Polymer Functional Layers for Perovskite Solar Cells
Abstract
1. Introduction
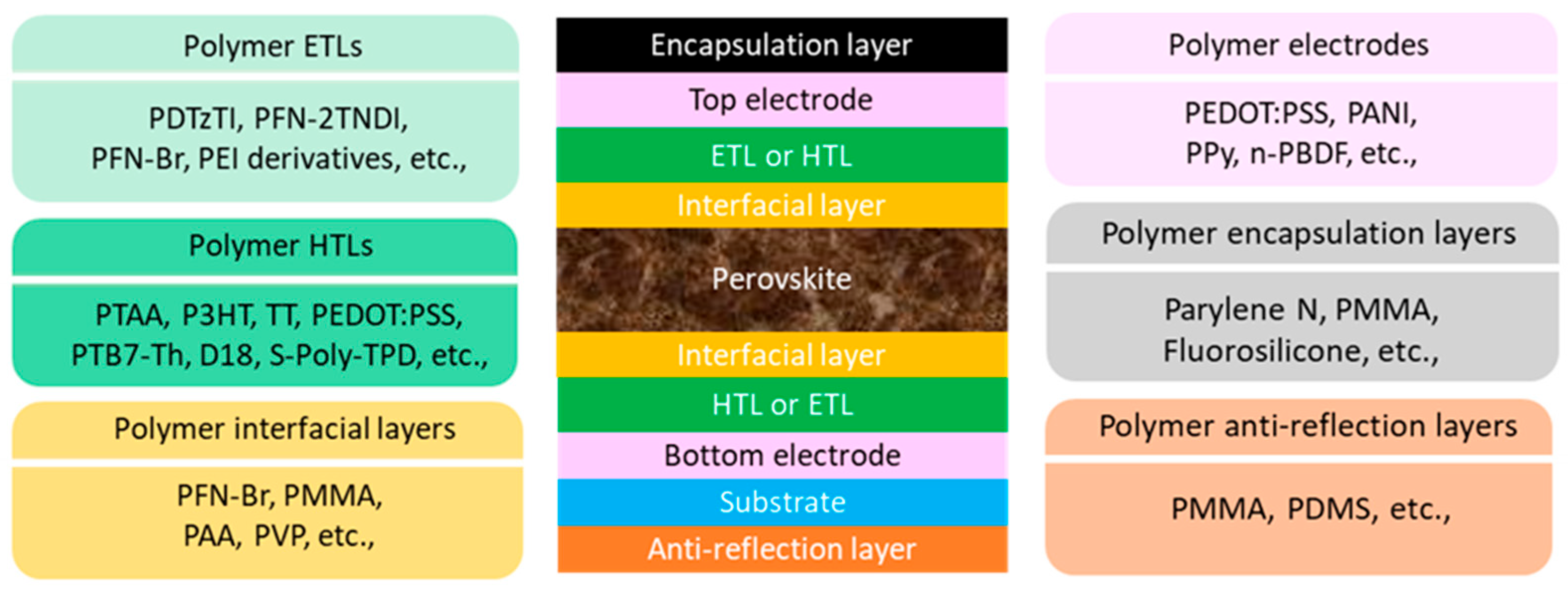
2. Polymers for PSCs
3. Polymers as ETLs for PSCs
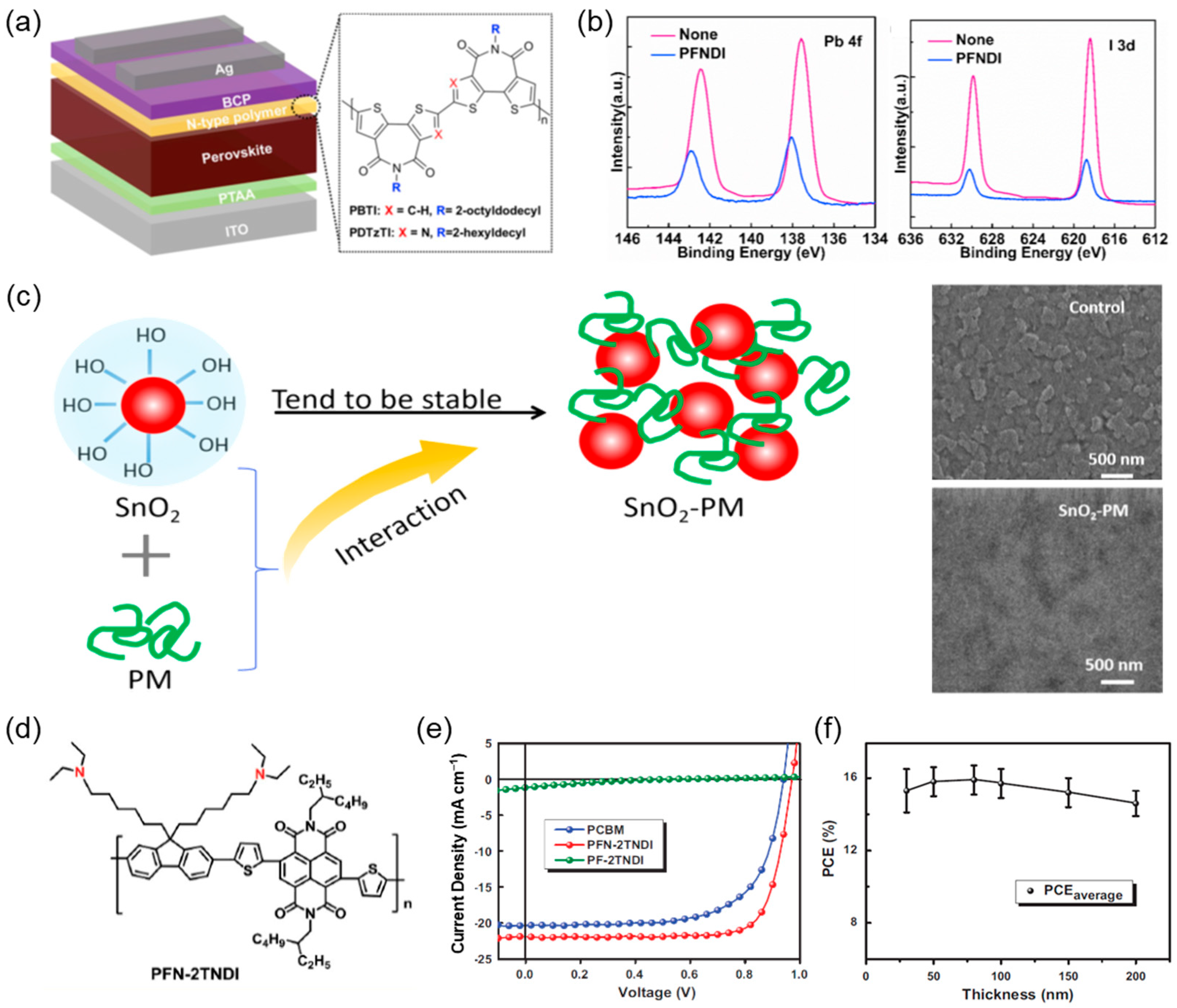
4. Polymers as HTLs for PSCs
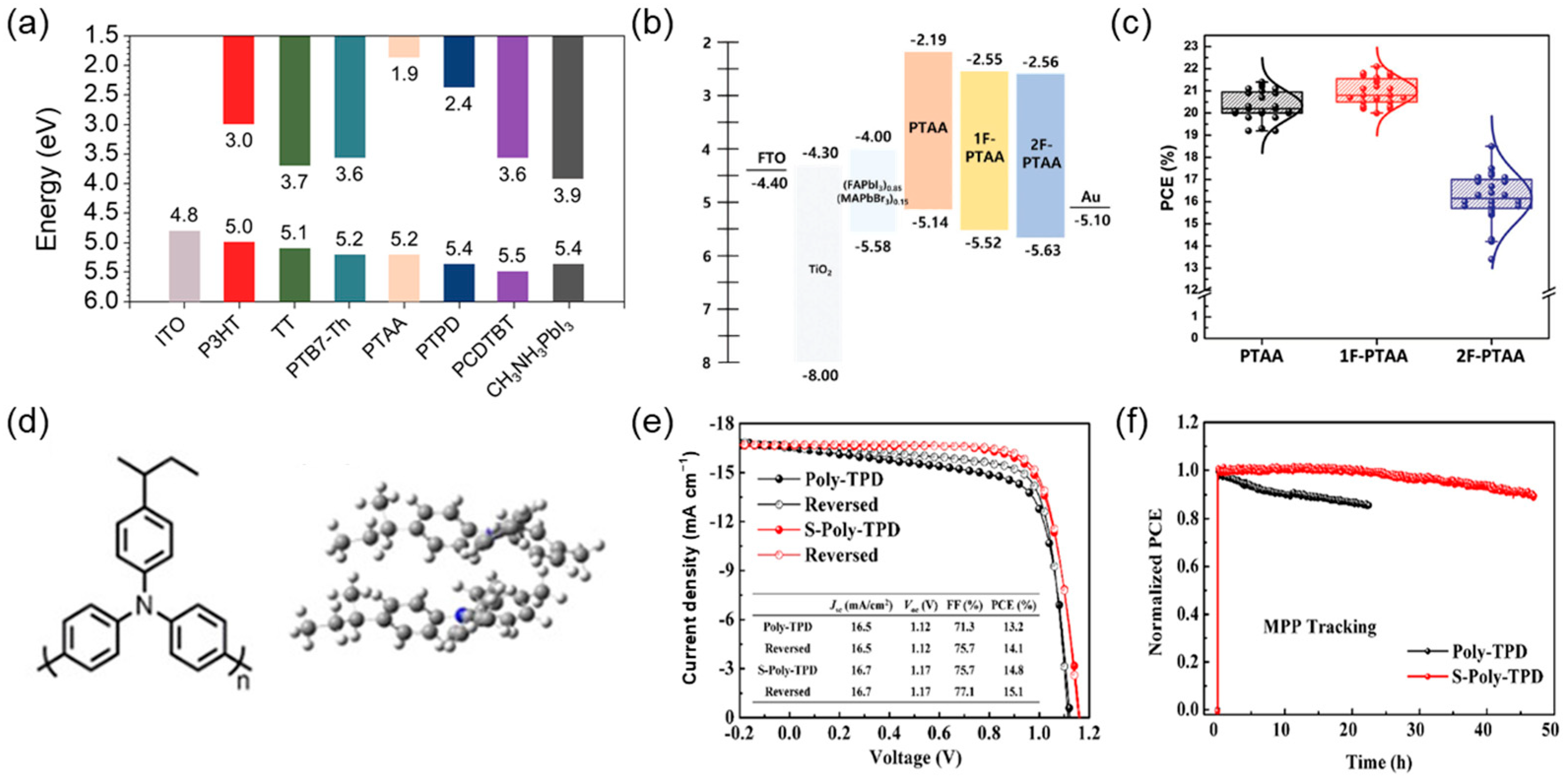
5. Polymers as Interfacial Layers for PSCs
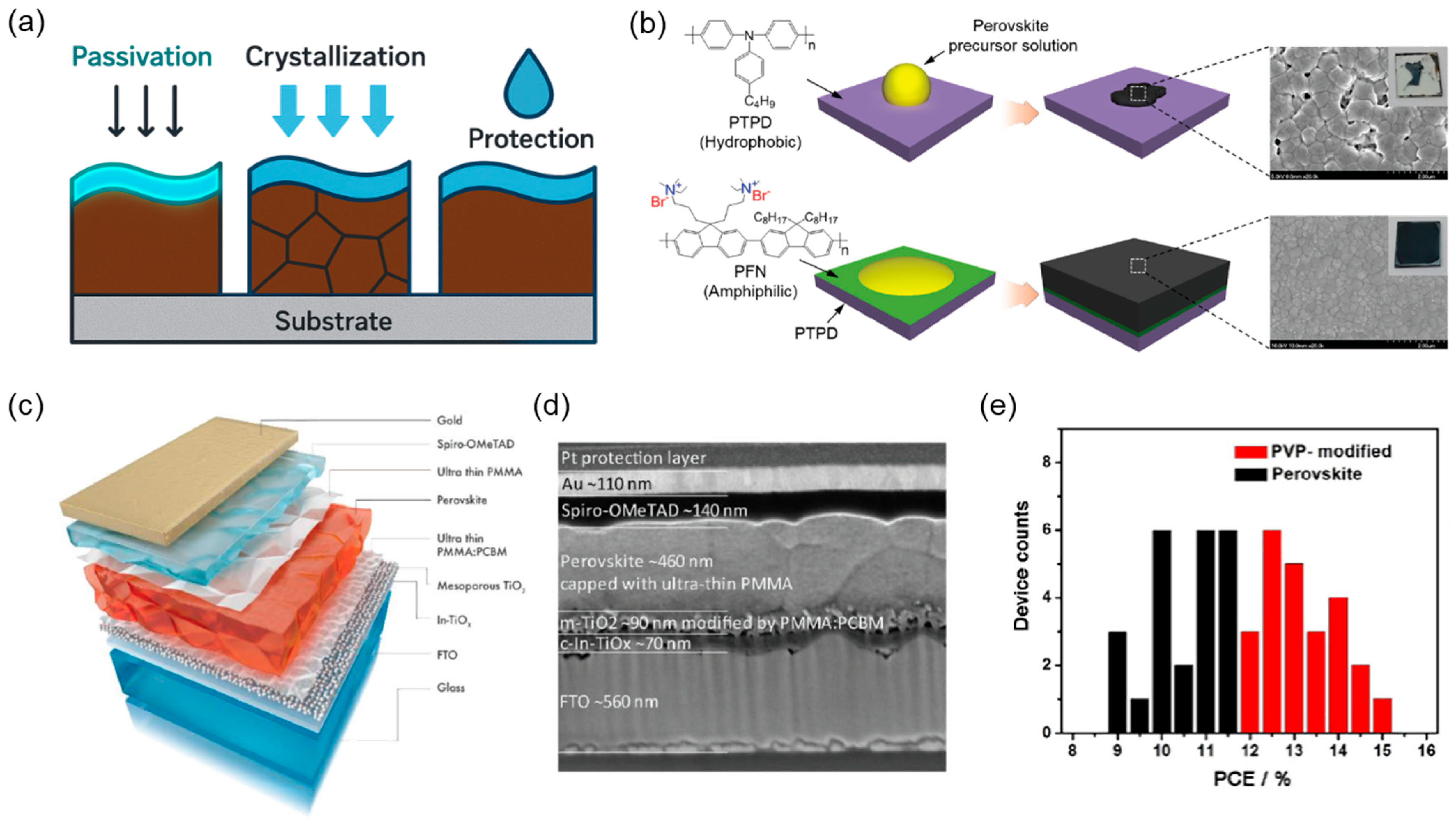
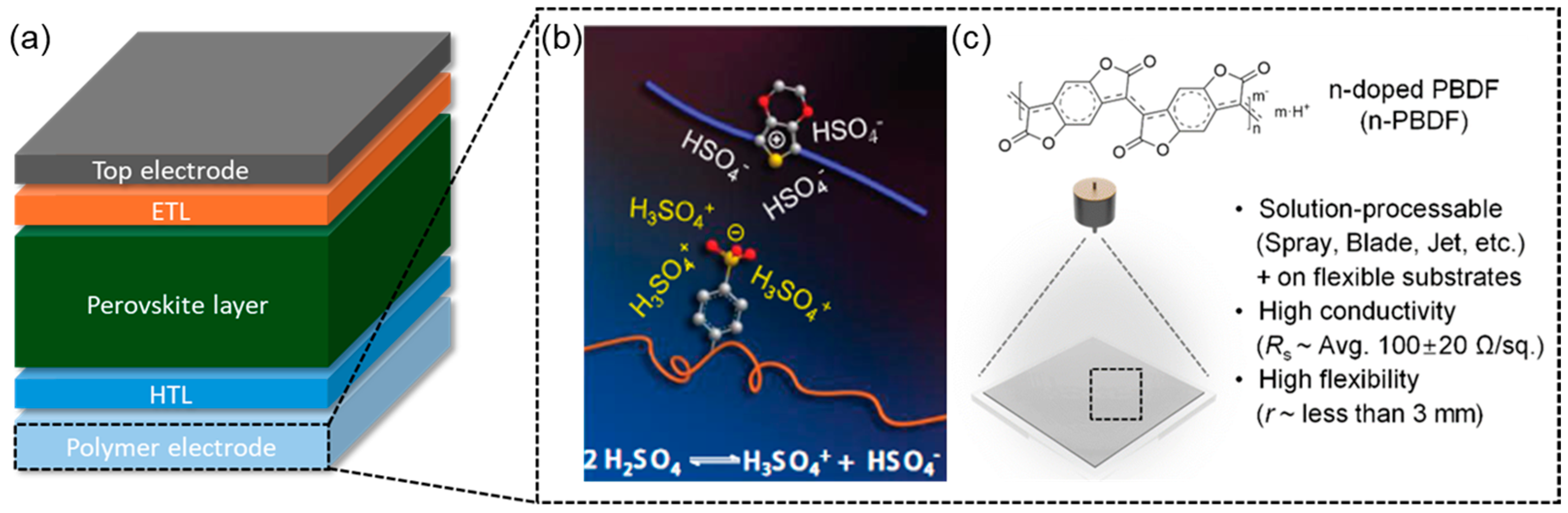
6. Polymers as Transparent Electrodes for PSCs
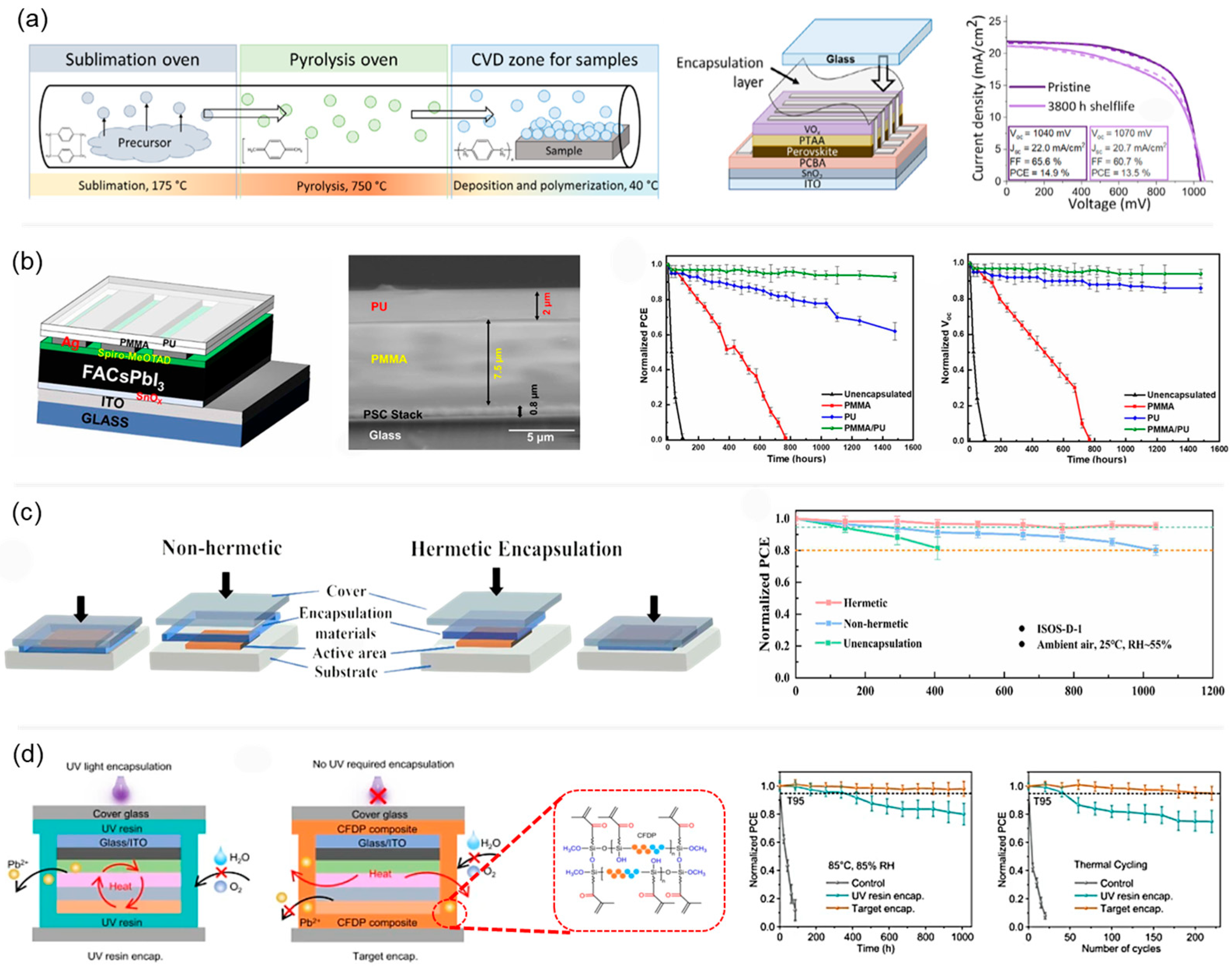
7. Polymers as Encapsulation Layers for PSCs
| Device Structure | Encapsulation Materials | Stability Test Condition | Performance | Ref. |
|---|---|---|---|---|
| Glass/ITO/SnO2/PCBA/MAPbI3/PTAA/VOx/Ag | Parylene-N + cover glass | Dark ambient (22–26 °C, 30–40% RH) | PCE retained 92% of initial value after 3800 h | [88] |
| Glass/ITO/SnOx/FA0.9Cs0.1PbI3/spiro-OMeTAD/Ag | PMMA/PU bilayer | Dark ambient (25 ± 3 °C, RH 80 ± 5%) | PCE retained 92% after 1500 h | [89] |
| FTO/SnO2/Cs0.05(FA0.85MA0.15)0.95Pb(Br0.15I0.85)3/Spiro-OMeTAD/Au | hermetic (Surlyn + glass) | Ambient air (25 °C, RH 55%), dark storage | PCE retained 95.3% after 1036 h | [90] |
| Glass/ITO/NiOx/Perovskite/PCBM/C60/BCP/Au+Cr | CFDP + cover glass | Thermal cycling (−40 °C to 85 °C, 220 cycles) | Retained 95% of initial PCE | [91] |
| Glass/FTO/c-TiO2/mp-TiO2/Cs0.10FA0.90Pb(I0.83Br0.17)3/HTL/Ag | Laser-assisted glass frit | 70 thermal cycles (−40 °C to 85 °C) + 50 h damp heat (85 °C, 85% RH) | Retained 97% of initial PCE after 50 h | [92] |
| Glass/ITO/NiOx/Cs0.17FA0.83Pb(I0.83Br0.17)3/PC60BM/SnO2/ZnSnO2/ITO/Ag | Ethylene vinyl acetate (EVA) polymer or ionomer Surlyn 5400 + glass | thermal cycling 200 cycles (−40 °C to 85 °C) | Retained 90% of initial PCE | [93] |
| Glass/ITO/NiO/Cs0.17FA0.83Pb(Br0.17I0.83)3/LiF/PCBM/SnO2/ZTO/ITO/Ag | EVA + glass, butyl rubber (edge seal) | damp heat test: 85 °C, 85% RH, 1000 h | PCE maintains over 90% after 1000 h | [94] |
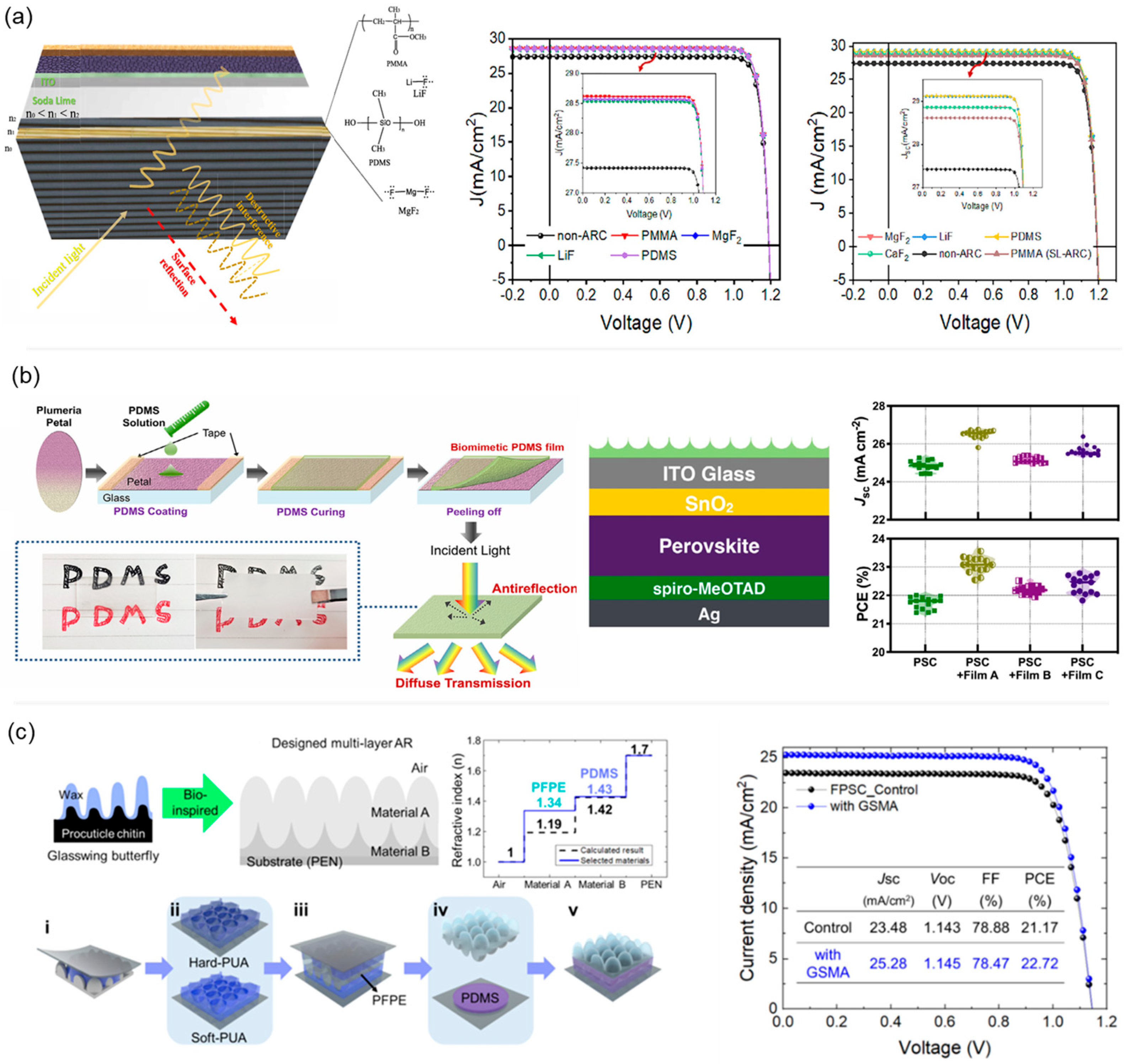
8. Polymers as Anti-Reflection Layers for PSCs
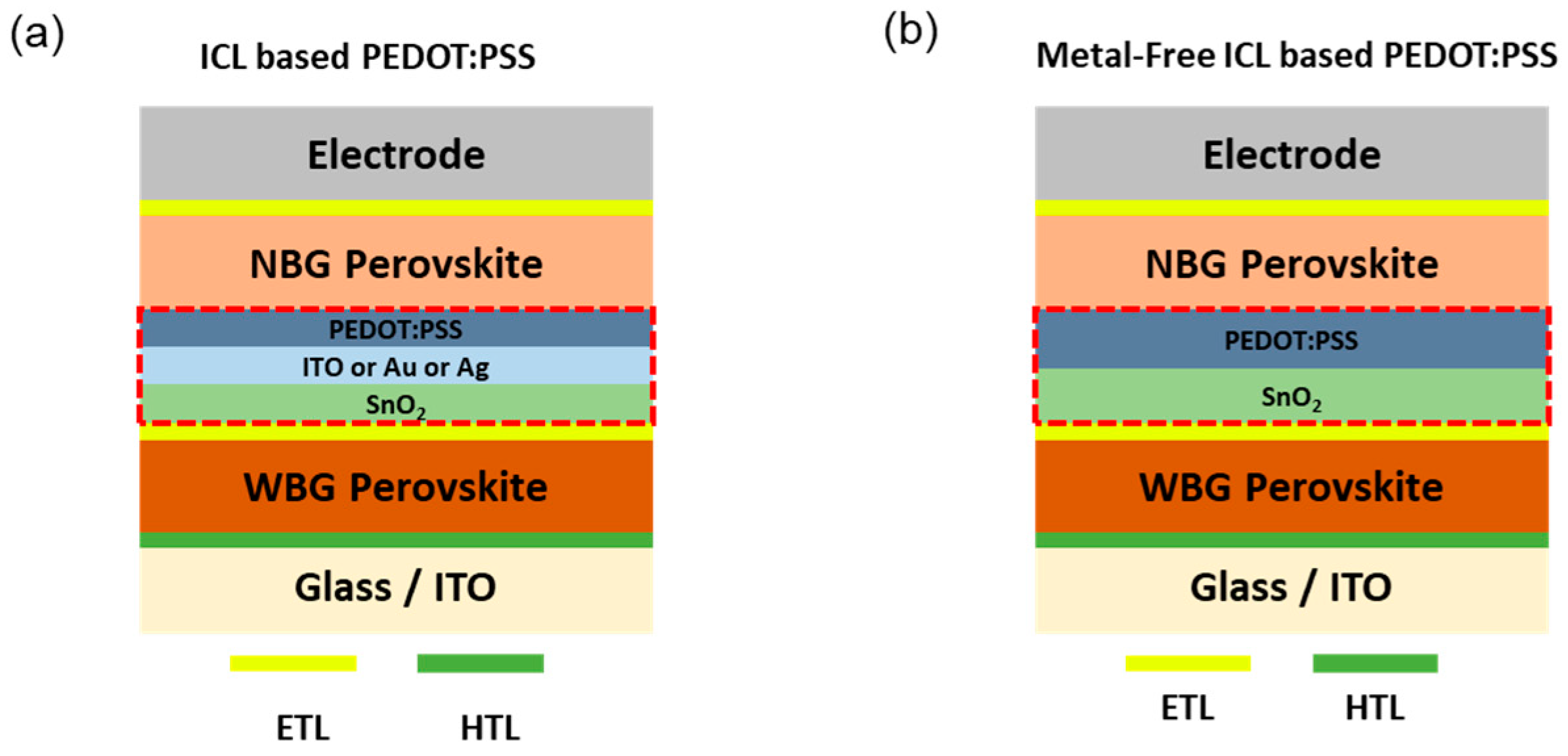
9. Polymers as Interconnecting Layers for Perovskite Tandem Cells
10. Challenges and Considerations for Large-Area Module Integration
11. Computational Modeling in Polymer Design
12. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PSC | Perovskite solar cells |
| PCE | Power conversion efficiency |
| Jsc | Short-circuit current density |
| Voc | Open-circuit voltage |
| DSSC | Dye-sensitized solar cells |
| HTL | Hole transport layer |
| ETL | Electron transport layer |
| Spiro-OMeTAD | 2,2′,7,7′-Tetrakis(N,N-di-p-methoxyphenylamine)-9,9′-spirobifluorene |
| PEDOT:PSS | Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) |
| PTAA | Poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine] |
| OSC | Organic solar cells |
| PANI | Polyaniline |
| PMMA | Poly(methyl methacrylate) |
| AR | Anti-reflection |
| PDMS | Polydimethylsiloxane |
| LED | Light-emitting diode |
| ICL | Interconnecting layer |
| ALD | Atomic layer deposition |
| P3HT | Poly(3-hexylthiophene) |
| PEI | Polyethylenimine |
| PVP | Poly(4-vinylpyridine) |
| PAA | Polyallylamine |
| PU | Polyurethane |
| n-PBDF | Poly(benzodifurandione) |
References
- Dastgeer, G.; Nisar, S.; Zulfiqar, M.W.; Eom, J.; Imran, M.; Akbar, K. A Review on Recent Progress and Challenges in High-Efficiency Perovskite Solar Cells. Nano Energy 2024, 132, 110401. [Google Scholar] [CrossRef]
- Seyisi, T.; Fouda-Mbanga, B.G.; Mnyango, J.I.; Nthwane, Y.B.; Nyoni, B.; Mhlanga, S.; Hlangothi, S.P.; Tywabi-Ngeva, Z. Major Challenges for Commercialization of Perovskite Solar Cells: A critical review. Energy Rep. 2025, 13, 1400–1415. [Google Scholar] [CrossRef]
- Kumar, N.S.; Naidu, K.C.B. A Review on Perovskite Solar Cells (PSCs), Materials and Applications. J. Mater. 2021, 7, 940–956. [Google Scholar]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The Emergence of Perovskite Solar Cells. Nat. Photon. 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Green, M.A.; Dunlop, E.D.; Yoshita, M.; Kopidakis, N.; Bothe, K.; Siefer, G.; Hao, X.; Jiang, J.Y. Solar Cell Efficiency Tables (Version 66). Prog. Photovolt. 2025, 33, 795–810. [Google Scholar] [CrossRef]
- Tang, X.; Yang, C.; Xu, Y.; Xia, J.; Li, B.; Li, M.; Zhou, Y.; Jiang, L.; Liu, H.; Ma, K.; et al. Enhancing the Efficiency and Stability of Perovskite Solar Cells via a Polymer Heterointerface Bridge. Nat. Photon. 2024, 19, 701–708. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Wang, B.; Tong, G.; Yang, J.; Shi, Y.; Chen, Z.; Ono, L.K.; Jiang, Y.; Qi, Y. Perovskite Solar Modules with High Efficiency Exceeding 20%: From Laboratory to Industrial Community. Joule 2025, 9, 102056. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, C.R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef]
- Heo, J.H.; Im, S.H.; Noh, J.H.; Mandal, T.N.; Lim, C.-S.; Chang, J.A.; Lee, Y.H.; Kim, H.-j.; Sarkar, A.; Nazeeruddin, M.K.; et al. Efficient Inorganic–Organic Hybrid Heterojunction Solar Cells Containing Perovskite Compound and Polymeric Hole Conductors. Nat. Photon. 2013, 7, 486–491. [Google Scholar] [CrossRef]
- Jung, H.S.; Park, N.-G. Perovskite Solar Cells: From Materials to Devices. Small 2015, 11, 10–25. [Google Scholar] [CrossRef]
- Zhang, H.; Park, N.-G. Progress and Issues in P-I-N Type Perovskite Solar Cells. DeCarbon 2024, 3, 100025. [Google Scholar] [CrossRef]
- Le, T.-H.; Driscoll, H.; Hou, C.-H.; Montgomery, A.; Li, W.; Stein, J.S.; Nie, W. Perovskite Solar Module: Promise and Challenges in Efficiency, Meta-Stability, and Operational Lifetime. Adv. Electron. Mater. 2023, 9, 2300093. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Y.; Jia, J.; Wang, H.; Yan, W.; Zhu, M. Perovskite Solar Cells: From Planar Designs to Fiber-Based Innovations. Wearable Electron. 2024, 1, 150–159. [Google Scholar] [CrossRef]
- Duan, L.; Liu, S.; Wang, X.; Zhang, Z.; Luo, J. Interfacial Crosslinking for Efficient and Stable Planar TiO2 Perovskite Solar Cells. Adv. Sci. 2024, 11, 2402796. [Google Scholar] [CrossRef]
- Yang, L.; Cappel, U.B.; Unger, E.L.; Karlsson, M.; Karlsson, K.M.; Gabrielsson, E.; Sun, L.; Boschloo, G.; Hagfeldt, A.; Johansson, E.M.J. Comparing Spiro-OMeTAD and P3HT Hole Conductors in Efficient Solid State Dye-Sensitized Solar Cells. Phys. Chem. Chem. Phys. 2012, 14, 779–789. [Google Scholar] [CrossRef]
- Marinova, N.; Valero, S.; Delgado, J.L. Organic and perovskite solar cells: Working Principles, Materials and Interfaces. J. Colloid Interface Sci. 2017, 488, 373–389. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, L.; Zhang, M.; Hameiri, Z.; Liu, X.; Bai, Y.; Hao, X. PTAA as Efficient Hole Transport Materials in Perovskite Solar Cells: A Review. Sol. RRL 2022, 6, 2200234. [Google Scholar] [CrossRef]
- Yi, J.; Zhang, G.; Yu, H.; Yan, H. Advantages, Challenges and Molecular Design of Different Material Types Used in Organic Solar Cells. Nat. Rev. Mater. 2024, 9, 46–62. [Google Scholar] [CrossRef]
- Stolterfoht, M.; Wolff, C.M.; Márquez, J.A.; Zhang, S.; Hages, C.J.; Rothhardt, D.; Albrecht, S.; Burn, P.L.; Meredith, P.; Unold, T.; et al. Visualization and Suppression of Interfacial Recombination for High-Efficiency Large-Area pin Perovskite Solar Cells. Nat. Energy 2018, 3, 847–854. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, Y.; Wang, H.; Ding, G.; Yang, F.; Xu, Y.; Yu, J.; Shao, Y. Shellac Protects Perovskite Solar Cell Modules under Real-World Conditions. Joule 2024, 8, 496–508. [Google Scholar] [CrossRef]
- Weerasinghe, H.C.; Macadam, N.; Kim, J.-E.; Sutherland, L.J.; Angmo, D.; Ng, L.W.T.; Scully, A.D.; Glenn, F.; Chantler, R.; Chang, N.L.; et al. The First Demonstration of Entirely Roll-to-Roll Fabricated Perovskite Solar Cell Modules under Ambient Room Conditions. Nat. Commun. 2024, 15, 1656. [Google Scholar] [CrossRef]
- Kang, B.; Yan, F. Emerging Strategies for the Large-Scale Fabrication of Perovskite Solar Modules: From Design to Process. Energy Environ. Sci. 2025, 18, 3917–3954. [Google Scholar] [CrossRef]
- Dai, X.; Deng, Y.; Brackle, C.H.V.; Chen, S.; Rudd, P.N.; Xiao, X.; Lin, Y.; Chen, B.; Huang, J. Scalable Fabrication of Efficient Perovskite Solar Modules on Flexible Glass Substrates. Adv. Energy Mater. 2020, 10, 1903108. [Google Scholar] [CrossRef]
- Jung, H.S.; Han, G.S.; Park, N.-G.; Ko, M.J. Flexible Perovskite Solar Cells. Joule 2019, 3, 1850–1880. [Google Scholar] [CrossRef]
- Lee, D.-K.; Park, N.-G. Materials and Methods for High-Efficiency Perovskite Solar Modules. Sol. RRL 2022, 6, 2100455. [Google Scholar] [CrossRef]
- Pathak, C.S.; Choi, H.; Kim, H.; Lim, J.; Cho, S.-K.; Ham, D.S.; Song, S. Recent Progress in Coating Methods for Large-Area Perovskite Solar Module Fabrication. Sol. RRL 2024, 8, 2300860. [Google Scholar] [CrossRef]
- Isakova, A.; Topham, P.D. Polymer Strategies in Perovskite Solar Cells. J. Polym. Sci. B Polym. Phys. 2017, 55, 549–568. [Google Scholar] [CrossRef]
- Ma, Y.; Ge, J.; Jen, A.K.-Y.; You, J.; Liu, S. Polymer Boosts High Performance Perovskite Solar Cells: A Review. Adv. Opt. Mater. 2024, 12, 2301623. [Google Scholar] [CrossRef]
- Wang, S.; Gong, X.Y.; Li, M.-X.; Li, M.-H.; Hu, J.-S. Polymers for Perovskite Solar Cells. J. Am. Chem. Soc. Au. 2024, 4, 3400–3412. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-S.; Choi, J.-M.; Lee, J.W.; Han, J.; Jeon, I.; Kim, H.D. Decoding Polymeric Additive-Driven Self-Healing Processes in Perovskite Solar Cells from Chemical and Physical Bonding Perspectives. Adv. Energy Mater. 2024, 14, 2304062. [Google Scholar] [CrossRef]
- Kim, K.; Han, J.; Maruyama, S.; Balaban, M.; Jeon, I. Role and Contribution of Polymeric Additives in Perovskite Solar Cells: Crystal Growth Templates and Grain Boundary Passivators. Sol. RRL 2021, 5, 2000783. [Google Scholar] [CrossRef]
- Fan, X.; Nie, W.; Tsai, H.; Wang, N.; Huang, H.; Cheng, Y.; Wen, R.; Ma, L.; Yan, F.; Xia, Y. PEDOT:PSS for Flexible and Stretchable Electronics: Modifications, Strategies, and Applications. Adv. Sci. 2019, 6, 190081376. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Park, N.-G.; Seok, S.I.; Saliba, M. All-Perovskite Tandem Solar Cells: From Fundamentals to Technological Progress. Energy Environ. Sci. 2024, 17, 4390–4425. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Merino, L.T.; Koc, M.; Aydin, E.; Zhumagali, S.; Haque, M.A.; Yazmaciyan, A.; Sharma, A.; Villalva, D.R.; Hernandez, L.H.; et al. Metal-Free Interconnecting Layer for Monolithic Perovskite/Organic Tandem Solar Cells with Enhanced Outdoor Stability. ACS Appl. Energy Mater. 2022, 5, 14035–14044. [Google Scholar] [CrossRef]
- Nejand, B.A.; Ritzer, D.B.; Hu, H.; Schackmar, F.; Moghadamzadeh, S.; Feeney, T.; Singh, R.; Laufer, F.; Schmager, R.; Azmi, R.; et al. Scalable Two-Terminal All-Perovskite Tandem Solar Modules with a 19.1% Efficiency. Nat. Energy 2022, 7, 620–630. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Choy, W.C.H. Efficient Interconnection in Perovskite Tandem Solar Cells. Small Methods 2020, 4, 2000093. [Google Scholar] [CrossRef]
- Lin, R.; Xiao, K.; Qin, Z.; Han, Q.; Zhang, C.; Wei, M.; Saidaminov, M.I.; Gao, Y.; Xu, J.; Xiao, M.; et al. Monolithic All-Perovskite Tandem Solar Cells with 24.8% Efficiency Exploiting Comproportionation to Suppress Sn(ii) Oxidation in Precursor Ink. Nat. Energy 2019, 4, 864–873. [Google Scholar] [CrossRef]
- Chen, W.; Shi, Y.; Wang, Y.; Feng, X.; Djurišić, A.B.; Woo, H.Y.; Guo, X.; He, Z. N-Type Conjugated Polymer as Efficient Electron Transport Layer for Planar Inverted Perovskite Solar Cells with Power Conversion Efficiency of 20.86%. Nano Energy 2020, 68, 104363. [Google Scholar] [CrossRef]
- Deng, C.; Wan, L.; Li, S.; Tao, L.; Wang, S.-n.; Zhang, W.; Fang, J.; Fu, Z.; Song, W. Naphthalene Diimide Based Polymer as Electron Transport Layer in Inverted Perovskite Solar Cells. Org. Electron. 2020, 87, 105959. [Google Scholar] [CrossRef]
- Li, D.; Sun, C.; Li, H.; Shi, H.; Shai, X.; Sun, Q.; Han, J.; Shen, Y.; Yip, H.-L.; Huang, F.; et al. Amino-Functionalized Conjugated Polymer Electron Transport Layers Enhance the UV-Photostability of Planar Heterojunction Perovskite Solar Cells. Chem. Sci. 2017, 8, 4587–4594. [Google Scholar] [CrossRef]
- Xu, Z.; Ng, C.H.; Zhou, X.; Li, X.; Zhang, P.; Teo, S.H. Polymer-Complexed SnO2 Electron Transport Layer for High-Efficiency n-i-p Perovskite Solar Cells. Nanoscale 2022, 14, 12090–12098. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wu, Z.; Yip, H.-L.; Zhang, H.; Jiang, X.-F.; Xue, Q.; Hu, Z.; Hu, Z.; Shen, Y.; Wang, M.; et al. Amino-Functionalized Conjugated Polymer as an Efficient Electron Transport Layer for High-Performance Planar-Heterojunction Perovskite Solar Cells. Adv. Energy Mater. 2015, 6, 1501534. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent Engineering for High-Performance Inorganic-Organic Hybrid Perovskite Solar Cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef]
- Ryu, S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Yang, W.S.; Seo, J.; Seok, S.I. Voltage Output of Efficient Perovskite Solar Cells with High Open-Circuit Voltage and Fill Factor. Energy Environ. Sci. 2014, 7, 2614–2618. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Z.; Li, B.; Li, X.; Gao, D.; Wu, X.; Zhu, Z. Exploring the Potential and Hurdles of Perovskite Solar Cells with p-i-n Structure. ACS Nano 2024, 18, 32299–32314. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, E.H.; Kim, G.; Kim, D.; Kim, B.J.; Seo, J. Sequentially Fluorinated PTAA Polymers for Enhancing VOC of High-Performance Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1801668. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, P.; Zhang, D.; Yang, G.; Yu, J. Enhanced Efficiency and Stability of p-i-n Perovskite Solar Cells Using PMMA Doped PTAA as Hole Transport Layers. Synth. Met. 2020, 265, 116428. [Google Scholar] [CrossRef]
- Xie, Y.-M.; Yao, Q.; Xue, Q.; Zeng, Z.; Niu, T.; Zhou, Y.; Zhuo, M.-P.; Tsang, S.-W.; Yip, H.-L.; Cao, Y. Subtle Side Chain Modification of Triphenylamine-Based Polymer Hole-Transport Layer Materials Produces Efficient and Stable Inverted Perovskite Solar Cells. Interdiscip. Mater. 2022, 1, 281–293. [Google Scholar] [CrossRef]
- Lee, J.; Kang, H.; Kim, G.; Back, H.; Kim, J.; Hong, S.; Park, B.; Lee, E.; Lee, K. Achieving Large-Area Planar Perovskite Solar Cells by Introducing an Interfacial Compatibilizer. Adv. Mater. 2017, 29, 1606363. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Walter, D.; Ren, Y.; Tebyetekerwa, M.; Wu, Y.; Duong, T.; Lin, Q.; Li, J.; Lu, T.; Mahmud, M.A.; et al. Nanoscale Localized Contacts for High Fill Factors in Polymer-Passivated Perovskite Solar Cells. Science 2021, 371, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Obrero-Perez, J.M.; Contreras-Bernal, L.; Nuñez-Galvez, F.; Castillo-Seoane, J.; Valadez-Villalobos, K.; Aparicio, F.J.; Anta, J.A.; Borras, A.; Sanchez-Valencia, J.R.; Barranco, A. Ultrathin Plasma Polymer Passivation of Perovskite Solar Cells for Improved Stability and Reproducibility. Adv. Energy Mater. 2022, 12, 2200812. [Google Scholar] [CrossRef]
- Yuan, B.; Li, C.; Yi, W.; Juan, F.; Yu, H.; Xu, F.; Li, C.; Cao, B. PMMA Passivated CsPbI2Br Perovskite Film for Highly Efficient and Stable Solar Cells. J. Phys. Chem. Solids 2021, 153, 110000. [Google Scholar] [CrossRef]
- Ferdowsi, P.; Ochoa-Martinez, E.; Alonso, S.S.; Steiner, U.; Saliba, M. Ultrathin Polymeric Films for Interfacial Passivation in Wide Band-Gap Perovskite Solar Cells. Sci. Rep. 2020, 10, 22260. [Google Scholar] [CrossRef]
- Kim, W.; Park, J.; Aggarwal, Y.; Sharma, S.; Choi, E.H.; Park, B. Highly Efficient and Stable Self-Powered Perovskite Photodiode by Cathode-Side Interfacial Passivation with Poly(Methyl Methacrylate). Nanomaterials 2023, 13, 619. [Google Scholar] [CrossRef]
- Peng, J.; Khan, J.I.; Liu, W.; Ugur, E.; Duong, T.; Wu, Y.; Shen, H.; Wang, K.; Dang, H.; Aydin, E.; et al. A Universal Double-Side Passivation for High Open-Circuit Voltage in Perovskite Solar Cells: Role of Carbonyl Groups in Poly(methyl methacrylate). Adv. Energy Mater. 2018, 8, 1801208. [Google Scholar] [CrossRef]
- Ochoa-Martinez, E.; Ochoa, M.; Ortuso, R.D.; Ferdowsi, P.; Carron, R.; Tiwari, A.N.; Steiner, U.; Saliba, M. Physical Passivation of Grain Boundaries and Defects in Perovskite Solar Cells by an Isolating Thin Polymer. ACS Energy Lett. 2021, 6, 2626–2634. [Google Scholar] [CrossRef]
- Chaudhary, B.; Kulkarni, A.; Jena, A.K.; Ikegami, M.; Udagawa, Y.; Kunugita, H.; Ema, K.; Miyasaka, T. Poly(4-Vinylpyridine)-Based Interfacial Passivation to Enhance Voltage and Moisture Stability of Lead Halide Perovskite Solar Cells. ChemSusChem 2017, 10, 2473–2479. [Google Scholar] [CrossRef]
- Zuo, L.; Guo, H.; Dequilettes, D.W.; Jariwala, S.; Marco, N.D.; Dong, S.; DeBlock, R.; Ginger, D.S.; Dunn, B.; Wang, M.; et al. Polymer-Modified Halide Perovskite Films for Efficient and Stable Planar Heterojunction Solar Cells. Sci. Adv. 2017, 3, e1700106. [Google Scholar] [CrossRef]
- Zhang, C.-C.; Li, M.; Wang, Z.-K.; Jiang, Y.-R.; Liu, H.-R.; Yang, Y.-G.; Gao, X.-Y.; Ma, H. Passivated Perovskite Crystallization and Stability in Organic-Inorganic Halide Solar Cells by Doping a Donor Polymer. J. Mater. Chem. A 2017, 5, 2572–2579. [Google Scholar] [CrossRef]
- Byranvand, M.M.; Behboodi-Sadabad, F.; Alrhman, E.A.; Trouillet, V.; Welle, A.; Ternes, S.; Hossain, I.M.; Khan, M.R.; Schwenzer, J.A.; Farooq, A.; et al. Chemical Vapor Deposited Polymer Layer for Efficient Passivation of Planar Perovskite Solar Cells. J. Mater. Chem. A 2020, 8, 20122–20132. [Google Scholar] [CrossRef]
- Xiong, H.; DeLuca, G.; Rui, Y.; Zhang, B.; Li, Y.; Zhang, Q.; Wang, H.; Reichmanis, E. Modifying Perovskite Films with Polyvinylpyrrolidone for Ambient-Air-Stable Highly Bendable Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 35385–35394. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; Wei, W.; Hao, Y.; Liu, S.; Ouyang, J.; Chang, J. Recent Progress of Electrode Materials for Flexible Perovskite Solar Cells. Nano-Micro Lett. 2022, 14, 117. [Google Scholar] [CrossRef]
- Guo, H.; Hou, F.; Ren, X.; Ning, X.; Wang, Y.; Yang, H.; Wei, J.; Guo, J.; Li, T.; Zhu, C.; et al. Recent Progresses on Transparent Electrodes and Active Layers Toward Neutral, Color Semitransparent Perovskite Solar Cells. Sol. RRL 2023, 7, 2300333. [Google Scholar] [CrossRef]
- Mujahid, M.; Chen, C.; Zhang, J.; Li, C.; Duan, Y. Recent advances in semitransparent perovskite solar cells. InfoMat 2020, 3, 101–124. [Google Scholar] [CrossRef]
- Wang, T.; Lu, K.; Xu, Z.; Lin, Z.; Ning, H.; Qiu, T.; Yang, Z.; Zheng, H.; Yao, R.; Peng, J. Recent Developments in Flexible Transparent Electrode. Crystals 2021, 11, 511. [Google Scholar] [CrossRef]
- Yelshibay, A.; Bukari, S.D.; Baptayev, B.; Balanay, M.P. Conducting Polymers in Solar Cells: Insights, Innovations, and Challenges. Organics 2024, 5, 640–669. [Google Scholar] [CrossRef]
- Kim, U.; Lee, J.; Lee, Y.S.; Choi, M. Mechanically Robust Transparent Conducting Electrodes for Flexible Perovskite Solar Cells. Korean J. Chem. Eng. 2024, 41, 3677–3692. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, W.; Guan, X.; Raza, H.; Zhang, S.; Zhang, Y.; Troshin, P.A.; Kuklin, S.A.; Liu, Z.; Chen, W. Rear Electrode Materials for Perovskite Solar Cells. Adv. Funct. Mater. 2022, 32, 2200651. [Google Scholar] [CrossRef]
- Gan, Y.; Sun, J.; Guo, P.; Jiang, H.; Li, J.; Zhu, H.; Fan, X.; Huang, L.; Wang, Y. Advances in the Research of Carbon Electrodes for Perovskite Solar Cells. Dalton Trans. 2023, 52, 16558. [Google Scholar] [CrossRef] [PubMed]
- Chae, E.C.; Seo, Y.-H.; Kang, B.J.; Oh, J.H.; Jung, Y.; Jang, J.; Kim, T.; Jo, Y.-R.; Kim, D.J.; Kim, T.-S.; et al. PTAA-Infiltrated Thin-Walled Carbon Nanotube Electrode with Hidden Encapsulation for Perovskite Solar Cells. EcoMat 2024, 6, e12495. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Papanastasiou, D.T.; Resende, J.; Bardet, L.; Sannicolo, T.; Jimenez, C.; Munoz-Rojas, D.; Nguyen, N.D.; Bellet, D. Advances in Flexible Metallic Transparent Electrodes. Small 2022, 18, e2106006. [Google Scholar] [CrossRef]
- Kim, N.; Kee, S.; Lee, S.H.; Lee, B.H.; Kahng, Y.H.; Jo, Y.-R.; Kim, B.-J.; Lee, K. Highly Conductive PEDOT:PSS Nanofibrils Induced by Solution-Processed Crystallization. Adv. Mater. 2014, 26, 2268–2272. [Google Scholar] [CrossRef]
- Zhu, T.; Yang, Y.; Yao, X.; Huang, Z.; Liu, L.; Hu, W.; Gong, X. Solution-Processed Polymeric Thin Film as the Transparent Electrode for Flexible Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 15456–15463. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Meng, X.; Zhang, L.; Zhang, Y.; Cai, Z.; Huang, Z.; Su, M.; Wang, Y.; Li, M.; Li, F.; et al. A Mechanically Robust Conducting Polymer Network Electrode for Efficient Flexible Perovskite Solar Cells. Joule 2019, 3, 2205–2218. [Google Scholar] [CrossRef]
- Kumar, P.; Lee, W.-J.; Tang, Y.; Yang, H.; Xu, W.; Rout, P.; You, L.; Romo, J.A.; Pradhan, B.; Mei, J.; et al. Enabling ITO-Free Perovskite Solar Cells through N-Doped Poly(benzodifurandione) (n-PBDF) Electrodes. EES Sol. 2025, 1, 529–535. [Google Scholar] [CrossRef]
- Chowdhury, T.A.; Zafar, M.A.B.; Islam, M.S.-U.; Shahinuzzaman, M.; Islam, M.A.; Khandaker, M.U. Stability of Perovskite Solar Cells: Issues and Prospects. RSC Adv. 2023, 13, 1787–1810. [Google Scholar] [CrossRef]
- Kahandal, S.S.; Tupke, R.S.; Bobade, D.S.; Kim, H.; Piao, G.; Sankapal, B.R.; Said, Z.; Pagar, B.P.; Pawar, A.C.; Kim, J.M.; et al. Perovskite Solar Cells: Fundamental Aspects, Stability Challenges, and Future Prospects. Prog. Solid State Chem. 2024, 74, 100463. [Google Scholar] [CrossRef]
- Dipta, S.S.; Uddin, A. Stability Issues of Perovskite Solar Cells: A Critical Review. Energy Technol. 2021, 9, 2100560. [Google Scholar] [CrossRef]
- Wang, D.; Wright, M.; Elumalai, N.K.; Uddin, A. Stability of Perovskite Solar Cells. Sol. Energy Mater. Sol. Cells 2016, 147, 255–275. [Google Scholar] [CrossRef]
- Uddin, A.; Upama, M.B.; Yi, H.; Duan, L. Encapsulation of Organic and Perovskite Solar Cells: A Review. Coatings 2019, 9, 65. [Google Scholar] [CrossRef]
- Ma, S.; Yuan, G.; Zhang, Y.; Yang, N.; Li, Y.; Chen, Q. Development of Encapsulation Strategies Towards the Commercialization of Perovskite Solar Cells. Energy Environ. Sci. 2022, 15, 13–55. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, Z.; Meng, X.; Yue, Y.; Ahmad, M.A.; Zhang, W.; Zhang, S.; Zhang, Y.; Liu, Z.; Chen, W. A Review on Encapsulation Technology from Organic Light Emitting Diodes to Organic and Perovskite Solar Cells. Adv. Funct. Mater. 2021, 31, 2100151. [Google Scholar] [CrossRef]
- Chu, Q.-Q.; Sun, Z.; Wang, D.; Cheng, B.; Wang, H.; Wong, C.-P.; Fang, B. Encapsulation: The Path to Commercialization of Stable Perovskite Solar Cells. Matter 2023, 6, 3838–3863. [Google Scholar] [CrossRef]
- Corsini, F.; Griffini, G. Recent Progress in Encapsulation Strategies to Enhance the Stability of Organometal Halide Perovskite Solar Cells. J. Phys. Energy 2020, 2, 31001. [Google Scholar] [CrossRef]
- Sutherland, L.J.; Weerasinghe, H.C.; Simon, G.P. A Review on Emerging Barrier Materials and Encapsulation Strategies for Flexible Perovskite and Organic Photovoltaics. Adv. Energy Mater. 2021, 11, 2101383. [Google Scholar] [CrossRef]
- Mu, L.; Wang, S.; Liu, H.; Li, W.; Zhu, L.; Wang, H.; Chen, H. Innovative Materials for Lamination Encapsulation in Perovskite Solar Cells. Adv. Funct. Mater. 2024, 35, 2415353. [Google Scholar] [CrossRef]
- Zagorovskaia, K.; Trepalin, O.; Krasionov, I.; Luchkin, S.; Krasnikov, D.V.; Nasibulin, A.G.; Boldyreva, A.G. Improved Operational Lifetime of MAPbI3 Solar Cells Encapsulated with Parylene-N. Sol. RRL 2025, 9, 2400833. [Google Scholar] [CrossRef]
- Raman, R.K.; Ganesan, S.; Alagumalai, A.; Menon, V.S.; Krishnan, S.; Thangavelu, S.A.G.; Krishnamoorthy, A. Facile and Scalable Bilayer Polymer Encapsulation to Achieve Long-Term Stability of Perovskite Solar Cells under Harsh Humidity Conditions. Sustain. Energ. Fuels 2024, 8, 1953–1965. [Google Scholar] [CrossRef]
- Yang, Y.; Song, X.; Liu, H.; Zhang, A.; Cao, J.; Wu, C. Encapsulation-Driven Stability in Perovskite Solar Cells: Suppressing Degradation through Hermetic Sealing. ACS Appl. Mater. Interfaces 2025, 17, 34119–34128. [Google Scholar] [CrossRef]
- Wang, T.; Yang, J.; Cao, Q.; Pu, X.; Li, Y.; Chen, H.; Zhao, J.; Zhang, Y.; Chen, X.; Li, X. Room Temperature Nondestructive Encapsulation via Self-Crosslinked Fluorosilicone Polymer Enables Damp Heat-Stable Sustainable Perovskite Solar Cells. Nat. Commun. 2023, 14, 1342. [Google Scholar] [CrossRef]
- Emami, S.; Martins, J.; Ivanou, D.; Mendes, A. Advanced Hermetic Encapsulation of Perovskite Solar Cells: The Route to Commercialization. J. Mater. Chem. A 2020, 8, 2654–2662. [Google Scholar] [CrossRef]
- Cheacharoen, R.; Rolston, N.; Harwood, D.; Bush, K.A.; Dauskardt, R.H.; McGehee, M.D. Design and Understanding of Encapsulated Perovskite Solar Cells to Withstand Temperature Cycling. Energy Environ. Sci. 2018, 11, 144–150. [Google Scholar] [CrossRef]
- Bush, K.A.; Palmstrom, A.F.; Yu, Z.J.; Boccard, M.; Cheacharoen, P.; Mailoa, J.P.; McMeekin, D.P.; Hoye, R.L.Z.; Bailie, C.D.; Leijtens, T.; et al. 23.6%-Efficient Monolithic Perovskite/Silicon Tandem Solar Cells with Improved Stability. Nat. Energy 2017, 2, 17009. [Google Scholar] [CrossRef]
- Ma, C.; Park, N.-G. A Realistic Methodology for 30% Efficient Perovskite Solar Cells. Chem 2020, 6, 1254–1264. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, S.; Song, H. Photon Management to Reduce Energy Loss in Perovskite Solar Cells. Chem. Soc. Rev. 2021, 50, 7250–7329. [Google Scholar] [CrossRef]
- Jung, S.-K.; Park, N.-G.; Lee, J.-W. Light Management in Perovskite Solar Cells. Mater. Today Energy 2023, 37, 101401. [Google Scholar] [CrossRef]
- Lei, Y.; Li, Y.; Jin, Z. Photon Energy Loss and Management in Perovskite Solar Cells. Energy Rev. 2022, 1, 100003. [Google Scholar] [CrossRef]
- Ji, C.; Liu, W.; Bao, Y.; Chen, X.; Yang, G.; Wei, B.; Yang, F.; Wang, X. Recent Applications of Antireflection Coatings in Solar Cells. Photonics 2022, 9, 906. [Google Scholar] [CrossRef]
- Shanmugam, N.; Pugazhendhi, R.; Elavarasan, R.M.; Kasiviswanathan, P.; Das, N. Anti-Reflective Coating Materials: A Holistic Review from PV Perspective. Energies 2020, 13, 2631. [Google Scholar] [CrossRef]
- Raut, H.K.; Ganesh, V.A.; Nair, A.S.; Ramakrishna, S. Anti-Reflective Coatings: A Critical, In-Depth Review. Energy Environ. Sci. 2011, 4, 779–3804. [Google Scholar] [CrossRef]
- Almuqoddas, E.; Budiawan, W.; Paramudita, I.; Shobih; Yuliarto, B.; Firdaus, Y. Near Fundamental Limit Performance of Inverted Perovskite Solar Cells with Anti-Reflective Coating Integration. Results Opt. 2024, 16, 100670. [Google Scholar] [CrossRef]
- Ma, S.; Tao, L.; Zhang, H.; Qi, C.; Ye, T.; Zhu, X.; Li, B.; Yun, D.-Q.; Sun, X.; Zheng, L. Optical Management of Perovskite Solar Cells by Biomimetic-Structured Polydimethylsiloxane Films. Adv. Eng. Mater. 2024, 26, 2400065. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, U.; Lee, J.; Lee, Y.S.; Choi, M.; Kang, S.M. Bioinspired Sticker-Type Multilayer Anti-Reflective Film for Flexible Perovskite Solar Cells. J. Energy Chem. 2025, 107, 540–547. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, Z.; Wei, H.; Xiao, X.; Ni, Z.; Chen, B.; Deng, Y.; Habisreutinger, S.N.; Chen, X.; Wang, K.; et al. Enhancing Electron Diffusion Length in Narrow-Bandgap Perovskites for Efficient Monolithic Perovskite Tandem Solar Cells. Nat. Commun. 2019, 10, 4498. [Google Scholar] [CrossRef]
- Koufi, A.; Ziat, Y.; Belkhanchi, H. First-Principles DFT and BoltzTraP Investigation of Multifunctional Properties of XNiH3 (X = Li, Na, K) Perovskite Hydrides: Thermoelectric and Hydrogen Storage Potential. Next Energy 2025, 9, 100402. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Kang, J.; Lee, J.-H.; Hong, S. Polymer Functional Layers for Perovskite Solar Cells. Polymers 2025, 17, 2607. https://doi.org/10.3390/polym17192607
Lee J, Kang J, Lee J-H, Hong S. Polymer Functional Layers for Perovskite Solar Cells. Polymers. 2025; 17(19):2607. https://doi.org/10.3390/polym17192607
Chicago/Turabian StyleLee, Jinho, Jaehyeok Kang, Jong-Hoon Lee, and Soonil Hong. 2025. "Polymer Functional Layers for Perovskite Solar Cells" Polymers 17, no. 19: 2607. https://doi.org/10.3390/polym17192607
APA StyleLee, J., Kang, J., Lee, J.-H., & Hong, S. (2025). Polymer Functional Layers for Perovskite Solar Cells. Polymers, 17(19), 2607. https://doi.org/10.3390/polym17192607






