pH-Sensitive Release of Functionalized Chiral Carbon Dots from PLGA Coatings on Titanium Alloys for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ti6Al4V Samples
2.2. Activation and Dopamine-Surface Modification
2.3. Carbon Dots Loading and Dip Coating on Metallic Surfaces
2.4. Surface Characterization
2.5. Degradation Tests
2.6. Release Profiles of Carbon Dots
2.7. Direct and Indirect Cytotoxicity Tests
2.7.1. Cell Culture
2.7.2. Indirect Viability Assay
2.7.3. Direct Viability Assay
2.8. Hemocompatibility Tests
2.9. Statistical Analysis
3. Results and Discussion
3.1. Surface Morphology and Wettability
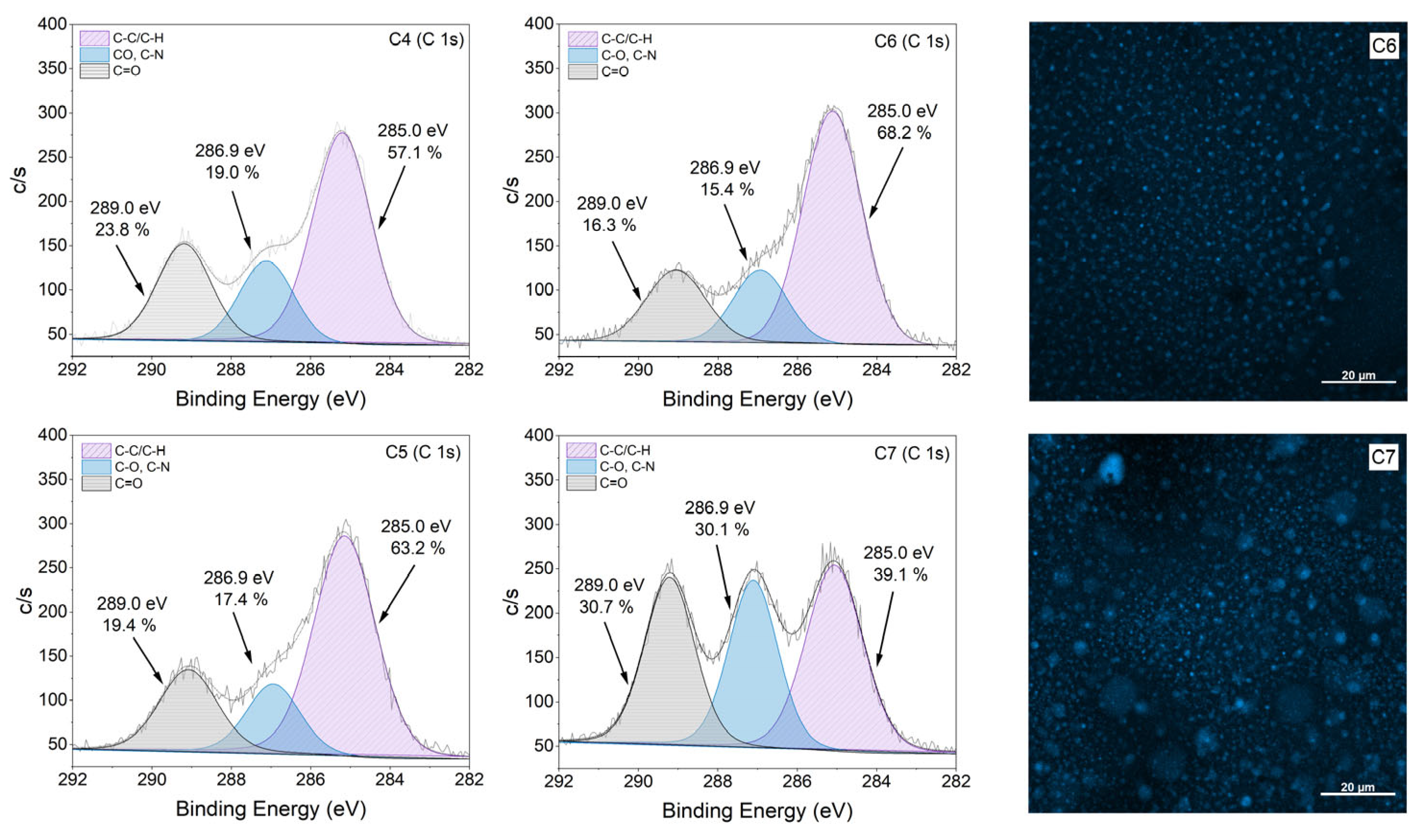
3.2. Effect of [CCDs-OH]/[CCDs-NH2] Incorporation
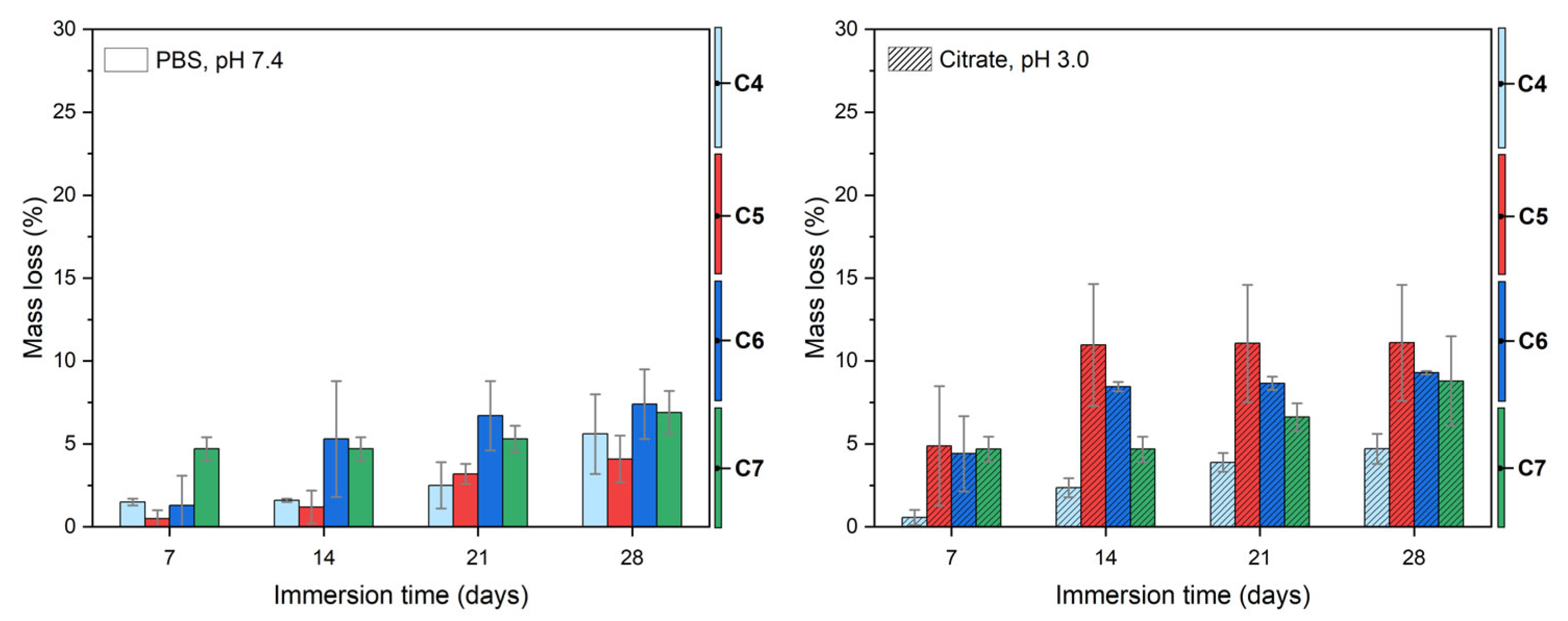
3.3. Coating Degradation Behavior
3.4. Carbon Dots Release Profiles
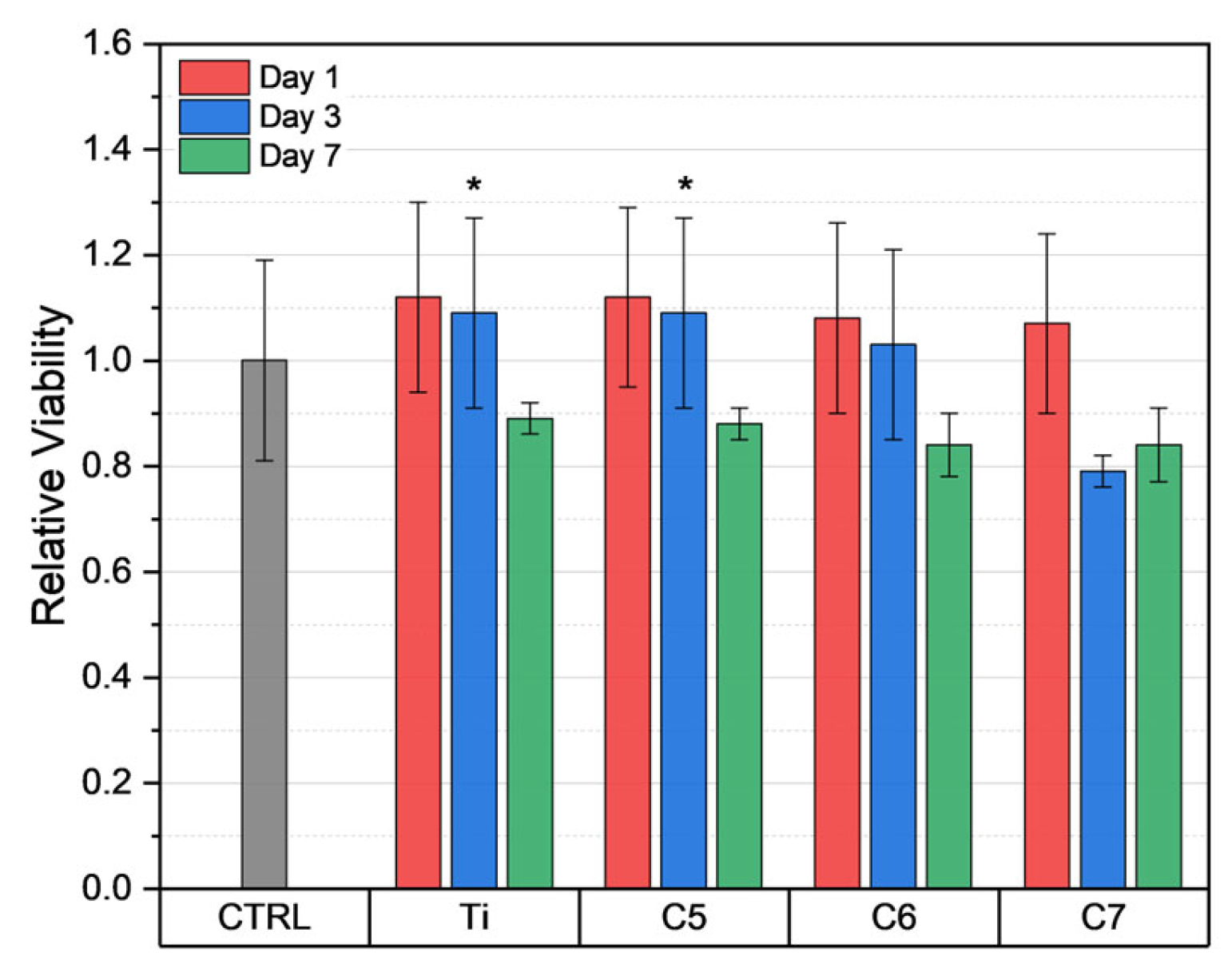
3.5. Biocompatibility
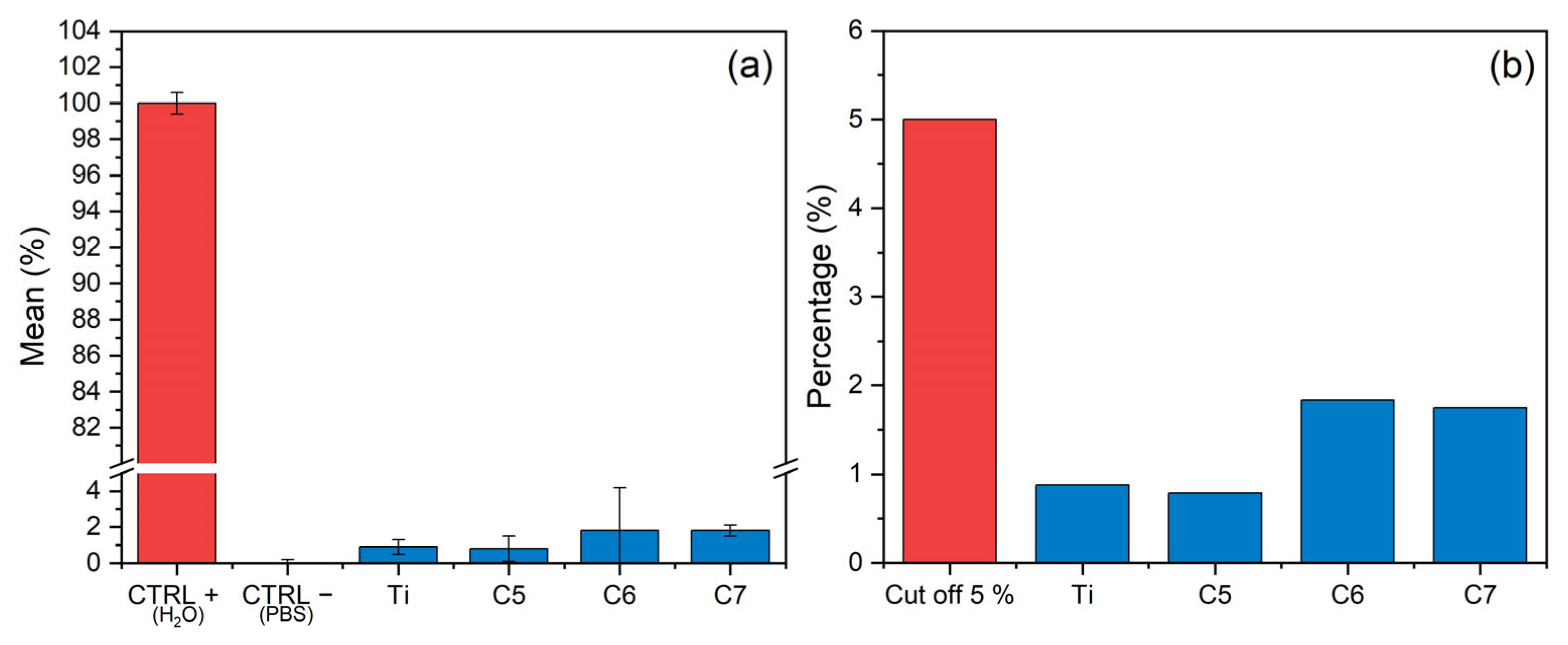
3.6. Hemocompatibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parisi, L.; Ghezzi, B.; Bianchi, M.G.; Toffoli, A.; Rossi, F.; Bussolati, O.; Macaluso, G.M. Titanium Dental Implants Hydrophilicity Promotes Preferential Serum Fibronectin over Albumin Competitive Adsorption Modulating Early Cell Response. Mater. Sci. Eng. C 2020, 117, 111307. [Google Scholar] [CrossRef]
- Mahmoudi, P.; Akbarpour, M.R.; Lakeh, H.B.; Jing, F.; Hadidi, M.R.; Akhavan, B. Antibacterial Ti–Cu Implants: A Critical Review on Mechanisms of Action. Mater. Today Bio 2022, 17, 100447. [Google Scholar] [CrossRef]
- Zaatreh, S.; Wegner, K.; Strauß, M.; Pasold, J.; Mittelmeier, W.; Podbielski, A.; Kreikemeyer, B.; Bader, R. Co-Culture of S. Epidermidis and Human Osteoblasts on Implant Surfaces: An Advanced In Vitro Model for Implant-Associated Infections. PLoS ONE 2016, 11, e0151534. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of Material Characteristics and/or Surface Topography on Biofilm Development. Clin. Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface Characteristics of Dental Implants: A Review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Han, A.; Tsoi, J.K.H.; Rodrigues, F.P.; Leprince, J.G.; Palin, W.M. Bacterial Adhesion Mechanisms on Dental Implant Surfaces and the Influencing Factors. Int. J. Adhes. Adhes. 2016, 69, 58–71. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Z.; Wang, Z.; Yan, X.; Duan, X.; Sun, H. Advanced Surface Modification Techniques for Titanium Implants: A Review of Osteogenic and Antibacterial Strategies. Front. Bioeng. Biotechnol. 2025, 13, 1549439. [Google Scholar] [CrossRef]
- Wu, Y.; Wan, K.; Lu, J.; Yuan, C.; Cui, Y.; Duan, R.; Yu, J. Research Progress on Surface Modification of Titanium Implants. Coatings 2025, 15, 229. [Google Scholar] [CrossRef]
- Rimondini, L.; Faré, S.; Brambilla, E.; Felloni, A.; Consonni, C.; Brossa, F.; Carrassi, A. The Effect of Surface Roughness on Early In Vivo Plaque Colonization on Titanium. J. Periodontol. 1997, 68, 556–562. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Padovani, G.C.; Feitosa, V.P.; Sauro, S.; Tay, F.R.; Durán, G.; Paula, A.J.; Durán, N. Advances in Dental Materials through Nanotechnology: Facts, Perspectives and Toxicological Aspects. Trends Biotechnol. 2015, 33, 621–636. [Google Scholar] [CrossRef]
- Vrček, I.V.; Žuntar, I.; Petlevski, R.; Pavičić, I.; Dutour Sikirić, M.; Ćurlin, M.; Goessler, W. Comparison of in Vitro Toxicity of Silver Ions and Silver Nanoparticles on Human Hepatoma Cells. Environ. Toxicol. 2016, 31, 679–692. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H.J. Nanosilver: A Nanoproduct in Medical Application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chatzimitakos, T.; Stalikas, C. Antimicrobial Properties of Carbon Quantum Dots. In Nanotoxicity: Prevention and Antibacterial Applications of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 301–315. ISBN 9780128199435. [Google Scholar]
- Wang, S.; Wang, D.; Wang, G.; Zhang, M.; Sun, Y.; Ding, J. Antibacterial Carbon Dots. Mater. Today Bio 2024, 30, 101383. [Google Scholar] [CrossRef] [PubMed]
- Manioudakis, J.; Victoria, F.; Thompson, C.A.; Brown, L.; Movsum, M.; Lucifero, R.; Naccache, R. Effects of Nitrogen-Doping on the Photophysical Properties of Carbon Dots. J. Mater. Chem. C Mater. 2019, 7, 853–862. [Google Scholar] [CrossRef]
- Hussen, N.H.; Hasan, A.H.; FaqiKhedr, Y.M.; Bogoyavlenskiy, A.; Bhat, A.R.; Jamalis, J. Carbon Dot Based Carbon Nanoparticles as Potent Antimicrobial, Antiviral, and Anticancer Agents. ACS Omega 2024, 9, 9849–9864. [Google Scholar] [CrossRef]
- Chen, X.; Yu, M.; Li, P.; Xu, C.; Zhang, S.; Wang, Y.; Xing, X. Recent Progress on Chiral Carbon Dots: Synthetic Strategies and Biomedical Applications. ACS Biomater. Sci. Eng. 2023, 9, 5548–5566. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.; Zhu, X. Preparation of Chiral Carbon Quantum Dots and Its Application. J. Fluoresc. 2024, 34, 1–13. [Google Scholar] [CrossRef]
- Victoria, F.; Manioudakis, J.; Zaroubi, L.; Findlay, B.; Naccache, R. Tuning Residual Chirality in Carbon Dots with Anti-Microbial Properties. RSC Adv. 2020, 10, 32202–32210. [Google Scholar] [CrossRef]
- Zhao, D.; Xu, M.; Dai, K.; Liu, H.; Jiao, Y.; Xiao, X. The Preparation of Chiral Carbon Dots and the Study on Their Antibacterial Abilities. Mater. Chem. Phys. 2023, 295, 127144. [Google Scholar] [CrossRef]
- Song, W.; Wang, X.; Nong, S.; Wang, M.; Kang, S.; Wang, F.; Xu, L. D-Cysteine-Derived Carbon Dots for Selective Discrimination, Imaging, and Synergistic Elimination of Gram-Positive Bacteria and Fungi. Adv. Funct. Mater. 2024, 34, 2402761. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current Development of Biodegradable Polymeric Materials for Biomedical Applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent Advances in Biodegradable Polymers for Sustainable Applications. npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Allison, S.D. Effect of Structural Relaxation on the Preparation and Drug Release Behavior of Poly(Lactic-Co-Glycolic) Acid Microparticle Drug Delivery Systems. J. Pharm. Sci. 2008, 97, 2022–2035. [Google Scholar] [CrossRef]
- Mohamed, F.; Van Der Walle, C.F. Engineering Biodegradable Polyester Particles with Specific Drug Targeting and Drug Release Properties. J. Pharm. Sci. 2008, 97, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Zolnik, B.S.; Burgess, D.J. Effect of Acidic PH on PLGA Microsphere Degradation and Release. J. Control. Release 2007, 122, 338–344. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, C.S.; Saylor, D.M.; Koo, D. Polymer Degradation and Drug Delivery in PLGA-Based Drug–Polymer Applications: A Review of Experiments and Theories. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Yang, X.; Ma, Q. Tannic Acid: A Crosslinker Leading to Versatile Functional Polymeric Networks: A Review. RSC Adv. 2022, 12, 7689–7711. [Google Scholar] [CrossRef]
- Gherasim, O.; Grumezescu, A.M.; Grumezescu, V.; Negut, I.; Dumitrescu, M.F.; Stan, M.S.; Nica, I.C.; Holban, A.M.; Socol, G.; Andronescu, E. Bioactive Coatings Based on Hydroxyapatite, Kanamycin, and Growth Factor for Biofilm Modulation. Antibiotics 2021, 10, 160. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). ISO 10993-5:2009; Biological Evaluation of Medical Devices, Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Liang, H.; Pei, Y.; Li, J.; Xiong, W.; He, Y.; Liu, S.; Li, Y.; Li, B. PH-Degradable Antioxidant Nanoparticles Based on Hydrogen-Bonded Tannic Acid Assembly. RSC Adv. 2016, 6, 31374–31385. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, X.; Fei, Z.; Fan, X.; Ma, L.; Wang, H.; Tian, C.; Zhang, B.; Luo, R.; Wang, Y.; et al. Ag-Incorporated Polydopamine/Tannic Acid Coating on Titanium With Enhanced Cytocompatible and Antibacterial Properties. Front. Bioeng. Biotechnol. 2022, 10, 877738. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.R.; Victoria, F.; Naccache, R. Microwave-Assisted Synthesis of Carbon Dots and Their Applications. J. Mater. Chem. C Mater. 2019, 7, 7175–7195. [Google Scholar] [CrossRef]
- Wang, H.; Ai, L.; Song, Z.; Nie, M.; Xiao, J.; Li, G.; Lu, S. Surface Modification Functionalized Carbon Dots. Chem.-A Eur. J. 2023, 29, e202302383. [Google Scholar] [CrossRef]
- Ge, G.; Li, L.; Wang, D.; Chen, M.; Zeng, Z.; Xiong, W.; Wu, X.; Guo, C. Carbon Dots: Synthesis, Properties and Biomedical Applications. J. Mater. Chem. B 2021, 9, 6553–6575. [Google Scholar] [CrossRef]
- Zheng, C.; An, X.; Gong, J. Novel PH Sensitive N-Doped Carbon Dots with Both Long Fluorescence Lifetime and High Quantum Yield. RSC Adv. 2015, 5, 32319–32322. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Hallaji, Z.; Najafi Nobar, S.; Bagheri, Z. Carbon Dots with PH-Responsive Fluorescence: A Review on Synthesis and Cell Biological Applications. Microchim. Acta 2020, 187, 150. [Google Scholar] [CrossRef]
- Sharma, V.; Tiwari, P.; Kaur, N.; Mobin, S.M. Optical Nanosensors Based on Fluorescent Carbon Dots for the Detection of Water Contaminants: A Review. Environ. Chem. Lett. 2021, 19, 3229–3241. [Google Scholar] [CrossRef]
- Wu, X.S.; Wang, N. Synthesis, Characterization, Biodegradation, and Drug Delivery Application of Biodegradable Lactic/Glycolic Acid Polymers. Part II: Biodegradation. J. Biomater. Sci. Polym. Ed. 2001, 12, 21–34. [Google Scholar] [CrossRef]
- Soylu, H.M.; Chevallier, P.; Copes, F.; Ponti, F.; Candiani, G.; Yurt, F.; Mantovani, D. A Novel Strategy to Coat Dopamine-Functionalized Titanium Surfaces With Agarose-Based Hydrogels for the Controlled Release of Gentamicin. Front. Cell Infect. Microbiol. 2021, 11, 678081. [Google Scholar] [CrossRef]
- Abouelmagd, S.A.; Meng, F.; Kim, B.K.; Hyun, H.; Yeo, Y. Tannic Acid-Mediated Surface Functionalization of Polymeric Nanoparticles. ACS Biomater. Sci. Eng. 2016, 2, 2294–2303. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of Poly (Lactic-Co-Glycolic Acid) and Progress of Poly (Lactic-Co-Glycolic Acid)-Based Biodegradable Materials in Biomedical Research. Pharmaceuticals 2023, 16, 454. [Google Scholar] [CrossRef]
- Ara, M.; Watanabe, M.; Imai, Y. Effect of Blending Calcium Compounds on Hydrolytic Degradation of Poly(Dl-Lactic Acid-Co-Glycolic Acid). Biomaterials 2002, 23, 2479–2483. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, M.; Ari, B.; Ram, M.K.; Sahiner, N. Nitrogen Doped Carbon-Dot Embedded Poly(Lactic Acid-Co-Glycolic Acid) Composite Films for Potential Use in Food Packing Industry and Wound Dressing. J. Compos. Sci. 2022, 6, 260. [Google Scholar] [CrossRef]
- Feng, Z.; Adolfsson, K.H.; Xu, Y.; Fang, H.; Hakkarainen, M.; Wu, M. Carbon Dot/Polymer Nanocomposites: From Green Synthesis to Energy, Environmental and Biomedical Applications. Sustain. Mater. Technol. 2021, 29, e00304. [Google Scholar] [CrossRef]
- Chen, J.; Guo, X.; Tan, R.; Huang, M.; Ren, J.; Liu, W.; Wang, M.; Li, B.; Ma, Z.; Zhang, Q. Achieving the Simultaneous Improvement of Degradation, Thermal, and Mechanical Properties of Polylactic Acid Composite Films by Carbon Quantum Dots. Compos. B Eng. 2025, 299, 112442. [Google Scholar] [CrossRef]
- Ren, J.; Weber, F.; Weigert, F.; Wang, Y.; Choudhury, S.; Xiao, J.; Lauermann, I.; Resch-Genger, U.; Bande, A.; Petit, T. Influence of Surface Chemistry on Optical, Chemical and Electronic Properties of Blue Luminescent Carbon Dots. Nanoscale 2019, 11, 2056–2064. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, F.; Hu, J.; Gao, W.; Zhang, M. A Mini Review on PH-Sensitive Photoluminescence in Carbon Nanodots. Front. Chem. 2021, 8, 605028. [Google Scholar] [CrossRef]
- Meierhofer, F.; Dissinger, F.; Weigert, F.; Jungclaus, J.; Müller-Caspary, K.; Waldvogel, S.R.; Resch-Genger, U.; Voss, T. Citric Acid Based Carbon Dots with Amine Type Stabilizers: PH-Specific Luminescence and Quantum Yield Characteristics. J. Phys. Chem. C 2020, 124, 8894–8904. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(Lactic-Co-Glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; National Center for Advancing Translational Sciences: Rockville, MD, USA, 2016. [Google Scholar]
- Azam, N.; Najabat Ali, M.; Javaid Khan, T. Carbon Quantum Dots for Biomedical Applications: Review and Analysis. Front. Mater. 2021, 8, 700403. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon Quantum Dots and Their Applications. Chem. Soc. Rev. 2014, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.T. Carbon Nanodots: Synthesis, Properties and Applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]




| Properties | Obtained Parameters | Technique |
|---|---|---|
| Average particle size | ~12 ± 3 nm (range 6–28 nm) | TEM |
| Morphology | quasi-spherical, uniform distribution | TEM |
| Optical response | absorption peaks at ~250 and 350 nm | UV-vis |
| Fluorescence | L- and D-cysCDs were at λexc = 350 nm | PL-spectroscopy |
| Quantum yield | ~14% (relative to quinine sulfate) | Photoluminescence |
| Chirality | mirror-image CD spectra for L- and D-cysCDs; distinct from precursors | Circular dichroism |
| Surface chemistry | –NH2, –COOH, –OH, S-containing groups | FTIR, XPS |
| Structure | predominantly amorphous at ~17° 2θ and sharp crystalline peaks | XRD |
| Thermal behavior | 2% loss from 30 to 100 °C, 5% loss from 100 to 150 °C, and 58% loss between 200 and 500 °C | TGA |
| Antibacterial activity | 4 mg/mL in E. coli for L-cysCDs 2 mg/mL in E. coli for D-cysCDs 2 mg/mL in M. luteus for L-cysCDs 0.5 mg/mL in M. luteus for D-cysCDs | MIC assays |
| Specimen | Type of Treatment |
|---|---|
| C0 | Untreated Ti6Al4V |
| C1 | Ti-polished (manual polishing) |
| C2 | Ti-OH (activation with NaOH) |
| C3 | Ti-Dopa (dopamine grafting) |
| C4 | Ti-Dopa-PLGA |
| C5 | Ti-Dopa-PLGA/TA/CaCl2 |
| C6 | Ti-Dopa-PLGA/TA/CaCl2-[CCDs-OH] |
| C7 | Ti-Dopa-PLGA/TA/CaCl2-[CCDs-NH2] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Muñoz, R.; Chevallier, P.; Copes, F.; Naccache, R.; Mantovani, D. pH-Sensitive Release of Functionalized Chiral Carbon Dots from PLGA Coatings on Titanium Alloys for Biomedical Applications. Polymers 2025, 17, 2667. https://doi.org/10.3390/polym17192667
López-Muñoz R, Chevallier P, Copes F, Naccache R, Mantovani D. pH-Sensitive Release of Functionalized Chiral Carbon Dots from PLGA Coatings on Titanium Alloys for Biomedical Applications. Polymers. 2025; 17(19):2667. https://doi.org/10.3390/polym17192667
Chicago/Turabian StyleLópez-Muñoz, Roberto, Pascale Chevallier, Francesco Copes, Rafik Naccache, and Diego Mantovani. 2025. "pH-Sensitive Release of Functionalized Chiral Carbon Dots from PLGA Coatings on Titanium Alloys for Biomedical Applications" Polymers 17, no. 19: 2667. https://doi.org/10.3390/polym17192667
APA StyleLópez-Muñoz, R., Chevallier, P., Copes, F., Naccache, R., & Mantovani, D. (2025). pH-Sensitive Release of Functionalized Chiral Carbon Dots from PLGA Coatings on Titanium Alloys for Biomedical Applications. Polymers, 17(19), 2667. https://doi.org/10.3390/polym17192667








