Electropolymerized PAA as a Functional Matrix for CeO2-NiO Hybrid Electrocatalysts for Efficient Water Oxidation
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
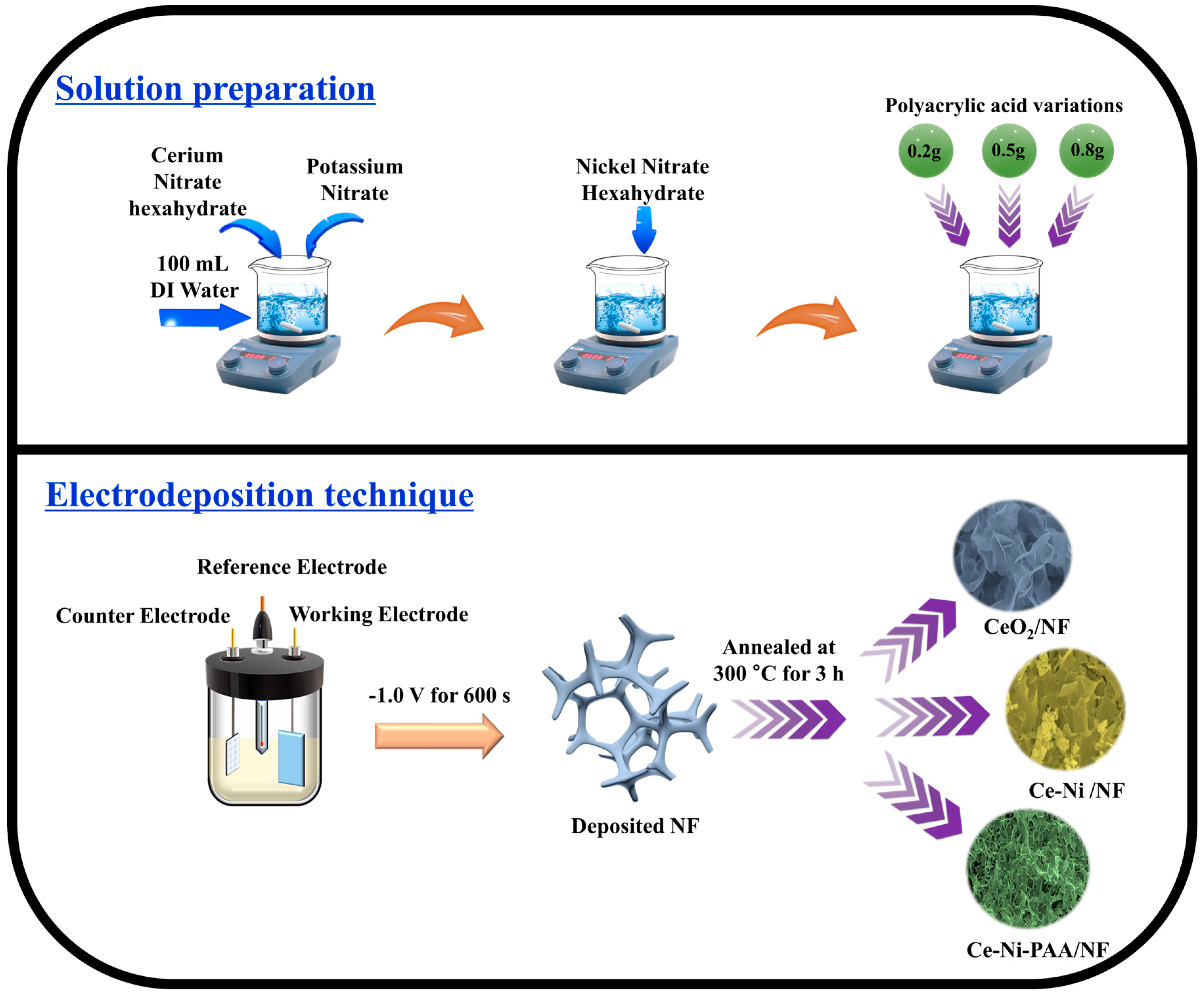
2.2. Synthesis of CeO2, CeO2-NiO, and CeO2-NiO-PAA Precursor Solution
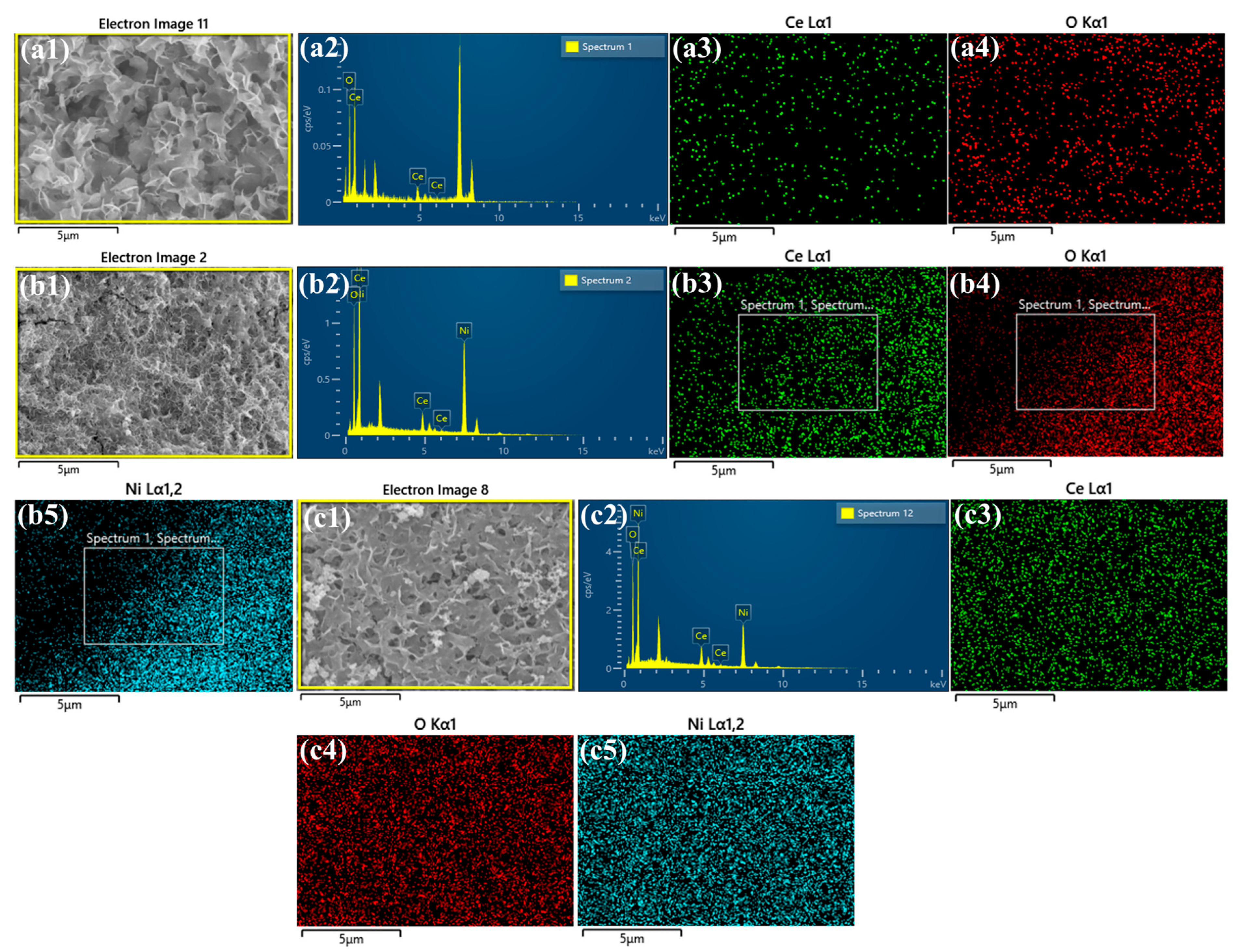
2.3. Material Characterization
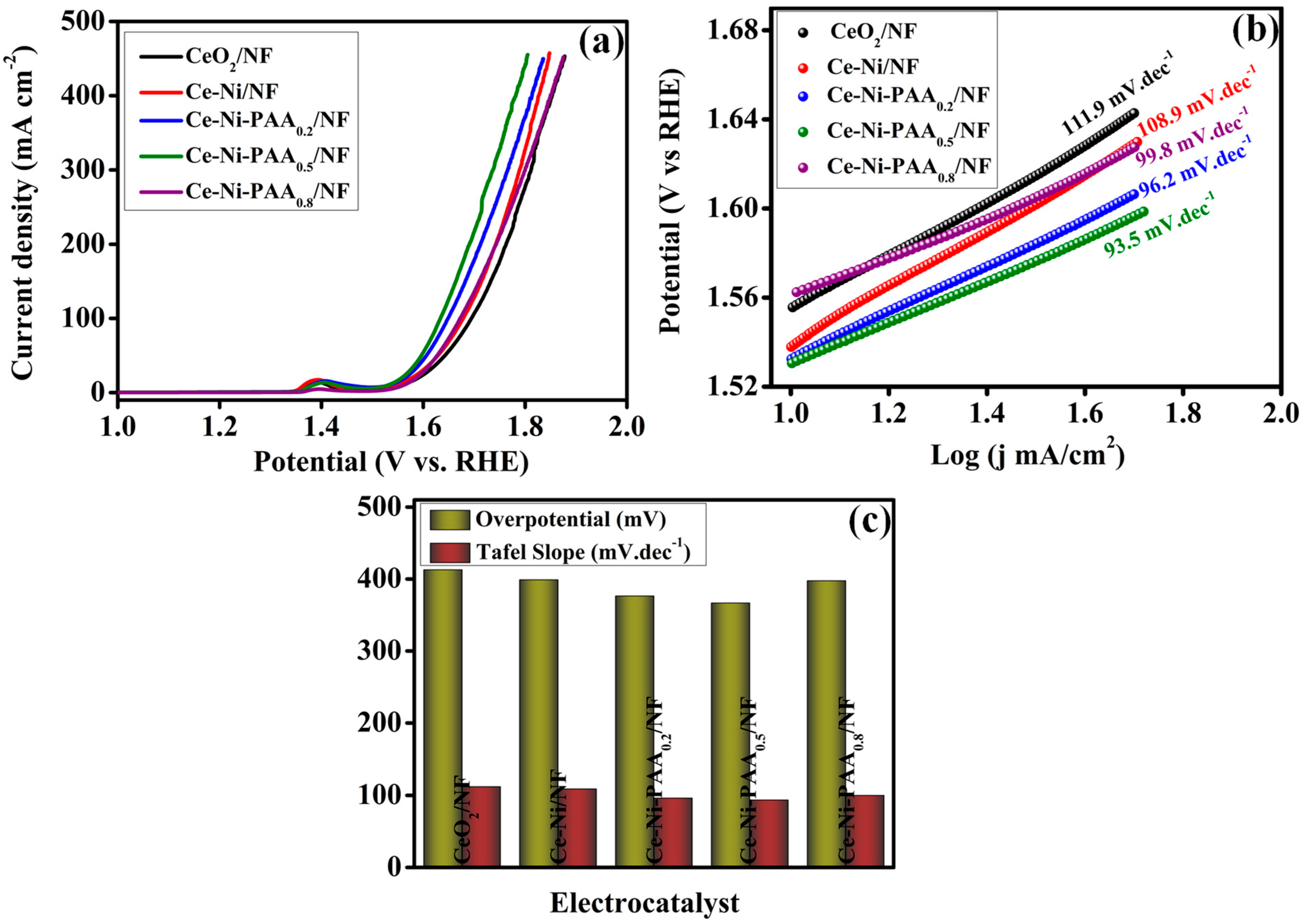
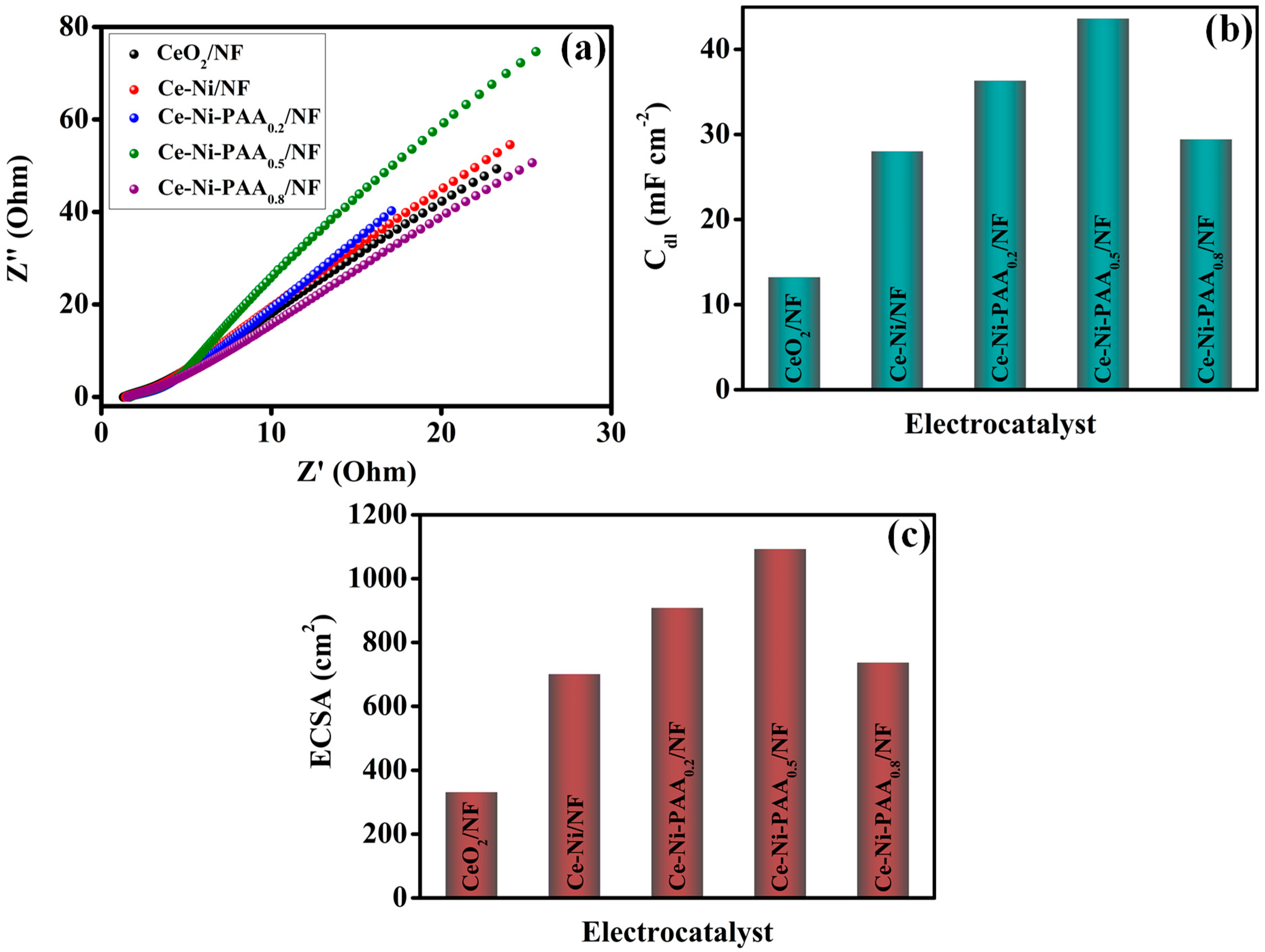
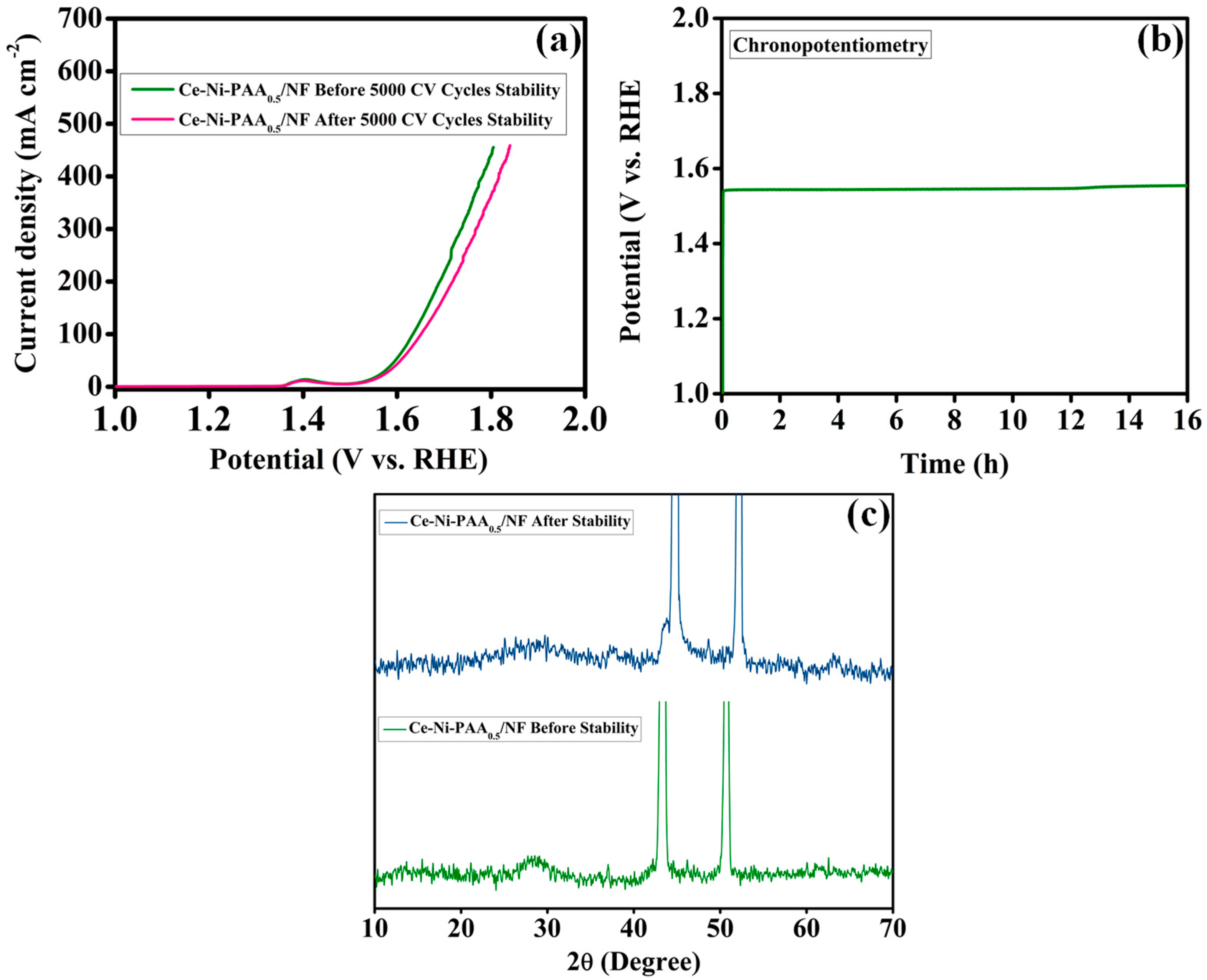
2.4. Electrochemical Analysis
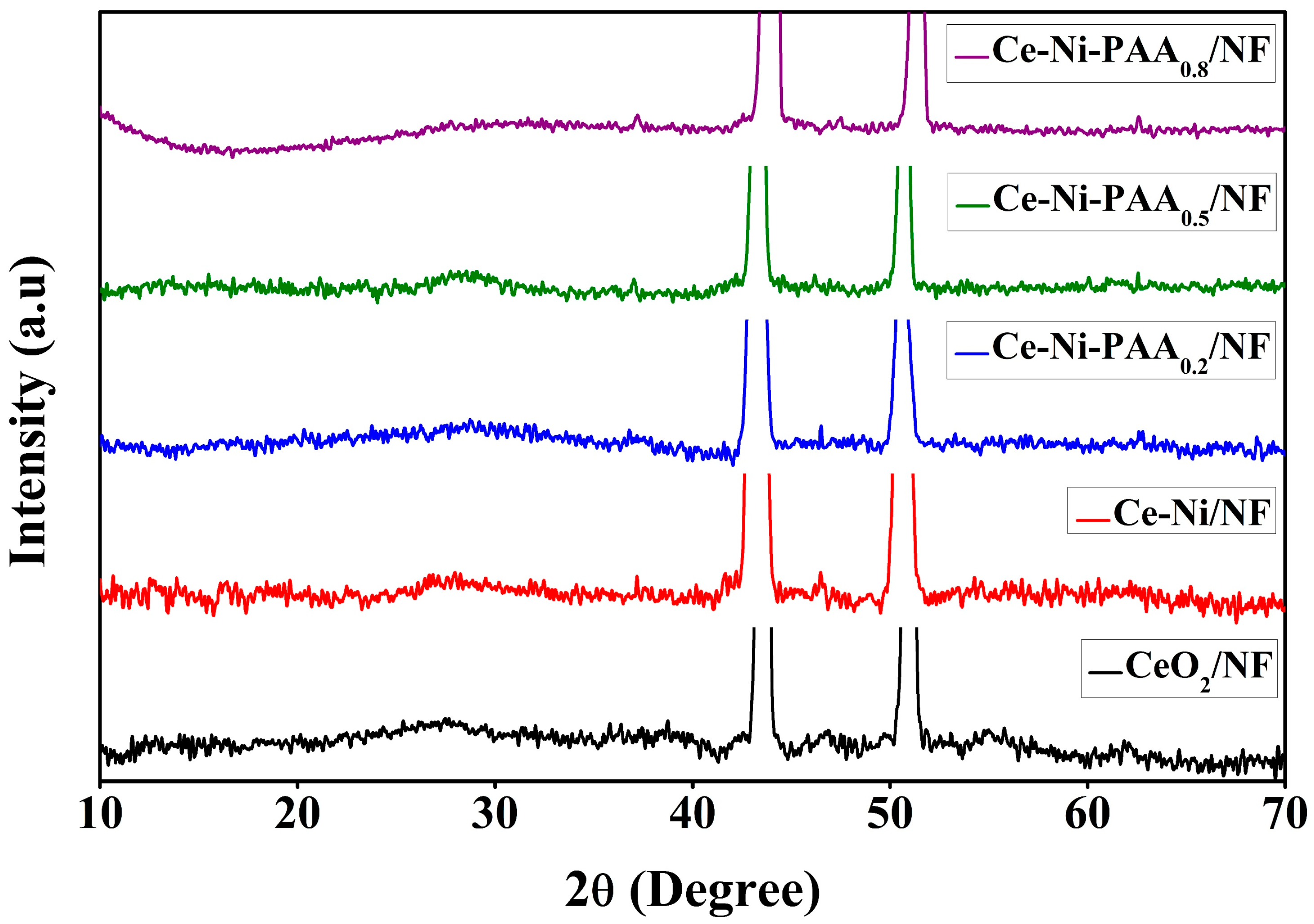
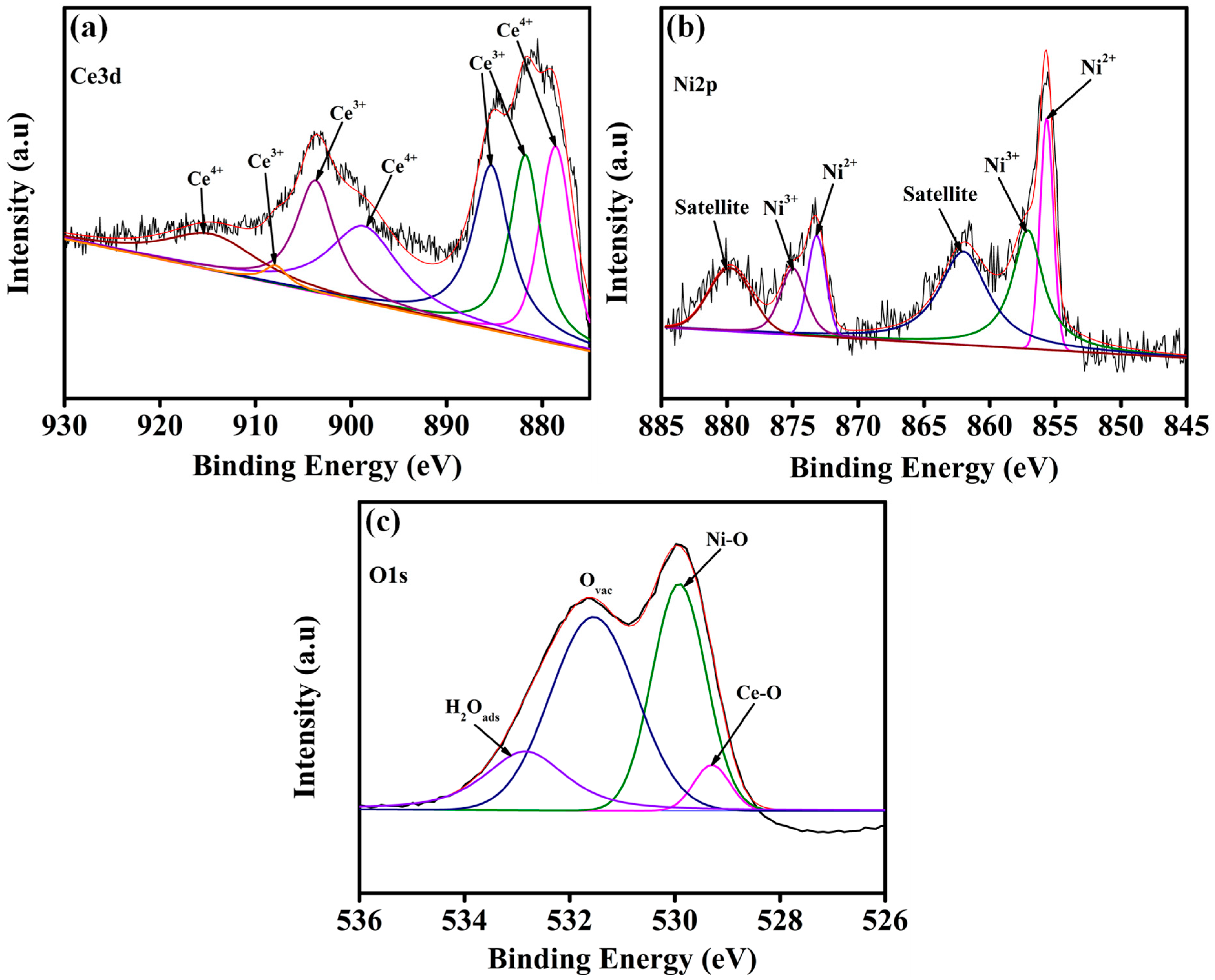
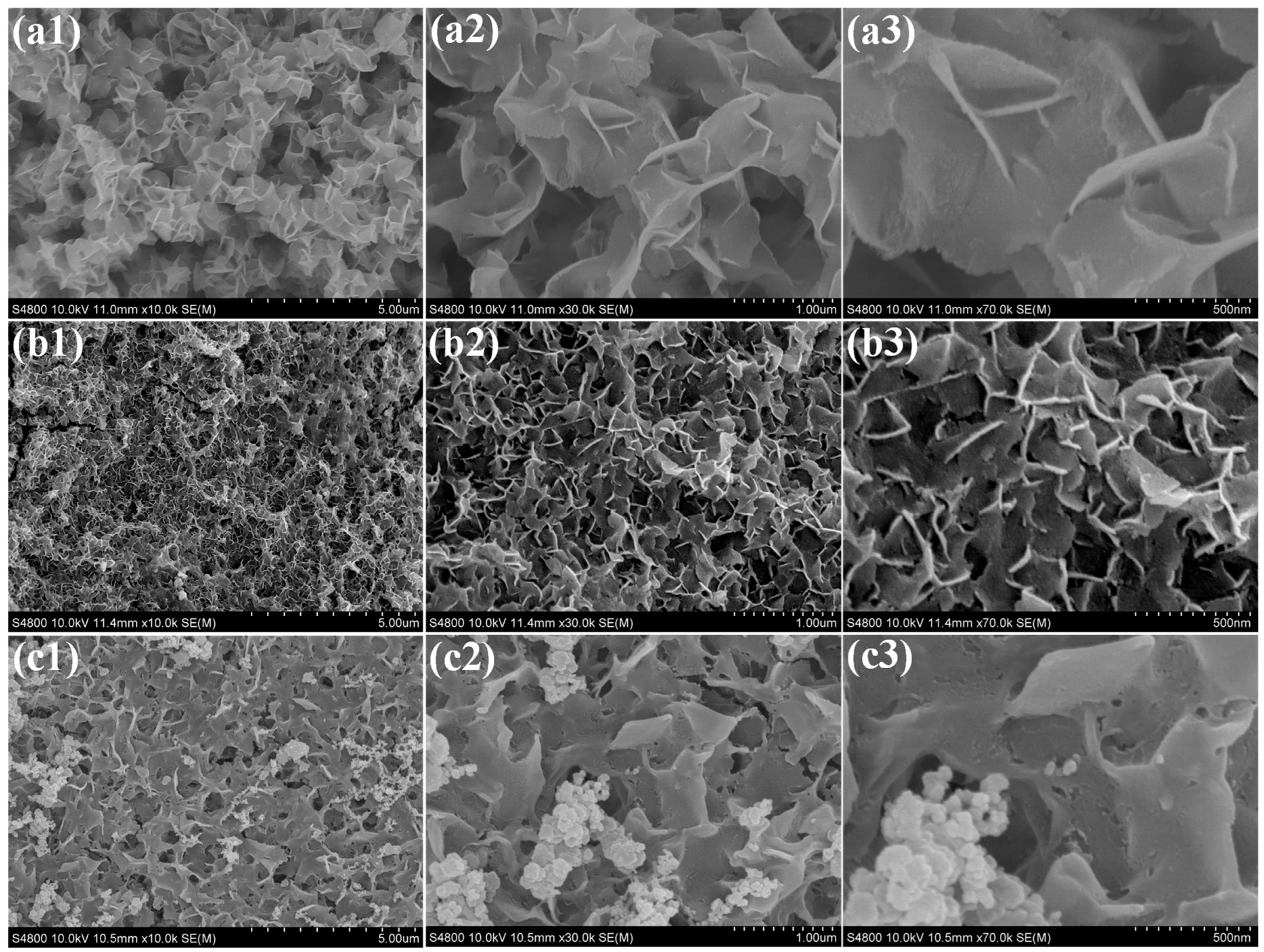
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Z.; Wu, Z.-S. Scalable production of high-performance electrocatalysts for electrochemical water splitting at large current densities. eScience 2025, 5, 100334. [Google Scholar] [CrossRef]
- Pan, D.; Yu, B.; Tressel, J.; Yu, S.; Saravanan, P.; Sangoram, N.; Ornelas-Perez, A.; Bridges, F.; Chen, S. Rapid Synthesis of Carbon-Supported Ru-RuO2 Heterostructures for Efficient Electrochemical Water Splitting. Adv. Sci. 2025, 12, 2414534. [Google Scholar]
- Lyu, L.M.; Chang, Y.C.; Li, H.J.; Wang, P.E.; Juang, R.H.; Lu, M.Y.; Li, C.S.; Kuo, C.H. Turning the Surface Electronic Effect Over Core-Shell CoS2─FexCo1-xS2 Nanooctahedra Toward Electrochemical Water Splitting in the Alkaline Medium. Adv. Sci. 2025, 12, 2411622. [Google Scholar] [CrossRef] [PubMed]
- Ranjith, B.; Gnanasekaran, L.; Karthika, P.; Rajabathar, J.R.; Al-Lohedan, H.A.; Kim, W.K.; Reddy, V.R.M.; Kapoor, M.; Singh, S.; Lavanyaj, M. Enhancing the activity of transition metal-based sulfides via synergistic effects for electrochemical overall water splitting. Int. J. Hydrogen Energy 2025, 112, 255–265. [Google Scholar] [CrossRef]
- Ye, S.H.; Shi, Z.X.; Feng, J.X.; Tong, Y.X.; Li, G.R. Activating CoOOH porous nanosheet arrays by partial iron substitution for efficient oxygen evolution reaction. Angew. Chem. Int. Ed. 2018, 57, 2672–2676. [Google Scholar] [CrossRef]
- Zhao, J.-W.; Li, C.-F.; Shi, Z.-X.; Guan, J.-L.; Li, G.-R. Boosting lattice oxygen oxidation of perovskite to efficiently catalyze oxygen evolution reaction by FeOOH decoration. Research 2020, 2020, 6961578. [Google Scholar] [CrossRef]
- Yu, J.; Cao, Q.; Feng, B.; Li, C.; Liu, J.; Clark, J.K.; Delaunay, J.-J. Insights into the efficiency and stability of Cu-based nanowires for electrocatalytic oxygen evolution. Nano Res. 2018, 11, 4323–4332. [Google Scholar] [CrossRef]
- Song, R.; Wang, X.; Ge, J. Recent progress of noble metal-based single-atom electrocatalysts for acidic oxygen evolution reaction. Curr. Opin. Electrochem. 2023, 42, 101379. [Google Scholar] [CrossRef]
- Hou, L.; Peng, X.; Lyu, S.; Li, Z.; Yang, B.; Zhang, Q.; He, Q.; Lei, L.; Hou, Y. Advancements in MXene-based nanohybrids for electrochemical water splitting. Chin. Chem. Lett. 2025, 36, 110392. [Google Scholar] [CrossRef]
- Lin, S.; Mandavkar, R.; Habib, M.A.; Dristy, S.A.; Joni, M.H.; Jeong, J.-H.; Lee, J. Fabrication of Ru-doped CuMnBP micro cluster electrocatalyst with high efficiency and stability for electrochemical water splitting application at the industrial-level current density. J. Colloid Interface Sci. 2025, 677, 587–598. [Google Scholar] [CrossRef]
- Vazhayil, A.; Vazhayal, L.; Thomas, J.; Thomas, N. A comprehensive review on the recent developments in transition metal-based electrocatalysts for oxygen evolution reaction. Appl. Surf. Sci. Adv. 2021, 6, 100184. [Google Scholar] [CrossRef]
- Han, L.; Dong, S.; Wang, E. Transition-metal (Co, Ni, and Fe)-based electrocatalysts for the water oxidation reaction. Adv. Mater. 2016, 28, 9266–9291. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Feng, Z.; Zhang, X.; Cui, L.; Liu, J. One-step achievement of Fe-doped and interfacial Ru nanoclusters co-engineered Ni (OH)2 electrocatalyst on Ni foam for promoted oxygen evolution reaction. J. Colloid Interface Sci. 2023, 638, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bao, X.; Chen, D.; Wang, Z.; Dewangan, N.; Li, M.; Xu, Z.; Wang, J.; Kawi, S.; Zhong, Q. A minireview on nickel-based heterogeneous electrocatalysts for water splitting. ChemCatChem 2019, 11, 5913–5928. [Google Scholar] [CrossRef]
- Kitiphatpiboon, N.; Chen, M.; Li, X.; Liu, C.; Li, S.; Wang, J.; Peng, S.; Abudula, A.; Guan, G. Heterointerface engineering of Ni3S2@ NiCo-LDH core-shell structure for efficient oxygen evolution reaction under intermittent conditions. Electrochim. Acta 2022, 435, 141438. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Han, G.H.; Hong, S.; Park, J.; Bang, J.; Kim, S.Y.; Ahn, S.H. Electrodeposition: An efficient method to fabricate self-supported electrodes for electrochemical energy conversion systems. In Exploration; Wiley Online Library: Hoboken, NJ, USA, 2022; p. 20210077. [Google Scholar]
- Abebe, E.M.; Ujihara, M. Simultaneous electrodeposition of ternary metal oxide nanocomposites for high-efficiency supercapacitor applications. ACS Omega 2022, 7, 17161–17174. [Google Scholar] [CrossRef]
- Miao, M.; Duan, H.; Luo, J.; Wang, X. Recent progress and prospect of electrodeposition-type catalysts in carbon dioxide reduction utilizations. Mater. Adv. 2022, 3, 6968–6987. [Google Scholar] [CrossRef]
- Minakshi, M.; Aughterson, R.; Sharma, P.; Sunda, A.P.; Ariga, K.; Shrestha, L.K. Micelle-Assisted Electrodeposition of γ-MnO2 on Lead Anodes: Structural and Electrochemical Insights. ChemNanoMat 2025, 2500270. [Google Scholar] [CrossRef]
- Minakshi, M.; Higley, S.; Baur, C.; Mitchell, D.R.; Jones, R.T.; Fichtner, M. Calcined chicken eggshell electrode for battery and supercapacitor applications. RSC Adv. 2019, 9, 26981–26995. [Google Scholar] [CrossRef]
- Biswal, A.; Panda, P.K.; Acharya, A.N.; Mohapatra, S.; Swain, N.; Tripathy, B.C.; Jiang, Z.-T.; Minakshi Sundaram, M. Role of additives in electrochemical deposition of ternary metal oxide microspheres for supercapacitor applications. Acs Omega 2020, 5, 3405–3417. [Google Scholar] [CrossRef]
- Somacescu, S.; Osiceanu, P.; Moreno, J.M.C.; Navarrete, L.; Serra, J.M. Mesoporous nanocomposite sensors based on Sn1−xCexO2−δ metastable solid solution with high percentage of Ce3+ valence state for selective detection of H2 and CO. Microporous Mesoporous Mater. 2013, 179, 78–88. [Google Scholar] [CrossRef]
- Gao, W.; Wen, D.; Ho, J.; Qu, Y. Incorporation of rare earth elements with transition metal–based materials for electrocatalysis: A review for recent progress. Mater. Today Chem. 2019, 12, 266–281. [Google Scholar] [CrossRef]
- Tian, L.; Liu, H.; Zhang, B.; Liu, Y.; Lv, S.; Pang, L.; Li, J. Ni and CeO2 nanoparticles anchored on cicada-wing-like nitrogen-doped porous carbon as bifunctional catalysts for water splitting. ACS Appl. Nano Mater. 2021, 5, 1252–1262. [Google Scholar] [CrossRef]
- Patel, K.B.; Mariyaselvakumar, M.; Vyas, G.; Chaudhari, J.C.; Patidar, R.; Srinivasan, K.; Srivastava, D.N.; Bhadu, G.R. Nickel oxide doped ceria nanoparticles (NiO@ CeO2) for boosting oxygen evolution reaction and enhancing stability. Appl. Surf. Sci. 2024, 649, 159212. [Google Scholar] [CrossRef]
- Ghosh, D.; Pradhan, D. Effect of cooperative redox property and oxygen vacancies on bifunctional OER and HER activities of solvothermally synthesized CeO2/CuO composites. Langmuir 2023, 39, 3358–3370. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, B.Y.; Zhao, B.; Wang, X. A review on noble-metal-free bifunctional heterogeneous catalysts for overall electrochemical water splitting. J. Mater. Chem. A 2016, 4, 17587–17603. [Google Scholar] [CrossRef]
- Qin, C.; Fan, A.; Ren, D.; Luan, C.; Yang, J.; Liu, Y.; Zhang, X.; Dai, X.; Wang, M. Amorphous NiMS (M: Co, Fe or Mn) holey nanosheets derived from crystal phase transition for enhanced oxygen evolution in water splitting. Electrochim. Acta 2019, 323, 134756. [Google Scholar] [CrossRef]
- Manzoor, S.; Mansha, M.; Ali, S.; Altaf, F.; Shams, A.; Khan, S.A. Facile synthesis of porous multiple hydroxyl and amine polymer@ NiO composite for stable and efficient electrochemical water splitting. J. Power Sources 2025, 657, 238229. [Google Scholar] [CrossRef]
- Bibi, H.; Mansoor, M.A.; Asghar, M.A.; Ahmad, Z.; Numan, A.; Haider, A. Facile hydrothermal synthesis of highly durable binary and ternary cobalt nickel copper oxides for high-performance oxygen evolution reaction. Int. J. Hydrogen Energy 2025, 107, 369–377. [Google Scholar] [CrossRef]
- Lin, C.; Zhao, Y.; Zhang, H.; Xie, S.; Li, Y.-F.; Li, X.; Jiang, Z.; Liu, Z.-P. Accelerated active phase transformation of NiO powered by Pt single atoms for enhanced oxygen evolution reaction. Chem. Sci. 2018, 9, 6803–6812. [Google Scholar] [CrossRef]
- Xin, X.; Zhang, Y.; Wang, R.; Wang, Y.; Guo, P.; Li, X. Hydrovoltaic effect-enhanced photocatalysis by polyacrylic acid/cobaltous oxide–nitrogen doped carbon system for efficient photocatalytic water splitting. Nat. Commun. 2023, 14, 1759. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liang, W.J.; Jian, J.X.; Li, C.B.; Chen, B.; Tung, C.H.; Wu, L.Z. Exceptional poly (acrylic acid)-based artificial [FeFe]-hydrogenases for photocatalytic H2 production in water. Angew. Chem. Int. Ed. 2013, 52, 8134–8138. [Google Scholar] [CrossRef] [PubMed]
- Biswal, A.; Sethy, P.K.; Swain, S.K. Change in orientation of polyacrylic acid and chitosan networks by imprintment of gold nanoparticles. Polym. Plast. Technol. Mater. 2021, 60, 182–194. [Google Scholar] [CrossRef]
- Amano, Y.; Nakagawa, Y.; Ohta, S.; Ito, T. Ion-responsive fluorescence resonance energy transfer between grafted polyacrylic acid arms of star block copolymers. Polymer 2018, 137, 169–172. [Google Scholar] [CrossRef]
- Hackett, A.J.; Malmström, J.; Travas-Sejdic, J. Grafting poly (acrylic acid) from PEDOT to control the deposition and growth of platinum nanoparticles for enhanced electrocatalytic hydrogen evolution. ACS Appl. Energy Mater. 2019, 2, 1436–1444. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, J.; Henzie, J.; Jiang, B.; Xia, W.; Chen, T.; Bando, Y.; Kang, Y.-M.; Hossain, M.S.A.; Sugahara, Y. Mesoporous iron-doped MoS2/CoMo2S4 heterostructures through organic–metal cooperative interactions on spherical micelles for electrochemical water splitting. ACS Nano 2020, 14, 4141–4152. [Google Scholar] [CrossRef]
- Panneerselvam, P.; Singh, C.; Jayaraj, S.K.; Doulassiramane, T.; Padmanaban, R.; Samal, A.K.; Mohan, S.; Jadhav, A.H. Unveiling the impact of oxygen vacancies in engineered bimetallic oxides for enhanced oxygen evolution reaction: Insights from experimental and theoretical approaches. J. Mater. Chem. A 2024, 12, 19149–19167. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, R.; Wu, J.; Xing, M.; Qiao, Z.; Zeng, X.; Wang, S.; Cao, D. Ni3N–CeO2 heterostructure bifunctional catalysts for electrochemical water splitting. Adv. Funct. Mater. 2023, 33, 2306786. [Google Scholar] [CrossRef]
- Bhosale, M.; Baby, N.; Magdum, S.S.; Murugan, N.; Kim, Y.A.; Thangarasu, S.; Oh, T.-H. Hierarchical nanoassembly of Ni3S2-MoS2 interconnected with CeO2 as a highly remarkable hybrid electrocatalyst for enhancing water oxidation and energy storage. J. Energy Storage 2024, 80, 110301. [Google Scholar] [CrossRef]
- Tang, C.; Zhong, L.; Xiong, R.; Xiao, Y.; Cheng, B.; Lei, S. Regulable in-situ autoredox for anchoring synergistic Ni/NiO nanoparticles on reduced graphene oxide with boosted alkaline electrocatalytic oxygen evolution. J. Colloid Interface Sci. 2023, 648, 181–192. [Google Scholar] [CrossRef]
- Bhosale, M.; Thangarasu, S.; Magdum, S.S.; Jeong, C.; Oh, T.-H. Enhancing the electrocatalytic performance of vanadium oxide by interface interaction with rGO and NiO nanostructures for electrochemical water oxidation. Int. J. Hydrogen Energy 2024, 54, 1449–1460. [Google Scholar] [CrossRef]
- Zhang, R.; Qiao, Q.; Yu, B.; Zhang, Z.; Wang, J.; Li, S.; Liu, Y.; Yuan, H.; Luo, J.; Wang, Y. Formation of CeO2/NiO composites with intergrowth superstructures for photocatalytic CO2 reduction. Nano Res. 2025, 18, 94907657. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Zhou, J.; Xu, H.; Wang, Z.; Liu, Y.; Liao, X.; Nie, M. CeO2 facilitates electron transfer at the Fe-Ni2 P heterointerface, enhancing the overall process of water splitting. J. Mater. Chem. A 2025, 13, 31329–31340. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, X.; Huang, B.; Xie, Z. CeO2-decorated Fe-doped Ni2P nanosheets for efficient electrocatalytic overall water splitting at high current densities. J. Alloys Compd. 2024, 981, 173672. [Google Scholar] [CrossRef]
- Baby, N.; Thangarasu, S.; Murugan, N.; Kim, Y.A.; Oh, T.-H. MOF derived Fe3O4/NiO decorated rGO-BN for efficient electrochemical water splitting. Int. J. Hydrogen Energy 2025, 130, 127–138. [Google Scholar] [CrossRef]
- Sha, W.; Song, Y.; Liu, P.; Wang, J.; Xu, B.; Feng, X.; Guo, J. Constructing multiple heterostructures on nickel oxide using rare-earth oxide and nickel as efficient bifunctional electrocatalysts for overall water splitting. ChemCatChem 2022, 14, e202101975. [Google Scholar] [CrossRef]
- Ghosh, D.; Manikanta Kumar, M.; Raj, C.R.; Pradhan, D. Bifunctional catalytic activity of solvothermally synthesized CeO2 nanosphere/NiO nanoflake nanocomposites. ACS Appl. Energy Mater. 2022, 5, 5666–5679. [Google Scholar] [CrossRef]
- Li, C.; Baek, J.-B. The promise of hydrogen production from alkaline anion exchange membrane electrolyzers. Nano Energy 2021, 87, 106162. [Google Scholar] [CrossRef]
- Yao, S.; Wei, H.; Zhang, Y.; Zhang, X.; Wang, Y.; Liu, J.; Tan, H.H.; Xie, T.; Wu, Y. Controlled growth of porous oxygen-deficient NiCo2 O4 nanobelts as high-efficiency electrocatalysts for oxygen evolution reaction. Catal. Sci. Technol. 2021, 11, 264–271. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Alfonso, D.; Tafen, D.N.; Lekse, J.; Wang, C.; Deng, X.; Lee, J.; Jang, H.; Lee, J.-S.; Kumar, S. Electrocatalytic oxygen evolution with an atomically precise nickel catalyst. ACS Catal. 2016, 6, 1225–1234. [Google Scholar] [CrossRef]
- Pan, U.N.; Singh, T.I.; Paudel, D.R.; Gudal, C.C.; Kim, N.H.; Lee, J.H. Covalent doping of Ni and P on 1T-enriched MoS2 bifunctional 2D-nanostructures with active basal planes and expanded interlayers boosts electrocatalytic water splitting. J. Mater. Chem. A 2020, 8, 19654–19664. [Google Scholar] [CrossRef]
- Chen, K.; Cao, Y.-H.; Yadav, S.; Kim, G.-C.; Han, Z.; Wang, W.; Zhang, W.-J.; Dao, V.; Lee, I.-H. Electronic structure reconfiguration of nickel–cobalt layered double hydroxide nanoflakes via engineered heteroatom and oxygen-vacancies defect for efficient electrochemical water splitting. Chem. Eng. J. 2023, 463, 142396. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhosale, M.; Morankar, P.J.; Lee, Y.; Seo, H.; Jeon, C.-W. Electropolymerized PAA as a Functional Matrix for CeO2-NiO Hybrid Electrocatalysts for Efficient Water Oxidation. Polymers 2025, 17, 2631. https://doi.org/10.3390/polym17192631
Bhosale M, Morankar PJ, Lee Y, Seo H, Jeon C-W. Electropolymerized PAA as a Functional Matrix for CeO2-NiO Hybrid Electrocatalysts for Efficient Water Oxidation. Polymers. 2025; 17(19):2631. https://doi.org/10.3390/polym17192631
Chicago/Turabian StyleBhosale, Mrunal, Pritam J. Morankar, Yeonsu Lee, Hajin Seo, and Chan-Wook Jeon. 2025. "Electropolymerized PAA as a Functional Matrix for CeO2-NiO Hybrid Electrocatalysts for Efficient Water Oxidation" Polymers 17, no. 19: 2631. https://doi.org/10.3390/polym17192631
APA StyleBhosale, M., Morankar, P. J., Lee, Y., Seo, H., & Jeon, C.-W. (2025). Electropolymerized PAA as a Functional Matrix for CeO2-NiO Hybrid Electrocatalysts for Efficient Water Oxidation. Polymers, 17(19), 2631. https://doi.org/10.3390/polym17192631





