Preparation of Concentrated PMMA Suspensions Stabilized by a Green Polysiloxane Surfactant
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
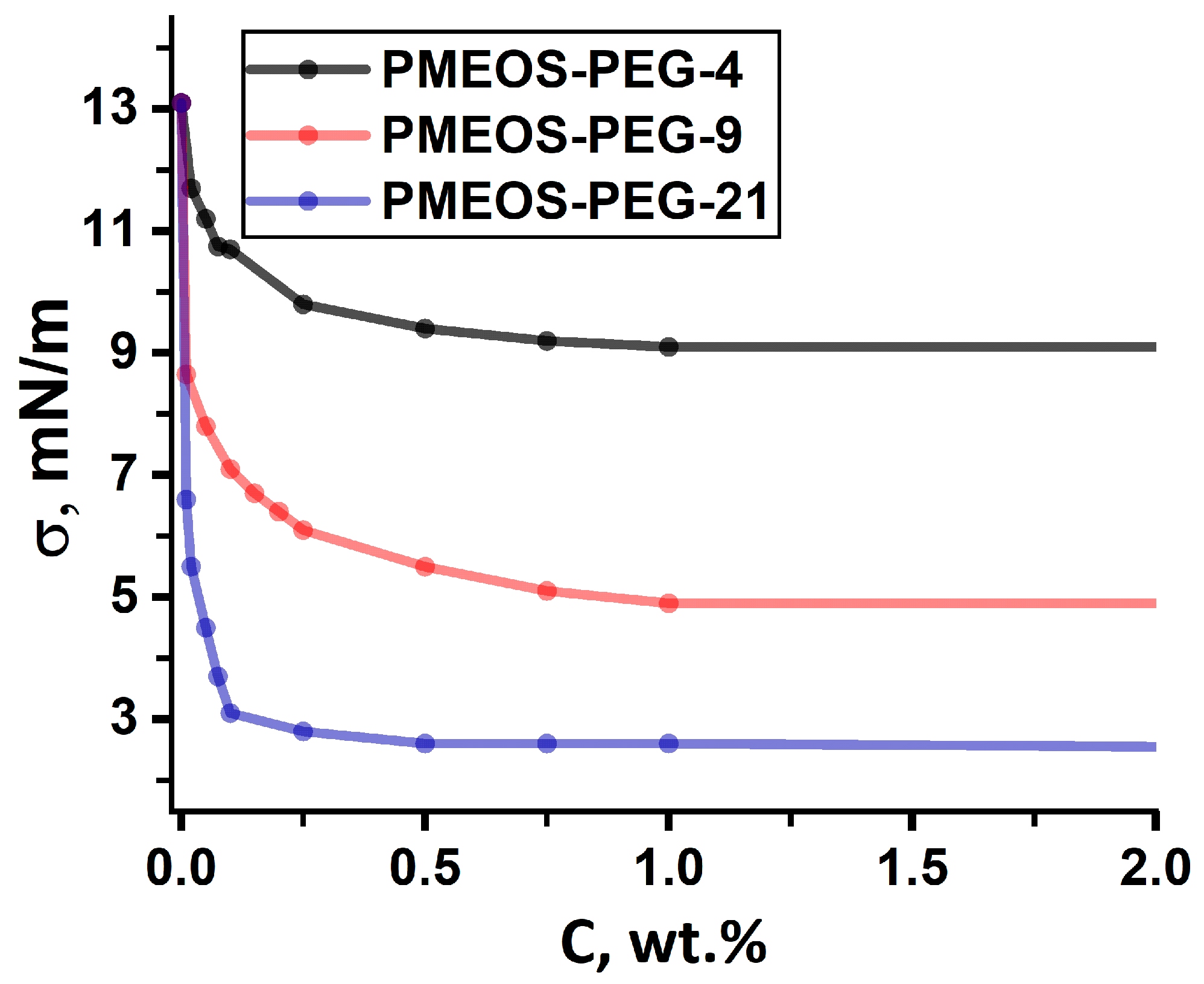
3.1. Colloid-Chemical Properties of PMEOS-PEGs at the MMA/Water Interface
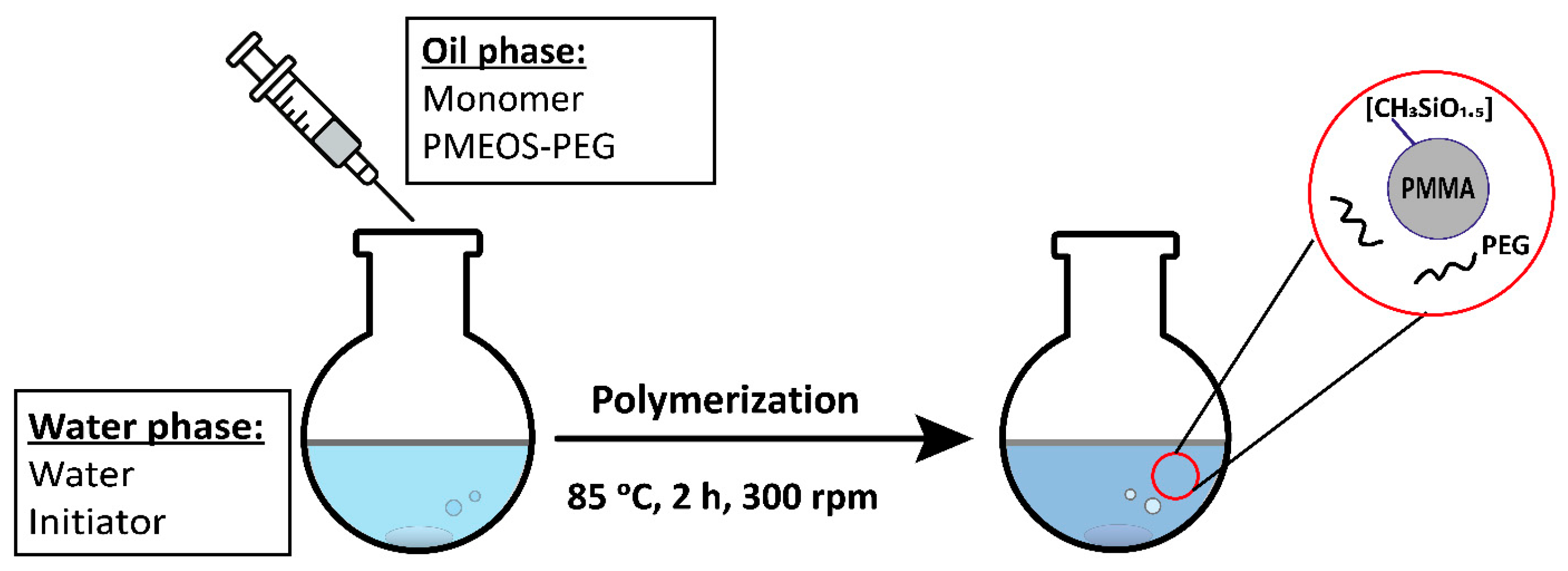
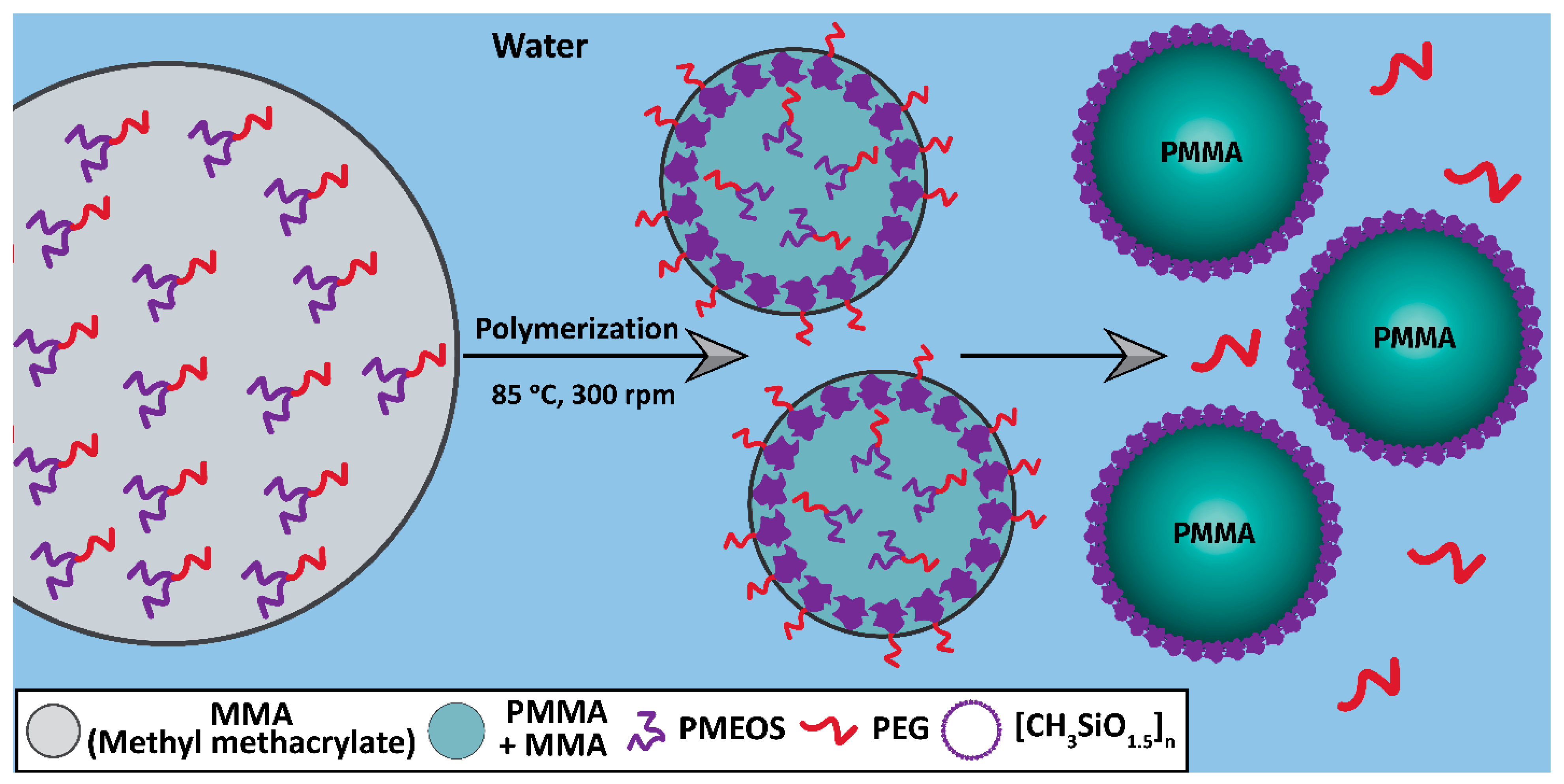
3.2. Synthesis of PMMA Suspensions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, K.K.; McCormick, A.V.; Francis, L.F. CryoSEM investigation of latex coatings dried in walled substrates. Langmuir 2012, 28, 10329–10333. [Google Scholar] [CrossRef] [PubMed]
- Fulmer, P.A.; Wynne, J.H. Development of broad-spectrum antimicrobial latex paint surfaces employing active amphiphilic compounds. ACS Appl. Mater. Interfaces 2011, 3, 2878–2884. [Google Scholar] [CrossRef]
- Kozakiewicz, J.; Ofat, I.; Trzaskowska, J. Silicone-containing aqueous polymer dispersions with hybrid particle structure. Adv. Colloid Interface Sci. 2015, 223, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.D.; Daniels, E.S. Emulsion Polymerisation and Latex Applications; iSmithers Rapra Publishing: Shawbury, UK, 2003; Volume 14. [Google Scholar]
- Takamura, K.; Urban, D. Polymer Dispersions and Their Industrial Applications; Wiley-InterScience: Charlotte, NC, USA, 2003. [Google Scholar]
- Bao, Y.; Ma, J.; Zhang, X.; Shi, C. Recent advances in the modification of polyacrylate latexes. J. Mater. Sci. 2015, 50, 6839–6863. [Google Scholar] [CrossRef]
- Li, H.; Yuan, J.; Qian, H.; Wu, L. Synthesis and properties of SiO2/P(MMA-BA) core–shell structural latex with siloxanes. Prog. Org. Coat. 2016, 97, 65–73. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, M. Preparation of fluorinated polysiloxane and its application in modified polyacrylates. J. Appl. Polym. Sci. 2024, 141, e55642. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Kiatkamjornwong, S.; Akkarakittimongkol, P.; Omi, S. Syntheses of acrylate core/shell imbiber beads by seeded suspension copolymerization and one-stage copolymerization for solvent absorption–desorption. J. Appl. Polym. Sci. 2002, 85, 670–682. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Liang, J.; Yuan, D.; Zhao, W. Research progress of poly (methyl methacrylate) microspheres: Preparation, functionalization and application. Eur. Polym. J. 2022, 175, 111379. [Google Scholar] [CrossRef]
- Zlotin, S.G.; Egorova, K.S.; Ananikov, V.P.; Akulov, A.A.; Varaksin, M.V.; Chupakhin, O.N.; Charushin, V.N.; Bryliakov, K.P.; Averin, A.D.; Beletskaya, I.P.; et al. The green chemistry paradigm in modern organic synthesis. Russ. Chem. Rev. 2023, 92, RCR5104. [Google Scholar] [CrossRef]
- Levshenko, E.N.; Grotskova, I.A.; Gusev, S.A.; Gusev, A.A.; Volkova, E.A. Polymeric microspheres as carriers of fluorescent labels during creation of three-dimensional model of vascular channel in test animals. Biotechnol. Russ. 2013, 29, 65–70. [Google Scholar]
- Voronkov, M.; Mileshkevich, V.; Yuzhelevskii, Y. Silikonovaya Svyaz’ (Silicone Bond); Siberian Branch of the Russian Academy of Sciences, Izd. Sib. Akad. Nauk, Irkutsk Institute of Organic Chemistry: Novosibirsk, Russia, 1976. [Google Scholar]
- Gritskova, I.A.; Prokopov, N.I.; Ezhova, A.A.; Chalykh, A.E.; Gusev, S.A.; Levachev, S.M.; Zubov, V.P.; Gomzyak, V.I.; Skopintsev, I.V.; Stuzhuk, A.N.; et al. New approaches to the synthesis and stabilization of polymer microspheres with a narrow size distribution. Polymers 2023, 15, 2464. [Google Scholar] [CrossRef]
- Adikanova, D.; Eligbaeva, G.Z.; Milushkova, E.; Gritskova, I.; Lobanova, N.; Prokopov, N.; Levachev, S.; Harlov, A.; Haddazh, M. The synthesis of high dispersed polstyrene suspensions with narrow particle distribution by sizes. Fine Chem. Technol. 2015, 10, 71–77. [Google Scholar]
- Sizov, V.E.; Zefirov, V.V.; Gallyamov, M.O.; Muzafarov, A.M. Organosilicone compounds in supercritical carbon dioxide. Polymers 2022, 14, 2367. [Google Scholar] [CrossRef] [PubMed]
- Gostenin, V.B.; Zubov, V.P.; Gritskova, I.A.; Shikhovtseva, I.S. One-step synthesis of polymer dispersions with large narrow-size-distribution particles via heterophase polymerization in the presence of surface-active water-insoluble organosilicon macromers. Polym. Int. 2022, 71, 656–661. [Google Scholar] [CrossRef]
- Gritskova, I.A.; Adikanova, D.B.; Papkov, V.S.; Prokopov, N.I.; Shragin, D.I.; Gusev, S.A.; Levachev, S.M.; Milushkova, E.V.; Ezhova, A.A.; Lukashevich, A.D. Polymerization of styrene in the presence of carboxyl-containing polydimethylsiloxane and its mixture with oxyethylated poly(propylene glycol). Polym. Sci. Ser. B 2016, 58, 163–167. [Google Scholar] [CrossRef]
- Gritskova, I.A.; Ezhova, A.A.; Chalikh, A.E.; Levachev, S.M.; Chvalun, S.N. Effect of the composition and structure of carbofunctional oligodimethylsiloxanes on their colloidal-chemical properties. Russ. Chem. Bull. 2019, 68, 132–136. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Z.; Zhu, X.; Möller, M. A facile one-step approach toward polymer@SiO2 core–shell nanoparticles via a surfactant-free miniemulsion polymerization technique. Macromolecules 2016, 49, 1552–1562. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Zhu, X.; Möller, M. One-pot formation of monodisperse polymer@SiO2 core–shell nanoparticles via surfactant-free emulsion polymerization using an adaptive silica precursor polymer. Polym. Chem. 2017, 8, 6263–6271. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Chen, Z.; Zhu, X.; Möller, M. Hybrid nanostructured particles via surfactant-free double miniemulsion polymerization. Nat. Commun. 2018, 9, 1918. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, Y.; Zhu, X.; Möller, M. Formation of monodisperse polymer@ SiO2 core–shell nanoparticles via polymerization in emulsions stabilized by amphiphilic silica precursor polymers: HLB dictates the reaction mechanism and particle size. Macromolecules 2019, 52, 5670–5678. [Google Scholar] [CrossRef]
- Borisov, K.; Kalinina, A.; Bystrova, A.; Muzafarov, A. Aerogel-like material based on PEGylated hyperbranched polymethylethoxysiloxane. Polymers 2023, 15, 4012. [Google Scholar] [CrossRef]
- Migulin, D.; Tatarinova, E.; Meshkov, I.; Cherkaev, G.; Vasilenko, N.; Buzin, M.; Muzafarov, A. Synthesis of the first hyperbranched polyorganoethoxysilsesquioxanes and their chemical transformations to functional core–shell nanogel systems. Polym. Int. 2016, 65, 72–83. [Google Scholar] [CrossRef]
- Griffin, W.C. Classification of surface-active agents by “HLB”. J. Soc. Cosmet. Chem. 1949, 1, 311–325. [Google Scholar]
- Meshkov, I.B.; Kalinina, A.A.; Kazakova, V.V.; Demchenko, A.I. Densely Cross-Linked Polysiloxane Nanogels. INEOS OPEN 2020, 3, 118–132. [Google Scholar] [CrossRef]
- Shragin, D.I.; Gritskova, I.A.; Kopylov, V.V.; Milushkova, E.V.; Zlydneva, L.A.; Levachev, S.M. Novel approach to synthesis of monodisperse polymeric microspheres: Heterophase polymerization of styrene and methyl methacrylate in presence of water-insoluble functional PDMSS. Silicon 2015, 7, 217–227. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Elsevier: Amsterdam, The Netherlands, 2017; pp. 43–58. [Google Scholar]
- Birdi, K. Surface and Colloid Chemistry: Principles and Applications; CRC Press: Cambridge, MA, USA, 2009. [Google Scholar]


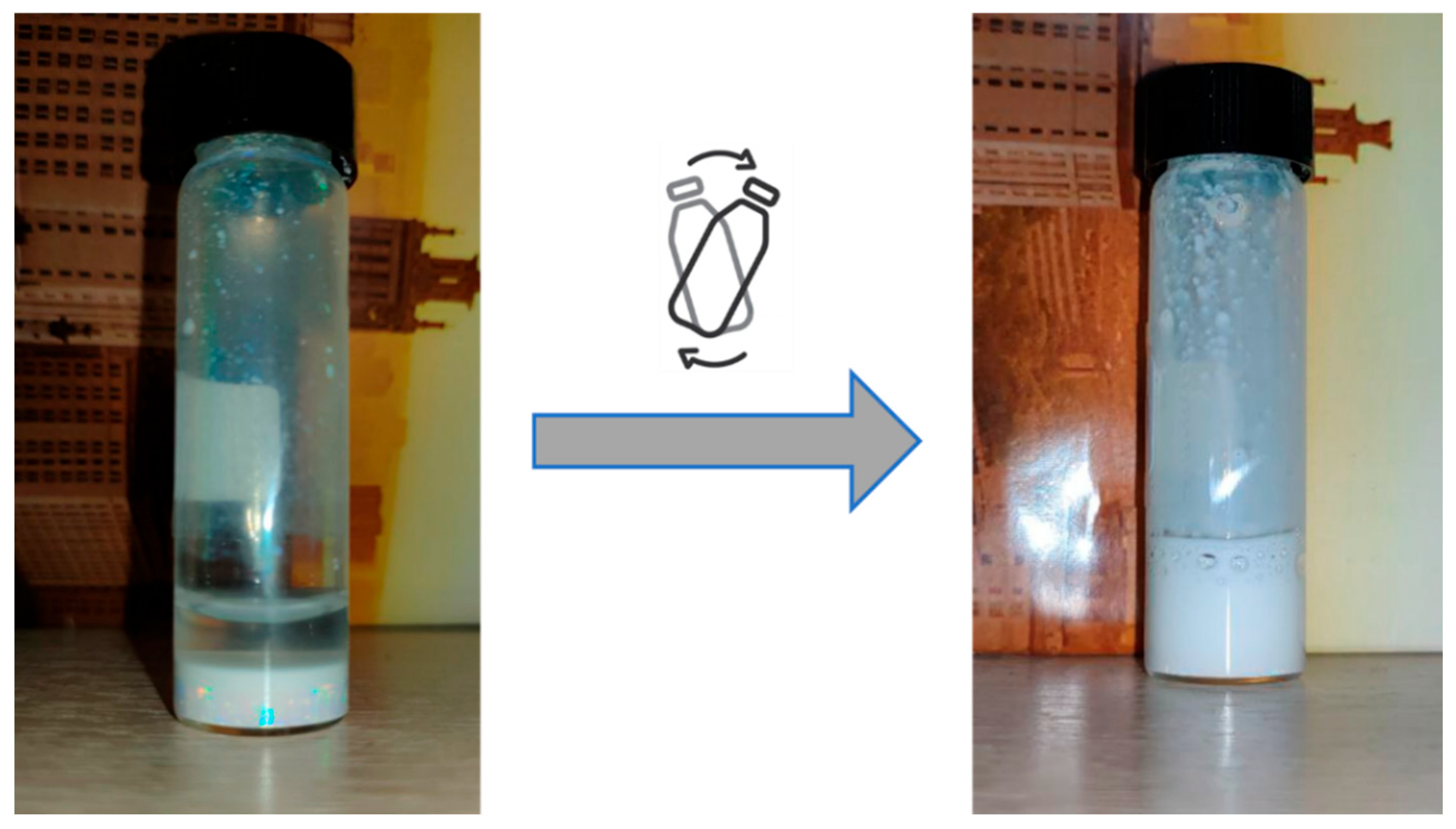
| Product | Ethoxy Group Substitution (mol%) | Mass of PMEOS (g) | Mass of PEG (g) | Yield (%)—(g) |
|---|---|---|---|---|
| PMEOS-PEG-4 | 4 | 25 | 2.8 | 99—43.7 |
| PMEOS-PEG-9 | 9 | 25 | 7.0 | 99—47.5 |
| PMEOS-PEG-21 | 21 | 25 | 14.5 | 99—55.2 |
| Sample | Mn(GPC) | Mw/Mn | Density, g/cm3 | HLB |
|---|---|---|---|---|
| PMEOS-PEG-4 | 1400 | 2.2 | 1.10 | 2.4 |
| PMEOS-PEG-9 | 1700 | 2.2 | 1.15 | 4.6 |
| PMEOS-PEG-21 | 2000 | 1.7 | 1.11 | 8.4 |
| Surfactant | σ, mN/m | CAC, wt.% | Гmax × 107, mol/m2 | g, (mJ·m)/mol | S0, Å2 | δ, nm |
|---|---|---|---|---|---|---|
| PMEOS-PEG-4 | 9.1 | 1 | 3.57 | 8.92 | 465 | 0.5 |
| PMEOS-PEG-9 | 4.9 | 1 | 4.46 | 39.28 | 372 | 0.7 |
| PMEOS-PEG-21 | 2.4 | 0.2 | 6.47 | 120.97 | 257 | 1.2 |
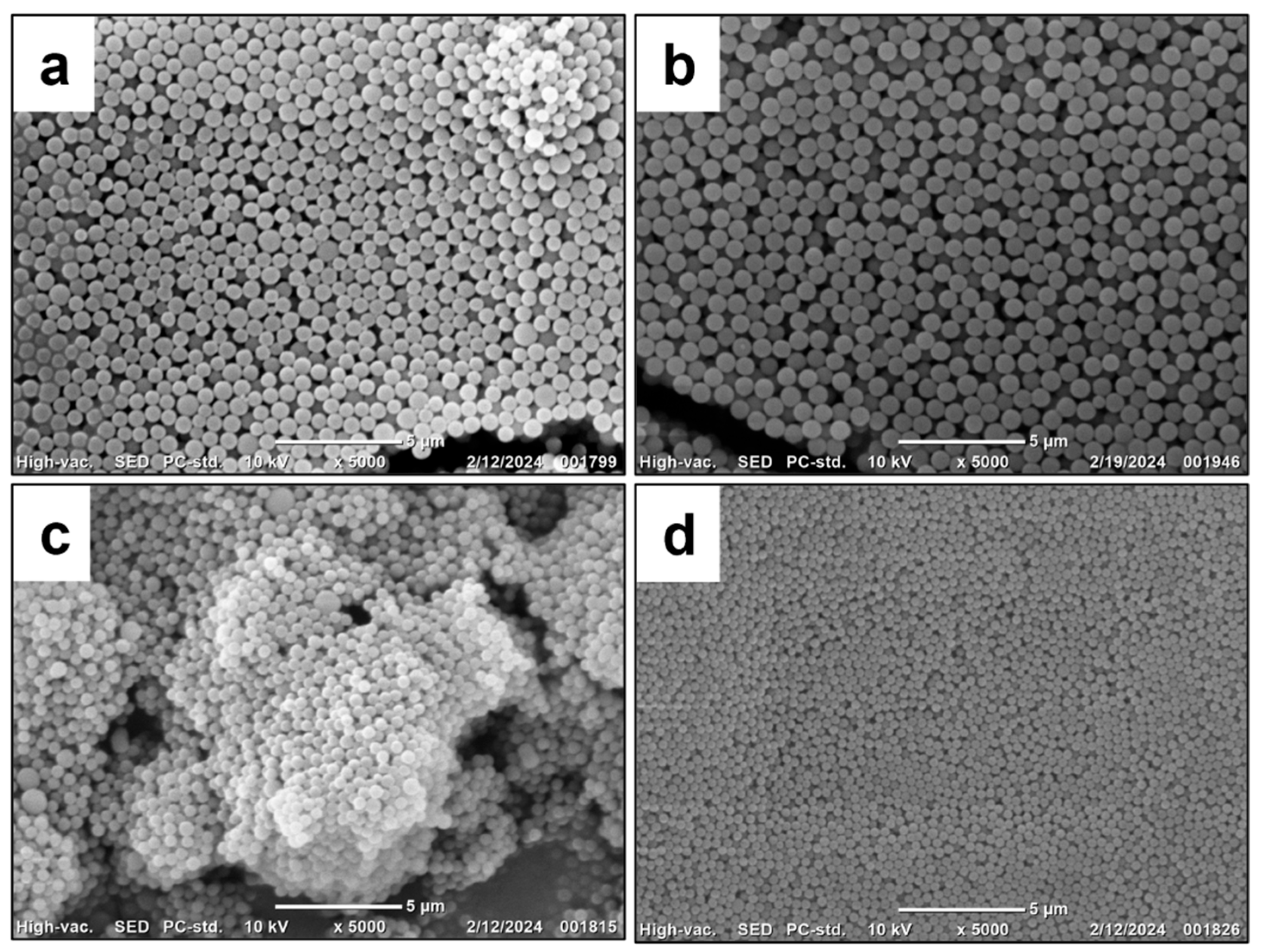
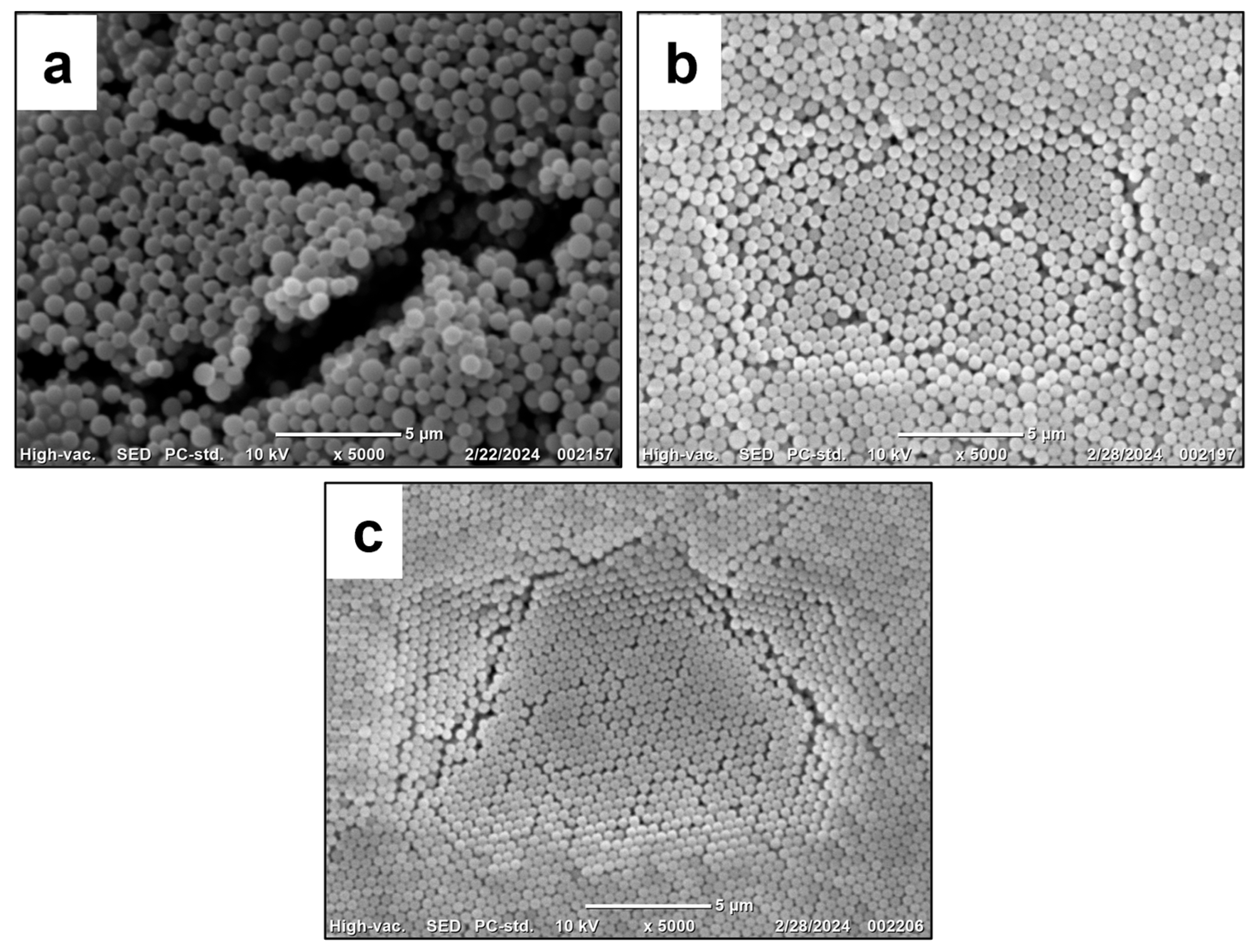
| Sample No. | Surfactant | Vm/VH2O | Csur, wt.% | Ddls ± SD, nm | Dsem ± SD, nm | PDIDLS | ζ, mV | P, % |
|---|---|---|---|---|---|---|---|---|
| 1 | No surfactant | 1:1 | - | Coagulation | ||||
| 2 | PMEOS-PEG-4 | 1:1 | 1 | |||||
| 3 | PMEOS-PEG-9 | 1:1 | ||||||
| 4 | PMEOS-PEG-21 | 1:1 | ||||||
| 5 | PMEOS-PEG-4 | 1:1 | 5 | 460 ± 10 | 490 ± 30 | 0.011 | −57.7 | 91 |
| 6 | PMEOS-PEG-9 | 1:1 | 530 ± 40 | 690 ± 50 | 0.008 | −54.5 | 92 | |
| 7 | PMEOS-PEG-21 | 1:1 | 840 ± 60 | 790 ± 80 | 0.027 | −51.7 | 94 | |
| 8 | No surfactant | 1:2 | - | Coagulation | ||||
| 9 | PMEOS-PEG-4 | 1:2 | 1 | |||||
| 10 | PMEOS-PEG-9 | 1:2 | ||||||
| 11 | PMEOS-PEG-21 | 1:2 | 580 ± 110 | 560 ± 40 | 0.038 | −52.6 | 98 | |
| 12 | PMEOS-PEG-4 | 1:2 | 5 | 590 ± 160 | 720 ± 60 | 0.073 | −55.4 | 95 |
| 13 | PMEOS-PEG-9 | 1:2 | 590 ± 60 | 500 ± 30 | 0.011 | −55.9 | 92 | |
| 14 | PMEOS-PEG-21 | 1:2 | 740 ± 120 | 510 ± 30 | 0.024 | −57.3 | 90 | |
| 15 | No surfactant | 1:4 | - | 430 ± 110 | 350 ± 30 | 0.063 | −50.7 | 88 |
| 16 | PMEOS-PEG-4 | 1:4 | 1 | 370 ± 20 | 310 ± 20 | 0.003 | −51.5 | 98 |
| 17 | PMEOS-PEG-9 | 1:4 | 510 ± 70 | 330 ± 50 | 0.021 | −54.8 | 99 | |
| 18 | PMEOS-PEG-21 | 1:4 | 520 ± 130 | 390 ± 30 | 0.056 | −53.2 | 94 | |
| Sample | Di, nm | ζ, mV |
|---|---|---|
| Freshly prepared | 590 ± 60 | −55.9 |
| After 3 months of storage | 580 ± 30 | −53.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borisova, D.; Borisov, K.; Kalinina, A.; Bystrova, A.; Gritskova, I.; Muzafarov, A. Preparation of Concentrated PMMA Suspensions Stabilized by a Green Polysiloxane Surfactant. Polymers 2025, 17, 2535. https://doi.org/10.3390/polym17182535
Borisova D, Borisov K, Kalinina A, Bystrova A, Gritskova I, Muzafarov A. Preparation of Concentrated PMMA Suspensions Stabilized by a Green Polysiloxane Surfactant. Polymers. 2025; 17(18):2535. https://doi.org/10.3390/polym17182535
Chicago/Turabian StyleBorisova, Diana, Kirill Borisov, Aleksandra Kalinina, Aleksandra Bystrova, Inessa Gritskova, and Aziz Muzafarov. 2025. "Preparation of Concentrated PMMA Suspensions Stabilized by a Green Polysiloxane Surfactant" Polymers 17, no. 18: 2535. https://doi.org/10.3390/polym17182535
APA StyleBorisova, D., Borisov, K., Kalinina, A., Bystrova, A., Gritskova, I., & Muzafarov, A. (2025). Preparation of Concentrated PMMA Suspensions Stabilized by a Green Polysiloxane Surfactant. Polymers, 17(18), 2535. https://doi.org/10.3390/polym17182535







