Synthesis and Characterization of Phosphorylated Cellulose Nanocrystals: Exploring Factors for Enhanced Thermal and Colloidal Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Isolation of Phosphorylated Cellulose Nanocrystals (P-CNCs)
2.3. Characterization of P-CNC
2.3.1. Surface Charge Determination by Conductometric Titration
2.3.2. Dynamic Light Scattering (DLS) and Zeta Potential
2.3.3. Transmission Electron Microscopy (TEM)
2.3.4. Fourier Transform Infrared Spectroscopy—FTIR
2.3.5. X-Ray Diffraction (XRD)
2.3.6. Thermal Stability Analysis of Nanoparticles
3. Results and Discussion
3.1. Influence of Reaction Conditions on Surface Charge and Zeta Potential on P-CNC Production
3.2. Characterization of P-CNCs Obtained
3.2.1. Morphological Analysis by TEM
3.2.2. FTIR Functional Group Analysis of P-CNCs
3.2.3. Hydrodynamic Behavior and Crystalline Structure of P-CNCs
3.2.4. Thermal Stability and Degradation Profile of P-CNCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Chen, Z.; Zhang, R.; Wang, G.; Suo, D.; Zhang, J. Preparation and Applications of the Cellulose Nanocrystal. Int. J. Polym. Sci 2019, 2019, 1767028. [Google Scholar] [CrossRef]
- Calle-Gil, R.; Castillo-Martínez, E.; Carretero-González, J. Cellulose Nanocrystals in Sustainable Energy Systems. Adv. Sustain. Syst. 2022, 6, 2100395. [Google Scholar] [CrossRef]
- Etale, A.; Onyianta, A.J.; Eloi, J.C.; Rowlandson, J.; Eichhorn, S.J. Phosphorylated cellulose nanocrystals: Optimizing production by decoupling hydrolysis and surface modification. Carbohydr. Polym. 2024, 325, 121560. [Google Scholar] [CrossRef]
- Dagnino, E.P.; Ehman, N.; Area, M.C. Recent Advances in Cellulose Nanocrystal Production from Green Methods. Processes 2025, 13, 790. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Surface chemistry, morphological analysis and properties of cellulose nanocrystals with gradiented sulfation degrees. Nanoscale 2014, 6, 5384–5393. [Google Scholar] [CrossRef]
- Li, M.-C.; Wu, Y.; Song, K.; Lee, S.; Qing, Y.; Wu, Q. Cellulose Nanoparticles: Structure-Morphology-Rheology Relationships. ACS Sustain. Chem. Eng. 2015, 3, 821–832. [Google Scholar] [CrossRef]
- Jordan, J.H.; Condon, B.D.; Easson, M.W. Alkali Hydrolysis of Sulfated Cellulose Nanocrystals: Optimization of Reaction Conditions and Tailored Surface Charge. Nanomaterials 2019, 9, 1232. [Google Scholar] [CrossRef]
- Aguayo, M.G.; Fernández-Pérez, A.; Reyes, G.; Oviedo, C.; Gacitúa, W.; Gonzalez, R.; Uyarte, O. Isolation and Characterization of Cellulose Nanocrystals from Rejected Fibers Originated in the Kraft Pulping Process. Polymers 2018, 10, 1145. [Google Scholar] [CrossRef] [PubMed]
- Vanderfleet, O.M.; Osorio, D.A.; Cranston, E.D. Optimization of cellulose nanocrystal length and surface charge density through phosphoric acid hydrolysis. Phil. Trans. R. Soc. A. 2018, 376, 20170041. [Google Scholar] [CrossRef]
- Aguayo, M.G.; Fernández-Pérez, A.; Oviedo, C.; Reyes, G.; Reyes-Contreras, P. Relationship between Structural Characteristics of Cellulose Nanocrystals Obtained from Kraft Pulp. Nanomaterials 2020, 10, 1775. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, S.; Heydari, A.; Fattahi, M. Isolation and Characterization of Cellulose Nanocrystals from Waste Cotton Fibers Using Sulfuric Acid Hydrolysis. Starch-Stärke 2022, 74, 2200159. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, D.; Wu, Q.; Zhou, L.; Li, M.-C. Cellulose Nanocrystals (CNCs) from Corn Stalk: Activation Energy Analysis. Materials 2017, 10, 80. [Google Scholar] [CrossRef]
- Maddalena, L.; Indias, J.M.; Bettotti, P.; Scarpa, M.; Carosio, F. Cellulose nanocrystals polyelectrolyte complexes as flame retardant treatment for cotton fabrics. Polym. Degrad. Stab. 2023, 220, 110646. [Google Scholar] [CrossRef]
- Kröger, M.; Badara, O.; Kontturi, E.; Hietala, S.; Schlapp-Hackl, I.; Pääkkönen, T. Efficient isolation method for highly charged phosphorylated cellulose nanocrystals. Biomacromolecules 2023, 24, 1318–1328. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. The potential of cellulose nanocrystals in tissue engineering strategies. Biomacromolecules 2014, 15, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Sunasee, R.; Hemraz, U. Synthetic Strategies for the Fabrication of Cationic Surface-Modified Cellulose Nanocrystals. Fibers 2018, 6, 15. [Google Scholar] [CrossRef]
- Arockiasamy, F.S.; Manoharan, B.; Santhi, V.M.; Prakalathan, K.; Periasamy, D.; Dhandapani, A.; Natarajan, V.; Krishnasamy, S.; Thiagamani, S.M.K.; Ilyas, R.A. Navigating the nano-world future: Harnessing cellulose nanocrystals from green sources for sustainable innovation. Heliyon 2025, 11, e41188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.P.; Cui, J.; Lynd, L.R.; Kuang, L.R. A Transition from Cellulose Swelling to Cellulose Dissolution by o-Phosphoric Acid: Evidence from Enzymatic Hydrolysis and Supramolecular Structure. Biomacromolecules 2006, 7, 644–648. [Google Scholar] [CrossRef]
- Sathitsuksanoh, N.; Zhu, Z.; Zhang, Y.H.P. Cellulose solvent-based pretreatment for corn stover and avicel: Concentrated phosphoric acid versus ionic liquid [BMIM] Cl. Cellulose 2012, 19, 1161–1172. [Google Scholar] [CrossRef]
- Ait Benhamou, A.; Sehaqui, H.; Nadifiyine, M.; Salim, M.H.; El Achaby, M.; Kassab, Z.; Moubarik, A. Extraction, characterization and chemical functionalization of phosphorylated cellulose derivatives from Giant Reed Plant. Cellulose 2021, 28, 4625–4642. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Reid, M.S.; Bras, J.; Heux, L.; Godoy-Vargas, J.; Panga, M.K.; Cranston, E.D. Insight into thermal stability of cellulose nanocrystals from new hydrolysis methods with acid blends. Cellulose 2019, 26, 507–528. [Google Scholar] [CrossRef]
- Lu, Q.; Tang, L.; Wang, S.; Lin, F.; Cai, Z.; Huang, B. Extraction of Cellulose Nanocrystals with a High Yield of 88% by Simultaneous Mechanochemical Activation and Phosphotungstic Acid Hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 2165–2172. [Google Scholar] [CrossRef]
- Wang, M.; Yin, G.Z.; Yang, Y.; Fu, W.; Palencia, J.L.D.; Zhao, J.; Wang, N.; Jiang, Y.; Wang, D.Y. Bio-based flame retardants to polymers: A review. Adv. Ind. Eng. Polym. Res. 2023, 6, 132–155. [Google Scholar] [CrossRef]
- Camarero Espinosa, S.; Kuhnt, T.; Foster, E.J.; Weder, C. Isolation of thermally stable cellulose nanocrystals by phosphoric acid hydrolysis. Biomacromolecules 2013, 14, 1223–1230. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, P. Bestimmung der Grosse und inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachr. Ges. Wiss. Göttingen 1918, 2, 8–100. [Google Scholar]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A review of water interactions, applications in composites, and water treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, L.; Cui, M.; Huang, R.; Qi, W.; Su, R. Pre-phosphorylation for facile production of phosphorylated cellulose nanocrystals with high charge content: An optimised design and life cycle assessment. Green Chem. 2023, 25, 5041–5050. [Google Scholar] [CrossRef]
- Lemke, C.H.; Dong, R.Y.; Michal, C.A.; Hamad, W.Y. New Insights into Nano-Crystalline Cellulose Structure and Morphology Based on Solid-State NMR. Cellulose 2012, 19, 1619–1629. [Google Scholar] [CrossRef]
- Varaksin, A.N.; Panov, V.G.; Katsnelson, B.A.; Minigalieva, I.A. Using various nonlinear response surfaces for mathematical description of the type of combined toxicity. Dose-Response 2018, 16, 1–10. [Google Scholar] [CrossRef]
- Leszczyńska, A.; Szefer, E.; Mičušík, M.; Omastová, M.; Pielichowski, K.; Radzik, P. Surface modification of cellulose nanocrystals with succinic anhydride. Polymers 2019, 11, 866. [Google Scholar] [CrossRef]
- Zhao, M.; Fujisawa, S.; Saito, T. Distribution and quantification of diverse functional groups on phosphorylated nanocellulose surfaces. Biomacromolecules 2021, 22, 5214–5222. [Google Scholar] [CrossRef]
- Liu, P.; Borrell, P.F.; Božič, M.; Kokol, V.; Oksman, K.; Mathew, A.P. Nanocelluloses and their Phosphorylated Derivatives for Selective Adsorption of Ag+, Cu2+ and Fe3+ from Industrial Effluents. J. Hazard. Mater. 2015, 294, 177–185. [Google Scholar] [CrossRef]
- Phan-Xuan, T.; Bordes, R.; Labrador, A.; Matic, A.; Thuresson, A.; Skepö, M. Aggregation behavior of aqueous cellulose nanocrystals: The effect of inorganic salts. Cellulose 2016, 23, 3653–3663. [Google Scholar] [CrossRef]
- Gan, I.; Chow, W.S. Tailoring chemical, physical, and morphological properties of sugarcane bagasse cellulose nanocrystals via phosphorylation method. J. Nat. Fibers 2021, 18, 1448–1459. [Google Scholar] [CrossRef]
- Kokol, V.; Božič, M.; Vogrinčič, R.; Mathew, A.P. Characterisation and properties of homo- and heterogenously phosphorylated nanocellulose. Carbohydr. Polym. 2015, 125, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Suflet, D.M.; Chitanu, G.C.; Popa, V.I. Phosphorylation of polysaccharides: New results on synthesis and characterisation of phosphorylated cellulose. React. Funct. Polym. 2006, 66, 1240–1249. [Google Scholar] [CrossRef]
- Bahloul, A.; Semlali, F.Z.; Oumam, M.; Hannache, H.; Kassab, Z.; El Achaby, M. Starch bio-nanocomposites based on phosphorylated and sulphated cellulose nanocrystals extracted from pepper plant residue: Effect of surface functionality on property improvements. Cellulose 2023, 30, 5051–5070. [Google Scholar] [CrossRef]
- Belasri, A.; Blilid, S.; El Assimi, T.; Lahcini, M.; El Kadib, A.; Beniazza, R. Soft phosphorylation of cellulose and starch for effective remediation of methylene blue dye and heavy metal-contaminated water. Int. J. Biol. Macromol. 2025, 309, 143107. [Google Scholar] [CrossRef]
- Petreuş, O.; Bubulac, T.; Petreuş, I.; Cazacu, G. Reactions of some phosphorus compounds with cellulose dissolved in aqueous alkaline solution. J. Appl. Polym. Sci. 2003, 90, 327–333. [Google Scholar] [CrossRef]
- Pecora, R. Dynamic light scattering measurement of nanometer particles in liquids. J. Nanopart. Res. 2000, 2, 123–131. [Google Scholar] [CrossRef]
- Zhang, C.; Jin, Z.; Zeng, B.; Wang, W.; Palui, G.; Mattoussi, H. Characterizing the Brownian diffusion of nanocolloids and molecular solutions: Diffusion-ordered NMR spectroscopy vs dynamic light scattering. J. Phys. Chem. B 2020, 124, 4631–4650. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Carrillo-Varela, I.; Retamal, R.; Pereira, M.; Mendonça, R.T. Structure and reactivity of cellulose from bleached kraft pulps of different Eucalyptus species upgraded to dissolving pulp. Cellulose 2019, 26, 5731–5744. [Google Scholar] [CrossRef]
- Kusmono; Affan, M.N. Isolation and characterization of nanocrystalline cellulose from ramie fibers via phosphoric acid hydrolysis. J. Nat. Fibers 2022, 19, 2744–2755. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues–wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Lin, L.; Chen, T.; Zhang, J.; Liu, S.; Li, Z.; Ouyang, P. Dissolution of Microcrystalline Cellulose in Phosphoric Acid—Molecular Changes and Kinetics. Molecules 2009, 14, 5027–5041. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.C.; Ferreira, F.F.; Rosa, D.S. X-ray powder diffraction and other analyses of cellulose nanocrystals obtained from corn straw by chemical treatments. Carbohydr. Polym. 2018, 193, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Rampazzo, R.; Alkan, D.; Gazzotti, S.; Ortenzi, M.A.; Piva, G.; Piergiovanni, L. Cellulose nanocrystals from lignocellulosic raw materials, for oxygen barrier coatings on food packaging films. Packag. Technol. Sci. 2017, 30, 645–661. [Google Scholar] [CrossRef]
- Rajan, K.; Djioleu, A.; Kandhola, G.; Labbé, N.; Sakon, J.; Carrier, D.J.; Kim, J.W. Investigating the effects of hemicellulose pre-extraction on the production and characterization of loblolly pine nanocellulose. Cellulose 2020, 27, 3693–3706. [Google Scholar] [CrossRef]
- Börjesson, M.; Westman, G. Crystalline Nanocellulose—Preparation, Modification, and Properties. Cellulose: Fundamental Aspects and Current Trends; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef]
- Ramires, E.C.; Dufresne, A. A review of cellulose nanocrystals and nanocomposites. Tappi J. 2011, 10, 9–16. [Google Scholar] [CrossRef]
- Aoki, D.; Nishio, Y. Phosphorylated cellulose propionate derivatives as thermoplastic flame resistant/retardant materials: Influence of regioselective phosphorylation on their thermal degradation behaviour. Cellulose 2010, 17, 963–976. [Google Scholar] [CrossRef]
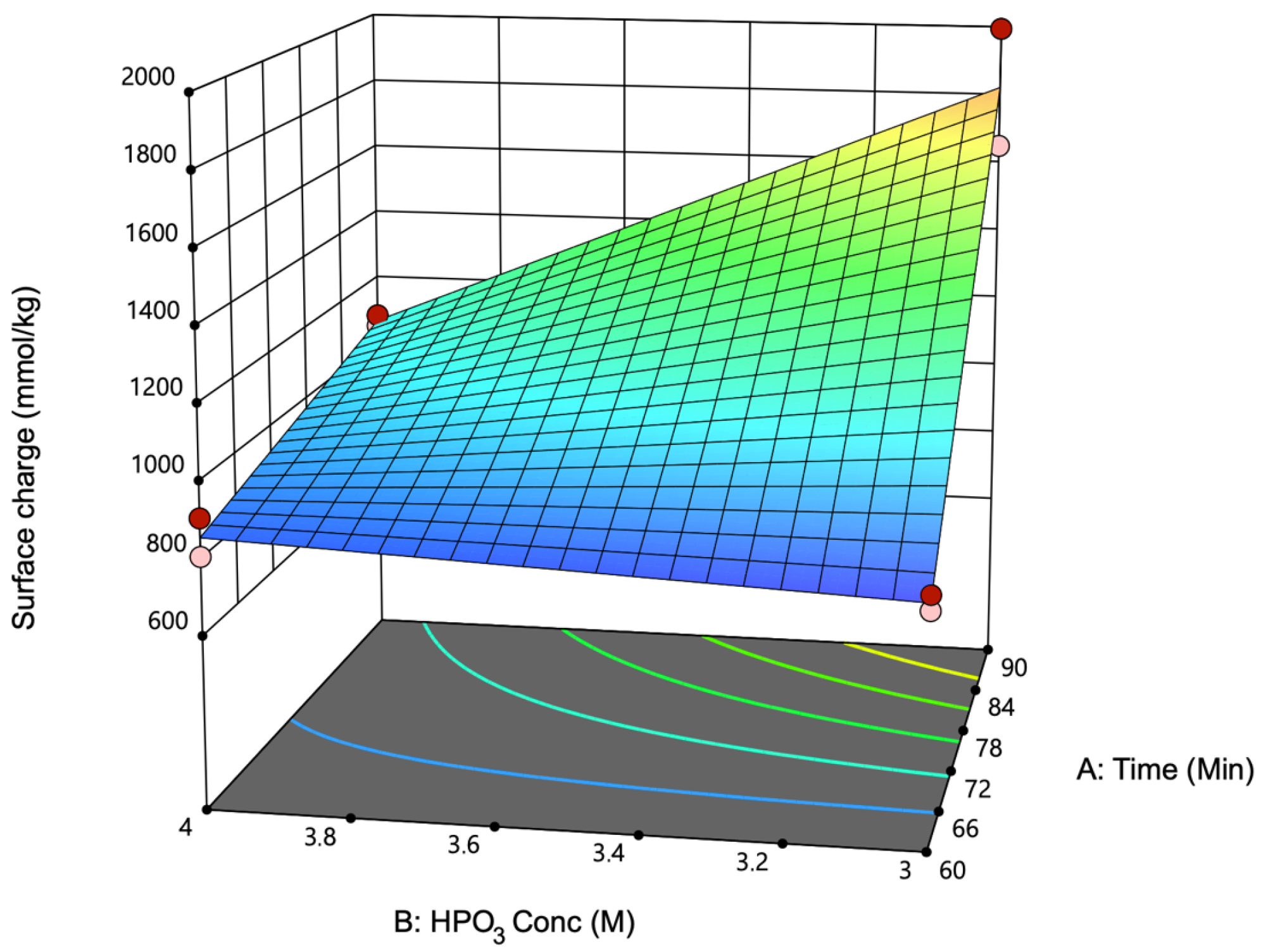
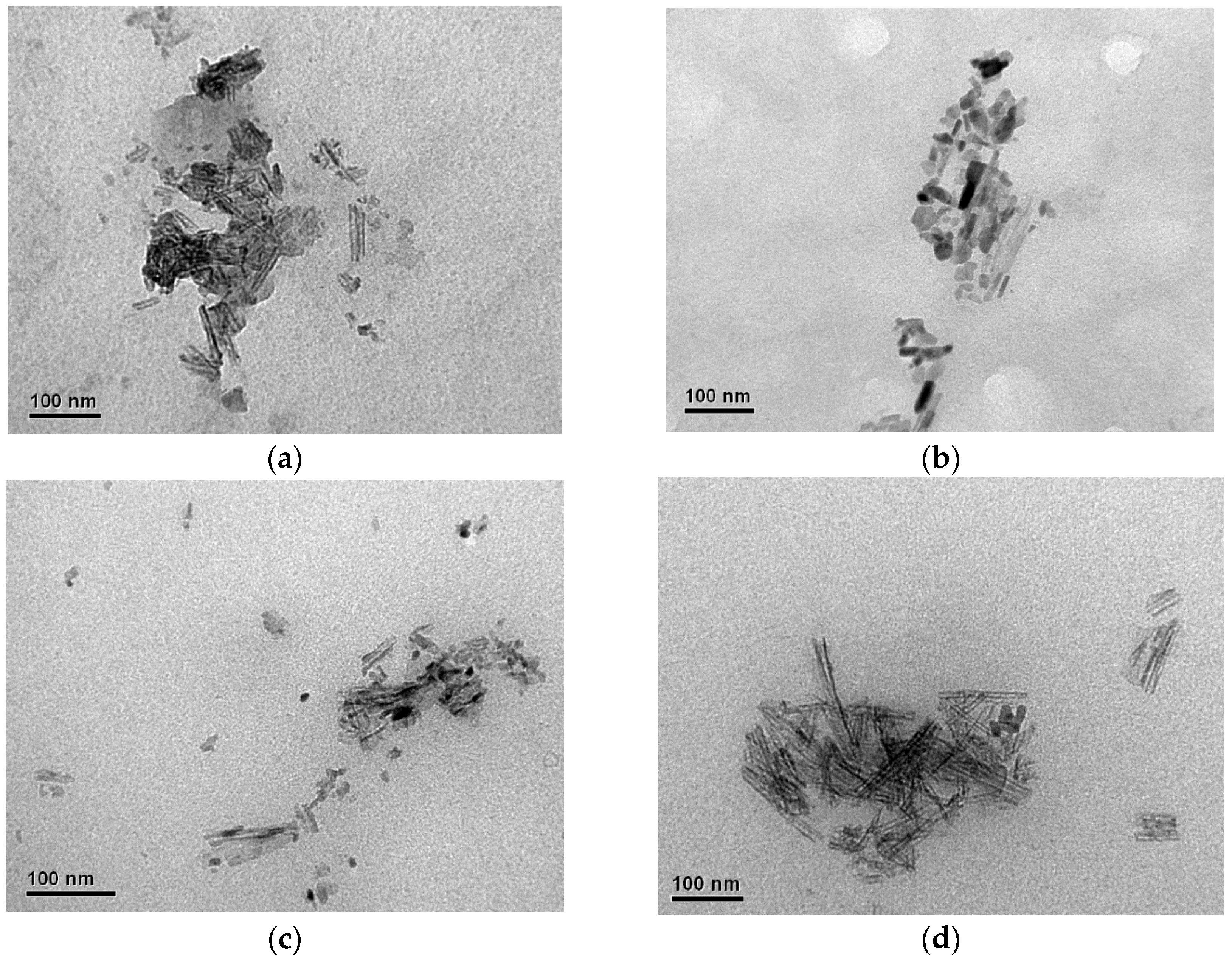


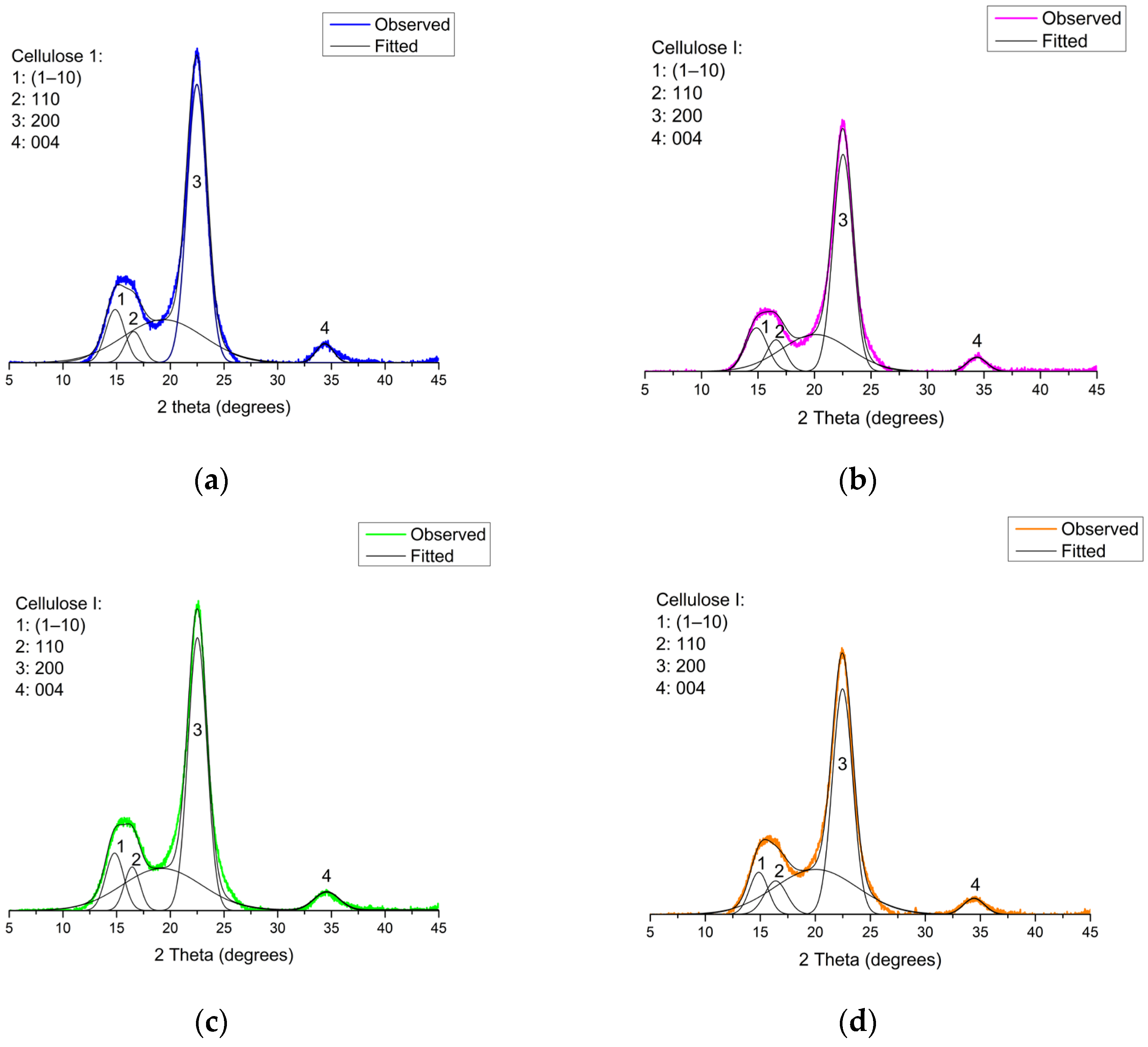

| Exp | Reaction Time (min) | HPO3 (M) | Surface Charge (mmol kg−1) | Zeta Potential (mV) |
|---|---|---|---|---|
| M1 | 60 | 3 | 757.29 | −42.00 ± 0.96 |
| M1R | 60 | 3 | 797.92 | −46.27 ± 1.56 |
| M2 | 60 | 4 | 808.70 | −30.17 ± 1.52 |
| M2R | 60 | 4 | 909.82 | −55.63 ± 0.71 |
| M3 | 90 | 3 | 1993.93 | −59.67 ± 0.50 |
| M3R | 90 | 3 | 1653.47 | −63.37 ± 0.90 |
| M4 | 90 | 4 | 1057.03 | −61.07 ± 0.50 |
| M4R | 90 | 4 | 1089.29 | −67.40 ± 0.71 |
| Exp | Particle Size (nm) * | Crystallinity Index (%) | Crystallite Size L200 (nm) |
|---|---|---|---|
| M1 | 45.17 ± 4.10 | 68.2 | 4.21 |
| M1R | 59.13 ± 2.64 | 71.3 | 4.41 |
| M2 | 47.50 ± 1.15 | 66.6 | 4.42 |
| M2R | 62.50 ± 5.76 | 68.4 | 4.64 |
| M3 | 39.67 ± 3.52 | 68.9 | 4.42 |
| M3R | 38.03 ± 4.29 | 67.8 | 4.64 |
| M4 | 34.40 ± 3.22 | 63.6 | 4.21 |
| M4R | 35.87 ± 7.13 | 65.3 | 4.64 |
| Exp | Maximum Degradation Temperature (°C) | Residue (%) at 600 °C |
|---|---|---|
| M1 | 345.4 | 19.54 |
| M1R | 305.9 | 25.02 |
| M2 | 347.0 | 15.21 |
| M2R | 346.8 | 16.99 |
| M3 | 341.7 | 14.48 |
| M3R | 329.9 | 25.46 |
| M4 | 346.7 | 22.03 |
| M4R | 342.1 | 10.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, D.; Aguayo, M.G.; Núñez-Decap, M.; Reyes-Contreras, P.; Teixeira Mendonça, R.; Reyes-González, I.; Opazo, B.; Valdebenito, F. Synthesis and Characterization of Phosphorylated Cellulose Nanocrystals: Exploring Factors for Enhanced Thermal and Colloidal Stability. Polymers 2025, 17, 2581. https://doi.org/10.3390/polym17192581
López D, Aguayo MG, Núñez-Decap M, Reyes-Contreras P, Teixeira Mendonça R, Reyes-González I, Opazo B, Valdebenito F. Synthesis and Characterization of Phosphorylated Cellulose Nanocrystals: Exploring Factors for Enhanced Thermal and Colloidal Stability. Polymers. 2025; 17(19):2581. https://doi.org/10.3390/polym17192581
Chicago/Turabian StyleLópez, Diego, María Graciela Aguayo, Mario Núñez-Decap, Pablo Reyes-Contreras, Regis Teixeira Mendonça, Isidora Reyes-González, Benjamín Opazo, and Fabiola Valdebenito. 2025. "Synthesis and Characterization of Phosphorylated Cellulose Nanocrystals: Exploring Factors for Enhanced Thermal and Colloidal Stability" Polymers 17, no. 19: 2581. https://doi.org/10.3390/polym17192581
APA StyleLópez, D., Aguayo, M. G., Núñez-Decap, M., Reyes-Contreras, P., Teixeira Mendonça, R., Reyes-González, I., Opazo, B., & Valdebenito, F. (2025). Synthesis and Characterization of Phosphorylated Cellulose Nanocrystals: Exploring Factors for Enhanced Thermal and Colloidal Stability. Polymers, 17(19), 2581. https://doi.org/10.3390/polym17192581








