Biaxial Stretching of PBAT/PLA Blends for Improved Mechanical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Design of Experiment
2.3. Film Preparation
2.4. Biaxial Stretching
2.5. Mechanical Testing
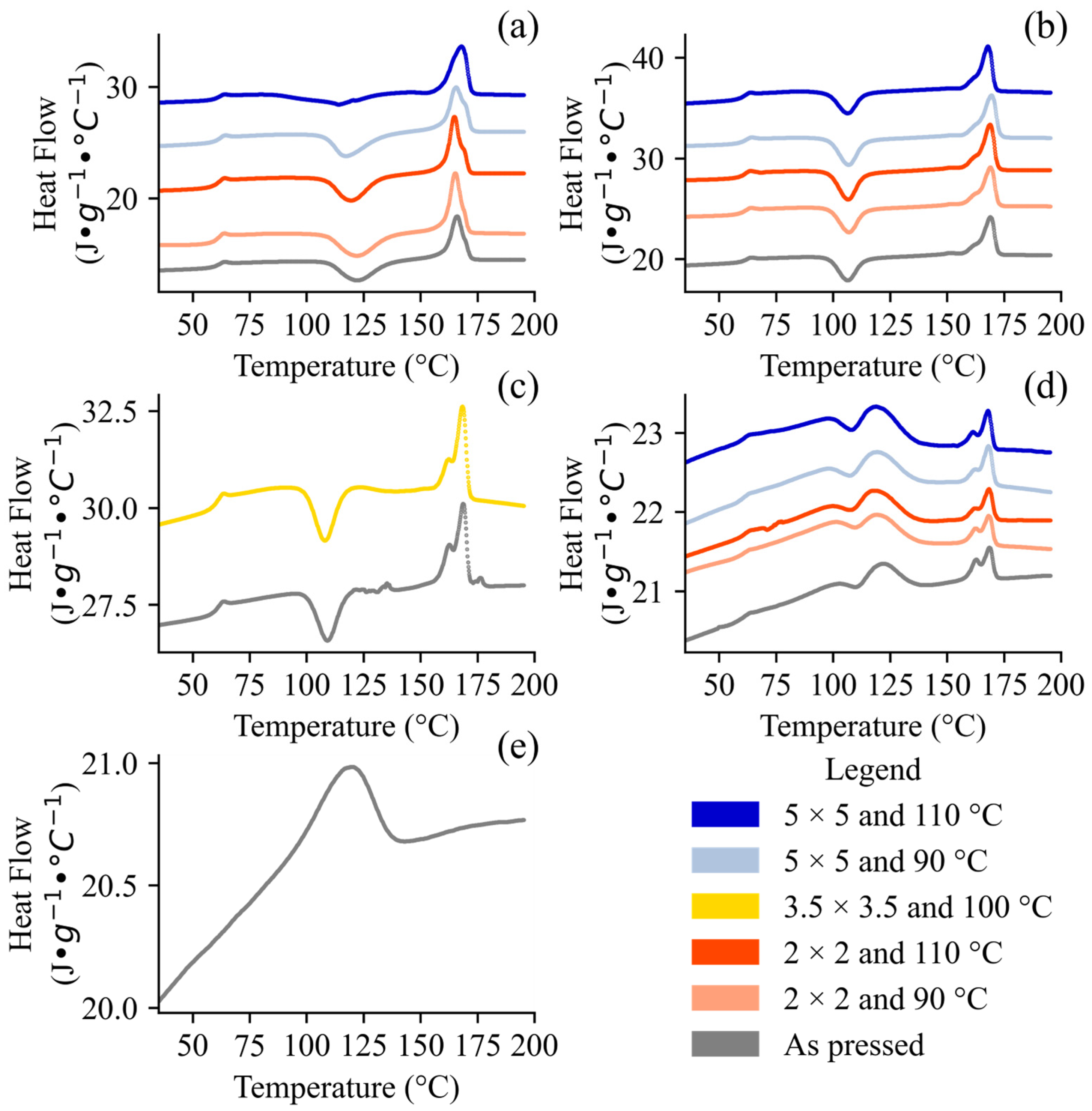
2.6. Differential Scanning Calorimetry (DSC)
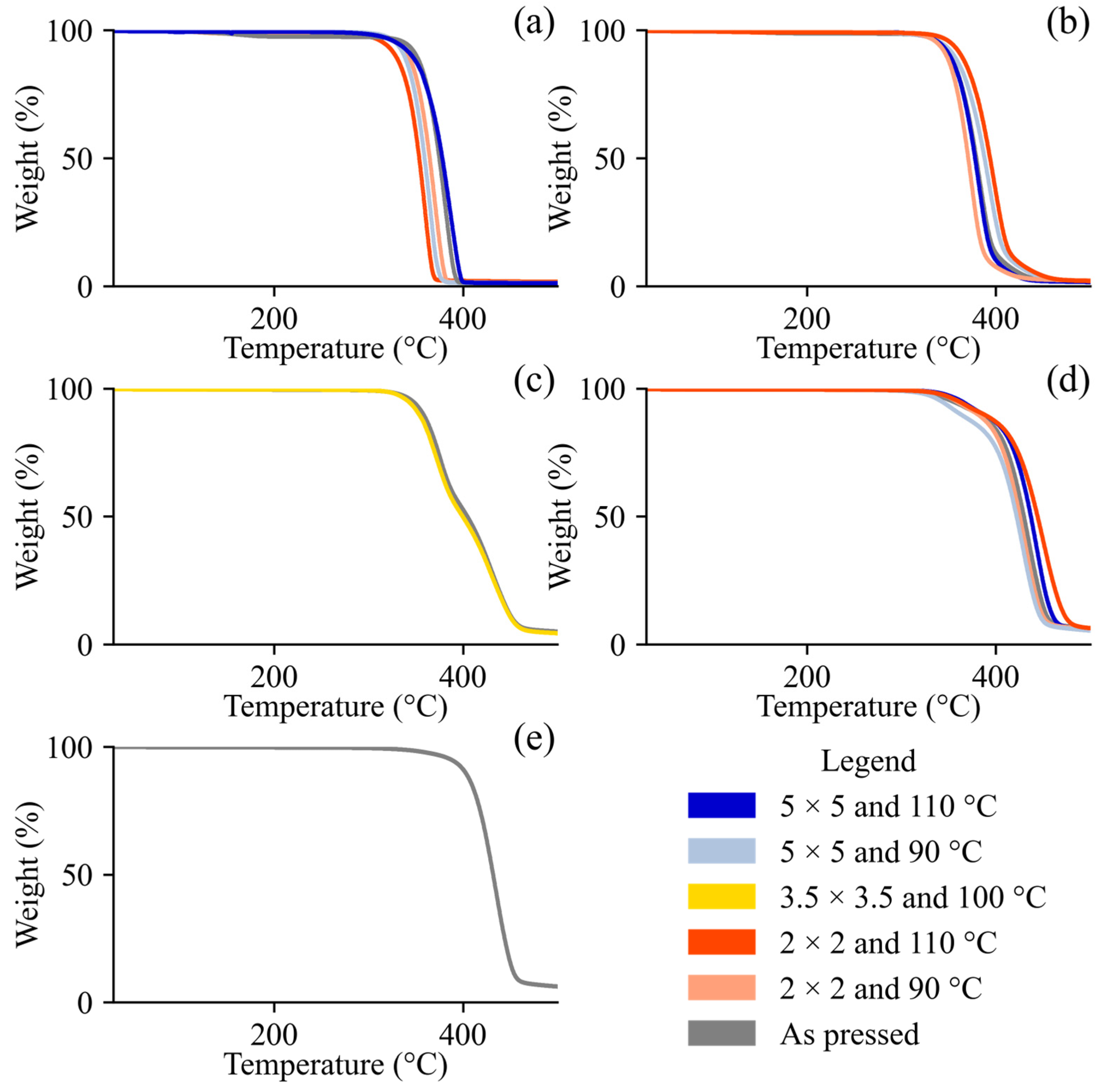
2.7. Thermogravimetric Analysis (TGA)
2.8. Scanning Electron Microscopy (SEM)
2.9. X-Ray Diffraction (XRD)
3. Results and Discussion
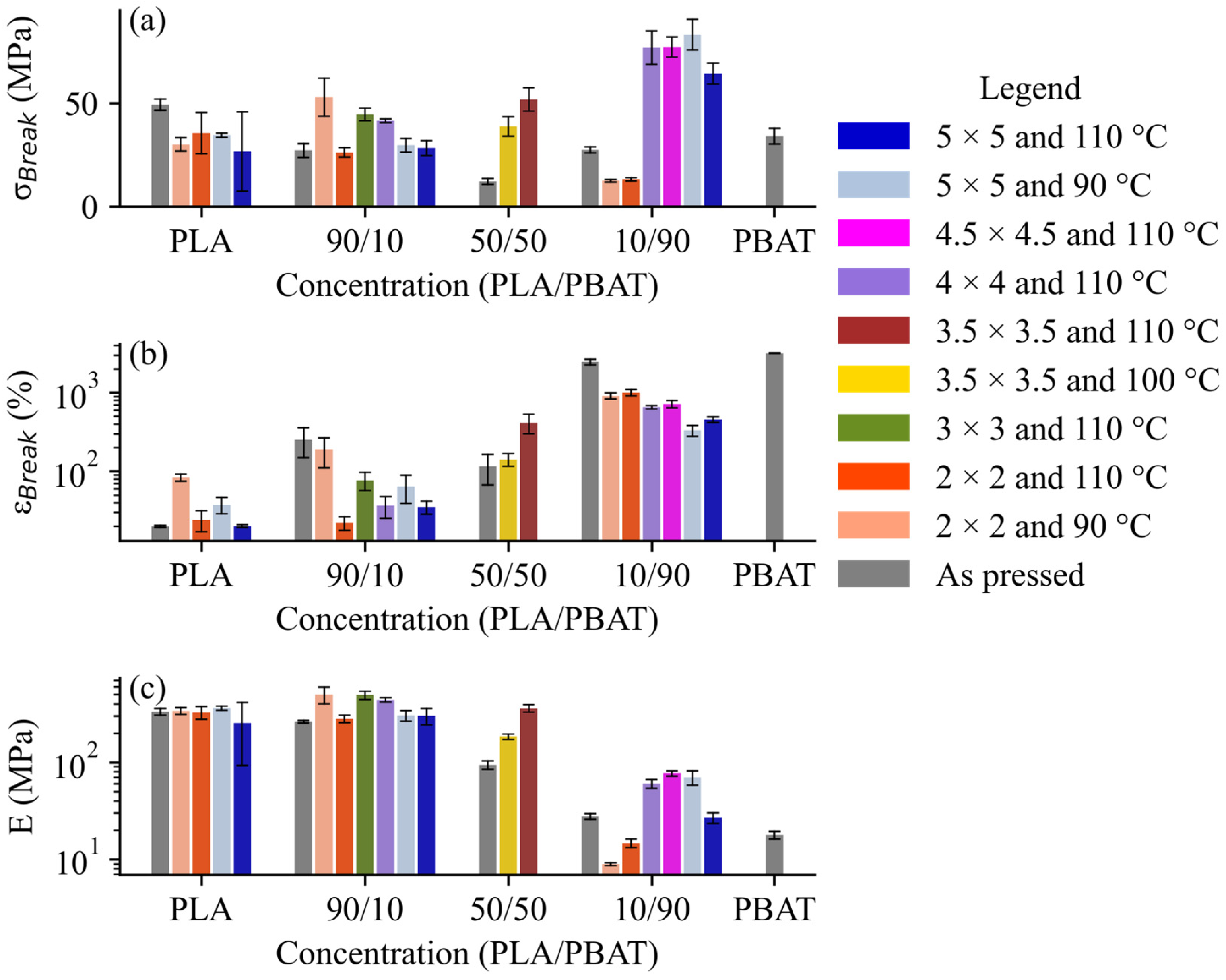
3.1. Mechanical Properties
3.2. Thermal Properties
3.3. X-Ray Diffraction
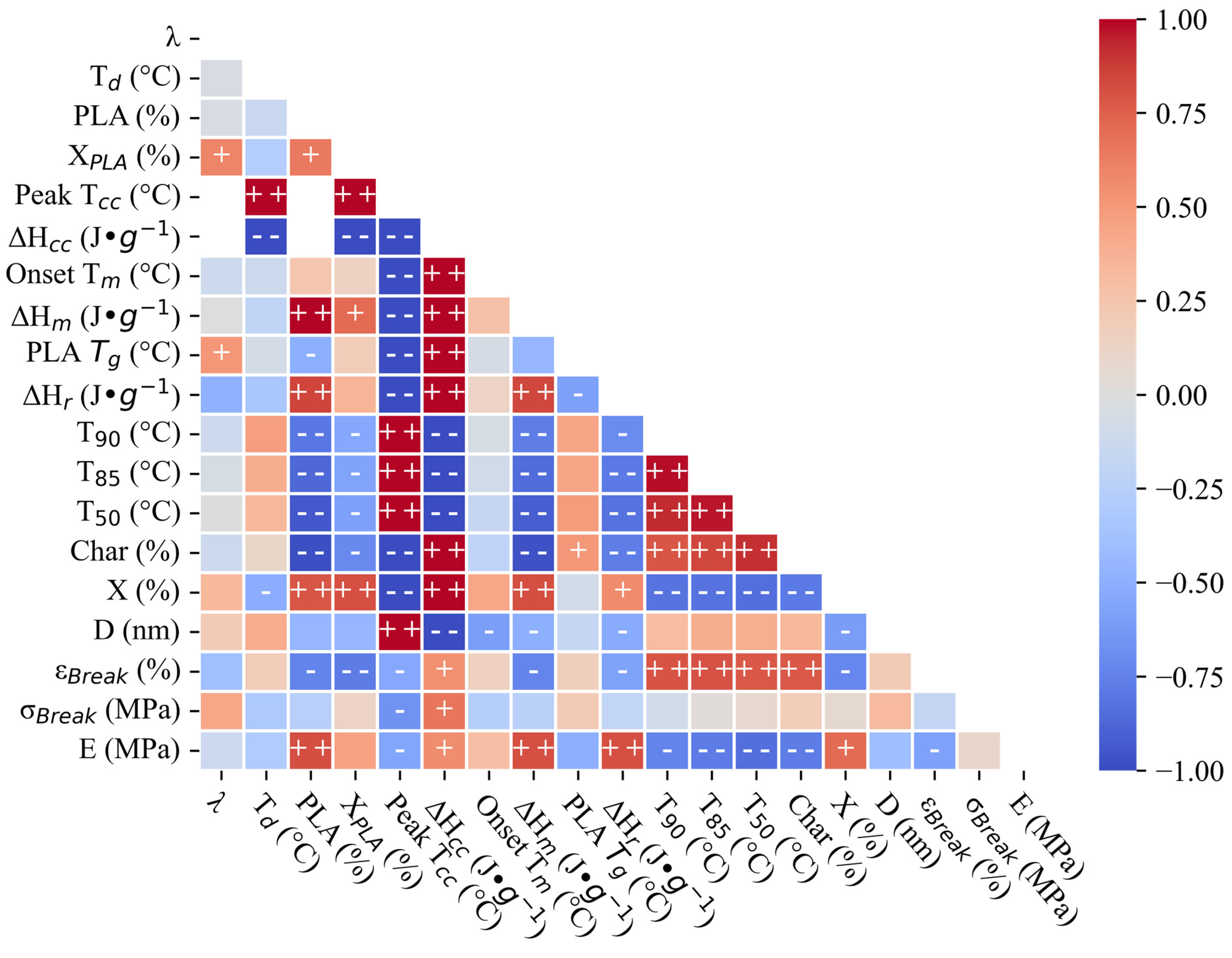
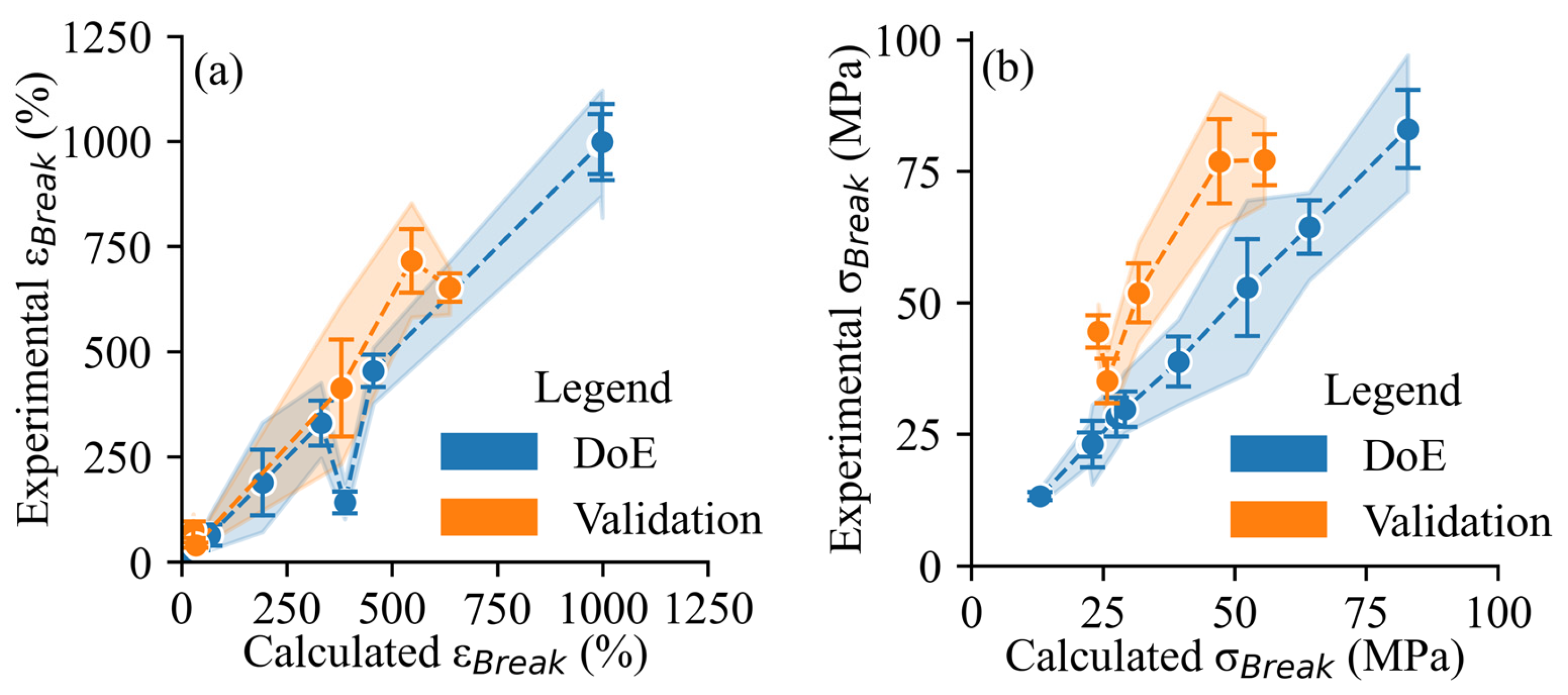
3.4. Exploratory Statistics and Regression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BA | Butylene adipate |
| BGU | Ben-Gurion University |
| BT | Butylene terephthalate |
| D | Crystallite size |
| DCM | Dichloromethane |
| DoE | Design of experiment |
| DSC | Differential scanning calorimetry |
| DTG | Derivative thermogravimetry |
| MD | Machine direction |
| Mn | Number average molecular weight |
| Mw | Weight average molecular weight |
| NJIT | New Jersey Institute of Technology |
| NSF | National Science Foundation |
| PBAT | Poly(butylene adipate-co-terephthalate) |
| PLA | Poly(lactic acid) |
| SEM | Scanning electron microscopy |
| T90 | Temperature at which 90% of mass remains (i.e., 10% mass loss) |
| T85 | Temperature at which 85% of mass remains (i.e., 15% mass loss), taken to be the onset of decomposition |
| T50 | Temperature at which 50% of mass remains (i.e., 50% mass loss) |
| TDecomp | Decomposition temperature |
| Tg | Glass transition temperature |
| TGA | Thermogravimetric analysis |
| TD | Transverse direction |
| ΔHcc | Enthalpy of cold crystallization |
| ΔH0fusion | Standard heat of fusion for infinitely thick crystalline form |
| ΔHm | Enthalpy of fusion |
| ΔHr | Enthalpic relaxation |
| εBreak | Elongation at break |
| λ | Draw ratio |
| σBreak | Stress at break |
| ΧPBAT | Fraction of crystalline PBAT |
| ΧPLA | Fraction of crystalline PLA |
| XRD | X-ray diffraction |
References
- Rodriguez, N.; Xing, F.; Gillor, O.; Guvendiren, M.; Axe, L. Methodology Development: Evaluation of Structural, Thermal, and Mechanical Properties of Poly(Lactic Acid)/Poly(Butylene Adipate-co-Terephthalate) Blends for Biodegradable Mulch. Polym. Bull. 2025, 82, 3685–3713. [Google Scholar] [CrossRef]
- Burford, T.; Rieg, W.; Madbouly, S. Biodegradable poly(butylene adipate-co-terephthalate) (PBAT). Phys. Sci. Rev. 2021, 8, 1127–1156. [Google Scholar] [CrossRef]
- Jian, J.; Zeng, X.; Huang, X. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Niu, D.; Li, J.; Xu, P.; Liu, T.; Yang, W.; Wang, Z.; Ma, P. High-performance and durable fibrous poly(glycolic acid)/poly(butylene adipate-co-terephthalate) blends by reactive compatibilization and solid-state drawing. Polym. Degrad. Stab. 2023, 210, 110293. [Google Scholar] [CrossRef]
- ASTM Standard D 6400-23; Standard Specification for Labeling of Plastics Designed to be Aerobically Composted in Municipal or Industrial Facilities. ASTM International: West Conshohocken, PA, USA, 2023.
- EN 13432; Packaging-Requirements for Packaging Recoverable Through Composting and Biodegradation-Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. European Committee for Standardization: Brussels, Belgium, 2000.
- EN 17033; Plastics-Biodegradable Mulch Films for Use in Agriculture and Horticulture-Requirements and Test Methods. European Committee for Standardization: Brussels, Belgium, 2018.
- Product Information Ecoflex® F Blend C1200. 2024, p. 3. Available online: https://download.basf.com/p1/8a8082587fd4b608017fd63230bf39c4/en/ecoflex%3Csup%3E%C2%AE%3Csup%3E_F_Blend_C1200_Product_Data_Sheet_English.pdf?view (accessed on 23 September 2025).
- AS 4736-2006; Standards for Compostable GreenPla Products. Standards Australia: Sydney, Australia, 2006.
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.A.; El-Khatib, S. Critical Review on Polylactic Acid: Properties, Structure, Processing, Biocomposites, and Nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Mohamed Zin, M.R.; Mahendrasingam, A.; Konkel, C.; Narayanan, T. Effect of d-isomer content on strain-induced crystallization behaviour of Poly(lactic acid) polymer under high speed uniaxial drawing. Polymer 2021, 216, 123422. [Google Scholar] [CrossRef]
- Crystallizing and Drying Ingeo Biopolymer; NatureWorks LLC: Plymouth, MN, USA; Available online: https://www.natureworksllc.com/~/media/Technical_Resources/Processing_Guides/ProcessingGuide_Crystallizing-and-Drying_pdf.pdf (accessed on 23 September 2025).
- Jariyasakoolroj, P.; Tashiro, K.; Wang, H.; Yamamoto, H.; Chinsirikul, W.; Kerddonfag, N.; Chirachanchai, S. Isotropically small crystalline lamellae induced by high biaxial-stretching rate as a key microstructure for super-tough polylactide film. Polymer 2015, 68, 234–245. [Google Scholar] [CrossRef]
- Su, S.; Duhme, M.; Kopitzky, R. Uncompatibilized PBAT/PLA Blends: Manufacturability, Miscibility and Properties. Materials 2020, 13, 4897. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, P.-P.; Ni, S.-H.; Xu, L.; Lin, H.; Zhong, G.-J.; Huang, H.-D.; Li, Z.-M. Superior Ductile and High-barrier Poly(lactic acid) Films by Constructing Oriented Nanocrystals as Efficient Reinforcement of Chain Entanglement Network and Promising Barrier Wall. Chin. J. Polym. Sci. 2022, 40, 1201–1212. [Google Scholar] [CrossRef]
- Gao, X.-R.; Li, Y.; Huang, H.-D.; Xu, J.-Z.; Xu, L.; Ji, X.; Zhong, G.-J.; Li, Z.-M. Extensional Stress-Induced Orientation and Crystallization can Regulate the Balance of Toughness and Stiffness of Polylactide Films: Interplay of Oriented Amorphous Chains and Crystallites. Macromolecules 2019, 52, 5278–5288. [Google Scholar] [CrossRef]
- França, D.C.; Almeida, T.G.; Abels, G.; Canedo, E.L.; Carvalho, L.H.; Wellen, R.M.R.; Haag, K.; Koschek, K. Tailoring PBAT/PLA/Babassu films for suitability of agriculture mulch application. J. Nat. Fibers 2019, 16, 933–943. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, G.; Bian, X.; Zeng, J.; Weng, Y. Biodegradation Behavior of Poly(Butylene Adipate-Co-Terephthalate) (PBAT), Poly(Lactic Acid) (PLA), and Their Blend in Freshwater with Sediment. Molecules 2020, 25, 3946. [Google Scholar] [CrossRef] [PubMed]
- Salehiyan, R.; Kim, M.C.; Xia, T.; Jin, S.; Nofar, M.; Chalmers, L.; Hyun, K. Enhanced re-processability of poly (butylene adipate-co-terephthalate) (PBAT) via chain extension toward a more sustainable end-of-life. Polym. Eng. Sci. 2024, 65, 1187–1199. [Google Scholar] [CrossRef]
- Weng, Y.-X.; Jin, Y.-J.; Meng, Q.-Y.; Wang, L.; Zhang, M.; Wang, Y.-Z. Biodegradation behavior of poly(butylene adipate-co-terephthalate) (PBAT), poly(lactic acid) (PLA), and their blend under soil conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, D.; Zhou, Z.; Wu, Y.; Li, H.; Zhao, C.; Li, Y. Biaxial Stretching of Polymer Nanocomposites: A Mini-Review. Front Mater. 2021, 8, 725422. [Google Scholar] [CrossRef]
- Ingeo™ Biopolymer 4032D Technical Data Sheet: Extrusion Grade for Higher Heat Products; NatureWorks LLC: Plymouth, MN, USA; Available online: https://www.natureworksllc.com/~/media/Files/NatureWorks/Technical-Documents/Technical-Data-Sheets/TechnicalDataSheet_4032D_general_pdf.pdf?la=en (accessed on 23 September 2025).
- Ingeo™ Biopolymer 4032D Technical Data Sheet: Biaxially Oriented Films–High Heat; NatureWorks LLC: Plymouth, MN, USA; Available online: https://www.natureworksllc.com/~/media/Technical_Resources/Technical_Data_Sheets/TechnicalDataSheet_4032D_films_pdf.pdf (accessed on 23 September 2025).
- Ortega, Z.; Douglas, P.; Hanna, P.R.; Garrett, G.; Clarke, A.; Cunningham, E.; Suárez, L. Characterization of PLA Sheets Prepared by Stretching under Different Conditions: Influence of Reprocessing and Establishing Optimal Conditions. Materials 2023, 16, 5114. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, T.; Yang, J.; Chen, F.; Fei, Y.; Zhong, M.; Kuang, T. Biomimetically Structured Poly(lactic acid)/Poly(butylene-adipate- co-terephthalate) Blends with Ultrahigh Strength and Toughness for Structural Application. ACS Appl. Polym. Mater. 2022, 4, 9351–9359. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, X.R.; Huang, H.D.; Xu, L.; Ji, X.; Zhong, G.J.; Lin, H.; Li, Z.M. Superhydrophobic, Self-Cleaning, and Robust Properties of Oriented Polylactide Imparted by Surface Structuring. ACS Sustain. Chem. Eng. 2021, 9, 6296–6304. [Google Scholar] [CrossRef]
- Yoksan, R.; Dang, K.M.; Boontanimitr, A.; Chirachanchai, S. Relationship between microstructure and performances of simultaneous biaxially stretched films based on thermoplastic starch and biodegradable polyesters. Int. J. Biol. Macromol. 2021, 190, 141–150. [Google Scholar] [CrossRef]
- Ouchiar, S.; Stoclet, G.; Cabaret, C.; Addad, A.; Gloaguen, V. Effect of biaxial stretching on thermomechanical properties of polylactide based nanocomposites. Polymer 2016, 99, 358–367. [Google Scholar] [CrossRef]
- Liu, X.; Mo, Z.; Cui, L.; Yu, C.; Zou, Z.; Liu, Y.; Zheng, W.; Tan, J. Effect of biaxial stretching on the microstructure evolution, optical, mechanical and oxygen barrier properties of biodegradable poly(lactic acid) (PLA)/poly(butylene adipate-co-terephthalate) (PBAT) films. Int. J. Biol. Macromol. 2023, 253, 126976. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Xu, C.; Huang, H.D.; Lin, H.; Zhong, G.J.; Li, Z.M. Polylactide Films with Superhydrophobicity and Superior Mechanical Properties Constructed by Combining Annealing Stretching and Nonsolvent-Induced Phase Separation. ACS Sustain. Chem. Eng. 2023, 11, 9498–9508. [Google Scholar] [CrossRef]
- Zhao, Y.; Lai, J.; Huang, Y.; Jiang, H.; Sun, Y.; Li, Y.; Li, Y.; Li, F.; Luo, Z.; Xie, D. Effect of molding on the structure and properties of poly(butylene adipate-co-terephthalate)/poly(propylene carbonate)/organically modified montmorillonite nanocomposites. Appl. Clay Sci. 2023, 234, 106854. [Google Scholar] [CrossRef]
- Menary, G.; Tan, C.; Harkin-Jones, E.; Armstrong, C.; Martin, P. Biaxial deformation and experimental study of PET at conditions applicable to stretch blow molding. Polym. Eng. Sci. 2012, 52, 671–688. [Google Scholar] [CrossRef]
- Lyu, J.S.; Han, J. Scale-up fabrication of a biodegradable PBAT/PLA composite film compatibilized with a chain extender for industrial agricultural mulch film application. Compos. Part C 2023, 12, 100397. [Google Scholar] [CrossRef]
- Shakoor, A.; Thomas, N.L. Talc as a nucleating agent and reinforcing filler in poly(lactic acid) composites. Polym. Eng. Sci. 2014, 54, 64–70. [Google Scholar] [CrossRef]
- Salaris, V.; García-Obregón, I.S.F.; López, D.; Peponi, L. Electrospun Nanofibers Reinforced with ZnO Nanoparticles and In Vitro Degradation Study. Nanomaterials 2023, 13, 2236. [Google Scholar] [CrossRef]
- Rampado, R.; Peer, D. Design of experiments in the optimization of nanoparticle-based drug delivery systems. J. Control. Release 2023, 358, 398–419. [Google Scholar] [CrossRef]
- Abdollahi Moghaddam, M.R.; Hesarinejad, M.A.; Javidi, F. Characterization and optimization of polylactic acid and polybutylene succinate blend/starch/wheat straw biocomposite by optimal custom mixture design. Polym. Test. 2023, 121, 108000. [Google Scholar] [CrossRef]
- Yan, D.; Wang, Z.; Guo, Z.; Ma, Y.; Wang, C.; Tan, H.; Zhang, Y. Study on the properties of PLA/PBAT composite modified by nanohydroxyapatite. J. Mater. Res. Technol. 2020, 9, 11895–11904. [Google Scholar] [CrossRef]
- Menossi, M.; Salcedo, F.; Rivilli, N.; Nicolini, A.T.; Alvarez, V.A.; Ludueña, L.N. Biodegradable Mulch Films Based on Starch/Poly (Lactic Acid)/Poly (ε-Caprolactone) Ternary Blends. J. Polym. Environ. 2023, 31, 2114–2137. [Google Scholar] [CrossRef]
- Sun, X.; Wang, K.; Yang, C.; Zhao, Y.; Yang, Y.; Li, F.; Xie, D. Evaluation of the ageing resistance and lifespan of talc-filled poly(butylene adipate-co-terephthalate)/polylactide (PBAT/PLA) films used for winter potato cultivation. Colloid Polym. Sci. 2023, 301, 1039–1049. [Google Scholar] [CrossRef]
- Palsikowski, P.A.; Kuchnier, C.N.; Pinheiro, I.F.; Morales, A. Biodegradation in Soil of PLA/PBAT Blends Compatibilized with Chain Extender. J. Polym. Environ. 2018, 26, 330–341. [Google Scholar] [CrossRef]
- Wang, H.; Wei, D.; Zheng, A.; Xiao, H. Soil burial biodegradation of antimicrobial biodegradable PBAT films. Polym. Degrad. Stab. 2015, 116, 14–22. [Google Scholar] [CrossRef]
- Leng, Y. Materials Characterization: Introduction to Microscopic and Spectroscopic Methods, 1st ed.; John Wiley & Sons: Weinheim, Germany, 2013; Incorporated. [Google Scholar]
- Righetti, M.C.; Gazzano, M.; Di Lorenzo, M.L.; Androsch, R. Enthalpy of melting of α′- and α-crystals of poly(l-lactic acid). Eur. Polym. J. 2015, 70, 215–220. [Google Scholar] [CrossRef]
- Chivrac, F.; Kadlecová, Z.; Pollet, E.; Avérous, L. Aromatic Copolyester-based Nano-biocomposites: Elaboration, Structural Characterization and Properties. J. Polym. Environ. 2006, 14, 393–401. [Google Scholar] [CrossRef]
- Velásquez, E.; Rojas, A.; Piña, C.; Galotto, M.J.; de Dicastillo, C.L. Development of Bilayer Biodegradable Composites Containing Cellulose Nanocrystals with Antioxidant Properties. Polymers 2019, 11, 1945. [Google Scholar] [CrossRef]
- Pan, H.; Li, Z.; Yang, J.; Li, X.; Ai, X.; Hao, Y.; Zhang, H.; Dong, L. The effect of MDI on the structure and mechanical properties of poly(lactic acid) and poly(butylene adipate-co-butylene terephthalate) blends. RSC Adv. 2018, 8, 4610–4623. [Google Scholar] [CrossRef]
- Li, X.; Yan, X.; Yang, J.; Pan, H.; Gao, G.; Zhang, H.; Dong, L. Improvement of compatibility and mechanical properties of the poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends and films by reactive extrusion with chain extender. Polym. Eng. Sci. 2018, 58, 1868–1878. [Google Scholar] [CrossRef]
- ASTM Standard D 638-22; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2022.
- Bianchi, M.; Dorigato, A.; Morreale, M.; Pegoretti, A. Evaluation of the Physical and Shape Memory Properties of Fully Biodegradable Poly(lactic acid) (PLA)/Poly(butylene adipate terephthalate) (PBAT) Blends. Polymers 2023, 15, 881. [Google Scholar] [CrossRef]
- Zhu, B.; Zhao, S.; Guo, J.; Song, K.; He, J.; Liu, S.; Zhou, X. Enhancing the mechanical properties of polylactic acid (PLA) composite films using Pueraria lobata root microcrystalline cellulose. Int. J. Biol. Macromol. 2024, 279, 135579. [Google Scholar] [CrossRef]
- Takkalkar, P.; Tobin, M.J.; Vongsvivut, J.; Mukherjee, T.; Nizamuddin, S.; Griffin, G.; Kao, N. Structural, thermal, rheological and optical properties of poly(lactic acid) films prepared through solvent casting and melt processing techniques. J. Taiwan Inst. Chem. Eng. 2019, 104, 293–300. [Google Scholar] [CrossRef]
- de Matos Costa, A.R.; Crocitti, A.; de Carvalho, L.H.; Carroccio, S.C.; Cerruti, P.; Santagata, G. Properties of Biodegradable Films Based on Poly(butylene Succinate) (PBS) and Poly(butylene Adipate-co-Terephthalate) (PBAT) Blends. Polymers 2020, 12, 2317. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, J.; Ding, Y.; Jiang, G.; Sun, L.; Lu, K. Effects of heating rate on thermal degradation behavior and kinetics of representative thermoplastic wastes. J. Environ. Manag. 2022, 314, 115071. [Google Scholar] [CrossRef]
- Reit, M.; Krug, N.; Zarges, J.; Heim, H. Evaluation of the Activation Energy for Pyrolytic Degradation of Poly-L-Lactide (PLA) During Artificially Accelerated Aging. Biopolymers 2025, 116, e23642. [Google Scholar] [CrossRef]
- Xiang, S.; Feng, L.D.; Bian, X.C.; Li, G.; Chen, X.S. Evaluation of PLA content in PLA/PBAT blends using TGA. Polym. Test. 2020, 81, 106211. [Google Scholar] [CrossRef]
- Kaoudom, K.; Suttiruengwong, S.; Seadan, M. Stretchability and Deformation Behavior of Polybutylene Adipate-co-terephthalate Blend Films. Suan Sunandha Sci. Technol. J. 2022, 9, 15–21. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, K.; Zhang, Z.-H.; Jing, M.-F.; Liu, C.-T.; Shen, C.-Y.; Wang, Y.-M. Enhanced Crystallization of Poly(butylene adipate-co-terephthalate) by a Self-assembly Nucleating Agent. Chin. J. Polym. Sci. 2024, 42, 663–674. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, L.; Li, C.; Sun, M.; Zhang, L.; Li, C. Modified cellulose nanocrystals based on SI-ATRP for enhancing interfacial compatibility and mechanical performance of biodegradable PLA/PBAT blend. Polym. Compos. 2022, 43, 3753–3764. [Google Scholar] [CrossRef]
- Guo, Z.; Song, W.; Wei, X.; Feng, Y.; Song, Y.; Guo, Z.; Cheng, W.; Miao, W.; Cheng, B.; Song, S. Effect of matrix composition on the performance of calcium carbonate filled poly(lactic acid)/poly(butylene adipate-co-terephthalate) composites. e-Polymers 2023, 23, 20230026. [Google Scholar] [CrossRef]
- Chen, P.-H.; Chen, C.-W.; Mao, H.-I.; Dai, C.-A.; Su, C.-S.; Tsai, J.-C.; Lin, F.-H. Bio-Based PLA/PBS/PBAT Ternary Blends with Added Nanohydroxyapatite: A Thermal, Physical, and Mechanical Study. Polymers 2023, 15, 4585. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, J.B.; Lee, D.Y. Selective Localization of Nanofiller on Mechanical Properties of Poly(lactic acid)/Poly(butylene adipate-co-terephthalate) Nanocomposites via the Surface Energy and Melt Blending Technique. Macromolecules 2022, 55, 3287–3300. [Google Scholar] [CrossRef]
- Mohammadi, M.; Heuzey, M.-C.; Carreau, P.J. Morphological, thermal, and mechanical properties of cellulose nanocrystal reinforced poly(lactic acid) and poly(butylene adipate-co-terephthalate): A comparative study on common and novel solvent casting methods. Polym. Compos. 2021, 42, 6688–6703. [Google Scholar] [CrossRef]
- Echeverría, C.; Limón, I.; Muñoz-Bonilla, A.; Fernández-García, M.; López, D. Development of Highly Crystalline Polylactic Acid with β-Crystalline Phase from the Induced Alignment of Electrospun Fibers. Polymers 2021, 13, 2860. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Han, L.; Zheng, B.; Bian, J.; Zhao, Y.; Pan, H.; Wang, M.; Zhang, H. Enhanced strength, toughness and heat resistance of poly (lactic acid) with good transparency and biodegradability by uniaxial pre-stretching. Int. J. Biol. Macromol. 2024, 278, 135222. [Google Scholar] [CrossRef]
- Byrn, S.R.; Zografi, G.; Chen, X. Differential Scanning Calorimetry and Thermogravimetric Analysis. In Solid State Properties of Pharmaceutical Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 124–141. [Google Scholar]
- Cai, Y.; Liu, S.; Fang, C.; Liu, Z.; He, Y.; Qu, J.-P. Strengthening-toughening pure poly(lactic acid) with ultra-transparency through increasing mesophase promoted by elongational flow field. Int. J. Biol. Macromol. 2023, 242, 125091. [Google Scholar] [CrossRef]
- Simmons, H.; Tiwary, P.; Colwell, J.E.; Kontopoulou, M. Improvements in the crystallinity and mechanical properties of PLA by nucleation and annealing. Polym. Degrad. Stab. 2019, 166, 248–257. [Google Scholar] [CrossRef]
- Byrn, S.R.; Zografi, G.; Chen, X.S. Amorphous Solids. In Solid State Properties of Pharmaceutical Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 69–88. [Google Scholar]
- Litauszki, K.; Petrény, R.; Haramia, Z.; Mészáros, L. Combined effects of plasticizers and D-lactide content on the mechanical and morphological behavior of polylactic acid. Heliyon 2023, 9, e14674. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, Z.; Guo, G. Biodegradation Assessment of Poly (Lactic Acid) Filled with Functionalized Titania Nanoparticles (PLA/TiO2) under Compost Conditions. Nanoscale Res. Lett. 2019, 14, 56. [Google Scholar] [CrossRef]
- Molinari, G.; Aliotta, L.; Gemmi, M.; Lazzeri, A.; Righetti, M.C. Constrained amorphous interphase in plasticized poly(lactic acid): Composition and tensile elastic modulus estimation. Polym. Test. 2024, 131, 108325. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Li, M.; Cao, W.; Liu, C.; Shen, C. Spectroscopic analysis of post drawing relaxation in poly(lactic acid) with oriented mesophase. Polym. Test. 2015, 43, 103–107. [Google Scholar] [CrossRef]
- Huang, F.; Wu, L.; Li, B.-G. Sulfonated biodegradable PBAT copolyesters with improved gas barrier properties and excellent water dispersibility: From synthesis to structure-property. Polym. Degrad. Stab. 2020, 182, 109391. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Lu, Y.; Hou, T.; Zhou, J.; Zhang, X.; Zhou, L.; Sun, M.; Xue, J.; Yang, B. Melting centrifugally spun ultrafine poly butylene adipate-co-terephthalate (PBAT) fiber and hydrophilic modification. RSC Adv. 2021, 11, 27019–27026. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Dong, Y.; Liu, Y.; Fan, S. The Effect of Stretching on the Crystal Structure and Crystal Orientation of PA510/SiO2 Films. Materials 2021, 14, 705. [Google Scholar] [CrossRef] [PubMed]
- Kalish, J.P.; Aou, K.; Yang, X.; Hsu, S.L. Spectroscopic and thermal analyses of α′ and α crystalline forms of poly(l-lactic acid). Polymer 2011, 52, 814–821. [Google Scholar] [CrossRef]
- Chaiwutthinan, P.; Chuayjuljit, S.; Srasomsub, S.; Boonmahitthisud, A. Composites of poly(lactic acid)/poly(butylene adipate-co-terephthalate) blend with wood fiber and wollastonite: Physical properties, morphology, and biodegradability. J. Appl. Polym. Sci. 2019, 136, 47543. [Google Scholar] [CrossRef]



| Property | PLA | PBAT | Ref. |
|---|---|---|---|
| Strength (εBreak) | High: up to ~90 MPa | Moderate: 14.5–61.3 MPa | [1,15,16,17,18] |
| Ductility (σBreak) | Low: 3–38% | High: 2–2500% | [1,16] |
| Glass transition temperature (Tg) | ~60 °C Brittle at ambient conditions | ~−30 °C Flexible at room temperature | [1,19] |
| Hydrophilicity | More hydrophilic (lower water contact angle) | Less hydrophilic | [20] |
| Crystallinity | Higher crystallinity, thus slower biodegradation | Lower crystallinity, thus faster biodegradation | [21] |
| Bulk density | Denser structure, thus slower degradation | Less dense, thus more readily degradable | [21] |
| Environmental degradation behavior | Slower when blended with PBAT Strongly influenced by molecular weight and stereoisomer composition | Degrades faster than PLA in blends (especially in freshwater sediment) | [20,21,22] |
| Run ID | PLA wt% | PBAT wt% | λ | Td (°C) |
|---|---|---|---|---|
| 1 D | 10 | 90 | 5 | 110 |
| 2 D | 10 | 90 | 5 | 90 |
| 3 D | 10 | 90 | 2 | 110 |
| 4 D | 10 | 90 | 2 | 90 |
| 5 D | 50 | 50 | 3.5 | 100 |
| 6 D | 90 | 10 | 5 | 110 |
| 7 D | 90 | 10 | 5 | 90 |
| 8 D | 90 | 10 | 2 | 110 |
| 9 D | 90 | 10 | 2 | 90 |
| 10 C | 100 | 0 | 5 | 110 |
| 11 C | 100 | 0 | 5 | 90 |
| 12 C | 100 | 0 | 2 | 110 |
| 13 C | 100 | 0 | 2 | 90 |
| 14 C | 100 | 0 | N/A | N/A |
| 15 C | 90 | 10 | N/A | N/A |
| 16 C | 50 | 50 | N/A | N/A |
| 17 C | 10 | 90 | N/A | N/A |
| 18 C | 0 | 100 | N/A | N/A |
| 19 V | 50 | 50 | 3.5 | 110 |
| 20 V | 90 | 10 | 3 | 110 |
| 21 V | 90 | 10 | 4 | 110 |
| 22 V | 10 | 90 | 4.5 | 110 |
| 23 V | 10 | 90 | 4 | 110 |
| Run ID | DSC | TGA | XRD | Tensile | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XPLA | Tcc | ΔHcc | Tm | ΔHm | Tg | ΔHr | T90 | T85 | T50 | Char | X | D | εBreak | σBreak | E | |
| % | °C | J·g−1 | °C | J·g−1 | °C | J·g−1 | °C | °C | °C | % | % | nm | % | MPa | MPa | |
| 1 D | 22.6 | * | * | 160.0 | 3.23 | 70.4 | 0.0071 | 386.8 | 405.0 | 437.0 | 4.3 | 19.4 | 46.3 | 453.9 | 64.3 | 26.7 |
| 2 D | 21.4 | * | * | 157.9 | 3.06 | 66.1 | 0.058 | 362.5 | 382.7 | 421.8 | 4.0 | 22.6 | 60.5 | 330.1 | 83.0 | 69.8 |
| 3 D | 17.3 | * | * | 162.2 | 2.48 | 64.6 | 0.055 | 387.0 | 406.3 | 444.1 | 4.4 | 8.7 | 44.0 | 998.5 | 13.2 | 14.6 |
| 4 D | 17.7 | * | * | 158.2 | 2.53 | 69.1 | * | 380.6 | 394.6 | 425.3 | 5.0 | 12.6 | 39.8 | 908.6 | 12.5 | 8.9 |
| 5 D | 22.2 | * | * | 159.4 | 15.85 | 67.5 | * | 353.2 | 360.4 | 399.7 | 3.3 | 31.8 | 29.4 | 141.7 | 38.8 | 184.1 |
| 6 D | 23.2 | * | * | 160.1 | 29.90 | 64.0 | 0.91 | 351.4 | 357.5 | 377.9 | 1.2 | 36.6 | 53.5 | 35.3 | 28.2 | 299.8 |
| 7 D | 28.0 | * | * | 162.4 | 35.98 | 67.9 | 1.32 | 355.7 | 363.2 | 387.9 | 1.4 | 51.0 | 18.9 | 64.1 | 29.7 | 302.3 |
| 8 D | 22.9 | 91.3 | 2.4 | 156.6 | 31.81 | 62.6 | 2.39 | 363.7 | 370.3 | 393.8 | 1.6 | 17.4 | 47.7 | 22.2 | 26.1 | 280.2 |
| 9 D | 22.0 | 88.2 | 3.8 | 162.3 | 32.07 | 63.0 | 2.62 | 347.7 | 353.0 | 370.7 | 1.9 | 43.0 | 30.0 | 189.0 | 52.9 | 495.8 |
| 10 C | 20.4 | 93.3 | 1.5 | 161.4 | 30.61 | 63.0 | 0.28 | 345.9 | 354.2 | 378.0 | 0.9 | 24.8 | 85.9 | 20.2 | 26.7 | 253.4 |
| 11 C | 25.6 | * | * | 158.3 | 36.60 | 67.3 | 1.07 | 337.9 | 342.7 | 359.3 | 1.0 | 57.8 | 17.7 | 37.6 | 34.6 | 360.2 |
| 12 C | 6.0 | 106.2 | 23.4 | 163.0 | 31.99 | 63.5 | 3.56 | 328.6 | 335.1 | 353.6 | 1.7 | 8.3 | 62.3 | 24.3 | 35.5 | 325.6 |
| 13 C | 12.6 | 91.7 | 16.3 | 162.9 | 34.28 | 62.3 | 4.11 | 342.3 | 347.6 | 364.7 | 1.2 | 14.1 | 64.5 | 83.5 | 30.1 | 337.3 |
| 14 C | 2.5 | 107.2 | 29.0 | 156.5 | 32.50 | 51.8 | 1.94 | 350.7 | 356.5 | 374.8 | 1.0 | 1.0 | 90.5 | 19.9 | 49.3 | 330.9 |
| 15 C | 4.0 | 102.0 | 23.5 | 159.6 | 28.68 | 56.3 | 2.25 | 352.0 | 358.0 | 379.1 | 1.6 | 1.1 | 118.0 | 252.7 | 27.1 | 261.3 |
| 16 C | 4.4 | 106.0 | 11.7 | 158.8 | 14.85 | 60.5 | 0.98 | 357.8 | 364.5 | 404.4 | 3.8 | 2.5 | 38.9 | 115.9 | 12.1 | 93.6 |
| 17 C | 14.7 | * | * | 161.6 | 2.10 | 60.3 | * | 381.5 | 398.0 | 429.7 | 4.9 | 5.6 | 29.6 | 2449.6 | 27.4 | 27.6 |
| 18 C | * | * | * | * | * | * | * | 402.3 | 409.5 | 431.8 | 4.8 | 6.1 | 32.5 | 3171.3 | 34.1 | 17.7 |
| 19 V | 20.9 | * | * | 161.4 | 14.93 | 66.1 | 0.56 | 343.7 | 353.3 | 392.1 | 4.5 | * | * | 413.8 | 51.8 | 358.9 |
| 20 V | 23.0 | * | * | 159.8 | 29.64 | 65.6 | 1.75 | 319.8 | 335.9 | 369.0 | 1.0 | * | * | 76.6 | 44.5 | 491.8 |
| 21 V | 22.6 | * | * | 161.8 | 29.05 | 67.1 | 1.05 | 333.3 | 341.8 | 364.1 | 1.2 | * | * | 36.5 | 41.5 | 438.7 |
| 22 V | 18.4 | 100.8 | 0.3 | 160.7 | 2.91 | 69.1 | * | 368.4 | 384.6 | 417.4 | 6.3 | * | * | 716.0 | 77.2 | 76.7 |
| 23 V | 23.5 | * | * | 160.6 | 3.35 | 66.6 | * | 367.6 | 384.4 | 421.7 | 6.0 | * | * | 652.4 | 76.9 | 59.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, N.; Gillor, O.; Guvendiren, M.; Axe, L. Biaxial Stretching of PBAT/PLA Blends for Improved Mechanical Properties. Polymers 2025, 17, 2651. https://doi.org/10.3390/polym17192651
Rodriguez N, Gillor O, Guvendiren M, Axe L. Biaxial Stretching of PBAT/PLA Blends for Improved Mechanical Properties. Polymers. 2025; 17(19):2651. https://doi.org/10.3390/polym17192651
Chicago/Turabian StyleRodriguez, Nikki, Osnat Gillor, Murat Guvendiren, and Lisa Axe. 2025. "Biaxial Stretching of PBAT/PLA Blends for Improved Mechanical Properties" Polymers 17, no. 19: 2651. https://doi.org/10.3390/polym17192651
APA StyleRodriguez, N., Gillor, O., Guvendiren, M., & Axe, L. (2025). Biaxial Stretching of PBAT/PLA Blends for Improved Mechanical Properties. Polymers, 17(19), 2651. https://doi.org/10.3390/polym17192651









