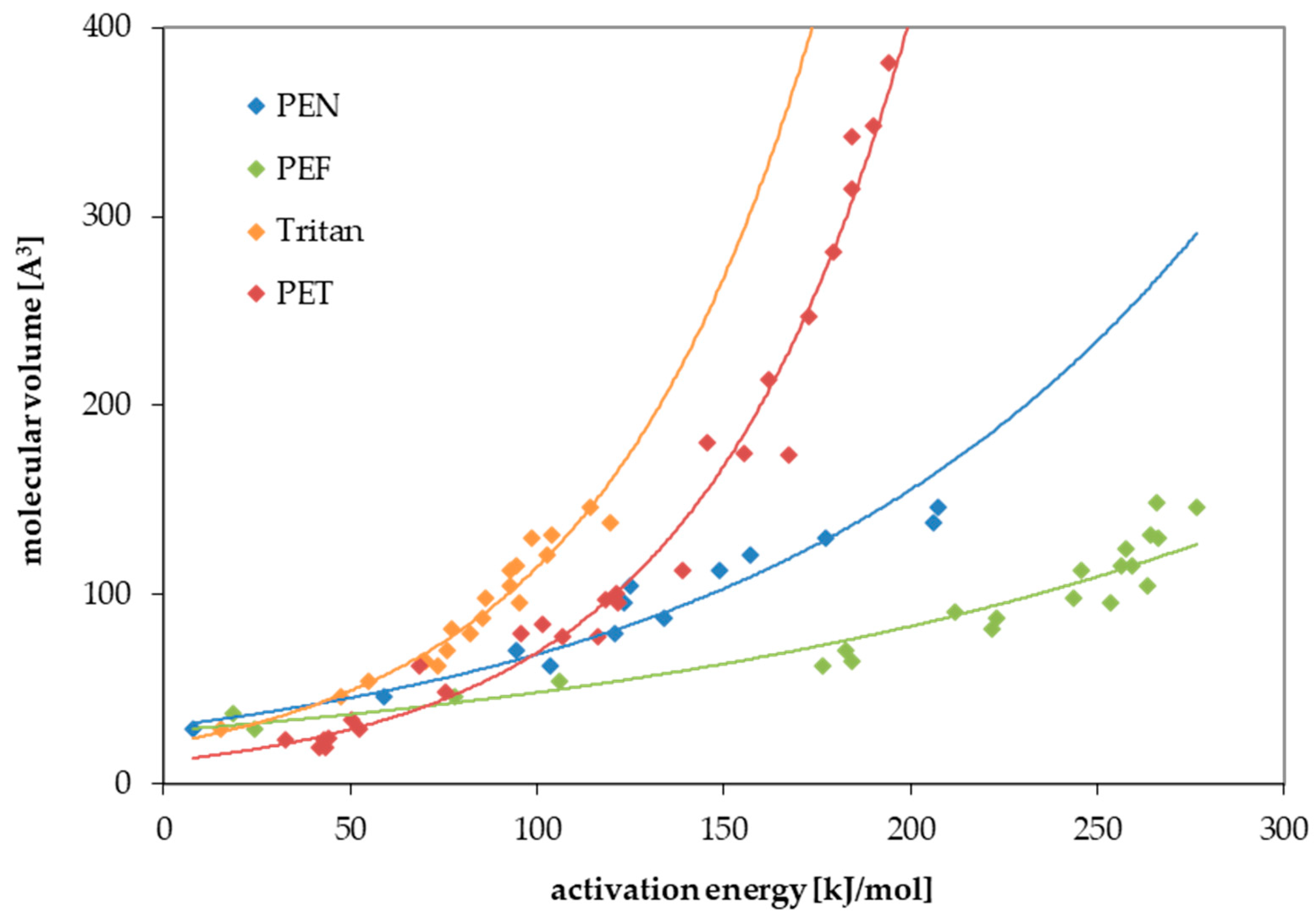
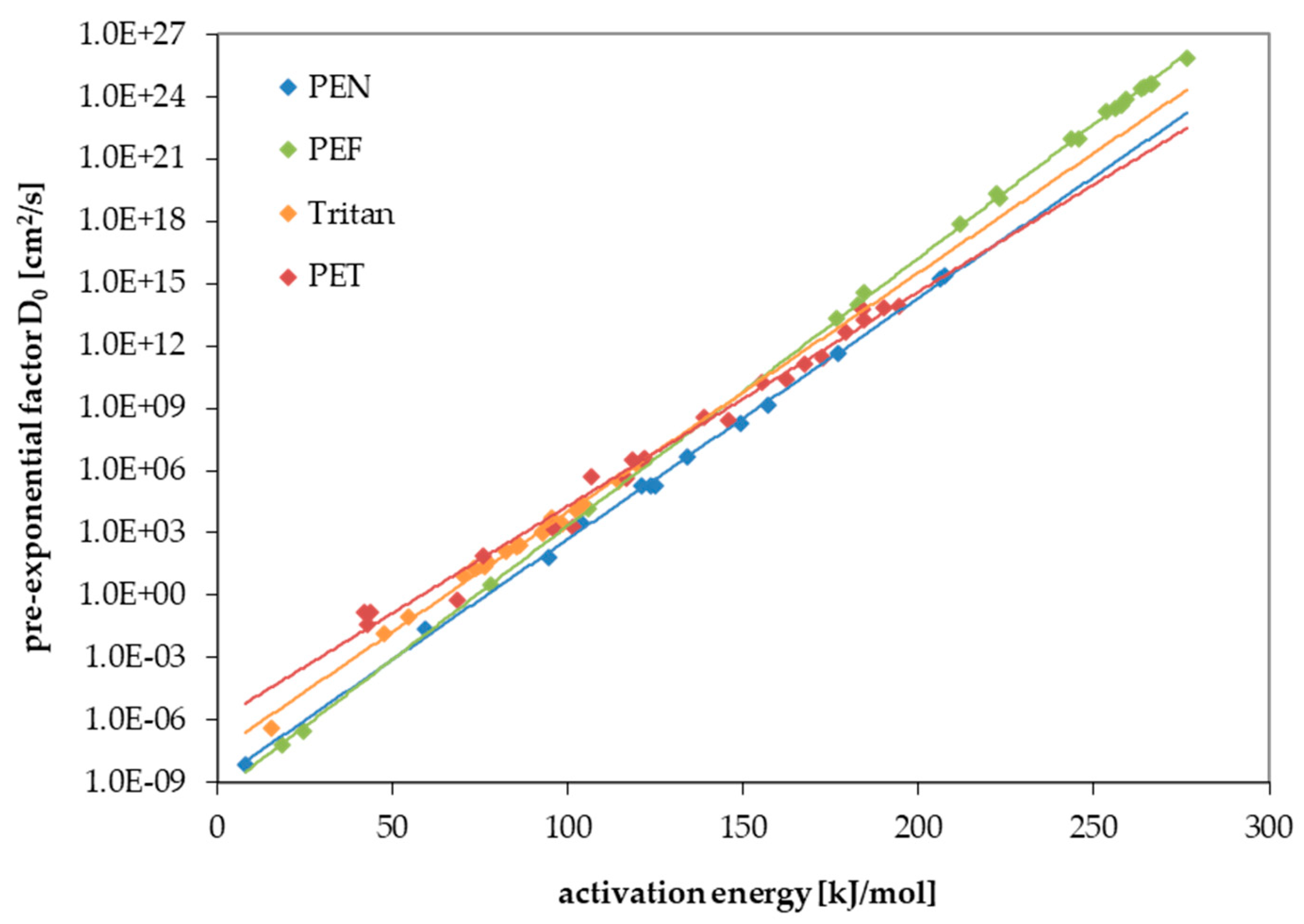
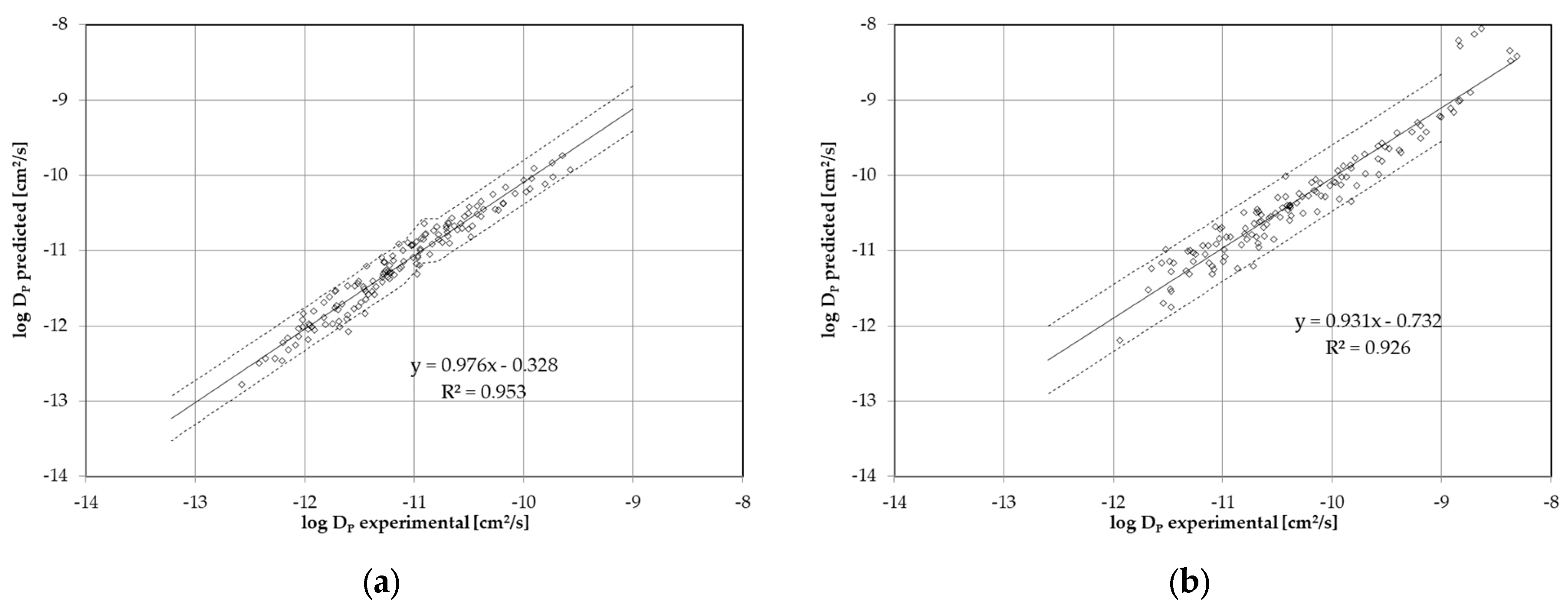
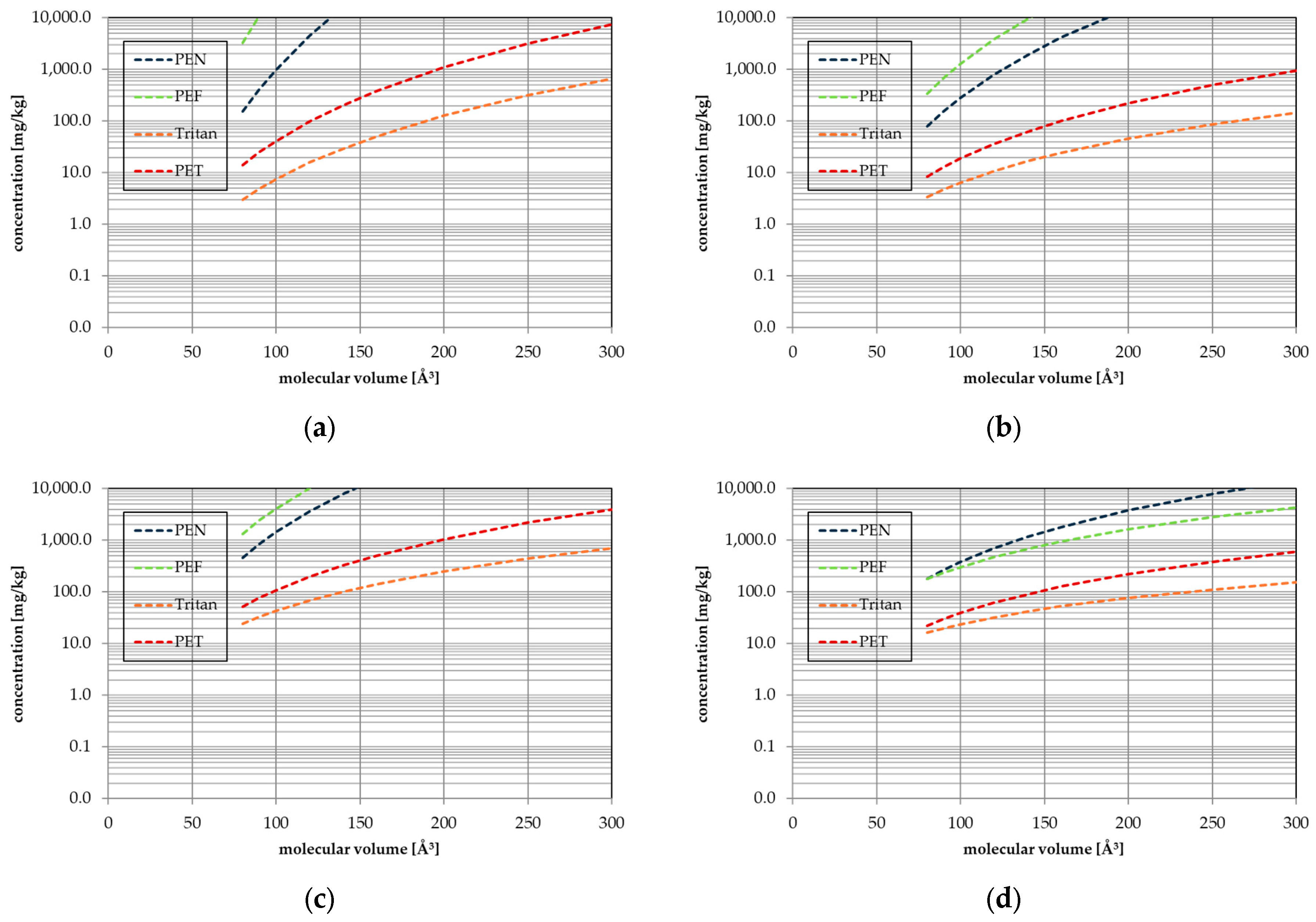
| Substance | Molecular Volume [Å3] | Activation Energy EA [kJ/mol] | Pre-Exponential Factor D0 [cm2/s] | Temperature Range, Number of Kinetic Points | Correlation Coefficient r2 of the Arrhenius Plot |
|---|---|---|---|---|---|
| methane | 26.64 | 24.5 | 2.82 × 10−7 | 90–120 °C, 6 points | 0.5043 |
| ethane | 45.76 | 77.9 | 3.25 × 100 | 90–120 °C, 6 points | 0.9206 |
| methanol | 37.21 | 18.6 | 6.73 × 10−8 | 100–120 °C, 5 points | 0.3751 |
| ethanol | 54.02 | 105.8 | 1.41 × 104 | 100–120 °C, 5 points | 0.9933 |
| acetone | 64.74 | 184.2 | 3.49 × 1014 | 100–120 °C, 5 points | 0.9941 |
| n-propane | 62.56 | 176.6 | 2.25 × 1013 | 100–120 °C, 5 points | 0.9847 |
| 1-propanol | 70.82 | 182.6 | 9.71 × 1013 | 100–120 °C, 5 points | 0.9958 |
| 2-butanone | 81.54 | 222.0 | 2.20 × 1019 | 100–120 °C, 5 points | 0.9949 |
| 1-butanol | 87.62 | 222.9 | 1.38 × 1019 | 100–120 °C, 5 points | 0.9912 |
| ethyl acetate | 90.53 | 211.8 | 7.78 × 1017 | 100–120 °C, 5 points | 0.9777 |
| n-pentane | 96.16 | 253.6 | 1.92 × 1023 | 105–120 °C, 4 points | 0.9716 |
| 2-pentanone | 98.34 | 243.5 | 8.82 × 1021 | 100–120 °C, 5 points | 0.9967 |
| 1-pentanol | 104.42 | 263.3 | 2.46 × 1024 | 105–120 °C, 4 points | 0.9867 |
| n-hexane | 113.0 | 245.5 | 9.78 × 1021 | 105–120 °C, 4 points | 0.9857 |
| hexanal | 115.1 | 256.3 | 2.83 × 1023 | 105–120 °C, 4 points | 0.9812 |
| 2-hexanone | 115.2 | 259.1 | 7.49 × 1023 | 100–120 °C, 5 points | 0.9973 |
| n-butyl acetate | 124.1 | 257.7 | 4.06 × 1023 | 105–120 °C, 4 points | 0.9761 |
| n-heptane | 129.8 | 266.4 | 4.42 × 1024 | 105–120 °C, 4 points | 0.9858 |
| 2-heptanone | 132.0 | 264.3 | 2.77 × 1024 | 100–120 °C, 5 points | 0.9962 |
| n-octane | 146.6 | 276.4 | 7.09 × 1025 | 105–120 °C, 4 points | 0.9871 |
| 2-octanone | 148.8 | 266.0 | 3.83 × 1024 | 105–120 °C, 4 points | 0.9937 |
| Substance | Molecular Volume [Å3] | Activation Energy EA [kJ/mol] | Pre-Exponential Factor D0 [cm2/s] | Temperature Range, Number of Kinetic Points | Correlation Coefficient r2 of the Arrhenius Plot |
|---|---|---|---|---|---|
| methane | 26.64 | 15.5 | 3.43 × 10−7 | 50–80 °C, 4 points | 0.8527 |
| ethane | 45.76 | 47.4 | 1.51 × 10−2 | 50–80 °C, 4 points | 0.9961 |
| ethanol | 54.02 | 54.7 | 8.73 × 10−2 | 80–100 °C, 5 points | 0.9745 |
| acetone | 64.74 | 70.1 | 9.07 | 50–100 °C, 6 points | 0.9973 |
| n-propane | 62.56 | 73.3 | 2.14 × 101 | 50–80 °C, 4 points | 0.9999 |
| 1-propanol | 70.82 | 76.1 | 2.58 × 101 | 80–100 °C, 5 points | 0.9947 |
| n-butane | 79.36 | 82.1 | 1.36 × 102 | 50–80 °C, 4 points | 0.9987 |
| 2-butanone | 81.54 | 77.3 | 4.16 × 101 | 50–100 °C, 6 points | 0.9973 |
| 1-butanol | 87.62 | 85.2 | 2.11 × 102 | 80–100 °C, 5 points | 0.9950 |
| n-pentane | 96.16 | 95.4 | 5.36 × 103 | 70–90 °C, 5 points | 0.9951 |
| 2-pentanone | 98.34 | 86.1 | 2.70 × 102 | 50–100 °C, 6 points | 0.9964 |
| 1-pentanol | 104.4 | 92.9 | 1.17 × 103 | 80–100 °C, 5 points | 0.9899 |
| n-hexane | 113.0 | 92.9 | 9.65 × 102 | 70–90 °C, 5 points | 0.9738 |
| 2-hexanone | 115.2 | 94.6 | 2.11 × 103 | 50–100 °C, 6 points | 0.9944 |
| 1-hexanol | 121.2 | 102.5 | 1.28 × 104 | 80–100 °C, 5 points | 0.9715 |
| n-heptane | 129.8 | 98.4 | 2.82 × 103 | 70–90 °C, 5 points | 0.9788 |
| 2-heptanone | 132.0 | 104.1 | 2.37 × 104 | 70–100 °C, 4 points | 0.9810 |
| 1-heptanol | 138.0 | 119.8 | 1.87 × 106 | 80–100 °C, 5 points | 0.9663 |
| n-octane | 146.6 | 114.4 | 2.94 × 105 | 75–90 °C, 4 points | 0.9927 |
| Parameter | PEF | Tritan |
|---|---|---|
| a [1/K] | 2.46 × 10−3 | 2.20 × 10−3 |
| b [cm2/s] | 3.09 × 10−10 | 3.14 × 10−8 |
| c [Å3] | 27.9 | 21.0 |
| d [1/K] | 4.54 × 10−5 | 1.41 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welle, F. Diffusion Behavior of Polyethylene Furanoate (PEF) and Tritan as Sustainable Polyester Packaging Materials. Polymers 2025, 17, 2674. https://doi.org/10.3390/polym17192674
Welle F. Diffusion Behavior of Polyethylene Furanoate (PEF) and Tritan as Sustainable Polyester Packaging Materials. Polymers. 2025; 17(19):2674. https://doi.org/10.3390/polym17192674
Chicago/Turabian StyleWelle, Frank. 2025. "Diffusion Behavior of Polyethylene Furanoate (PEF) and Tritan as Sustainable Polyester Packaging Materials" Polymers 17, no. 19: 2674. https://doi.org/10.3390/polym17192674
APA StyleWelle, F. (2025). Diffusion Behavior of Polyethylene Furanoate (PEF) and Tritan as Sustainable Polyester Packaging Materials. Polymers, 17(19), 2674. https://doi.org/10.3390/polym17192674






