Improved Bioactivity of Titanium-Based Surfaces Fabricated by Laser Melting Deposition by Functionalization with 3D Polymeric Microstructures Produced by Laser Direct Writing via Two-Photon Polymerization
Abstract
1. Introduction
2. Materials and Methods
2.1. Powders for LMD Fabrication of Ti-Bases Substrates
2.2. IP-Dip Photoresist
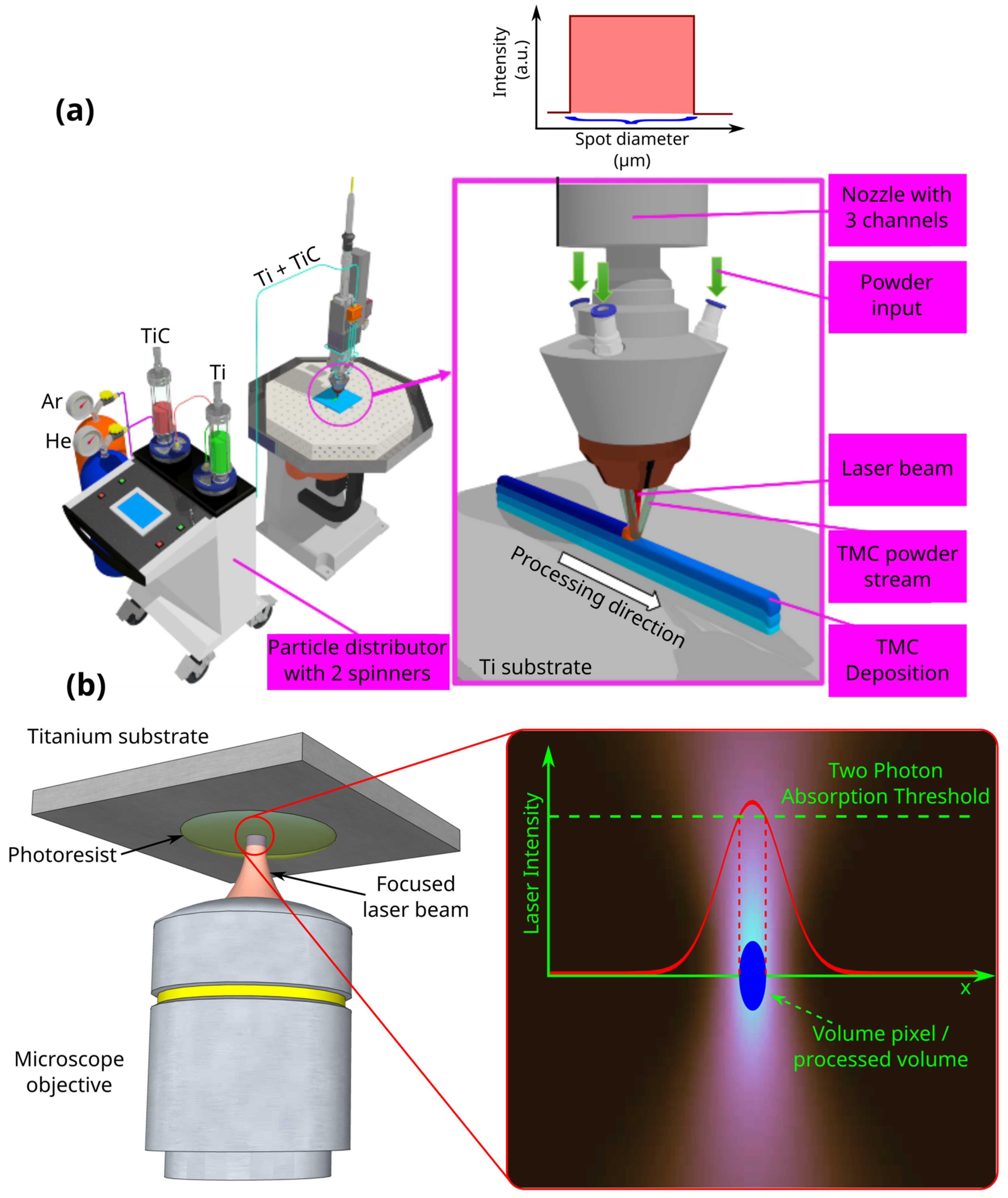
2.3. LMD Fabrication of Ti-Based Substrates
2.4. Laser Direct Writing via Two-Photon Polymerization (LDW via TPP)
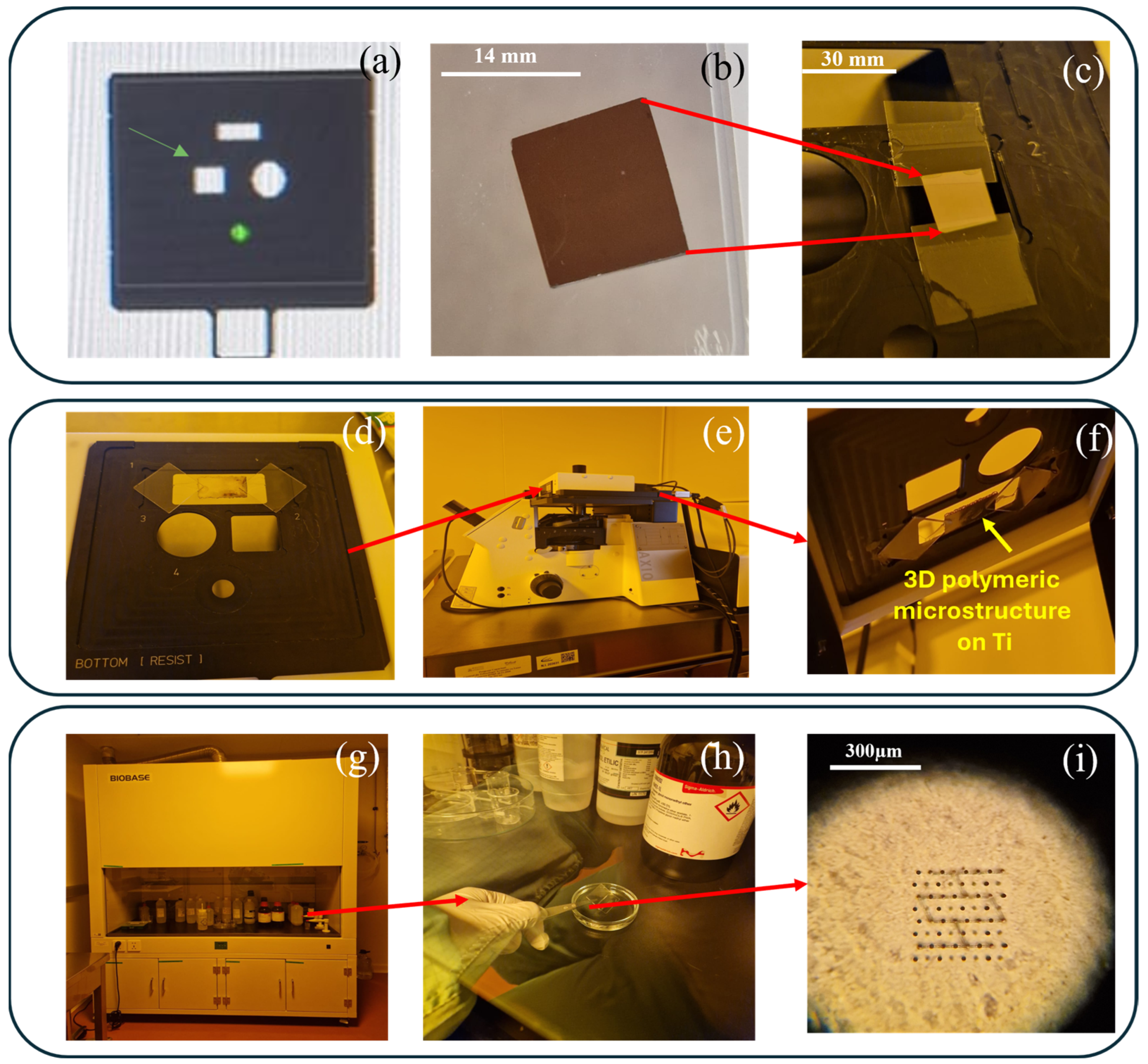
2.5. Experimental Setup for Functionalization of Ti-Base Substrates with 3D Polymeric Microstructures
2.6. Morpho-Physical Characterization of Ti-Based Substrates
2.7. Morpho-Structural Analysis of the 3D Microstructures Imprinted on the Ti-Baseed Surfaces
2.8. In Vitro Evaluation of the 3D Microstructure Printed on Ti Surfaces
2.8.1. Cell Seeding
2.8.2. Preparation of Samples for Morpho-Structural Analysis
2.9. Simulation of the Traction Forces Exercised by Cells Cultured onto the 3D Microstructures
2.9.1. Finite Element Analysis of the Cells Cultured onto the 3D Microstructures
2.9.2. FEBio Studio
2.9.3. Preparing the Geometry and Choosing the Materials for FEB Studio
3. Results and Discussions
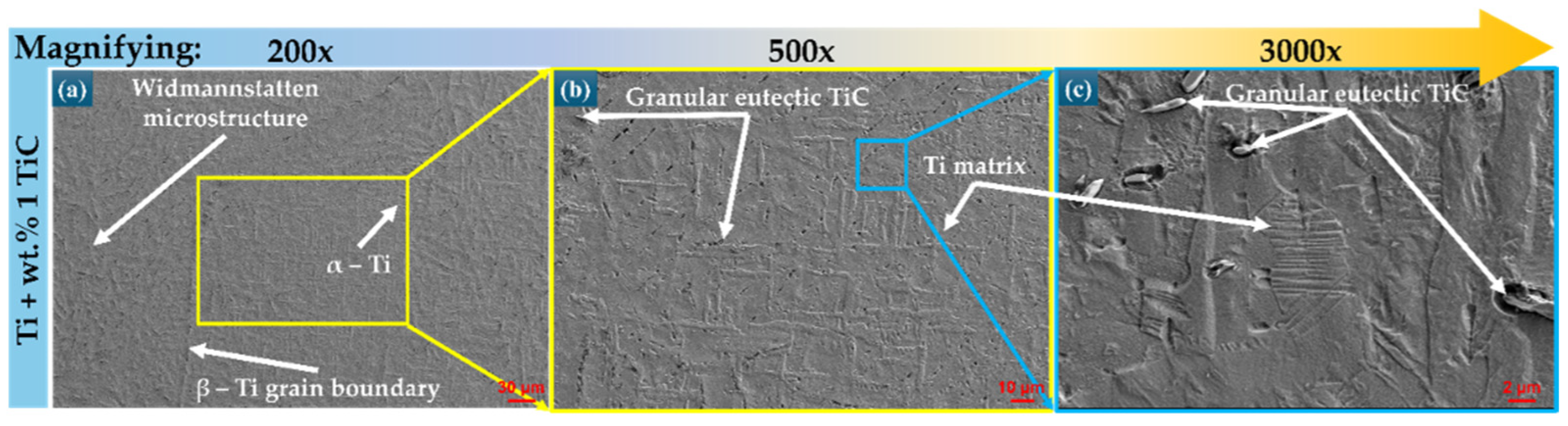
3.1. Morphological Investigation of Ti-Based Substrates
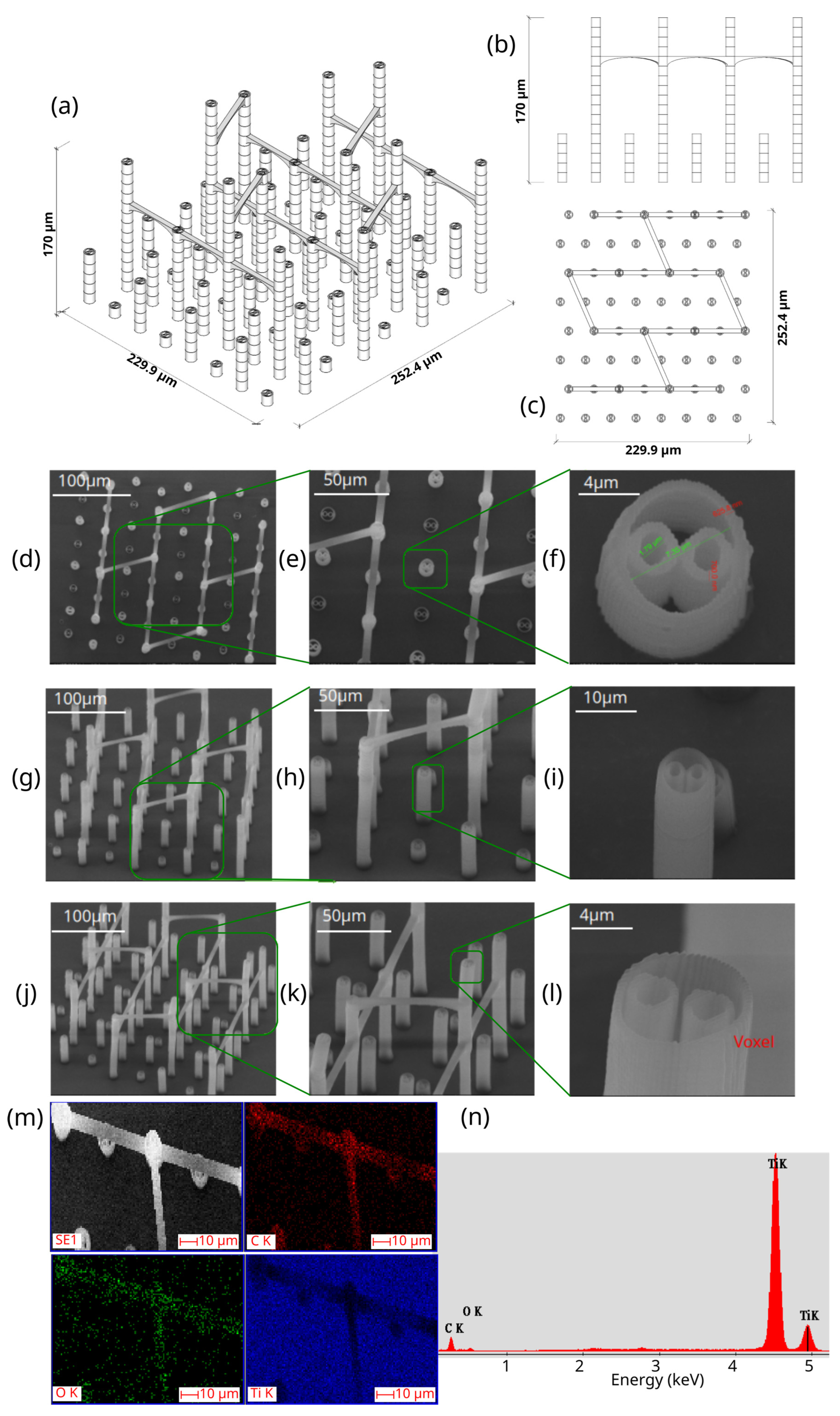
3.2. Morpho-Structural Characterization of the 3D Microstructure Printed on Ti Surfaces
- -
- Total volume ~9,853,200 µm3
- -
- Structure volume ~202,413 µm3
- -
- Empty volume ~9,650,787 µm3
- -
- Porosity ~97.9%
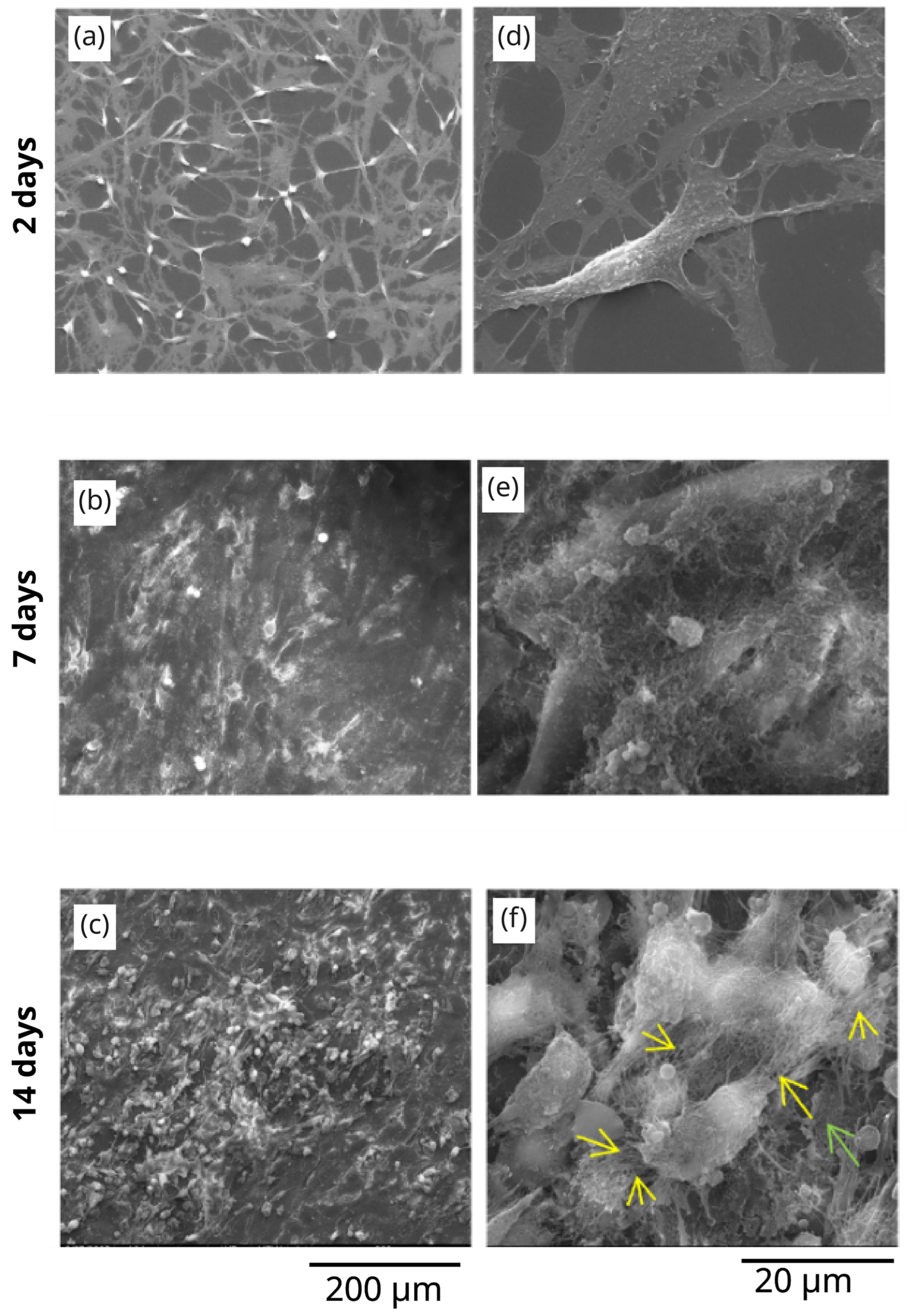
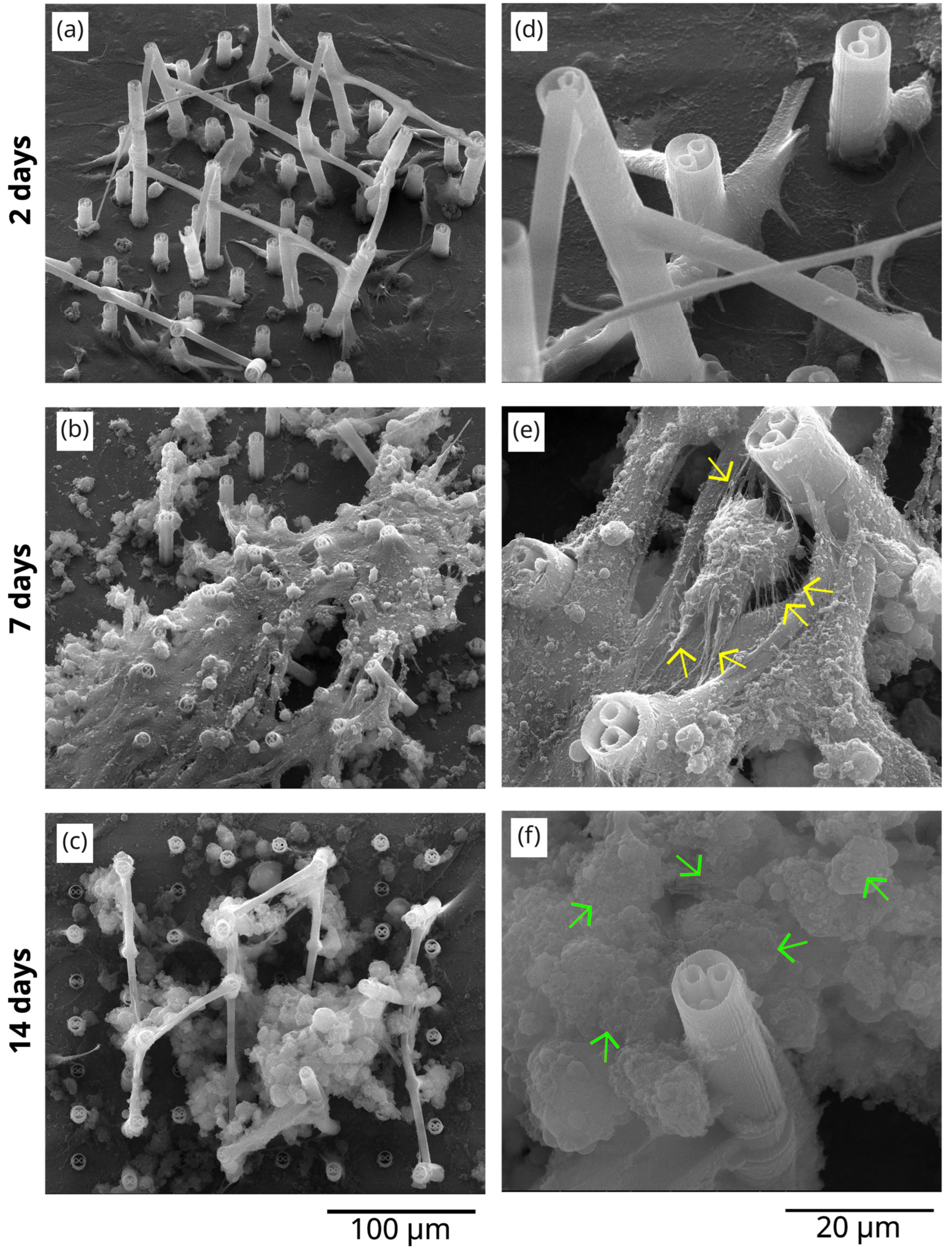
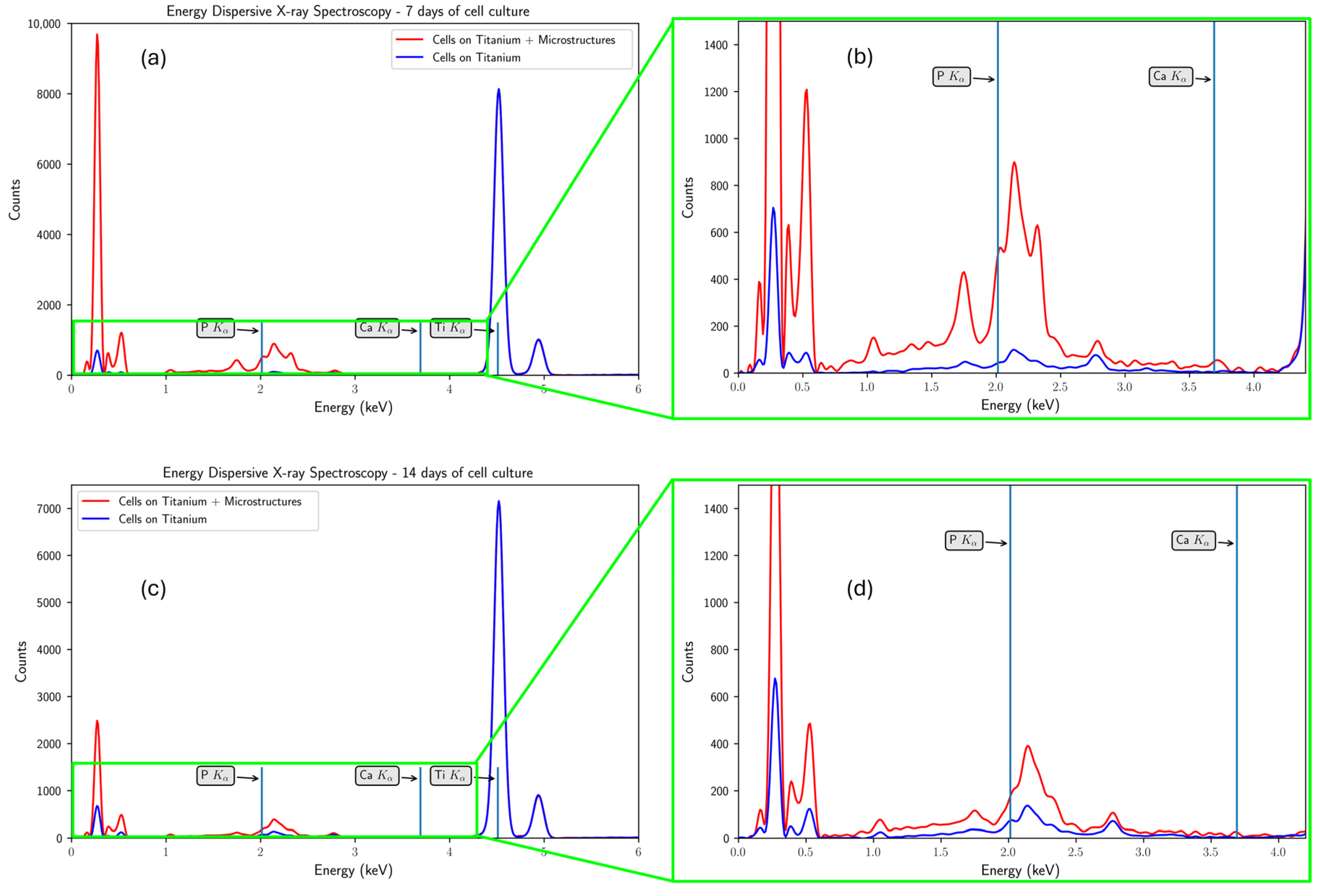
3.3. The In Vitro Biocompatibility Assessment of the 3D Microstructure Printed on Ti Surfaces
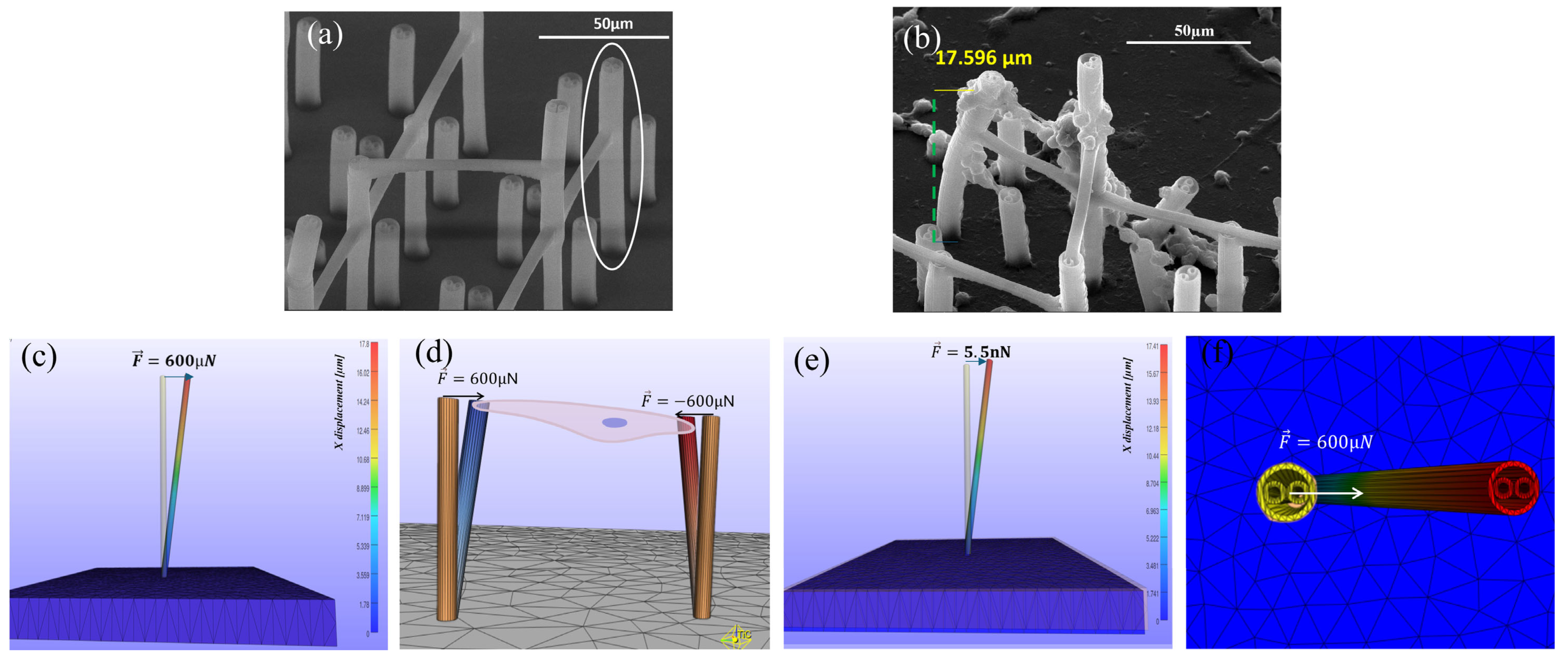
3.4. Traction Forces Exercised by Cells onto 3D Microstructures Using Finite Element Analysis in FEBio Studio
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Ott, S.M. Cortical or Trabecular Bone: What’s the Difference? Am. J. Nephrol. 2018, 47, 373–375. [Google Scholar] [CrossRef]
- Kang, M.J.; Hong, H.S.; Chung, S.J.; Lee, Y.A.; Shin, C.H.; Yang, S.W. Body composition and bone density reference data for Korean children, adolescents, and young adults according to age and sex: Results of the 2009–2010 Korean National Health and Nutrition Examination Survey (KNHANES). J. Bone Miner. Metab. 2016, 34, 429–439. [Google Scholar] [CrossRef]
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral. Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef]
- Tan, A.E.; Brogan, W.F.; McComb, H.K.; Henry, P.J. Secondary Alveolar Bone Grafting—Five-Year Periodontal and Radio-graphic Evaluation in 100 Consecutive Cases. Cleft Palate Craniofac. J. 1996, 33, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Chan, H.L.; Galindo-Moreno, P.; Enlayef, B.; Suarez-Lopesc del Amo, F.; Wang, F.; Wang, H.L. Alveolar Bone Architecture: A Systematic Review and Meta-Analysis. J. Periodontol. 2015, 86, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Goiato, M.C.; Santiago Junior, J.F.; Pellizzer, E.P.; Moreno, A.; Villa, L.M.R.; Fiuza de Carvalho Dekon, S.; Perri de Carvalho, P.S.; dos Santos, D.M. Systemic Trans- and Postoperative Evaluations of Patients Undergoing Dental Implant Surgery. Clinics 2016, 71, 156–162. [Google Scholar] [CrossRef]
- Ballard, D.H.; Mills, P.; Duszak, R.; Weisman, J.A.; Rybicki, F.J.; Woodard, K.P. Medical 3D Printing Cost-Savings in Orthopedic and Maxillofacial Surgery: Cost Analysis of Operating Room Time Saved with 3D Printed Anatomic Models and Surgical Guides. Acad. Radiol. 2020, 27, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Signor, F.; Noal Alves, S.; Lima, D.D.; Bevilaqua, W.L.; Mareze, P.H.; Daudt, N.F. Molten Salt Shielding Sintering of Porous Ti and Ti-Nb Alloys Fabricated from Metal Injection Molding Feedstocks. J. Mater. Eng. Perform. 2025, 1059–9495. [Google Scholar] [CrossRef]
- Chang, C.; Yao, G.; Cox, S.C.; Zhang, X.; Sheng, L.; Liu, M.; Cheng, W.; Lu, Y.; Yan, X. From macro-, through meso- to micro-scale: Densification behavior, deformation response and microstructural evolution of selective laser melted Mg-RE Alloy. J. Magnes. Alloys 2025, 13, 3947–3963. [Google Scholar] [CrossRef]
- Heinl, P.; Muller, L.; Korner, C.; Singer, R.F.; Muller, F.A. Cellular Ti-6Al-4V structures with interconnected macro porosity for bone implants fabricated by selective electron beam melting. Acta Biomater. 2008, 4, 1536–1544. [Google Scholar] [CrossRef]
- Duta, L.; Neamtu, J.; Melinte, R.P.; Zureigat, O.Z.; Popescu-Pelin, G.; Chioibasu, D.; Oktar, F.N.; Popescu, A.C. In Vivo Assessment of Bone Enhancement in the Case of 3D-Printed Implants Functionalized with Lithium-Doped Biological-Derived Hydroxyapatite Coatings: A Preliminary Study on Rabbits. Coatings 2020, 10, 992. [Google Scholar] [CrossRef]
- Chioibasu, D.; Achim, A.; Popescu, C.; Stan, G.E.; Pasuk, I.; Enculescu, M.; Iosub, S.; Duta, L.; Popescu, A. Prototype Orthopedic Bone Plates 3D Printed by Laser Melting Deposition. Materials 2019, 12, 906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.C.; Lu, H.B.; Mickel, C.; Eckert, J. Ductile ultrafine-grained Ti-based alloys with high yield strength. Appl. Phys. Lett. 2007, 91, 051906. [Google Scholar] [CrossRef]
- Carman, A.; Zhang, L.C.; Ivasishin, O.M.; Savvakin, D.G.; Matviychuk, M.V.; Pereloma, E.V. Role of alloying elements in microstructure evolution and alloying elements behaviour during sintering of a near-β titanium alloy. Mat. Sci. Eng. A 2011, 528, 1686–1693. [Google Scholar] [CrossRef]
- Kennedy, S.M.; Vasanthanathan, A.; Amudhan, K. Exploring the frontiers of metal additive manufacturing in orthopaedic implant development. MethodsX 2024, 13, 103056. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Zhao, J.; Hussain, M.; Wang, M. Additive Manufacturing in Orthopedics: A Review. ACS Biomater. Sci. Eng. 2022, 8, 1367–1380. [Google Scholar] [CrossRef]
- Chowdhury, S.; Oliullah, S.; Hossen, Z.; Manik, M.H.; Hurayra, A.; Al Mahmud, Z.; Hossain, N.; Mobarak, H.; Islam, D. Additive manufacturing in bone science: A cutting-edge review of its potential and progress. Med. Nov. Technol. Devices 2025, 27, 100379. [Google Scholar] [CrossRef]
- Cong, B.; Zhang, H. Innovative 3D printing technologies and advanced materials revolutionizing orthopedic surgery: Current applications and future directions. Front. Bioeng. Biotechnol. 2025, 13, 1542179. [Google Scholar] [CrossRef] [PubMed]
- Mobarak, H.; Islam, A.; Hossain, N.; Mahbum, Z.; Rayhan, T.; Nishi, N.J.; Chowdhury, M.A. Recent advances of additive manufacturing in implant fabrication—A review. Appl. Surf. Sci. Adv. 2023, 18, 100462. [Google Scholar] [CrossRef]
- Meng, M.; Wang, J.; Huang, H.; Liu, X.; Zhang, J.; Li, Z. 3D printing metal implants in orthopedic surgery: Methods, applications and future prospects. J. Ortho Transl. 2023, 42, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Alontseva, D.; Azamatov, B.; Safarova, Y.; Voinarovych, S.; Nazenova, G. A Brief Review of Current Trends in the Additive Manufacturing of Orthopedic Implants with Thermal Plasma-Sprayed Coatings to Imprive the Implant Surface Biocompatibility. Coatings 2023, 13, 1175. [Google Scholar] [CrossRef]
- Veronesi, F.; Brogini, S.; De Luca, A.; Bellini, D.; Casagranda, V.; Fini, M.; Giavaresi, G. Cell Adhesion and Initial Bone Matrix Deposition on Titanium-Based Implants with Chitosan–Collagen Coatings: An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 4810. [Google Scholar] [CrossRef]
- Abakay, E.; Armagan, M.; Avcu, Y.Y.; Guney, M.; Yousif, B.F.; Avcu, E. Advances in improving tribological performance of titanium alloys and titanium matrix composites for biomedical applications: A critical review. Front. Mater. 2024, 11, 1452288. [Google Scholar] [CrossRef]
- Mahmood, M.A.; Chioibasu, D.; Sajjad, U.; Mihai, S.; Tiseanu, I.; Popescu, A.C. Printed layers height calibration curve and porosity in laser melting deposition of Ti6Al4V combining experiments, mathematical modelling and deep neural network. Rapid Prototyp. J. 2024, 30, 415–429. [Google Scholar] [CrossRef]
- Chioibasu, D.; Mihai, S.; Mahmood, M.A.; Lungu, M.; Porosnicu, I.; Sima, A.; Dobrea, C.; Tiseanu, I.; Popescu, A.C. Use of X-ray Computed Tomography for Assessing Defects in Ti Grade 5 Parts Produced by Laser Melting Deposition. Metals 2020, 10, 1408. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, M.; Liu, L.; Xu, R.; Huang, Z.; Shi, Z.; Liu, J.; Li, Z.; Li, X.; Hao, P.; et al. Femtosecond laser treat-ment promotes the surface bioactivity and bone ingrowth of Ti6Al4V bone scaffolds. Front. Bioeng. Biotechnol. 2022, 10, 962483. [Google Scholar] [CrossRef]
- Kampleitner, C.; Changi, K.; Felfel, R.M.; Scotchford, C.A.; Sottile, V.; Kluger, R.; Hoffmann, O.; Grant, D.M.; Epstein, M.M. Preclinical biological and physicochemical evaluation of two-photon engineered 3D biomimetic copolymer scaffolds for bone healing. Biomater. Sci. 2020, 8, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, Z.Q.; Lin, B.; Liu, M.C.; Zhang, Y.; Liu, S.J.; Li, Y.; Zhao, Q. Two-photon polymerization-based 3D micro-scaffolds toward biomedical devices. Chem. Eng. J. 2024, 493, 152469. [Google Scholar] [CrossRef]
- Otuka, A.J.G.; Tomazio, N.B.; Paula, K.T.; Mendonca, C.R. Two-Photon Polymerization: Functionalized Micro-structures, Micro-Resonators, and Bio-Scaffolds. Polymers 2021, 13, 1994. [Google Scholar] [CrossRef] [PubMed]
- Paun, I.A.; Mustaciosu, C.C.; Mihailescu, M.; Calin, B.S.; Sandu, A.M. Magnetically-driven 2D cells organization on superparamagnetic micromagnets fabricated by laser direct writing. Sci. Rep. 2020, 10, 16418. [Google Scholar] [CrossRef]
- Peltola, S.M.; Melchels, F.P.; Grijpma, D.W.; Kellomaki, M. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 2008, 40, 268–280. [Google Scholar] [CrossRef]
- Purtov, J.; Verch, A.; Rogin, P.; Hensel, R. Improved development procedure to enhance the stability of micro-structures created by two-photon polymerization. Microelectron. Eng. 2018, 194, 45–50. [Google Scholar] [CrossRef]
- O’Halloran, S.; Pandit, A.; Heise, A.; Kellett, A. Two-Photon Polymerization: Fundamentals, Materials, and Chemical Modification Strategies. Adv. Sci. 2022, 10, 2204072. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Park, S.; McLamb, M.; Lata, M.; Schoche, S.; Childers, D.; Aggarwal, I.D.; Poutous, M.K.; Boreman, G.; Hofmann, T. UV to NIR optical properties of IP-Dip, IP-L, and IP-S after two-photon polymerization determined by spectroscopic ellipsometry. Opt. Mat. Express 2019, 9, 4318–4328. [Google Scholar] [CrossRef]
- Durisova, J.; Pudis, D.; Goraus, M.; Gaso, P. IP-Dip photoresist surfaces for photonic applications prepared by laser lithography and studied by AFM. Appl. Surf. Sci. 2018, 461, 108–112. [Google Scholar] [CrossRef]
- Diamantopoulou, M.; Karathanasopoulos, N.; Mohr, D. Stress-strain response of polymers made through two-photon lithography: Micro-scale experiments and neural network modeling. Addit. Manuf. 2021, 47, 102266. [Google Scholar] [CrossRef]
- Paun, I.A.; Mustaciosu, C.C.; Popescu, R.C.; Calin, B.S.; Mihailescu, M. Collagen/Chitosan Functionalization of Complex 3D Structures Fabricated by Laser Direct Writing via Two-Photon Polymerization for Enhanced Osteogenesis. Int. J. Mol. Sci. 2020, 21, 6426. [Google Scholar] [CrossRef]
- Fischer, E.R.; Hansen, B.T.; Nair, V.; Hoyt, F.H.; Schwartz, C.L.; Dorward, D.W. Scanning Electron Microscopy. Curr. Protoc. 2024, 4, e1034. [Google Scholar] [CrossRef]
- Norman, J.; Mukundan, V.; Bernstein, D.; Pruitt, B.L. Microsystems for Biomechanical Measurements. Pediatr. Res. 2008, 63, 576–583. [Google Scholar] [CrossRef]
- Maas, S.A.; Ellis, B.J.; Ateshian, G.A.; Weiss, J.A. FEBio: Finite Elements for Biomechanics. J. Biomech. Eng. 2012, 134, 011005. [Google Scholar] [CrossRef]
- Muhammad, A.; Ali, M.A.H.; Shanono, I.H. ANSYS—A bibliometric study. Mat. Today Proc. 2020, 26, 1005–1009. [Google Scholar] [CrossRef]
- Muller, J.H. Computational modelling of knee implants. In Computational Modelling of Biomechanics and Biotribology in the Musculoskeletal System; Jin, Z., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2014; pp. 417–446. [Google Scholar] [CrossRef]
- Xie, C.; Ye, J.; Liang, R.; Yao, X.; Wu, X.; Koh, Y.; Wei, W.; Zhang, X.; Ouyang, H. Advanced Strategies of Biomimetic Tissue-Engineered Grafts for Bone Regeneration. Adv. Healthc. Mater. 2021, 10, e2100408. [Google Scholar] [CrossRef]
- Paun, I.A.; Calin, B.S.; Mustaciosu, C.C.; Mihailescu, M.; Moldovan, A.; Crisan, O.; Leca, A.; Luculescu, C.R. 3D Superparamagnetic Scaffolds for Bone Mineralization under Static Magnetic Field Stimulation. Materials 2019, 12, 2834. [Google Scholar] [CrossRef]
- Yadav, S.; Nath, A.; Suresh, R.; Vasudevan, A.K.; Balakrishnan, B.; Peter, M.R. Advances in Implant Surface Technology: A Biological Perspective. Cureus 2025, 17, e78264. [Google Scholar] [CrossRef]
- Maglio, M.; Fini, M.; Sartori, M.; Codispoti, G.; Borsari, V.; Dallari, D.; Ambretti, S.; Rocchi, M.; Tschon, M. An Advanced Human Bone Tissue Culture Model for the Assessment of Implant Osteointegration In Vitro. Int. J. Mol. Sci. 2024, 25, 5322. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral. Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Chen, K.; Yao, H.; Chen, C.; Lu, X.; Ding, P.; Wang, M.; Hua, X.; Shan, A. Chemical Reaction and Bonding Mechanism at the Polymer-Metal Interface. Surf. Interf. Appl. 2022, 14, 27383–27396. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.S. Chemistry and adhesion of metal-polymer interfaces. Appl. Surf. Sci. 1990, 41–42, 559–566. [Google Scholar] [CrossRef]
- Zou, X.; Huang, L.; Chen, L.; Jiang, M.; Zhang, S.; Wang, M.; Hua, X.; Shan, A. Surface Structuring via Additive Manufacturing to Improve the Performance of Metals and Polymer Joints. Metals 2021, 11, 567. [Google Scholar] [CrossRef]
- Katayama, S.; Kawahito, Y. Laser direct joining of metal and plastic. Scr. Mater. 2008, 59, 1247–1250. [Google Scholar] [CrossRef]
- Renault, O.; Deleuze, P.M.; Courtin, J.; Bure, T.R.; Gauthier, N.; Nolot, E.; Robert-Goumet, C.; Pauly, N.; Martinez, E.; Artyushkova, K. New directions in the analysis of buried interfaces for device technology by hard X-ray photoemission. Faraday Discuss. 2022, 236, 288–310. [Google Scholar] [CrossRef] [PubMed]
- Hinder, S.J.; Lowe, C.; Maxted, J.T.; Watts, J.F. A ToF-SIMS investigation of a buried polymer/polymer interface exposed by ultra-low-angle microtomy. Surf. Interf. Anal. 2004, 36, 1575–1581. [Google Scholar] [CrossRef]
- Zhao, W.; Michalik, D.; Ferguson, S.; Hofstetter, W.; Lemaitre, J.; von Rechenberg, B.; Bowen, P. Rapid evaluation of bioactive Ti-based surfaces using an in vitro titration method. Nat. Commun. 2019, 10, 2062. [Google Scholar] [CrossRef]
- Addison, W.N.; Nelea, V.; Chicatun, F.; Chien, Y.C.; Tran-Khanh, N.; Buschmann, M.D.; Nazhat, S.N.; Kaartinen, M.T.; Vali, H.; Tecklenburg, M.M.; et al. Extracellular matrix mineralization in murine MC3T3-E1 osteoblast cultures: An ultrastructural, compositional and comparative analysis with mouse bone. Bone 2015, 71, 244–256. [Google Scholar] [CrossRef]
- Califano, J.P.; Reinhart-King, C.A. Substrate Stiffness and Cell Area Predict Cellular Traction Stresses in Single Cells and Cells in Contact. Cell Mol. Bioeng. 2010, 3, 68–75. [Google Scholar] [CrossRef]
- Sun, M.; Chi, G.; Xu, J.; Tan, Y.; Xu, J.; Lv, S.; Xu, Z.; Xia, Y.; Li, L.; Li, Y. Extracellular matrix stiffness controls osteogenic differentiation of mesenchymal stem cells mediated by integrin α5. Stem Cell Res. Ther. 2018, 9, 52. [Google Scholar] [CrossRef]
- Sugawara, Y.; Ando, R.; Kamioka, H.; Ishihara, Y.; Murshid, S.A.; Hashimoto, K.; Kataoka, N.; Tsujioka, K.; Kajiya, F.; Yamashiro, T.; et al. The alteration of a mechanical property of bone cells during the process of changing from osteoblasts to osteocytes. Bone 2008, 43, 19–24. [Google Scholar] [CrossRef]
- Cheng, C.W.; Solorio, L.D.; Alsberg, E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol. Adv. 2014, 32, 462–484. [Google Scholar] [CrossRef]
- Campagna, R.; Schiavoni, V.; Marchetti, E.; Salvolini, E.; Frontini, A.; Sampalmieri, F.; Bambini, F.; Meme, L. In Vitro Studo of the Proliferation of MG63 Cells Cultured on Five Different Titanium Surfaces. Materials 2024, 17, 2208. [Google Scholar] [CrossRef] [PubMed]
- Cytion: Cells for Innovation. Available online: https://www.cytion.com/MG-63-Cells/300441 (accessed on 14 September 2025).
- Pautke, C.; Schieker, M.; Tischer, T.; Kolk, A.; Neth, P.; Mutschler, W.; Milz, S. Characterization of Osteosarcoma Cell Lines MG-63, Saos02 and U-2 OS in Comparison to Human Osteoblasts. Anticancer Res. 2004, 24, 3743–3748. [Google Scholar]
- Liu, Y.; Luo, D.; Wang, T. Hierarchical Structures of Bone and Bioinspired Bone Tissue Engineering. Small 2016, 12, 4611–4632. [Google Scholar] [CrossRef]
- Bottini, M.; Mebarek, S.; Anderson, K.L.; Strzelecka-Kiliszek, A.; Bozycki, L.; Simão, A.M.S.; Bolean, M.; Ciancaglini, P.; Pikula, J.B.; Pikula, S.; et al. Matrix vesicles from chondrocytes and osteoblasts: Their biogenesis, properties, functions and biomimetic models. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Millán, J.L. The role of phosphatases in the initiation of skeletal mineralization. Calcif. Tissue Int. 2013, 93, 299–306. [Google Scholar] [CrossRef]
- Blair, H.C.; Larrouture, Q.C.; Tourkova, I.L.; Liu, L.; Bian, J.H.; Stolz, D.B.; Nelson, D.J.; Schlesinger, P.H. Support of bone mineral deposition by regulation of pH. Am. J. Physiol. Cell Physiol. 2018, 315, C587–C597. [Google Scholar] [CrossRef] [PubMed]
- Penido, M.G.; Alon, U.S. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ren, A.; Lv, W.; Aazmi, A.; Qin, C.; Liang, X.; Xu, X.; Yu, M.; Li, Q.; Yang, H. Multiphoton Polymerization-Based Micro/Nanomanufacturing Toward Precision Medicine. Engineering 2025, 49, 35–60. [Google Scholar] [CrossRef]
- Jaiswal, A.; Rastogi, C.K.; Rani, S.; Singh, G.P.; Saxena, S.; Shukla, S. Two decades of two-photon lithography: Materials science perspective for additive manufacturing of 2D/3D nano-microstructures. iScience 2023, 26, 106374. [Google Scholar] [CrossRef]
- Son, A.I.; Opfermann, J.D.; McCue, C.; Ziobro, J.; Abrahams, J.H.; Jones, K.; Morton, P.D.; Ishii, S.; Oluigbo, C.; Krieger, A.; et al. An Implantable Micro-Caged Device for Direct Local Delivery of Agents. Sci. Rep. 2017, 7, 17624. [Google Scholar] [CrossRef]
- Otchy, T.M.; Michas, C.; Lee, B.; Gopalan, K.; Nerurkar, V.; Gleick, J.; Semu, D.; Darkwa, L.; Holinski, B.J.; Chew, D.J.; et al. Printable microscale interfaces for long-term peripheral nerve mapping and precision control. Nat. Commun. 2020, 11, 4191. [Google Scholar] [CrossRef]
| Crystal Structure | Hardness [HV] | Thermal Conductivity [W/m·K] | Melting Temperature [°C] | Density [kg/m3] | Material |
|---|---|---|---|---|---|
| α—HCP; β—BCC | 117–202 | 17 | 1650–1670 | 4500 | Ti |
| FCC | 2500 | 21 | 3160 | 4940 | TiC |
 [min] |  [N] |  |  [rpm] |  | Medium | Step |
|---|---|---|---|---|---|---|
| Until plane | 25 | Synchronous rotation | 250 | H2O | SiC—paper P320 | Planar grinding |
| 2:00 | 25 | Synchronous rotation | 250 | H2O | SiC—paper P600 | Grinding |
| 5:00 | 25 | Synchronous rotation | 120 | Dia complete Poly, 9 µm | Beta | Pre-polishing |
| 10:00 (H2O during final 0:30) | 30 | Counter rotation | 120 | Eposil F, 0.1 µm | Omega | Final polishing |
| 0:45 | Kroll’s reagent | Etching |
| Refraction Index | Poisson Coefficient | Durity [MPa] | Young Modulus [GPa] | Density (Solid) [g/cm3] | Density (Liquid) [g/cm3] |
|---|---|---|---|---|---|
| 1.52 | 0.35 | 152 | 1.55 | 1.2 | 1.14–1.19 |
| 5 | 4 | 3 | 2 | 1 | Floor |
|---|---|---|---|---|---|
| 170 | 130 | 90 | 50 | 10 | Height [µm] |
| 78.5 | 78.5 | 78.5 | 78.5 | 78.5 | Surface [µm2] |
| 17:1 | 13:1 | 9:1 | 5:1 | 1:1 | Aspect ratio |
| Porosity | Designed Structure | Floors | Porosity | Designed Structure | Floors |
|---|---|---|---|---|---|
| 94.8% | 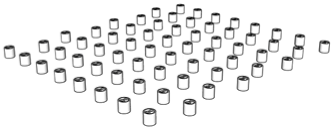 | 1st floor | 94.8% | 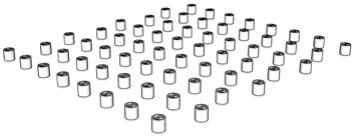 | 1st floor |
| 96.2% | 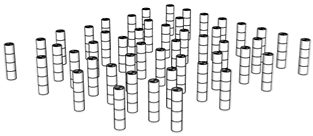 | Only 2nd floor | 95.9% | 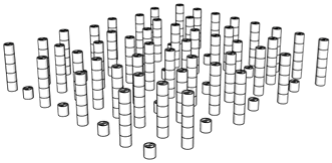 | 1st + 2nd floors |
| 98.2% | 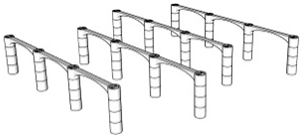 | Only 3rd floor | 96.9% | 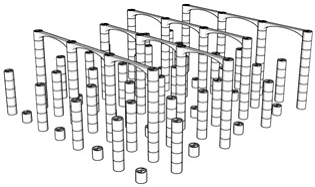 | 1st + 2nd + 3rd floors |
| 98.7% | 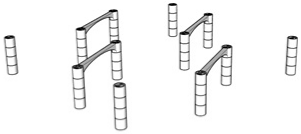 | Only 4th floor | 97.4% | 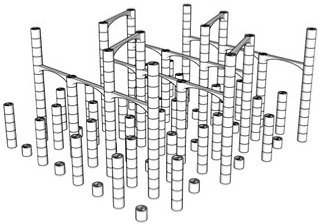 | 1st + 2nd + 3rd + 4th floors |
| 99.6% | 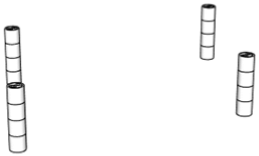 | Only 5th floor | 97.9% | 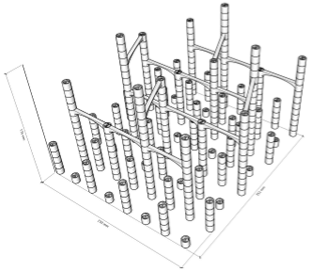 | All floors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calin, B.S.; Popescu, R.C.; Ghita, R.G.; Tanasa, E.; Mihai, S.; Paun, I.A. Improved Bioactivity of Titanium-Based Surfaces Fabricated by Laser Melting Deposition by Functionalization with 3D Polymeric Microstructures Produced by Laser Direct Writing via Two-Photon Polymerization. Polymers 2025, 17, 2620. https://doi.org/10.3390/polym17192620
Calin BS, Popescu RC, Ghita RG, Tanasa E, Mihai S, Paun IA. Improved Bioactivity of Titanium-Based Surfaces Fabricated by Laser Melting Deposition by Functionalization with 3D Polymeric Microstructures Produced by Laser Direct Writing via Two-Photon Polymerization. Polymers. 2025; 17(19):2620. https://doi.org/10.3390/polym17192620
Chicago/Turabian StyleCalin, Bogdan Stefanita, Roxana Cristina Popescu, Roxana Gabriela Ghita, Eugenia Tanasa, Sabin Mihai, and Irina Alexandra Paun. 2025. "Improved Bioactivity of Titanium-Based Surfaces Fabricated by Laser Melting Deposition by Functionalization with 3D Polymeric Microstructures Produced by Laser Direct Writing via Two-Photon Polymerization" Polymers 17, no. 19: 2620. https://doi.org/10.3390/polym17192620
APA StyleCalin, B. S., Popescu, R. C., Ghita, R. G., Tanasa, E., Mihai, S., & Paun, I. A. (2025). Improved Bioactivity of Titanium-Based Surfaces Fabricated by Laser Melting Deposition by Functionalization with 3D Polymeric Microstructures Produced by Laser Direct Writing via Two-Photon Polymerization. Polymers, 17(19), 2620. https://doi.org/10.3390/polym17192620







