Study of the Influence of Melamine and Expanded Graphite on Selected Properties of Polyurethane Foams Based on Uracil Derivatives
Abstract
1. Introduction
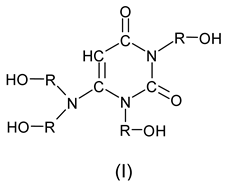
2. Materials and Methods
2.1. Materials
2.2. Oligoetherol Preparation
2.3. Foams Preparation
2.4. Analytical Methods
3. Results and Discussion
3.1. Oligoetherol Synthesis
3.2. Polyurethane Foams Synthesis
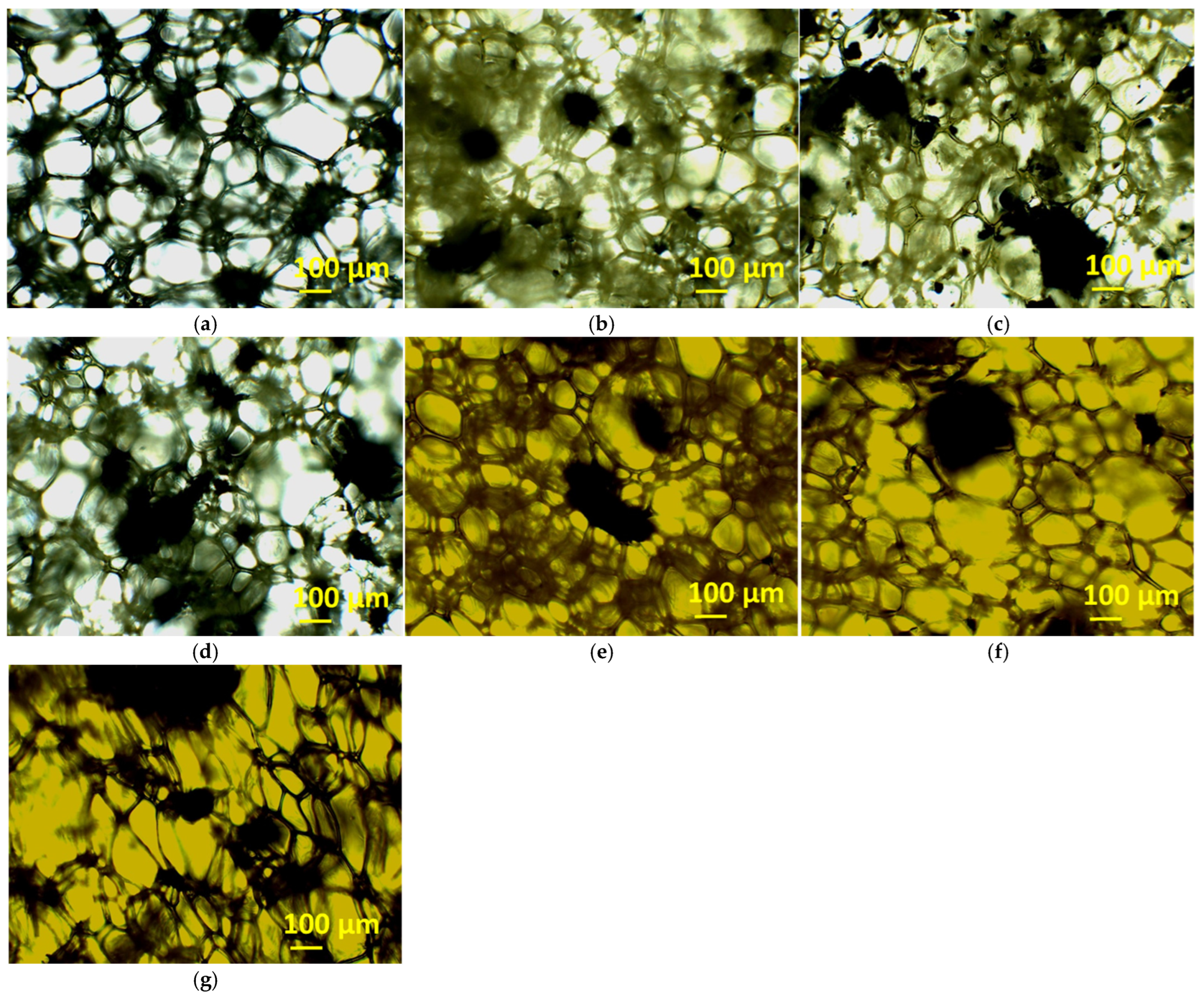
3.3. Properties of Foams
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6-AU | 6-aminouracil |
| EC | ethylene carbonate |
| EG | expanded graphite |
| LOI | limiting oxygen index |
| p-MDI | polymeric diphenylmethane 4,4′-diisocyanate |
| PO | propylene oxide |
| TEA | triethylamine |
References
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethane; Rapra Technology Limited: Shawbury, UK, 2005; pp. 1–22. ISBN 978-1-84735-035-0. [Google Scholar]
- Paciorek-Sadowska, J.; Czupryński, B.; Liszkowska, J. New polyol for production of rigid polyurethane-polyisocyanurate foams. Part 2: Preparation of rigid polyurethane-polyisocyanurate foams with the new polyol. J. Appl. Polym. Sci. 2010, 118, 2250–2256. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Czupryński, B.; Liszkowska, J. Boron-containing fire retardant rigid polyurethane-polyisocyanurate foams: Part II—Preparation and evaluation. J. Fire Sci. 2015, 33, 48–68. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J. Polyol Containing Boron Atoms as a Compound Which Reduces Flammability of Rigid Polyurethane-Polyisocyanurate Foams. Aspects of Polyurethanes.; InTech: Rijeka, Croatia, 2017; pp. 111–130. [Google Scholar] [CrossRef]
- Chmiel, E.; Lubczak, J.; Stagraczyński, R. Modification of polyurethane foams with 1,3,5-triazine ring and boron. Macromol. Res. 2017, 25, 317–324. [Google Scholar] [CrossRef]
- Chmiel-Szukiewicz, E. Improved thermally stable oligoetherols from 6-aminouracil, ethylene carbonate and boric acid. Open Chem. 2019, 17, 1080–1086. [Google Scholar] [CrossRef]
- Chmiel-Szukiewicz, E. Hardly flammable polyurethane foams with 1,3-pyrimidine ring and boron atoms. Polymers 2021, 13, 1603. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Peng, Z.; Chen, Y.; Li, G.; Wang, L.; Tang, Y.; Pang, R.; Khan, Z.U.H.; Wan, P. Preparation and characterization of flame retardant polyurethane foams containing phosphorus–nitrogen-functionalized lignin. RSC Adv. 2014, 4, 55271–55279. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.; Yang, R. The Preparation and Properties of Flame-Retardant Polyisocyanurate-Polyurethane Foams Based on Two DOPO Derivatives. J. Fire Sci. 2016, 34, 431–444. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, S. Synthesis and Properties of Rigid Polyurethane Foams Synthesized from Modified Urea-Formaldehyde Resin. Constr. Build. Mater. 2019, 202, 718–726. [Google Scholar] [CrossRef]
- Liu, L.; Lv, R. Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam. e-Polymers 2019, 19, 235–243. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, M. Addition of anti-flaming agents in castor oil based rigid polyurethane foams: Studies on mechanical and flammable behaviour. Mater. Res. Express 2020, 7, 015333. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Nando, G.B.; Naik, Y.P.; Singha, N.K. Halogen-free flame retardant PUF: Effect of melamine compounds on mechanical, thermal and flame retardant properties. Polym. Degrad. Stability. 2010, 95, 1138–1145. [Google Scholar] [CrossRef]
- Lubczak, J.; Lubczak, R. Thermally resistant polyurethane foams with reduced flammability. J. Cell. Plast. 2017, 54, 561–576. [Google Scholar] [CrossRef]
- Lubczak, R.; Broda, D.; Kus-Liśkiewicz, M.; Szczęch, D.; Bobko, E.; Dębska, B.; Szpiłyk, M.; Lubczak, J. Flame retardant polyurethane foams with starch unit. Polym. Test. 2021, 104, 107395. [Google Scholar] [CrossRef]
- Ahir, M.; Bodhak, C.; Gupta, R.K. Harnessing enhanced flame retardancy in rigid polyurethane composite foams through hemp seed oil-derived natural fillers. Polymers 2024, 16, 1584. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; de Souza, F.M.; Dawsey, T.; Gupta, R.K. Recent Advancements in Flame-Retardant Polyurethane Foams: A Review. Ind. Eng. Chem. Res. 2022, 61, 15046–15065. [Google Scholar] [CrossRef]
- Shi, L.; Li, Z.M.; Yang, M.B.; Yin, B.; Zhou, Q.M.; Tian, C.R.; Wang, J.H. Expandable graphite for halogen-free flame-retardant of high-density rigid polyurethane foams. Polym. Plast. Technol. Eng. 2005, 44, 1323–1337. [Google Scholar] [CrossRef]
- Hu, X.-M.; Wang, D.-M. Enhanced fire behavior of rigid polyurethane foam by intumescent flame retardants. J. Appl. Polym. Sci. 2013, 129, 238–246. [Google Scholar] [CrossRef]
- Cheng, J.-J.; Shi, B.-B.; Zhou, F.-B.; Chen, X.-Y. Effects of inorganic fillers on the flame-retardant and mechanical properties of rigid polyurethane foams. J. Appl. Polym. Sci. 2014, 131, 40253. [Google Scholar] [CrossRef]
- Li, Y.; Zou, J.; Zhou, S.; Chen, Y.; Zou, H.; Liang, M.; Luo, W. Effect of expandable graphite particle size on the flame retardant, mechanical, and thermal properties of water-blown semi-rigid polyurethane foam. J. Appl. Polym. Sci. 2014, 131, 39885. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Y.; Liu, F.; Shen, J.; Wu, R.; Xiang, B. Preparation Method of Expansible Graphite Modified High-Flame-Retardant Rigid Polyurethane Foam. Chinese Patent CN105218782, 2016. Available online: https://pl.espacenet.com/publicationDetails/biblio?FT=D&date=20160106&DB=EPODOC&locale=pl_PL&CC=CN&NR=105218782A&KC=A&ND=8 (accessed on 17 August 2025).
- Gama, N.V.; Silva, R.; Mohseni, F.; Davarpanah, A.; Amaral, V.S.; Ferreira, A.; Barros-Timmons, A. Enhancement of physical and reaction to fire properties of crude glycerol polyurethane foams filled with expanded graphite. Polym. Test. 2018, 69, 199–207. [Google Scholar] [CrossRef]
- Tomiak, F.; Schneider, K.; Schoeffel, A.; Rathberger, K.; Drummer, D. Expandable graphite as a multifunctional flame-retarding additive for highly filled thermal conductive polymer formulations. Polymers 2022, 14, 1613. [Google Scholar] [CrossRef] [PubMed]
- Hejna, A. Expandable graphite as intumescent flame retardant for polyurethane foams: Mini review and application guidelines. Environ. Chem. Safety. 2025, 1, 9600003. [Google Scholar] [CrossRef]
- Wei, M.; Murphy, D.; Barry, C.; Mead, J. Halogen-free flame retardants for wire and cable applications. Rubber Chem. Technol. 2010, 83, 282–302. [Google Scholar] [CrossRef]
- Luan, J.; Chen, C.; Lan, F.; Ji, G.; Dong, C.; Lu, Z. A halogen-free flame retardant with P/N and optimization for cotton fabrics tensile properties. Int. J. Biol. Macromol. 2024, 283, 137662. [Google Scholar] [CrossRef]
- Patel, R.; Chaudhary, M.L.; Patel, Y.N.; Chaudhari, K.; Gupta, R.K. Fire-Resistant Coatings: Advances in Flame-Retardant Technologies, Sustainable Approaches, and Industrial Implementation. Polymers 2025, 17, 1814. [Google Scholar] [CrossRef]
- Chmiel-Szukiewicz, E. New foamed plastic based polyetherols obtained from 6-aminouracil, ethylene carbonate and propylene oxide. J. Appl. Polym. Sci. 2013, 127, 1595–1600. [Google Scholar] [CrossRef]
- Kijowska, D.; Wołowiec, S.; Lubczak, J. Kinetics and mechanism of initial steps of synthesis of polyetherols from melamine and ethylene carbonate. J. Appl. Polym. Sci. 2004, 93, 294–300. [Google Scholar] [CrossRef]
- Brojer, Z.; Hertz, Z.; Penczek, P. Epoxy Resins; WNT: Warszawa, Poland, 1972; pp. 462–463. (In Polish) [Google Scholar]
- PN-EN ISO 845:2010; Cellular Plastics and Rubbers—Determination of Apparent (Bulk) Density. Polish Committee for Standardization: Warsaw, Poland, 2010.
- PN-EN ISO 2896:1987; Cellular Plastics, Rigid—Determination of Water Absorption. Polish Committee for Standardization: Warsaw, Poland, 1987.
- PN-EN ISO 2796:1986; Cellular Plastics, Rigid—Test of Dimensional Stability. Polish Committee for Standardization: Warsaw, Poland, 1986.
- PN-EN 13165:2009; Thermal Insulation Products for Buildings—Factory Made Rigid Polyurethane Foam (PU) Products—Specification. Polish Committee for Standardization: Warsaw, Poland, 2009.
- PN-EN ISO 844-1978; Cellular Plastics, Compression Test for Rigid Materials. Polish Committee for Standardization: Warsaw, Poland, 1978.
- PN ISO 4589-2:1999; Plastics—Determination of Burning Behaviour by Oxygen Index—Ambient-Temperature Test. Polish Committee for Standardization: Warsaw, Poland, 1999.
- ISO 9772:2012; Cellular Plastics. Determination of Horizontal Burning Characteristics of Small Specimens Subjected to a Small Flame. Polish Committee for Standardization: Warsaw, Poland, 2012.
- PN-EN ISO 60695-2002; Fire Hazard Testing—Part 11-10: Test flames—Methods of Test flames 50 W horizontal and vertical alignment of the sample. Polish Committee for Standardization: Warsaw, Poland, 2002.
- Wirpsza, Z. Polyurethanes: Chemistry, Technology, Application; WNT: Warsaw, Poland, 1991; p. 240. (In Polish) [Google Scholar]
- Jiao, L.; Xiao, H.; Wang, Q.; Sun, J. Thermal degradation characteristics of rigid polyure-thane foam and the volatile products analysis with TG-FTIR-MS. Polym. Degrad. Stab. 2013, 98, 2687–2696. [Google Scholar] [CrossRef]
- Ketata, N.; Sanglar, C.; Waton, H.; Alamercery, S.; Delolme, F.; Raffin, G.; Grenier-Loustalot, M.F. Thermal Degradation of Polyurethane Bicomponent Systems in Controlled Atmospheres. Polym. Polym. Compos. 2005, 13, 1–26. [Google Scholar] [CrossRef]
- PN-EN ISO 3582-2002; Flexible Cellular Polymeric Materials—Laboratory Characteristics of Small Specimens Subject to a Small Flame. Polish Committee for Standardization: Warsaw, Poland, 2002. (In Polish)
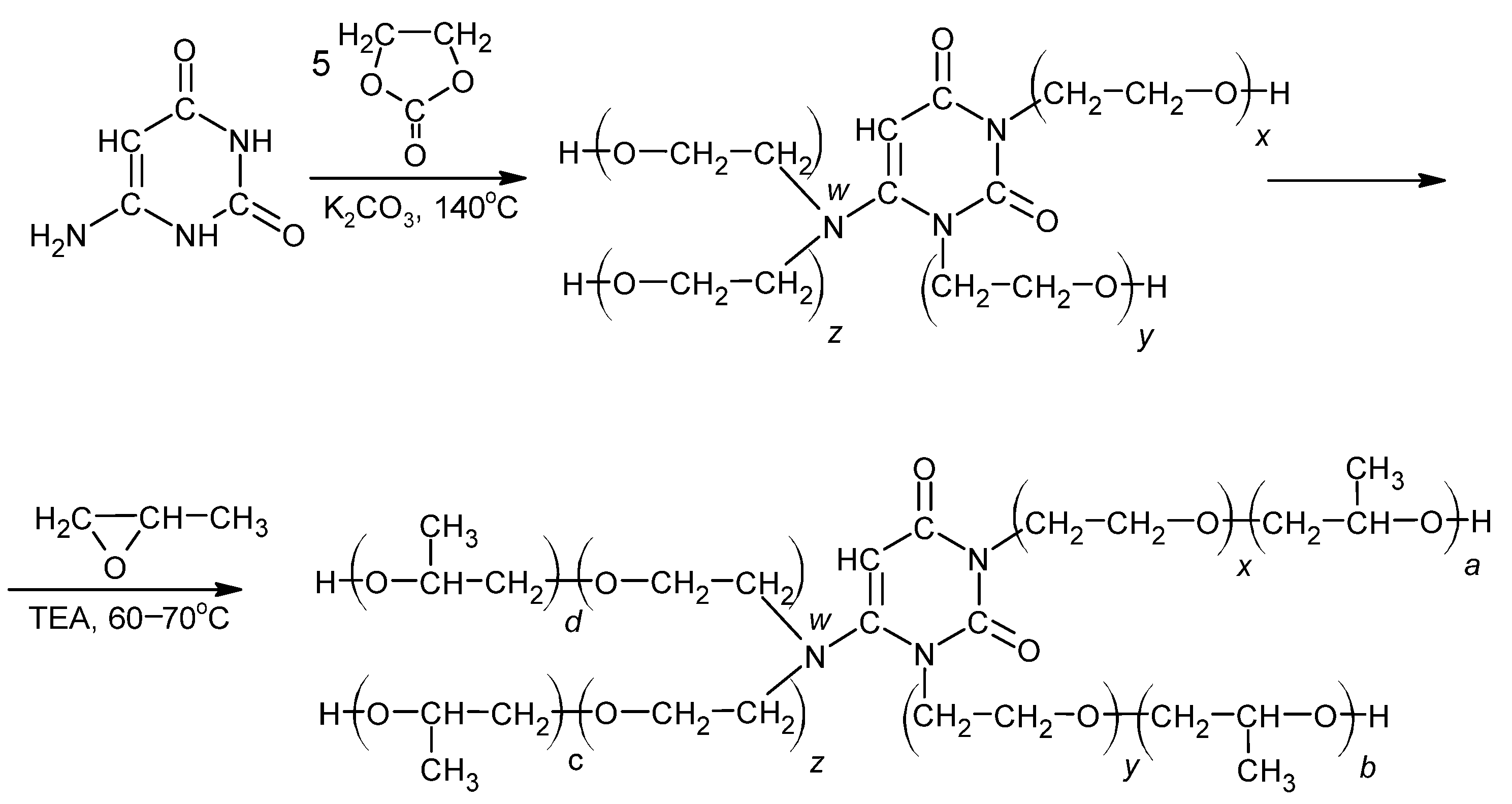
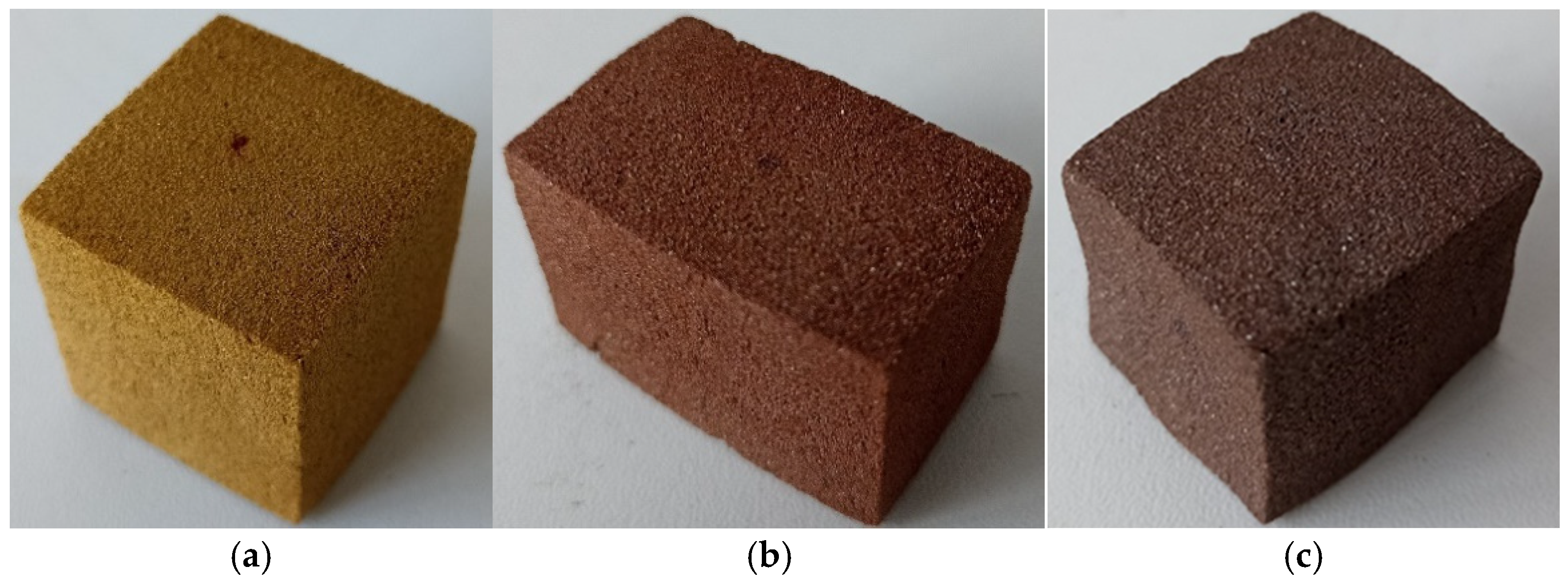

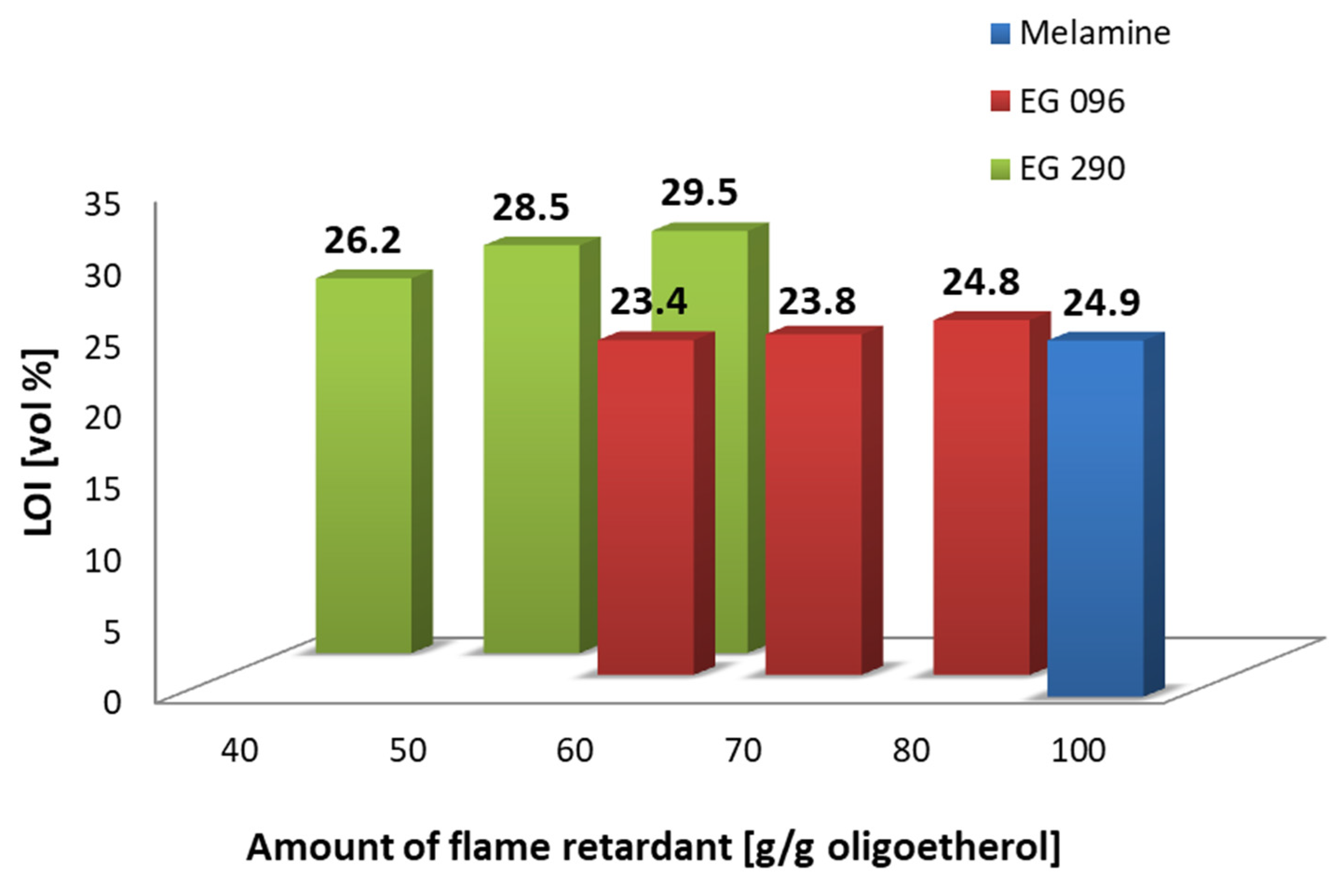
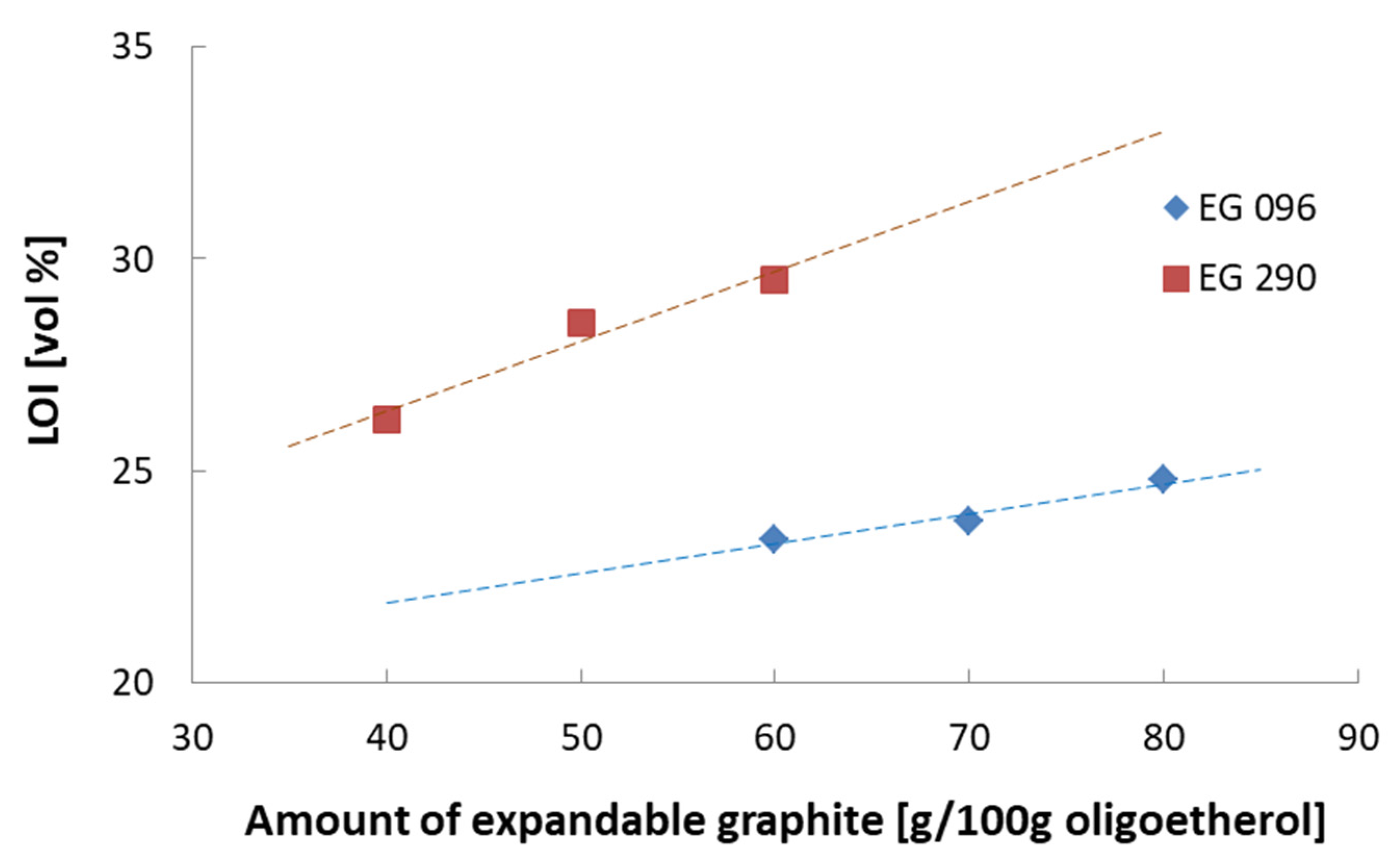
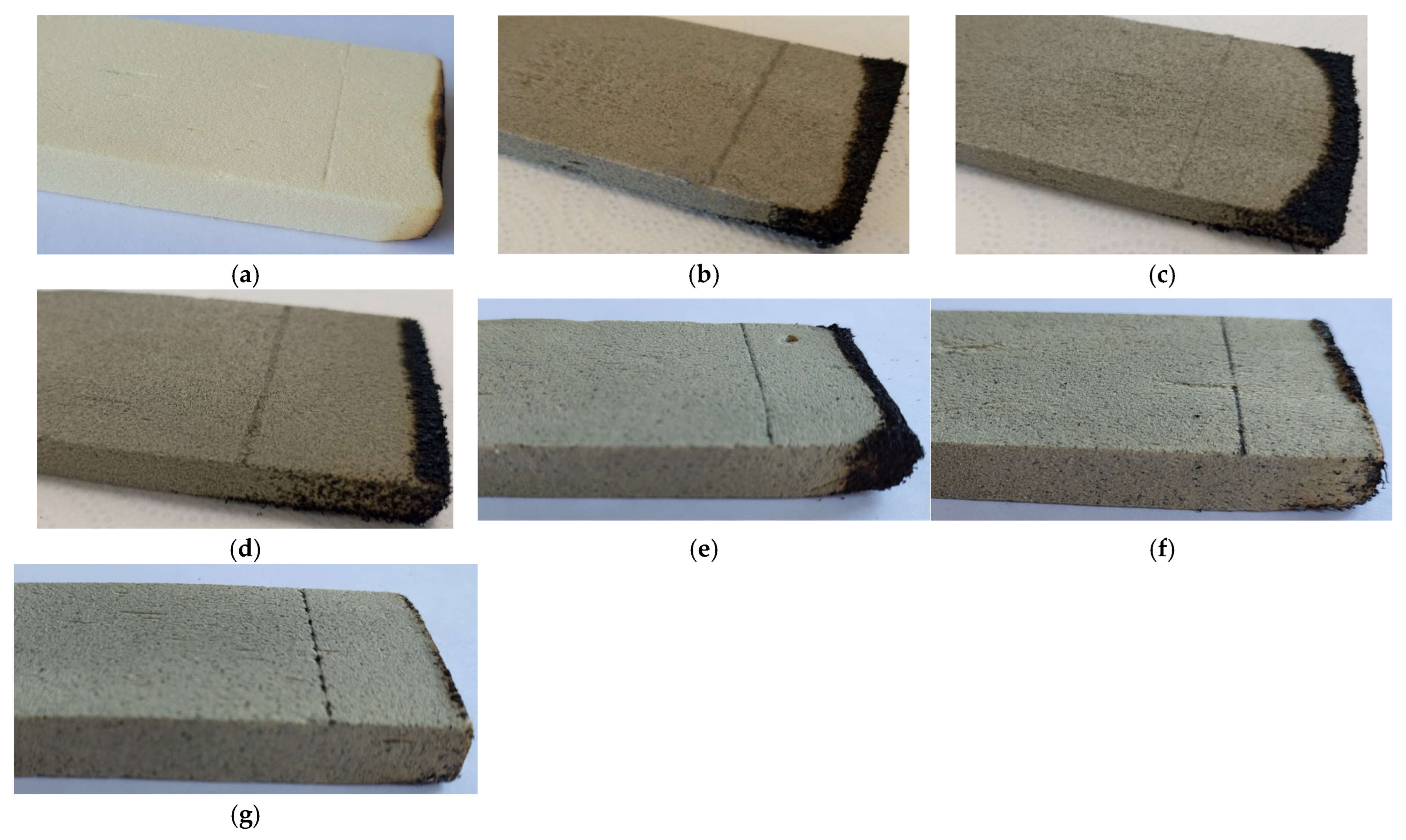
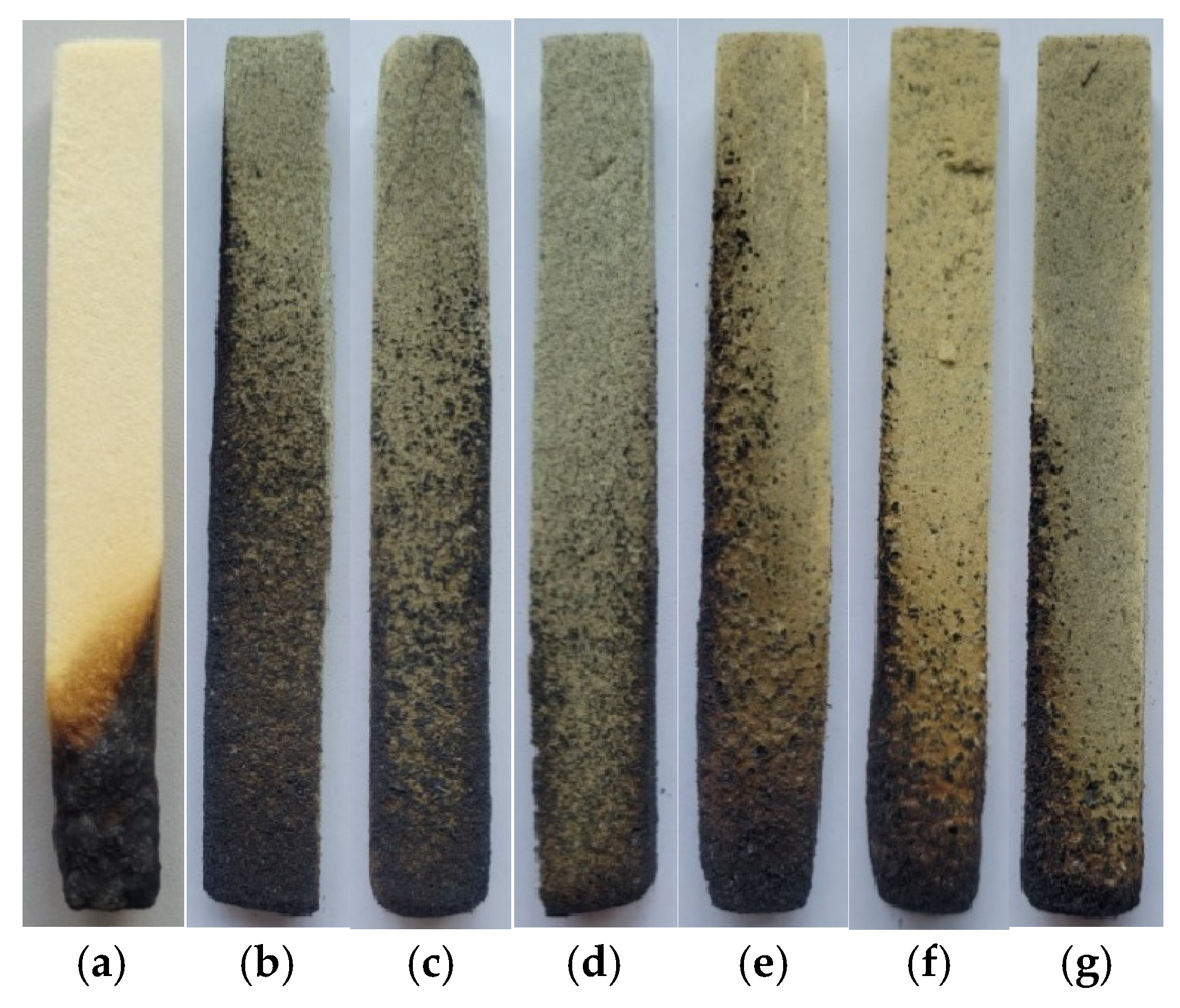
| Flame Retardant | Composition Number | Composition [g/100 g Oligoetherol 6-AU:EC:PO = 1:5:3] | Foaming Process | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Flame Retardant | p-MDI | Water | TEA | Silicon | Cream Time [s] | Growing Time [s] | Tack-Free Time [s] | ||
| Melamine | 1 | 100 | 144 | 3 | 0.27 | 3.50 | 19 | 35 | 2 |
| EG 096 | 2 | 60 | 144 | 3 | 0.61 | 3.84 | 24 | 32 | 1 |
| 3 | 70 | 144 | 3 | 0.67 | 3.84 | 25 | 38 | 1 | |
| 4 | 80 | 144 | 3 | 0.94 | 3.84 | 23 | 30 | 1 | |
| EG 290 | 5 | 40 | 144 | 3 | 0.54 | 3.84 | 26 | 31 | 5 |
| 6 | 50 | 144 | 3 | 0.54 | 3.84 | 26 | 31 | 5 | |
| 7 | 60 | 144 | 3 | 0.54 | 3.84 | 24 | 33 | 1 | |
| Composition Number (Amount and Kind of Flame Retardant) | Density [kg/m3] | Absorption of Water [vol.%] | Thermal Conductivity Coefficient λ [W/m∙K] | ||
|---|---|---|---|---|---|
| After 5 min | After 3 h | After 24 h | |||
| 1 (100% melamine) | 76 | 1.10 | 1.89 | 3.41 | 0.033 |
| 2 (60% EG 096) | 66 | 0.70 | 2.10 | 4.10 | 0.034 |
| 3 (70% EG 096) | 65 | 0.70 | 2.00 | 3.40 | 0.036 |
| 4 (80% EG 096) | 60 | 0.90 | 1.50 | 2.40 | 0.037 |
| 5 (40% EG 290) | 52 | 1.30 | 2.50 | 4.50 | 0.028 |
| 6 (50% EG 290) | 51 | 1.60 | 2.50 | 3.90 | 0.033 |
| 7 (60% EG 290) | 52 | 1.20 | 2.10 | 2.70 | 0.033 |
| foam without flame retardant [29] | 44 | 4.10 | 7.38 | 9.11 | - |
| Composition Number (Amount and Kind of Flame Retardant) | Linear Dimensions Stability at Temperature 150 °C | |||||
|---|---|---|---|---|---|---|
| Length Increase [%] | Width Increase [%] | Depth Increase [%] | ||||
| After 20 h | After 40 h | After 20 h | After 40 h | After 20 h | After 40 h | |
| 1 (100% melamine) | −1.24 | −1.55 | −1.11 | −1.24 | −1.17 | −1.45 |
| 2 (60% EG 096) | −0.37 | −1.12 | −1.11 | −2.52 | 0.14 | −0.20 |
| 3 (70% EG 096) | −0.49 | −1.14 | −0.66 | −1.36 | −0.43 | −1.53 |
| 4 (80% EG 096) | −0.55 | −1.10 | 1.29 | −1.48 | −0.54 | −1.62 |
| 5 (40% EG 290) | −1.49 | −2.12 | −2.06 | −2.47 | 0.30 | −1.10 |
| 6 (50% EG 290) | −1.87 | −2.24 | −2.23 | −2.97 | 0.81 | −0.01 |
| 7 (60% EG 290) | −1.57 | −2.34 | −1.76 | −2.77 | 0.84 | 0.14 |
| foam without flame retardant [29] | 0.00 | −1.17 | −0.05 | −0.75 | 0.00 | 1.52 |
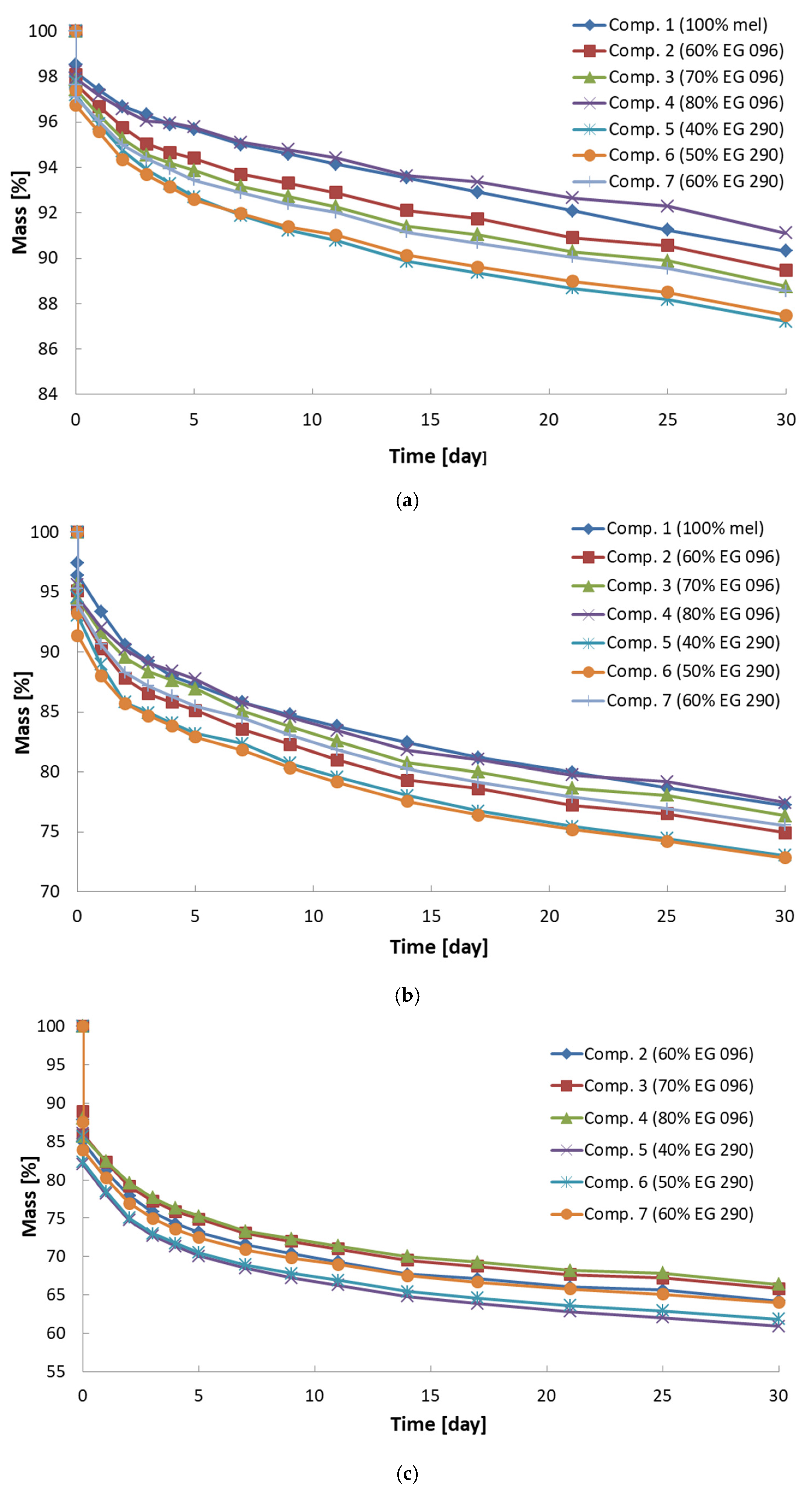
| Composition Number (Amount and Kind of Flame Retardant) | Mass Loss After 30 Days Exposure in Temperature [wt.%] | Thermal Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| 150 °C | 175 °C | 200 °C | T5% [°C] | T10% [°C] | T25% [°C] | T50% [°C] | Tmax [°C] | |
| 1 (100% melamine) | 9.7 | 22.8 | - | 229 | 260 | 307 | 387 | 315 |
| 2 (60% EG 096) | 10.5 | 25.1 | 35.8 | 161 | 177 | 207 | 276 | 290 |
| 3 (70% EG 096) | 11.2 | 23.7 | 34.2 | 160 | 178 | 210 | 280 | 285 |
| 4 (80% EG 096) | 8.9 | 22.5 | 33.6 | 162 | 180 | 218 | 295 | 290 |
| 5 (40% EG 290) | 12.8 | 27.0 | 39.1 | 179 | 235 | 294 | 380 | 315 |
| 6 (50% EG 290) | 12.5 | 27.2 | 38.2 | 202 | 242 | 300 | 388 | 315 |
| 7 (60% EG 290) | 11.4 | 24.5 | 36.0 | 249 | 276 | 318 | 415 | 315 |
| foam without flame retardant [29] | 7.9 | 24.7 | 37.8 | - | 200 | 260 | 400 | - |
| Composition Number (Amount and Kind of Flame Retardant) | Compression Strength | |||
|---|---|---|---|---|
| Before Exposition | After Exposition at | |||
| σ10 [MPa] | 150 °C | 175 °C | 200 °C | |
| σ10 [MPa] | σ10 [MPa] | σ10 [MPa] | ||
| 1 (100% melamine) | 0.270 | 0.330 | 0.114 | - |
| 2 (60% EG 096) | 0.280 | 0.320 | 0.610 | 0.780 |
| 3 (70% EG 096) | 0.250 | 0.360 | 0.530 | 0.980 |
| 4 (80% EG 096) | 0.220 | 0.260 | 0.510 | 0.810 |
| 5 (40% EG 290) | 0.300 | 0.470 | 0.710 | 1.740 |
| 6 (50% EG 290) | 0.250 | 0.430 | 0.700 | 1.430 |
| 7 (60% EG 290) | 0.260 | 0.360 | 0.630 | 1.220 |
| foam without flame retardant [29] | 0.220 | - | - | - |
| Composition Number (Amount and Kind of Flame Retardant) | Horizontal Burning Tests | Vertical Burning Test | |||||
|---|---|---|---|---|---|---|---|
| Linear Burning Rate [mm/s] | Rating | Burning Time After First Flame Application [s] | Burning Time After Second Flame Application [s] | Combustion Up to Holding Clamp (Specimens Completely Burned) | Dripping of Burning Specimens, Ignition of Cotton Batting | Rating | |
| 1 (100% melamine) | the foam goes out immediately | HF-1 | 1 | 0 | No | No | V-0 |
| 2 (60% EG 096) | the foam goes out immediately | HF-1 | 0 | 0 | No | No | V-0 |
| 3 (70% EG 096) | the foam goes out immediately | HF-1 | 0 | 0 | No | No | V-0 |
| 4 (80% EG 096) | the foam goes out immediately | HF-1 | 0 | 0 | No | No | V-0 |
| 5 (40% EG 290) | the foam goes out immediately | HF-1 | 0 | 0 | No | No | V-0 |
| 6 (50% EG 290) | the foam goes out immediately | HF-1 | 0 | 0 | No | No | V-0 |
| 7 (60% EG 290) | the foam goes out immediately | HF-1 | 0 | 0 | No | No | V-0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmiel-Szukiewicz, E.; Paciorek-Sadowska, J. Study of the Influence of Melamine and Expanded Graphite on Selected Properties of Polyurethane Foams Based on Uracil Derivatives. Polymers 2025, 17, 2610. https://doi.org/10.3390/polym17192610
Chmiel-Szukiewicz E, Paciorek-Sadowska J. Study of the Influence of Melamine and Expanded Graphite on Selected Properties of Polyurethane Foams Based on Uracil Derivatives. Polymers. 2025; 17(19):2610. https://doi.org/10.3390/polym17192610
Chicago/Turabian StyleChmiel-Szukiewicz, Elżbieta, and Joanna Paciorek-Sadowska. 2025. "Study of the Influence of Melamine and Expanded Graphite on Selected Properties of Polyurethane Foams Based on Uracil Derivatives" Polymers 17, no. 19: 2610. https://doi.org/10.3390/polym17192610
APA StyleChmiel-Szukiewicz, E., & Paciorek-Sadowska, J. (2025). Study of the Influence of Melamine and Expanded Graphite on Selected Properties of Polyurethane Foams Based on Uracil Derivatives. Polymers, 17(19), 2610. https://doi.org/10.3390/polym17192610







