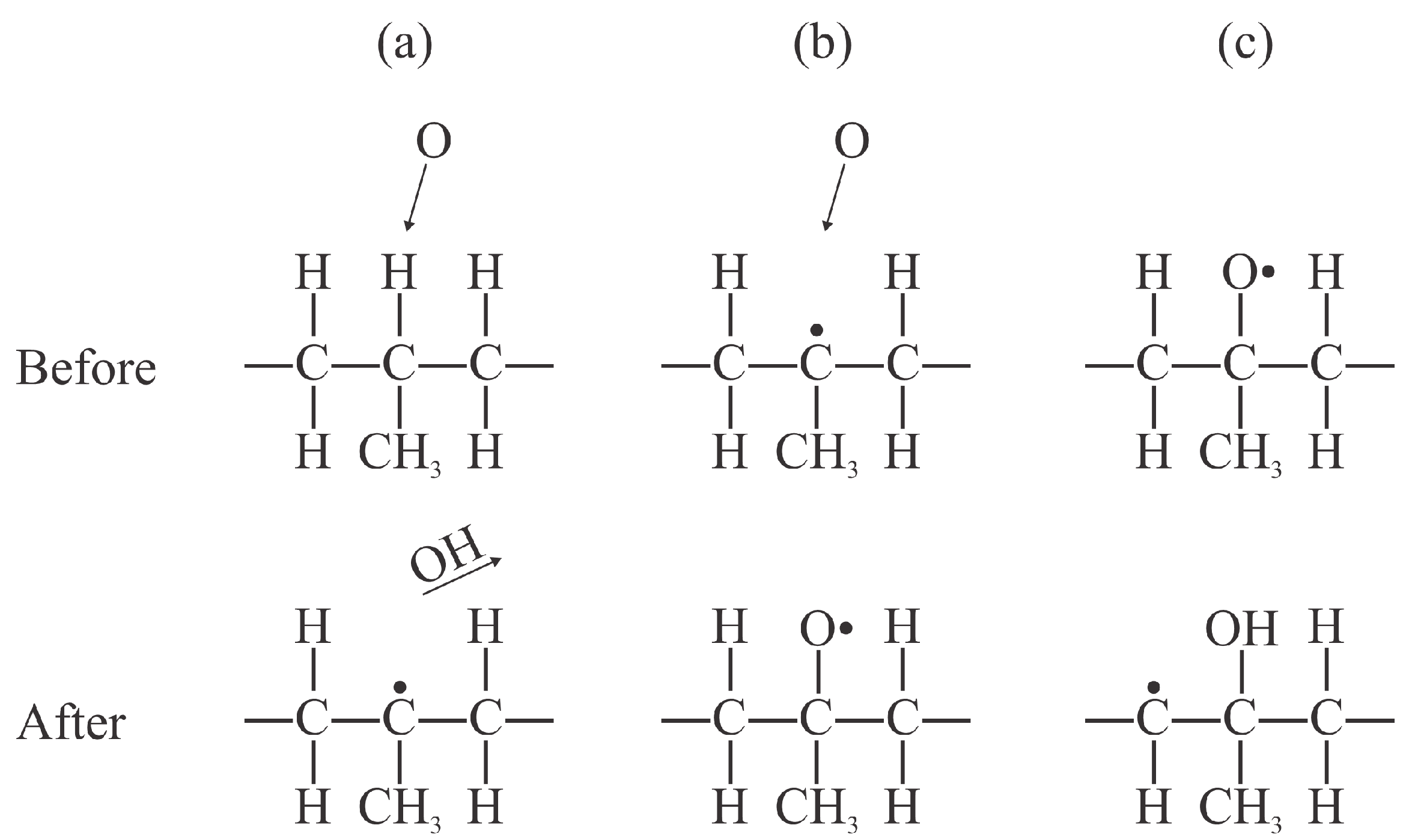1. Introduction
Polypropylene (PP) is a commonly used polymer with broad applications from electronics to the food industry and medical products [
1,
2,
3]. Its wide application arises from its low cost, good chemical inertness, and ease of manufacturing various products, from textiles to three-dimensional objects. The chemical inertness may be an obstacle in numerous applications, such as when a polypropylene product is printed, painted, or joined to another object by gluing. Good chemical inertness causes poor interaction of the coating on the PP product, and the absence of adhesion forces is attributed to the lack of polar functional groups on the PP surface. The adhesion of the coating could be increased by treating the PP surface with polar functional groups. Several methods for the surface functionalization of PP products have been reported [
4], including the thermo-oxidative chemical method [
5], thermo-molecular adhesion [
6], flaming, side-chain crystalline block copolymer [
7], and photochemical metallization [
8]. An often used method is treatment with gaseous plasma. Namely, plasma treatment does not require any chemicals and produces no waste, and thus is regarded as ecologically benign. Both atmospheric and low-pressure plasmas are useful for modifying polymer surface properties. Atmospheric pressure plasmas are also cost-effective because the discharges are powered by inexpensive generators and vacuum pumps are not needed, albeit one drawback may be the inability to sustain homogeneous non-equilibrium plasma in a large volume. Accordingly, such plasmas are not very useful for treating three-dimensional products [
9]. Even though this limitation does not apply to low-pressure plasmas, several technological challenges still arise when seeking to apply such plasmas on an industrial scale [
10].
Plasma treatment of PP was first reported decades ago. Early scientific articles include [
11,
12,
13], and perhaps the first theory on surface kinetics on the atomic scale was provided by a team led by Kushner [
14]. Later, several authors reported plasma functionalization using various experimental setups. Knowledge concerning the surface kinetics upon plasma treatment of PP has expanded over the decades. Today, it is generally accepted that plasma treatment causes numerous effects, including surface functionalization with polar functional groups. Other reactions include chain scission, cross-linking, modification of a surface film’s mechanical properties, formation of low-molecular-weight fragments, formation of volatile molecules with high vapor pressure even at room temperature, and etching, which typically modifies the surface morphology [
6,
15,
16,
17,
18,
19,
20,
21]. All these reactions influence the performance of plasma-treated PP. The intensity of various reactions occurring on the PP surface or subsurface region depends on the plasma parameters, which, in turn, depend on how the experimental setup was configured by the authors. Unfortunately, the vast majority of authors who reported the surface functionalization of PP by gaseous plasma treatment did not even mention the plasma parameters, let alone the intensity of a particular surface reaction versus the fluxes and/or fluences of the plasma species. The plasma species capable of causing the surface modification of PP include positively charged ions and neutral radicals, such as atoms, highly excited molecules, and energetic photons. The lack of detailed knowledge on the interaction of plasma species with the PP surface is not simply due to inadequate plasma characterization but also the lack of techniques for real-time monitoring of the surface composition and structure during plasma treatment. Namely, the vast majority of authors did not integrate the plasma into instruments for surface and thin film characterization. Instead, they treated polymer samples in a plasma reactor, exposed them to air, and performed surface characterization several minutes or even hours after the plasma treatment. Any fast surface reactions will occur between the plasma treatment and the surface characterization, and thus some information on the surface kinetics will be lost before the plasma-treated material is characterized. Spontaneous reactions cause a relaxation of the plasma-induced surface effects and, thus, aging. Aging is generally attributed to hydrophobic recovery, that is, the spontaneous loss of the hydrophilic character of the plasma-treated PP.
Aging is particularly apparent in systems with high potential energy or large gradients in the concentrations of various elements and functional groups. The laws of thermodynamics facilitate spontaneous relaxation until the most stable form has been reached. Gaseous plasma is a source of particles holding large potential and/or kinetic energy, and the vast majority of their interaction with the solid material occurs right on the surface such that the plasma treatment causes gradients and, thus, a thermodynamically unfavorable surface finish. Despite the aging of polypropylene being unavoidable, it is manageable to a tolerable extent, as shown in this review article.
The vast majority of authors used one of the oldest and most reliable methods for detecting the aging phenomenon of plasma-treated polymers: a water droplet. Only a few authors relied on more sophisticated methods, and some results are contradictory. It is hence impossible to draw any correlation between these methods and the water-droplet technique.
2. Gaseous Plasma
Gaseous plasma is the most common state of matter in the universe. In thermally equilibrium plasma, the distribution of excited states of gaseous molecules follows the Boltzmann and Maxwell laws, which means the concentration of plasma species with high potential or kinetic energy is only achievable at high gas temperatures. Polymers degrade at high temperatures and thermal plasma has limited use as it can only tailor the surface properties of PP when applied in very short pulses.
Non-equilibrium plasma is more useful for tailoring the surface properties of polymers. It is usually sustained by a gaseous discharge. A sufficiently high electric field is applied, and the charged particles accelerate and gain energy from that field. Molecular ions quickly lose their kinetic energy in elastic collisions with neutral molecules, radicals, and atoms, while electrons maintain their energy and their temperature (or, better, distribution over the kinetic energy) remains much larger than the temperature of other particles. In fact, the electron temperature in most plasmas of use for treating polymers is several 10,000 K. Such a high electron temperature does not cause significant heating of polymer samples exposed to non-equilibrium gaseous plasma for two reasons: first, the electron density is typically much smaller than the density of gaseous molecules and radicals, and second, they are retarded from the surface due to the negative surface potential, which is spontaneously formed upon the treatment of any object with non-equilibrium plasma. The role of free electrons in plasma is the excitation of molecules, which may be excited to electronic and rovibrational states upon inelastic collisions with such electrons. Energetically sufficient electrons may cause the dissociation of gaseous molecules, and electrons from the high-energy tail of their distribution function will also cause ionization. Ionization is crucial for sustaining gaseous plasma since the production of electrons upon ionization collisions between fast electrons and neutral particles should compensate for the loss of electrons by neutralization with positively charged ions.
The density of neutral plasma species with high potential energy (radicals, electronically excited molecules in metastable states, and atoms) is generally orders of magnitude larger than the electron density because the loss in the gas phase or on surfaces is often marginal compared to the neutralization of the charged particles. Electrons from the high-energy tail of their energy distribution also excite energetic states that are unstable, and relax almost immediately by electric dipole radiation. Therefore, non-equilibrium gaseous plasma is also a rich source of radiation, which is often predominant in the vacuum ultraviolet (VUV) range of the spectrum; as in, below approximately 200 nm. Such photons are energetic enough to cause bond scission in polymer materials. The concentration of electrons capable of exciting radiative energetic states is low and therefore the flux of VUV photons is much smaller than that of the reactive plasma particles suitable for chemical interaction with the PP samples. However, it is not negligible and causes significant modification of the polymer surfaces and subsurface film of thickness, which corresponds to their penetration depth.
Non-equilibrium plasma is usually sustained by an electrical discharge, in either a reactor in a controlled atmosphere or ambient conditions. The adjustable (also sometimes known as ‘external’) parameters include the peculiarities of the plasma reactor and the discharge configuration, discharge voltage, current, power, types of gases and their flow rates, and gas pressure. The discharge causes the formation of plasma, which is characterized by plasma parameters such as the electron density and temperature (or electron energy distribution function), density of positively and negatively charged molecules and their radicals, and density of metastable atoms and molecules. Plasma species impinge on the polymer surface, and thus the polymer is subjected to a flux of such species.
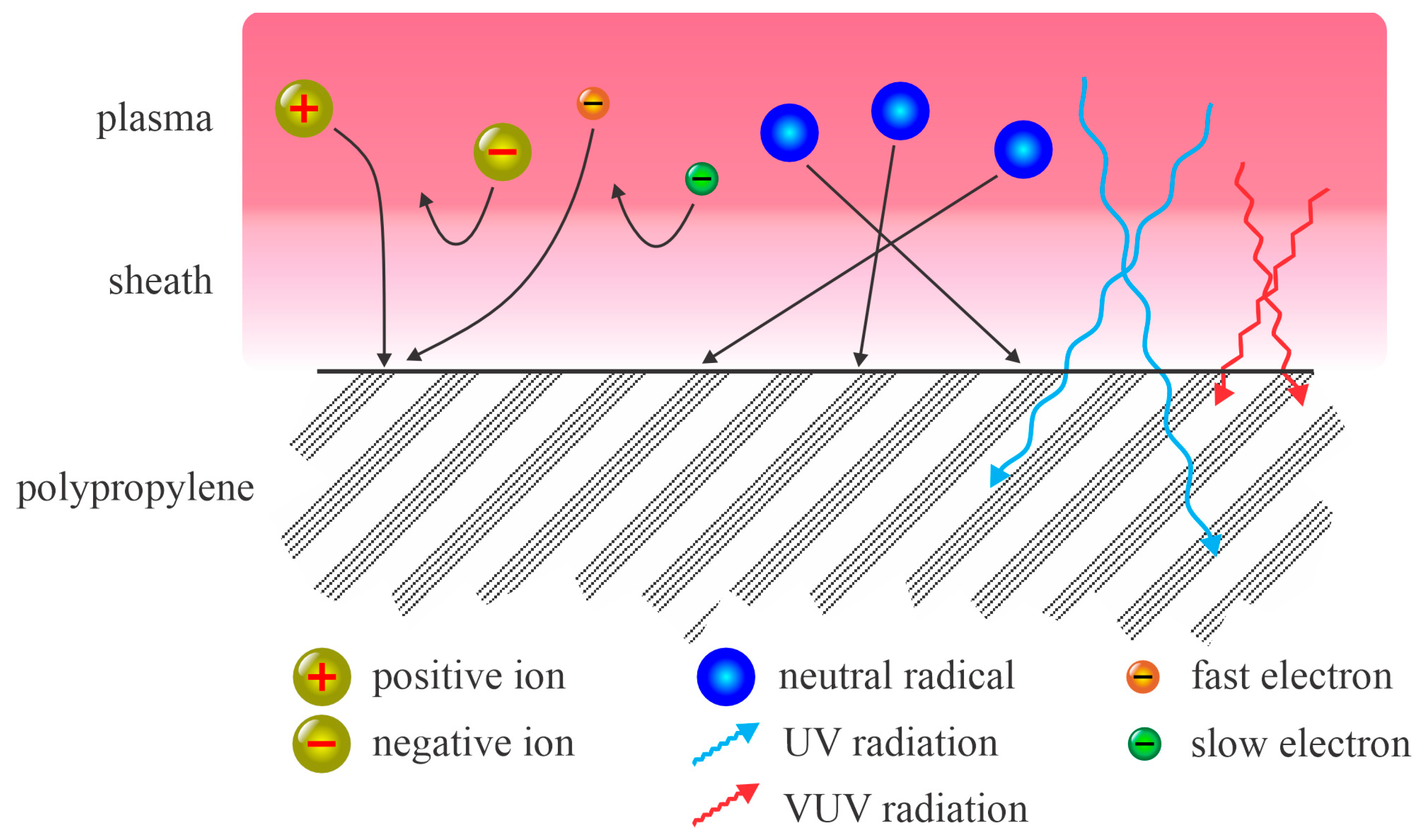
The treatment of PP with non-equilibrium plasma is illustrated in
Figure 1. A sheath exists between the plasma and the polymer surface. The electrons are much faster in the plasma than any other charged particles. Accordingly, the first effect after turning on the discharge is the formation of a negative charge on the polymer surface facing the plasma. As a result, the electric field retards the negatively charged plasma species and accelerates the positive ions. The positively charged ions are thus accelerated across the sheath and bombard the polymer’s surface. Their kinetic energy, however, is low, typically about 10 eV less when they collide with slow neutrals while passing the sheath. The electrons are retarded by the potential across the sheath and therefore only those from the high-energy tail can reach the surface. This explains why their influence on polymer modification is typically minor. The same applies to negatively charged ions, which are present in significant concentrations in plasmas sustained in electronegative gases. Since their kinetic energy in the plasma is low, much lower than the electron energy, they are unlikely to reach the polymer surface. The neutral species usually move randomly in both the plasma and the sheath; hence, they impinge on the surface in all directions. Their temperature in non-equilibrium plasma is low and remains low within the sheath, which means they do not have substantial kinetic energy while impinging on the polymer surface. Metastables may release their potential energy upon colliding with a polymer surface. Finally, radiation arising from the plasma influences the polymer if the photon energy is above the threshold for bond scission. The penetration depth of radiation exceeds that of the other plasma species.
Figure 1 indicates that the fluxes of positively charged ions, neutral radicals, and UV/VUV radiation govern the polymer surface finish.
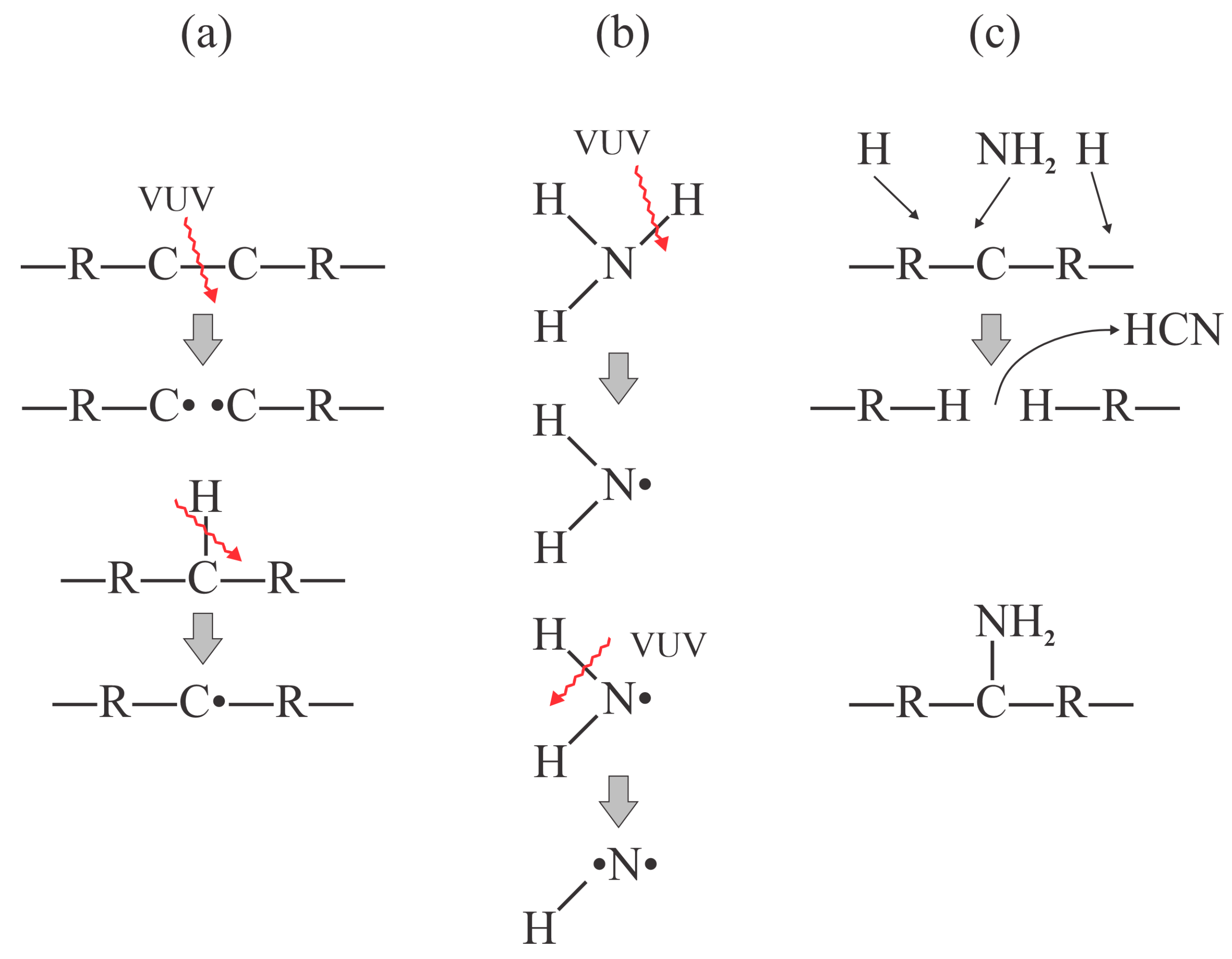
Numerous surface reactions occur when treating polypropylene with plasma species. As early as in 2003, Dorai et al. identified about 20 different surface reactions caused by oxygen species [
14]. The initial reactions while treating polypropylene with oxygen plasma are the subtraction of an H atom and the formation of an OH radical, which desorbs from the surface. The resulting dangling bond is likely to be occupied by another oxygen atom from the gas phase. According to Dorai et al., the dangling bond is sufficiently reactive to interact with ozone and even neutral oxygen molecules in the ground electronic state [
14]. The initial reactions likely to occur upon interaction of PP with oxygen plasma are shown in
Figure 2. Once the surface is saturated with hydroxyl groups, other groups are likely to be formed upon further exposure to O atoms, and the bond scission in the polymer backbone is likely to occur in the surface film with a high oxygen concentration, which leads to etching (the release of CO
2 and H
2O molecules).
4. Review of Articles Reporting the Hydrophobic Recovery of Plasma-Treated PP
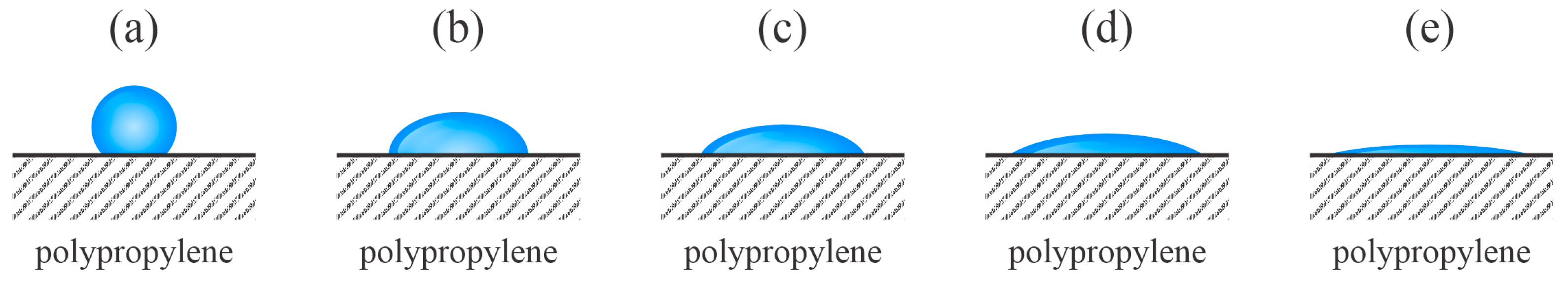
One of the first studies on the aging of plasma-treated PP samples treated with gaseous plasma was conducted by Behnisch et al. [
24]. The PP samples were first cleaned in an ethanol ultrasound bath and then exposed to non-equilibrium plasma treatments. A set of experiments was performed using solely oxygen plasma. Another set of samples was first treated with hydrogen plasma, and then with oxygen plasma. Hydrogen plasma is a rich source of VUV radiation [
25]. The plasma reactor was evacuated using a rotary pump, and the ultimate pressure was 0.1 Pa. The plasma was sustained by a capacitively coupled radiofrequency (RF) electrodeless discharge, and the plasma reactor was made of a dielectric material (Pyrex glass). The density of the charged particles was negligible with the position of the samples in the plasma reactor as they were mounted far from the glowing plasma in the volume between the externally mounted electrodes. Indeed, the main plasma species that influenced the PP wettability in [
24] were neutral radicals (H or O atoms) and quite weak radiation, which arose from the glowing plasma. The treatment time was 10 min for hydrogen plasma and 5 min for oxygen plasma. Interestingly, both treatments in oxygen plasma only and the subsequent treatments in hydrogen and oxygen plasma caused the same wettability since the WCA decreased from the initial 90° to approximately 50° immediately after the plasma treatments. The authors explained the moderately low WCA by bond scission rather than surface functionalization. Bond scission is a consequence of the absorption of VUV radiation. The plasma-treated samples were washed in distilled water in an ultrasonic bath, which increased the WCA to approximately 80° for both sets of samples. The authors explained the hydrophobic recovery upon ultrasound treatment by removing weakly bonded low-molecular-density fragments that formed upon the plasma treatment. Later, numerous authors treated PP samples, with all reporting surface functionalization with polar oxygen-containing functional groups after treatment with oxygen plasma [
26]. Even though hydrogen plasma should not cause oxidation of the polymer samples, the comparable WCA obtained by pre-treatment in hydrogen plasma can be explained by the presence of a residual atmosphere in the treatment chamber, which is water vapor in the case of hermetically tight vacuum systems. Water molecules dissociate under plasma conditions, and OH radicals readily interact chemically with the polyolefin surfaces, forming surface hydroxyl (and other) groups [
27]. Oxygen plasma is a much weaker source of VUV radiation than hydrogen and thus bond scission by energetic photons should be less intense in the case of oxygen plasma treatment [
28]. Given these facts, the results reported by Behnisch et al. [
24] can be interpreted as displayed in
Figure 4. The effect of VUV irradiation on surface hydrophilization and hydrophobic recovery was later elaborated by Truica-Marasescu et al. [
29]. They used a VUV lamp to treat PP foils.
Novak et al. [
30] used PP foils of different crystallinity, biaxially oriented and extruded, to study the long-term hydrophobic recovery after plasma treatment. The samples were treated in plasma sustained at atmospheric pressure by corona discharge in air. Regrettably, details of the discharge configuration, power, and treatment time were not disclosed. The samples were not washed after plasma treatment. The WCA after plasma treatment was approximately 68° and 50° for biaxially oriented and extruded PP, respectively. Aging was pronounced in the first month of being stored in ambient conditions, and after 1 year, the final WCA stabilized at 75° and 80°. The polar component of the surface free energy (PCSFE) was measured during the prolonged aging. The plasma treatment enabled PCSFE values of 9 and 14 mJ/m
2 for the biaxially oriented and extruded samples, respectively. Aging was much more pronounced for the extruded samples since the PCSFE stabilized at approximately 6 and 3 mJ/m
2 after several months of being stored under ambient conditions for biaxially oriented and extruded samples, respectively. This is one of the few articles to report variation in hydrophobic recovery for the same material, but with different crystallinities. The adhesion force of the polyvinyl acetate rose linearly as the polar component of the surface free energy increased, indicating the beneficial effect of plasma treatment on the adhesion of the two polymers. Similar results were published in [
31].
Guimond and Wertheimer also used corona discharge in the air to sustain plasma and treat biaxially oriented PP foils [
32]. The energy dissipated on the polymer surface varied by up to approximately 400 mJ/cm
2. The WCA of the untreated samples was about 85°, rising with increasing discharge energy, and stabilized at approximately 65° after an energy dose of ~30 mJ/cm
2. The concentration of oxygen in the surface film, as probed by XPS (several nanometers), was approximately 5, 9, and 11 at.% at the energy doses of 10, 100, and 430 mJ/cm
2, respectively. Such nonlinear behavior is typical for plasma-treated polymers because of the surface saturation with polar functional groups at moderate doses of plasma radicals and etching and nanostructuring at larger doses. The authors [
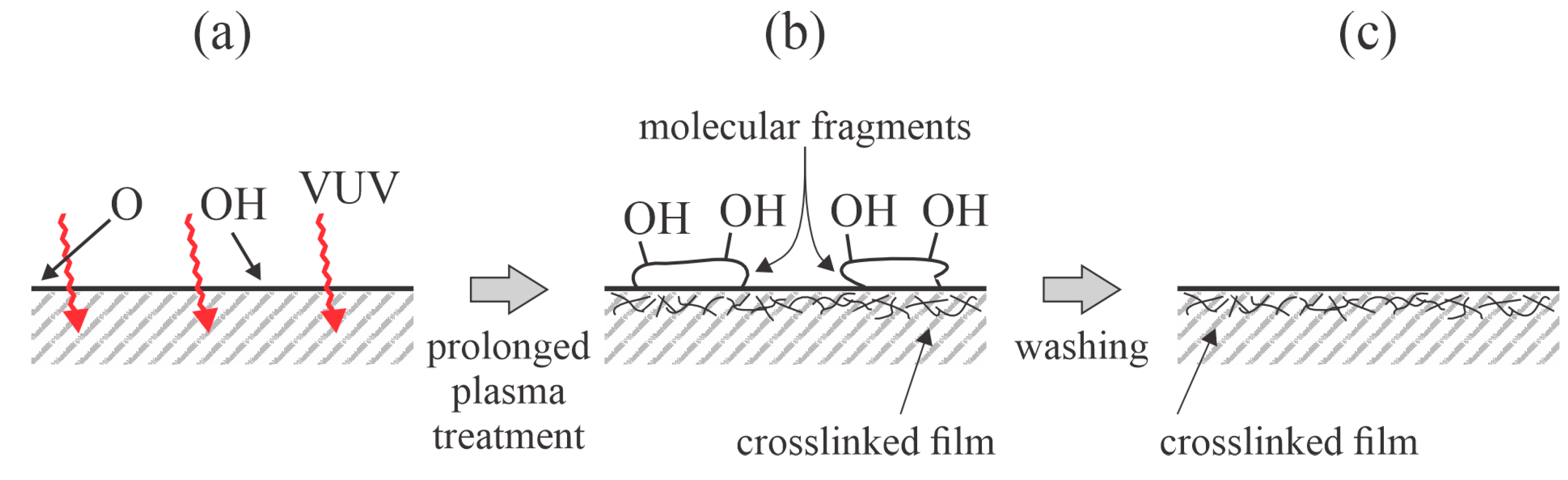
32] washed PP foils in deionized water after plasma treatment. The wettability of the washed samples was worse than that of the unwashed samples. The concentration of oxygen on the washed samples was almost equal (approximately 4 at.%) for all samples, irrespective of the energy dissipated on the surface upon plasma treatment. The authors explained this effect by the removal of weakly bonded low-molecular-density fragments that formed upon the plasma treatment, similar to Behnisch et al. [
24]. Interestingly, the loss of hydrophilicity after washing was practically the same as that reported by Behnisch et al. [
24]. The WCA of the washed PP samples was about 77°, irrespective of the energy dose upon plasma treatment. The effects of washing, as shown in
Figure 4c, also apply to [
32], albeit the discharge parameters were completely different in [
32] than in [
24]. Hydrophobic recovery was studied for unwashed samples kept in separate Petri dishes at atmospheric pressure, 23 °C, and 50% relative humidity. The loss of wettability was almost independent of the energy dose between 200 and 600 mJ/cm
2. The WCA increased to 75° within 1 day and to 78° within 3 months.
The team [
33] compared the effects of nitrogen plasma treatment of PP foils with those obtained by irradiation with VUV photons from a Kr resonant lamp operating at 123.6 nm in an ammonia atmosphere at 40 Pa. The nitrogen plasma was sustained at atmospheric pressure by a DBD discharge operating at a frequency of a few kHz and a peak voltage of 3.7 kV. The selected energy surface density was 0.9, 1.4, 1.9, and 2.8 J/cm
2, namely, much higher than in [
32]. The surface free energy of the untreated PP foils was 27 mJ/m
2 and increased to 37, 44, and 57 mJ/m
2 after treating the samples at energy densities of 0.3, 1, and 2 J/cm
2, respectively. They found that VUV radiation was beneficial, which is similar to Behnisch’s pioneering study [
24]. The authors [
33] reported surface energy as high as 65 mN/m, and the concentration of nitrogen, as determined by XPS, as high as 24 at.% after treating the PP samples with VUV radiation for 2800 s. Shorter treatment times led to significantly different results. Treatment with VUV radiation in an ammonia atmosphere for 500 s caused marginal surface effects. The pioneering work on VUV-stimulated surface hydrophilization showed promising results in terms of stability since the hydrophobic recovery was relatively small. The surface energy of 65 mN/m quickly dropped to 60 mN/m but remained as high as 55 mN/m even after storing the samples under ambient conditions for 3 months. Powerful VUV sources are available these days and thus a treatment time of 1 s is sufficient [
34]. The effects reported in [
33] are presented in
Figure 5. The first dissociation energy of ammonia is 2.6 eV [
35]; therefore, VUV photons with an energy of 10 eV can be absorbed and cause the formation of NH
x radicals. Since the NH
3 pressure is quite low, many photons reach the PP surface, causing bond scission in the surface film. The NH
x radicals occupy the dangling bonds and form stable nitrogen-containing functional groups. Therefore, the modified film is more than a monolayer thick, which explains the large nitrogen concentration as determined by XPS, the large surface energy as determined by WCA, and the slow hydrophobic recovery.
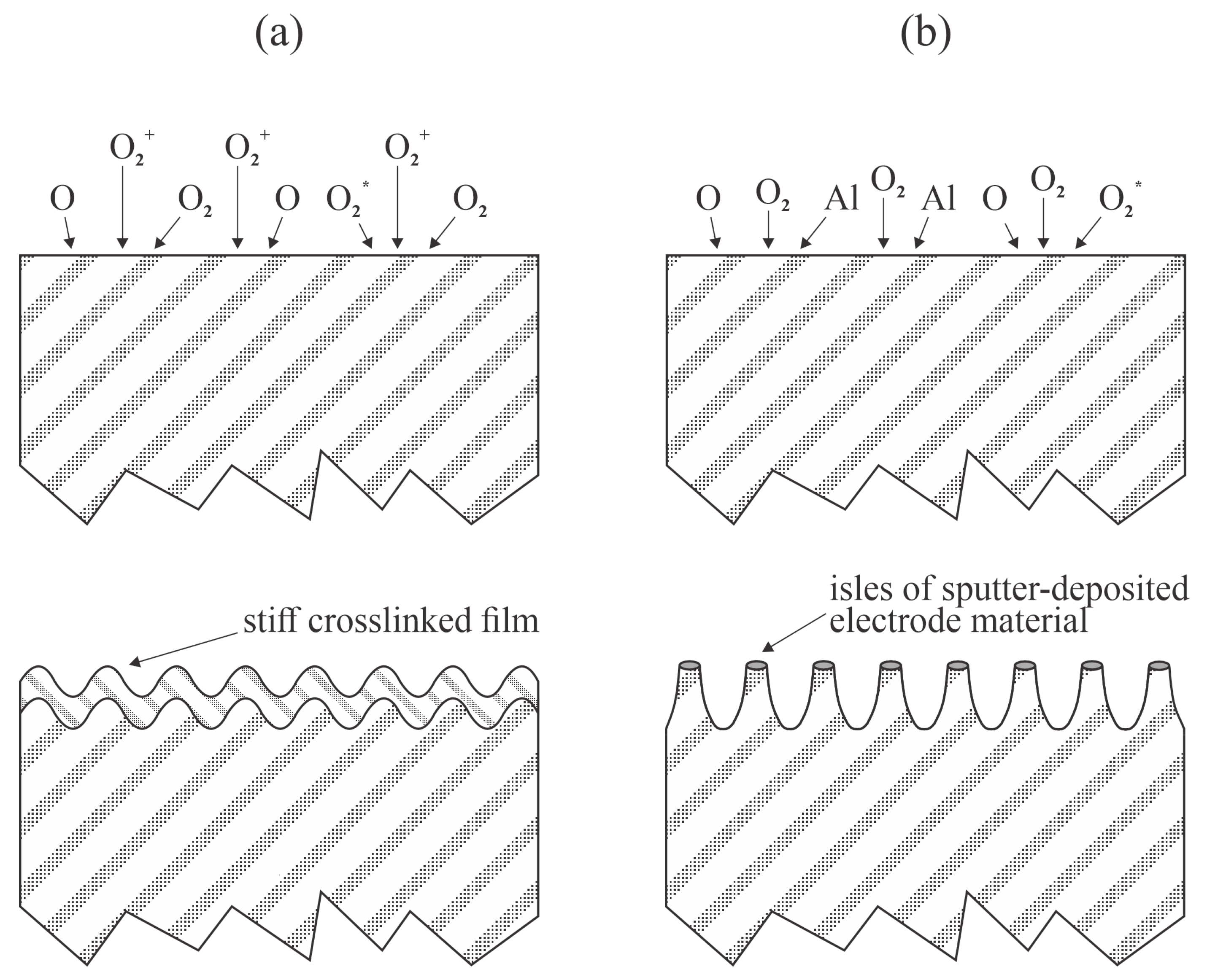
Ren et al. [
36] treated PP foils and nonwoven fabrics with low-pressure oxygen plasma sustained by a capacitively coupled RF discharge at 40 kHz. The oxygen pressure was kept constant at 15 Pa, whereas the discharge power was varied between 200 and 600 W, and the treatment time was up to 8 min. The WCA was measured for foils only given that the droplets were absorbed in the oxygen plasma-treated fabrics. At a discharge power of 600 W, the WCA decreased from 100° to approximately 30° after 1 min of plasma treatment, remained fairly unchanged for another 6 min, and dropped to 10° after treatment for 8 min. At a fixed treatment time of 4 min, the WCA was 40° at 200 W and approximately 30° for discharge powers between 300 and 600 W. XPS characterization was performed 2 days after the plasma treatment. The surface film of the plasma-treated samples contained about 30 and 25 at.% of oxygen for foils and PP fabrics, respectively. No statistically significant differences in composition were observed 22 days after the plasma treatment. Significant hydrophobic recovery was observed with both the foils and fabrics. The WCA on the foil increased to 57° and 71° after 12 days and 1 month of storage in ambient conditions, respectively. Interestingly, the water droplets were absorbed in the fabrics made from PP fibers with a rich morphology on the micrometer scale, even after having been stored for 1 month. Ren et al. [
36] clearly showed that the hydrophobic recovery of PP is not correlated with the loss of oxygen on the polymer surface. The paper by Ren et al. [
36] is the only one to have reported very low WCA on PP foils (10°), and explained it by increasing roughness after prolonged treatment. Unfortunately, the team did not report the discharge voltage to estimate the kinetic energy of ions impinging on the electrodes, or the position of the foils. If the foils were placed onto an electrode of a low-frequency RF discharge, a DC self-biasing would occur, and the foil would be subjected to bombardment by energetic ions, as displayed in
Figure 1. Namely, the sheath is likely to be almost collisionless at a pressure of 15 Pa, where the mean free path is about 1 mm [
37]. Prolonged bombardment with positive ions is likely to cause nanostructuring, as explained in detail by Bruce et al. [
38]. Alternatively, bombardment of the powered electrode by positively charged ions causes sputtering. The sputtered atoms condense on the surfaces, forming unevenly distributed metal-oxide clusters that locally prevent polymer etching. As an outcome, a nanostructured surface is formed, as elaborated by Tsougeni et al. [
39] and Palumbo et al. [
40]. Both effects are illustrated in
Figure 6.
Encinas et al. [
41] treated PP foils with gaseous plasma at a pressure of 2 bar. Plasma was sustained using a rotating torch powered by a 20 kV voltage source operating at a frequency of 17 kHz (Plasma Treat GmbH, Steinhagen, Germany) and expanded to the ambient pressure in which the polymer foils were placed. Plasma-treated foils were stored in dust-free air at a relative humidity of approximately 50% and a temperature of 25 °C. The untreated PP exhibited a surface free energy of 22 mJ/m
2 (corresponding to a WCA of approximately 100°), and the plasma treatment caused an increase to 50 mJ/m
2 (the WCA dropped to ~42°). While this surface free energy (SFE) persisted for a few days of storage, prolonged aging (21 and 32 days) caused the SFE to increase. Such a rise in the surface energy following storage has not been reported by other authors and has yet to be explained. All other authors reported a decrease in surface energy following storage under ambient conditions. A large standard deviation was reported for PP foils aged for different periods of up to about 1 week. The WCA after 1 day of aging was approximately 45°, which decreased to 40° after 1 week. The lowest WCA of about 20° was reported for a sample stored for 1 month. This is one of the few scientific articles to report a larger SFE (lower WCA) for aged PP foils than for as-treated foils. The unexpected behavior of the surface wettability was supported by XPS measurements. The authors found that the concentration of the hydroxyl groups was approximately 12 at.%, even for an untreated sample. Plasma treatment caused an increase in the concentration to 30 at.%, and the formation of C=O as well as O–C=O functional groups among the concentration of about 10 at.%. The highest concentration of oxygen, as calculated from the survey spectra, was reported for samples stored for 21 days.
Chen et al. [
42] treated PP foils with oxygen plasma sustained by a capacitively coupled RF discharge at a pressure of 60 Pa. The aging of plasma-treated samples was studied for samples stored in either in a vacuum desiccator or distilled water. The samples aged in water were dried well before the water contact angle was measured. The WCA of the untreated PP sample was 103°. The plasma treatment caused a gradual decrease in the WCA when the plasma was sustained at a discharge power of 20 W, and it was more rapid when sustained at 80 W. The final WCA after 10 min of plasma treatment was approximately 90° for all discharge powers. A discharge power and treatment time of 80 W and 120 s, respectively, were found to be optimal because the lowest WCA of 80° was obtained. However, aging was studied for samples that exhibited a WCA of 56° immediately after plasma treatment. When stored in air, these samples exhibited weak hydrophobic recovery within the first week, but prolonged storage in air did not influence the wettability as the WCA remained at approximately 70° for a period between 10 and 90 days after the plasma treatment. Somewhat different behavior in surface wettability was observed for plasma-treated PP samples stored in distilled water. The sample was immersed in water for a very short time, dried in a vacuum, and probed for wettability. Even brief immersion in water led to an increase in the WCA from 56 to 73°. Storage in water for a few days caused further hydrophobic recovery because the WCA for the samples stored in water increased to approximately 80° within 1 week. Thereafter, it remained constant for the next 80 days. The rapid hydrophobic recovery of PP samples stored in water may be explained by the removal of highly functionalized molecular fragments, as displayed in
Figure 4.
The behavior of washed PP samples reported by Chen et al. [
42] was observed to be similar in Jokinen et al. [
43]. Jokinen used a low-pressure plasma reactor powered by a microwave (MW) discharge at a frequency of 2.45 GHz at a power of 500 W. The gas flow rate was 800 sccm, but neither the gas pressure nor the pumping speed of the vacuum pump was provided. The WCA of the untreated PP foils was 101°, which decreased to ~60° after treatment with oxygen plasma for 10 min. The samples immersed in water exhibited very fast and total hydrophobic recovery, which may be explained by the removal of the surface-modified moieties upon washing (
Figure 4). Jokinen et al. [
43] also treated the same PP samples with nitrogen plasma and found somewhat better wettability since the WCA after treating for 10 min dropped to 43°. Still, hydrophobic recovery was faster for these samples. All washed samples eventually assumed the WCA typical for non-treated samples, even after being stored for a few days. The samples stored in air did not exhibit total hydrophobic recovery because the WCA remained at 85–90° even after aging for 120 days.
Kalapat et al. [
44] treated biaxially oriented PP foils with atmospheric pressure plasma sustained by a corona discharge in the air. The WCA was measured on samples treated at different discharge powers, normalized on the surface area between about 1.5 and 76 J/cm
2. The WCA for the untreated samples was 100° and decreased to 72, 65, and 55° after receiving energy doses of 1.5, 3.8, and 7.6 J/cm
2, respectively. No significant aging was observed after the samples were stored in ambient conditions for up to 3 months. The stability of the moderately hydrophilic character of these samples is difficult to explain, especially since Novak et al. [
30] used a similar discharge but reported a fast hydrophobic recovery.
Ozkaya et al. [
45] used an MW discharge in the electron cyclotron resonance mode to sustain the plasma, which is useful for the surface hydrophilization of PP films. The films were deposited onto gold-coated silicon wafers via spin coating. The samples were mounted in a vacuum chamber and exposed to oxygen ions extracted from the plasma using an appropriate electrically biased grid. The ion kinetic energy after impinging on the polymer samples was 250 eV, and the flux at the beam center was approximately 5 × 10
17 m
−2s
−1. The evolution of the surface morphology must be as illustrated in
Figure 6a. The oxygen content on the PP surface, as probed by XPS, increased with higher doses of oxygen ions. AFM-based chemical force microscopy revealed an increased adhesion force after receiving an oxygen ion fluence of approximately 5 × 10
17 m
−2. Nevertheless, the adhesion force was not significantly different from that of the untreated sample at other fluences, up to 3 × 10
19 m
−2.
Kostov et al. [
46] used two different discharges for treating PP foils: an atmospheric-pressure plasma jet sustained in argon and a dielectric barrier discharge (DBD) sustained in air. The plasma jet was operated at a voltage of 10 kV, frequency of 37 kHz, and treatment time of 1 min. DBD was operated at 12 kV and 20 kHz. XPS was used to determine the changes in the surface composition. The untreated samples contained 2 at.% of oxygen, and the high-resolution C1s peak revealed that oxygen was bonded in the hydroxyl groups. Treatment with the plasma jet caused an increase in the oxygen content to 27 at.%, and approximately one-third of the oxygen was bonded to the O–C=O groups. The lowest achievable WCA was 52°. Rinsing in distilled water for 1 min caused significant changes in the surface composition because the HR XPS C1s peak corresponding to O–C=O vanished and the peak corresponding to the C=O group was close to the detection limit. The authors concluded that only molecular fragments were heavily oxidized after the plasma treatment and were removed by rinsing with distilled water. The effect is shown in
Figure 4. Similar results were reported for PP treated by DBD, except that the oxygen concentration after the plasma treatment was only ~16 at.%. Interestingly, the rinsing was not as effective as for the samples treated by the plasma jet since the concentration of the remaining hydroxyl groups was almost twice that of those treated with the plasma jet. The much higher degree of functionalization while using Ar plasma can be explained by the VUV radiation arising from the plasma [
47]. Radiation causes bond scission and the formation of numerous dangling bonds in the surface film. The dangling bonds interact with oxygen from the effluent gas or water vapor, which desorbs from the tube in which the jet was sustained [
48].
Lindner et al. used atmospheric pressure corona discharge in air to treat PP foils [
49]. The discharge energy normalized to the surface area was in the range of 1–5 J/cm
2. The WCA of the untreated samples was approximately 100° and decreased to about 72° independent of the normalized discharge energy. Gradual hydrophobic recovery was observed upon aging under ambient conditions. However, the measured WCAs were significantly scattered, and the error bars were large. The average WCAs after aging for 1 day, week, and month were approximately 72, 75, and 77°, respectively. Half a year of aging led to a further increase in WCA to approximately 80°.
Matoušek et al. [
50] treated PP foils in air plasma. The DBD discharge was powered by a generator at a frequency of 3 kHz, voltage of 20 kV, and power of 120 W. The selected treatment times were 1, 2, and 3 s. The water contact angle of the untreated sample was approximately 99°. The WCA dropped after the plasma treatment to 81, 75, and 70° for treatment times of 1, 2, and 3 s, respectively. An increase in wettability is expected for short treatment times. Of greater interest is the report on the hydrophobic recovery. The WCA for all three samples decreased 1 day after the plasma treatment and assumed values of 67, 69, and 67°, respectively. Such an increase in wettability after storing plasma-treated polymers for 1 day under ambient conditions was reported by Encinas et al. [
41]. The XPS results, on the other hand, showed a gradual decrease in the oxygen concentration during aging, which contradicts what was reported by Ren et al. [
36]. About 6 at.% of oxygen was found on the surface of an untreated PP foil, and 17% just after treatment. The oxygen concentration decreased to 12 at.% after 2 months of aging, irrespective of the plasma treatment duration. Aging was established to be detrimental to the adhesion of the solvent-based paint because it was within the limits of the experimental error for the untreated samples. The reported adhesion of water-based paint followed a path that is difficult to explain.
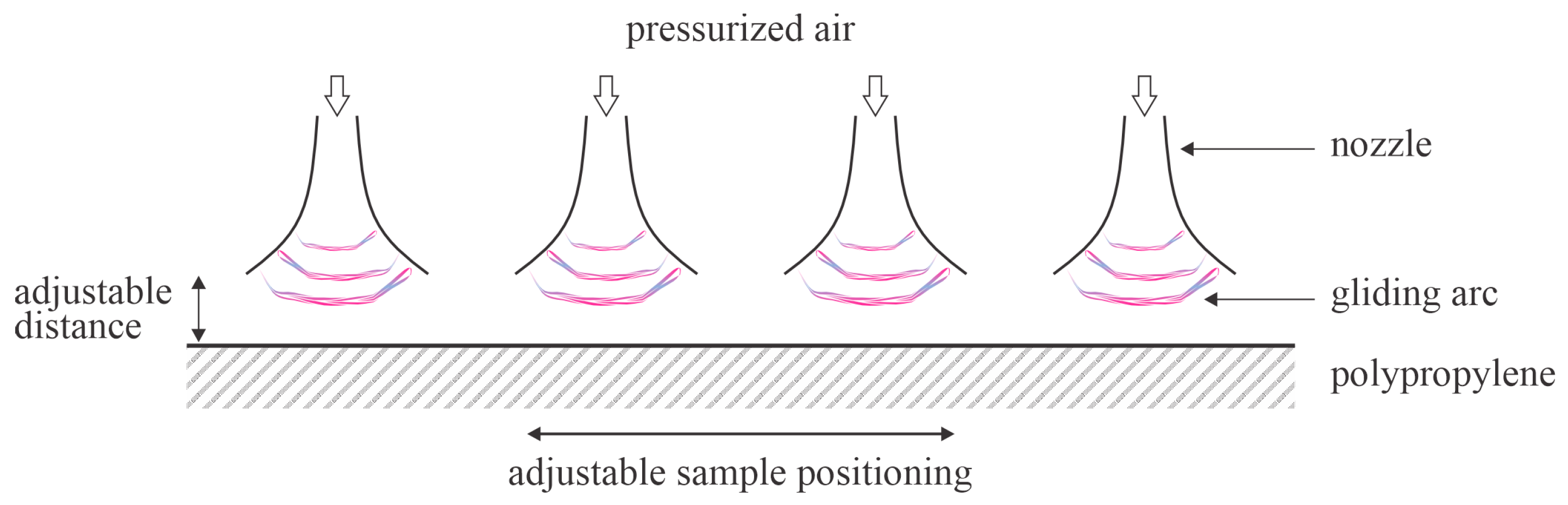
Pichal et al. [
51] treated thick PP foils with plasma in air at atmospheric pressure, sustained by a gliding arc in a specific configuration. Four linearly placed gliding arcs were mounted parallel to each other so the total length of the plasma in contact with the polymer was about 20 cm. The discharge power was about 1 kW, but the PP sample was placed away from the arcs; the thermal effects were thus suppressed. The samples were mounted on a conveyor belt and moved at various speeds from 0.05 to 0.2 m/s. The described configuration enabled the plasma treatment time of the PP plate to be approximately 1 s at a belt speed of 200 mm/s. The gliding arcs occurred at the exhaust of the pressurized air nozzle. Dry air was pressurized to 6.5 bar and delivered to the arcs at a flow rate of 70 L/s. As expected, the surface wettability of the PP samples decreased with increasing distance from the main discharges and with increasing conveyor belt speed. The WCA of the untreated PP sample was 102°. The minimal WCA after plasma treatment of 25° was reported for the distance between the arc discharge PP plate of 5 mm at a conveyor belt speed of 50 mm/s. This is namely the lowest value of the water contact angle reported by any team that published experimental results on the hydrophobic recovery of PP treated for reasonable times. Further, such a low WCA persisted for a few days in the air at 23 °C and 50% humidity. The error bars were moderate at approximately ±10°. Hydrophobic recovery became significant only after storing the plasma-treated sample for 10 days, whereas 110 days of storage resulted in serious, yet incomplete recovery since the WCA was approximately 85°. Samples treated at a higher conveyor belt speed did not perform well because the WCA after plasma treatment was close to 50°, which is a value typically reported by most authors. The methods disclosed by Pichal et al. [
51] thus enable almost optimal wettability of PP samples and are scalable virtually to any dimensions. One drawback of this method is its high energy consumption. The method is illustrated in
Figure 7.
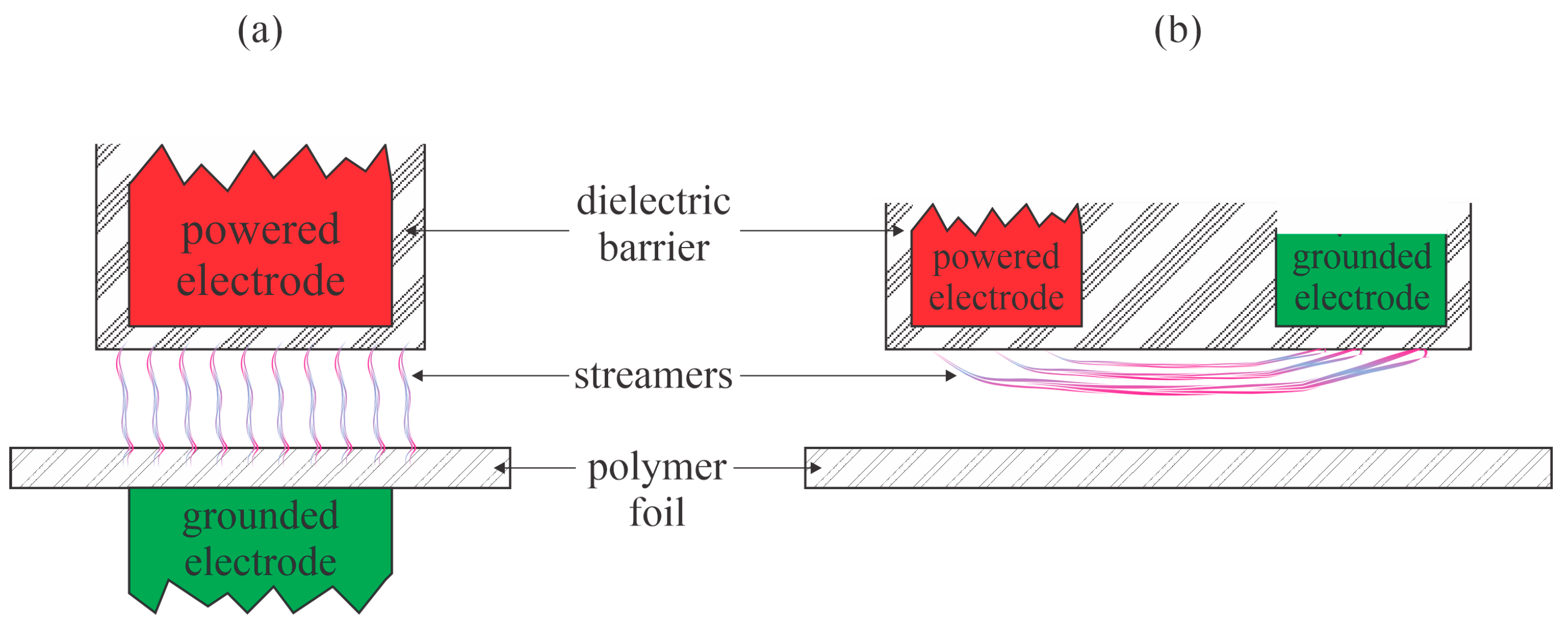
Šramkova et al. [
20] performed hydrophilization of PP foils with atmospheric pressure plasma sustained in the air by a couple of discharges: a conventional DBD and a coplanar DBD. The WCA of the untreated samples was 105°. The main difference between these discharges is that the discharge streamers hit the surface of the polymer samples upon plasma treatment while using standard DBD, and did not hit the polymer surface in the case of coplanar discharges. Thus, the classical DBD always causes thermal damage, including microcraters at positions where they hit the polymer surface, whereas the coplanar discharges enable gentler surface treatment because the major reactants are molecular radicals that move randomly. The treatment time was 1–10 s. The conventional DBD caused a minimal WCA of approximately 68° at a treatment time of 3 s. The coplanar DBD, however, caused a monotonous decrease in the WCA, which was 87, 63, 60, and 53° after treating the PP foils for 1, 3, 5, and 10 s, respectively. Modest hydrophobic recovery was reported after storing the plasma-treated samples under ambient conditions for up to 1 month. A comparison of the standard and coplanar DBD is provided in
Figure 8. Configuration (
Figure 8b) prevents polymer damage by streamers, but the distance between the electrodes and the polymer foil should be adjusted carefully due to the large vertical gradients in the concentration of the reactive gaseous species.
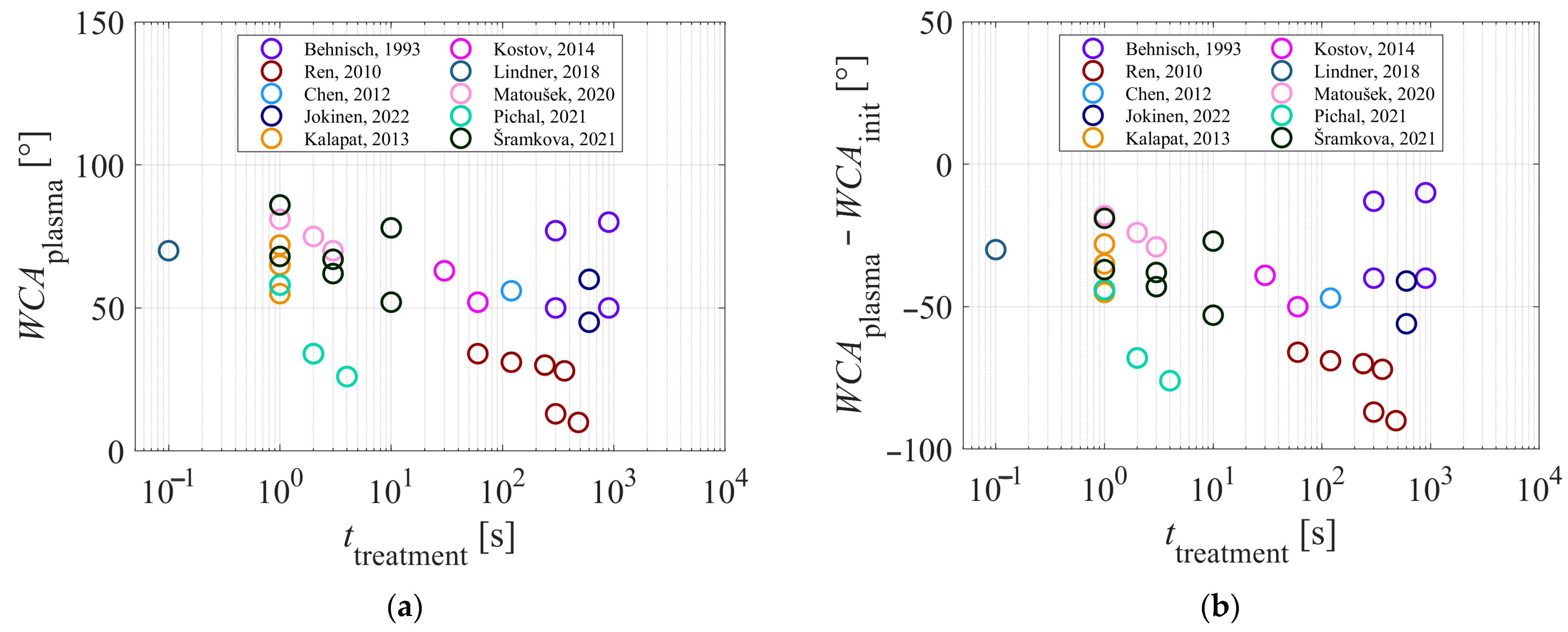
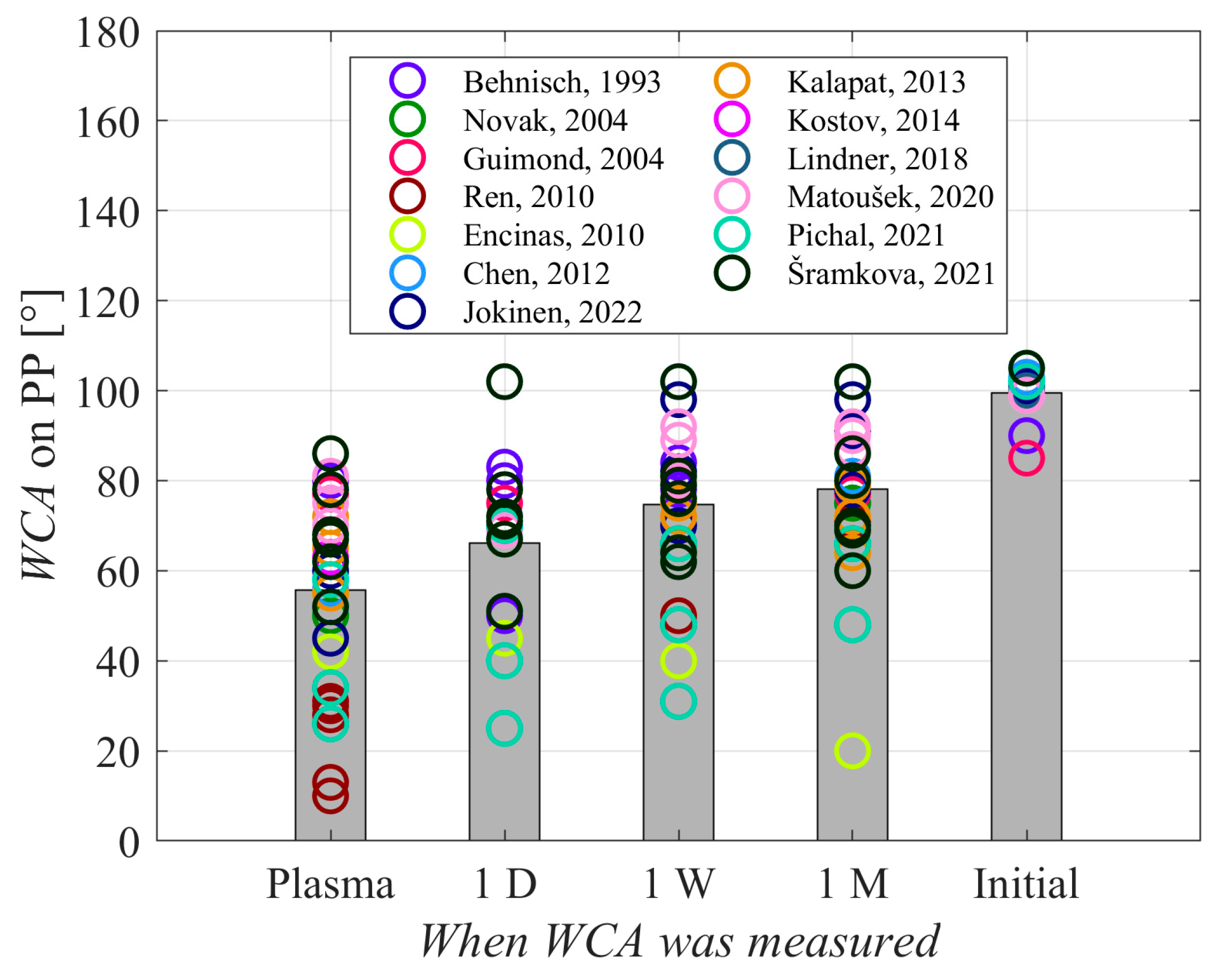
Table 1 presents a summary of the key results reported in the reviewed articles. All authors reported the type of discharge selected for sustaining gaseous plasma, as well as the gas or gas mixture. While many authors also reported the discharge power, it should be stressed that the samples were usually much smaller than the area of the plasma-surface interface and thus discharge power alone might not be the relevant parameter. The authors also reported the treatment time, and the WCA before and after plasma treatment. A few authors also studied the hydrophobic recovery of plasma-treated polypropylene samples.