Preparation and Characterization of a High-Performance Foam Extinguishing Agent with Sulfobetaine and Polyoxyethylene Ether for Solid Fires
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Foam Solution Preparation
2.3. Key Physical Property Testing of Foam Solution
2.3.1. Viscosity Measurement of Foam Solutions
2.3.2. Wettability Test of Foam Solutions
2.3.3. Surface Tension Measurement of Foam Solutions
2.3.4. Foam Expansion Ratio Test
2.4. Key Foam Physical Property Parameter Testing
2.4.1. Foam Wetting Performance Test
2.4.2. Foam Viscosity Test
2.4.3. Foam Adhesion Test
- Vertical Angle Foam Adhesion Test
- 2.
- Inclined Angle Foam Adhesion Test
2.5. Microscopic and Fire Extinguishing Experiments
2.5.1. Foam Fire Extinguishing Performance Test
2.5.2. Foam Microscopic Observation
3. Results and Discussion
3.1. Introduction to Experimental Components
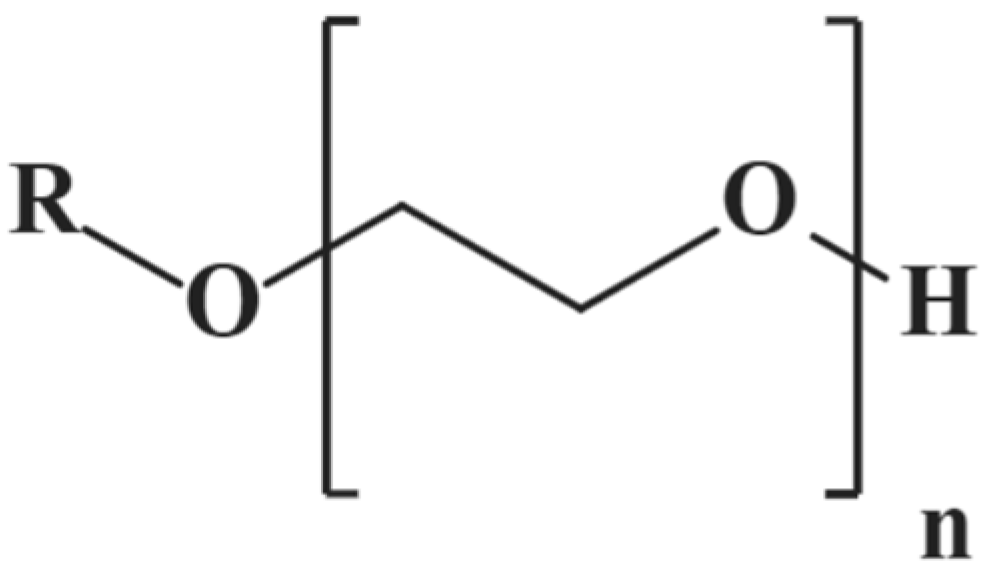
3.1.1. Introduction to Polyoxyethylene Ether
3.1.2. Introduction to Sulfobetaine
3.2. Analysis of Foam Solution Properties
3.2.1. Analysis of Viscosity Testing of Foam Solutions
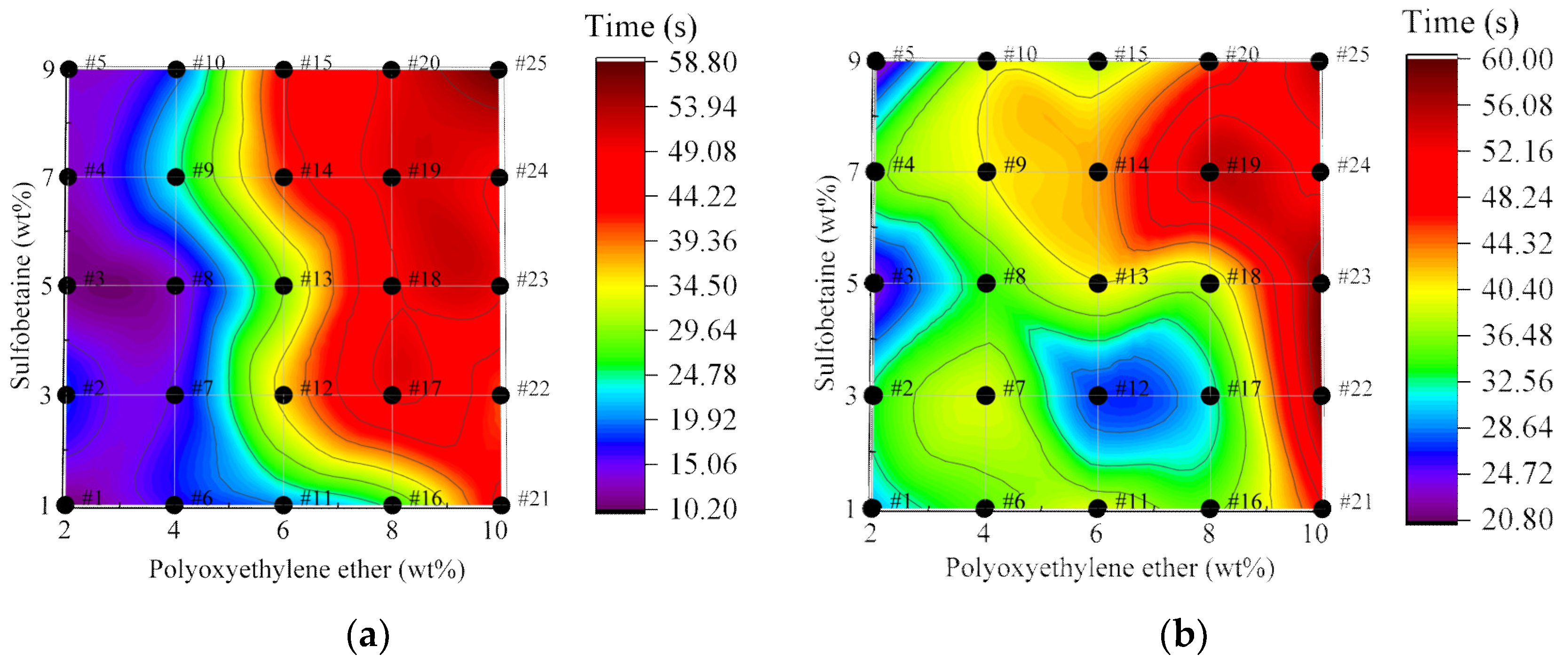
3.2.2. Analysis of Wetting Time of Foam Solutions
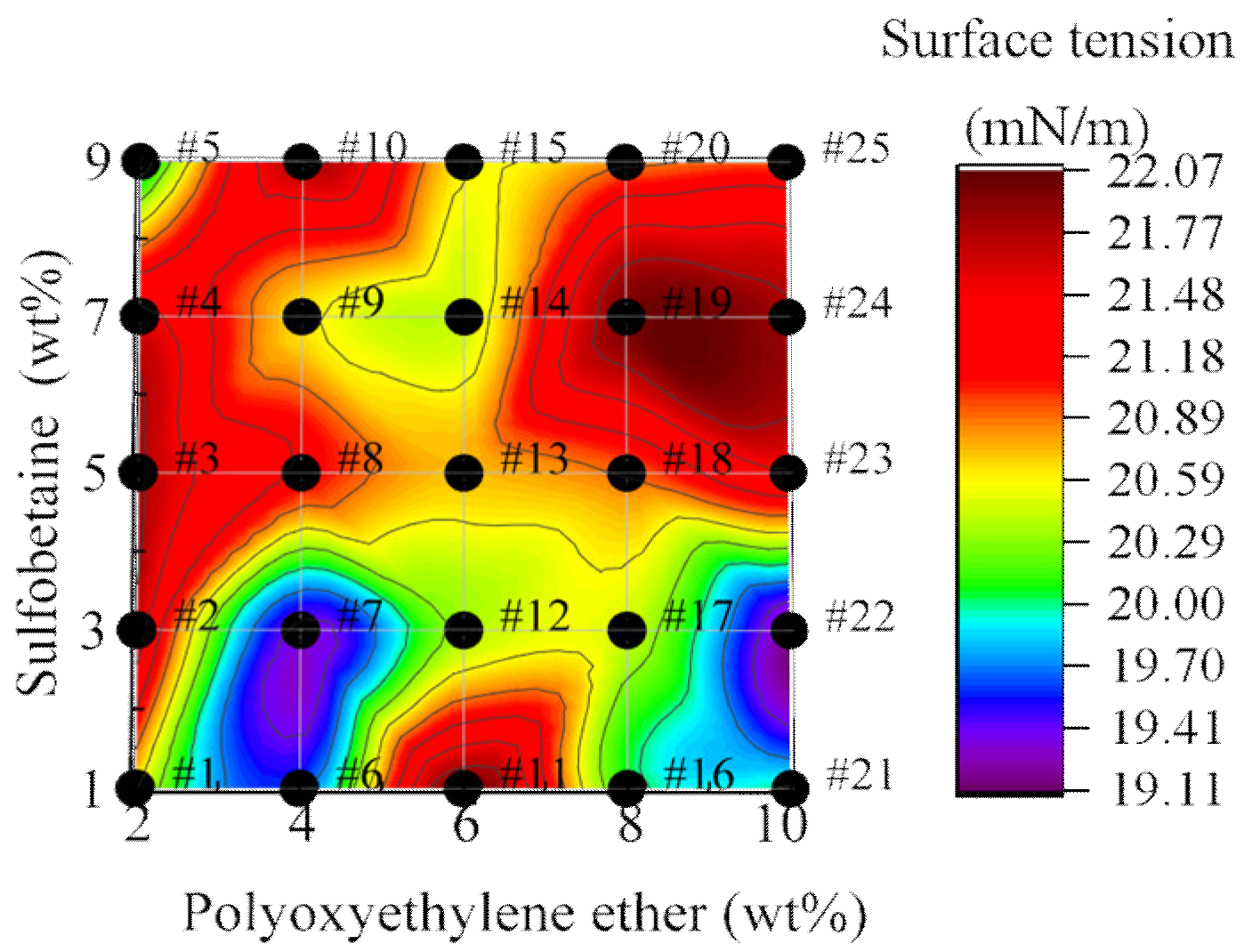
3.2.3. Analysis of Surface Tension of Foam Solutions
3.3. Analysis of Foam Property Test Results
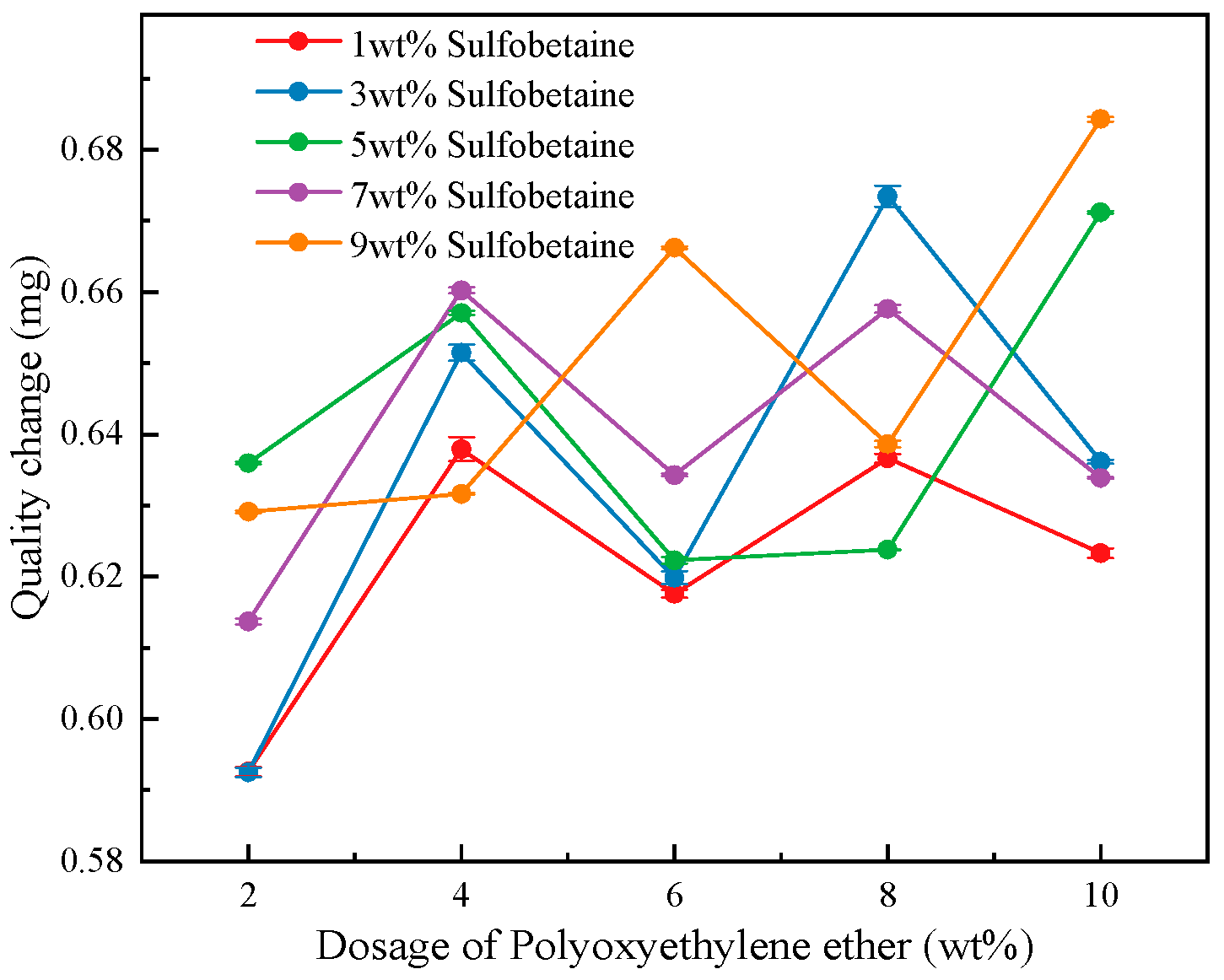
3.3.1. Analysis of Foam Wetting Performance Test
3.3.2. Analysis of Foam Viscosity Test
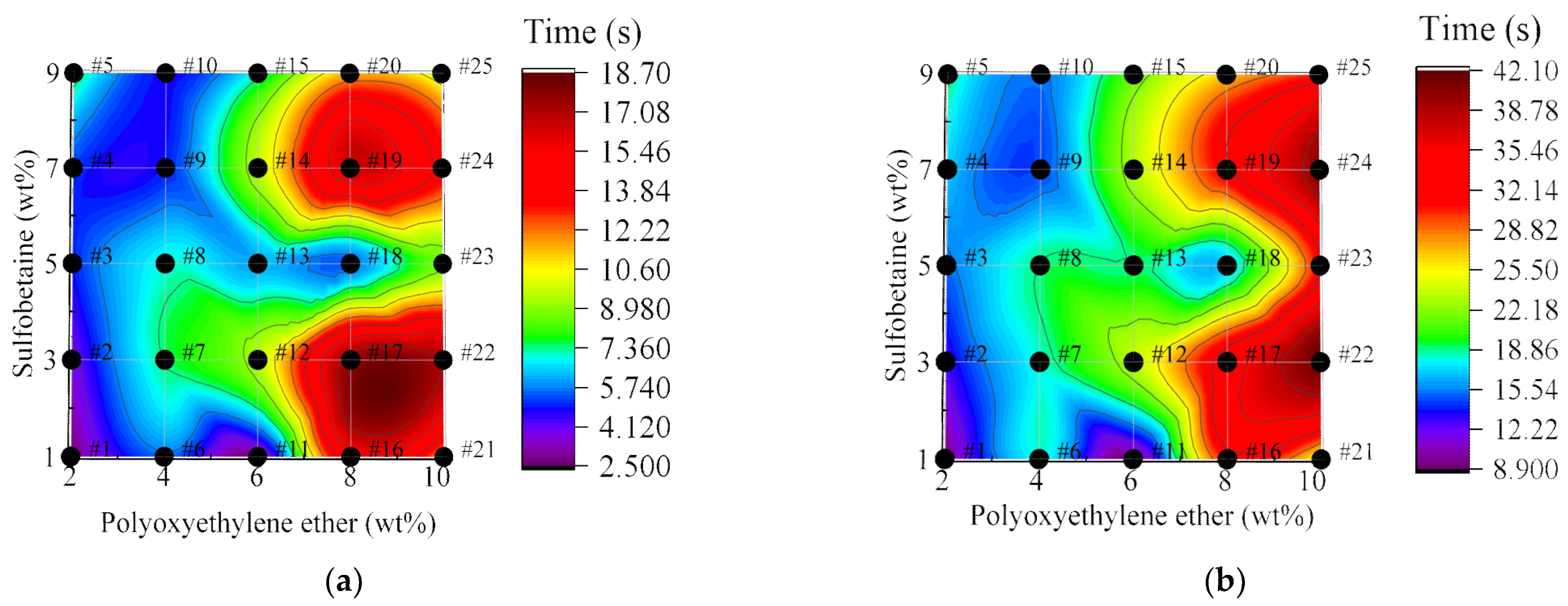
3.3.3. Analysis of Foam Adhesion Test
3.4. Foam Extinguishing and Microscopic Experiments
3.4.1. Analysis of Foam Extinguishing Experiments
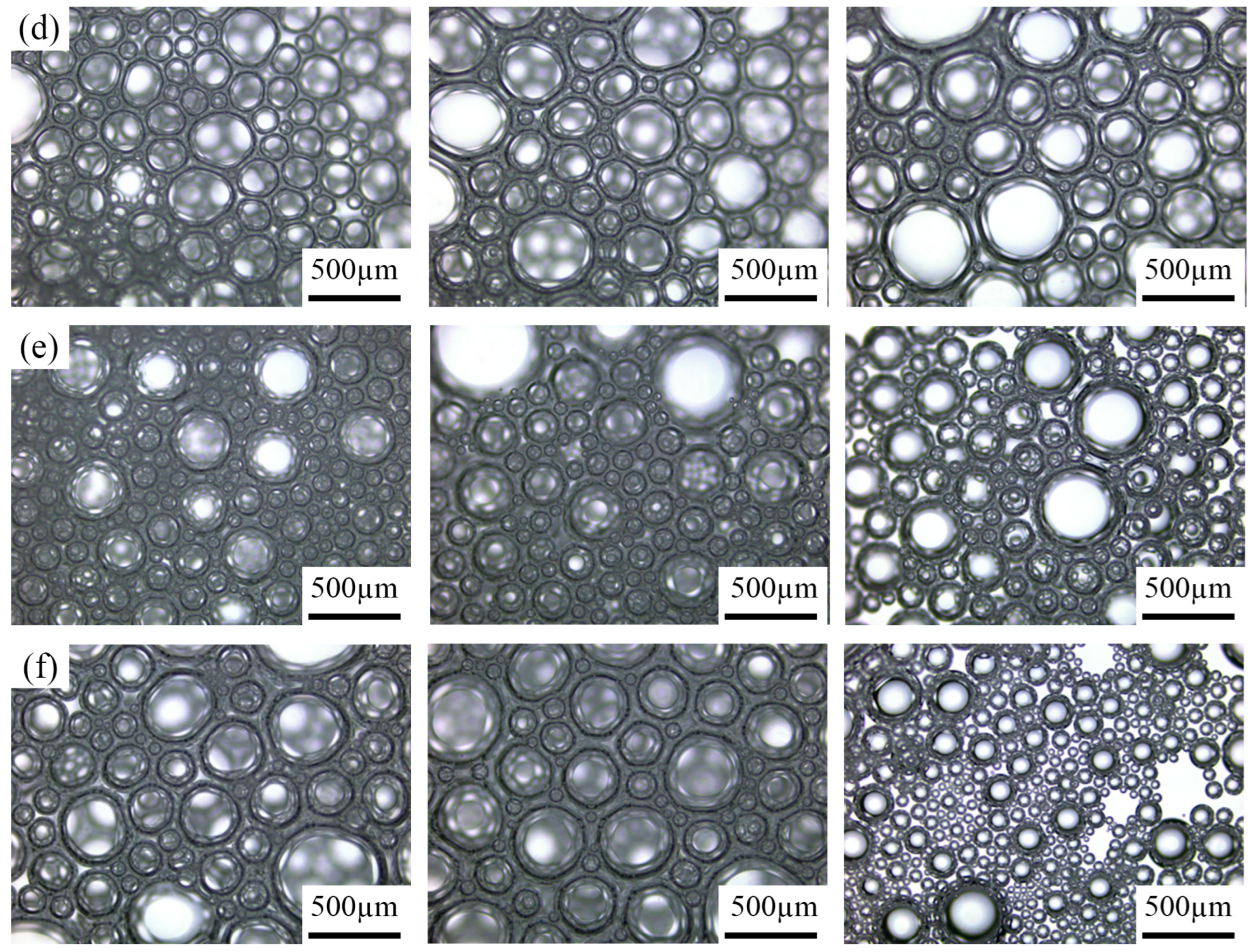
3.4.2. Analysis of Foam Microscopic Observation
4. Conclusions
- Synergistic effect of Sulfobetaine and Polyoxyethylene ether on viscosity and wettability. The combination of Sulfobetaine and Polyoxyethylene ether significantly enhances solution viscosity. The functional groups of the two surfactants interact to form hydrogen bonds and a strong hydration layer, increasing intermolecular steric hindrance. Additionally, the hydrophobic alkyl chain of Polyoxyethylene ether can associate with the hydrophobic tail of Sulfobetaine, further contributing to viscosity enhancement. The Sulfobetaine–Polyoxyethylene ether mixture also markedly improves foam wettability. Polyoxyethylene ether enables the molecular layer to conform to the solid surface topology, promoting effective wetting. The ether oxygen atoms in Polyoxyethylene ether interact strongly via hydrogen bonding with the sulfonic groups of Sulfobetaine, forming a dense composite interfacial film. The aggregation of the long hydrophobic chains at the interface generates a hydrophobic layer that effectively repels water molecules and reinforces the foam film strength.
- Interplay between viscosity and wettability. A negative feedback effect exists between foam solution viscosity and wettability. At Sulfobetaine loadings of 3 wt% and 9 wt%, the mixture achieves optimal synergistic effects in both intermolecular interactions and surface viscosity enhancement. Wettability reaches its maximum when the concentration of Polyoxyethylene ether is maintained above 8 wt%. The surface tension of all foam solutions is relatively uniform, ranging from 19.0 to 22.5 mN/m, in accordance with national standards and adequate for practical applications.
- Adhesiveness–wettability interaction. A similar negative feedback effect is observed between foam adhesiveness and wettability. The overall trend of foam adhesiveness follows that of the solution viscosity, with superior adhesion observed at 3 wt% and 9 wt% Sulfobetaine. Adhesion tests on inclined substrates revealed that the variation in foam adhesion time under inclined conditions is similar to that observed under vertical conditions; however, compared with the vertical orientation, the adhesion time is further extended, and foams on the underside of the board exhibit longer adhesion times than those on the upper side. Foam wettability shows minor deviations from the solution behavior but remains optimal at Polyoxyethylene ether loadings of 8–10 wt%.
- Foam performance in fire suppression and microscopic observations. Fire-extinguishing experiments identified the optimal formulations: a wetting-type foam suitable for rapid cooling in liquid fires can be prepared with 8 wt% Polyoxyethylene ether and 5 wt% Sulfobetaine, whereas an adhesion-type foam capable of sustained attachment to solid combustible surfaces is obtained with 8 wt% Polyoxyethylene ether and 9 wt% Sulfobetaine. Microscopic observations indicate that Polyoxyethylene ether addition increases the liquid film thickness, enhances film strength, and slows drainage, while Sulfobetaine addition mitigates foam size variation, reduces pressure differences between bubbles, and delays coalescence. The foam with excellent fire suppression performance formed a stable, dense foam layer that slowly dissipated over time, providing a stable covering effect.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Feng, H.; Hong, J.; Wang, G. Enhanced foaming ability of thermoplastic polyurethane by crystallization during mold-opening foam injection molding with supercritical N2 as blowing agents. J. Mater. Res. Technol. 2023, 22, 2489–2501. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, G.; Ding, H.; Wang, H.; Li, J.; Yu, R.; Gao, Y.; Wang, P. Study on the inhibition performance of double network physicochemical nanocomposite gel inhibitor on coal spontaneous combustion. Fuel 2023, 350, 128697. [Google Scholar] [CrossRef]
- Wang, Q.G.; Tuo, L.N.; Zhou, G.; Zhang, Y.Y.; Geng, X.; Zhang, F.S.; Li, Y.H. Effect of silicones and polymers on the wetting and foaming properties of anionic and nonionic hydrocarbon surfactants. Environ. Sci. Pollut. Res. 2022, 29, 81713–81725. [Google Scholar] [CrossRef] [PubMed]
- Li, W.X.; Zhu, L.W.; Zhang, F.L.; Han, C.R.; Xiang, H.; Jiang, J.X. Synergism and foam stability in a binary mixture of tea saponin derived from Camellia oleifera Abel and Sodium Lauryl Ether Sulfate. Surf. Interfaces 2022, 29, 101707. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.; Qiu, K.; Bo, B.; Li, Q.; Lu, S. Role of salts in fire extinguishing performance of aqueous film-forming foam (AFFF). Case Stud. Therm. Eng. 2023, 49, 103159. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Z.; Zhang, M.; Wu, L. Application of Alkyl Amidopropyl Betaine in Fire Fighting Foam Extinguishing Agent. MATEC Web Conf. 2023, 380, 01012. [Google Scholar] [CrossRef]
- Li, F.; Yu, X.; Zong, R. Experimental study on the foam performance and fire extinguishing performance of aqueous foam stabilized by alkyl polyglucoside and urea. IOP Conf. Ser. Earth Environ. Sci. 2022, 983, 012085. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Liu, S.; Nie, X.; Zhang, Y.L.; Dong, K.L.; Wang, J.F. Experimental and Molecular Simulation Research on the Effect of Metal Ions on the Stability of SDS Foam. Energy Fuels 2022, 36, 521–526. [Google Scholar] [CrossRef]
- Sheng, Y.; Li, Y.; Yan, C.; Peng, Y. Polymer Foam Stabilizers and SiO2 Nanoparticles on the Foam Properties of Fluorocarbon/Hydrocarbon Mixed Systems. Polym. Mater. Sci. Eng. 2023, 39, 33. [Google Scholar]
- Qiu, Y.; Wang, Y.; Liu, Y.; Zhang, L.; Chen, Y.; Li, C.; Wu, T.; Wang, C. Development of fiber compound foaming agent and experimental study on application performance of foamed lightweight soil. Appl. Rheol. 2023, 33, 20230108. [Google Scholar] [CrossRef]
- Dinesh; Wang, H.B.; Pham, D.H.; Kim, J. Anionic surfactant effect on the structural and thermal insulation properties of crosslinked-cellulose nanofiber foam and its superhydrophobic treatment. Int. J. Biol. Macromol. 2024, 283, 137934. [Google Scholar] [CrossRef]
- Liu, D.; Zeng, W.; Yang, K.; Shi, X.; Wei, X.; Zhang, T.; Sun, S. Foam Property and CO2/N2 Response Performance of Anionic Surfactant/Tertiary Amine Compound Foam. Oilfield Chem. 2022, 39, 331–337. [Google Scholar]
- Yang, Y.; Peng, M.; Sha, M.; Fang, J.; Zhang, D.; Pan, R.; Jiang, B. Study on aqueous film-forming foam extinguishing agent based on fluorocarbon cationic–hydrocarbon anionic surfactants mixture system. J. Surfactants Deterg. 2022, 25, 205–216. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Gao, F.; Zhu, X.; Liu, Q.; Jia, X. One-step preparation and characterization of an imidazolium-contained fluorocarbon surfactant and its potential application in aqueous film-forming foam. J. Chem. Res. 2024, 48, 17475198241275875. [Google Scholar] [CrossRef]
- Lou, M.; Jia, H.; Lin, Z.; Zeng, D.; Huo, J. Study on fire extinguishing performance of different foam extinguishing agents in diesel pool fire. Results Eng. 2023, 17, 100874. [Google Scholar] [CrossRef]
- Jia, X.; Bo, H.; He, Y. Synthesis and characterization of a novel surfactant used for aqueous film-forming foam extinguishing agent. Chem. Pap. 2019, 73, 1777–1784. [Google Scholar] [CrossRef]
- Yang, Y.-W.; Fang, J.-Q.; Sha, M.; Zhang, D.; Pan, R.-M.; Jiang, B. Study on foam extinguishing agents based on hydrocarbon and perfluorinated branched short-chain fluorocarbon surfactants mixed system. Chem. Pap. 2021, 75, 6241–6255. [Google Scholar] [CrossRef]
- Wang, H.; Bai, S.; Zhu, X.; Gao, F.; Wang, P.; Zhang, Y.; Liu, Z. Synthesis and Characterization of a Novel Imidazolium Short-Chain Fluorocarbon Surfactant as a Potential Agent for Aqueous Film Forming Foam. ChemistrySelect 2023, 8, e202302185. [Google Scholar] [CrossRef]
- Geng, D.Q.; Li, J.H.; Li, H.Y.; Huang, W.Z. Effects of Particle Combinations With Different Wettability on Foam Structure and Stability. Front. Mater. 2021, 8, 699187. [Google Scholar] [CrossRef]
- Wang, Q.G.; Geng, X.; Li, Y.H.; Zhang, F.S.; Tuo, L.; Zhang, Y.Y. Effect of nanoparticles and silicone surfactants on the foam properties and wettability of dust removal foam. Chem. Eng. Sci. 2023, 281, 119147. [Google Scholar] [CrossRef]
- Gholipour-Sangelaji, K.; Hosseini-Nasab, S.M.; Hormozi, F. Experimental Study on Mechanisms of Hybrid Effects of Different Surfactant Types on Nano-Particle Wettability to Enhance Foam Stability. Acs Omega 2025, 10, 28836–28847. [Google Scholar] [CrossRef]
- Eknapakul, T.; Lapawea, K.; Musikajareon, S.; Nakajima, H.; Chanlek, N.; Meevasana, W. Surface Analysis on Wettability of Nickel Foams. Chiang Mai J. Sci. 2020, 47, 1092–1101. [Google Scholar]
- Ajayi, A.A.; Pandurangan, M.T.; Kanny, K.; Ramachandran, V. Thermal and Wettability Properties of Nanoclay-Filled Epoxy-Based Foam Composite as Lightweight Material. Mater. Perform. Charact. 2023, 12, 293–306. [Google Scholar] [CrossRef]
- Besson, S.; Debrégeas, G.; Cohen-Addad, S.; Höhler, R. Dissipation in a Sheared Foam: From Bubble Adhesion to Foam Rheology. Phys. Rev. Lett. 2008, 101, 214504. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Z.; Wang, Q.G.; Zhang, J.P.; Li, Y.; He, H.Q.; Huang, G.J. Foamed crumb rubber asphalt binder: Preparation, rheological properties and adhesion characteristics. J. Clean. Prod. 2023, 396, 136516. [Google Scholar] [CrossRef]
- Dai, B.W.; Liu, Q.; Jin, F.; Huang, W.; Jiang, B.L.; Huang, Z.D.; Song, Y.R.; Chen, H.B. Adhesion behaviors and kinetics at silicone foam/metal interfaces. Polym. Degrad. Stab. 2024, 225, 110772. [Google Scholar] [CrossRef]
- Nie, W.; Tian, Q.F.; Niu, W.J.; Li, R.X.; Bao, Q.; Zhang, X.H.; Yan, X.; Lian, J. Synergistic effect of binary mixture of anionic nonionic surfactant on inhibiting coal dust pollution: Experiment and simulation. J. Environ. Chem. Eng. 2023, 11, 110099. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Qiao, M.; Zhao, H.X.; Ran, Q.P.; Yuan, S.L. Effect of mixed surfactants on foam stabilization: A molecular dynamics simulation. J. Mol. Liq. 2022, 365, 120096. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, X.; Yan, L.; Kang, W. Fire-extinguishing performance and mechanism of aqueous film-forming foam in diesel pool fire. Case Stud. Therm. Eng. 2020, 17, 100578. [Google Scholar] [CrossRef]
- Dlugogorski, B.Z.; Schaefer, T.H. Compatibility of aqueous film-forming foams (AFFF) with sea water. Fire Saf. J. 2021, 120, 103288. [Google Scholar] [CrossRef]
- Ping, P.; Li, B.; Chen, J.; He, X.; Wang, D.; Zhang, J.; Kong, D. Effect of temperature on stability and film thinning behavior of aqueous film forming foam. J. Mol. Liq. 2023, 370, 120978. [Google Scholar] [CrossRef]
- Yan, L.; Wang, N.; Guan, J.; Wei, Z.; Xiao, Q.; Xu, Z. Comparative Study of the Suppression Behavior and Fire-Extinguishing Mechanism of Compressed-Gas Aqueous Film-Forming Foam in Diesel Pool Fires. Fire 2023, 6, 269. [Google Scholar] [CrossRef]
- GB 27897-2011; Class A Foam Extinguishing Agent. Standardization Administration of China: Beijing, China, 2011.
- Sheng, Y. Research on Foam Fire Extinguishing Agent with Hydrocarbon and Silicone Surfactant Compound System as the Base Agent. 2018. Available online: https://d.wanfangdata.com.cn/thesis/ChhUaGVzaXNOZXdTMjAyNDA5MjAxNTE3MjUSCFkzNDAwMzk2Ggh5bGR5aDdvMQ%3D%3D (accessed on 30 August 2025).
- Jiang, N. Study on Water-Resistant Aqueous Film-Forming Foam Extinguishing Agent Based on Short Chain Carbon-Fluorine-Hydrocarbon Complex System. 2021. Available online: https://d.wanfangdata.com.cn/thesis/ChhUaGVzaXNOZXdTMjAyNDA5MjAxNTE3MjUSCFkzODI3NzExGghuaXF4YzF2dg%3D%3D (accessed on 30 August 2025).
- Lu, Q.; Bao, Z.; Chen, T.; Zhang, X.; Fu, X. Experimental Study on the Fire Extinguishing Performance of Class A Foam Extinguishing Agent. Fire Sci. Technol. 2013, 2, 177–179. [Google Scholar] [CrossRef]
- Zhou, Y. A Brief Analysis of Fire Extinguishing Test Methods in Fire Extinguisher Inspection. Fire Prot. Technol. Prod. Inf. 2008, 8, 5–7. [Google Scholar] [CrossRef]
- Fu, X.C.; Ye, H.L.; Bao, Z.M.; Chen, T.; Xu, G.; Hu, N.Y.; Wang, R.J. The Development and Outlook of Class A Foam Fire Extinguishing Agents. Fire Sci. Technol. 2008, 27, 590–592. [Google Scholar] [CrossRef]
- Chen, W.; Lu, J. Compressed air Class A foam system technology and its application in fire engines. Strait Sci. 2014, 9, 53–54+63. [Google Scholar] [CrossRef]
- Ma, L.; Fan, X.; Wei, G.; Sheng, Y.; Liu, S.; Liu, X. Preparation and characterization of antioxidant gel foam for preventing coal spontaneous combustion. Fuel 2023, 338, 127270. [Google Scholar] [CrossRef]









| Foam Performance | Surface Tension (nN/m) | Expansion Ratio | Fire Extinguishing Time (s) | |
| Foam Category | ||||
| Wetting-type foam (this study) | 20.91 | 8.2 | 12 | |
| Adhesive-type foam (this study) | 20.83 | 7.8 | 20 | |
| Fluorocarbon cationic–hydrocarbon anionic surfactant foam [13] | 15.33 | 7 | 28 | |
| Alkylamide propyl betaine foam [6] | 17.1 | 7.5 | 114 | |
| Fluorocarbon surfactant foam containing imidazole [14] | 17.5 | 10.5 | 65 | |
| 6% AFFF foam extinguishing agent [15] | 22.5 | 25.75 | 42 | |
| 6% PF foam extinguishing agent [15] | 32.5 | 17.78 | 50 | |
| Novel aqueous film-forming foam extinguishing agent [16] | 16.58 | 9.8 | 65 | |
| Hydrocarbon–perfluorinated branched short-chain fluorocarbon surfactant foam [17] | 19.18 | 11.5 | 31 | |
| Imidazolium short-chain fluorocarbon surfactant foam [18] | 17.81 | 9.7 | 75 | |
| Group | SAS | AES | Polyoxyethylene Ether | Sulfobetaine | Butyl Glycol | Deionized Water |
|---|---|---|---|---|---|---|
| #1 | 15 | 5 | 2 | 1 | 20 | 57 |
| #2 | 15 | 5 | 2 | 3 | 20 | 55 |
| #3 | 15 | 5 | 2 | 5 | 20 | 53 |
| #4 | 15 | 5 | 2 | 7 | 20 | 51 |
| #5 | 15 | 5 | 2 | 9 | 20 | 49 |
| #6 | 15 | 5 | 4 | 1 | 20 | 55 |
| #7 | 15 | 5 | 4 | 3 | 20 | 53 |
| #8 | 15 | 5 | 4 | 5 | 20 | 51 |
| #9 | 15 | 5 | 4 | 7 | 20 | 49 |
| #10 | 15 | 5 | 4 | 9 | 20 | 47 |
| #11 | 15 | 5 | 6 | 1 | 20 | 53 |
| #12 | 15 | 5 | 6 | 3 | 20 | 51 |
| #13 | 15 | 5 | 6 | 5 | 20 | 49 |
| #14 | 15 | 5 | 6 | 7 | 20 | 47 |
| #15 | 15 | 5 | 6 | 9 | 20 | 45 |
| #16 | 15 | 5 | 8 | 1 | 20 | 51 |
| #17 | 15 | 5 | 8 | 3 | 20 | 49 |
| #18 | 15 | 5 | 8 | 5 | 20 | 47 |
| #19 | 15 | 5 | 8 | 7 | 20 | 45 |
| #20 | 15 | 5 | 8 | 9 | 20 | 43 |
| #21 | 15 | 5 | 10 | 1 | 20 | 49 |
| #22 | 15 | 5 | 10 | 3 | 20 | 47 |
| #23 | 15 | 5 | 10 | 5 | 20 | 45 |
| #24 | 15 | 5 | 10 | 7 | 20 | 43 |
| #25 | 15 | 5 | 10 | 9 | 20 | 41 |
| Dosage | Polyoxyethylene Ether (wt%) | Sulfobetaine (wt%) | Viscosity (mPa·s) | Standard Deviation | Wetting Time (s) | Standard Deviation | Expansion Ratio | Fire Extinguishing Time (s) |
|---|---|---|---|---|---|---|---|---|
| #4 | 2 | 7 | 2.27 | 0.052 | 7.57 | 0.0651 | 4.9 | 13 |
| #8 | 4 | 5 | 1.27 | 0.0458 | 7.65 | 0.0757 | 7.6 | 35 |
| #9 | 4 | 7 | 1.28 | 0.07 | 6.51 | 0.0557 | 8.3 | 26 |
| #14 | 6 | 7 | 1.28 | 0.017 | 6.28 | 0.0651 | 8 | 15 |
| #16 | 8 | 1 | 1.67 | 0.02 | 6.23 | 0.0569 | 8.2 | 18 |
| #17 | 8 | 3 | 1.97 | 0.0346 | 6.50 | 0.0451 | 4.9 | 30 |
| #18 | 8 | 5 | 1.24 | 0.02 | 6.45 | 0.0601 | 8.2 | 12 |
| #19 | 8 | 7 | 1.18 | 0.0346 | 5.65 | 0.0601 | 8.1 | 33 |
| #20 | 8 | 9 | 1.89 | 0.02 | 5.98 | 0.0702 | 7.8 | 20 |
| #24 | 10 | 7 | 1.16 | 0.0625 | 5.31 | 0.06 | 8.3 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Cheng, L.; Zhang, L.; Ma, L.; Deng, J.; Zhao, A.; Jiang, X.; Wang, F. Preparation and Characterization of a High-Performance Foam Extinguishing Agent with Sulfobetaine and Polyoxyethylene Ether for Solid Fires. Polymers 2025, 17, 2579. https://doi.org/10.3390/polym17192579
Ma H, Cheng L, Zhang L, Ma L, Deng J, Zhao A, Jiang X, Wang F. Preparation and Characterization of a High-Performance Foam Extinguishing Agent with Sulfobetaine and Polyoxyethylene Ether for Solid Fires. Polymers. 2025; 17(19):2579. https://doi.org/10.3390/polym17192579
Chicago/Turabian StyleMa, Huizhong, Liang Cheng, Lan Zhang, Liyang Ma, Jia Deng, Ao Zhao, Xin Jiang, and Fei Wang. 2025. "Preparation and Characterization of a High-Performance Foam Extinguishing Agent with Sulfobetaine and Polyoxyethylene Ether for Solid Fires" Polymers 17, no. 19: 2579. https://doi.org/10.3390/polym17192579
APA StyleMa, H., Cheng, L., Zhang, L., Ma, L., Deng, J., Zhao, A., Jiang, X., & Wang, F. (2025). Preparation and Characterization of a High-Performance Foam Extinguishing Agent with Sulfobetaine and Polyoxyethylene Ether for Solid Fires. Polymers, 17(19), 2579. https://doi.org/10.3390/polym17192579






