Environmental Applications of Chitosan Derivatives and Chitosan Composites
Abstract
1. Introduction
2. Modification Strategies and Design Principles for Enhanced Environmental Performance of Chitosan
2.1. Modern Strategies for Modifying Chitosan to Enhance Stability, Selectivity, and Efficiency
2.2. Fundamental Design Principles Guiding the Development of High-Performance Chitosan Composites
2.3. Key Mechanisms Underlying Pollutant Removal via Adsorption, Catalysis, Membrane Filtration, and Flocculation
2.4. Practical Challenges Related to Scalability, Regeneration, Lifecycle Sustainability, and Real-World Implementation
2.5. Emerging Trends: Circular Economy Integration, Seafood Waste Valorisation, and Digital Optimization Using Artificial Intelligence
3. Practical Environmental Applications of Chitosan Derivatives and Composites
3.1. Water Treatment
3.1.1. Heavy Metal Removal
3.1.2. Dye Removal
3.1.3. Organic Pollutant Adsorption
3.2. Waste Management
3.2.1. Biodegradable Packaging
3.2.2. Composting Additives
3.3. Soil Remediation
3.3.1. Heavy Metal Immobilization
3.3.2. Pesticide Remediation
3.4. Air Purification
3.5. Air Filtration
3.6. Antimicrobial Activity
3.7. Oil Spill Cleanup
3.8. Other Environmental Applications
4. Discussion
5. Limitations and Future Perspectives
6. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DD | Degree of deacetylation |
| MW | Molecular weight |
| CMCS | Carboxymethyl chitosan |
| ECH | Epichlorhydrin |
| BADGE | Bisphenol A diglycidyl ether |
| PEI | Polyethyleneimide |
| ChNS | Chitosan-nanosilica composites |
| ChSG | Chitosan-silica gel composites |
| GO | Graphene oxide |
| CNTs | Carbon nanotubes |
| NS | Nanosilica |
| SG | Silica gel |
| GA | Glutaraldehyde |
| CBZ | Carbamazepine |
| CIP | Ciprofloxacin |
| SEM | Scanning electron microscopy |
| PDA | Polydopamine |
| TPP | Tripolyphosphate |
| Car/CSAs | Chitosan/carbon ratio in aerogel composites |
| MB | Methylene blue |
| CSGO | Chitosan and graphene oxide membrane |
| MMMs | Mixed matrix membranes |
| MOFs | Metal–organic frameworks |
| LCA | Lifecycle analysis |
| AI | Artificial intelligence |
| ML | Machine learning |
| ANFIS | Adaptive neuro-fuzzy inference systems |
| ANNs | Artificial neural networks |
| RSM | Response surface methodology |
| EDTA | Ethylenediaminetetraacetic acid |
| MIPs | Molecularly imprinted polymers |
| VOCs | Volatile organic compounds |
References
- Maraveas, C. Production of Sustainable and Biodegradable Polymers from Agricultural Waste. Polymers 2020, 12, 1127. [Google Scholar] [CrossRef] [PubMed]
- Ateş, B.; Köytepe, S.; Ulu, A.; Gürses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.O.; Strieder, M.M.; Silva, L.F.O.; dos Reis, G.S.; Dotto, G.L. Advanced Technologies in Water Treatment: Chitosan and Its Modifications as Effective Agents in the Adsorption of Contaminants. Int. J. Biol. Macromol. 2024, 270, 132307. [Google Scholar] [CrossRef]
- Bashir, S.M.; Rather, G.A.; Patrício, A.B.; Haq, Z.; Sheikh, A.A.; Shah, M.Z.U.H.; Singh, H.; Khan, A.A.; Imtiyaz, S.; Ahmad, S.B.; et al. Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications. Materials 2022, 15, 6521. [Google Scholar] [CrossRef] [PubMed]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef]
- Cheba, B.A. Chitosan: Properties, Modifications and Food Nanobiotechnology. Procedia Manuf. 2020, 46, 652. [Google Scholar] [CrossRef]
- Lamm, M.E.; Li, K.; Ker, D.; Zhao, X.; Hinton, H.; Copenhaver, K.; Tekinalp, H.; Ozcan, S. Exploiting Chitosan to Improve the Interface of Nanocellulose Reinforced Polymer Composites. Cellulose 2022, 29, 3859. [Google Scholar] [CrossRef]
- Rasheed, T.; Hassan, A.A.; Bilal, M.; Hussain, T.; Rızwan, K. Metal-Organic Frameworks Based Adsorbents: A Review from Removal Perspective of Various Environmental Contaminants from Wastewater. Chemosphere 2020, 259, 127369. [Google Scholar] [CrossRef]
- da Silva Alves, D.C.; Healy, B.; Pinto, L.A.d.A.; Cadaval, T.R.S., Jr.; Breslin, C.B. Recent Developments in Chitosan-Based Adsorbents for the Removal of Pollutants from Aqueous Environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef]
- Sheraz, M.; Xiao, S.; Siddiqui, A.; Hu, S.; Song, Z. Research Advances in Natural Polymers for Environmental Remediation. Polymers 2025, 17, 559. [Google Scholar] [CrossRef]
- Vidal, J.L.; Jin, T.; Lam, E.; Kerton, F.M.; Moores, A. Blue Is the New Green: Valorization of Crustacean Waste. Curr. Res. Green. Sustain. Chem. 2022, 5, 100330. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.; et al. Characterization of Chitin and Chitosan Derived from Hermetia Illucens, a Further Step in a Circular Economy Process. Sci. Rep. 2022, 12, 6613. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.M.; Jian, G.; Li, H.; Miller, Q.R.S.; Wolcott, M.P.; Fernandez, C.A.; Nassiri, S. Impact of Chitin Nanofibers and Nanocrystals from Waste Shrimp Shells on Mechanical Properties, Setting Time, and Late-Age Hydration of Mortar. Sci. Rep. 2022, 12, 20539. [Google Scholar] [CrossRef] [PubMed]
- Popović, N.T.; Lorencin, V.; Strunjak-Perović, I.; Čož-Rakovac, R. Shell Waste Management and Utilization: Mitigating Organic Pollution and Enhancing Sustainability. Appl. Sci. 2023, 13, 623. [Google Scholar] [CrossRef]
- El-araby, A.; Ghadraoui, L.E.; Errachidi, F. Physicochemical Properties and Functional Characteristics of Ecologically Extracted Shrimp Chitosans with Different Organic Acids during Demineralization Step. Molecules 2022, 27, 8285. [Google Scholar] [CrossRef]
- Mathaba, M.; Daramola, M.O. Effect of Chitosan’s Degree of Deacetylation on the Performance of PES Membrane Infused with Chitosan during AMD Treatment. Membranes 2020, 10, 52. [Google Scholar] [CrossRef]
- Agasty, A.; Wiśniewska, A.; Kalwarczyk, T.; Koynov, K.; Hołyst, R. Macroscopic Viscosity of Polymer Solutions from the Nanoscale Analysis. ACS Appl. Polym. Mater. 2021, 3, 2813. [Google Scholar] [CrossRef]
- Khouri, N.G.; Bahú, J.O.; Blanco-Llamero, C.; Severino, P.; Concha, V.O.C.; Souto, E.B. Polylactic Acid (PLA): Properties, Synthesis, and Biomedical Applications—A Review of the Literature. J. Mol. Struct. 2024, 1309, 138243. [Google Scholar] [CrossRef]
- Xi, Z.; Feng, J.; Lu, X. Interfacial Colloidal Performance and Adhesive Strength of an Environmentally Friendly Cellulose-Microcrystal-Based Adhesive Substance. Am. J. Nanosci. 2020, 6, 24–33. [Google Scholar] [CrossRef]
- Fatullayeva, S.S.; Tagiyev, D.B.; Zeynalov, N.A.; Mammadova, S.; Aliyeva, E. Recent Advances of Chitosan-Based Polymers in Biomedical Applications and Environmental Protection. J. Polym. Res. 2022, 29, 259. [Google Scholar] [CrossRef]
- Cao, W.; Yan, J.; Liu, C.; Zhang, J.; Wang, H.; Gao, X.; Yan, H.; Niu, B.; Li, W. Preparation and Characterization of Catechol-Grafted Chitosan/Gelatin/Modified Chitosan-AgNP Blend Films. Carbohydr. Polym. 2020, 247, 116643. [Google Scholar] [CrossRef]
- Chen, X.; Wilson, L.D. An Overview of the Design of Chitosan-Based Fiber Composite Materials. J. Compos. Sci. 2021, 5, 160. [Google Scholar] [CrossRef]
- El-araby, A.; Janati, W.; Ullah, R.; Erċışlı, S.; Errachidi, F. Chitosan, Chitosan Derivatives, and Chitosan-Based Nanocomposites: Eco-Friendly Materials for Advanced Applications (a Review). Front. Chem. 2024, 11, 1327426. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.; Journot, C.M.A.; Gerber-Lemaire, S. Chitosan Functionalization: Covalent and Non-Covalent Interactions and Their Characterization. Polymers 2021, 13, 4118. [Google Scholar] [CrossRef]
- Fernandez-Zelaia, P.; Kirka, M.M.; Dryepondt, S.; Gussev, M.N. Crystallographic Texture Control in Electron Beam Additive Manufacturing via Conductive Manipulation. Mater. Des. 2020, 195, 109010. [Google Scholar] [CrossRef]
- Bhagath, S.; Vivek, A.; Krishna, V.V.; Mittal, S.S.; Balachandran, M. Synthesis and Characteristics of MMT Reinforced Chitosan Nanocomposite. Mater. Today Proc. 2020, 46, 4487. [Google Scholar] [CrossRef]
- Bolan, S.; Hou, D.; Wang, L.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Li, R.; Wang, B.; Xu, J.; Wang, T.; et al. The Potential of Biochar as a Microbial Carrier for Agricultural and Environmental Applications. Sci. Total Environ. 2023, 886, 163968. [Google Scholar] [CrossRef]
- Haghighi, P.; Hekmati, M.; Ziyadi, H.; Ghasemi, E.; Esmaeili, D. Hibiscus Sabdariffa Extract Modified Magnetic Polymer Nanocomposite for Azo Dyes Removal from Aqueous Samples. Mater. Chem. Phys. 2021, 267, 124608. [Google Scholar] [CrossRef]
- Bashir, T.; Dutta, J.; Masarat, S.; Kyzas, G.Z. Formulation and Characterization of Lignin Modified Chitosan Beads. Adsorption 2024, 30, 947. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, F.; Xu, K.; Che, Y.; Qi, M.; Song, C. Modified Magnetic Chitosan Materials for Heavy Metal Adsorption: A Review. RSC Adv. 2023, 13, 6713. [Google Scholar] [CrossRef]
- Prasad, R.; Nisha, P.V.; Khan, H.; Jarin, T. Sustainable Regeneration of Transformer Oil through Composite Adsorbent of Chitosan and Sepiolite: A Biorefinery Approach. Energy Source Part A 2024, 46, 10861–10884. [Google Scholar] [CrossRef]
- Olafadehan, O.A.; Bello, V.E.; Amoo, K.O. Production and Characterization of Composite Nanoparticles Derived from Chitosan, CTAB and Bentonite Clay. Chem. Pap. 2022, 76, 5063. [Google Scholar] [CrossRef]
- Wu, Z.; Ye, X.; Liu, H.; Zhang, H.; Liu, Z.; Guo, M.; Li, Q.; Li, J. Interactions between Adsorbents and Adsorbates in Aqueous Solutions. Pure Appl. Chem. 2020, 92, 1655. [Google Scholar] [CrossRef]
- Xu, X.; Li, R.; Chen, J.; Yang, J.; Wu, Y.; Liu, J.; Huang, Y.; Chen, S.; Ye, X.; Wang, W. Enhancing the Phosphate Adsorption of a Polyallylamine Resin in Alkaline Environments by Lanthanum Oxalate Modification. ACS Omega 2022, 7, 19743. [Google Scholar] [CrossRef] [PubMed]
- Thambiliyagodage, C.; Jayanetti, M.; Mendis, A.; Ekanayake, G.; Liyanaarachchi, H.; Vigneswaran, S. Recent Advances in Chitosan-Based Applications—A Review. Materials 2023, 16, 2073. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, M.; Jankowska, K.; Klak, M.; Wszoła, M. Chitosan as an Underrated Polymer in Modern Tissue Engineering. Nanomaterials 2021, 11, 3019. [Google Scholar] [CrossRef]
- Rokhati, N.; Prasetyaningrum, A.; Aji, R.W.; Hamada, N.A. The Use of Chitosan as Non-Toxic Flocculant for Harvesting Microalgae Spirulina sp. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Surakarta, Indonesia, 29–30 September 2020; IOP Publishing: Bristol, UK, 2021; Volume 828, p. 12009. [Google Scholar]
- Vincent, I.; Lee, E.-C.; Kim, H.-M. Solutions to the Water Flooding Problem for Unitized Regenerative Fuel Cells: Status and Perspectives. RSC Adv. 2020, 10, 16844. [Google Scholar] [CrossRef]
- Priya; Naveen; Kaur, K.; Sidhu, A.K. Green Synthesis: An Eco-Friendly Route for the Synthesis of Iron Oxide Nanoparticles. Front. Nanotechnol. 2021, 3, 655062. [Google Scholar] [CrossRef]
- Song, J.; Cao, X.; Huang, Z. Diatomite-Chitosan Composite with Abundant Functional Groups as Efficient Adsorbent for Vanadium Removal: Key Influencing Factors and Influence of Surface Functional Groups. J. Mol. Liq. 2022, 367, 120428. [Google Scholar] [CrossRef]
- Castiello, C.; Junghanns, P.; Mergel, A.M.; Jacob, C.; Ducho, C.; Valente, S.; Rotili, D.; Fioravanti, R.; Zwergel, C.; Mai, A. GreenMedChem: The Challenge in the next Decade toward Eco-Friendly Compounds and Processes in Drug Design. Green. Chem. 2023, 25, 2109. [Google Scholar] [CrossRef]
- Agrawal, K.; Goktas, P.; Holtkemper, M.; Beecks, C.; Kumar, N. AI-Driven Transformation in Food Manufacturing: A Pathway to Sustainable Efficiency and Quality Assurance. Front. Nutr. 2025, 12, 1553942. [Google Scholar] [CrossRef]
- Michalik, R.; Wandzik, I. A Mini-Review on Chitosan-Based Hydrogels with Potential for Sustainable Agricultural Applications. Polymers 2020, 12, 2425. [Google Scholar] [CrossRef] [PubMed]
- Abouelnaga, A.M.; Mansour, A.M.; Hammad, A.B.A.; Nahrawy, A.M.E. Optimizing Magnetic, Dielectric, and Antimicrobial Performance in Chitosan-PEG-Fe2O3@NiO Nanomagnetic Composites. Int. J. Biol. Macromol. 2024, 260, 129545. [Google Scholar] [CrossRef]
- Sundaramoorthy, N.; Ramya, G.; Thasneem, A.L.; Kaviarasi, D.; Hemapriya, J.; Aswini, R.; Vijayanand, S. Biomedical Applications of Chitosan and Its Derivatives—A Review. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 3636–3643. [Google Scholar] [CrossRef]
- Pathak, K.; Misra, S.K.; Sehgal, A.; Singh, S.; Bungău, S.; Najda, A.; Gruszecki, R.; Behl, T. Biomedical Applications of Quaternized Chitosan. Polymers 2021, 13, 2514. [Google Scholar] [CrossRef] [PubMed]
- Ekapakul, N.; Sinthuvanich, C.; Ajiro, H.; Choochottiros, C. Bioactivity of Star-Shaped Polycaprolactone/Chitosan Composite Hydrogels for Biomaterials. Int. J. Biol. Macromol. 2022, 212, 420. [Google Scholar] [CrossRef]
- Ding, S.; Wang, Y.; Li, J.; Chen, S. Progress and Prospects in Chitosan Derivatives: Modification Strategies and Medical Applications. J. Mater. Sci. Technol. 2020, 89, 209. [Google Scholar] [CrossRef]
- Hu, J.; Chen, K.; Xiang, M.; Wei, J.; Zeng, Y.; Qin, Y.; Zhang, L.; Zhang, W. A Novel Sponge Composite of Chitosan-Sodium Tripolyphosphate-Melamine for Anionic Dye Orange II Removal. Int. J. Biol. Macromol. 2024, 270, 132056. [Google Scholar] [CrossRef] [PubMed]
- Federer, C.; Kurpiers, M.; Bernkop–Schnürch, A. Thiolated Chitosans: A Multi-Talented Class of Polymers for Various Applications. Biomacromolecules 2021, 22, 24–56. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, K.; Zhao, L.; Peng, H.; Gong, Y. EDAC-Modified Chitosan/Imidazolium-Polysulfone Composite Beads for Removal of Cr(VI) from Aqueous Solution. Int. J. Biol. Macromol. 2024, 278, 134876. [Google Scholar] [CrossRef]
- Węgrzynowska-Drzymalska, K.; Grebicka, P.; Młynarczyk, D.T.; Chełminiak-Dudkiewicz, D.; Kaczmarek, H.; Gośliński, T.; Ziegler-Borowska, M. Crosslinking of Chitosan with Dialdehyde Chitosan as a New Approach for Biomedical Applications. Materials 2020, 13, 3413. [Google Scholar] [CrossRef]
- Pedige, M.P.H.; Asoh, T.; Hsu, Y.; Uyama, H. Stimuli-Responsive Composite Hydrogels with Three-Dimensional Stability Prepared Using Oxidized Cellulose Nanofibers and Chitosan. Carbohydr. Polym. 2021, 278, 118907. [Google Scholar] [CrossRef]
- Hastuti, B.; Siswanta, D.; Mudasir, M.; Triyono, T. Adsorption and Characterization of Pb(II) Ion onto Porogen Adsorbent Membrane (CC-Pec-BADGENa): Kinetic, Equilibrium and Thermodynamic Studies. Rasayan J. Chem. 2020, 13, 2325. [Google Scholar] [CrossRef]
- Moon, S.H.; Hwang, H.J.; Jeon, H.R.; Park, S.J.; Bae, I.S.; Yang, Y.J. Photocrosslinkable Natural Polymersin Tissue Engineering. Front. Bioeng. Biotechnol. 2023, 11, 1127757. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.T.T.; Giang, N.T.K.; Ha, N.N.; Lan, P.T. Understanding the Interaction in Cellulose–Chitosan Composite and Its Adsorption Ability for Nickel (II): A Theoretical Investigation. Mol. Simul. 2023, 49, 1303–1310. [Google Scholar] [CrossRef]
- Yan, Y.; Nagappan, S.; Yoo, J.; Nechikkattu, R.; Park, S.S.; Ha, C. Polyethyleneimine-Grafted Polysilsesquioxane Hollow Spheres for the Highly Efficient Removal of Anionic Dyes and Selective Adsorption of Cr(VI). J. Environ. Chem. Eng. 2021, 9, 104814. [Google Scholar] [CrossRef]
- Zhang, Y.; Mo, Y.; Vincent, T.; Faur, C.; Guibal, E. Boosted Cr(VI) Sorption Coupled Reduction from Aqueous Solution Using Quaternized Algal/alginate@PEI Beads. Chemosphere 2021, 281, 130844. [Google Scholar] [CrossRef]
- Motlagh, L.; Shabani, S.; Ghaderzadeh, S. Adsorption of Thiophene Using Chitosan Functionalized Silica as a Biopolymer Composite. Adsorption 2024, 30, 1153–1160. [Google Scholar] [CrossRef]
- Seidi, F.; Saeb, M.R.; Huang, Y.; Akbari, A.; Xiao, H. Thiomers of Chitosan and Cellulose: Effective Biosorbents for Detection, Removal and Recovery of Metal Ions from Aqueous Medium. Chem. Rec. 2021, 21, 1876. [Google Scholar] [CrossRef]
- Lončarević, A.; Ivanković, M.; Rogina, A. Electrosprayed Chitosan–Copper Complex Microspheres with Uniform Size. Materials 2021, 14, 5630. [Google Scholar] [CrossRef]
- Azhar, A.S.; Kamis, W.Z.W.; Hamid, H.A.; Kassim, N.F.A.; Isa, N. Removal of Tartrazine Dye Using Kyllinga Brevifolia Extract And Silver Nanoparticles As Catalysts. J. Phys. Conf. Ser. 2021, 2129, 12033. [Google Scholar] [CrossRef]
- Sharifi, M.J.; Nouralishahi, A.; Hallajisani, A.; Askari, M. Magnetic Chitosan Nanocomposites as Adsorbents in Industrial Wastewater Treatment: A Brief Review. Cell Chem. Technol. 2021, 55, 185. [Google Scholar] [CrossRef]
- Pérez-Moreno, A.; Reyes-Peces, M.V.; Vilches-Pérez, J.I.; Fernández-Montesinos, R.; Pinaglia-Tobaruela, G.; Salido, M.; de la Rosa-Fox, N.; Piñero, M. Effect of Washing Treatment on the Textural Properties and Bioactivity of Silica/Chitosan/TCP Xerogels for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 8321. [Google Scholar] [CrossRef]
- He, X.; Mao, H.; Wang, S.; Tian, Z.; Zhou, T.; Cai, L. Fabrication of Chitosan/Phenylboronic Acid/SiO2 Hydrogel Composite Silk Fabrics for Enhanced Adsorption and Controllable Release on Luteolin. Int. J. Biol. Macromol. 2023, 248, 125926. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.S.; Afroze, S.; Кутербекoв, K.A.; Kabyshev, A.; Бекмырза, K.; Haque, M.N.; Islam, S.N.; Hossain, M.A.; Hassan, M.; Roy, H.; et al. Advanced Applications of Carbonaceous Materials in Sustainable Water Treatment, Energy Storage, and CO2 Capture: A Comprehensive Review. Sustainability 2023, 15, 8815. [Google Scholar] [CrossRef]
- Simonescu, C.M.; Tătăruş, A.; Culiță, D.C.; Stănică, N.; Ionescu, I.A.; Butoi, B.; Banici, A.-M. Comparative Study of CoFe2O4 Nanoparticles and CoFe2O4-Chitosan Composite for Congo Red and Methyl Orange Removal by Adsorption. Nanomaterials 2021, 11, 711. [Google Scholar] [CrossRef]
- Guo, X.; Wang, L.; Wang, L.; Huang, Q.; Bu, L.; Wang, Q. Metal-Organic Frameworks for Food Contaminant Adsorption and Detection. Front. Chem. 2023, 11, 1116524. [Google Scholar] [CrossRef]
- Sacco, P.; Pedroso-Santana, S.; Kumar, Y.; Joly, N.; Martin, P.; Bocchetta, P. Ionotropic Gelation of Chitosan Flat Structures and Potential Applications. Molecules 2021, 26, 660. [Google Scholar] [CrossRef] [PubMed]
- García-Castro, M.; Moscoso, A.; Sarabia, F.; López-Romero, J.M.; Contreras-Cáceres, R.; Díaz, A. Nanoscale Biocompatible Structures Generated from Fluorinated Tripodal Phenylenes on Gold Nanoprisms. ChemistryOpen 2022, 11, e202200007. [Google Scholar] [CrossRef]
- Berillo, D.; Al-Jwaid, A.K.; Caplin, J. Polymeric Materials Used for Immobilisation of Bacteria for the Bioremediation of Contaminants in Water. Polymers 2021, 13, 1073. [Google Scholar] [CrossRef]
- Fabiano, A.; Beconcini, D.; Migone, C.; Piras, A.M.; Zambito, Y. Quaternary Ammonium Chitosans: The Importance of the Positive Fixed Charge of the Drug Delivery Systems. Int. J. Mol. Sci. 2020, 21, 6617. [Google Scholar] [CrossRef]
- De Freitas, E.D.; de Moura, C.F.; Kerwald, J.; Beppu, M.M. An Overview of Current Knowledge on the Properties, Synthesis and Applications of Quaternary Chitosan Derivatives. Polymers 2020, 12, 2878. [Google Scholar] [CrossRef] [PubMed]
- Darie-Niţă, R.N.; Irimia, A.; Doroftei, F.; Ştefan, L.M.; Iwańczuk, A.; Trusz, A. Bioactive and Physico-Chemical Assessment of Innovative Poly(Lactic Acid)-Based Biocomposites Containing Sage, Coconut Oil, and Modified Nanoclay. Int. J. Mol. Sci. 2023, 24, 3646. [Google Scholar] [CrossRef]
- Qu, B.; Luo, Y. A Review on the Preparation and Characterization of Chitosan-Clay Nanocomposite Films and Coatings for Food Packaging Applications. Carbohydr. Polym. Tech. 2021, 2, 100102. [Google Scholar] [CrossRef]
- Prado-Audelo, M.L.D.; Caballero-Florán, I.H.; Sharifi-Rad, J.; Mendoza-Muñoz, N.; González-Torres, M.; Urbán-Morlán, Z.; Florán, B.; Cortés, H.; Leyva-Gómez, G. Chitosan-Decorated Nanoparticles for Drug Delivery. J. Drug Deliv. Sci. Tech. 2020, 59, 101896. [Google Scholar] [CrossRef]
- Saheed, I.O.; Oh, W.; Suah, F.B.M. Chitosan Modifications for Adsorption of Pollutants—A Review. J. Haz. Mater. 2020, 408, 124889. [Google Scholar] [CrossRef]
- Sahajwalla, V.; Hossain, R. Rethinking Circular Economy for Electronics, Energy Storage, and Solar Photovoltaics with Long Product Life Cycles. MRS Bull. 2023, 48, 375. [Google Scholar] [CrossRef]
- Nikita, K.; Vijayakumar, V.; Nam, S.Y. Chitosan-Based Membranes: A Comprehensive Review of Nanofiltration, Pervaporation, and Ion Exchange Applications. Polysaccharides 2025, 6, 31. [Google Scholar] [CrossRef]
- Tanjung, F.A.; Kuswardani, R.A.; Idumah, C.I.; Siregar, J.P.; Karim, A. Characterization of Mechanical and Thermal Properties of Esterified Lignin Modified Polypropylene Composites Filled with Chitosan Fibers. Mater. Polym. Compos. 2022, 30, 09673911221082482. [Google Scholar] [CrossRef]
- Picos-Corrales, L.A.; Morales-Burgos, A.M.; Ruelas-Leyva, J.P.; Crini, G.; García-Armenta, E.; Jiménez-Lam, S.A.; Ayón-Reyna, L.E.; Rocha-Alonzo, F.; Calderón-Zamora, L.; Osuna-Martínez, U.; et al. Chitosan as an Outstanding Polysaccharide Improving Health-Commodities of Humans and Environmental Protection. Polymers 2023, 15, 526. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, H.; Zheng, G.; Mei, C.; Cai, L.; Le, Q.V.; Xia, C. Multilayered Nanocomposites Prepared through Quadruple-Layering Approach towards Enhanced Mechanical Performance. Molecules 2022, 27, 4852. [Google Scholar] [CrossRef]
- Badagliacco, D.; Fiore, V.; Sanfilippo, C.; Valenza, A. Effectiveness of Sodium Acetate Treatment on the Mechanical Properties and Morphology of Natural Fiber-Reinforced Composites. J. Compos. Sci. 2021, 6, 5. [Google Scholar] [CrossRef]
- Chen, C.-M.; Chang, H.-L.; Lee, C. Improvement Prediction on the Dynamic Performance of Epoxy Composite Used in Packaging by Using Nano-Particle Reinforcements in Addition to 2-Hydroxyethyl Methacrylate Toughener. Materials 2021, 14, 4193. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, T. Synthesis and Characterization of New Chitosan-Based Nanocomposite Gel and Its Application towards Dye Removal. arXiv 2021. [Google Scholar] [CrossRef]
- Gan, P.G.; Sam, S.T.; Omar, M.S.; Abdullah, M.F. Effect of Glutaraldehyde as Crosslinker on the Properties of Cellulose Nanocrystal/Chitosan Films. In Proceedings of the IOP Conference Series Materials Science and Engineering, Pahang, Malaysia, 30 March 2020; IOP Publishing: Bristol, UK, 2020; Volume 957, p. 12038. [Google Scholar]
- Jin, L.; Chen, Q.; Hu, X.; Chen, H.; Lu, Y.; Zhang, Y.; Zhou, H.; Bai, Y. Enhanced Mechanical Strength and Antibacterial Properties of Chitosan/Graphene Oxide Composite Fibres. Cellulose 2022, 29, 3889. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, L.; Zhang, X.; Chen, L.; Cai, Q.; Yang, X. Hierarchical and Heterogeneous Hydrogel System as a Promising Strategy for Diversified Interfacial Tissue Regeneration. Biomat Sci. 2020, 9, 1547. [Google Scholar] [CrossRef]
- Lakshmi, D.S.; Radha, K.; Castro-Muñoz, R.; Tańczyk, M. Emerging Trends in Porogens toward Material Fabrication: Recent Progresses and Challenges. Polymers 2022, 14, 5209. [Google Scholar] [CrossRef] [PubMed]
- Zaman, H.G.; Baloo, L.; Kutty, S.R.; Aziz, K.; Altaf, M.; Ashraf, A.; Aziz, F. Insight into Microwave-assisted Synthesis of the chitosan-MOF Composite: Pb(II) Adsorption. Environ. Sci. Pollut. Res. Int. 2022, 30, 6216–6233. [Google Scholar] [CrossRef]
- Hartoyo, A.P.P.; Aulia, F.; Muhammad, D.N.; Puspitasari, E.; Setiawan, Y.; Sudrajat, D.J.; Naufal, H.; Carolina, A.; Solikhin, A. Peatland Plants Growth Performance of Valorized Oil Palm Microfibers Infiltrated in Chitosan/NPK and Chitosan/PMAA/NPK Composite. Bioresour. Technol. Rep. 2023, 23, 101580. [Google Scholar] [CrossRef]
- Wu, Q.; Xiao, B.; Li, Y.; Yao, R.; Jin, D.; Lei, Y.; Yang, D.; Zhu, J. Bioactive Chitosan/Polydopamine Nanospheres Coating on Carbon Fiber towards Strengthening Epoxy Composites. Int. J. Biol. Macromol. 2024, 275, 133568. [Google Scholar] [CrossRef]
- Ali, A.; Andriyana, A. Properties of Multifunctional Composite Materials Based on Nanomaterials: A Review. RSC Adv. 2020, 10, 16390. [Google Scholar] [CrossRef]
- Jalageri, M.B.; Kumar, G.C.M. Graphene Oxide Reinforced Polyvinyl Alcohol/Chitosan Composite Hydrogel for Cartilage Regeneration. Polym. Bull. 2024, 81, 10915. [Google Scholar] [CrossRef]
- Politaeva, N.; Yakovlev, A.V.; Yakovleva, E.V.; Chelysheva, V.; Tarantseva, K.; Efremova, S.; Mukhametova, L.; Ilyashenko, S. Graphene Oxide-Chitosan Composites for Water Treatment from Copper Cations. Water 2022, 14, 1430. [Google Scholar] [CrossRef]
- Yasin, A.; Hussain, T.; Shuaib, U.; Mubarik, F.E.; Amjad, M.; Ahmad, S.; Shakir, I. Alcohothermal Synthesis and Characterization of Chitosan Supported Nickel Cobaltite Composite for Enhanced Photocatalytic and Antibacterial Activity. Bionanoscience 2024, 14, 1033–1043. [Google Scholar] [CrossRef]
- Bujnicki, B.; Sowiński, P.; Makowski, T.; Krasowska, D.; Pokora-Sobczak, P.; Shkyliuk, I.; Drabowicz, J.; Piórkowska, E. Microbiologically Pure Cotton Fabrics Treated with Tetrabutylammonium OXONE as Mild Disinfection Agent. Materials 2022, 15, 7749. [Google Scholar] [CrossRef]
- Zhang, C.; Hui, D.; Du, C.; Sun, H.; Wei, P.; Pu, X.; Li, Z.; Sun, J.; Zhou, C. Preparation and Application of Chitosan BioMaterials in Dentistry. Int. J. Biol. Macromol. 2020, 167, 1198. [Google Scholar] [CrossRef]
- Nike, D.U.; Fadilah, N.I.M.; Sallehuddin, N.; Azlan, A.Y.H.N.; Imran, F.-H.; Maarof, M.; Fauzi, M.B. Genipin-Crosslinking Effects on Biomatrix Development for Cutaneous Wound Healing: A Concise Review. Front. Bioeng. Biotechnol. 2022, 10, 865014. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A.; Olewnik-Kruszkowska, E.; Reczyńska, K.; Pamuła, E. Is Dialdehyde Chitosan a Good Substance to Modify Physicochemical Properties of Biopolymeric Materials? Int. J. Mol. Sci. 2021, 22, 3391. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Y.; Tang, Q.; Li, J.; Dou, X.; Gou, D.; Liu, T. Starch–Chitosan Composite Films for the Effective Removal of Protein in Water. Biomass Convers. Biorefinery 2023, 14, 16403–16413. [Google Scholar] [CrossRef]
- Shrigandhi, G.D.; Kothavale, B.S. Biodegradable Composites for Filament Winding Process. Mater. Today Proc. 2021, 42, 2762. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Katapodis, P.; Leontiou, A.; Moschovas, D.; Karydis-Messinis, A. Development of ZnO/Na-Montmorillonite Hybrid Nanostructures Used for PVOH/ZnO/Na-Montmorillonite Active Packaging Films Preparation via a Melt-Extrusion Process. Nanomaterials 2020, 10, 1079. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, X.; Yu, Z.; Sun, C.; Shan, T.; Zhang, Z. Non-Crosslinked Systems Modulate the Gel Behavior and Structural Properties of Chitosan/Silica Composite Aerogels. Int. J. Biol. Macromol. 2024, 264, 130630. [Google Scholar] [CrossRef]
- Dobrzyński, W.; Piszko, P.J.; Kiryk, J.; Kiryk, S.; Michalak, M.; Kotela, A.; Kensy, J.; Świenc, W.; Grychowska, N.; Matys, J.; et al. Dental Resin Composites Modified with Chitosan: A Systematic Review. Mar. Drugs 2025, 23, 199. [Google Scholar] [CrossRef]
- Taylor, T.S.; Appiah–Effah, E.; Akodwaa-Boadi, K.; Obeng, E.; Ofei-Quartey, M.N.L. Engineered Column Treatment of Greywater Using Raw and Pyrolyzed Coconut Husk Powder. Front. Environ. Sci. 2023, 11, 1077379. [Google Scholar] [CrossRef]
- Pavlidis, G.; Zotou, I.; Karasali, H.; Marousopoulou, A.; Bariamis, G.; Nalbantis, I.; Tsihrintzis, V.A. Experiments on Pilot-Scale Constructed Floating Wetlands Efficiency in Removing Agrochemicals. Toxics 2022, 10, 790. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, T.R.; Prelot, B. Adsorption Processes for the Removal of Contaminants from Wastewater. In Elsevier eBooks; Elsevier BV: Amsterdam, The Netherlands, 2020; p. 161. [Google Scholar]
- Eninanç, O.; Baybaş, D.; Ulusoy, U. Ternary Composite of Polyacrylamide-Chitosan-Montmorillonite: Characterization and Adsorptive Features for Thorium and BSA. J. Mol. Struct. 2024, 1321, 140065. [Google Scholar] [CrossRef]
- Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M.; Anitha, K. Recent Advances in Heavy Metal Removal by Chitosan Based Adsorbents. Carbohydr. Polym. 2020, 251, 117000. [Google Scholar] [CrossRef]
- Moktar, A.; Daaou, M.; Bendraoua, A.; Morsli, A. Study of Cu2+, Zn2+ and Mn2+ Ions Adsorption Using Asphaltenes-Chitosan Composite Beads. Mater. Today Commun. 2022, 33, 104928. [Google Scholar] [CrossRef]
- Gahrouei, A.E.; Rezapour, A.; Pirooz, M.; Pourebrahimi, S. From Classic to Cutting-Edge Solutions: A Comprehensive Review of Materials and Methods for Heavy Metal Removal from Water Environments. Desalin Water Treat. 2024, 319, 100446. [Google Scholar] [CrossRef]
- Riyanti, F.; Hariani, P.L.; Fatma, F.; Yuliasari, N.; Said, M.; Ramadiati, T. Synthesis of Chitosan-SiO2 Composite for Adsorption Methyl Dyes from Solution. In Proceedings of the IOP Conference Series Materials Science and Engineering, Bandar Lampung, Indonesia, 23–25 September 2019; IOP Publishing: Bristol, UK, 2020; Volume 857, p. 12001. [Google Scholar]
- Kausar, A.; Ijaz, S.; Rafaqat, M.; Dahshan, A.; Latif, A.; Bibi, S.; Al-Kadhi, N.S.; Alissa, S.A.; Nazir, A.; Iqbal, M. Chitosan-Cellulose Composite for the Adsorptive Removal of Anionic Dyes: Experimental and Theoretically Approach. J. Mol. Liq. 2023, 391, 123347. [Google Scholar] [CrossRef]
- Lujanienė, G.; Novikau, R.; Joel, E.F.; Karalevičiūtė, K.; Šemčuk, S.; Mažeika, K.; Talaikis, M.; Pakštas, V.; Tumėnas, S.; Mažeika, J.; et al. Preparation of Graphene Oxide-Maghemite-Chitosan Composites for the Adsorption of Europium Ions from Aqueous Solutions. Molecules 2022, 27, 8035. [Google Scholar] [CrossRef]
- Li, L.; Zhao, L.; Ma, J.; Tian, Y. Preparation of Graphene Oxide/Chitosan Complex and Its Adsorption Properties for Heavy Metal Ions. Green. Process Synth. 2020, 9, 294. [Google Scholar] [CrossRef]
- Smržová, D.; Szatmáry, L.; Ecorchard, P.; Machálková, A.; Maříková, M.; Salačová, P.; Straka, M. Carbon and Zeolite-Based Composites for Radionuclide and Heavy Metal Sorption. Heliyon 2022, 8, e12293. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.-B.; Zeng, J.; Zhang, Z.; Ma, S.; Tang, C.; Xu, J. Preparation of Polyethyleneimine-Modified Chitosan/Ce-UIO-66 Composite Hydrogel for the Adsorption of Methyl Orange. Carbohydr. Polym. 2022, 299, 120079. [Google Scholar] [CrossRef] [PubMed]
- Burdzy, K.; Chen, Y.-G.; Lv, G.; Chen, S.-H.; Kołodyńska, D. Application of Ion Exchangers with the N-Methyl-D-Glucamine Groups in the V(V) Ions Adsorption Process. Materials 2022, 15, 1026. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-Z.; Chu, R.; Zhang, Q.; Usman, M.; Haider, F.U.; Cai, L. Nano Zero-Valent Iron Loaded Corn-Straw Biochar for Efficient Removal of Hexavalent Chromium: Remediation Performance and Interfacial Chemical Behaviour. RSC Adv. 2022, 12, 26953. [Google Scholar] [CrossRef]
- Finčur, N.; Sfîrloagă, P.; Putnik, P.; Despotović, V.; Lazarević, M.; Uzelac, M.M.; Abramović, B.; Vlăzan, P.; Ianăşi, C.; Alapi, T.; et al. Removal of Emerging Pollutants from Water Using Environmentally Friendly Processes: Photocatalysts Preparation, Characterization, Intermediates Identification and Toxicity Assessment. Nanomaterials 2021, 11, 215. [Google Scholar] [CrossRef]
- Hsu, C.; Ajaj, Y.; Mahmoud, Z.H.; Ghadir, G.K.; Alani, Z.K.; Hussein, M.M.; Hussein, S.A.; Karim, M.M.; Al-khalidi, A.; Abbas, J.K.; et al. Adsorption of Heavy Metal Ions Use Chitosan/Graphene Nanocomposites: A Review Study. Results Chem. 2024, 7, 101332. [Google Scholar] [CrossRef]
- Leonardi, M.; Caruso, G.; Carroccio, S.; Boninelli, S.; Curcuruto, G.; Zimbone, M.; Allegra, M.; Torrisi, B.; Ferlito, F.; Miritello, M. Smart Nanocomposites of Chitosan/Alginate Nanoparticles Loaded with Copper Oxide as Alternative Nanofertilizers. Environ. Sci. Nano 2021, 8, 174. [Google Scholar] [CrossRef]
- Jeong, E.; Woo, H.; Moon, Y.; Lee, D.Y.; Jung, M.-J.; Lee, Y.; Bae, J.-S. Self-Cleaning Polyester Fabric Prepared with TiOF2 and Hexadecyltrimethoxysilane. Polymers 2021, 13, 387. [Google Scholar] [CrossRef]
- Taha, A.; Da’na, E. Phyto-Assisted Assembly of Metal Nanoparticles in Chitosan Matrix Using S. argel Leaf Extract and Its Application for Catalytic Oxidation of Benzyl Alcohol. Polymers 2022, 14, 766. [Google Scholar] [CrossRef]
- Grzybek, P.; Jakubski, Ł.; Dudek, G. Neat Chitosan Porous Materials: A Review of Preparation, Structure Characterization and Application. Int. J. Mol. Sci. 2022, 23, 9932. [Google Scholar] [CrossRef]
- Liang, H.; Lv, C.; Chen, H.; Wu, L.; Hou, X. Facile Synthesis of Chitosan Membranes for Visible-Light-Driven Photocatalytic Degradation of Tetracycline Hydrochloride. RSC Adv. 2020, 10, 45171. [Google Scholar] [CrossRef] [PubMed]
- Nalatambi, S.; Oh, K.S.; Yoon, L.W.; Tee, L.H. Evaluation of Antibacterial Property and Greywater Treatment Performance Using Composite Chitosan/Graphene Oxide Membrane. Mater. Chem. Phys. 2022, 295, 127160. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, X.; Ji, Y.; Hao, Y.; Liao, S.; Cui, Z.; Li, J.; Younas, M.; He, B. pH-Responsive Nanofiltration Membrane Containing Chitosan for Dye Separation. J. Membr. Sci. 2021, 635, 119445. [Google Scholar] [CrossRef]
- Hao, S.; Jia, Z.; Wen, J.; Li, S.; Peng, W.; Huang, R.; Xu, X. Progress in Adsorptive Membranes for Separation—A Review. Sep. Purif. Technol. 2020, 255, 117772. [Google Scholar] [CrossRef]
- Liu, S.; Mukai, Y. Selective Adsorption and Separation of Proteins by Ligand-Modified Nanofiber Fabric. Polymers 2021, 13, 2313. [Google Scholar] [CrossRef]
- Ola, A.T.T.; Rahmat, R.; Fahri, A.N.; Heryanto, H.; Mutmainna, I.; Sesa, E.; Tahir, D. Synergistic Effect of Chitosan and Activated Carbon (AC) in Suppressing Recombination Charge of Composite Ca2Fe2O5 −AC/ Chitosan for High Photodegradation of Fipronil Wastewater. J. Polym. Environ. 2022, 30, 3218–3229. [Google Scholar] [CrossRef]
- Kaurin, T.; Čurlin, M.; Šaravanja, A.; Vojnović, B.; Pušić, T. Design of Chitosan-Polyester Composites to Reduce Particulate Contamination of Washing Wastewater. Water 2023, 15, 2418. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Q.; Kou, J.; Sun, C.; Li, H. Aggregation Mechanism of Colloidal Kaolinite in Aqueous Solutions with Electrolyte and Surfactants. PLoS ONE 2020, 15, e0238350. [Google Scholar] [CrossRef]
- Panda, P.K.; Dash, P.; Yang, J.; Chang, Y. Development of Chitosan, Graphene Oxide, and Cerium Oxide Composite Blended Films: Structural, Physical, and Functional Properties. Cellulose 2022, 29, 2399. [Google Scholar] [CrossRef]
- Xu, Y.; Gan, K.; Liang, S.; Liu, H.; Wang, Q. Investigation and Optimization of Chitosan Performance in Flocculating Kaolin Suspensions Using a Real-Time Suspending Solid Concentration Measuring Method. Water 2021, 13, 513. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Jiang, W.; Wu, M.; Li, Z. Comprehensive Review of Floc Growth and Structure Using Electrocoagulation: Characterization, Measurement, and Influencing Factors. Chem. Eng. J. 2021, 417, 129310. [Google Scholar] [CrossRef]
- Bhatt, P.; Joshi, S.; Bayram, G.M.U.; Khati, P.; Şimşek, H. Developments and Application of Chitosan-Based Adsorbents for Wastewater Treatments. Environ. Res. 2023, 226, 115530. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Eberhart, M.S.; Martinson, A.B.F.; Tiede, D.M.; Mulfort, K.L. Molecularly Functionalized Electrodes for Efficient Electrochemical Water Remediation. ChemSusChem 2021, 14, 3267. [Google Scholar] [CrossRef]
- Costa, C.G.; Bom, L.F.R.P.; Martins, C.R.; da Silva, C.F.; de Moraes, M.A. (Bio)Composites of Chitin/Chitosan with Natural Fibers. In Elsevier eBooks; Elsevier BV: Amsterdam, The Netherlands, 2020; p. 273. [Google Scholar]
- Junceda-Mena, I.; García-Junceda, E.; Revuelta, J. From the Problem to the Solution: Chitosan Valorization Cycle. Carbohydr. Polym. 2023, 309, 120674. [Google Scholar] [CrossRef]
- Tonndorf, R.; Aibibu, D.; Cherif, C. Isotropic and Anisotropic Scaffolds for Tissue Engineering: Collagen, Conventional, and Textile Fabrication Technologies and Properties. Int. J. Mol. Sci. 2021, 22, 9561. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, C.; Kaneti, Y.V.; Eguchi, M.; Lin, J.; Yamauchi, Y.; Na, J. Practical MOF Nanoarchitectonics: New Strategies for Enhancing the Processability of MOFs for Practical Applications. Langmuir 2020, 36, 4231. [Google Scholar] [CrossRef] [PubMed]
- Biehl, P.; Schacher, F.H. Surface Functionalization of Magnetic Nanoparticles Using a Thiol-Based Grafting-Through Approach. Surfaces 2020, 3, 116–131. [Google Scholar] [CrossRef]
- Alqarni, L.S.; Alghamdi, M.D.; Alshahrani, A.A.; Nassar, A. Green Nanotechnology: Recent Research on Bioresource-Based Nanoparticle Synthesis and Applications. J. Chem. 2022, 1, 4030999. [Google Scholar] [CrossRef]
- Gamage, A.; Jayasinghe, N.; Thiviya, P.; Wasana, M.L.D.; Merah, O.; Madhujith, T.; Koduru, J.R. Recent Application Prospects of Chitosan Based Composites for the Metal Contaminated Wastewater Treatment. Polymers 2023, 15, 1453. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.K.; Naglah, A.M. Nanoarchitectonics of Chitosan/Glutaraldehyde/Zinc Oxide as a Novel Composite for the Efficient Removal of Eriochrome Black T Dye from Aqueous Media. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2022. [Google Scholar] [CrossRef]
- Lamanna, L.; Giacoia, G.; Friuli, M.; Leone, G.; Carlucci, N.; Russo, F.; Sannino, A.; Demitri, C. Oil–Water Emulsion Flocculation through Chitosan Desolubilization Driven by pH Variation. ACS Omega 2023, 8, 20708. [Google Scholar] [CrossRef]
- Li, C.; Shi, M.; Xu, D.; Liao, Q.; Liu, G.; Guo, Y.; Zhang, H.; Zhu, H. Fabrication of Photo-Fenton Self-Cleaning PVDF Composite Membrane for Highly Efficient Oil-in-Water Emulsion Separation. RSC Adv. 2022, 12, 35543. [Google Scholar] [CrossRef]
- Salman, S.; Sheikh, C.; Hasan, M.; Hasan, N.; Kubra, K.T.; Rehan, A.I.; Awual, E.; Rasee, A.I.; Waliullah, R.; Hossain, M.S.; et al. Chitosan-Coated Cotton Fiber Composite for Efficient Toxic Dye Encapsulation from Aqueous Media. Appl. Surf. Sci. 2023, 622, 157008. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Cheng, Q.; Wang, C.; Li, H.; Han, X.; Fan, Z.; Su, G.; Pan, D.; Li, Z. Research Progress of Adsorption and Removal of Heavy Metals by Chitosan and Its Derivatives: A Review. Chemosphere 2021, 279, 130927. [Google Scholar] [CrossRef]
- Su, X. Electrochemical Separations for Metal Recycling. Electrochem. Soc. Interf. 2020, 29, 55. [Google Scholar] [CrossRef]
- Datiles, W.C.P.; Herrera, M.U.; Manalo, R.D.; Maguyon-Detras, M.C.; Futalan, C.M.; Balela, M.D.L. Kapok-Cotton Carbon Sponges for Oil Recovery. In Proceedings of the IOP Conference Series Earth and Environmental Science, Virtual, 2–4 April 2024; IOP Publishing: Bristol, UK, 2021; Volume 812, p. 12014. [Google Scholar]
- Patel, H. Elution Profile of Cationic and Anionic Adsorbate from Exhausted Adsorbent Using Solvent Desorption. Sci. Rep. 2022, 12, 1665. [Google Scholar] [CrossRef]
- Rimu, S.H.; Rahman, M.M. Insight of Chitosan-Based Nanocomposite for Removal of Hexavalent Chromium from Wastewater—A Review. Int. J. Environ. Anal. Chem. 2020, 102, 6801. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, S. A Waste Recycling Method Based on the Life Cycle Analysis of Products. Mob. Int. Syst. 2022, 1, 3590826. [Google Scholar] [CrossRef]
- Alves, D.I.; Barreiros, M.P.; Fangueiro, R.; Ferreira, D.P. Valorization of Textile Waste: Non-Woven Structures and Composites. Front. Environ. Sci. 2024, 12, 1365162. [Google Scholar] [CrossRef]
- Majiya, H.; Clegg, F.; Sammon, C. Bentonite-Chitosan Composites or Beads for Lead (Pb) Adsorption: Design, Preparation, and Characterisation. Appl. Clay Sci. 2023, 246, 107180. [Google Scholar] [CrossRef]
- Cataldo, S.; Muratore, N.; Giannici, F.; Bongiorno, D.; Chiodo, V.; Maisano, S.; Pettignano, A. Hydrocarbons Removal from Synthetic Bilge Water by Adsorption onto Biochars of Dead Posidonia Oceanica. Environ. Sci. Pollut. Res. 2022, 29, 90231. [Google Scholar] [CrossRef] [PubMed]
- Belgis, B. Industrial Application of Chitosan as Promising Material for Wastewater Purification: A Review. CSID J. Infrastruct. Dev. 2020, 3, 51–63. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, K.; Zhu, A.; Meng, Z.; Li, W.; Qin, C. Preparation of Bentonite/Chitosan Composite for Bleaching of Deteriorating Transformer Oil. Polymers 2020, 12, 60. [Google Scholar] [CrossRef]
- Hameed, A.Z.; Raj, S.A.; Kandasamy, J.; Baghdadi, M.A.; Shahzad, M.A. Chitosan: A Sustainable Material for Multifarious Applications. Polymers 2022, 14, 2335. [Google Scholar] [CrossRef]
- Macheca, A.D.; Mutuma, B.K.; Adalima, J.L.; Midheme, E.; Lucas, L.H.M.; Ochanda, V.K.; Mhlanga, S.D. Perspectives on Plastic Waste Management: Challenges and Possible Solutions to Ensure Its Sustainable Use. Recycling 2024, 9, 77. [Google Scholar] [CrossRef]
- Hossain, S.; Hossain, F. Chitosan: An Effective Material for Textile Waste Water Management. Int. J. Adv. Res. 2020, 8, 26. [Google Scholar] [CrossRef]
- Konstantopoulos, G.; Koumoulos, E.P.; Charitidis, C.A. Digital Innovation Enabled Nanomaterial Manufacturing; Machine Learning Strategies and Green Perspectives. Nanomaterials 2022, 12, 2646. [Google Scholar] [CrossRef]
- Willenbacher, M.; Scholten, J.; Wohlgemuth, V. Machine Learning for Optimization of Energy and Plastic Consumption in the Production of Thermoplastic Parts in SME. Sustainability 2021, 13, 6800. [Google Scholar] [CrossRef]
- Jia, F.; Yin, S.; Chen, L.; Chen, X. The Circular Economy in the Textile and Apparel Industry: A Systematic Literature Review. J. Clean. Prod. 2020, 259, 120728. [Google Scholar] [CrossRef]
- Rai, S.; Pokhrel, P.; Udash, P.; Chemjong, M.; Bhattarai, N.; Thuanthong, A.; Nalinanon, S.; Nirmal, N.P. Chitin and Chitosan from Shellfish Waste and Their Applications in Agriculture and Biotechnology Industries. Crit. Rev. Biotechnol. 2025, 1, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, D.S.; Ravindran, L.; Mary, P.J.M.; Rathika, G.; Sreekala, M.S.; Munisamy, S. Valorization of Crustacean Shells: Preparation, Characterization, and Chemical Modification of Nano-Chitin Biopolymer Membrane for Application in Water Purification. Waste Biomass Valorization 2024, 15, 6067–6090. [Google Scholar] [CrossRef]
- Chavez, M.Y. The Sustainability of Industrial Insect Mass Rearing for Food and Feed Production: Zero Waste Goals through by-Product Utilization. Curr. Opin. Insect Sci. 2021, 48, 44. [Google Scholar] [CrossRef] [PubMed]
- Luangapai, F.; Iwamoto, S. Influence of Blending and Layer-by-Layer Assembly Methods on Chitosan–Gelatin Composite Films Enriched with Curcumin Nanoemulsion. Int. J. Biol. Macromol. 2023, 249, 126061. [Google Scholar] [CrossRef]
- Tan, Y.N.; Lee, P.P.; Chen, W.N. Microbial Extraction of Chitin from Seafood Waste Using Sugars Derived from Fruit Waste-Stream. AMB Express 2020, 10, 17. [Google Scholar] [CrossRef]
- Arnold, N.D.; Brück, W.M.; Garbe, D.; Brück, T. Enzymatic Modification of Native Chitin and Conversion to Specialty Chemical Products. Mar. Drugs 2020, 18, 93. [Google Scholar] [CrossRef]
- Islam, M.; Huang, Y.; Islam, S.; Fan, B.; Tong, L.; Wang, F. Influence of the Degree of Hydrolysis on Functional Properties and Antioxidant Activity of Enzymatic Soybean Protein Hydrolysates. Molecules 2022, 27, 6110. [Google Scholar] [CrossRef]
- Shen, N.; Yang, M.; Xie, C.; Pan, J.; Pang, K.; Zhang, H.; Wang, Y.; Jiang, M. Isolation and Identification of a Feather Degrading Bacillus Tropicus Strain Gxun-17 from Marine Environment and Its Enzyme Characteristics. BMC Biotechnol. 2022, 22, 11. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Wang, C.-L.; Wang, S. Microbial Conversion of Shrimp Heads to Proteases and Chitin as an Effective Dye Adsorbent. Polymers 2020, 12, 2228. [Google Scholar] [CrossRef]
- Venugopal, V.; Sasidharan, A. Functional Proteins through Green Refining of Seafood Side Streams. Front. Nutr. 2022, 9, 974447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, L.; Li, C.; Xie, X.; Li, G.; Hu, Z.; Li, S. Research Progress of Chitosan-Based Biomimetic Materials. Mar. Drugs 2021, 19, 372. [Google Scholar] [CrossRef]
- Pal, K.; Bharti, D.; Sarkar, P.; Anis, A.; Kim, D.; Chałas, R.; Maksymiuk, P.; Stachurski, P.; Jarzębski, M. Selected Applications of Chitosan Composites. Int. J. Mol. Sci. 2021, 22, 10968. [Google Scholar] [CrossRef]
- Zewude, D.A.; Izawa, H.; Ifuku, S. Optimization of Chitin Nanofiber Preparation by Ball Milling as Filler for Composite Resin. J. Compos. Sci. 2022, 6, 197. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An Overview on Engineering the Surface Area and Porosity of Biochar. Sci. Total Environ. 2020, 763, 144204. [Google Scholar] [CrossRef]
- Shobuke, H.; Matsumoto, T.; Hirosawa, F.; Miyagawa, M.; Takaba, H. Estimation of Adsorbed Amounts in Organoclay by Machine Learning. ACS Omega 2023, 8, 1146–1153. [Google Scholar] [CrossRef]
- Nighojkar, A.; Zimmermann, K.; Ateia, M.; Barbeau, B.; Mohseni, M.; Krishnamurthy, S.; Dixit, F.; Kandasubramanian, B. Application of Neural Network in Metal Adsorption Using BioMaterials (BMs): A Review. Environ. Sci. Adv. 2022, 2, 11. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Watwe, V.S.; Chavan, T.B.; Kulkarni, S.D. Artificial Neural Networking for Remediation of Methylene Blue Dye Using Fuller’s Earth Clay. Curr. Res. Green. Sustain. Chem. 2021, 4, 100131. [Google Scholar] [CrossRef]
- Cheng, Q.; Chunhong, Z.; Qianglin, L. Development and Application of Random Forest Regression Soft Sensor Model for Treating Domestic Wastewater in a Sequencing Batch Reactor. Sci. Rep. 2023, 13, 9149. [Google Scholar] [CrossRef] [PubMed]
- Ateş, A.; Demirel, H.; Altıntığ, E.; Bozdag, D.; Usta, Y.; Özçelik, T.O. Artificial Neural Network Modeling of the Removal of Methylene Blue Dye Using Magnetic Clays: An Environmentally Friendly Approach. Processes 2024, 12, 2262. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, J.; Miller-Hooks, E.; Zhou, W.; Chen, C.; Lee, L.H.; Chew, E.P.; Li, H. Analytics with Digital-Twinning: A Decision Support System for Maintaining a Resilient Port. Decis. Support Syst. 2021, 143, 113496. [Google Scholar] [CrossRef]
- Torres, K.; Álvarez-Hornos, F.J.; Gabaldón, C.; Marzal, P. Start-Up of Chitosan-Assisted Anaerobic Sludge Bed Reactors Treating Light Oxygenated Solvents under Intermittent Operation. Int. J. Environ. Res. Public Health 2021, 18, 4986. [Google Scholar] [CrossRef]
- Cordeiro, M.; Ferreira, J.C. Beyond Traceability: Decentralised Identity and Digital Twins for Verifiable Product Identity in Agri-Food Supply Chains. Appl. Sci. 2025, 15, 6062. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Abakah, E.J.A.; Le, T.N.L.; Hiz, D.I.L. la Markov-Switching Dependence between Artificial Intelligence and Carbon Price: The Role of Policy Uncertainty in the Era of the 4th Industrial Revolution and the Effect of COVID-19 Pandemic. Technol. Forecast. Soc. Change 2020, 163, 120434. [Google Scholar] [CrossRef]
- Ababneh, S.; Al-Araidah, O.; Almomani, M.A. An Analytic Hierarchy Process Model for Selecting Adsorbent for Heavy Metal Ion Removal from Wastewater. Water SA 2020, 46, 493–499. [Google Scholar] [CrossRef]
- Fournier-Tombs, É. Local Transplantation, Adaptation, and Creation of AI Models for Public Health Policy. Front. Artif. Intell. 2023, 6, 1085671. [Google Scholar] [CrossRef]
- Titirici, M.; Baird, S.G.; Sparks, T.D.; Yang, S.M.; Brandt-Talbot, A.; Hosseinaei, O.; Harper, D.P.; Parker, R.; Vignolini, S.; Berglund, L.A.; et al. The Sustainable Materials Roadmap. J. Phys. Mater. 2022, 5, 32001. [Google Scholar] [CrossRef]
- Khattab, H.; Gawish, A.A.; Gomaa, S.; Hamdy, A.; El-hoshoudy, A.N. Assessment of Modified Chitosan Composite in Acidic Reservoirs through Pilot and Field-Scale Simulation Studies. Sci. Rep. 2024, 14, 10634. [Google Scholar] [CrossRef]
- Yan, X.; Ge, H. Preparation of Metal Organic Frameworks Modified Chitosan Composite with High Capacity for Hg(II) Adsorption. Int. J. Biol. Macromol. 2023, 232, 123329. [Google Scholar] [CrossRef]
- Na, Y.; Lee, J.; Lee, S.H.; Kumar, P.; Kim, J.H.; Patel, R. Removal of Heavy Metals by Polysaccharide: A Review. Polym. Plast. Tech. Mat. 2020, 59, 1770. [Google Scholar] [CrossRef]
- Cao, X.; Zhou, C.; Wang, S.; Man, R. Adsorption Properties for La(III), Ce(III), and Y(III) with Poly(6-Acryloylamino-Hexyl Hydroxamic Acid) Resin. Polymers 2020, 13, 3. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Kim, S.; Igalavithana, A.D.; Hashimoto, Y.; Choi, Y.-E.; Mukhopadhyay, R.; Sarkar, B.; Ok, Y.S. Fe(III) Loaded Chitosan-Biochar Composite Fibers for the Removal of Phosphate from Water. J. Haz. Mater. 2021, 415, 125464. [Google Scholar] [CrossRef]
- Saleh, N.J.; Sulttan, S.; Khazm, A.J. Removal of Hexavalent Chromium Ions from Aqeous Medium Using Chitosan Magnetic Nanocomposite Modified with Hexamine. Diyala J. Eng. Sci. 2020, 13, 34. [Google Scholar] [CrossRef]
- Sareło, P.; Wiśniewska-Wrona, M.; Sikora, M.; Mielan, B.; Gerasymchuk, Y.; Wędzyńska, A.; Boiko, V.; Hreniak, D.; Szymonowicz, M.; Sobieszczańska, B.; et al. Development and Evaluation of Graphene Oxide-Enhanced Chitosan Sponges as a Potential Antimicrobial Wound Dressing for Infected Wound Management. Int. J. Mol. Sci. 2025, 26, 7403. [Google Scholar] [CrossRef]
- Kiralj, A.; Tomić, Ž.; Hadnadjev-Kostic, M.; Lukić, N.; Vulić, T.; Grahovac, J.; Jokić, A. Application of the Adsorbent CR-100 for Ammonium Removal: Thermodynamic and Kinetic Studies. Croat. Chem. Acta 2021, 94, 201–212. [Google Scholar] [CrossRef]
- Cojocaru, C.; Humelnicu, A.C.; Pascariu, P.; Samoilă, P. Artificial Neural Network and Molecular Modeling for Assessing the Adsorption Performance of a Hybrid Alginate-Based Magsorbent. J. Mol. Liq. 2021, 337, 116406. [Google Scholar] [CrossRef]
- Lei, C.; Wang, C.; Chen, W.; He, M.; Huang, B. Polyaniline@magnetic Chitosan Nanomaterials for Highly Efficient Simultaneous Adsorption and In-Situ Chemical Reduction of Hexavalent Chromium: Removal Efficacy and Mechanisms. Sci. Total Environ. 2020, 733, 139316. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Calderón, J.; Santos, M.V.; Zaritzky, N. Synthesis, Characterization and Application of Cross-Linked Chitosan/Oxalic Acid Hydrogels to Improve Azo Dye (Reactive Red 195) Adsorption. React. Funct. Polym. 2020, 155, 104699. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Dreiss, C.A. Hydrogel Design Strategies for Drug Delivery. Curr. Opin. Colloid. Interface Sci. 2020, 48, 1. [Google Scholar] [CrossRef]
- Shi, P.; Hu, X.; Duan, M. A UIO-66/Tannic Acid/Chitosan/Polyethersulfone Hybrid Membrane-like Adsorbent for the Dynamic Removal of Dye and Cr (VI) from Water. J. Clean. Prod. 2021, 290, 125794. [Google Scholar] [CrossRef]
- Shoemaker, B.A.; Domingues, T.S.; Haji-Akbari, A. Ideal Conductor Model: An Analytical Finite-Size Correction for Nonequilibrium Molecular Dynamics Simulations of Ion Transport through Nanoporous Membranes. J. Chem. Theor. Comput. 2022, 18, 7142. [Google Scholar] [CrossRef]
- Gomri, M.; Abderrazak, H.; Chabbah, T.; Souissi, R.; Saint-Martin, P.; Casabianca, H.; Chatti, S.; Merçier, R.; Errachid, A.; Hammami, M.A.; et al. Adsorption Characteristics of Aromatic Pollutants and Their Halogenated Derivatives on Bio-Based Poly (Ether-Pyridine)s. J. Environ. Chem. Eng. 2020, 8, 104333. [Google Scholar] [CrossRef]
- Santillo, C.; Wang, Y.; Buonocore, G.G.; Gentile, G.; Verdolotti, L.; Kačiulis, S.; Xia, H.; Lavorgna, M. Hybrid Graphenene Oxide/Cellulose Nanofillers to Enhance Mechanical and Barrier Properties of Chitosan-Based Composites. Front. Chem. 2022, 10, 926364. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, M.M.; Hammad, S.F.; Abdella, A.A.; Mansour, F.R. Chitosan-Based Molecular Imprinted Polymer for Extraction and Spectrophotometric Determination of Ketorolac in Human Plasma. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 241, 118668. [Google Scholar] [CrossRef]
- Shi, B.; Hao, Z.; Du, Y.; Jia, M.; Xie, S. Mechanical and Barrier Properties of Chitosan-Based Composite Film as Food Packaging: A Review. Bioresources 2024, 19, 4001. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, G.; Zhou, C.; Pan, Q.; He, Z.; Wang, C.; Liu, Y.; Song, S.; Yu, L.; Qu, Y.; et al. Amphiphilic Chitosan/Carboxymethyl Gellan Gum Composite Films Enriched with Mustard Essential Oil for Mango Preservation. Carbohydr. Polym. 2022, 300, 120290. [Google Scholar] [CrossRef]
- Yan, R.; Liu, M.; Zeng, X.; Du, Q.; Wu, Z.; Guo, Y.; Tu, M.; Pan, D. Preparation of Modified Chitosan-Based Nano-TiO2–Nisin Composite Packaging Film and Preservation Mechanism Applied to Chilled Pork. Int. J. Biol. Macromol. 2024, 269, 131873. [Google Scholar] [CrossRef] [PubMed]
- Haddar, A.; Sellami, E.; Bouazizi, O.; Sila, A.; Bougatef, A. Biodegradable Levan/Chitosan Composite Films: Development and Application in Beef Filet Packaging. Foods 2025, 14, 2133. [Google Scholar] [CrossRef]
- Mustika, R.D.; Wardana, A.A. Nanocomposite Coating Based on Chitosan and ZnO Nanoparticles to Maintain the Storage Quality of Meatball. Food Res. 2020, 4, 1867. [Google Scholar] [CrossRef]
- Silva, F.A.G.S.; Dourado, F.; Gama, M.; Poças, F. Nanocellulose Bio-Based Composites for Food Packaging. Nanomater 2020, 10, 2041. [Google Scholar]
- Rkhaila, A.; Abla, E.H.; Idrissi, M.B.; Ounine, K. The Amendment with Chitin and/or Chitosan Improves the Germination and Growth of Lycopersicon esculentum L., Capsicum annuum L. and solanum MELONGENA L. Ind. J. Agric. Res. 2020, 54, 420–428. [Google Scholar] [CrossRef]
- Gumelar, M.D.; Hamzah, M.; Hidayat, A.S.; Saputra, D.A.; Idvan. Utilization of Chitosan as Coating Material in Making NPK Slow Release Fertilizer. Macromol. Symp. 2020, 391, 1900188. [Google Scholar] [CrossRef]
- Clough, T.J.; Cardenas, L.M.; Friedl, J.; Wolf, B. Nitrous Oxide Emissions from Ruminant Urine: Science and Mitigation for Intensively Managed Perennial Pastures. Curr. Opin. Environ. Sust. 2020, 47, 21. [Google Scholar] [CrossRef]
- Ren, X.; Zhu, B.; Bah, H.; Raza, S.T. How Tillage and Fertilization Influence Soil N2O Emissions after Forestland Conversion to Cropland. Sustainability 2020, 12, 7947. [Google Scholar] [CrossRef]
- Shahrokh, V.; Martínez-Martínez, S.; Faz, Á.; Zornoza, R.; Acosta, J.A. Efficiency of Large-Scale Aided Phytostabilization in a Mining Pond. Environ. Geochem. Health 2023, 45, 4665. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, Κ.; Mikulewicz, M. The Combined Rhizoremediation by a Triad: Plant-Microorganism-Functional Materials. Environ. Sci. Poll. Res. 2023, 30, 90500–90521. [Google Scholar] [CrossRef]
- Adamczuk, A.; Józefaciuk, G. Impact of Chitosan on the Mechanical Stability of Soils. Molecules 2022, 27, 2273. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Marzec-Grządziel, A.; Makowska, M.; Kolasa-Więcek, A.; Ranjitha, J.; Kałuża, T.; Pilarski, K. The Use of Chitosan/Perlite Material for Microbial Support in Anaerobic Digestion of Food Waste. Materials 2025, 18, 3504. [Google Scholar] [CrossRef]
- Antunes, J.C.; Domingues, J.; Miranda, C.S.; Silva, A.F.G.; Homem, N.C.; de Amorim, M.T.P.; Felgueiras, H.P. Bioactivity of Chitosan-Based Particles Loaded with Plant-Derived Extracts for Biomedical Applications: Emphasis on Antimicrobial Fiber-Based Systems. Mar. Drugs 2021, 19, 359. [Google Scholar] [CrossRef] [PubMed]
- Khubiev, O.M.; Egorov, A.R.; Kirichuk, A.A.; Khrustalev, V.N.; Tskhovrebov, A.G.; Kritchenkov, A.S. Chitosan-Based Antibacterial Films for Biomedical and Food Applications. Int. J. Mol. Sci. 2023, 24, 10738. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Rodrigues, C.; Valente, S.; Pimenta, C.; Pires, J.R.A.; Alves, M.M.; Santos, C.; Coelhoso, I.M.; Fernando, A.L.A. da C. Eco-Friendly ZnO/Chitosan Bionanocomposites Films for Packaging of Fresh Poultry Meat. Coatings 2020, 10, 110. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan Based Nanocomposite Films and Coatings: Emerging Antimicrobial Food Packaging Alternatives. Trends Food Sci. Technol. 2020, 97, 196. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; de Farias, B.S.; Cadaval, T.R.S.; de Almeida Pinto, L.A.; Diaz, P.S. Chitosan–Based Nanofibers for Enzyme Immobilization. Int. J. Biol. Macromol. 2021, 183, 1959. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-F.; Chuang, C.-Y.; Yang, S. Evaluation of the Bioaerosol Inactivation Ability of Chitosan-Coated Antimicrobial Filters. Int. J. Environ. Res. Public Health 2021, 18, 7183. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, S.; Pramanik, J.; Sivamaruthi, B.S.; Jayeoye, T.J.; Prajapati, B.G.; Chaiyasut, C. Chitosan-Based Composites: Development and Perspective in Food Preservation and Biomedical Applications. Polymers 2023, 15, 3150. [Google Scholar] [CrossRef] [PubMed]
- Mondal, I.H.; Ahmed, F.; Roknuzzaman; Huda, N.; Habib, A. Antimicrobial Activity of Chitosan and Its Derivatives Exhausted Cotton Fabrics as Ecofriendly Antimicrobial Agents. J. Text. Eng. Fash. Technol. 2020, 6, 77–80. [Google Scholar] [CrossRef]
- Toan, L.V.; Thong, N.H.; Quan, D.; Huan, P.V.; Trang, T.T.; Thuy, V.T.P.; Giang, N.T.P.; Tam, P.D.; Hung, N.V.; Pham, V.-H. Synthesis of Polyethylene Glycol–Chitosan–Nano Ag Composites and Their Antibacterial Properties. J. Appl. Spectrosc. 2022, 89, 482. [Google Scholar] [CrossRef]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial Nanoparticles: Current Landscape and Future Challenges. RSC Pharm. 2024, 1, 388. [Google Scholar] [CrossRef]
- Chylińska, M.; Kaczmarek, H. N-Halamine Hydantoin-Containing Chitosan: Synthesis, Characterization, Thermal and Photolytic Stability Studies. Molecules 2020, 25, 3728. [Google Scholar] [CrossRef]
- Dumitru, C.D.; Ilie, C.-I.; Neacşu, I.A.; Motelică, L.; Oprea, O.; Totan, A.; Pițuru, S.; Bălașea, B.V.; Marinescu, F.; Andronescu, E. Antimicrobial Composite Films Based on Alginate–Chitosan with Honey, Propolis, Royal Jelly and Green-Synthesized Silver Nanoparticles. Int. J. Mol. Sci. 2025, 26, 6809. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Nie, W.; Zheng, H.; Wang, Q.; Long, Y. Study on Oil Absorption Properties of Magnetic Chitosan Stearic Acid Compound. J. Phys. Conf. Ser. 2021, 2076, 12031. [Google Scholar] [CrossRef]
- Wen, H.; Raza, S.; Wang, P.; Zhu, Z.; Zhang, J.; Huang, W.; Liang, L.; Hu, H.; Deng, L.; Liu, C. Robust Super Hydrophobic Cotton Fabrics Functionalized with Ag and PDMS for Effective Antibacterial Activity and Efficient Oil–Water Separation. J. Environ. Chem. Eng. 2021, 9, 106083. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, F.; Pan, K.; Zhao, Y.; Ma, L.; Wei, S. Studies on Kinetics, Isotherms, Thermodynamics and Adsorption Mechanism of Methylene Blue by N and S Co-Doped Porous Carbon Spheres. Nanomaterials 2021, 11, 1819. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, Y.; Du, Q.; Chen, B.; Chen, K.; Zhang, Y.; Wang, M.; Sun, Y.; Zhao, S.; Jing, Z.; et al. Efficient Adsorption of Congo Red by MIL-53(Fe)/Chitosan Composite Hydrogel Spheres. Microporous Mesoporous Mater. 2023, 348, 112404. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, W.; Zhang, Y.; Zhang, B.; Jin, Y.; Chen, S.; Tang, S.; Su, Y.; Yu, X.; Chen, G. Chitosan/Magnetic Biochar Composite with Enhanced Reusability: Synergistic Effect of Functional Groups and Multilayer Structure. Arab. J. Chem. 2024, 17, 105746. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.-F.; Zeng, J.; Zhang, Y.; Zhang, Z.-B.; Zhang, Z.-J.; Ma, S.; Tang, C.-M.; Xu, J.-Q. Chitosan/Polyethyleneimine Magnetic Hydrogels for Adsorption of Heavy Metal Ions. Iran. Polym. J. 2022, 31, 1273–1282. [Google Scholar] [CrossRef]
- Patel, P.K.; Pandey, L.M.; Uppaluri, R.V.S. Cyclic Desorption Based Efficacy of Polyvinyl Alcohol-Chitosan Variant Resins for Multi Heavy-Metal Removal. Int. J. Biol. Macromol. 2023, 242, 124812. [Google Scholar] [CrossRef]
- Gupta, A.; Chauhan, V.S.; Sankararamakrishnan, N. Preparation and Evaluation of Iron–Chitosan Composites for Removal of As(III) and As(V) from Arsenic Contaminated Real Life Groundwater. Water Res. 2009, 43, 3862–3870. [Google Scholar] [CrossRef]
- Ozudogru, Y.; Tekne, E. Adsorption of Methylene Blue from Aqueous Solution Using Spent Coffee/Chitosan Composite. J. Water Chem. Technol. 2023, 45, 234–245. [Google Scholar] [CrossRef]
- Billah, R.E.K.; Ayouch, I.; Abdellaoui, Y.; Kassab, Z.; Khan, M.A.; Agunaou, M.; Soufiane, A.; Otero, M.; Jeon, B.-H. A Novel Chitosan/Nano-Hydroxyapatite Composite for the Adsorptive Removal of Cd(II) from Aqueous Solution. Polymers 2023, 15, 1524. [Google Scholar] [CrossRef]
- Salah, T.A.; Mohammad, A.M.; Hassan, M.A.; El-Anadouli, B.E. Development of Nano-Hydroxyapatite/Chitosan Composite for Cadmium Ions Removal in Wastewater Treatment. J. Taiwan Inst. Chem. Eng. 2014, 45, 1571–1577. [Google Scholar] [CrossRef]
- Prando, J.; Reinehr, I.L.; Visioli, L.J.; Paulino, A.T.; Enzweiler, H. Photolysis, Photocatalysis, and Sorption of Caffeine in Aqueous Media in the Presence of Chitosan Membrane and Chitosan/TiO2 Composite Membrane. Processes 2015, 13, 2439. [Google Scholar] [CrossRef]

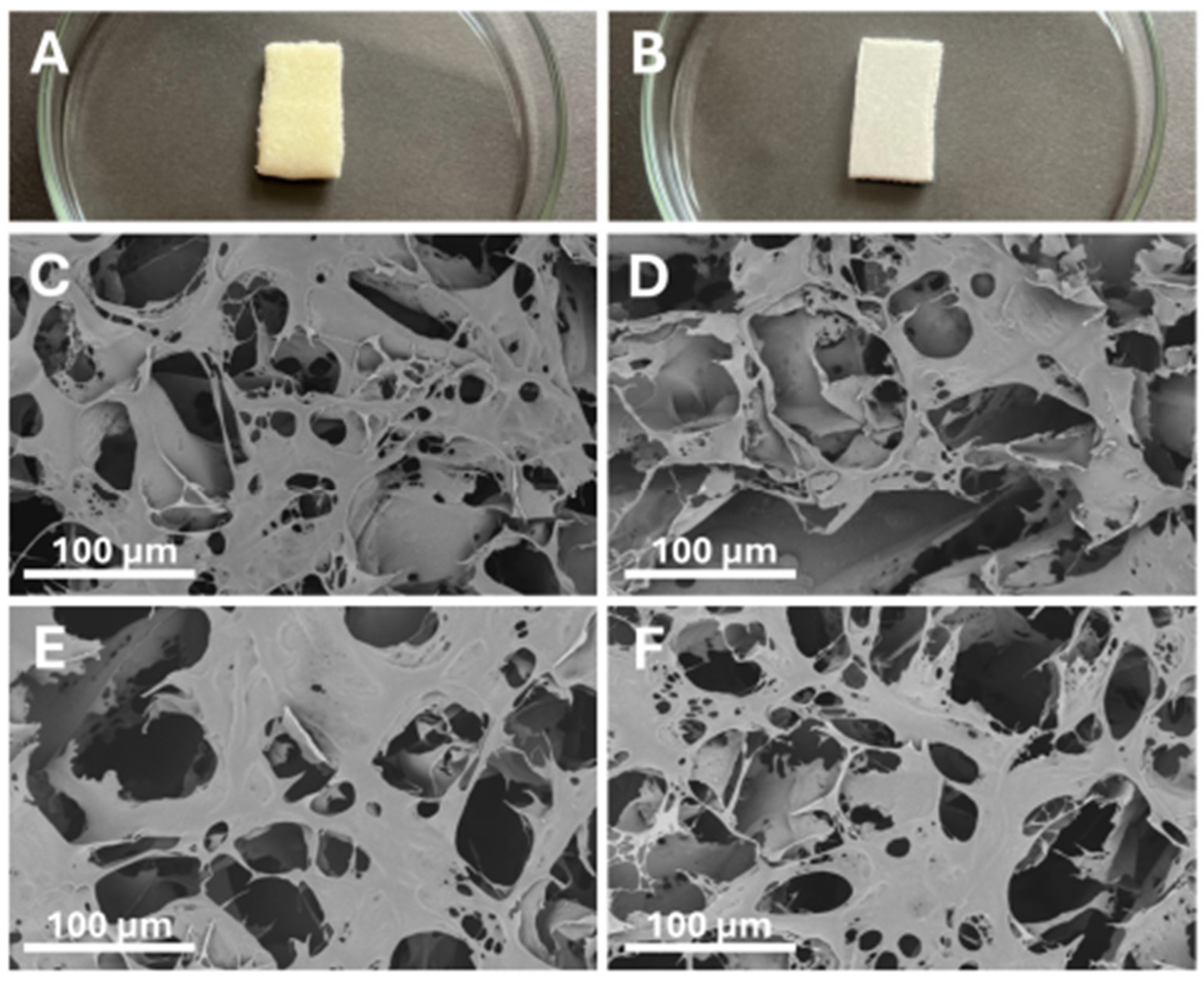

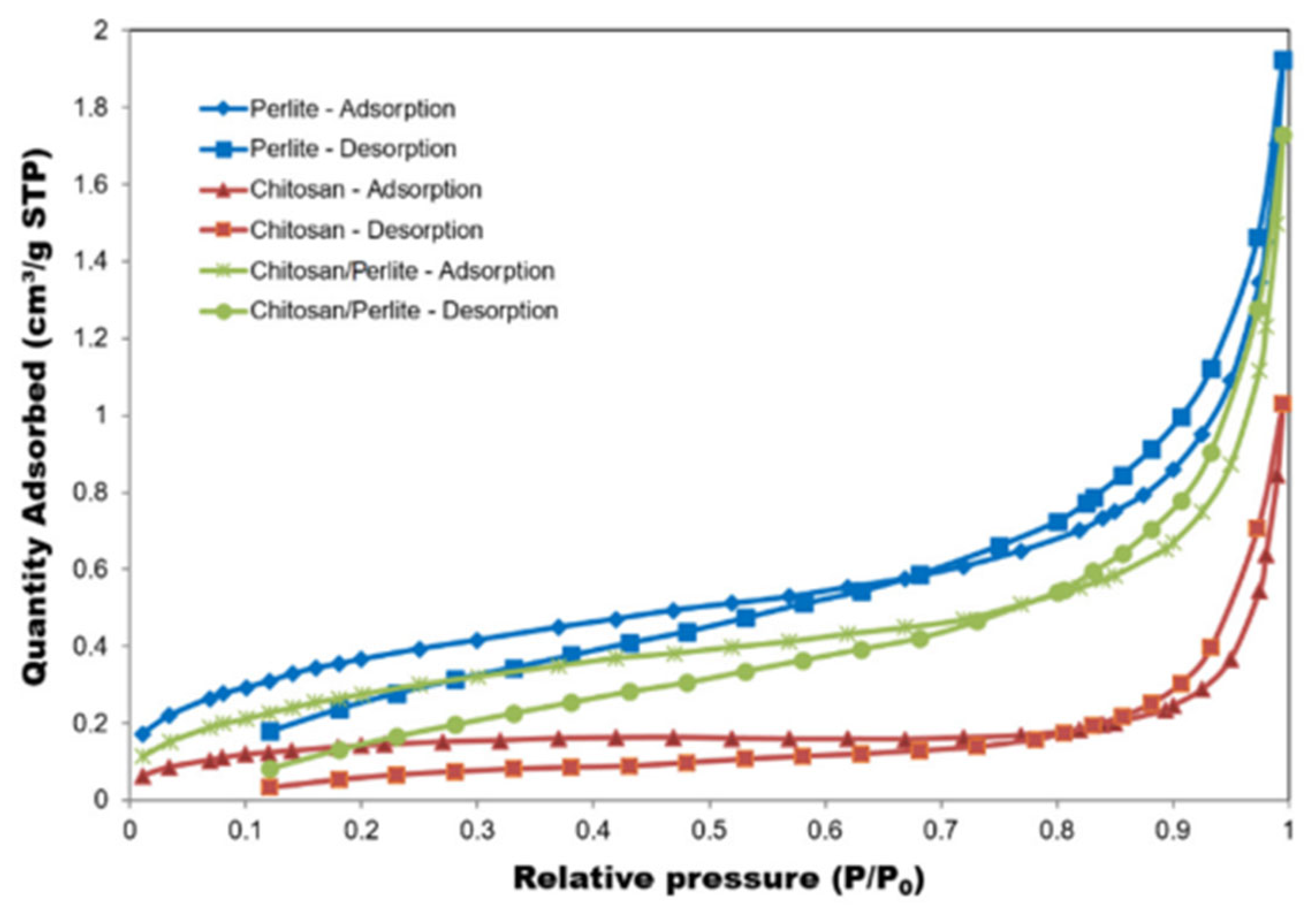
| Impact Category | Crustacean Chitosan + Chemical Modification | Fungal Chitosan + Enzymatic/Green Modification |
|---|---|---|
| Raw Material Source | Shrimp/crab shells (waste stream, seasonal, geographically limited) | Aspergillus niger, Mucor rouxii (cultivated, consistent supply) |
| Deacetylation Process | Concentrated NaOH, 90–120 °C, 3–6 h | Enzymatic (chitin deacetylase), 40–60 °C, mild pH |
| Energy Consumption | High (8–12 kWh/kg)—due to high-temperature alkaline treatment | Medium (5–7 kWh/kg)—lower thermal demand and reduced chemical load |
| Carbon Footprint (CO2-eq) | ~5–7 kg CO2/kg—high emissions from NaOH production and waste treatment | ~2–3 kg CO2/kg (~30–40% lower)—lower chemical use, biodegradable reagents |
| Chemical Waste | High—alkaline effluent, high chemical oxygen demand, acid washes for regeneration | Low—biodegradable enzymes, citric acid, or genipin; less hazardous byproducts |
| Water Use | High—multiple washing steps to remove proteins, minerals, and residual alkali | Moderate to low—closed-loop fermentation possible, less washing required |
| Modification Method | Glutaraldehyde, epichlorohydrin—toxic, non-biodegradable | Moderate to low—closed-loop fermentation possible, less washing required |
| Reusability | Moderate (3–5 cycles, >20% capacity loss)—structural degradation from acid/base regeneration | Moderate to high—improved stability with green crosslinking, less leaching |
| End-of-Life Biodegradability | High (pure chitosan); reduced in composites with synthetic polymers or nanoparticles | High; potentially enhanced by cleaner synthesis and absence of persistent toxicants |
| Nanoparticle Leaching Risk | Moderate to high—Fe3O4, TiO2, ZnO in composites may leach under acidic conditions or after reuse | Similar risk if nanomaterials are used, but lower if green composites are designed |
| Adsorbent | Target Pollutant | Max. Adsorption Capacity (mg/g) | Regeneration Cycles | Remaining Efficiency After Last Cycle (%) | Eluent Used | Key Stability Features | Ref. |
|---|---|---|---|---|---|---|---|
| MIL-53(Fe)/Chitosan hydrogel spheres | Congo Red (CR) | 590.8 | 3 | ~85% | ethanol | High porosity, good structural integrity | [241] |
| MWSBC-0.5 (magnetic straw-based composite) | Cr(VI) | 80.79 | 7 | 78.6% | HCl | Magnetic separation; stable at pH 5 | [242] |
| PEI-functionalized chitosan hydrogel | Pb(II) | ~100 | 4 | ~81% | 1 M HCl | Swelling-resistant; reusable | [243] |
| Chitosan-MOF composite | Pb(II) | 98 | 5 | >80% | 0.5 M Na2SO4 | Good cyclic performance | [90] |
| CTS-STPP-MS | Orange II | 948 | 5 | 97.85 | NaOH | Easy separation, regenerable | [49] |
| PVA-CS aerogel | Cu(II) | 111.85 | 3 | N/A | 0.1 M Na2EDTA | Robust film structure | [244] |
| Ch-Fe composite | As(V) | 16.1 | 2 | N/A | N/A | Enhanced Fe-NP immobilization | [245] |
| Coffee-chitosan (50:50) | Methylene Blue (MB) | 75.76 | Not tested | N/A | N/A | Natural, low-cost composite | [246] |
| HAp/CTS composite | Cd(II) | 126.58 | 10 | 56.5 | DI water | Thermally stable; good ion exchange | [247] |
| CS/n-HAp composite | Cd(II) | 122 | Not tested | N/A | N/A | Biocompatible; stable in aqueous media | [248] |
| Sample | Degree of Swelling (%) | |||
|---|---|---|---|---|
| H2O | pH 6 | pH 7 | pH 8 | |
| Chitosan | 481.59 ± 74.26 a | 138.20 ± 6.51 b | 171.17 ± 40.92 b | 180.50 ± 38.53 b |
| Composite | 376.25 ± 8.31 a | 115.42 ± 10.11 c | 158.81 ± 7.77 b | 131.20 ± 5.95 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehocký, M. Environmental Applications of Chitosan Derivatives and Chitosan Composites. Polymers 2025, 17, 2583. https://doi.org/10.3390/polym17192583
Lehocký M. Environmental Applications of Chitosan Derivatives and Chitosan Composites. Polymers. 2025; 17(19):2583. https://doi.org/10.3390/polym17192583
Chicago/Turabian StyleLehocký, Marián. 2025. "Environmental Applications of Chitosan Derivatives and Chitosan Composites" Polymers 17, no. 19: 2583. https://doi.org/10.3390/polym17192583
APA StyleLehocký, M. (2025). Environmental Applications of Chitosan Derivatives and Chitosan Composites. Polymers, 17(19), 2583. https://doi.org/10.3390/polym17192583






