Modification of Urea-Formaldehyde Resin with Triethylenetetramine: Effect on Adhesive Properties and Plywood Strength
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adhesive Mixtures Preparation and Testing
2.3. Plywood Manufacturing and Testing
2.4. Statistical Analysis
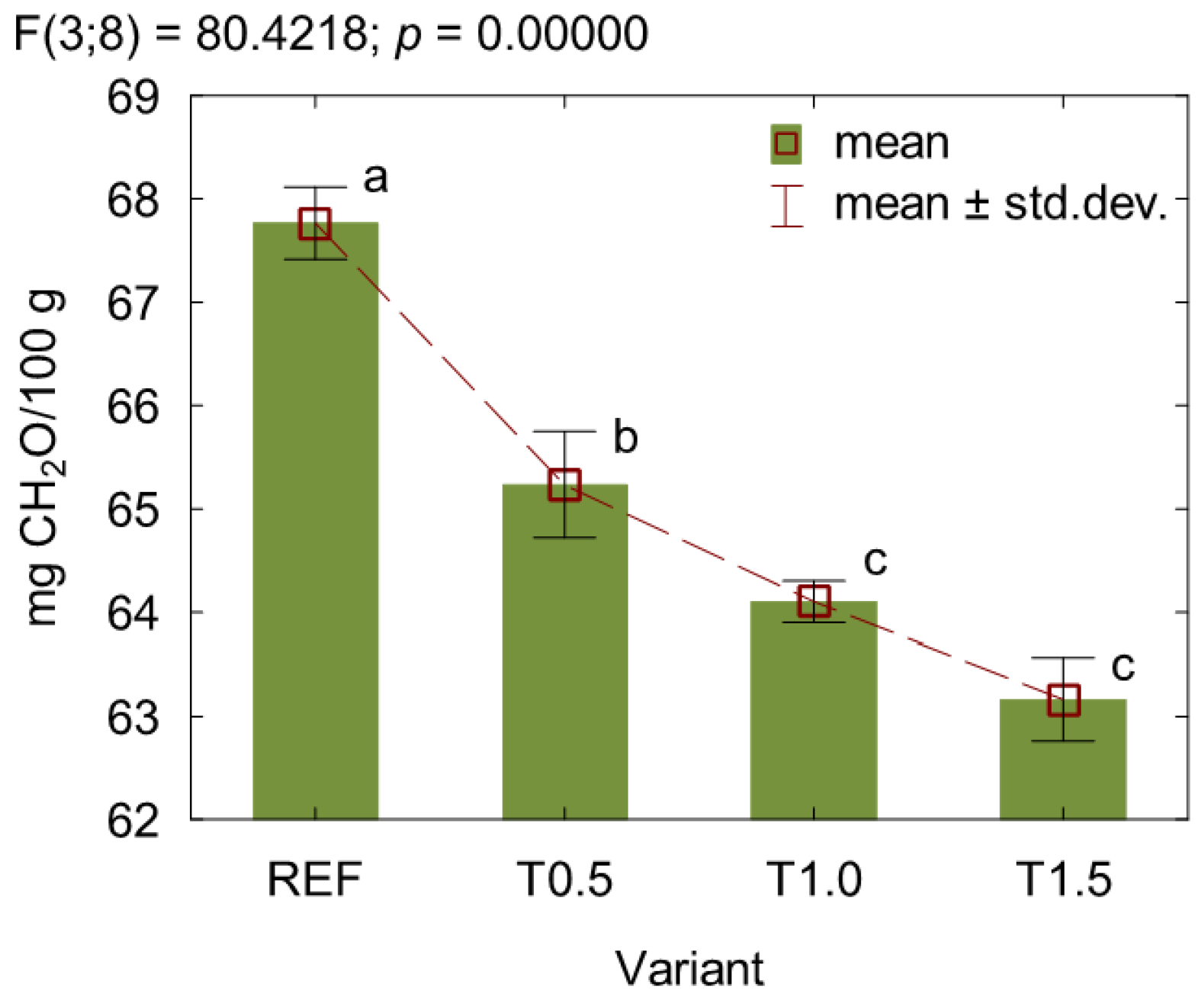
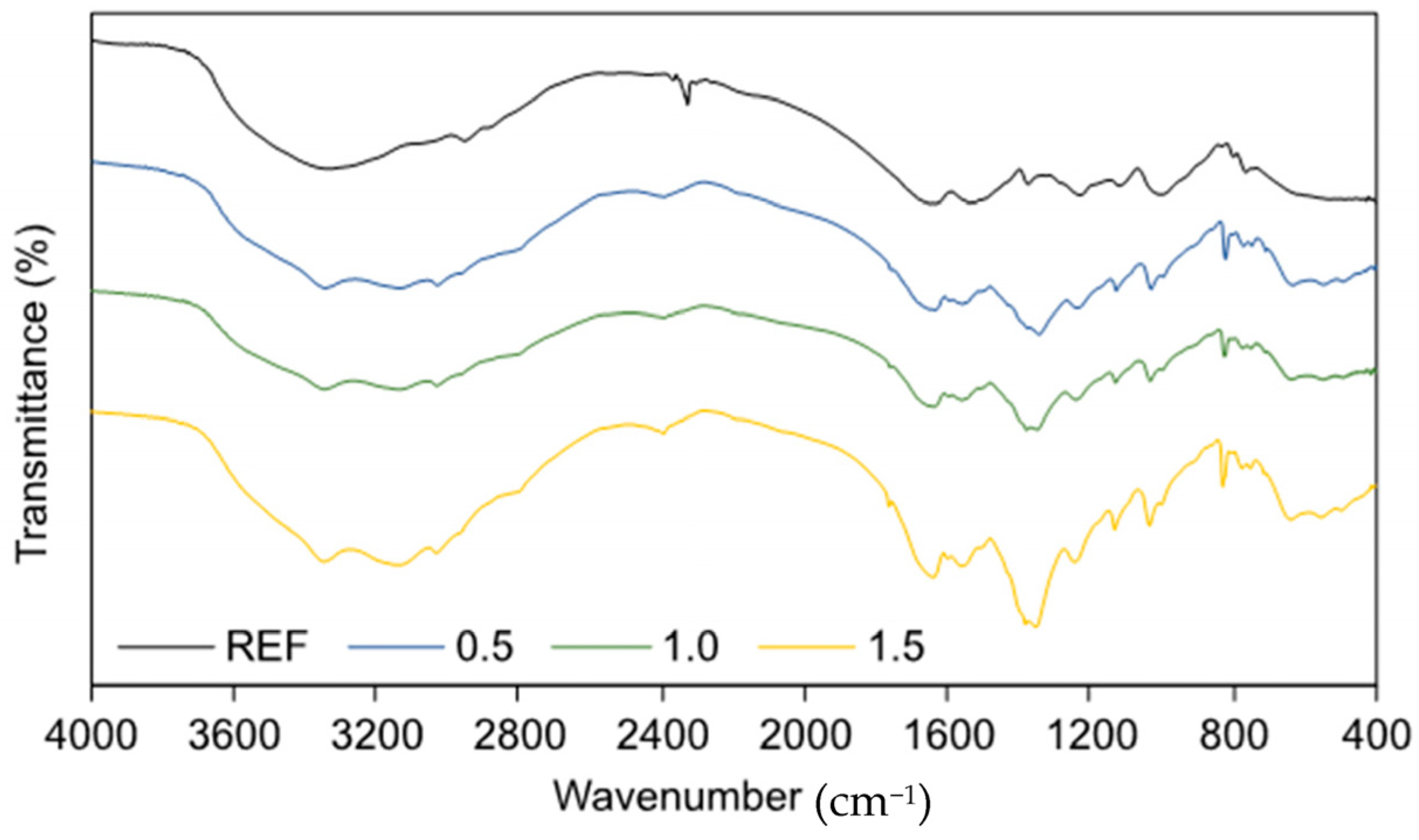
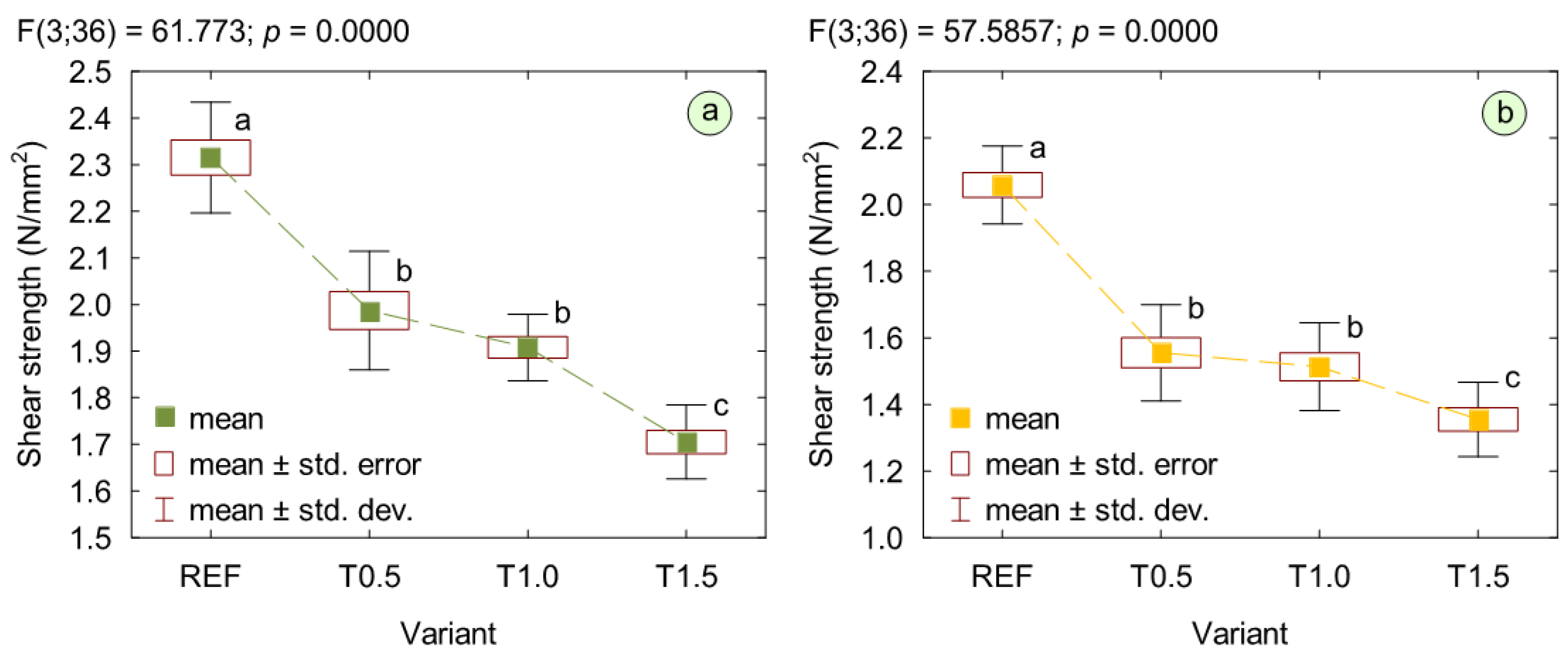
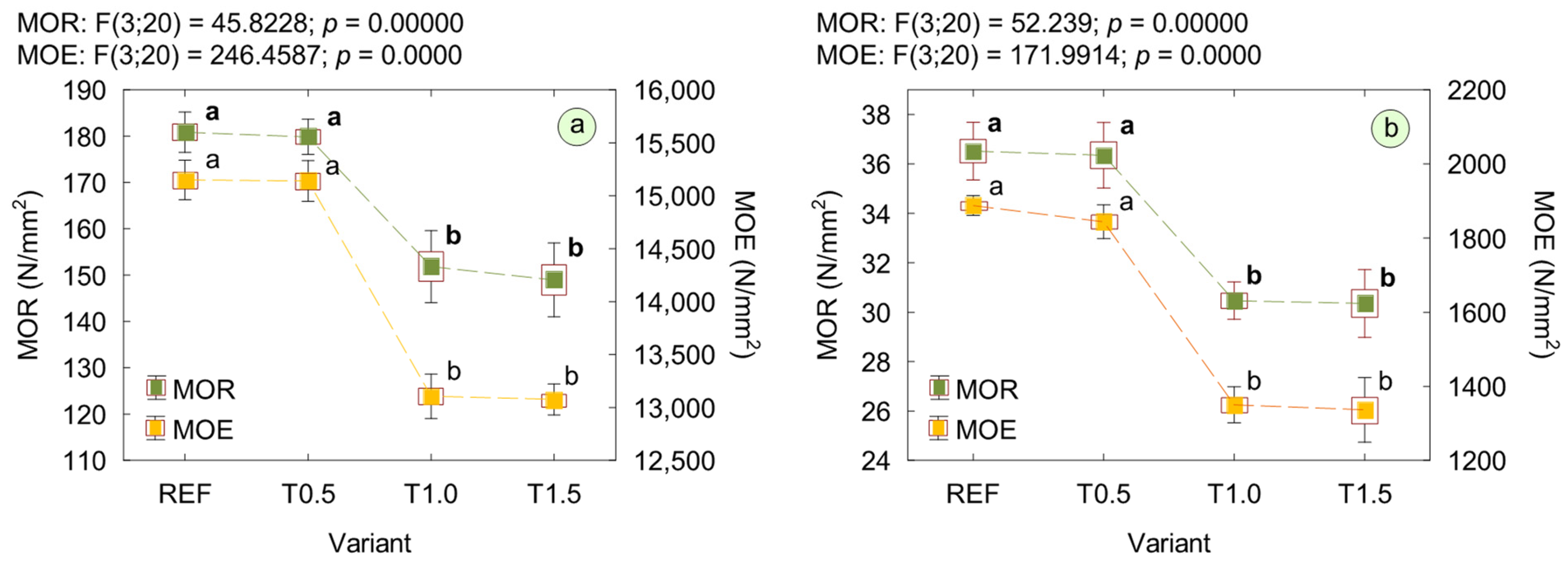
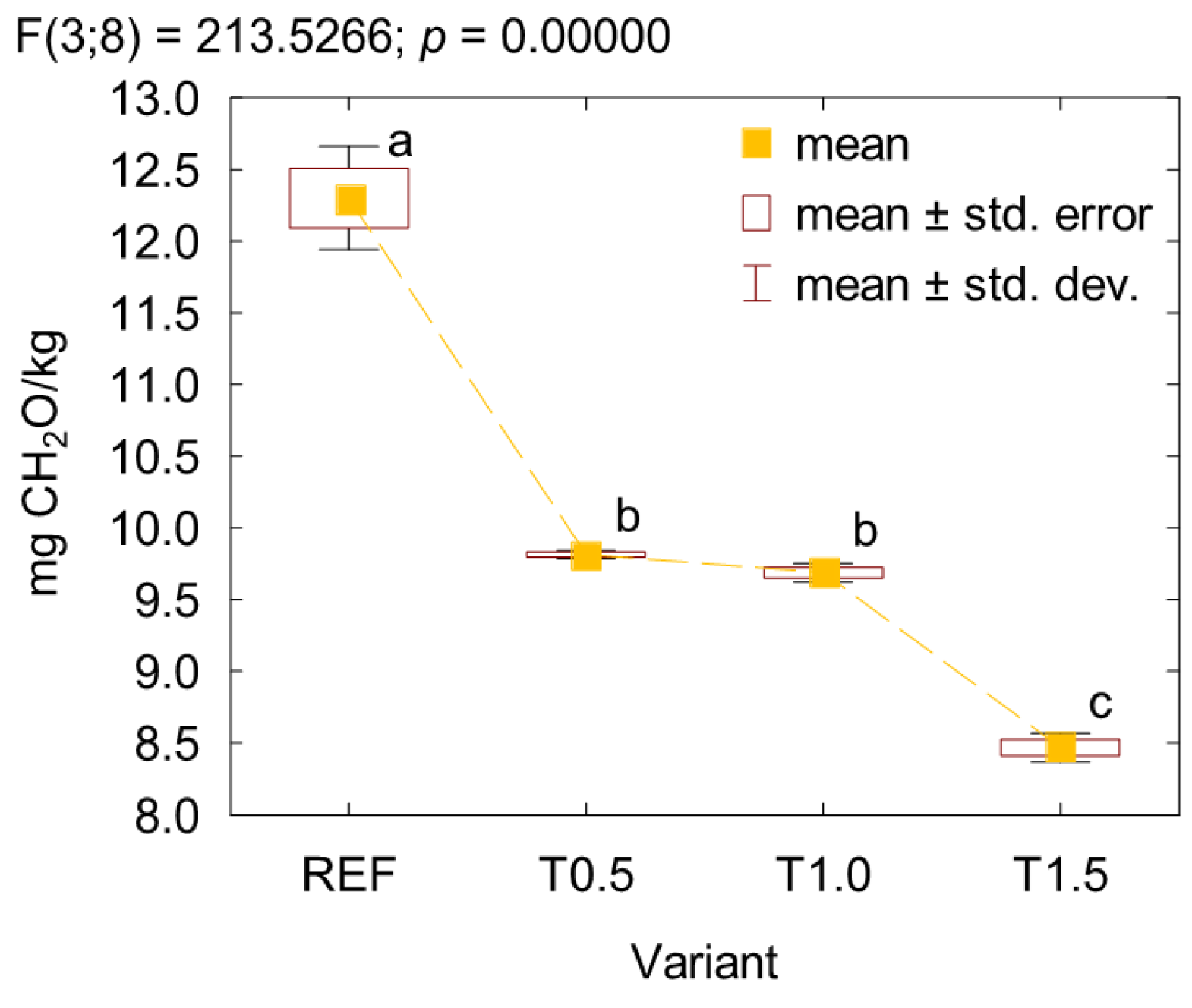
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. FAO Yearbook of Forest Products; Food and Agriculture Organization: Rome, Italy, 2020. [Google Scholar]
- Bekhta, P.; Bryn, O.; Sedliacik, J.; Novák, I. Effect of Different Fire Retardants on Birch Plywood Properties. Acta Fac. Xylologiae Zvolen. Res. Publica Slovaca 2016, 58, 59. [Google Scholar]
- Dukarska, D.; Kawalerczyk, J.; Kmieciak, J. Modified Pine Needles as a Formaldehyde Scavenger for Urea-Formaldehyde Resin in Plywood Production. Eur. J. Wood Wood Prod. 2023, 82, 147–158. [Google Scholar] [CrossRef]
- Muñoz, F.; Moya, R.; Muñoz, F.; Moya, R. Effect of Nanoclay-Treated UF Resin on the Physical and Mechanical Properties of Plywood Manufactured with Wood from Tropical Fast Growth Plantations. Maderas. Cienc. Tecnol. 2018, 20, 11–24. [Google Scholar] [CrossRef]
- Jorda, J.; Kain, G.; Barbu, M.-C.; Petutschnigg, A.; Král, P. Influence of Adhesive Systems on the Mechanical and Physical Properties of Flax Fiber Reinforced Beech Plywood. Polymers 2021, 13, 3086. [Google Scholar] [CrossRef] [PubMed]
- Cakiroglu, E.; Demir, A.; Aydin, I. Comparison of Birch and Beech Wood in Terms of Economic and Technological Properties for Plywood Manufacturing. Drv. Ind. 2019, 70, 169–174. [Google Scholar] [CrossRef]
- Setter, C.; Zidanes, U.L.; De Novais Miranda, E.H.; Brito, F.M.S.; Mendes, L.M.; Junior, J.B.G. Influence of Wood Species and Adhesive Type on the Performance of Multilaminated Plywood. Environ. Sci. Pollut. Res. 2021, 28, 50835–50846. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, X.; Shi, R.; Gao, Q.; Li, J.; Li, L. Determination of Formaldehyde and TVOC Emission Behavior from Interior Use Plywood Using Various Post Heat Treatment Processes. J. Appl. Polym. Sci. 2017, 134, 44909. [Google Scholar] [CrossRef]
- Dorieh, A.; Selakjani, P.P.; Shahavi, M.H.; Pizzi, A.; Movahed, S.G.; Pour, M.F.; Aghaei, R. Recent Developments in the Performance of Micro/Nanoparticle-Modified Urea-Formaldehyde Resins Used as Wood-Based Composite Binders: A Review. Int. J. Adhes. Adhes. 2022, 114, 103106. [Google Scholar] [CrossRef]
- Kaden, D.A.; Mandin, C.; Nielsen, G.D.; Wolkoff, P. Formaldehyde. In WHO Guidelines for Indoor Air Quality: Selected Pollutants; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Dunky, M. Urea–Formaldehyde (UF) Adhesive Resins for Wood. Int. J. Adhes. Adhes. 1998, 18, 95–107. [Google Scholar] [CrossRef]
- Bekhta, P.; Sedliacik, J.; Saldan, R.; Novak, I. Effect of Different Hardeners for Urea-Formaldehyde Resin on Properties of Birch Plywood. Acta Fac. Xylologiae Zvolen. Res. Publica Slovaca 2016, 58, 65. [Google Scholar]
- Reingruber, H.; Pontel, L.B. Formaldehyde Metabolism and Its Impact on Human Health. Curr. Opin. Toxicol. 2018, 9, 28–34. [Google Scholar] [CrossRef]
- Protano, C.; Buomprisco, G.; Cammalleri, V.; Pocino, R.N.; Marotta, D.; Simonazzi, S.; Cardoni, F.; Petyx, M.; Iavicoli, S.; Vitali, M. The Carcinogenic Effects of Formaldehyde Occupational Exposure: A Systematic Review. Cancers 2021, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, L.; Barbosa, E.; Charao, M.F.; Brucker, N. Formaldehyde Toxicity Reports from in Vitro and in Vivo Studies: A Review and Updated Data. Drug Chem. Toxicol. 2022, 45, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Kristak, L.; Antov, P.; Bekhta, P.; Lubis, M.A.R.; Iswanto, A.H.; Reh, R.; Sedliacik, J.; Savov, V.; Taghiyari, H.R.; Papadopoulos, A.N.; et al. Recent Progress in Ultra-Low Formaldehyde Emitting Adhesive Systems and Formaldehyde Scavengers in Wood-Based Panels: A Review. Wood Mater. Sci. Eng. 2023, 18, 763–782. [Google Scholar] [CrossRef]
- Chen, L.; Long, Y.; Huang, B.; Sun, C.; Kang, S.; Wang, Y. Technological Innovations Review in Reducing Formaldehyde Emissions through Adhesives and Formaldehyde Scavengers for Building Materials. Environ. Res. 2025, 273, 121242. [Google Scholar] [CrossRef]
- Hassannejad, H.; Shalbafan, A.; Rahmaninia, M. Reduction of Formaldehyde Emission from Medium Density Fiberboard by Chitosan as Scavenger. J. Adhes. 2018, 96, 797–813. [Google Scholar] [CrossRef]
- Ghani, A.; Bawon, P.; Ashaari, Z.; Wahab, M.W.; Hua, L.S.; Chen, L.W. Addition of Propylamine as Formaldehyde Scavenger for Urea Formaldehyde-Bonded Particleboard. Wood Res. 2017, 62, 329–334. [Google Scholar]
- Ghani, A.; Ashaari, Z.; Bawon, P.; Lee, S.H. Reducing Formaldehyde Emission of Urea Formaldehyde-Bonded Particleboard by Addition of Amines as Formaldehyde Scavenger. Build. Environ. 2018, 142, 188–194. [Google Scholar] [CrossRef]
- Kawalerczyk, J.; Walkiewicz, J.; Woźniak, M.; Dziurka, D.; Mirski, R. The Effect of Urea-Formaldehyde Adhesive Modification with Propylamine on the Properties of Manufactured Plywood. J. Adhes. 2023, 99, 1427–1440. [Google Scholar] [CrossRef]
- Boran, S.; Usta, M.; Gümüşkaya, E. Decreasing Formaldehyde Emission from Medium Density Fiberboard Panels Produced by Adding Different Amine Compounds to Urea Formaldehyde Resin. Int. J. Adhes. Adhes. 2011, 31, 674–678. [Google Scholar] [CrossRef]
- Gao, W.; Du, G.; Kamdem, P. Influence of Ammonium Pentaborate (APB) on the Performance of Urea Formaldehyde (UF) Adhesives for Plywood. J. Adhes. 2015, 91, 186–196. [Google Scholar] [CrossRef]
- Valyova, M.; Ivanova, Y.; Koynov, D. Investigation of Free Formaldehyde Quantity in Production of Plywood with Modified Urea-Formaldehyde Resin. Int. J. Wood Des. Technol. 2017, 6, 72–76. [Google Scholar]
- EN 827; Adhesives—Determination of Conventional Solids Content and Constant Mass Solids Content. Slovenski Inštitut za Standardizacijo: Ljubljana, Slovenia, 2005.
- PN-C-89352-3; Kleje-Kleje do Drewna-Metody Badań—Oznaczanie Czasu Żelowania. Polish Committee for Standardization: Warsaw, Poland, 1996.
- EN 120; Wood Based Panels—Determination of Formaldehyde Content—Extraction Method Called the Perforator Method. European Committee for Standardization: Brussels, Belgium, 1994.
- Dziurka, D.; Mirski, R. Properties of Liquid and Polycondensed UF Resin Modified with pMDI. Drv. Ind. 2014, 65, 115–119. [Google Scholar] [CrossRef]
- EN 314-1; Plywood–Bond Quality–Test Methods. European Committee for Standardization: Brussels, Belgium, 2004.
- EN 310; Wood-Based Panels—Determination of Modulus of Elasticity in Bending and of Bending Strength. European Committee for Standardization: Brussels, Belgium, 1994.
- EN 717-3; Wood-Based Panels—Determination of Formaldehyde Release—Part 3: Formaldehyde Release by the Flask Method. European Committee for Standardization: Brussels, Belgium, 1996.
- Kim, M.; Park, B.-D. Effects of Molecular Weight of Urea–Formaldehyde Resins on Wettability and Adhesion at Wood Surface, Interphase, and Plywood. Wood Sci. Technol. 2022, 56, 1675–1703. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Li, J.; Gao, Q. Development of a High-Performance Adhesive with a Microphase, Separation Crosslinking Structure Using Wheat Flour and a Hydroxymethyl Melamine Prepolymer. Polymers 2019, 11, 893. [Google Scholar] [CrossRef]
- Soubam, T.; Gupta, A.; Jamari, S.S. Eco-Friendly Bio-Based Adhesive for Plywood from Natural Rubber Latex (NRL)-Blended Isocyanate Cross-Linked Starch. Environ. Sci. Pollut. Res. 2022, 30, 124610–124618. [Google Scholar] [CrossRef]
- Chen, F.; Liu, S.; Yu, J. Efficient Removal of Gaseous Formaldehyde in Air Using Hierarchical Titanate Nanospheres with in Situ Amine Functionalization. Phys. Chem. Chem. Phys. 2016, 18, 18161–18168. [Google Scholar] [CrossRef]
- Nomura, A.; Jones, C.W. Enhanced Formaldehyde-vapor Adsorption Capacity of Polymeric Amine-incorporated Aminosilicas. Chem. Eur. J. 2014, 20, 6381–6390. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Yu, Y.; Song, J. Improve Performance of Soy Flour-Based Adhesive with a Lignin-Based Resin. Polymers 2017, 9, 261. [Google Scholar] [CrossRef]
- Su, J.-F.; Huang, Z.; Yuan, X.-Y.; Wang, X.-Y.; Li, M. Structure and Properties of Carboxymethyl Cellulose/Soy Protein Isolate Blend Edible Films Crosslinked by Maillard Reactions. Carbohydr. Polym. 2010, 79, 145–153. [Google Scholar] [CrossRef]
- Wang, W.H.; Li, X.P.; Zhang, X.Q. A Soy-Based Adhesive from Basic Modification. Pigment Resin Technol. 2008, 37, 93–97. [Google Scholar] [CrossRef]
- Ghahri, S.; Mohebby, B.; Pizzi, A.; Mirshokraie, A.; Mansouri, H.R. Improving Water Resistance of Soy-Based Adhesive by Vegetable Tannin. J. Polym. Environ. 2018, 26, 1881–1890. [Google Scholar] [CrossRef]
- Wu, S.; Jiang, H.; Lu, J. Adsorptive Performance and Mechanism Exploration of L-Lysine Functionalized Celluloses for Enhanced Removal of Pb(II) from Aqueous Medium. Int. J. Biol. Macromol. 2023, 242, 124997. [Google Scholar] [CrossRef]
- Ghani, A.; Bawon, P.; Lee, S.H.; Ashaari, Z. Physico-Mechanical Properties and Formaldehyde Emission of Rubberwood Particleboard Made with UF Resin Admixed with Ammonium and Aluminium-Based Hardeners. Pertanika J. Sci. Technol. 2019, 27, 473–488. [Google Scholar]
- Li, J.; Zhang, Y. Morphology and Crystallinity of Urea-Formaldehyde Resin Adhesives with Different Molar Ratios. Polymers 2021, 13, 673. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, J.E. The C N Stretching Frequency in the Infrared Spectra of Schiff’s Base Complexes—I. Copper Complexes of Salicylidene Anilines. Spectrochim. Acta Part A Mol. Spectrosc. 1967, 23, 183–187. [Google Scholar] [CrossRef]
- Amirou, S.; Zhang, J.; Essawy, H.A.; Pizzi, A.; Zerizer, A.; Li, J.; Delmotte, L. Utilization of Hydrophilic/Hydrophobic Hyperbranched Poly(Amidoamine)s as Additives for Melamine Urea Formaldehyde Adhesives. Polym. Compos. 2015, 36, 2255–2264. [Google Scholar] [CrossRef]
- Yuan, L.; Liang, G.-Z.; Xie, J.-Q.; Guo, J.; Li, L. Thermal Stability of Microencapsulated Epoxy Resins with Poly (Urea–Formaldehyde). Polym. Degrad. Stab. 2006, 91, 2300–2306. [Google Scholar] [CrossRef]
- Roucoules, V.; Fail, C.A.; Schofield, W.C.E.; Teare, D.O.H.; Badyal, J.P.S. Diels−Alder Chemistry on Alkene Functionalized Films. Langmuir 2005, 21, 1412–1415. [Google Scholar] [CrossRef]
- Qi, W.; Lei, Y.; Xu, W.; Sha, J.; Zhao, S.; Tian, Y.; Wang, X. Preparation of Carbon Microspheres from Lignin–Urea–Formaldehyde Resin for Application in High-Performance Supercapacitor. Wood Sci. Technol. 2022, 56, 367–387. [Google Scholar] [CrossRef]
- Kawalerczyk, J.; Dziurka, D.; Dukarska, D.; Mirski, R. Effect of Lysine-Functionalized Nanocellulose on Urea-Formaldehyde Adhesive Performance in Particleboard Production. J. Wood Chem. Technol. 2025, 45, 231–243. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Kim, H.-J. Relationship between Curing Activation Energy and Free Formaldehyde Content in Urea-Formaldehyde Resins. J. Adhes. Sci. Technol. 2013, 27, 598–609. [Google Scholar] [CrossRef]
- Xing, C.; Zhang, S.Y.; Deng, J.; Wang, S. Urea–Formaldehyde-resin Gel Time as Affected by the pH Value, Solid Content, and Catalyst. J. Appl. Polym. Sci. 2007, 103, 1566–1569. [Google Scholar] [CrossRef]
- Lubis, M.A.R.; Hidayat, W.; Zaini, L.H.; Park, B.D. Effects of Hydrolysis on the Removal of Cured Urea-Formaldehyde Adhesive in Waste Medium-Density Fiberboard. J. Sylva Lestari 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Costa, N.A.; Pereira, J.; Ferra, J.; Cruz, P.; Martins, J.; Magalhães, F.D.; Mendes, A.; Carvalho, L.H. Scavengers for Achieving Zero Formaldehyde Emission of Wood-Based Panels. Wood Sci. Technol. 2013, 47, 1261–1272. [Google Scholar] [CrossRef]
- Hrázskỳ, J.; Král, P. Assessing the Bending Strength and Modulus of Elasticity in Bending of Exterior Foiled Plywoods in Relation to Their Construction. J. For. Sci. 2005, 51, 77–94. [Google Scholar] [CrossRef]

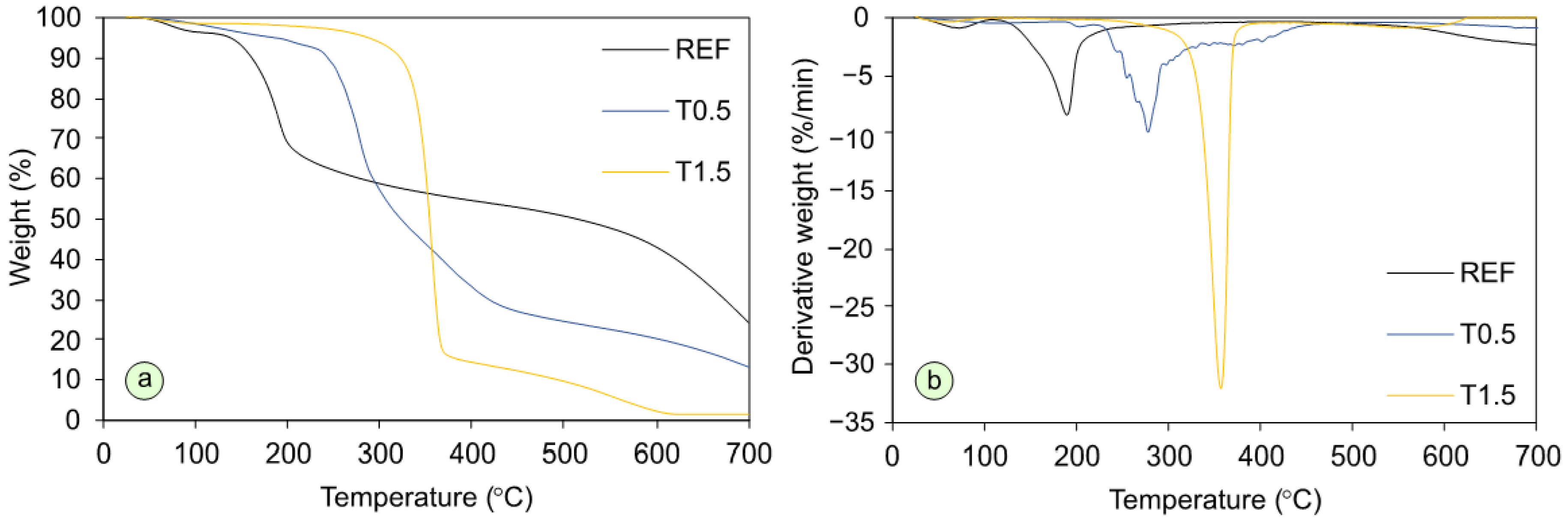
| Variant | Weight Loss (%) | Resiude at 700 °C (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100 °C | 150 °C | 200 °C | 250 °C | 300 °C | 350 °C | 400 °C | 500 °C | 600 °C | ||
| REF | 3.55 | 7.03 | 31.16 | 37.90 | 41.23 | 43.55 | 45.43 | 49.28 | 56.98 | 24.28 |
| T0.5 | 1.55 | 3.72 | 1.06 | 11.94 | 42.94 | 56.25 | 66.86 | 75.43 | 79.69 | 13.27 |
| T1.5 | 1.39 | 1.49 | 2.02 | 2.98 | 6.19 | 39.33 | 85.58 | 90.3 | 97.70 | 1.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawalerczyk, J.; Dukarska, D.; Góral, B.; Antov, P.; Dziurka, D.; Mirski, R. Modification of Urea-Formaldehyde Resin with Triethylenetetramine: Effect on Adhesive Properties and Plywood Strength. Polymers 2025, 17, 2652. https://doi.org/10.3390/polym17192652
Kawalerczyk J, Dukarska D, Góral B, Antov P, Dziurka D, Mirski R. Modification of Urea-Formaldehyde Resin with Triethylenetetramine: Effect on Adhesive Properties and Plywood Strength. Polymers. 2025; 17(19):2652. https://doi.org/10.3390/polym17192652
Chicago/Turabian StyleKawalerczyk, Jakub, Dorota Dukarska, Błażej Góral, Petar Antov, Dorota Dziurka, and Radosław Mirski. 2025. "Modification of Urea-Formaldehyde Resin with Triethylenetetramine: Effect on Adhesive Properties and Plywood Strength" Polymers 17, no. 19: 2652. https://doi.org/10.3390/polym17192652
APA StyleKawalerczyk, J., Dukarska, D., Góral, B., Antov, P., Dziurka, D., & Mirski, R. (2025). Modification of Urea-Formaldehyde Resin with Triethylenetetramine: Effect on Adhesive Properties and Plywood Strength. Polymers, 17(19), 2652. https://doi.org/10.3390/polym17192652







