Application of Polymers in Hydraulic Fracturing Fluids: A Review
Abstract
1. Introduction
1.1. Hydraulic Fracturing
1.2. Fracturing Fluids
2. Polymer Used for Hydraulic Fracturing
2.1. Classification Based on Origin and Source
- Natural polymers come from natural sources like plants and animals. Examples include guar gum, cellulose such as CMC, and microbials such as Xanthan.
- Synthetic polymers are synthesized through chemical processes. Examples include polyacrylamide (PAM) and derivatives.
- Semi-synthetic polymers are modified natural polymers, such as cellulose acetate or rayon, which are chemically treated to enhance their properties [41].
2.2. Other Classification
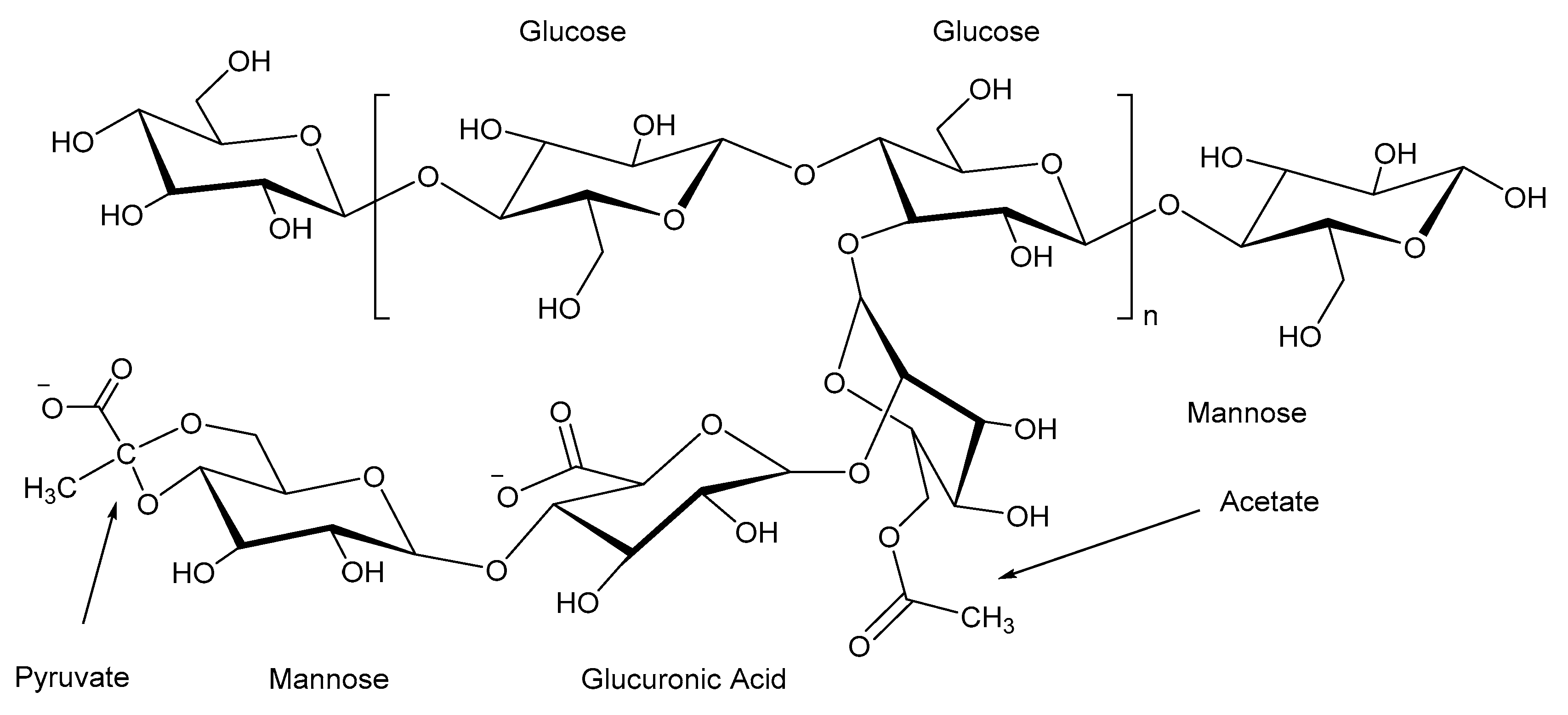
2.3. Guar Gum
2.4. Hydroxypropyl Guar
2.5. Carboxymethyl Hydroxypropyl Guar
2.6. Carboxymethyl Cellulose
2.7. Xanthan Gum
2.8. Polymer Cost
3. Other Fracturing Fluid Polymeric Additives
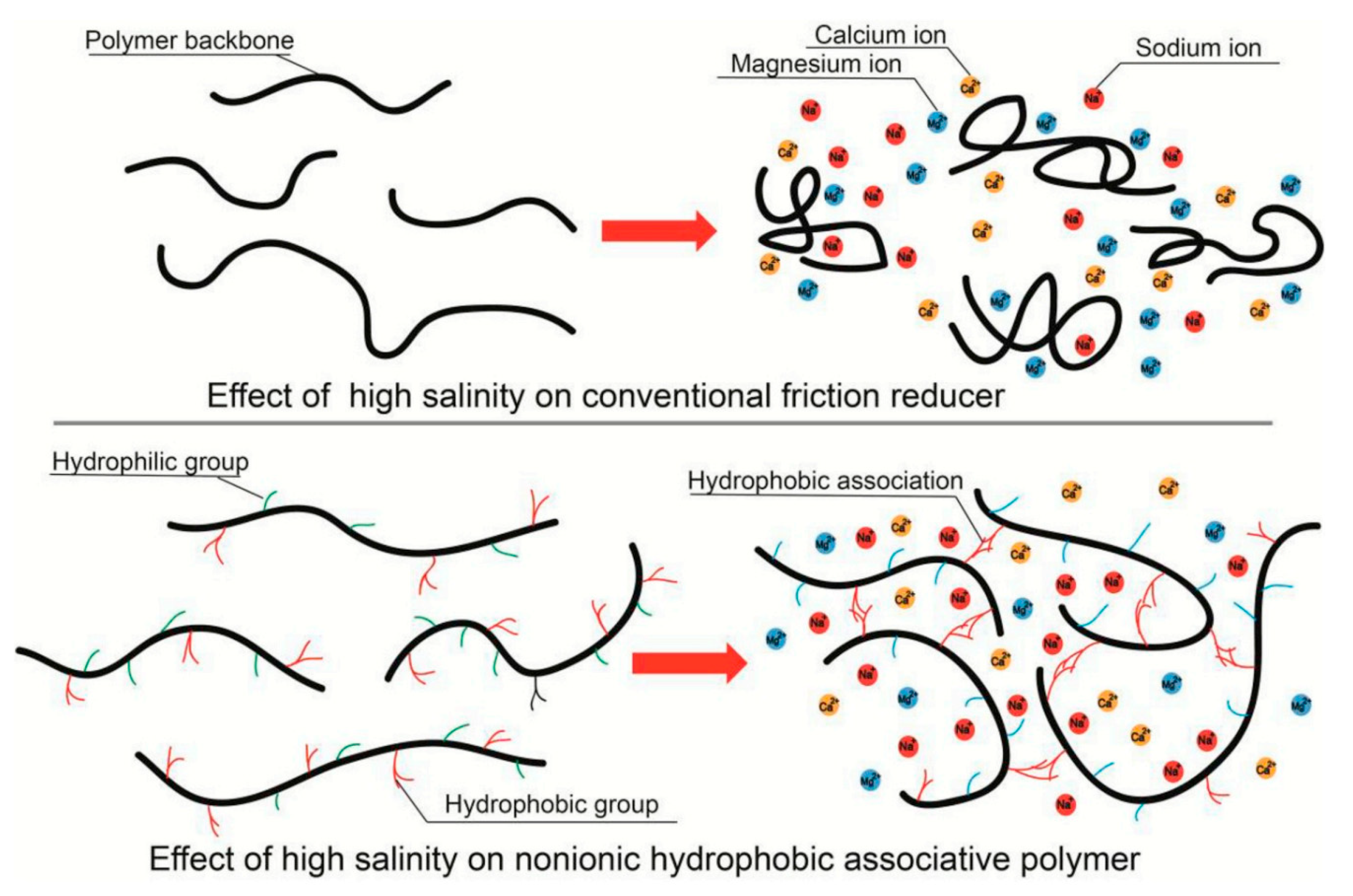
3.1. Friction Reducers
3.2. Scale Inhibitors
3.3. Comparison of Cross-Linked System to Slick Water System
4. Polymers’ Interaction with Fracturing Fluid Components
4.1. Hydration
Hydration in Saline Waters
4.2. Polymers Cross-Linking
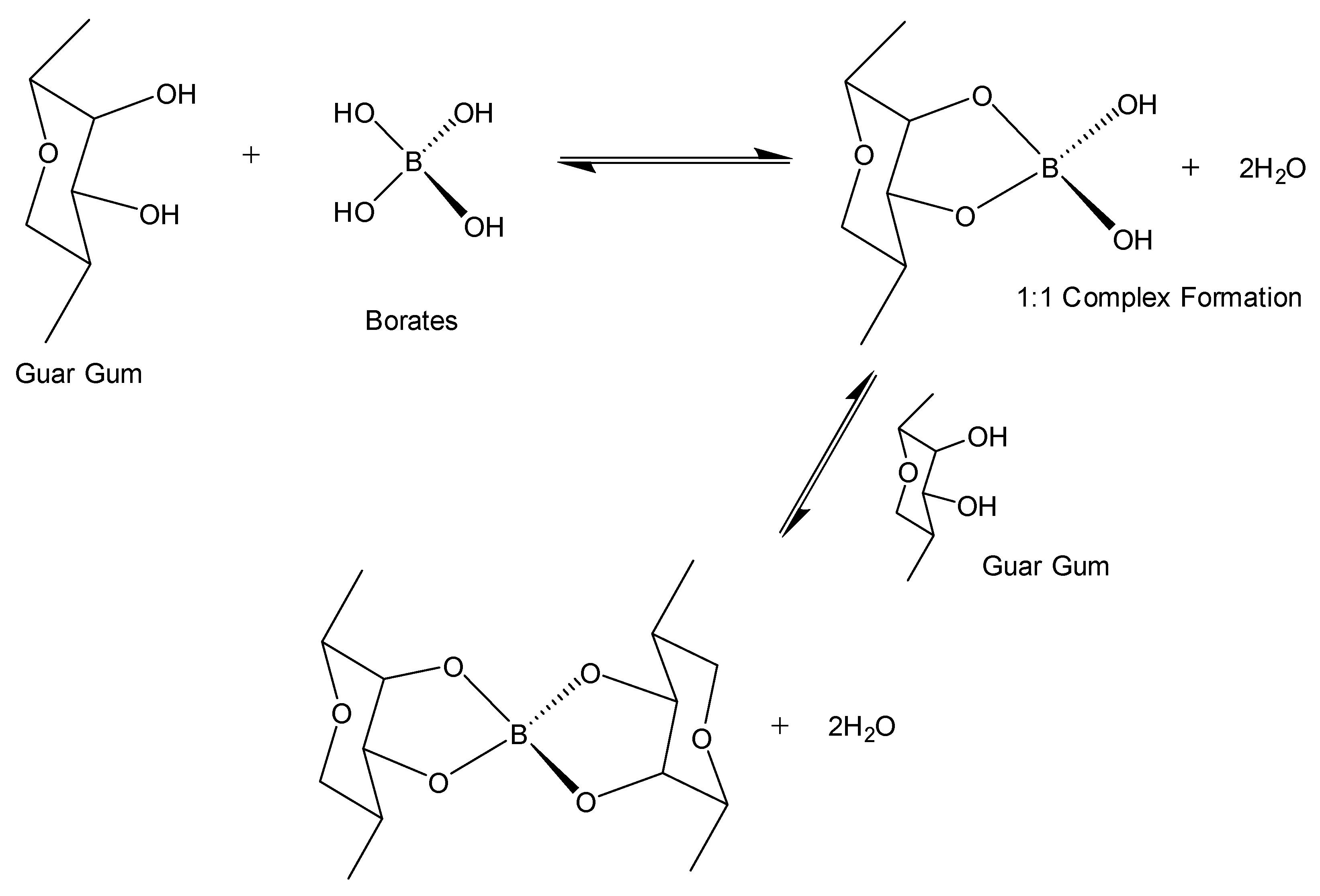
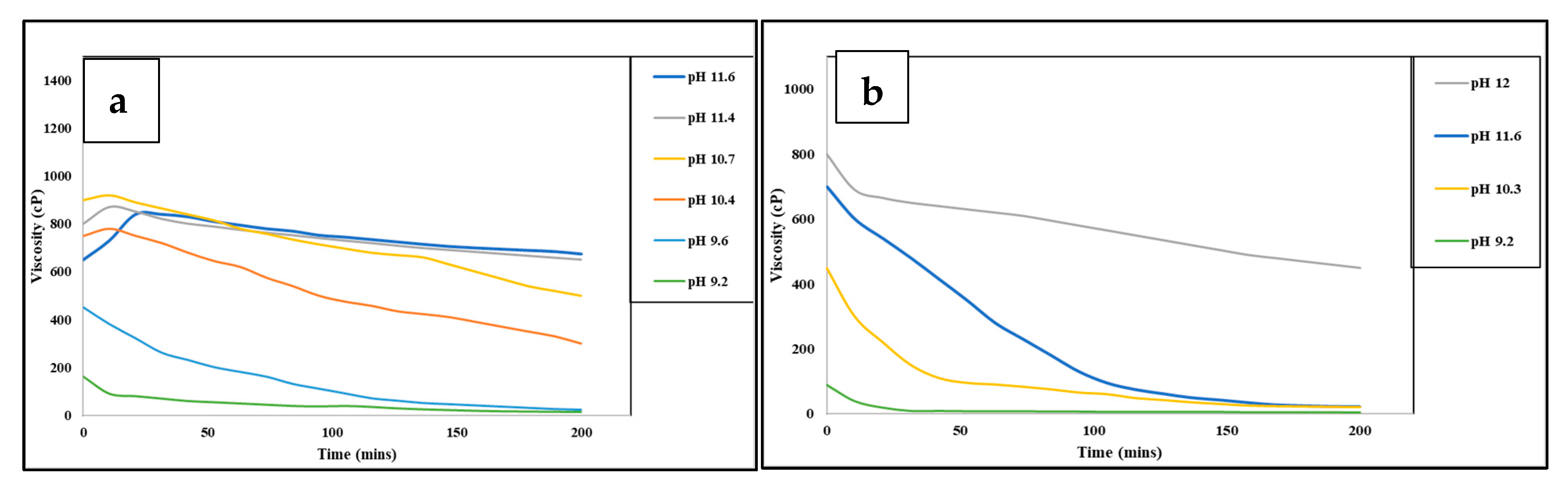
4.2.1. Salt Cross-Linkers
4.2.2. Metal Cross-Linkers
4.3. Polymer Breaking
4.3.1. Oxidative Breakers
4.3.2. Enzymatic Breakers
4.3.3. Delayed Breakers
5. Formation Damage and Environmental Issues
5.1. Formation Damage from Polymers
5.2. Environmental Effects
6. Recommendations and Prospects
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Rukavishnikov, V.S.; Minaev, K.M. Synthetic Polymers: A Review of Applications in Drilling Fluids. Pet. Sci. 2024, 21, 475–518. [Google Scholar] [CrossRef]
- Oseh, J.O.; Mohd, N.M.N.A.; Gbadamosi, A.O.; Agi, A.; Blkoor, S.O.; Ismail, I.; Igwilo, K.C.; Igbafe, A.I. Polymer Nanocomposites Application in Drilling Fluids: A Review. Geoenergy Sci. Eng. 2023, 222, 211416. [Google Scholar] [CrossRef]
- Agi, A.; Junin, R.; Gbonhinbor, J.; Onyekonwu, M. Natural Polymer Flow Behaviour in Porous Media for Enhanced Oil Recovery Applications: A Review. J. Pet. Explor. Prod. Technol. 2018, 8, 1349–1362. [Google Scholar] [CrossRef]
- Castro, R.H.; Llanos, S.; Rodríguez, J.; Quintero, H.I.; Manrique, E. Polymers for Eor Application in High Temperature and High Viscosity Oils: Rock–Fluid Behavior. Energies 2020, 13, 5944. [Google Scholar] [CrossRef]
- Musa, T.A.; Ibrahim, A.F.; Nasr-El-Din, H.A.; Hassan, A.M. New Insights into Guar Gum as Environmentally Friendly Polymer for Enhanced Oil Recovery in High-Salinity and High-Temperature Sandstone Reservoirs. J. Pet. Explor. Prod. Technol. 2021, 11, 1905–1913. [Google Scholar] [CrossRef]
- Abbas, S.; Sanders, A.W.; Donovan, J.C.; Oil, D. Applicability of Hydroxyethylcellulose Polymers for Chemical EOR. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 2–4 July 2013; pp. 2–4. [Google Scholar]
- Couto, M.R.; Gudiña, E.J.; Ferreira, D.; Teixeira, J.A.; Rodrigues, L.R. The Biopolymer Produced by Rhizobium Viscosum CECT 908 Is a Promising Agent for Application in Microbial Enhanced Oil Recovery. New Biotechnol. 2019, 49, 144–150. [Google Scholar] [CrossRef]
- Eiroboyi, I.; Ikiensikimama, S.S.; Oriji, B.A.; Okoye, I.P. The Effect of Monovalent and Divalent Ions on Biodegradable Polymers in Enhanced Oil Recovery. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 5–7 August 2019. [Google Scholar]
- Ogunkunle, T.F.; Oni, B.A.; Afolabi, R.O.; Fadairo, A.S.; Ojo, T.; Adesina, O. Comparative Analysis of the Performance of Hydrophobically Associating Polymer, Xanthan and Guar Gum as Mobility Control Agent, in Enhanced Oil Recovery Application. J. King Saud Univ.-Eng. Sci. 2022, 34, 402–407. [Google Scholar] [CrossRef]
- Anyaezu, T.V.; Musaab, M.; Salehi, S. Experimental Investigation of PAM/PEI Polymer Mud for Reducing Lost Circulation in High-Temperature Formations. Geothermics 2023, 114, 102786. [Google Scholar] [CrossRef]
- Rajabi, M.S.; Moradi, R.; Andrade, L.O. Chemically Crosslinked Polyvinyl Alcohol for Water Shut-off and Conformance Control Treatments during Oil Production: The Effect of Silica Nanoparticles. J. Appl. Polym. Sci. 2023, 140, e53382. [Google Scholar] [CrossRef]
- Lakatos, I.; Szentes, G.; Toro, M.; Karaffa, Z.; Vago, A. Mitigation of Formation Damage Caused by Chemical Overdosing in Water Shut-Off Treatments. In Proceedings of the SPE International Conference and Exhibition on Formation Damage Control, Lafayette, LA, USA, 19–21 February 2020; pp. 19–21. [Google Scholar]
- Al-Muntasheri, G.A.; Nasr-El-Din, H.A.; Hussein, I.A. A Rheological Investigation of a High Temperature Organic Gel Used for Water Shut-off Treatments. J. Pet. Sci. Eng. 2007, 59, 73–83. [Google Scholar] [CrossRef]
- Reddy, B.R.; Eoff, L.S. Gellable Treatment Fluids Comprising Quaternary Ammonium Salt Gel-Time Modifiers and Methods for Use Thereof. U.S. Patent 9,150,781, 6 October 2015. [Google Scholar]
- El-Karsani, K.S.M.; Al-Muntasheri, G.A.; Sultan, A.S.; Hussein, I.A. Gelation of a Water-Shutoff Gel at High Pressure and High Temperature: Rheological Investigation. SPE J. 2015, 20, 1103–1112. [Google Scholar] [CrossRef]
- Othman, A.; Aljawad, M.S.; Al-Ramadan, M.; Kamal, M.S.; Al-Taq, A.; Al-Mohsin, A. Evaluation of a Novel Emulsion Preparation Method for Reducing Water-Cut in Production. In Proceedings of the Society of Petroleum Engineers—ADIPEC 2024, Abu Dhabi, United Arab Emirates, 4–7 November 2024; Society of Petroleum Engineers: Richardson, TX, USA, 2024. [Google Scholar]
- Economides, M.J.; Nolte, K.G. Reservoir Stimulation; Wiley: Hoboken, NJ, USA, 2000; Volume 3, ISBN 978-0471491927. [Google Scholar]
- Das, P.; Rahim, Z. Evaluate Fracturing Fluid Performance for Hydraulic Stimulation in Pre-Khuff Sandstone Reservoirs of Ghawar Gas Field. In Proceedings of the Society of Petroleum Engineers—SPE Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 21–24 April 2014. [Google Scholar] [CrossRef]
- Samuel, M.; Polson, D.; Graham, D.; Kordziel, W.; Waite, T.; Waters, G.; Vinod, P.S.; Fu, D.; Downey, R. Viscoelastic Surfactant Fracturing Fluids: Applications in Low Permeability Reservoirs. In Proceedings of the SPE Rocky Mountain Regional/Low-Permeability Reservoirs Symposium and Exhibition, Denver, CO, USA, 12–15 March 2000; pp. 1–7. [Google Scholar] [CrossRef]
- Gandossi, L.; Von EStroff, U. An Overview of Hydraulic Fracturing and Other Formation Stimulation Technologies for Shale Gas Production; Publications Office of the European Union: Luxembourg, 2015. [Google Scholar]
- Hoffman, A.; Olsson, G.; Lindström, A. Shale Gas and Hydraulic Fracturing: Framing the Water Issue; SIWI: Stockholm, Sweden, 2014; ISBN 9789198186017. [Google Scholar]
- Cook, T.; Perrin, J.; Van Wagener, D. Hydraulically Fractured Horizontal Wells Account for Most New Oil and Natural Gas Wells; U.S. Energy Information Administration: Washington, DC, USA, 2018. [Google Scholar]
- Gaurina-Međimurec, N.; Brkić, V.; Topolovec, M.; Mijić, P. Fracturing Fluids and Their Application in the Republic of Croatia. Appl. Sci. 2021, 11, 2807. [Google Scholar] [CrossRef]
- Fink, J. Petroleum Engineer’s Guide to Oil Field Chemicals and Fluids, 3rd ed.; Gulf Professional Publishing: Houston, TX, USA, 2021; ISBN 9780128037355. [Google Scholar]
- Barati, R.; Liang, J.T. A Review of Fracturing Fluid Systems Used for Hydraulic Fracturing of Oil and Gas Wells. J. Appl. Polym. Sci. 2014, 131, 1–11. [Google Scholar] [CrossRef]
- Harris, P.C. Fracturing-Fluid Additives. J. Pet. Technol. 1988, 40, 1277–1279. [Google Scholar] [CrossRef]
- Wilson, A. A Comparison Between Seawater-Based and Freshwater-Based Fracturing Fluids. J. Pet. Technol. 2017, 69, 46–47. [Google Scholar] [CrossRef]
- Almubarak, T.; AlKhaldi, M.; Ng, J.H.; Nasr-El-Din, H.A. Design and Application of High-Temperature Raw-Seawater-Based Fracturing Fluids. SPE J. 2019, 24, 1929–1946. [Google Scholar] [CrossRef]
- Harris, P.C.; van Batenburg, D. Comparison of Freshwater- and Seawater-Based Borate-Crosslinked Fracturing Fluids. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 16–19 February 1999; pp. 671–675. [Google Scholar] [CrossRef]
- Langford, M.; Holland, B.; Green, C.A.; Bocaneala, B.; Norris, M. Offshore Horizontal Well Fracturing: Operational Optimisation in the Southern North Sea. In Proceedings of the Society of Petroleum Engineers—SPE Offshore Europe Conference and Exhibition, OE 2013, Aberdeen, UK, 3–6 September 2013; Volume 2, pp. 104–113. [Google Scholar] [CrossRef]
- Powell, R.J.; McCabe, M.A.; Slabaugh, B.F.; Terracina, J.M.; McPike, T. Gulf of Mexico Frac-and-Pack Treatments Using a New Fracturing Fluid System. World Oil 1998, 219, 69–73. [Google Scholar] [CrossRef]
- Denney, D. Fracturing-Fluid Effects on Shale and Proppant Embedment. J. Pet. Technol. 2012, 64, 59–61. [Google Scholar] [CrossRef]
- Patil, P.; Muthusamy, R.; Pandya, N. Novel Controlled-Release Breakers for High-Temperature Fracturing. In Proceedings of the Society of Petroleum Engineers—North Africa Technical Conference and Exhibition 2013, NATC 2013, Cairo, Egypt, 15–17 April 2013; Volume 1, pp. 599–604. [Google Scholar] [CrossRef]
- Sangaru, S.S.; Yadav, P.; Huang, T.; Agrawal, G.; Chang, F.F. Surface Modified Nanoparticles as Internal Breakers for Viscoelastic Surfactant Based Fracturing Fluids for High Temperature Operations. In Proceedings of the Society of Petroleum Engineers—SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition 2017, Dammam, Saudi Arabia, 24–27 April 2017; pp. 2317–2323. [Google Scholar] [CrossRef]
- Xue, Y.; Voordouw, G. Control of Microbial Sulfide Production with Biocides and Nitrate in Oil Reservoir Simulating Bioreactors. Front. Microbiol. 2015, 6, 1387. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Raju, S. A State of Review on Hydraulic Fracturing Fluid Properties Using Nanoparticles Application in an Unconventional Reservoir. Int. J. Eng. Res. Technol. 2020, 9, 247–257. [Google Scholar]
- Reynolds, M.A. A Technical Playbook for Chemicals and Additives Used in the Hydraulic Fracturing of Shales. Energy Fuels 2020, 34, 15106–15125. [Google Scholar] [CrossRef]
- Baker, R.O.; Bora, R.; Schechter, D.S.; McDonald, P.; Knight, W.H.; Leonard, P.; Rounding, C. Development of a Fracture Model for Spraberry Field, Texas USA. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 30 September–3 October 2001; pp. 2701–2716. [Google Scholar] [CrossRef]
- Hassan, A.; Mahmoud, M.; Bageri, B.S.; Aljawad, M.S.; Kamal, M.S.; Barri, A.A.; Hussein, I.A. Applications of Chelating Agents in the Upstream Oil and Gas Industry: A Review. Energy Fuels 2020, 34, 15593–15613. [Google Scholar] [CrossRef]
- Liang, F.; Sayed, M.; Al-Muntasheri, G.A.; Chang, F.F.; Li, L. A Comprehensive Review on Proppant Technologies. Petroleum 2016, 2, 26–39. [Google Scholar] [CrossRef]
- Fried, J.R. Polymer Science and Technology, 3rd ed.; Prentice Hall Press: Hoboken, NJ, USA, 2014; ISBN 0137039557. [Google Scholar]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A Review of Water Interactions, Applications in Composites, and Water Treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef]
- Othman, A.; Aljawad, M.S.; Mahmoud, M.; Kamal, M.S.; Patil, S.; Bataweel, M. Chelating Agents Usage in Optimization of Fracturing Fluid Rheology Prepared from Seawater. Polymers 2021, 13, 2111. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of Xanthan Gum as Polysaccharide in Tissue Engineering: A Review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Uranta, K.G.; Rezaei Gomari, S.; Russell, P.; Hamad, F. Application of Polymer Integration Technique for Enhancing Polyacrylamide (PAM) Performance in High Temperature and High Salinity Reservoirs. Heliyon 2019, 5, e02113. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liang, Z.; Zhang, R.; Zhou, S.; Yang, H.; Chen, Y.; Zhang, J.; Yin, H.; Yu, D. Research Advances in Superabsorbent Polymers. Polymers 2024, 16, 501. [Google Scholar] [CrossRef] [PubMed]
- Berradi, A.; Aziz, F.; Achaby, M.E.; Ouazzani, N.; Mandi, L. A Comprehensive Review of Polysaccharide-Based Hydrogels as Promising Biomaterials. Polymers 2023, 15, 2908. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Z. Tailoring the Swelling-Shrinkable Behavior of Hydrogels for Biomedical Applications. Adv. Sci. 2023, 10, 2303326. [Google Scholar] [CrossRef]
- Luo, J.; Demchuk, Z.; Zhao, X.; Saito, T.; Tian, M.; Sokolov, A.P.; Cao, P.F. Elastic Vitrimers: Beyond Thermoplastic and Thermoset Elastomers. Matter 2022, 5, 1391–1422. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Mészáros, L.; Bárány, T. Ground Tyre Rubber (GTR) in Thermoplastics, Thermosets, and Rubbers. J. Mater. Sci. 2013, 48, 1–38. [Google Scholar] [CrossRef]
- Kauffman, G.B. Polymer Chemistry: An Introduction, 3rd Edition. Chem. Educ. 2000, 5, 97–98. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Nezafat, Z.; Shafiei, N.; Soleimani, F. Polysaccharides in Food Industry. In Biopolymer-Based Metal Nanoparticle Chemistry for Sustainable Applications: Volume 2: Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 47–96. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar Gum: Processing, Properties and Food Applications—A Review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Sharahi, M.; Bahrami, S.H.; Karimi, A. A Comprehensive Review on Guar Gum and Its Modified Biopolymers: Their Potential Applications in Tissue Engineering. Carbohydr. Polym. 2025, 347, 122739. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ye, J. Rheological and Fracturing Characteristics of a Cationic Guar Gum. Int. J. Biol. Macromol. 2023, 224, 196–206. [Google Scholar] [CrossRef]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Guar Gum as a Promising Starting Material for Diverse Applications: A Review. Int. J. Biol. Macromol. 2016, 88, 361–372. [Google Scholar] [CrossRef]
- Ihejirika, B.; Dosunmu, A.; Eme, C. Performance Evaluation of Guar Gum as a Carrier Fluid for Hydraulic Fracturing. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 4–6 August 2015; p. 178297. [Google Scholar]
- Weaver, J.; Gdanski, R.; Karcher, A. Guar Gum Degradation: A Kinetic Study. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 5–7 February 2003. [Google Scholar]
- Cooke, C.E. Effect of Fracturing Fluids on Fracture Conductivity. J. Pet. Technol. 1975, 27, 1273–1282. [Google Scholar] [CrossRef]
- Prabhanjan, H.; Gharia, M.M.; Srivastava, H.C. Guar Gum Derivatives. Part I: Preparation and Properties. Carbohydr. Polym. 1989, 11, 279–292. [Google Scholar] [CrossRef]
- Montgomery, C. Fracturing Fluid Components. In Effective and Sustainable Hydraulic Fracturing; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Wang, S.; Tang, H.; Guo, J.; Wang, K. Effect of PH on the Rheological Properties of Borate Crosslinked Hydroxypropyl Guar Gum Hydrogel and Hydroxypropyl Guar Gum. Carbohydr. Polym. 2016, 147, 455–463. [Google Scholar] [CrossRef]
- Xiong, Z.; Fu, F.; Zou, Z.; Li, X.; Tao, S.; Li, Y. Development and Application of Guar Gum Crosslinked Gel with Adjustable Gelation Time for Total Loss Treatment. Petroleum 2023, 9, 621–628. [Google Scholar] [CrossRef]
- Fan, H.; Gong, Z.; Wei, Z.; Chen, H.; Fan, H.; Geng, J.; Kang, W.; Dai, C. Understanding the Temperature-Resistance Performance of a Borate Cross-Linked Hydroxypropyl Guar Gum Fracturing Fluid Based on a Facile Evaluation Method. RSC Adv. 2017, 7, 53290–53300. [Google Scholar] [CrossRef]
- Al-Mohammed, A.M.; Nasr-El-Din; Hisham, A.; Al-Fuwaires, O.A.; Al-Aamri, A. Degradation of High PH Borate Gels. In Proceedings of the International Petroleum Technology Conference, Dubai, United Arab Emirates, 4–6 December 2007. [Google Scholar]
- Zhong, C.; Du, P.; Deng, Y.; Wu, Y.; He, J. Coupled Computational and Laboratory Investigations of Interface Interactions between Binary Polymers, Hydroxypropyl Guar, and Potassium Formate for Crosslinked Hydraulic Fracturing Fluids. SPE J. 2024, 29, 4651–4667. [Google Scholar] [CrossRef]
- Zhang, L.M.; Zhou, J.F.; Hui, P.S. A Comparative Study on Viscosity Behavior of Water-Soluble Chemically Modified Guar Gum Derivatives with Different Functional Lateral Groups. J. Sci. Food Agric. 2005, 85, 2638–2644. [Google Scholar] [CrossRef]
- Alohaly, M.; Binghanim, A.; Rahal, R.; Rahim, S. Seawater Fracturing Fluid Development Challenges: A Comparison between Seawater-Based and Freshwater-Based Fracturing Fluids Using Two Types of Guar Gum Polymers. In Proceedings of the Society of Petroleum Engineers—SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 25–28 April 2016. [Google Scholar] [CrossRef]
- Almubarak, T.; Li, L.; Ng, J.H.; Nasr-El-Din, H.; AlKhaldi, M. New Insights into Hydraulic Fracturing Fluids Used for High-Temperature Wells. Petroleum 2021, 7, 70–79. [Google Scholar] [CrossRef]
- Othman, A.; Aljawad, M.S.; Kamal, M.S.; Mahmoud, M.; Patil, S.; Alkhowaildi, M. Rheological Study of Seawater-Based Fracturing Fluid Containing Polymer, Crosslinker, and Chelating Agent. ACS Omega 2022, 7, 31318–31326. [Google Scholar] [CrossRef]
- Othman, A.; Kamal, M.S.; Aljawad, M.S.; Mahmoud, M.; Sultan, A.; Kalgaonkar, R. Study of Cross-Linked Gel and Chelating Agent Compatibility in High Salinity Environments. Energy Fuels 2024, 38, 608–616. [Google Scholar] [CrossRef]
- Idris, R.; Rosli, N.R.; Mohammadian, E.; Hasan, N. Rheological Studies of High-Density Fracturing Fluid Using High-Density Brine and Carboxymethyl Hydroxypropyl Guar (CMHPG) for High-Temperature Well Condition. Arch. Mater. Sci. Eng. 2023, 120, 49–59. [Google Scholar] [CrossRef]
- Ming, H.; Lu, Y.; Qiu, X.; Shu, Y.; Wang, S. Development and Field Application of a Novel Cellulose Fracturing Fluid. In Proceedings of the SPE Asia Pacific Hydraulic Fracturing Conference, Beijing, China, 24–26 August 2016. [Google Scholar]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and Sustainable Pretreatment Methods for Cellulose Extraction from Lignocellulosic Biomass and Its Applications: A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, Y.; Wang, X.; Duan, Y.; Che, M.; Lu, Y. Study and Application of Novel Cellulose Fracturing Fluid in Ordos Basin. IOP Conf. Ser. Earth Environ. Sci. 2018, 170, 022145. [Google Scholar] [CrossRef]
- Abdulhameed, A.; Mbuvi, H.M.; Changamu, E.O.; Maingi, F.M. Microwave Synthesis of Carboxymethylcellulose (CMC) from Rice Husk. IOSR J. Appl. Chem. 2019, 12, 33–42. [Google Scholar] [CrossRef]
- Scheffer, G.; Berdugo-Clavijo, C.; Sen, A.; Gieg, L.M. Enzyme Biotechnology Development for Treating Polymers in Hydraulic Fracturing Operations. Microb. Biotechnol. 2021, 14, 953–966. [Google Scholar] [CrossRef]
- Nsengiyumva, E.M.; Alexandridis, P. Xanthan Gum in Aqueous Solutions: Fundamentals and Applications. Int. J. Biol. Macromol. 2022, 216, 583–604. [Google Scholar] [CrossRef]
- Daiana Preichardt, L.; Klaic, P. Xanthan Gum Application in Food. In Xanthan Gum Applications and Research Studies; Nova Publishers: Hauppauge, NY, USA, 2016; ISBN 978-1-53610-010-5. [Google Scholar]
- Gall, B.L.; Sattler, A.R.; Maloney, D.R.; Raible, C.J. Permeability Damage to Natural Fractures Caused by Fracturing Fluid Polymers. In Proceedings of the SPE Rocky Mountain Petroleum Technology Conference/Low-Permeability Reservoirs Symposium, Casper, WY, USA, 11–13 May 1988. [Google Scholar]
- Rupert Neate Beans Mean High Profits for Guar Farmers of Rajasthan. Available online: https://www.theguardian.com/environment/2012/dec/18/fracking-guar-beans-profit-rajasthan-india (accessed on 21 July 2025).
- Chemical and Material Carboxymethylhydroxypropyl Guar (CMHPG) Market. Available online: https://pmarketresearch.com/chemi/carboxymethyl-hydroxypropyl-guar-cmhpg-market/?utm_source=chatgpt.com (accessed on 21 July 2025).
- Zhong, C.; Chen, R.; Liu, B.; Pu, S.; Hou, D. Trends in Polyacrylamide Utilization and Treatment for Hydraulic Fracturing. npj Mater. Sustain. 2024, 2, 15. [Google Scholar] [CrossRef]
- Chemanalyst. Chemical Price Analysis, Chemical Latest Prices. Available online: https://www.chemanalyst.com/Pricing/Pricingoverview (accessed on 10 September 2025).
- IMARC. Guar Gum Prices, Trend, Chart, Demand, Market Analysis, News, Historical and Forecast Data Report 2025 Edition. Available online: https://www.imarcgroup.com/guar-gum-pricing-report?utm_source=chatgpt.com (accessed on 21 July 2025).
- Sareen, A.; Zhou, J.; Cruz, C.; Sun, H.; Qu, Q.; Li, L.; Hughes, B. Successful Slickwater Fracturing in Ultrahigh TDS Produced Water by Novel Environmentally Preferred Friction Reducer. In Proceedings of the International Petroleum Technology Conference, Kuala Lumpur, Malaysia, 10–12 December 2014; pp. 10–12. [Google Scholar]
- Ghosh, B.; Abdelrahim, M.; Ghosh, D.; Belhaj, H. Delayed Breaker Systems to Remove Residual Polymer Damage in Hydraulically Fractured Reservoirs. ACS Omega 2021, 6, 31646–31657. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhao, J.; Mao, J.; Tan, H.; Zhang, Y.; Song, Z. Review of Friction Reducers Used in Slickwater Fracturing Fluids for Shale Gas Reservoirs. J. Nat. Gas Sci. Eng. 2019, 62, 302–313. [Google Scholar] [CrossRef]
- Aften, C.W.; Watson, W.P.; Chemicals, K. Improved Friction Reducer for Hydraulic Fracturing. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 19–21 January 2009; pp. 19–21. [Google Scholar]
- Aften, C.W. Study of Friction Reducers for Recycled Stimulation Fluids in Environmentally Sensitive Regions. In Proceedings of the SPE Eastern Regional Meeting, Morgantown, WV, USA, 12–14 October 2010; pp. 12–14. [Google Scholar]
- Olajire, A.A. A Review of Oilfield Scale Management Technology for Oil and Gas Production. J. Pet. Sci. Eng. 2015, 135, 723–737. [Google Scholar] [CrossRef]
- FQE-Chemicals Water Formed Scale. Available online: https://fqechemicals.com/water-formed-scale/ (accessed on 25 May 2025).
- Vo, L.K.; Cortez, J.; Hoeman, K.; Rahal, R.; Biyani, M. Scale Inhibition: A Challenge and a Mitigation Study in Fracturing with High-Brine Water. In Proceedings of the SPE/CSUR Unconventional Resources Conference, Manama, Bahrain, 6–9 March 2017. [Google Scholar]
- Cheremisov, K.; Oussoltsev, D.; Butula, K.K.; Gaifullin, A.; Faizullin, I.; Senchenko, D.; Neft, G. First Application of Scale Inhibitor During Hydraulic Fracturing Treatments in Western Siberia. In Proceedings of the SPE International Oilfield Scale Conference, Aberdeen, UK, 28–29 May 2008; pp. 28–29. [Google Scholar]
- Korlepara, N.K.; Ruthala, S.; Deva, A.; Kosireddy, K. Rheological Study of Slickwater Fluid Systems Consisting of High-Vis Friction Reducers Additives for Hydraulic Fracturing Applications. In Proceedings of the 2nd Edition of Indian Oil & Gas Chemistry, Chemicals and Additives Conference, Ahmedabad, India, 16–17 September 2019. [Google Scholar]
- Kotb, A.; Almubarak, T.; Nasr-El-Din, H.A. Surfactant and Friction Reducer Interaction in High Salinity Slickwater Fracturing. In Proceedings of the Society of Petroleum Engineers—SPE International Hydraulic Fracturing Technology Conference and Exhibition, IHFT 2022, Muscat, Oman, 11–13 January 2022; Society of Petroleum Engineers: Richardson, TX, USA, 2022. [Google Scholar]
- Harris, P.C.; Harms, W.M.; Norman, L.; Services, H. Study of Continuously Mixed Crosslinked Fracturing Fluids with a Recirculating Flow-Loop Viscometer. SPE Prod. Eng. 1989, 4, 430–434. [Google Scholar] [CrossRef]
- Bai, H.; Zhou, F.; Liu, X.; Xin, X.; Zhao, H.; Ding, Z.; Wang, Y.; Wang, X.; Li, X.; Li, W.; et al. Study on PH-Responsive Delayed, Cross-Linking and Weighted Fracturing Fluid. Molecules 2024, 29, 5847. [Google Scholar] [CrossRef]
- Du, J.; Liu, J.; Zhao, L.; Liu, P.; Chen, X.; Wang, Q.; Yu, M. Water-Soluble Polymers for High-Temperature Resistant Hydraulic Fracturing: A Review. J. Nat. Gas Sci. Eng. 2022, 104, 104673. [Google Scholar] [CrossRef]
- Gupta, D.V.S.; Carman, P.S.; Nguyen, S. Novel Chelation Opens the Door for Redeployment of Sea Water Based Fracturing Fluids in High Temperature Wells. In Proceedings of the SPE International Symposium on Formation Damage Control, Lafayette, LA, USA, 7–9 February 2018. [Google Scholar] [CrossRef]
- Li, L.; Al-Muntasheri, G.A.; Liang, F. A Review of Crosslinked Fracturing Fluids Prepared with Produced Water. Petroleum 2016, 2, 313–323. [Google Scholar] [CrossRef]
- Prakash, C.; Raykov, T.; Koalsa, B.; Belakshe, R.; Janiczek, P. Hydraulic Fracturing Application of New Seawater-Based Clean Fluid. In Proceedings of the Society of Petroleum Engineers—Abu Dhabi International Petroleum Exhibition and Conference 2016, Abu Dhabi, United Arab Emirates, 7–10 November 2016; pp. 1–20. [Google Scholar] [CrossRef]
- Othman, A.; Aljawad, M.S.; Kamal, M.S.; Mahmoud, M.; Patil, S.; Kalgaonkar, R. Individual Seawater Ions’ Impact on the Rheology of Crosslinked Polymers in the Presence of a Chelating Agent. Energy Fuels 2023, 37, 7328–7338. [Google Scholar] [CrossRef]
- de Kruijf, A.S.; Roodhart, L.P.; Davies, D.R. Relation between Chemistry and Flow Mechanics of Borate-Crosslinked Fracturing Fluids. SPE Prod. Facil. 1993, 8, 165–169. [Google Scholar] [CrossRef]
- Budiman, O.; Alajmei, S. Seawater-Based Fracturing Fluid: A Review. ACS Omega 2023, 8, 41022–41038. [Google Scholar] [CrossRef] [PubMed]
- Stringfellow, W.T.; Domen, J.K.; Camarillo, M.K.; Sandelin, W.L.; Borglin, S. Physical, Chemical, and Biological Characteristics of Compounds Used in Hydraulic Fracturing. J. Hazard. Mater. 2014, 275, 37–54. [Google Scholar] [CrossRef]
- Bishop, M.; Shahid, N.; Yang, J.; Barron, A.R. Determination of the Mode and Efficacy of the Cross-Linking of Guar by Borate Using MAS11B NMR of Borate Cross-Linked Guar in Combination with Solution11B NMR of Model Systems. Dalton Trans. 2004, 2621–2634. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, J.; Zuo, Z.; Liao, M.; Peng, P. Preparation and Property Evaluation of a Temperature-Resistant Zr-Crosslinked Fracturing Fluid. J. Ind. Eng. Chem. 2021, 96, 121–129. [Google Scholar] [CrossRef]
- Harris, P.C. Chemistry and Rheology of Borate-Crosslinked Fluids at Temperatures to 300F. J. Pet. Technol. 1993, 45, 264–269. [Google Scholar] [CrossRef]
- Bahamdan, A. Hydrophobic Guar Gum Derivatives Prepared by Controlled Grafting Processes for Hydraulic Fracturing Applications. Ph.D. Thesis, Louisiana State University, Baton Rouge, LA, USA, 1989. [Google Scholar]
- Jain, N.; Garg, K.; Chandra Karmakar, N.; Kumar Palei, S.; Karmakar, N.C.; Palei, S.K. Guar Gum in Hydraulic Fracturing in Indian Shale Mines. In Proceedings of the Present Technology and Safety Scenario in Mining and Allied Industries, Varanasi, India, 25–27 February 2013. [Google Scholar]
- Almubarak, T.; Ng, J.H.; Nasr-El-Din, H.A.; Almubarak, M.; AlKhaldi, M. Zirconium Crosslinkers: Understanding Performance Variations in Crosslinked Fracturing Fluids. In Proceedings of the Offshore Technology Conference Asia (OTAC), Kuala Lumpur, Malaysia, 2–6 November 2020. [Google Scholar] [CrossRef]
- Chauhan, G.; Verma, A.; Doley, A.; Ojha, K. Rheological and Breaking Characteristics of Zr-Crosslinked Gum Karaya Gels for High-Temperature Hydraulic Fracturing Application. J. Pet. Sci. Eng. 2019, 172, 327–339. [Google Scholar] [CrossRef]
- Kyaw, A.; Syabila, B.; Azahar, B.N.; Tunio, S.Q. Fracturing Fluid (Guar Polymer Gel) Degradation Study by Using Oxidative and Enzyme Breaker. Res. J. Appl. Sci. Eng. Technol. 2012, 4, 1667–1671. [Google Scholar]
- Fuller, M.J. An Innovative Approach to Gel Breakers for Hydraulic Fracturing. JPT J. Pet. Technol. 2017, 69, 48–51. [Google Scholar] [CrossRef]
- Armstrong, C.D.; Stevens, R.F.; Le, H.; Stephenson, C.; Qu, Q. The next Generation of Regenerative Catalytic Breakers for Use in Alkaline and High-Temperature Fracturing Fluids. In Proceedings of the SPE International Symposium on Formation Damage Control, Lafayette, LA, USA, 10–12 February 2010; Volume 2, pp. 603–615. [Google Scholar] [CrossRef]
- Alfakher, B.; Al-Taq, A.; Aldarweesh, S.; Alhamad, L.; Aramco, S. Fracturing Fluid Design: A Closer Look at Breaker and Surfactant Selection. In Proceedings of the SPE Middle East Oil & Gas Show and Conference, Event Canceled, 28 November–1 December 2021. [Google Scholar]
- Fei, G.; Liu, B.; Guo, L.; Chang, Y.; Xue, B. Development and Evaluation of Mesoporous SiO2 Nanoparticle-Based Sustained-Release Gel Breaker for Clean Fracturing Fluids. Polymers 2025, 17, 2078. [Google Scholar] [CrossRef]
- Brannon, H.D.; Tjon-Joe-Pin, R.M.; Services, B.J.; Usa, C. Application of Polymeric Damage Removal Treatment Results in Multi-Fold Well Productivity Improvement: A Case Study. In Proceedings of the SPE Middle East Oil Show, Manama, Bahrain, 11–14 March 1995. [Google Scholar]
- Lee, D.S.; Elsworth, D.; Yasuhara, H.; Weaver, J.D.; Rickman, R. Experiment and Modeling to Evaluate the Effects of Proppant-Pack Diagenesis on Fracture Treatments. J. Pet. Sci. Eng. 2010, 74, 67–76. [Google Scholar] [CrossRef]
- Karazincir, O.; Li, Y.; Zaki, K.; Williams, W.; Wu, R.; Tan, Y.; Rijken, P.; Allan, C. Measurement of Reduced Permeability at Fracture Face Due to Proppant Embedment and Depletion-Part II. In Proceedings of the SPE Annual Technical Conference and Exhibition, Calgary, AB, Canada, 30 September–2 October 2019; Volume 2, p. 196204. [Google Scholar]
- Dong, K.; Wang, M.; Zhang, C. Effect of Wettability of Ceramic Proppant Surface in Guar Gum Solution on the Oil Flow Efficiency in Fractures. Petroleum 2019, 5, 388–396. [Google Scholar] [CrossRef]
- Lee, D.S.; Yasuhara, H. An Evaluation of the Effects of Fracture Diagenesis on Hydraulic Fracturing Treatment. Geosyst. Eng. 2013, 16, 113–118. [Google Scholar] [CrossRef]
- Da, Q.; Yao, C.; Zhang, X.; Li, L.; Lei, G. Reservoir Damage Induced by Water-Based Fracturing Fluids in Tight Reservoirs: A Review of Formation Mechanisms and Treatment Methods. Energy Fuels 2024, 38, 18093–18115. [Google Scholar] [CrossRef]
- Chen, M.; Yan, M.; Kang, Y.; Fang, S.; Liu, H.; Wang, W.; Shen, J.; Chen, Z. Shale Formation Damage during Fracturing Fluid Imbibition and Flowback Process Considering Adsorbed Methane. Energies 2022, 15, 9176. [Google Scholar] [CrossRef]
- Khan, H.J.; Spielman-Sun, E.; Jew, A.D.; Bargar, J.; Kovscek, A.; Druhan, J.L. A Critical Review of the Physicochemical Impacts of Water Chemistry on Shale in Hydraulic Fracturing Systems. Environ. Sci. Technol. 2021, 55, 1377–1394. [Google Scholar] [CrossRef]
- Lijun, Y.; Benbin, X.; Jian, Y.; Yili, K.; Huifen, H.; Liang, W.; Bin, Y. Mechanism of Fracture Damage Induced by Fracturing Fluid Flowback in Shale Gas Reservoirs. Nat. Gas Ind. B 2019, 6, 366–373. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, Y.; Wang, K.; Zhang, C. The Adsorption Behavior of Hydroxypropyl Guar Gum onto Quartz Sand. J. Mol. Liq. 2018, 258, 10–17. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, L.; Davletshin, A.; Li, Z.; You, J.; Tan, S. Application of Polysaccharide Biopolymer in Petroleum Recovery. Polymers 2020, 12, 1860. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Plicht, C.; Buchmann, C.; Knott, M.; Meyer, M.; Müller-Schüssele, S.; Strieth, D.; Prosenc, M.H.; Steinmetz, H.; Jungkunst, H.F.; et al. Plastic Problem Solved? Environmental Implications of Synthetic Hydrophilic Polymers across Ecosystem Boundaries. TrAC—Trends Anal. Chem. 2024, 181, 118000. [Google Scholar] [CrossRef]
- Al-Hajri, S.; Negash, B.M.; Rahman, M.M.; Haroun, M.; Al-Shami, T.M. Perspective Review of Polymers as Additives in Water-Based Fracturing Fluids. ACS Omega 2022, 7, 7431–7443. [Google Scholar] [CrossRef]
- McLaughlin, M.C.; Borch, T.; Blotevogel, J. Spills of Hydraulic Fracturing Chemicals on Agricultural Topsoil: Biodegradation, Sorption, and Co-Contaminant Interactions. Environ. Sci. Technol. 2016, 50, 6071–6078. [Google Scholar] [CrossRef]
- He, Y.; Sun, C.; Zhang, Y.; Folkerts, E.J.; Martin, J.W.; Goss, G.G. Developmental Toxicity of the Organic Fraction from Hydraulic Fracturing Flowback and Produced Waters to Early Life Stages of Zebrafish (Danio rerio). Environ. Sci. Technol. 2018, 52, 3820–3830. [Google Scholar] [CrossRef]
- Almubarak, T.; Ng, J.H.C.; Alkhaldi, M.; Panda, S.; Nasr-El-din, H.A. Insights on Potential Formation Damage Mechanisms Associated with the Use of Gel Breakers in Hydraulic Fracturing. Polymers 2020, 12, 2722. [Google Scholar] [CrossRef]
- Berdugo-Clavijo, C.; Scheffer, G.; Sen, A.; Gieg, L.M. Biodegradation of Polymers Used in Oil and Gas Operations: Towards Enzyme Biotechnology Development and Field Application. Polymers 2022, 14, 1871. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.; Alsulaimani, M.; Aljawad, M.S.; Sangaru, S.S.; Kamal, M.S.; Mahmoud, M. The Synergetic Impact of Anionic, Cationic, and Neutral Polymers on VES Rheology at High-Temperature Environment. Polymers 2022, 14, 1145. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Chen, Y.; Wu, L.; Luo, P.; Zhang, X.; Yang, Z.; Chen, X. Research Progress on Nanoparticle-Enhanced Surfactant-Based Fracturing Fluids: A Comprehensive Review. Chem. Eng. Res. Des. 2025, 220, 29–48. [Google Scholar] [CrossRef]
- Grieser, B.; Calvin, J.; Dulin, J. Halliburton, formerly Lessons Learned: Refracs from 1980 to Present. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 9–11 February 2016; pp. 9–11. [Google Scholar]
- Kamal, M.S.; Mohammed, M.; Mahmoud, M.; Elkatatny, S. Development of Chelating Agent-Based Polymeric Gel System for Hydraulic Fracturing. Energies 2018, 11, 1663. [Google Scholar] [CrossRef]
- Azizov, E.; Quintero, H.J.; Saxton, K.; Sessarego, S. Carboxymethylcellulose a Cost Effective Alternative to Guar, CMHPG and Surfactant-Based Fluid Systems. In Proceedings of the SPE/CSUR Unconventional Resources Conference, Calgary, AB, Canada, 20–22 October 2015; pp. 20–22. [Google Scholar]
- Baker Hughes. AquaPerm Linear-Gel Fracturing Fluid System. Available online: https://www.bakerhughes.com/completions/stimulation-and-fracturing/fracturing-services/aquaperm-lineargel-fracturing-fluid-system (accessed on 31 July 2025).
- Blamble, E.A.; Pyncheon, J. Guar Replacement with Synthetic Polymers-Utica Shale Case Histories. In Proceedings of the SPE International Conference & Exhibition on Formation Damage Control, Lafayette, LA, USA, 24–26 February 2016; pp. 24–26. [Google Scholar]
- Xin, H.; Fang, B.; Yu, L.; Lu, Y.; Xu, K.; Li, K. Rheological Performance of High-Temperature-Resistant, Salt-Resistant Fracturing Fluid Gel Based on Organic-Zirconium-Crosslinked HPAM. Gels 2023, 9, 151. [Google Scholar] [CrossRef]
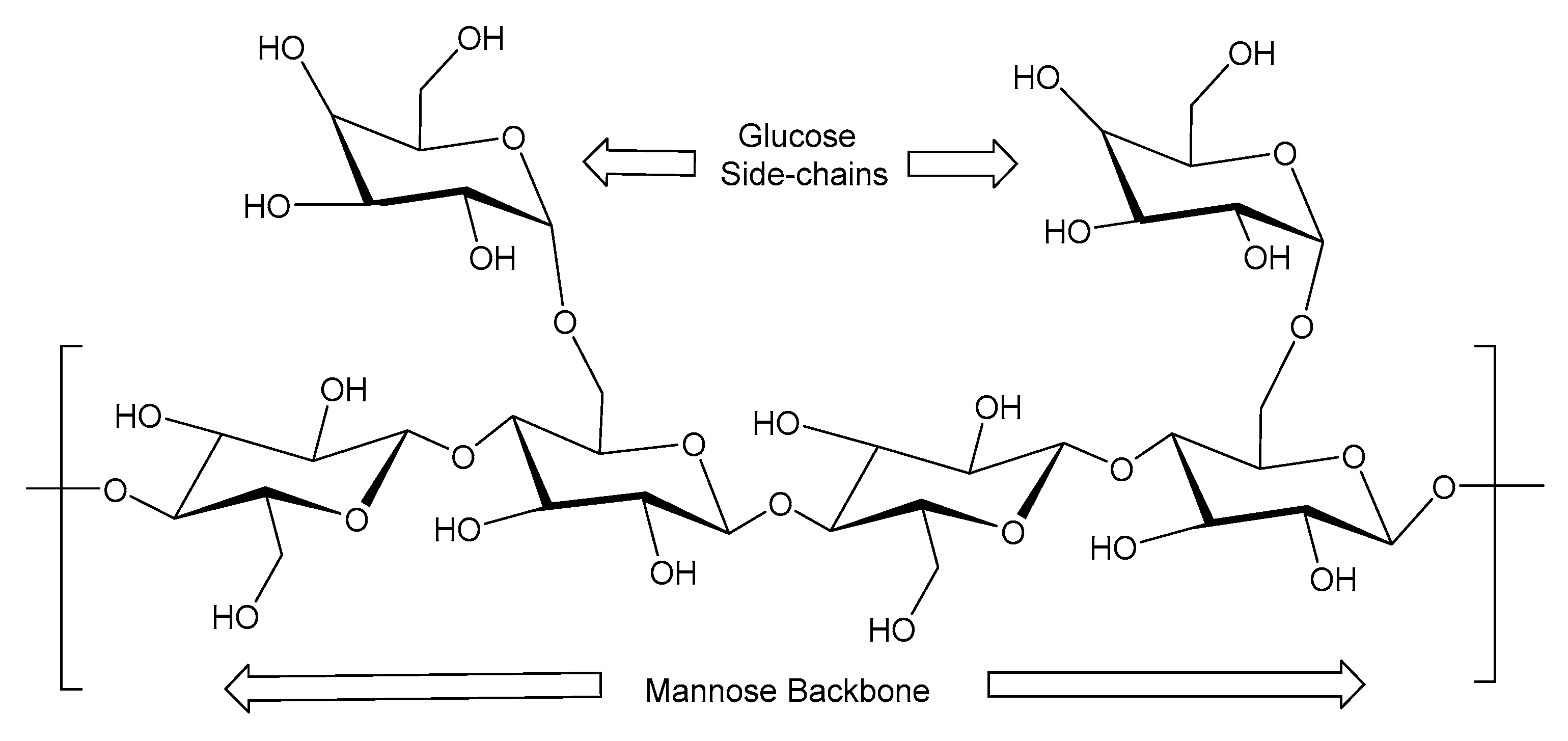
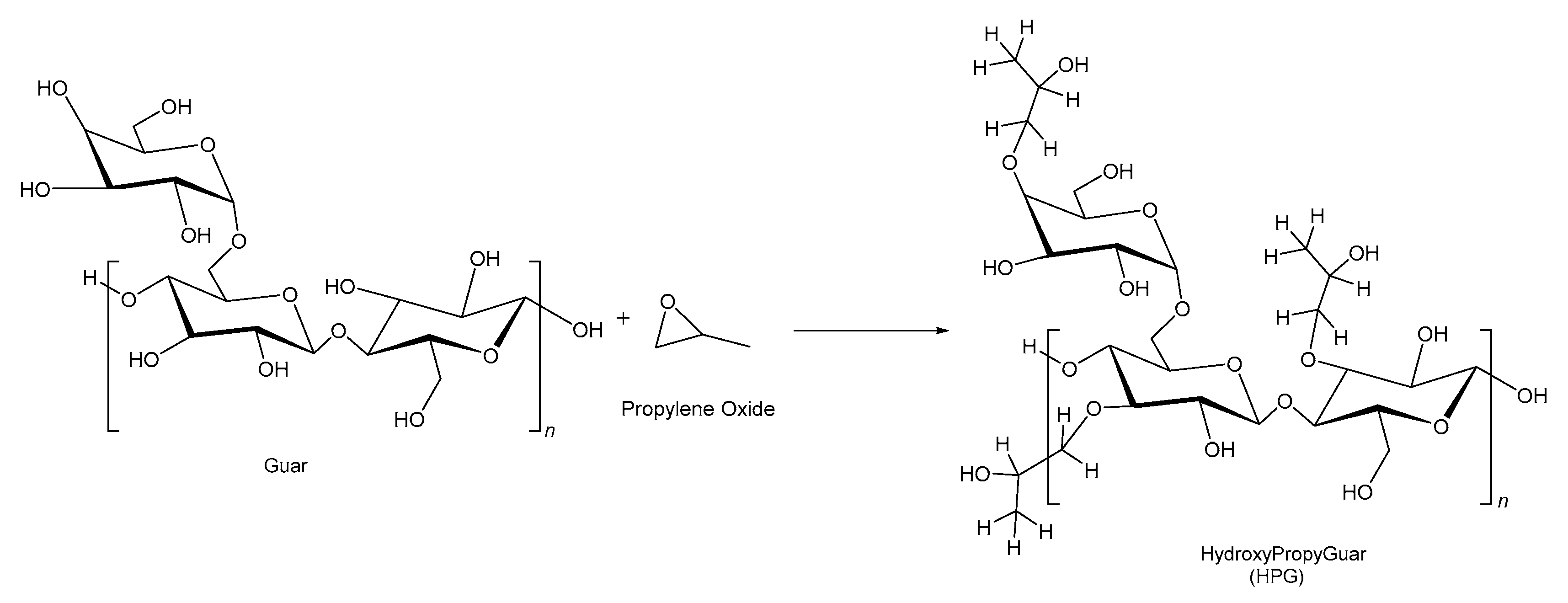
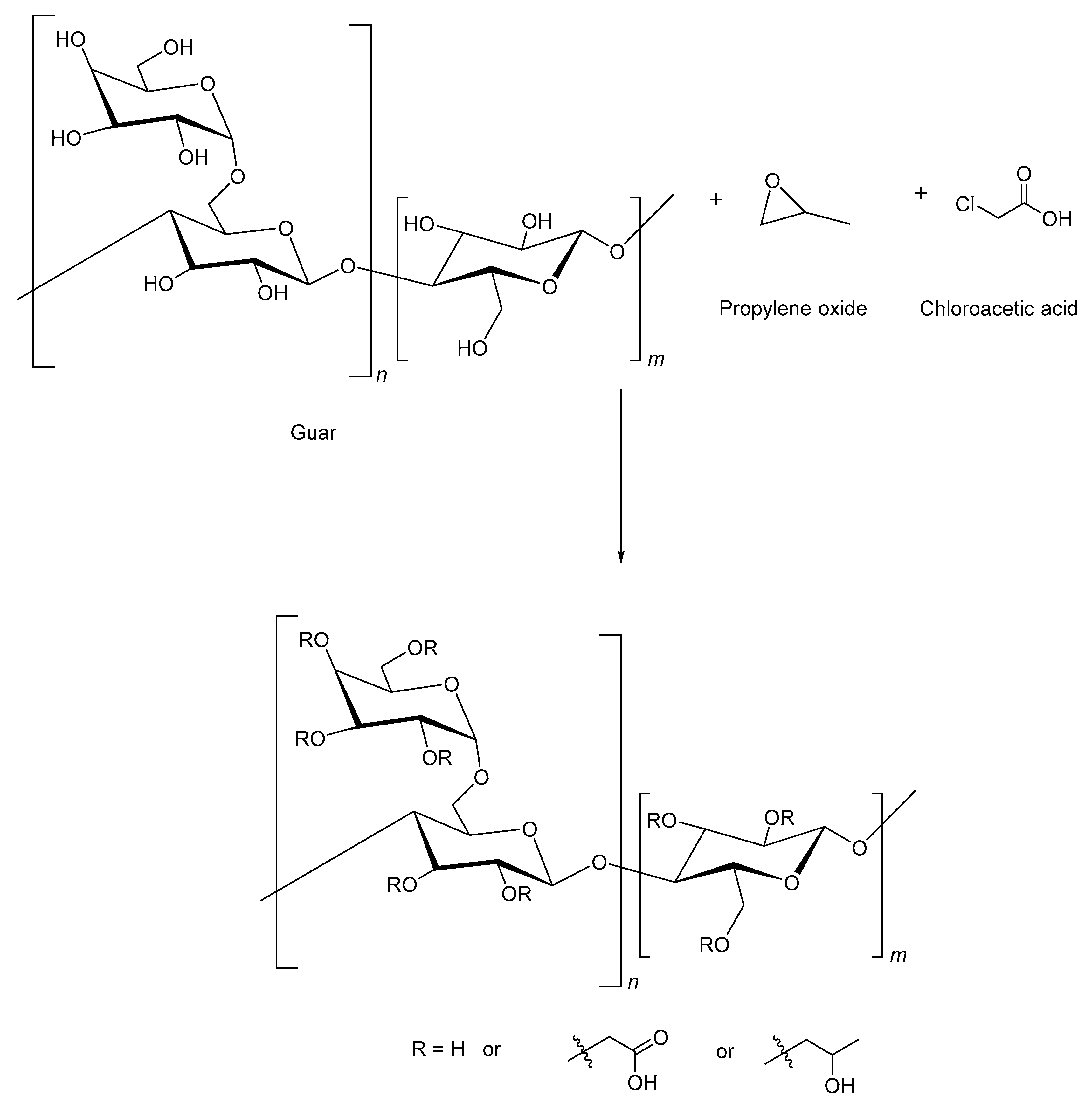
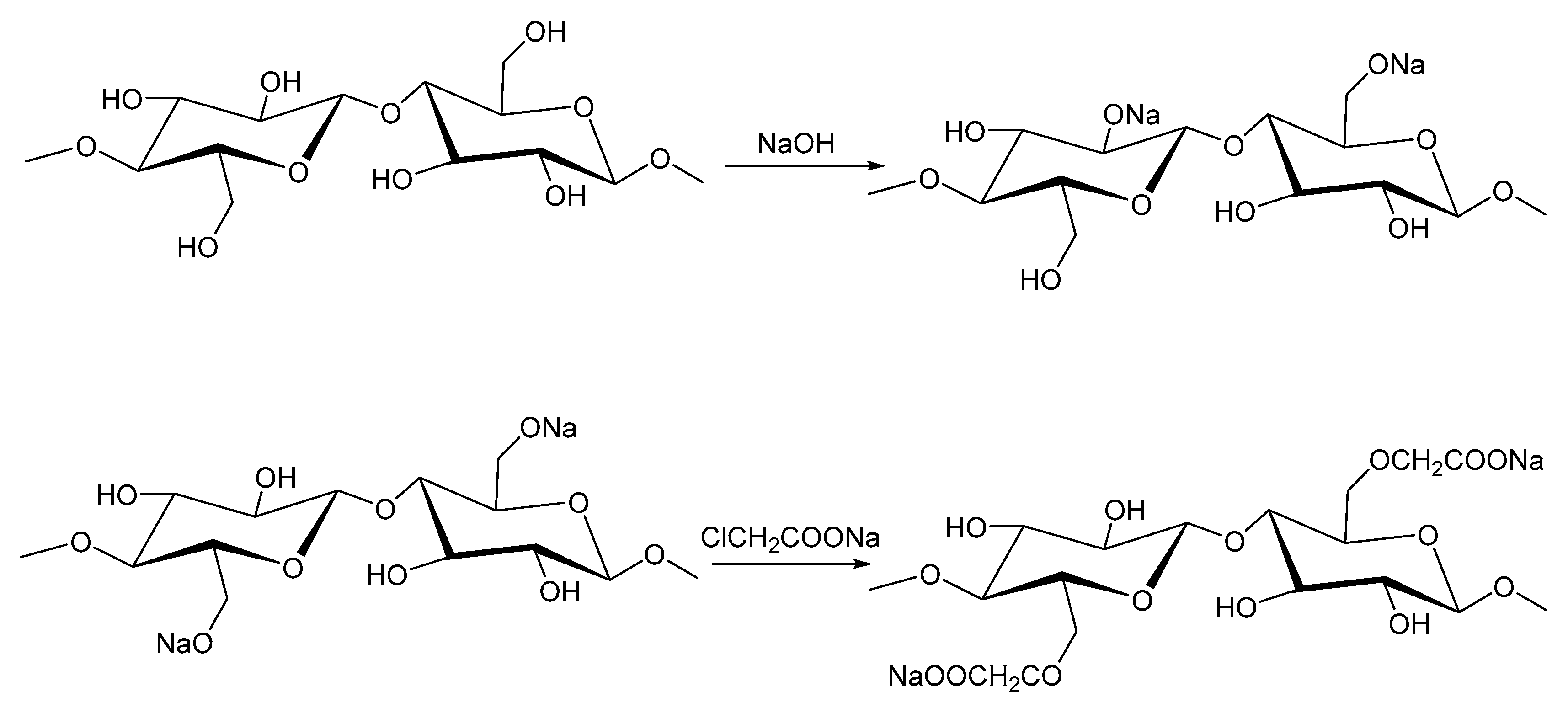

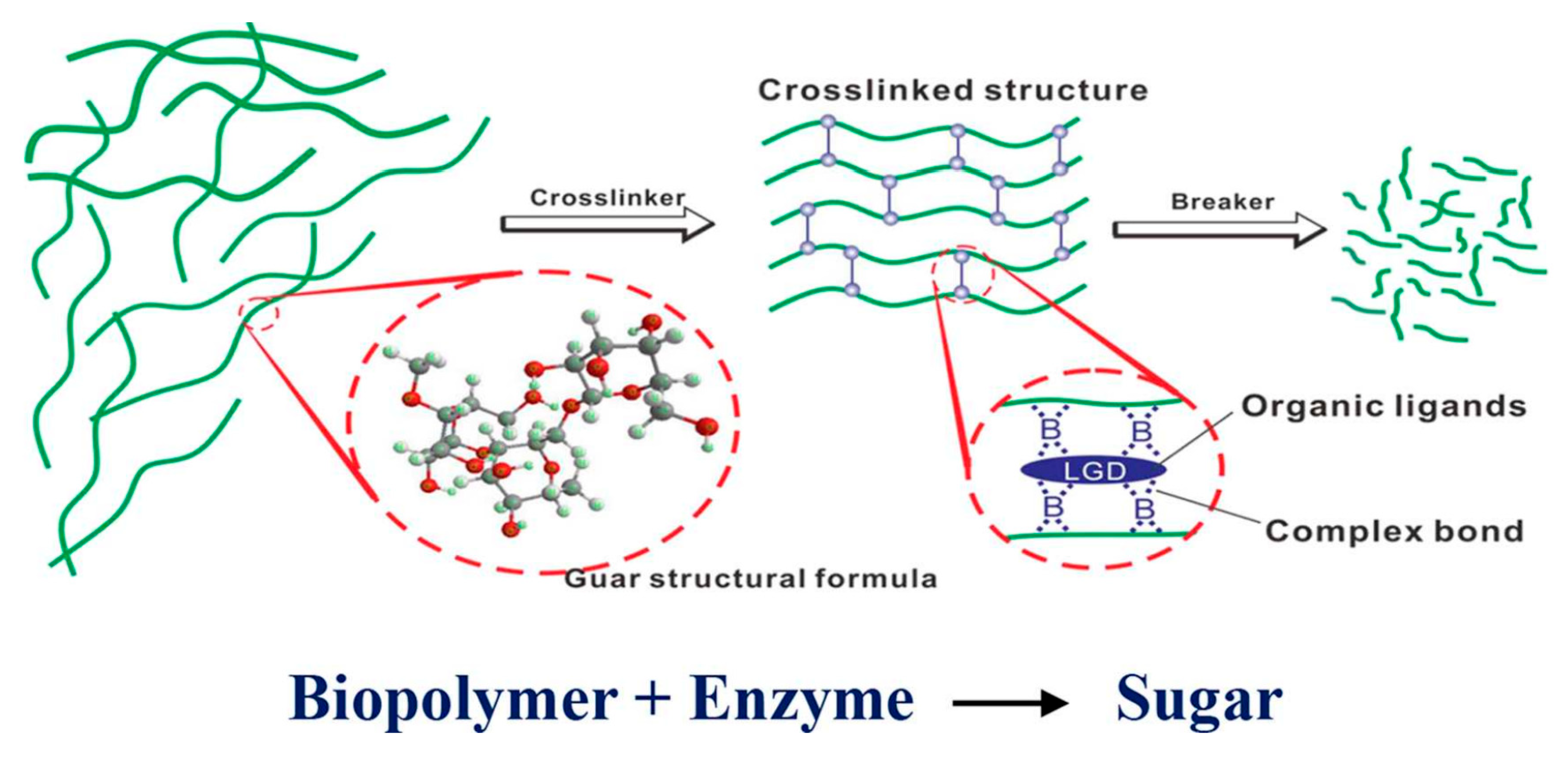
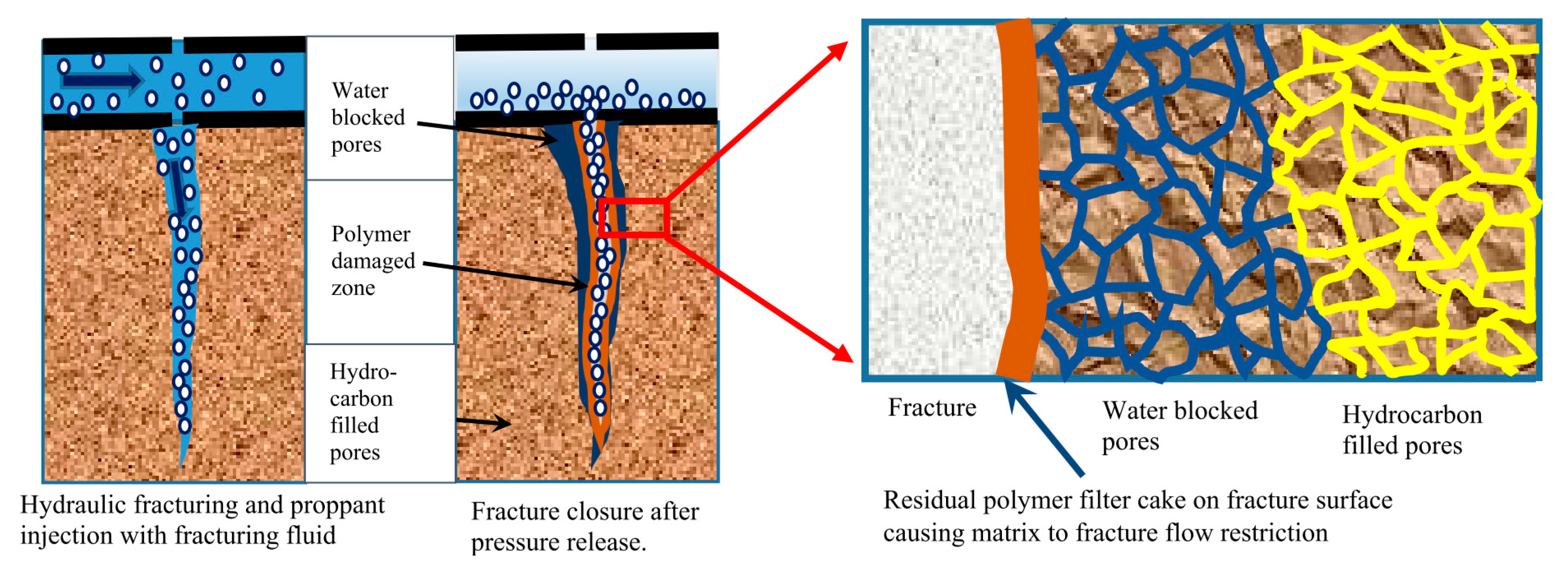
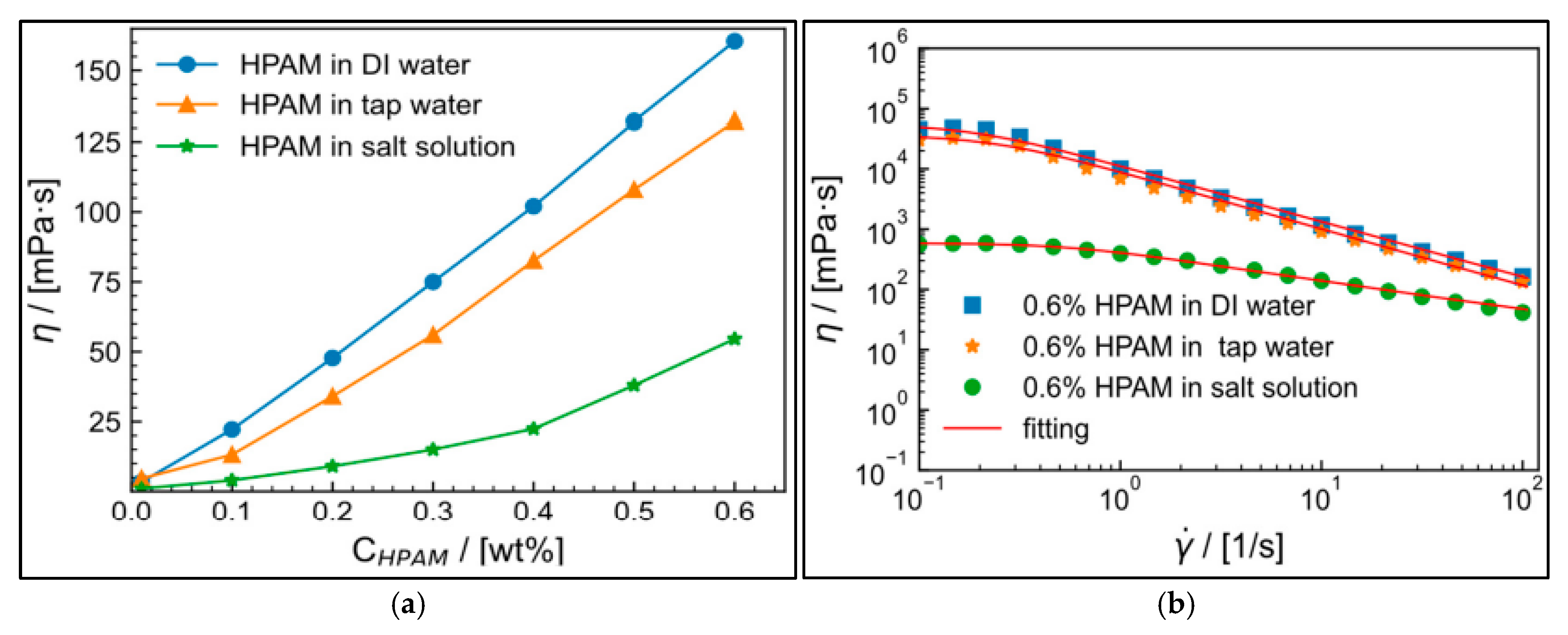
| Source | Polymer | General Properties | Applications |
|---|---|---|---|
| Plant-Based | Cellulose [42] | Linear polysaccharide | Hydraulic fracturing Textile industry (cotton, rayon) |
| Insoluble, good interaction with water | Paper manufacturing | ||
| High tensile strength | Biofuels | ||
| Guar Derivatives [43] | Branched polysaccharide | Hydraulic fracturing Drilling fluids | |
| Hydrophilic | Food stabilizer | ||
| Thickening and gelling properties | Pharmaceutical excipient | ||
| Microbial-Based | Xanthan Gum [44] | Branched polysaccharide | Food thickener |
| Water-soluble | Oil drilling fluid stabilizer | ||
| Viscosity-enhancing properties | Cosmetic emulsifier | ||
| Synthetic-Based | PAM [45] | Water-soluble synthetic polymer | Hydraulic fracturing, EOR, Water shut-off |
| High viscosity in aqueous solutions | Water treatment and as an additive in fracturing operations | ||
| Thermal stability | Soil conditioning | ||
| PAA (Polyacrylic acid) [46] | Water-soluble | Hydraulic Fracturing, Water treatment | |
| High water absorption capacity | Superabsorbent materials | ||
| pH-responsive | EOR |
| Polymer | Gel Loading Viscosity | Preparation | Thermal Ranges | Salinity Tolerance | Performance, Residues and Cost |
|---|---|---|---|---|---|
| Guar | 25–50 pptg 10–30 cP (linear) 500–2000 cP (cross-linked) | Grinding, Hydration, Cross-linking | Limited stability above 120 °C, improved with modifications | Low (<40,000 ppm, freshwater) | Cost-effective, high viscosity, struggles in high salinity and high temperature, 13% residues damage the formation. |
| HPG | 25–50 pptg 20–50 cP (linear) Up to 1000 cP (cross-linked) | Remove insoluble oxidation, neutralization, washing, and drying | Stable up to 150 °C, retains viscosity better than guar | Moderate (up to 100,000 ppm) | Moderate cost, improved thermal stability, suitable for medium salinity, 3% residues, damage less compared to guar. |
| CMHPG | 20–40 pptg 50–100 cP (linear) Up to 5000 cP (cross-linked) | Treating guar with propylene oxide and chloroacetic acid in the presence of NaOH, washing, drying | Stable up to 177 °C with stabilizers | High (up to 200,000 ppm) | Moderate-high cost, excellent for high salinity and high-temperature wells, minimal residue of 1%. |
| CMC | 10–20 pptg 10–30 cP (linear) 500–1000 cP (cross-linked) | Alkalization, Etherification, Drying | Stable up to 100 °C, degrades at higher temperatures | High (up to brine levels >150,000 ppm) | Moderate cost, good proppant transport, but limited carrying capacity in high-shear environments. Low residue < 1%. |
| Xanthan Gum | 1–3 pptg 20–50 cP (linear) 500–5000 cP (cross-linked) | Fermentation, Hydration, Cross-linking | Stable up to 120 °C, degrades above 75 °C | High (up to brine levels >150,000 ppm) | Moderate cost, poor thermal stability, excellent for saline and low-shear-rate conditions, low residue <1%. |
| Polymer | Approx. Price (USD/kg) | Notes |
|---|---|---|
| Guar gum | 1.9 (US), 1.4 (China) | The most recent pricing information is obtained from (IMARC, 2025) [85]. Previously reported at $2/kg [61] |
| HPG | 5.00 | Slightly more expensive than raw guar gum |
| CMHPG | 10.00 | Highest among guar derivatives |
| CMC | 5.00 | Offers good performance at moderate cost |
| Xanthan gum | 3.00 | Moderate cost, common in high-salinity and low-shear environments |
| PHPA (partially hydrolyzed polyacrylamide) | 0.17 | Low-cost additive, used in WBDF and as support in fracturing fluids |
| PAM | 2.44 (US), 0.83 (China) | Price varies by region; widely used in EOR and water treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, A.; Aljawad, M.S.; Kalgaonkar, R.; Kamal, M.S. Application of Polymers in Hydraulic Fracturing Fluids: A Review. Polymers 2025, 17, 2562. https://doi.org/10.3390/polym17182562
Othman A, Aljawad MS, Kalgaonkar R, Kamal MS. Application of Polymers in Hydraulic Fracturing Fluids: A Review. Polymers. 2025; 17(18):2562. https://doi.org/10.3390/polym17182562
Chicago/Turabian StyleOthman, Amro, Murtada Saleh Aljawad, Rajendra Kalgaonkar, and Muhammad Shahzad Kamal. 2025. "Application of Polymers in Hydraulic Fracturing Fluids: A Review" Polymers 17, no. 18: 2562. https://doi.org/10.3390/polym17182562
APA StyleOthman, A., Aljawad, M. S., Kalgaonkar, R., & Kamal, M. S. (2025). Application of Polymers in Hydraulic Fracturing Fluids: A Review. Polymers, 17(18), 2562. https://doi.org/10.3390/polym17182562








