3.3. XPS Spectra of Li and LiAs
X-ray photoelectron spectroscopy (XPS) was employed to investigate the elemental composition and chemical functionalities of lignin (Li) and lignin esterified with aspartic acid (LiAs). The survey spectra (
Figure 4) confirm that both materials are predominantly composed of carbon (C) and oxygen (O), with minor contributions from sulfur (S). The Lignoboost lignin (Li) sample exhibited relative atomic concentrations of 77.50% C, 21.64% O, and 0.85% S (
Table 2), consistent with previously reported values for technical lignins.
The high-resolution deconvolution of the O 1s spectrum of Li (
Table 3) revealed three primary contributions: the peak at 531.9 eV (10.01%) was attributed to oxygen in carbonyl environments (C=O, such as ketones or quinones), the dominant peak at 533.3 eV (76.98%) corresponded to hydroxyl and ether functionalities (C–OH/C–O), and the peak at 534.3 eV (13.01%) was associated with peroxide or adsorbed oxygen species (O–O) [
29].
Similarly, the C 1s spectrum of Li (
Table 4) exhibited signals at 285.0 eV (C–C/C–H, 57.94%), 286.5 eV (C–O/C–OH, 35.24%), 287.5 eV (C=O, 5.33%), and 289.0 eV (O–C=O, 1.49%) [
13]. These assignments reflect the structural complexity of lignin, which includes aromatic rings, aliphatic chains, and a variety of oxygenated functionalities.
Upon esterification with aspartic acid, the XPS data for LiAs indicated clear chemical modifications. The appearance of a N 1s peak at 398.0 eV in the LiAs spectrum (
Table 5) confirmed the incorporation of nitrogen, a signature of the amino acid moiety. The O 1s peak in LiAs, centered around 531.0 eV, showed increased intensity and relative concentration (22.98%) compared to Li, suggesting a higher content of carbonyl-containing groups—likely ester and amide functionalities introduced during the reaction. The C 1s spectrum of LiAs also shifted, showing increased intensity at 286.0 eV, indicative of an enhanced presence of oxygenated carbons (e.g., C–O, C–N, C=O).
Together, these spectroscopic changes, particularly the rise in carbonyl-associated oxygen signals and the introduction of nitrogen, strongly support the successful esterification of lignin with aspartic acid. These findings are in agreement with complementary FTIR and 13C NMR analyses and confirm the structural transformation of Li into LiAs through the formation of ester and amide linkages.
3.5. FTIR Spectra of Materials
The FTIR spectra of the Gu/DexS formulations, both with and without the incorporation of lignin, esterified lignin, and
Rosa canina extract, reveal a series of characteristic absorption bands that provide detailed insights into the chemical composition and molecular interactions within these composites (
Figure 6). All samples exhibit a broad band in the 3300–3400 cm
−1 region, which is attributed to O–H stretching vibrations arising from polysaccharides, phenolic groups, and residual moisture. In the Gu/DexS and Gu/DexS/RC samples, the pronounced nature of this band indicates an extensive hydrogen bonding network, with the addition of
Rosa canina extract further modulating these interactions through its polyphenolic hydroxyl groups.
Complementary to this, absorptions near 2920 cm−1 are observed, corresponding to the C–H stretching vibrations of the polysaccharide backbone, while a band between 1640 and 1650 cm−1 is ascribed to bound water and potential overlap with amide or aromatic ring stretching vibrations. Notably, the DexS-containing matrices display a distinct set of peaks between 1200 and 1250 cm−1, which arise from the asymmetric stretching of sulfate groups.
Upon the incorporation of lignin—either in its native form or esterified with aspartic acid—additional spectral features emerge. Samples containing lignin display intensified signals in the 1500–1600 cm
−1 region, which are consistent with aromatic ring vibrations inherent to lignin (
Figure 6C,D). Moreover, in formulations with esterified lignin, more pronounced bands near 1700–1740 cm
−1 are evident, reflecting the formation of new ester and carboxylic functionalities introduced through the reaction with aspartic acid (
Figure 6E,F). The presence of
Rosa canina extract further enhances the intensity of the broad O–H band, underscoring the contribution of extra hydroxyl groups from its polyphenolic compounds. In these RC-containing formulations, an additional signal between 1716 and 1741 cm
−1 is observed and assigned to symmetric C=O stretching vibrations in ketones, aldehydes, and carboxylic acids (
Figure 6B,D,F).
Additional absorption features further delineate the complex chemical environment within these materials. The spectra reveal O–H stretching at around 3340 cm
−1, C–H stretching of CH
2 groups between 2850 and 2927 cm
−1, and C–H bending vibrations occurring from 1320 to 1425 cm
−1, alongside C–C stretching near 1200 cm
−1. Specific carbohydrate linkages are also identified, with bands at 870 cm
−1 and 930 cm
−1 corresponding to 1–4 and 1–6 linkages of galactose and mannose, respectively [
31]. Moreover, two characteristic bands at 1236 cm
−1 and 820 cm
−1 are attributed to the asymmetric stretching of sulfate groups in ester sulfate and the symmetric stretching of C–O–SO
3− groups in the DexS structure, respectively [
32]. Subtle shifts and changes in intensity within the fingerprint region (900–1200 cm
−1) further suggest interactions among the hydroxyl, carboxyl, and sulfate groups of the matrix and the phenolic or carbonylic functionalities of the extract.
Overall, the FTIR analysis confirms the successful incorporation of both native and esterified lignin as well as Rosa canina extract into the Gu/DexS-based formulations. The observed vibrational changes provide valuable insight into the chemical modifications and intermolecular interactions within these composite materials, thereby contributing to a comprehensive understanding of their structural properties.
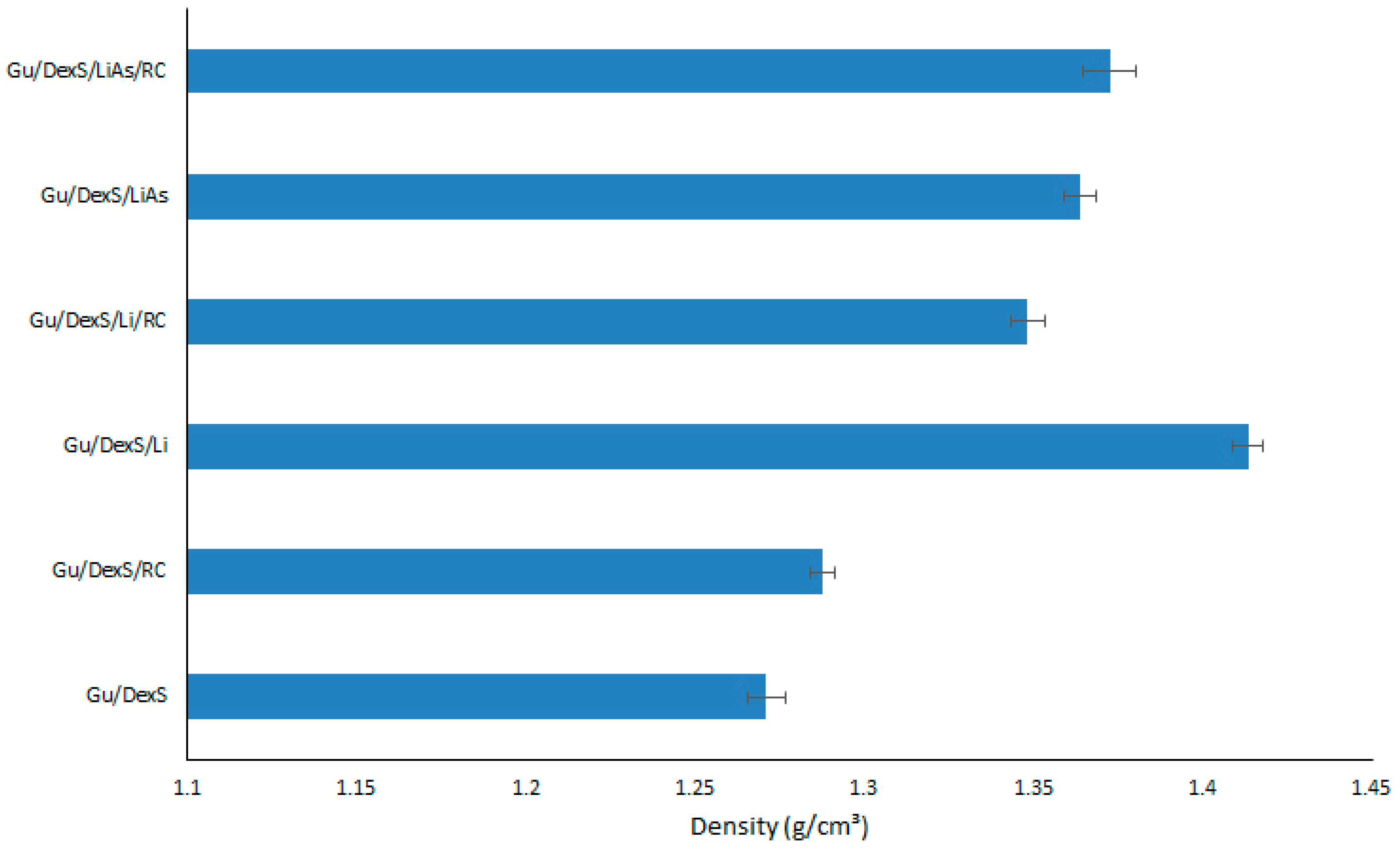
3.6. Density of Materials
The densities of the obtained materials are presented in
Figure 7. In general, when fillers are added to a polymeric matrix, the material’s density often increases because fillers are typically denser than the polymeric matrix itself. Additionally, fillers can enhance the packing efficiency within the material, reducing void spaces and making the composite more compact. This increase in density affects the material’s mechanical properties, including strength or rigidity [
33].
Material containing Li (Gu/DexS/Li) presented the highest density (1.41 g/cm
3) due to the inherent properties of lignin itself. Lignin is a complex, high-molecular-weight biopolymer, with a relatively dense and rigid structure compared to other organic polymers. The aromatic rings and phenolic groups from the Li structure [
34] contribute to a higher molecular mass and stronger intermolecular forces, making it more compact and less flexible.
Materials containing LiAs (Gu/DexS/LiAs and Gu/DexS/LiAs/RC) exhibit a slight decrease in density (1.36 and 1.37 g/cm3) compared to those containing unmodified Li (Gu/DexS/Li and Gu/DexS/Li/RC with density values of 1.41 and 1.34 g/cm3). This decrease in density can be logically attributed to the chemical and structural alterations introduced during the esterification process.
Lignin is inherently a dense, aromatic biopolymer, characterized by rigid phenylpropanoid units and extensive intermolecular interactions, including π-π stacking and hydrogen bonding. These features contribute to a tightly packed and compact molecular arrangement. However, the esterification of lignin with aspartic acid disrupts this native architecture. The addition of aspartic acid introduces bulky, hydrophilic side chains that increase steric hindrance and reduce the degree of molecular packing. This structural modification not only interferes with aromatic stacking but also introduces greater conformational flexibility and spatial irregularity within the polymer network.
Furthermore, the enhanced polarity of the LiAs-modified structure may facilitate the retention of small amounts of water or promote the formation of microvoids, both of which can contribute to a lower overall material density. Thus, the observed reduction in density for LiAs-based composites is consistent with the molecular reorganization and increased free volume induced by esterification, highlighting the significant impact of chemical modification on the physical properties of lignin-based systems.
The presence of RC extract also influences material density. Gu/DexS/RC materials present a lower density (1.28 g/cm
3) compared to Gu/DexS/Li. RC extract consists of bioactive compounds [
35], which have a lower molecular weight and are less dense than lignin’s rigid, aromatic polymer structure. These materials disrupt the packing efficiency of the polymeric matrix, resulting in a less dense overall structure.
3.8. Mechanical Properties of Materials
The mechanical properties of the Gu/DexS-based materials exhibit significant variations depending on the presence of lignin (Li), esterified lignin (LiAs), and
Rosa canina (RC) extract, as observed in the tensile strength and Young’s modulus evaluations (
Table 7,
Figure 9).
The base formulation, Gu/DexS, serves as a reference, displaying a tensile strength of approximately 1.0 MPa and a Young’s modulus of 1.42 ± 0.15 MPa. The incorporation of RC extract (Gu/DexS/RC) results in a considerable enhancement, increasing the tensile strength to about 1.3 MPa and elevating the Young’s modulus to 3.18 ± 0.96 MPa. This represents an increase of over 120%, which suggests that the polyphenolic compounds in RC contribute to intermolecular hydrogen bonding within the matrix, reinforcing the structural integrity of the film. The observed improvement is consistent with previous studies reporting increased tensile strength and antioxidant activity upon the introduction of polyphenol-rich grape seed and green tea extracts [
37].
The addition of lignin (Gu/DexS/Li) further improves mechanical performance, with Young’s modulus increasing to approximately 1.7 MPa and tensile strength reaching 1.5 MPa. Lignin molecules tend to form particle-like structures that contribute to a more rigid phase within the polymeric matrix, strengthening the network and enhancing stiffness and tensile strength [
38,
39]. Consequently, the Gu/DexS/Li material exhibits an 18.26% reduction in elongation at break compared to Gu/DexS, which is indicative of its increased stiffness. A more pronounced effect is observed when lignin is combined with RC extract (Gu/DexS/Li/RC), where tensile strength increases to 1.8 MPa and Young’s modulus reaches 2.83 ± 0.19 MPa, confirming a synergistic effect between lignin and polyphenols that further reinforces the composite through additional hydrogen bonding.
A substantial shift in mechanical behavior is observed upon the esterification of lignin with aspartic acid (LiAs). The Gu/DexS/LiAs formulation exhibits an increase in rigidity by approximately 30–40%, leading to a tensile strength of 0.67 ± 0.32 MPa and a significantly reduced elongation at break of 203.4 ± 10.9% (
Table 7). These findings can be attributed to electrostatic interactions between the negatively charged aspartate groups of lignin ester and the polymeric matrix, which contains negatively charged sulfate groups from DexS. The increased rigidity is further reflected in the Young’s modulus of 2.2 MPa, marking a 45% improvement compared to Gu/DexS.
The most pronounced enhancement in mechanical properties is observed when both esterified lignin and
Rosa canina extract are present in the Gu/DexS/LiAs/RC formulation. This composite exhibits a tensile strength of 1.49 ± 0.17 MPa, which is more than double that of Gu/DexS/LiAs alone, and a Young’s modulus of 3.74 ± 0.08 MPa, representing an overall increase of over 160% relative to Gu/DexS. The enhanced stiffness can be explained by the combined effects of additional hydrogen bonding between the hydroxyl groups of the matrix and the polyphenolic compounds in RC, as well as the increased crosslinking capacity introduced by esterified lignin. Previous studies have suggested that such polyphenolic-rich extracts contribute to the formation of denser polymer networks via interfacial adhesion, further reinforcing mechanical stability [
40].
The mechanical performance of the developed Gu/DexS-based materials was evaluated in comparison with the literature data on polysaccharide-based hydrogels designed for dermal or biomedical use. The optimized formulation of Gu/DexS/LiAs/RC exhibited a tensile strength of 1.49 MPa and an elongation at break of 237.8%, values significantly superior to those typically reported for commercial hydrogel dressings, which range between 0.03–0.3 MPa for tensile strength and 10–100% for elongation at break, depending on composition and crosslinking density [
27,
31,
34]. These results indicate that the incorporation of both lignin and
Rosa canina extract led to enhanced polymer chain interactions, improving both the resistance to mechanical stress and the elasticity of the final hydrogel network. The obtained mechanical parameters suggest that these hybrid materials can meet or exceed the structural requirements of current skin-compatible biomaterials, and may be particularly suitable for flexible wound coverings or dermal patches.
Overall, the results confirm that, while lignin alone provides a moderate increase in mechanical properties due to its role in crosslinking and network stabilization, the combination of esterified lignin and RC extract leads to a significantly stronger and stiffer material. The synergistic effect of these components enhances tensile properties, making the resulting biocomposite a promising candidate for applications requiring robust mechanical performance.
3.9. Material Morphology
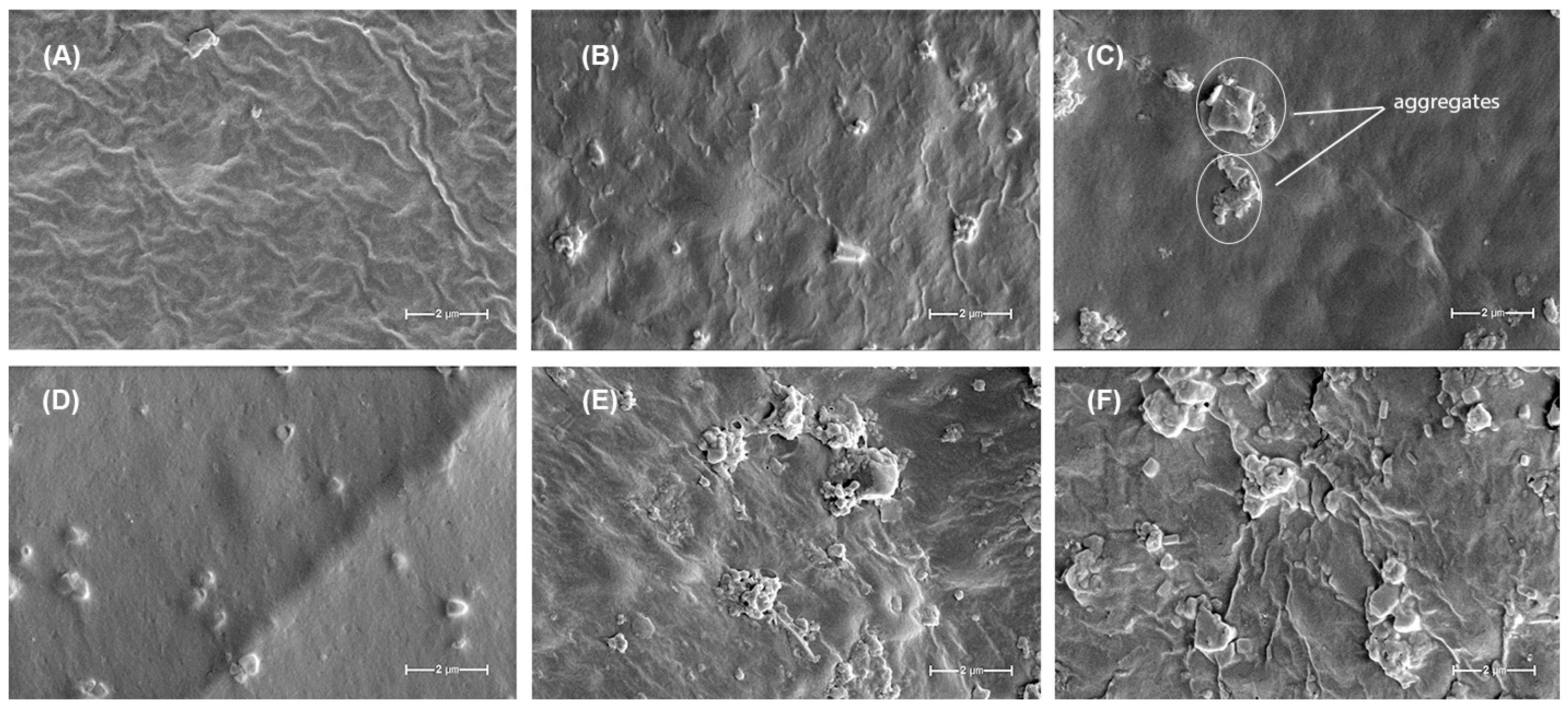
The SEM images provide valuable insights into the morphological characteristics of the Gu/DexS-based materials and their correlation with the mechanical properties and component interactions.
The reference material, Gu/DexS (
Figure 10A), exhibits a relatively smooth and homogeneous surface with slight wrinkles, indicative of an organized polymeric network. This morphology is consistent with the moderate mechanical strength observed for this formulation, where the polysaccharide-based matrix provides a uniform structure but lacks reinforcement.
Upon the incorporation of
Rosa canina extract (Gu/DexS/RC,
Figure 10B), the surface becomes slightly rougher, with minor agglomerations and irregularities. This suggests the integration of polyphenolic compounds, likely through hydrogen bonding interactions with the polymeric matrix. These interactions lead to an increase in Young’s modulus and tensile strength, as previously observed, reinforcing the film without significantly disrupting its homogeneity.
The addition of lignin (Gu/DexS/Li,
Figure 10C) results in a noticeable change in surface morphology, characterized by increased roughness and the appearance of distinct particulate aggregates, as highlighted in the image. These aggregates reflect localized clustering of lignin within the polymer matrix, which likely contributes to the observed enhancement in mechanical strength. While this structural reinforcement improves tensile resistance, the heterogeneous distribution of these domains may also introduce local stress concentrations, potentially explaining the moderate decrease in elongation at break compared to the lignin-free formulation.
When both lignin and
Rosa canina extract are present (Gu/DexS/Li/RC,
Figure 10D), the surface appears somewhat smoother compared to Gu/DexS/Li but retains small dispersed particles. This suggests a better dispersion of lignin within the matrix, likely facilitated by interactions with the polyphenolic compounds from the extract. The mechanical properties reflect this synergy, as the material exhibits improved tensile strength and modulus while maintaining a more balanced structural integrity.
A major shift in morphology is observed in the Gu/DexS/LiAs sample (
Figure 10E), where large agglomerates and morphological heterogeneity become evident. The esterification of lignin with aspartic acid introduces additional functional groups, leading to stronger electrostatic interactions with the negatively charged DexS matrix. This results in a denser and stiffer structure, as evidenced by the significant increase in Young’s modulus. However, the reduced elongation at break and breaking length indicate that this enhanced rigidity compromises flexibility, leading to a more brittle material.
The most heterogeneous morphology is observed in the Gu/DexS/LiAs/RC sample (
Figure 10F), where large irregular aggregates are dispersed throughout the matrix. This suggests strong interactions among the esterified lignin,
Rosa canina extract, and the polysaccharide matrix, leading to significant reinforcement and increased morphological heterogeneity. The mechanical properties confirm this trend, with the highest Young’s modulus and breaking length values, indicating a rigid and mechanically robust material. However, the excessive aggregation may contribute to localized stress points, which could affect the material’s overall flexibility.
Overall, the SEM analysis confirms that lignin, esterified lignin, and Rosa canina extract interact differently with the Gu/DexS matrix, affecting both mechanical properties and morphology. While lignin and RC extract improve mechanical performance through hydrogen bonding and dispersion effects, the esterification of lignin with aspartic acid introduces strong electrostatic interactions that lead to a denser but more brittle structure. The combination of esterified lignin and RC extract results in the most rigid material, confirming that chemical modifications and polyphenol interactions play a critical role in determining the final properties of the composite films.
3.10. RC Extract Release from Materials
The mathematical modeling of drug release from polymeric matrices based on natural polysaccharides provides a framework to understand and predict the underlying transport mechanisms. The Higuchi model [
41], one of the earliest and most widely used, is typically expressed as Equation (6):
where
Q is the amount of drug released at time
t, and
kH is the Higuchi dissolution constant. This parameter
kH combines characteristics of drug diffusivity, solubility, and matrix porosity, indicating how rapidly the drug migrates out of the polymer. When the release kinetics follow Fickian diffusion and the matrix remains relatively intact, a good fit to the Higuchi equation implies that diffusion is the dominant process controlling drug release.
In cases where the release profile exhibits a sigmoidal shape due to variable rates of swelling or erosion in natural polysaccharide matrices, the Makoid–Banakar model [
42] can be applied. It is generally written as Equation (7):
where
F(
t) is the fraction of drug released,
a is a scaling factor related to the maximum release,
k is the release rate constant, and
n denotes the shape of the release curve. Here,
a reflects the asymptotic extent of release,
k provides insight into how quickly the transition from slow to accelerated release occurs, and
n controls the steepness or sigmoid form of the curve. These parameters become especially relevant in natural polysaccharide systems that may exhibit an initial lag phase followed by a distinct acceleration in drug release once the polymer begins to swell or degrade.
The Weibull model [
43] is a versatile mathematical tool widely employed to describe the kinetics of drug release from polymeric matrices. Its flexible form allows for the characterization of various release mechanisms, ranging from diffusion-dominated processes to degradation-controlled systems. The general equation (Equation (8)) of the Weibull model is as follows:
where
y—cumulative drug release at time
x;
A—maximum cumulative release (asymptotic value);
k—release rate constant, indicating the speed of drug release;
xc—lag time before significant drug release begins;
d—shape parameter, which determines the nature of the release curve.
By fitting experimental data to the Weibull model, the values of A, k, xc, and d provide valuable insights into the drug release profile: A—indicates the total amount of drug that can be released; k—reflects the speed of release, influenced by the matrix’s structure and drug properties; xc—identifies any delay in release, such as a hydration phase or lag due to matrix swelling; d—reveals the dominant release mechanism, distinguishing between diffusion and matrix degradation.
The model accommodates various types of polymeric matrices, including hydrogels, crosslinked networks, and composite materials. Its adaptability allows it to describe systems with single or combined release mechanisms.
The Weibull model is often used to fit experimental release data, enabling the determination of the kinetics and mechanisms of drug release. For example, a low d value (<1) indicates diffusion-dominated release, common in hydrophilic polymeric matrices.
By comparing k and d values across different formulations, researchers can evaluate the influence of polymer composition, crosslinking density, and drug–polymer interactions on release behavior.
When matrix erosion or structural disintegration governs release rather than diffusion alone, the Hixson–Crowell cube-root model [
44] is often appropriate. It is typically expressed as Equation (9):
where
Q0 is the initial amount of drug in the dosage form,
Q is the remaining amount of drug at time
t, and
kHC is the Hixson–Crowell constant; this equation underscores how changes in surface area drive drug liberation. The constant
kHC represents the rate at which the dosage form diminishes in size, capturing how quickly erosion of the polysaccharide matrix exposes a fresh surface area for drug release.
Another sigmoidal model often used for complex release kinetics is the Gompertz function, written in the dissolution context as Equation (10):
where
b and
α determine the shape and rate of the release curve. The parameter
b generally signifies the scale of the release process, sometimes interpreted as relating to the position of the inflection point, whereas
α influences how quickly the release curve rises after an initial lag phase. When analyzing natural polysaccharide matrices, changes in polymer swelling or crosslinking can manifest in shifts in
b and
α, thereby indicating whether the system is prone to early rapid swelling or a delayed onset of drug release.
A versatile semi-empirical model is the Korsmeyer–Peppas equation [
45], usually given as Equation (11):
where
kKP is the release rate constant, and
n is the release exponent. This exponent
n classifies the release mechanism: if
n ≈ 0.5, Fickian diffusion is typically dominant; if
n is closer to 1.0, erosion or polymer relaxation strongly influences the release; values in between often indicate anomalous transport. In many natural polysaccharides that can undergo swelling and partial erosion, the Korsmeyer–Peppas model is invaluable for distinguishing which process—diffusion, swelling, or erosion—takes precedence. The constant
kKP then provides a measure of how quickly the drug traverses the matrix, guiding formulation scientists in adjusting polymer properties to achieve the desired release rate.
Collectively, these models and their parameters depict how the microstructure of natural polysaccharides, as well as their swelling, erosion, and diffusion characteristics, influence the course of drug release. By comparing experimental dissolution profiles with predicted curves and identifying which parameters shift in response to formulation changes, it becomes possible to design polymeric systems with optimized release patterns suitable for a wide range of therapeutic applications.
To identify the most appropriate mathematical model describing the release of
Rosa canina extract from the polymeric materials (Gu/DexS/RC, Gu/DexS/Li/RC, and Gu/DexS/LiAs/RC), the abovementioned mathematical models were evaluated. Model performance was compared using both the reduced chi-square (
red-χ2) and the coefficient of determination (R
2) as shown in
Table 8.
Overall, the Makoid–Banakar model exhibited the most consistent goodness of fit across all three tested materials. In the Gu/DexS/Li/RC system, for instance, it yielded a reduced chi-square of 1.8442 and an R2 of 0.98799, outperforming the Weibull model (red-χ2 = 2.14893, R2 = 0.98694), as well as the other four models tested. In the Gu/DexS/LiAs/RC system, the Makoid–Banakar model again provided the best fit (red-χ2 = 2.19185, R2 = 0.99221), with notably lower chi-square and higher R2 compared to the next-best alternative (Weibull). Although Weibull demonstrated a slightly lower red-χ2 for Gu/DexS/RC (3.77609 vs. 5.65783 for Makoid–Banakar), Makoid gave consistently high R2 values (0.99292 in Gu/DexS/RC and above 0.98 in the other two systems), leading to superior overall performance when all samples were evaluated collectively.
Furthermore, a rank-sum approach was used to account for performance differences among the three systems. Each model was ranked based on its red-χ2 in each system; then, the ranks were summed. Makoid had the lowest total rank sum (i.e., it was either first or second place in each polymeric material), thus reinforcing its robust and reliable fit across all formulations. Given these findings, and considering that the Makoid–Banakar model uses a moderate number of parameters (which helps mitigate overfitting risks), Makoid was selected as the single most appropriate model to describe release kinetics from the three polymeric materials in this study.
The release kinetic of RC extract from tested materials according to the Makoid–Banakar model is depicted in
Figure 11.
In the Gu/DexS/RC formulation, the Makoid–Banakar parameters were k ≈ 5.84, a ≈ 0.00293, and n ≈ 0.635, indicative of a relatively brisk initial release (higher k) and a moderate exponent (n) that underlines both diffusion-driven kinetics and matrix relaxation effects. When lignin was incorporated into the Gu/DexS blend (Gu/DexS/Li/RC), k dropped by about 25% (to ≈4.38), reflecting a slower initial rate of RC extract release. The exponential decay factor a also showed a marked reduction of roughly 74% (to ≈0.00076), suggesting a more sustained deceleration of release over time. This behavior can be attributed to the increased hydrophobicity and structural density introduced by lignin, which limits water penetration and thus dampens the overall release rate. Concomitantly, the exponent n shifted to ≈0.451, highlighting a stronger reliance on diffusion processes relative to polymer relaxation, as the lignin-containing network restricts chain mobility.
Further modifications in the matrix composition were evident in Gu/DexS/LiAs/RC, where the esterification of lignin with aspartic acid altered the release profile once again. The rate constant k fell to ≈3.24, a clear sign that the composite became even less permissive to rapid RC release, yet a rose to ≈0.00123, partially restoring the influence of exponential decay. This indicates that the aspartic acid moieties, with their polar groups, somewhat balance out lignin’s hydrophobic character, allowing moderate re-swelling or structural reorganization. The exponent n remained close to 0.57, suggesting that the combined effects of diffusion and matrix relaxation persisted but were tempered by the interplay of the various components in the polymeric network. Altogether, the numerical values of k, a, and n show that Gu and DexS alone support faster and somewhat diffusion-limited release, while the addition of lignin slows the initial release and prolongs drug availability, and lignin-aspartic acid conjugates introduce intermediate hydrophilic–hydrophobic interactions that modulate both the rate and mechanism of release. The ability of the Makoid–Banakar model to incorporate a power-law component and an exponential decay term, each corresponding to distinct release mechanisms, highlights its superior performance in describing how Gu, DexS, Li, LiAs, and RC interact within the polymeric network to govern the overall release process.
3.11. Anti-Inflammatory Activity of Materials
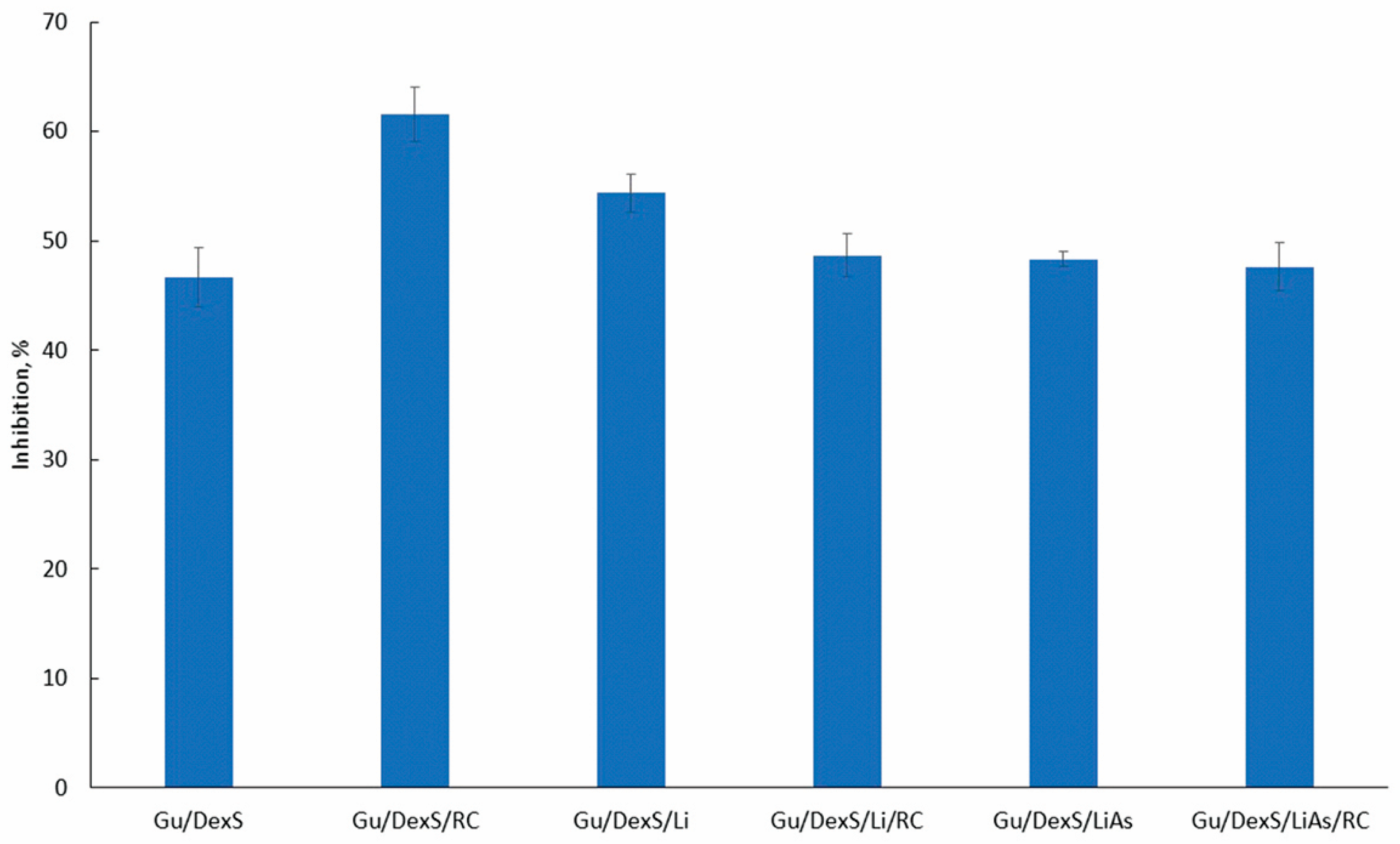
The anti-inflammatory activity of the Gu/DexS-based materials was evaluated using the egg albumin denaturation assay. The results (
Figure 12) indicate that all formulations incorporating fillers exhibited the notable inhibition of protein denaturation (>45%). The strongest activity was recorded for Gu/DexS/RC (61.58%), followed by Gu/DexS/Li (54.36%) and Gu/DexS/Li/RC (48.70%), confirming the beneficial role of
Rosa canina extract (RC) and lignin (Li) in mitigating protein aggregation under stress conditions.
The superior performance of Gu/DexS/RC is attributed to the phenolic and flavonoid content of RC extract (
Section 2.4 and
Section 2.5) that contributes to its antioxidant and anti-inflammatory properties [
29]. The relatively smooth surface morphology of Gu/DexS/RC observed in the SEM micrographs (
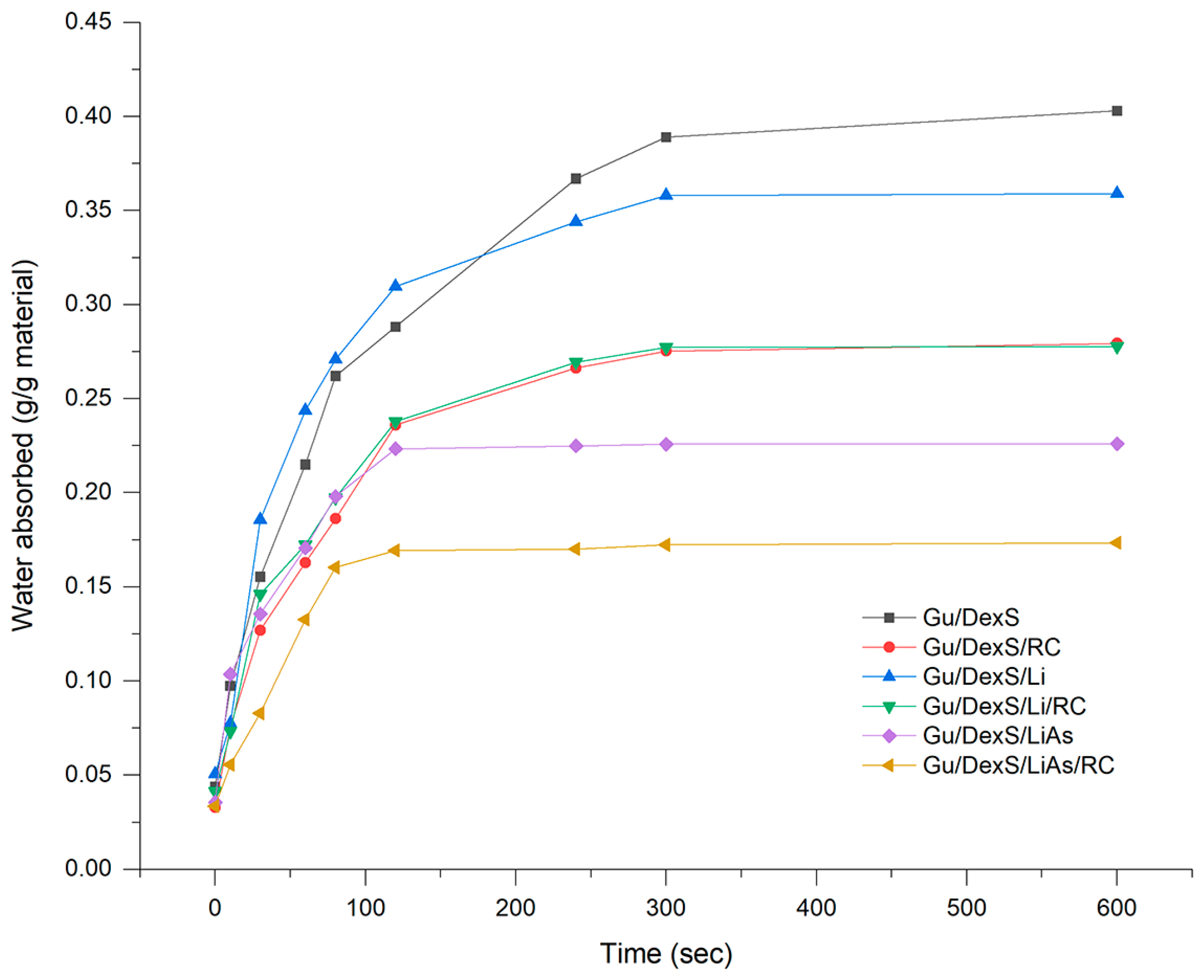
Figure 10B) supports the good dispersion of the extract in the matrix, facilitating homogeneous release and consistent surface bioactivity. Moreover, its moderate swelling capacity (0.23 g/g,
Figure 8) supports rapid hydration, enhancing active compound diffusion to the application site.
Incorporating unmodified lignin (Gu/DexS/Li) also contributed to anti-inflammatory effects (54.36%) due to lignin’s intrinsic phenolic antioxidant activity [
6]. However, the lower swelling ratio (0.35 g/g) and higher density (3.60 g/cm
3,
Figure 8) compared to Gu/DexS/RC suggest a more compact matrix, likely delaying active release. The mechanical reinforcement observed (Young’s modulus ~ 1.70 MPa,
Table 7 and
Figure 9) correlates with the rougher, aggregated morphology seen in the SEM images (
Figure 10C), which may limit matrix flexibility but improve sustained activity.
Interestingly, Gu/DexS/Li/RC showed slightly reduced inhibition (48.70%) compared to RC or Li alone. This suggests potential competitive interactions between polyphenols and lignin at the molecular level, possibly hindering optimal release or surface availability. These findings align with the observed mechanical stiffening (Young’s modulus ~ 2.83 MPa) and increased matrix density (2.33 g/cm3), both of which may restrict the diffusion of actives.
The esterification of lignin with aspartic acid (LiAs) aimed to enhance compatibility with the hydrophilic matrix and introduce additional bioactivity. Although aspartic acid is known to reduce oxidative stress [
36], the Gu/DexS/LiAs/RC formulation presented a modest inhibition effect (~46%), slightly lower than Gu/DexS/Li. This may be due to the denser, phase-separated morphology (
Figure 10F), which can entrap active compounds and reduce immediate bioavailability.
Drug release kinetics further explain this observation. According to the Makoid–Banakar model (
Table 8), the rate constant (k) decreased significantly from Gu/DexS/RC (5.84) to Gu/DexS/Li/RC (4.38) and Gu/DexS/LiAs/RC (3.24), indicating delayed RC extract release. Additionally, the exponential decay factor (a) and release exponent (n) dropped across the same series, reflecting a shift from rapid to more diffusion-controlled release (n values: 0.635 → 0.451 → 0.573). These trends correlate with the reduced swelling ratios and increasing matrix rigidity, confirming that the delayed but sustained release profile in lignin-containing systems contributes to prolonged, though less immediate, anti-inflammatory action.
Overall, anti-inflammatory efficacy across the materials is determined by a fine balance between structural compactness, filler chemistry, mechanical behavior, and drug release dynamics. Gu/DexS/RC offers the fastest and most potent activity due to rapid swelling and RC diffusion, while Gu/DexS/Li and Gu/DexS/LiAs provide sustained effects through reinforced matrices and delayed release, making them valuable for prolonged skin therapies.
3.12. In Vitro Biocompatibility of Materials
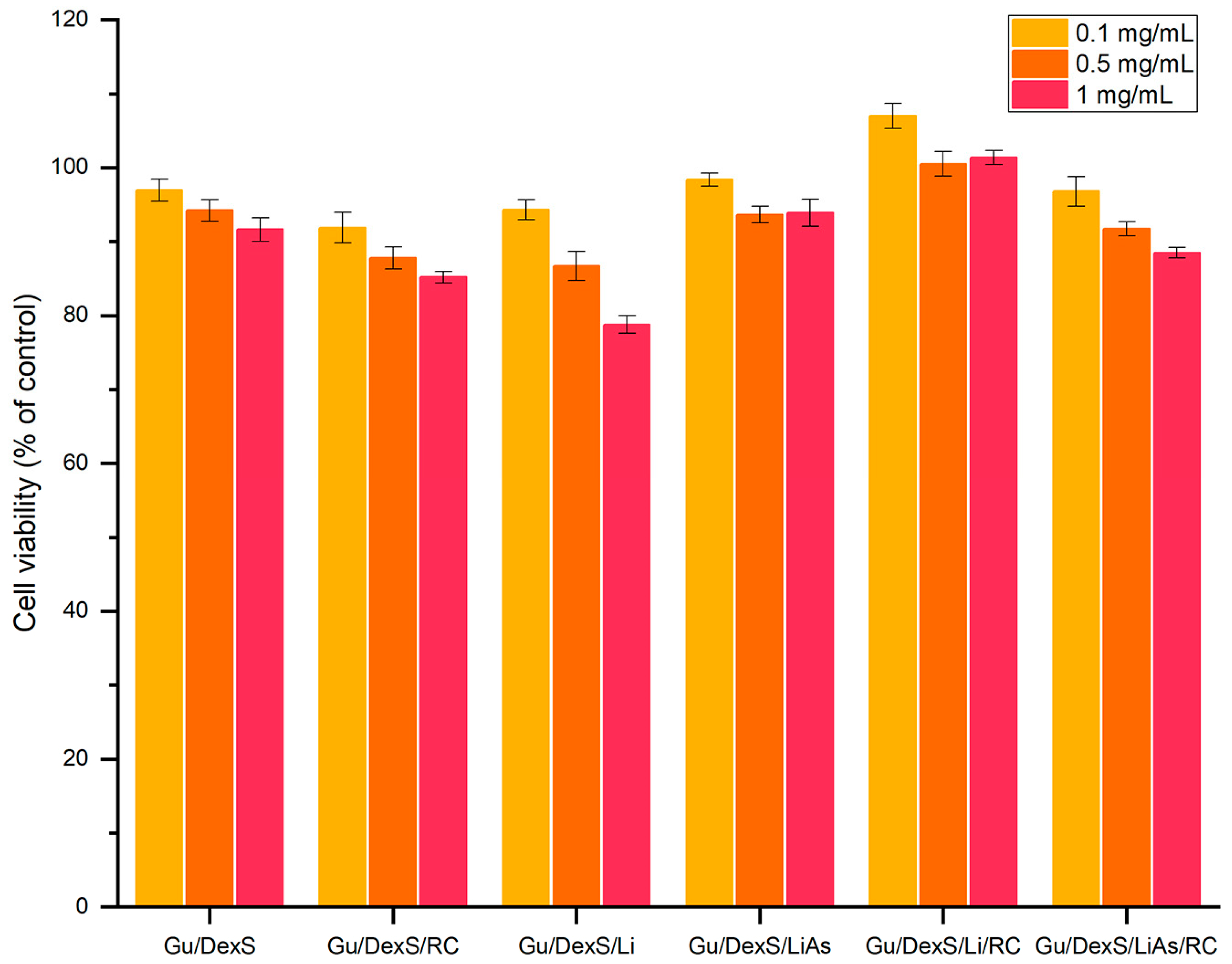
The in vitro biocompatibility of the Gu/DexS-based materials was assessed using the MTS assays on human gingival fibroblasts. All tested formulations exhibited high cell viability across concentrations of 0.1, 0.5, and 1 mg/mL, with values consistently above 80% (
Figure 13), confirming their cytocompatibility and potential for dermatological use.
The reference matrix, Gu/DexS, showed excellent viability (92–97%), attributed to the known non-toxic, hydrophilic nature of polysaccharides, which promote favorable cell adhesion and proliferation [
46]. The SEM images (
Figure 10A) revealed a smooth, uniform surface, while mechanical testing (
Figure 9) indicated moderate stiffness (Young’s modulus ~ 1.42 MPa), supporting its ability to maintain a soft, compliant interface conducive to cell growth.
The addition of
Rosa canina extract slightly reduced viability at higher concentrations (85% at 1 mg/mL), likely due to the polyphenolic content exerting mild pro-oxidative or dose-dependent effects [
47]. Despite this, Gu/DexS/RC maintained robust cellular response while achieving the highest anti-inflammatory effect (61.58% inhibition of albumin denaturation,
Figure 12), indicating a balanced bioactivity–biocompatibility profile.
Materials containing unmodified lignin (Gu/DexS/Li) exhibited lower cell viability at 1 mg/mL (~79%), likely due to the hydrophobic, aromatic character of lignin and its rougher surface morphology (
Figure 10C), which may hinder cell adhesion and nutrient exchange. Mechanical reinforcement (Young’s modulus ~ 1.70 MPa) further reflects this rigidity, possibly compromising cellular spreading.
By contrast, the esterification of lignin with aspartic acid (LiAs) significantly improved biocompatibility. Gu/DexS/LiAs showed cell viability of 94–98%, a direct result of increased polarity and hydrophilicity conferred by amino and carboxylic groups in LiAs [
48,
49]. Though the SEM images (
Figure 10E) displayed visible morphological heterogeneity, the improved chemical affinity with the Gu/DexS matrix likely reduced interfacial tension, supporting better cellular interaction. The associated mechanical properties (Young’s modulus ~ 2.2 MPa) reflect a denser but more functionally integrated matrix.
The most favorable biological response was observed for Gu/DexS/LiAs/RC, which combined high cell viability (88–100%) with strong anti-inflammatory activity (46.23%) and enhanced mechanical performance (Young’s modulus ~ 3.74 MPa) (
Figure 9). The SEM micrographs (
Figure 10F) revealed heterogeneous morphology with large aggregates, suggesting strong intermolecular interactions. Despite this structural density, drug release kinetics (
Table 8) remained controlled (
k ≈ 3.24;
n ≈ 0.57, Makoid–Banakar model), allowing the sustained delivery of RC bioactive compounds and reducing cytotoxic peaks.
The pseudo-second-order swelling behavior observed for all materials (
Figure 8,
Table 6) further supports their favorable hydration kinetics, gradually increasing water uptake without sudden volumetric expansion, thus preserving the integrity of the cell–material interface over time [
23].
In summary, the biocompatibility of the tested materials is closely tied to their structural and functional features. The integration of LiAs and RC extract within the Gu/DexS matrix creates a balance between mechanical integrity, sustained bioactive release, and cellular tolerance. The Gu/DexS/LiAs/RC formulation emerges as the most promising candidate for skin-contact biomaterials, combining reinforced structure, antioxidant and anti-inflammatory bioactivity, and excellent cytocompatibility.