Structure and Properties of Sprayed Polyurethane Bio-Based Foams Produced Under Varying Fabrication Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Polyurethane System
2.1.2. The Synthesis Process Information
2.2. Methods
- Sampling methodology
- Scanning Electron Microscopy (SEM) and image analysis
- Thermogravimetric analysis (TGA)
- Differential scanning calorimetry (DSC)
- Fourier Transform Infrared Spectroscopy (FT-IR)
- Apparent density
- Physicomechanical properties
- Thermal Conductivity
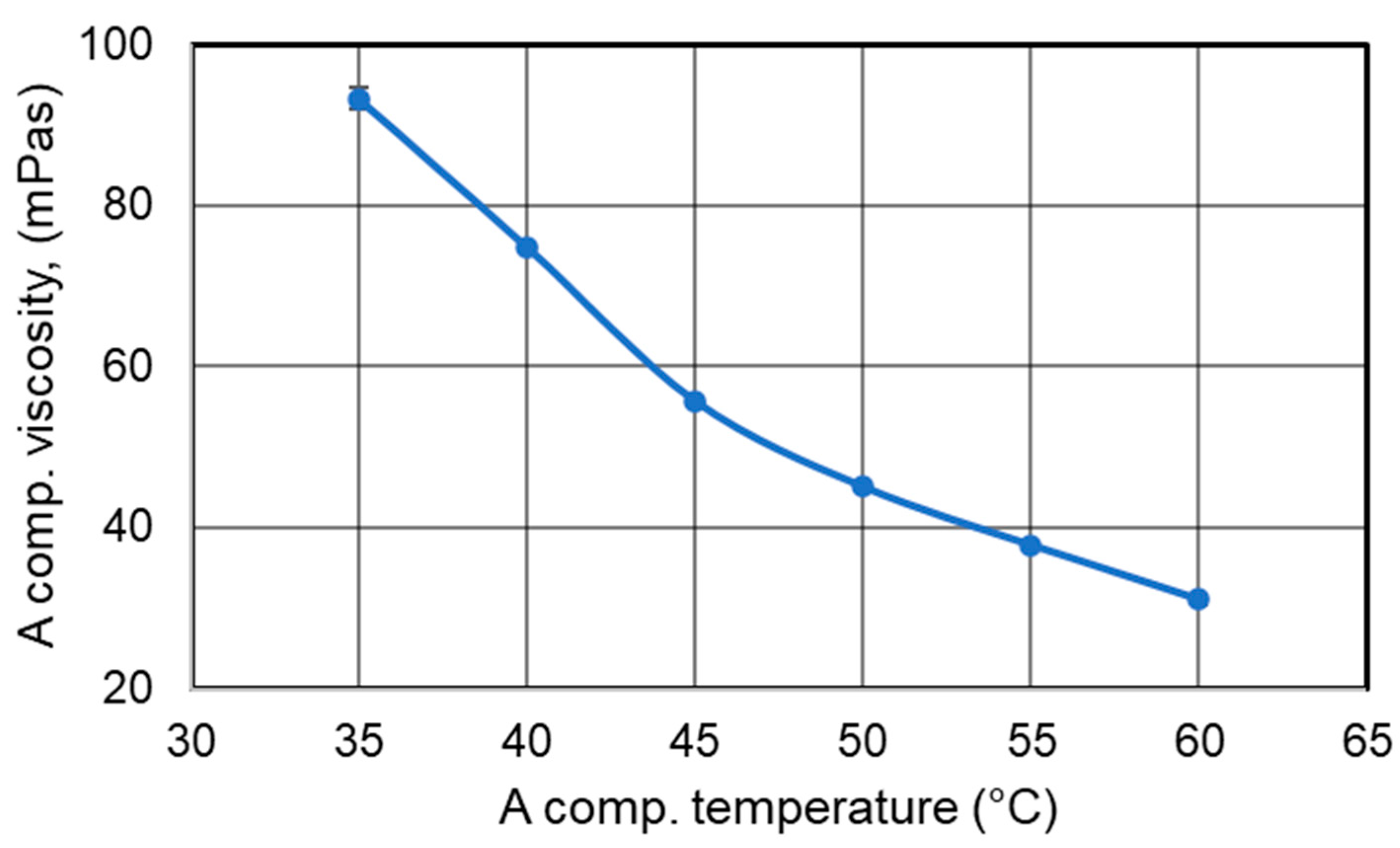
- Viscosity Testing
- Surface temperature
3. Results and Discussion
3.1. Evaluation of Foaming Process Parameters for Polyurethane Foams
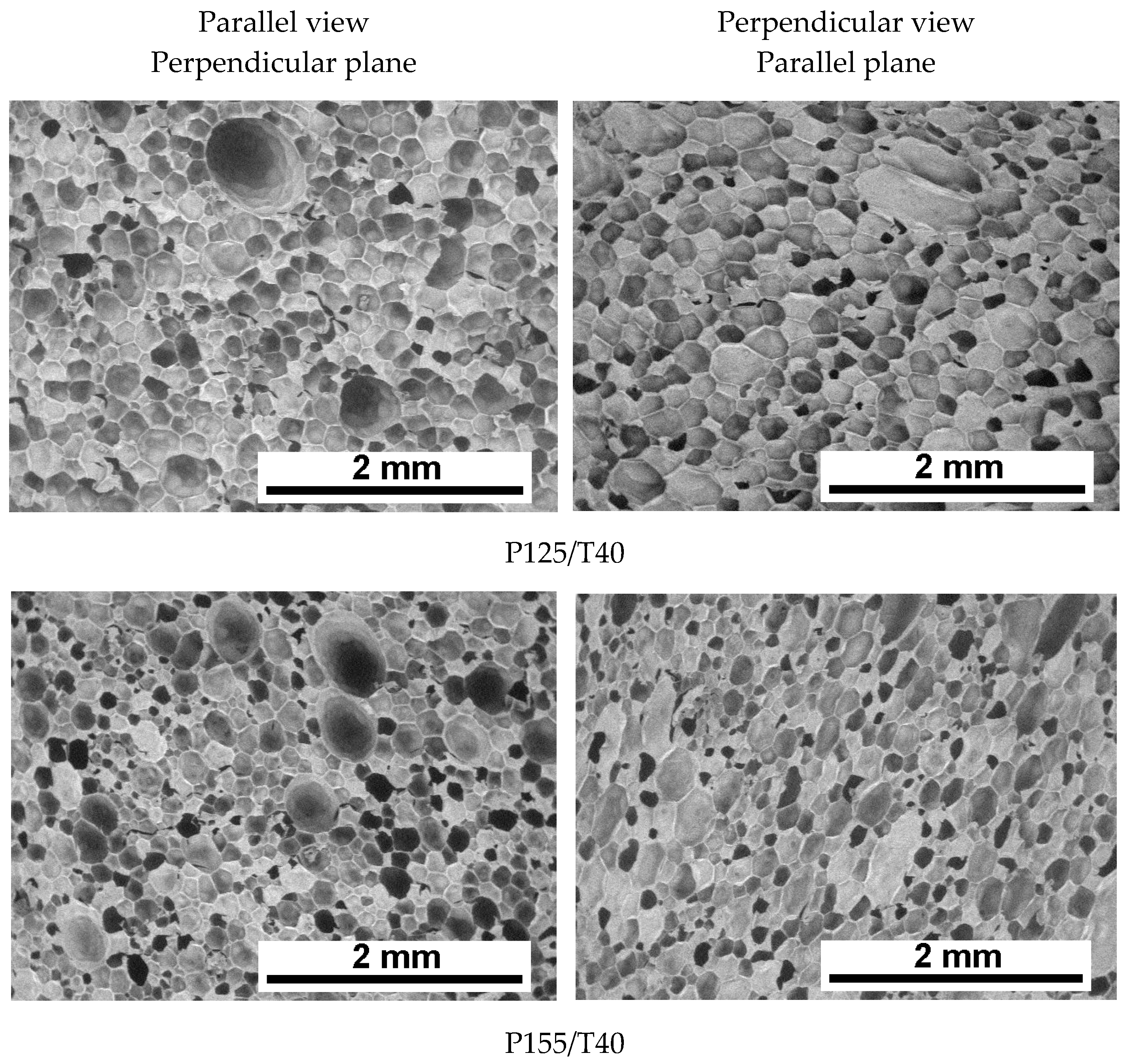
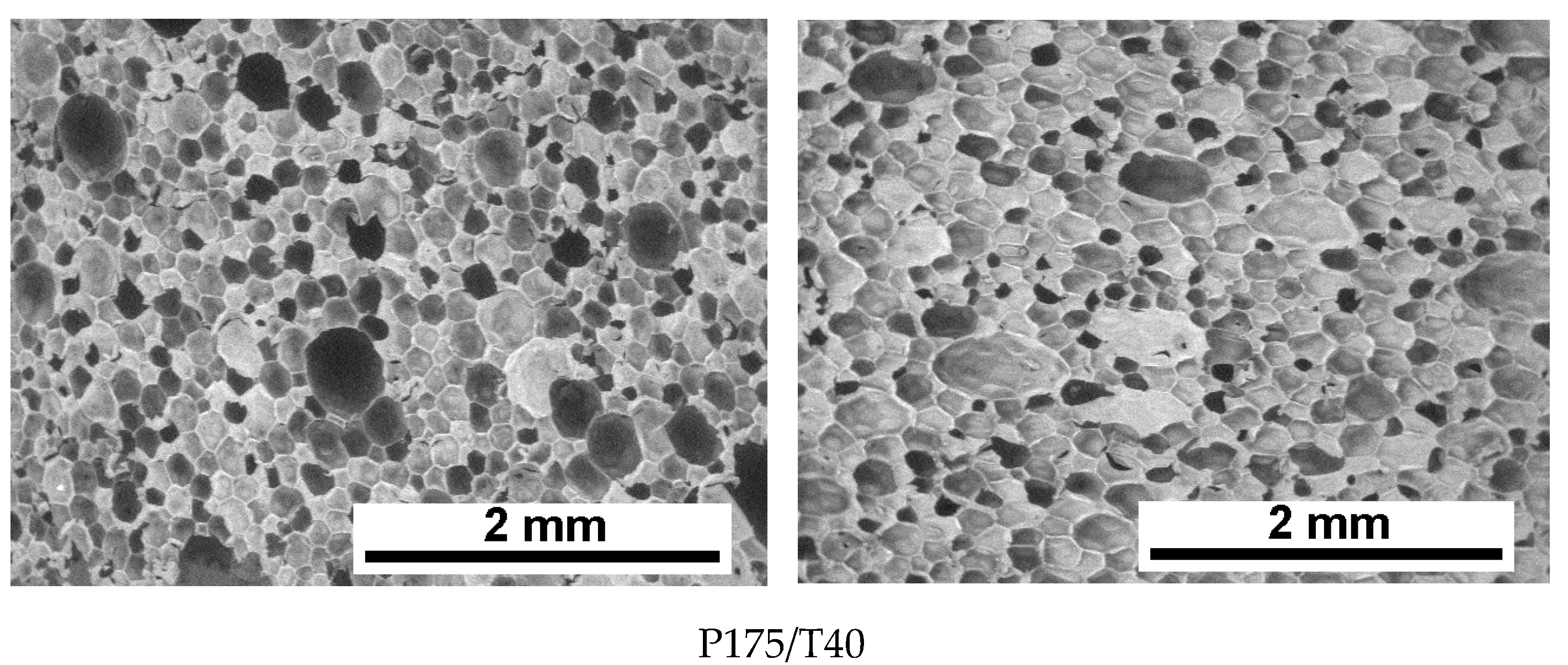
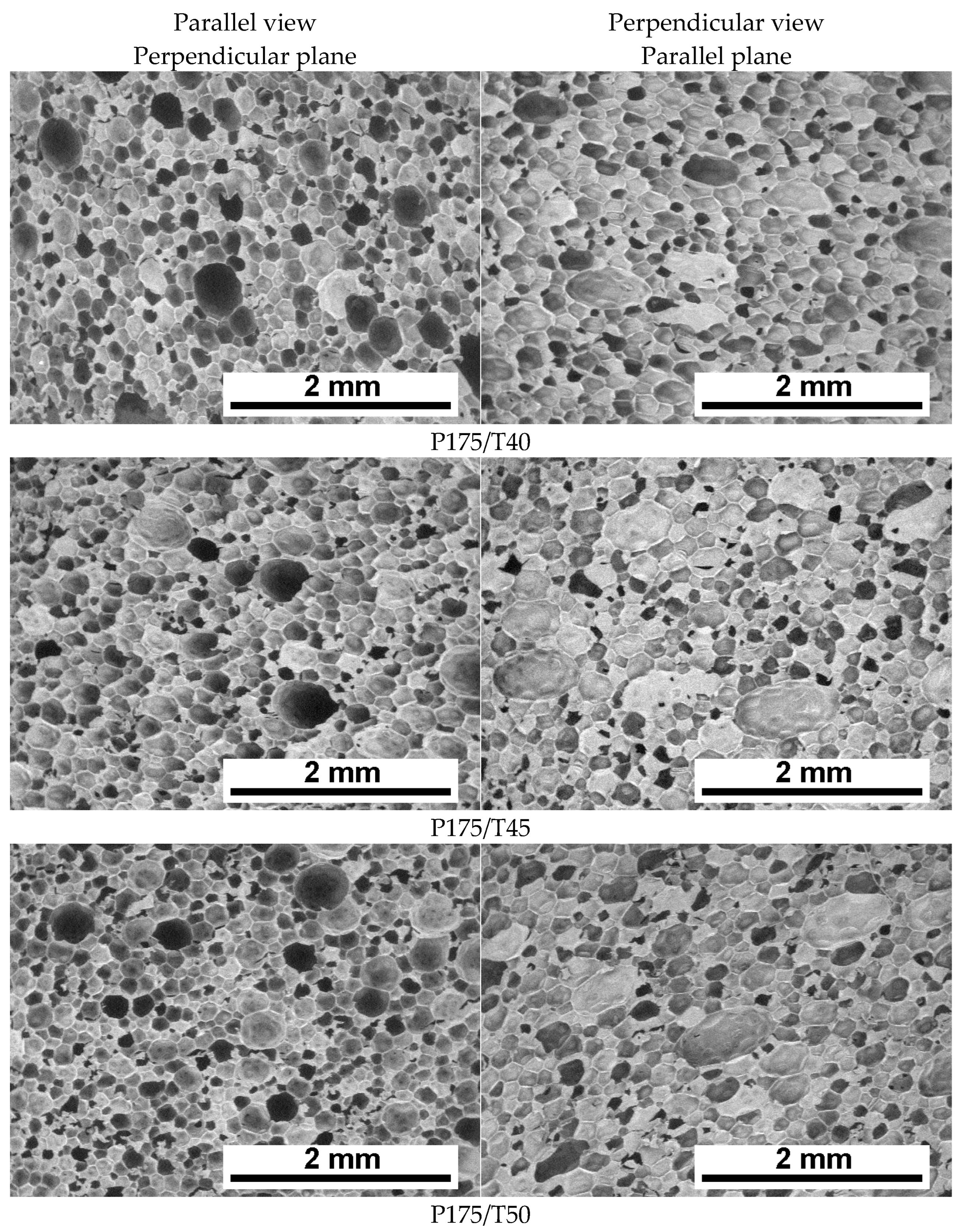
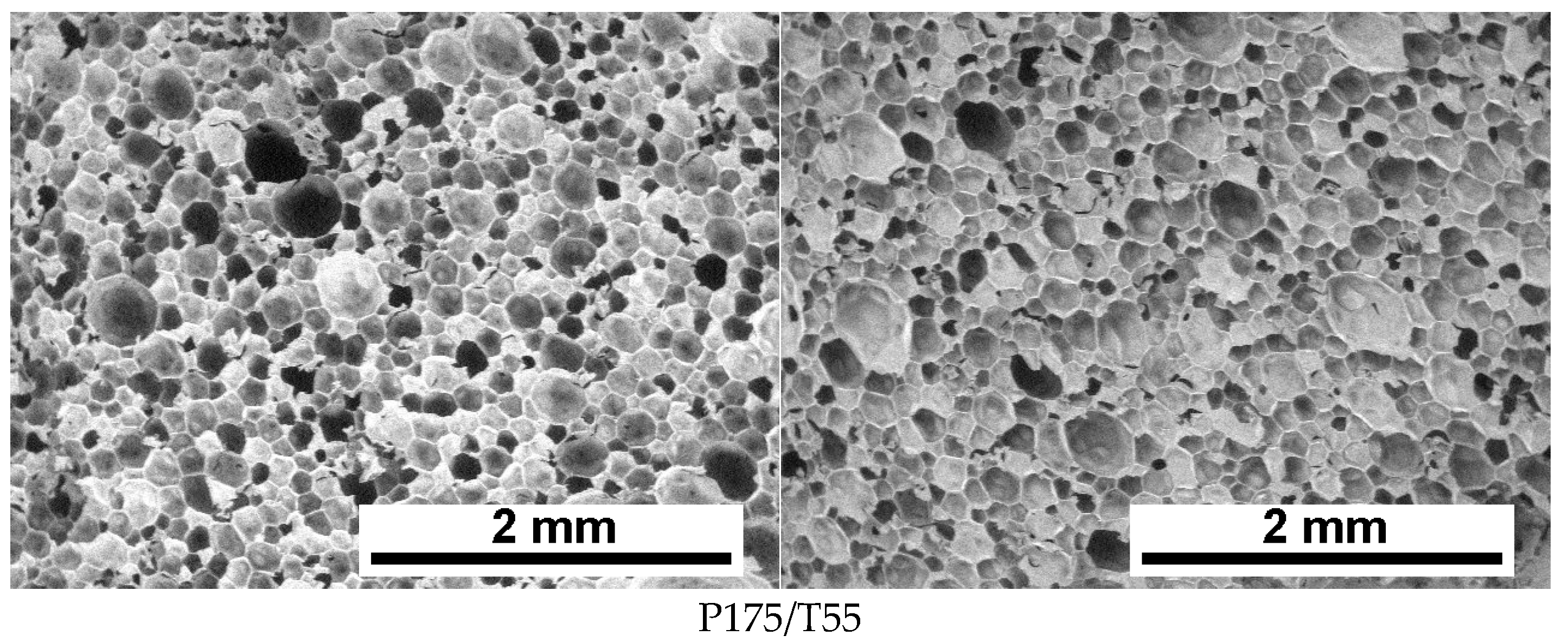
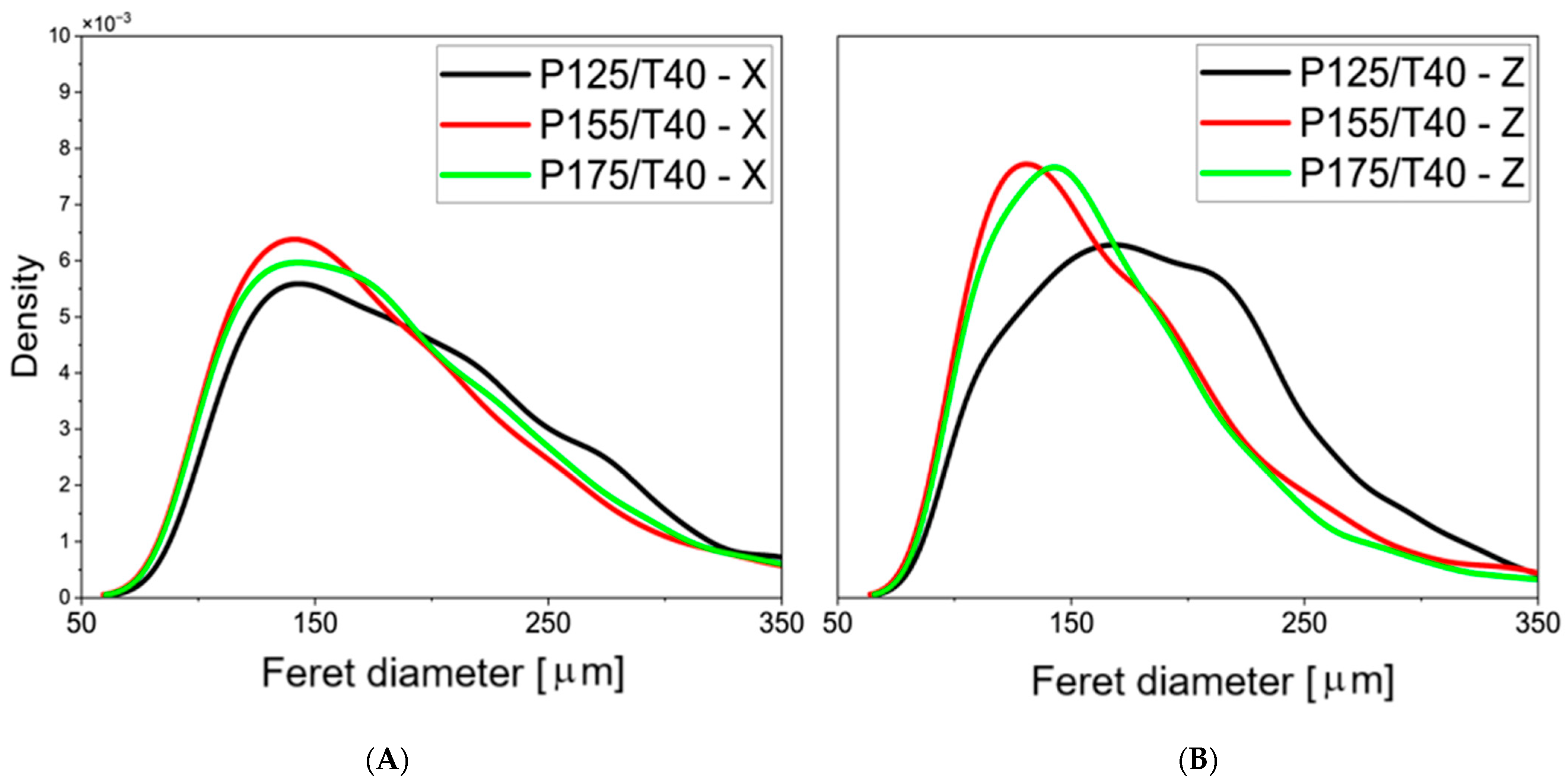
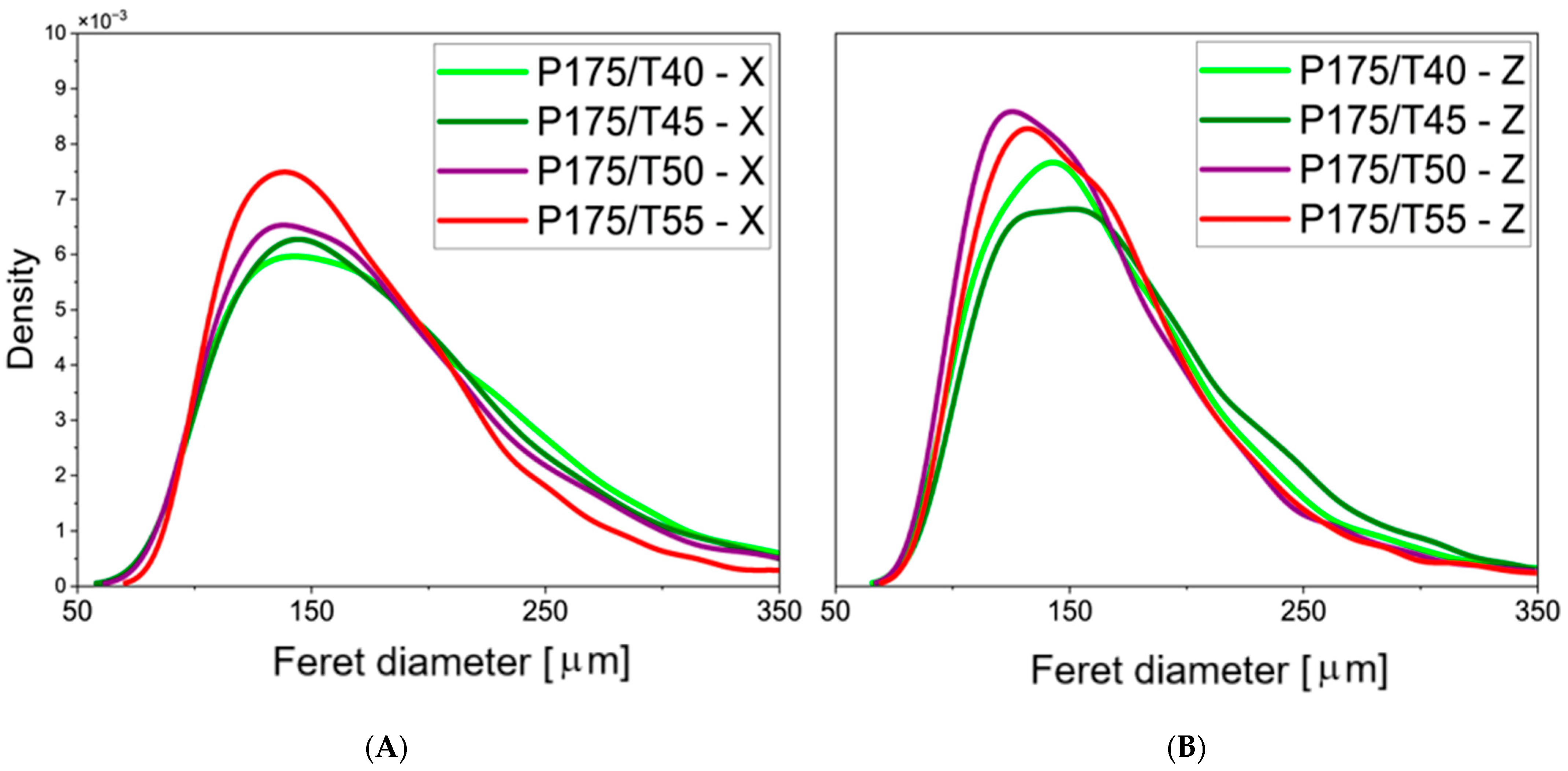
3.2. Cellular Structure Analysis of the Foams
3.3. Thermal Analysis of the Foams
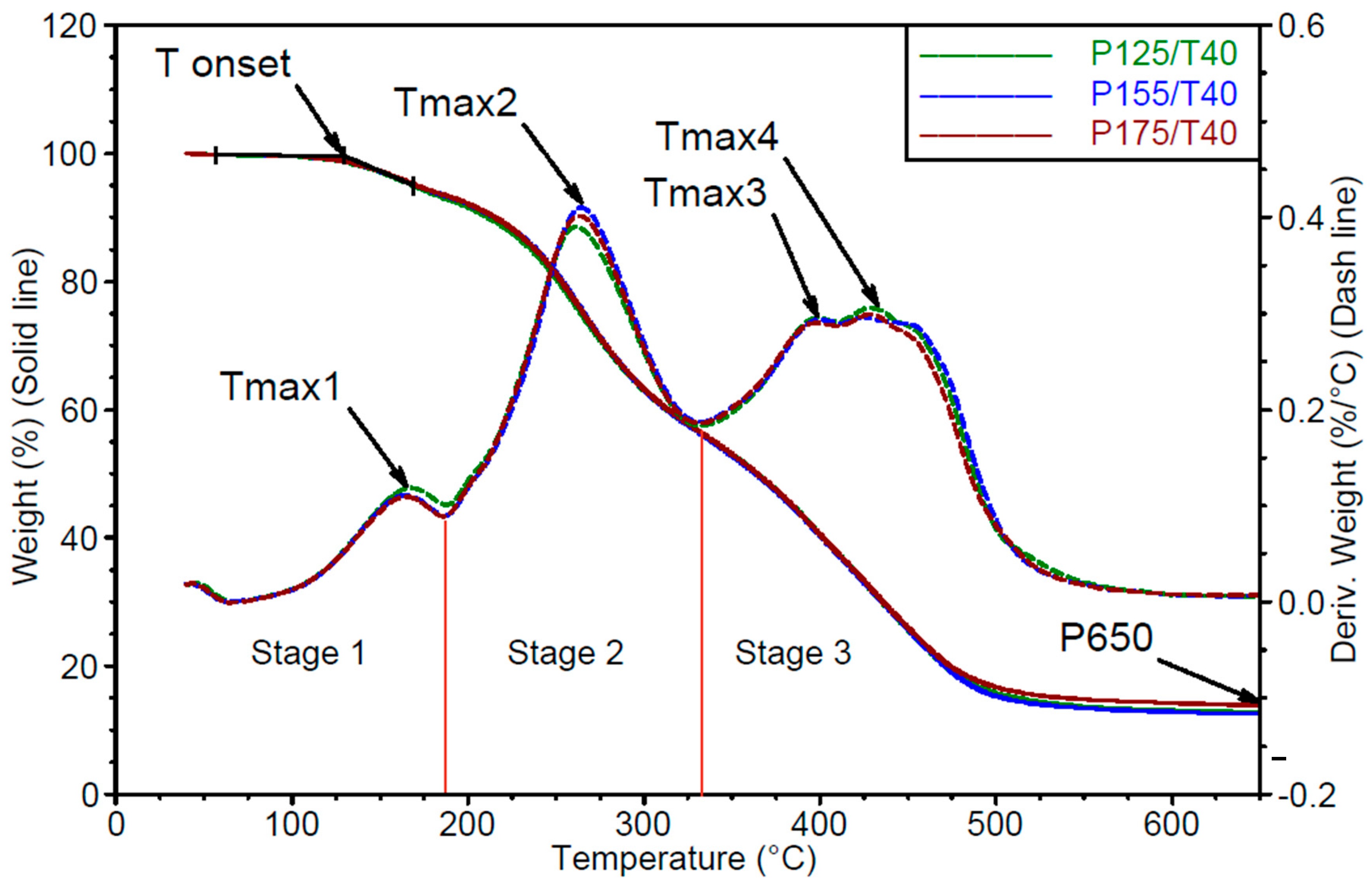
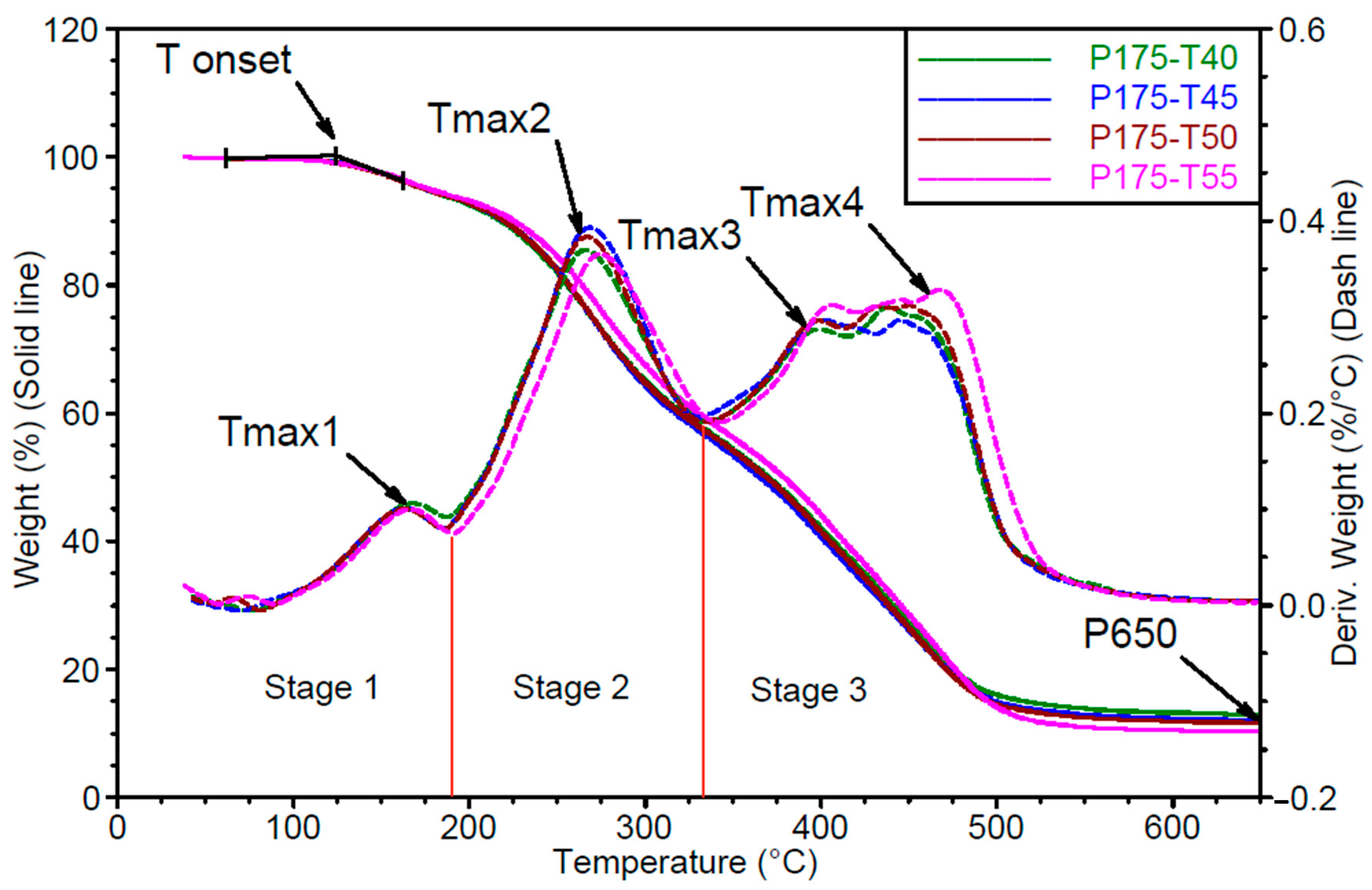
- Thermogravimetric analysis
- Stage 1: up to approximately 200 °C. In the first stage, the mass loss corresponds to the release of unbound, low-boiling-point components such as water, as well as the decomposition of polyols and isocyanates. In the tested materials, the degradation process begins during the first stage, where a peak corresponding to the maximum degradation rate Vmax1 is observed at temperature Tmax1.
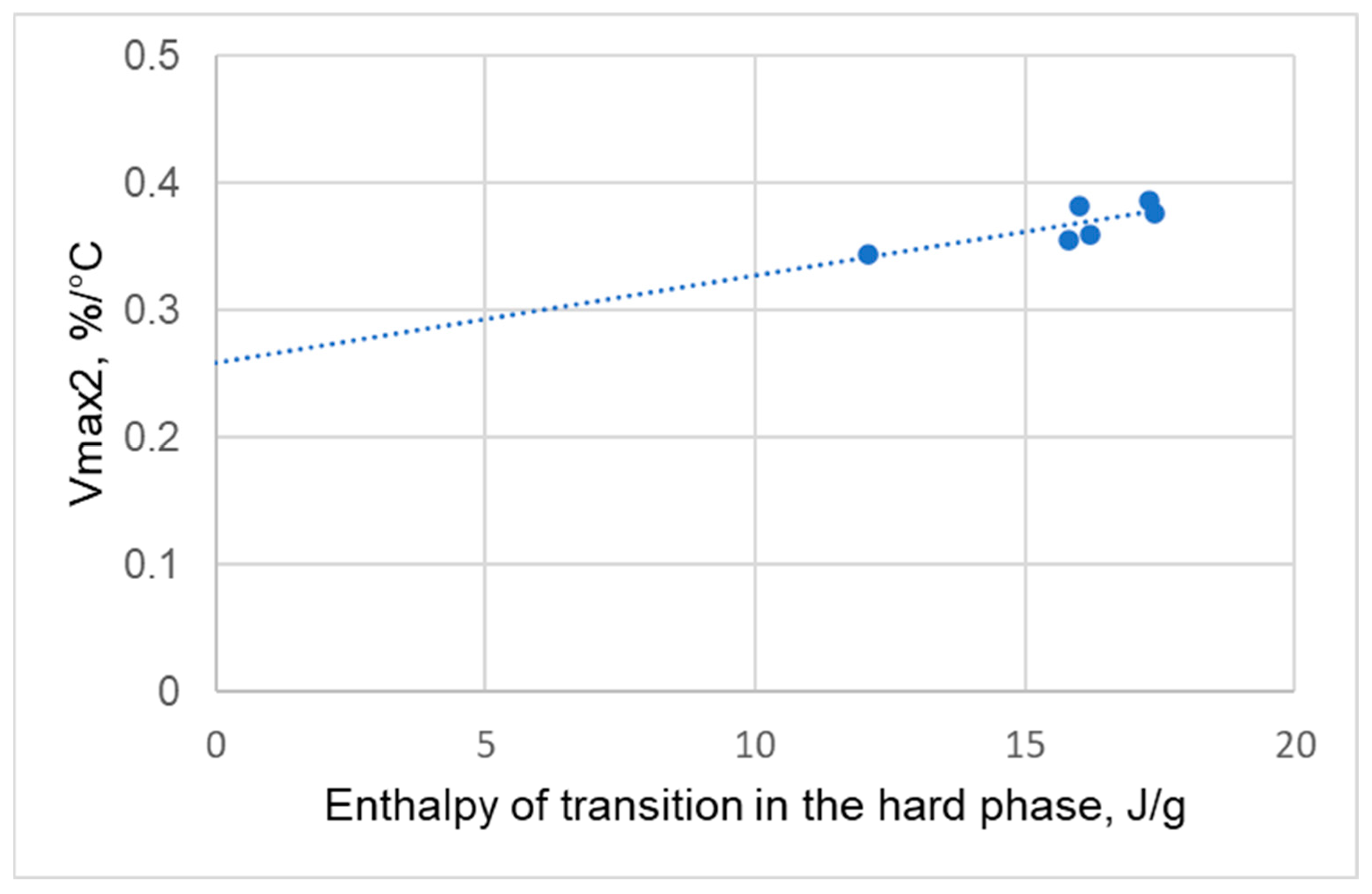
- Stage 2: from 200 to 340 °C. In the second stage, degradation is mainly associated with the breakdown of both the hard and soft segments, with mass loss resulting from the cleavage of polyol and polyisocyanate bonds. Urethane bond scission in polyurethane polymers typically occurs in the range of 250–300 °C [30,31,32,33,34]. The second degradation stage is characterized by a single peak of maximum degradation rate Vmax2 at temperature Tmax2.
- Stage 3: from 340 to 650 °C. In the third stage, mass loss results from the degradation of aromatic products originating from the hard and soft segments, which are generated during the second stage. This phase also involves the decomposition of secondary and tertiary amines [31]. In the third stage, two distinct peaks are recorded, corresponding to maximum degradation rates Vmax3 and Vmax4, observed at temperatures Tmax3 and Tmax4, respectively.
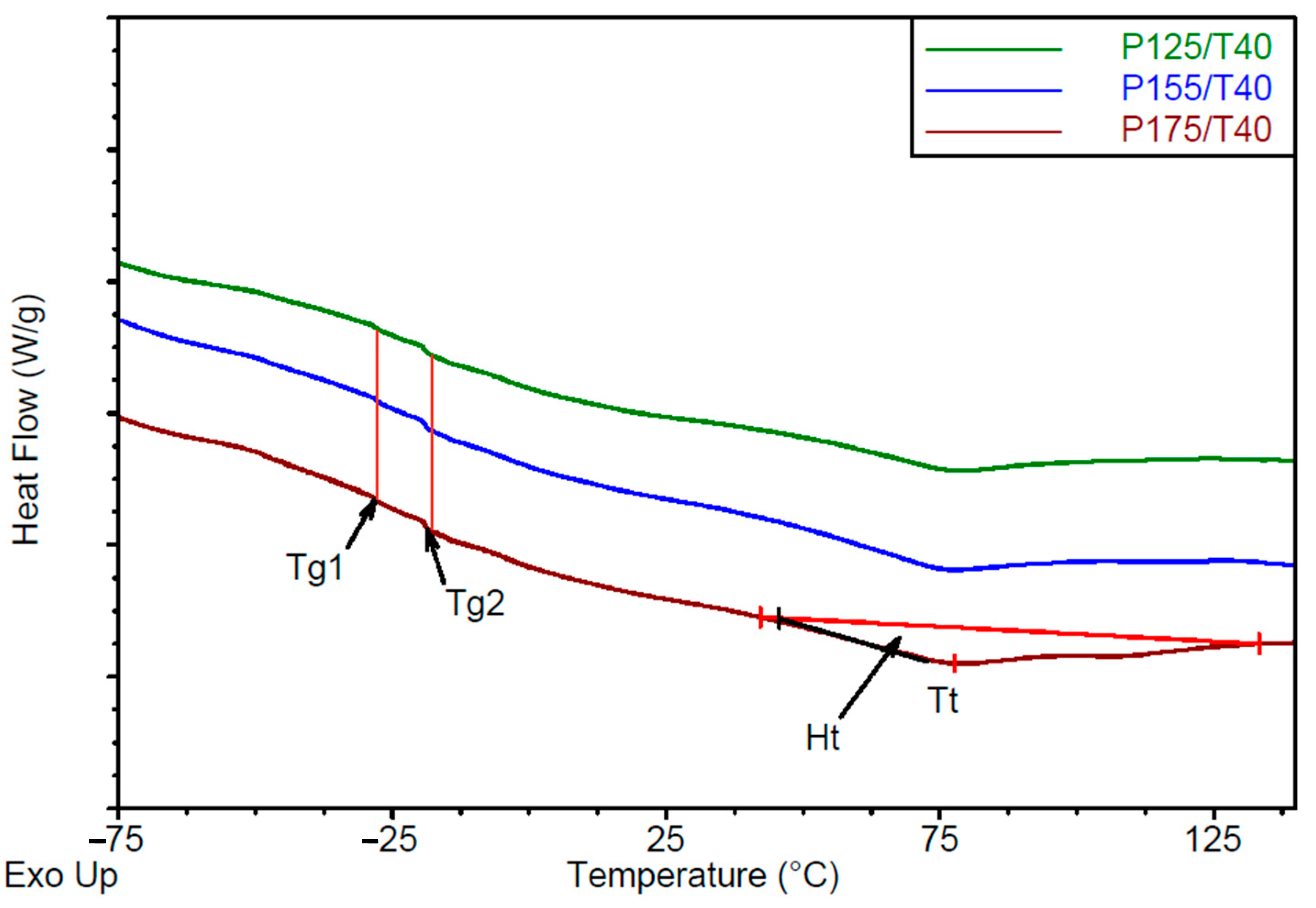
- Differential Scanning Calorimetry analysis
- Thermal conductivity
- Linear thermal stability
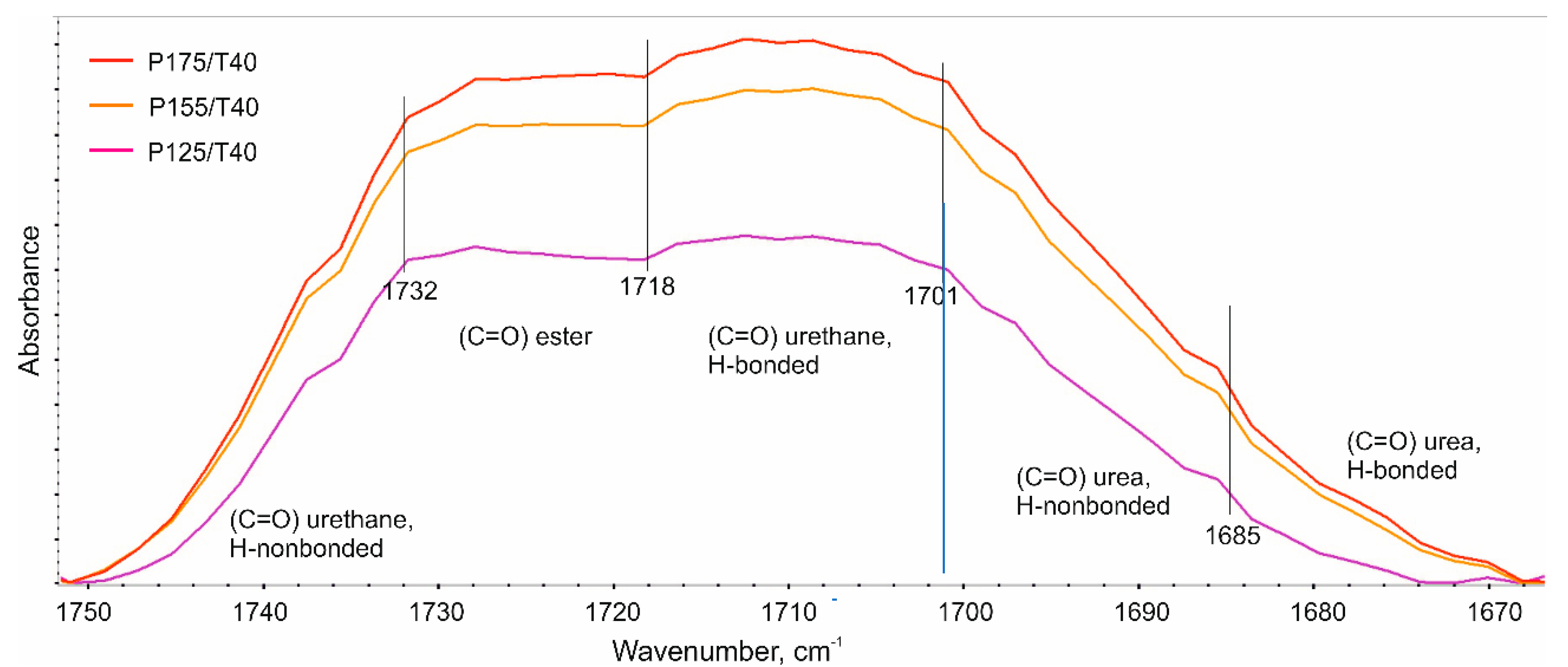
3.4. Analysis of the Chemical Composition of the Foams
3.5. Analysis of Physico-Mechanical Properties of the Foams
4. Conclusions
- a higher cell density and a corresponding reduction in average pore size,
- a significant increase in the closed-cell content was also observed,
- an increase in the degradation rate,
- a decrease in the amount of ash after degradation at 650 °C,
- an increase in the enthalpy of hard phase transformation,
- an increase in the thermal conductivity coefficient,
- an increase in the degree of phase separation,
- a higher cell density and a corresponding reduction in average pore size,
- a significant increase in the closed-cell content was also observed,
- the degradation rate in subsequent stages of foam decomposition remains unchanged,
- the amount of ash after degradation at 650 °C remains unchanged,
- a tendency towards a decrease in the enthalpy of transformation in the hard phase is observed,
- the thermal conductivity coefficient remains unchanged,
- a decrease in linear thermal stability,
- a tendency towards a decrease in the degree of phase separation is observed,
- a not significantly decrease in apparent density,
- a decrease in compressive strength,
- a tendency towards a decrease in friability and water absorption is observed.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Polyurethane Foam Insulation Materials Market Size, Share, Competitive Landscape and Trend Analysis Report, by Type Application—Global Opportunity Analysis and Industry Forecast, 2015–2023; Allied Market Research: Portland, OR, USA, 2017.
- Insulation Products Market by Insulation Type (Thermal, Acoustic), Material Type (Mineral Wool, Polyurethane Foam, Flexible Elastomeric Foam), End-Use (Building & Construction, Industrial, Transportation, Consumer)—Global Forecast to 2029; MarketsandMarkets: Pune, India, 2024.
- Kirpluks, M.; Vanags, E.; Abolins, A.; Michalowski, S.; Fridrihsone, A.; Cabulis, U. High functionality bio-polyols from tall oil and rigid polyurethane foams formulated solely using bio-polyols. Materials 2020, 13, 1985. [Google Scholar] [CrossRef]
- Directive (EU) 2023/1791. EUR-Lex. Available online: https://eur-lex.europa.eu (accessed on 1 September 2025).
- Al-Moameri, H.H.; Nabhan, B.J.; Tawfeeq Wasmi, M.; Abdulrehman, M.A. Impact of blowing agent blends on polyurethane foams thermal and mechanical properties. AIP Conf. Proc. 2020, 2213, 20015. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A. The influence of rapeseed oil-based polyols on the foaming process of rigid polyurethane foams. Ind. Crops Prod. 2016, 89, 182–187. [Google Scholar] [CrossRef]
- Prociak, A.; Rokicki, G.; Ryszkowska, J.L. Materiały Poliuretanowe; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2016. [Google Scholar]
- Wirpsza, Z. Poliuretany: Chemia, Technologia, Zastosowanie; Wydawnictwo Naukowo-Techniczne: Warsaw, Poland, 1991. [Google Scholar]
- Zhang, C.; Luo, Y.; Zhou, L.; Chen, S. The foaming dynamic characteristics of polyurethane foam. J. Cell. Plast. 2019, 56, 279–295. [Google Scholar] [CrossRef]
- Kabakci, E.; Sayer, G.; Suvaci, E.; Uysal, O.; Güler, I.; Kaya, M. Processing-structure-property relationship in rigid polyurethane foams. J. Appl. Polym. Sci. 2017, 134, 44870. [Google Scholar] [CrossRef]
- Węgrzyk, G.; Grzęda, D.J.; Ryszkowska, J. The effect of mixing pressure in a high-pressure machine on morphological and physical properties of free-rising rigid polyurethane foams—A case study. Materials 2023, 16, 857. [Google Scholar] [CrossRef]
- Węgrzyk, G.; Grzęda, D.; Bulanda, K.; Oleksy, M.; Ryszkowska, J. A new method for determining the parameters of mechanical mixing using the example of polyurethane rigid foam synthesis. Constr. Build. Mater. 2024, 416, 135205. [Google Scholar] [CrossRef]
- Özdemir, B.; Akar, F. Effects of composition and temperature of initial mixture on the formation and properties of polyurethane foam. Adv. Polym. Technol. 2018, 37, 2520–2527. [Google Scholar] [CrossRef]
- Wang, J.; Ji, S.Y.; Xing, J. Effect of process conditions on the structure and properties of rigid polyurethane foam cells. Polyurethane Ind. 2009, 24, 32–35. [Google Scholar]
- European Commission. Plastics in a Circular Economy. Available online: https://ec.europa.eu (accessed on 1 September 2025).
- Carme, M.; Ferrer, C.; Babb, D.; Ryan, A.J. Characterisation of polyurethane networks based on vegetable-derived polyol. Polymer 2008, 49, 4991–5000. [Google Scholar] [CrossRef]
- Mazurek, M.M.; Tomczyk, K.; Auguścik, M.; Ryszkowska, J.; Rokicki, G. Influence of the soft segment length on the properties of water-cured poly(carbonate-urethane-urea)s. Polym. Adv. Technol. 2015, 26, 57–67. [Google Scholar] [CrossRef]
- ISO 845:2006; Cellular Plastics and Rubbers—Determination of Apparent Density. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 844:2007; Rigid Cellular Plastics—Determination of Compression Properties. International Organization for Standardization: Geneva, Switzerland, 2007.
- ISO 4590:2016; Rigid Cellular Plastics—Determination of the Volume Percentage of Open Cells and of Closed Cells. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 2796:1986; Rigid Cellular Plastics—Test for Dimensional Stability. International Organization for Standardization: Geneva, Switzerland, 1986.
- PN-EN ISO 29767:2019-08; Rigid Cellular Plastics—Determination of Water Absorption. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 8301:1991; Thermal Insulation—Determination of Steady-State Thermal Resistance and Related Properties—Heat Flow Meter Apparatus. International Organization for Standardization: Geneva, Switzerland, 1991.
- Bartczak, P.; Kowalski, M.; Nowak, A. Closed-cell polyurethane spray foam obtained with novel TiO2–ZnO hybrid fillers—Mechanical, insulating properties and microbial purity. J. Build. Eng. 2023, 65, 105760. [Google Scholar] [CrossRef]
- Oppon, C.; Hackney, P.M.; Shyha, I.; Birkett, M. Effect of varying mixing ratios and pre-heat temperature on the mechanical properties of polyurethane (PU) foam. Procedia Eng. 2015, 132, 701–708. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Deng, Y.; Zhang, P. A review of research on the effect of temperature on the properties of polyurethane foams. Polymers 2022, 14, 4586. [Google Scholar] [CrossRef]
- Soloveva, O.V.; Solovev, S.A.; Vankov, Y.V.; Shakurova, R.Z. Experimental studies of the effective thermal conductivity of polyurethane foams with different morphologies. Processes 2022, 10, 2257. [Google Scholar] [CrossRef]
- Kurańska, M.; Cabulis, U.; Prociak, A.; Polaczek, K.; Uram, K.; Kirpluks, M. Scale-up and testing of polyurethane bio-foams as potential cryogenic insulation materials. Materials 2022, 15, 3469. [Google Scholar] [CrossRef] [PubMed]
- Peyrton, J.; Avérous, L. Structure-properties relationships of cellular materials from biobased polyurethane foams. Mater. Sci. Eng. R Rep. 2021, 145, 100608. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, Z.; Huang, H.; Chen, H. Thermal degradation of polyurethane based on IPDI. J. Anal. Appl. Pyrolysis 2009, 84, 89–94. [Google Scholar] [CrossRef]
- Ketata, N.; Trabelsi, H.; Ghorbel, A. Thermal degradation of polyurethane bicomponent systems in controlled atmospheres. Polym. Polym. Compos. 2005, 13, 1–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, Z.; Huang, H.; Chen, H. A degradation study of waterborne polyurethane based on TDI. Polym. Test. 2009, 28, 264–269. [Google Scholar] [CrossRef]
- Datta, J.; Włoch, M. Recycling of polyurethanes. In Polyurethane Polymers: Blends and Interpenetrating Polymer Networks; Elsevier: Amsterdam, The Netherlands, 2017; pp. 323–358. [Google Scholar] [CrossRef]
- Zakharyan, E.M.; Maksimov, A.L. Pyrolysis of polyurethanes: Process features and composition of reaction products. Russ. J. Appl. Chem. 2022, 95, 191–255. [Google Scholar] [CrossRef]
- Jarfelt, U.; Ramnäs, O. Thermal conductivity of polyurethane foam—Best performance. In Proceedings of the 10th International Symposium on District Heating and Cooling, Section 6a: Heat Distribution Pipe Properties, Hannover, Germany, 3–5 September 2006. [Google Scholar]
- Liu, H.; Zhao, X. Thermal conductivity analysis of high porosity structures with open and closed pores. Int. J. Heat Mass Transf. 2022, 183, 122089. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Li, H.; Chen, Z. Novel polyurethane viscoelastic foam modified with discarded luffa seed oil in accordance with cleaner production. J. Clean. Prod. 2022, 341, 130795. [Google Scholar] [CrossRef]
- Auguścik-Królikowska, M.; Kowalska, A.; Nowak, B. Composites of open-cell viscoelastic foams with blackcurrant pomace. Materials 2021, 14, 934. [Google Scholar] [CrossRef] [PubMed]
- Oliwa, R.; Malinowska, K.; Baran, W. Effects of various types of expandable graphite and blackcurrant pomace on the properties of viscoelastic polyurethane foams. Materials 2021, 14, 1801. [Google Scholar] [CrossRef]
- Carriço, C.S.; Fraga, T.; Pasa, V.M.D. Production and characterization of polyurethane foams from a simple mixture of castor oil, crude glycerol and untreated lignin as bio-based polyols. Eur. Polym. J. 2016, 85, 53–61. [Google Scholar] [CrossRef]
- El-Kabbany, F.; Taha, F.S.; Hafez, M. IR spectroscopic analysis of polymorphism in C13H14N4O. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Pretsch, T.; Jacob, L.; Muller, W. Hydrolytic degradation and functional stability of a segmented shape memory poly(ester urethane). Polym. Degrad. Stab. 2009, 94, 61–70. [Google Scholar] [CrossRef]
- Kairyt, A.; Kremensas, A.; Balciunas, G.; Członka, S.; Strakowska, A. Closed-cell rigid polyurethane foams based on low functionality polyols: Research of dimensional stability and standardised performance properties. Materials 2020, 13, 1438. [Google Scholar] [CrossRef]
- Huntsman Building Solutions. Declaration of Performance: HEATLOK HFO PRO 3.0. Available online: https://huntsmanbuildingsolutions.com/pl-PL/sites/pl_pl/files/2022-01/DoP-HEATLOK%20HFO%20PRO-3.0%20_PL.pdf (accessed on 1 September 2025).
- Huntsman Building Solutions. Declaration of Performance: HEATLOK EZ 2.0. Available online: https://huntsmanbuildingsolutions.com/pl-PL/sites/pl_pl/files/2022-01/DoP-Heatlok%20EZ-2.0%20EN_PL.pdf (accessed on 1 September 2025).
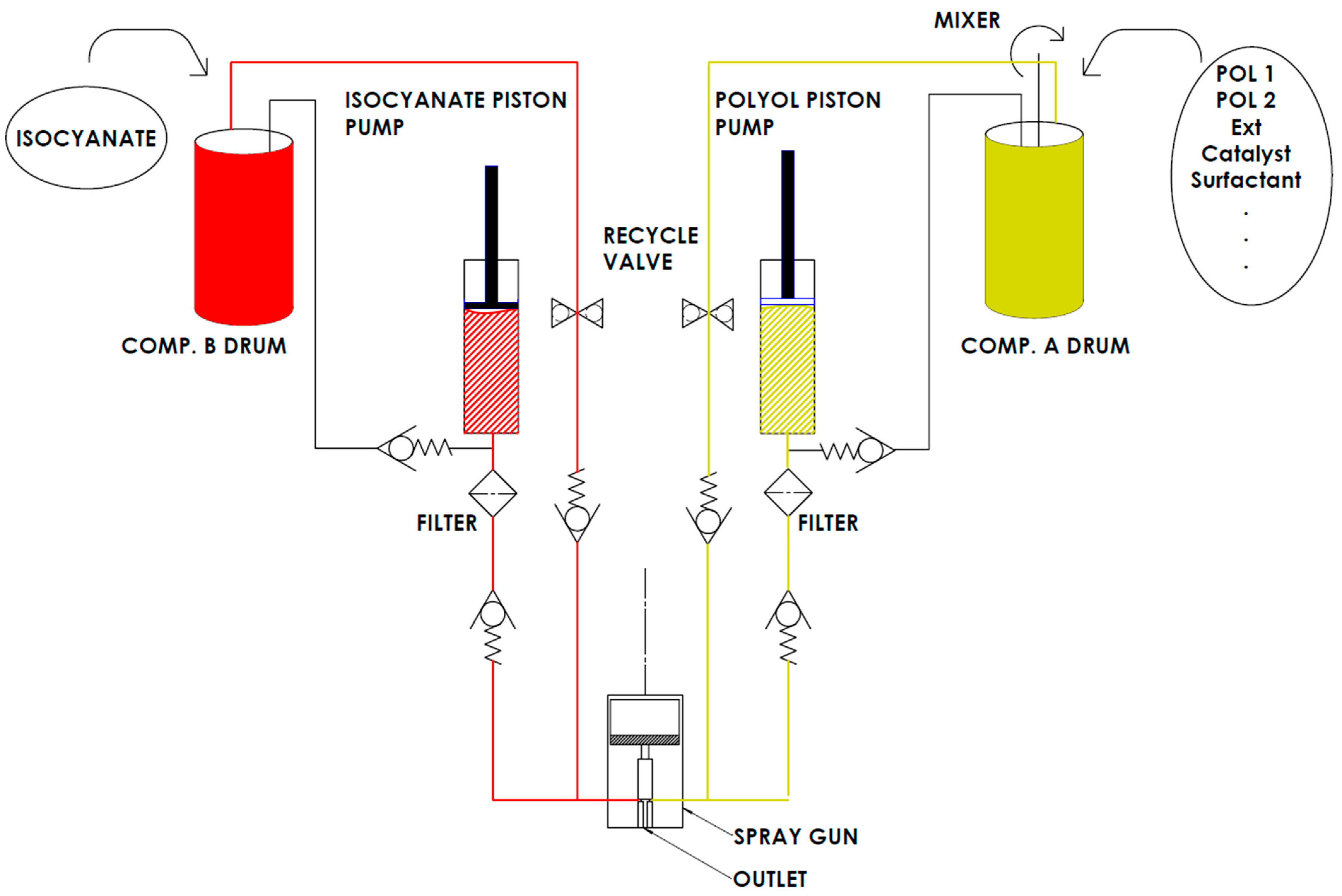


| Components | Producer | Amount, wt. % |
|---|---|---|
| Component A | ||
| Tail oil–based polyol (symbol TT) | Polylabs, Riga, Latvia | 80 |
| Polyol Lupranol 3300 | BASF, Ludwigshafen, Germany | 15 |
| Diethylene glycol (DEG) | Sigma-Aldrich, St. Louis, MO, USA | 5 |
| Tris(1-chloro-2-propyl) phosphate (TCPP)–flame retardant | Albermarle (Louvain-la-Neuve, Belgium) | 15 |
| Water–blowing agent | PCC Rokita, Brzeg dolny, Poland | 3.75 |
| Catalysts | Evonik Industries AG, Essen, Germany | 9.00 |
| Surfactant | Evonik Industries AG, Essen, Germany | 1.50 |
| Component B | ||
| MDI based isocyanate–pMDI | BASF, Ludwigshafen, Germany | 159 |
| Process Parameters | |||||||
|---|---|---|---|---|---|---|---|
| Sample name | P125/T40 | P155/T40 | P175/T40 | P175/T45 | P175/T50 | P175/T55 | |
| Component A and B temperature, °C | A | 40 ± 2 | 40 ± 2 | 40 ± 2 | 45 ± 2 | 50 ± 2 | 55 ± 2 |
| B | 40 ± 2 | 40 ± 2 | 40 ± 2 | 45 ± 2 | 50 ± 2 | 55 ± 2 | |
| Component A and B pressure, MPa | A | 12.5 ± 0.5 | 15.5 ± 0.5 | 17.5 ± 0.5 | 17.5 ± 0.5 | 17.5 ± 0.5 | 17.5 ± 0.5 |
| B | 12.5 ± 0.5 | 15.5 ± 0.5 | 17.5 ± 0.5 | 17.5 ± 0.5 | 17.5 ± 0.5 | 17.5 ± 0.5 | |
| Hydraulic pressure, bar | 40 | 50 | 60 | 60 | 60 | 60 | |
| Start time, s | 3.5 | 3.5 | 3.8 | 2.3 | 1.2 | 1.0 | |
| Thickness of the foam, cm | 3.3 ± 0.5 | 4.2 ± 0.7 | 3.0 ± 0.6 | 4.2 ± 0.5 | 4.5 ± 0.7 | 7.2 ± 0.9 | |
| Temperature on the surface of the foam, °C | 101 ± 2 | 103 ± 2 | 107 ± 2 | 116 ± 2 | 118 ± 2 | 119 ± 2 | |
| Spraying Aluminium Surface Parameters | |||||||
| Nominal thickness of surface material, mm | 4 ± 0.1 | ||||||
| Spraying orientation, horizontal/vertical | Horizontal | ||||||
| Surface temperature, °C | 25 ± 2 | ||||||
| Mixing Pressure | Mean Pore Diameter, dx [μm] | Mean Pore Diameter, dz [μm] | Cell Density, dx [1·mm−2] | Cell Density, dz [1·mm−2] | Content of Closed Cells [%] |
|---|---|---|---|---|---|
| P125/T40 | 152 ± 58 | 149 ± 53 | 46.7 | 49.5 | 89.6 ± 1.9 |
| P155/T40 | 142 ± 59 | 135 ± 57 | 52.1 | 57.6 | 90.3 ± 0.7 |
| P175/T40 | 145 ± 59 | 133 ± 51 | 50.7 | 61.0 | 93.5 ± 0.3 |
| P175/T45 | 145 ± 64 | 134 ± 49 | 49.5 | 60.4 | 93.3 ± 1.7 |
| P175/T50 | 140 ± 56 | 126 ± 49 | 54.7 | 67.6 | 93.4 ± 1.6 |
| P175/T55 | 129 ± 45 | 127 ± 46 | 66.1 | 68.0 | 94.5 ± 0.3 |
| Sample | TOnset, °C | Tmax1, °C | Vmax1, %/°C | Tmax2, °C | Vmax2, %/°C |
|---|---|---|---|---|---|
| P125/T40 | 132 ± 1.2 | 173 ± 1.6 | 0.12 ± 0.001 | 289 ± 1.7 | 0.34 ± 0.018 |
| P155/T40 | 132 ± 0.9 | 171 ± 1.2 | 0.10 ± 0.000 | 280 ± 2.2 | 0.36 ± 0.007 |
| P175/T40 | 132 ± 2.9 | 169 ± 1.2 | 0.11 ± 0.001 | 271 ± 4.0 | 0.39 ± 0.020 |
| P175/T45 | 125 ± 1.6 | 166 ± 1.7 | 0.10 ± 0.004 | 271 ± 3.1 | 0.38 ± 0.014 |
| P175/T50 | 128 ± 0.9 | 166 ± 1.4 | 0.10 ± 0.001 | 270 ± 2.2 | 0.38 ± 0.003 |
| P175/T55 | 130 ± 2.4 | 166 ± 1.7 | 0.10 ± 0.002 | 272 ± 1.2 | 0.36 ± 0.006 |
| Sample | Tmax3, °C | Vmax3, %/°C | Tmax4, °C | Vmax4, %/°C | P650, % |
|---|---|---|---|---|---|
| P125/T40 | 400 ± 3.3 | 0.19 ± 0.010 | 433 ± 3.6 | 0.19 ± 0.026 | 22.1 ± 1.5 |
| P155/T40 | 397 ± 1.9 | 0.24 ± 0.021 | 455 ± 8.4 | 0.24 ± 0.051 | 18.0 ± 3.4 |
| P175/T40 | 403 ± 2.8 | 0.30 ± 0.009 | 449 ± 9.5 | 0.32 ± 0.007 | 12.0 ± 0.8 |
| P175/T45 | 403 ± 2.4 | 0.30 ± 0.002 | 454 ± 6.3 | 0.32 ± 0.013 | 12.0 ± 0.5 |
| P175/T50 | 402 ± 3.1 | 0.30 ± 0.006 | 454 ± 4.2 | 0.32 ± 0.003 | 11.2 ± 1.0 |
| P175/T55 | 403 ± 3.3 | 0.31 ± 0.005 | 459 ± 4.9 | 0.32 ± 0.009 | 12.4 ± 1.5 |
| Sample | Tg1 [°C] | Tg2 [°C] | Tt [°C] | DHt [Jg−1] |
|---|---|---|---|---|
| P125/T40 | −28.1 ± 0.1 | −19.0 ± 0.1 | 76.8 ± 0.3 | 12.1 ± 0.2 |
| P155/T40 | −28.1 ± 0.1 | −19.2 ± 0.0 | 75.8 ± 0.1 | 15.8 ± 0.5 |
| P175/T40 | −28.1 ± 0.1 | −19.0 ± 0.1 | 77.4 ± 0.1 | 17.3 ± 0.8 |
| P175/T45 | −28.1 ± 0.1 | −19.0 ± 0.1 | 76.6 ± 0.1 | 17.4 ± 0.7 |
| P175/T50 | −27.9 ± 0.3 | −19.0 ± 0.1 | 76.0 ± 0.7 | 16.0 ± 0.4 |
| P175/T55 | −28.0 ± 0.2 | −19.0 ± 0.1 | 76.4 ± 1.0 | 16.2 ± 0.6 |
| Sample | λ [mW·m−1∙K−1] | Thermal Stability [%] |
|---|---|---|
| P125/T40 | 18.55 ± 0.02 | 0.51 ± 0.11 |
| P155/T40 | 19.76 ± 0.02 | 0.32 ± 0.22 |
| P175/T40 | 21.82 ± 0.03 | 0.53 ± 0.49 |
| P175/T45 | 22.17 ± 0.09 | 0.45 ± 0.37 |
| P175/T50 | 22.30 ± 0.06 | 0.17 ± 0.07 |
| P175/T55 | 21.54 ± 0.08 | 0.25 ± 0.23 |
| P125/T40 | P155/T40 | P175/T40 | P175/T45 | P175/T50 | P175/T55 | |
|---|---|---|---|---|---|---|
| Wavenumbers [cm−1] | Bond (Vibration) | |||||
| 3305 | 3306 | 3304 | 3307 | 3307 | 3307 | N-H (stretching) |
| 2925 | 2925 | 2925 | 2925 | 2925 | 2925 | C-H (asymmetric stretching) |
| 2854 | 2853 | 2853 | 2852 | 2854 | 2854 | C-H (symmetric stretching) |
| 1705 | 1705 | 1705 | 1706 | 1705 | 1705 | C=O (stretching) |
| 1595 | 1595 | 1595 | 1595 | 1595 | 1595 | C=C (stretching) |
| 1510 | 1510 | 1510 | 1510 | 1510 | 1510 | N-H (bending) |
| 1453 | 1453 | 1453 | 1453 | 1453 | 1453 | C-H (deformation) |
| 1411 | 1411 | 1411 | 1411 | 1411 | 1411 | PIR (deformation) |
| 1307 | 1307 | 1307 | 1307 | 1307 | 1307 | C-H (streching) |
| 1217 | 1217 | 1217 | 1217 | 1217 | 1217 | C-N (stretching) |
| 1055 | 1056 | 1056 | 1056 | 1054 | 1056 | C-O (stretching) |
| Sample | DPS |
|---|---|
| P125/T40 | 0.49 ± 0.5 |
| P155/T40 | 0.60 ± 0.4 |
| P175/T40 | 0.63 ± 0.6 |
| P175/T45 | 0.64 ± 0.7 |
| P175/T50 | 0.61 ± 0.4 |
| P175/T55 | 0.62 ± 0.6 |
| Sample | Apparent Density [kg·m−3] | Compressive Strength | Friability [%] | Water Absorption, [kg·m−2] | |
|---|---|---|---|---|---|
| Para, kPa | Perp, kPa | ||||
| P125/T40 | 45.5 ± 1.43 | 248 ± 1.8 | 228 ± 9.4 | 0.68 ± 0.16 | 9.6 ± 0.3 |
| P155/T40 | 45.1 ± 1.16 | 254 ± 6.7 | 232 ± 8.1 | 0.43 ± 0.14 | 12.1 ± 0.6 |
| P175/T40 | 45.1 ± 0.56 | 255 ± 2.9 | 223 ± 2.1 | 0.53 ± 0.12 | 7.8 ± 0.8 |
| P175/T45 | 45.0 ± 0.08 | 242 ± 7.6 | 202 ± 5.5 | 0.31 ± 0.03 | 11.4 ± 0.1 |
| P175/T50 | 44.4 ± 0.67 | 251 ± 5.2 | 179 ± 2.5 | 0.37 ± 0.17 | 12.9 ± 0.3 |
| P175/T55 | 44.0 ± 1.21 | 225 ± 8.9 | 165 ± 8.6 | 0.36 ± 0.23 | 8.9 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Węgrzyk, G.; Grzęda, D.; Leszczyńska, M.; Vēvere, L.; Cābulis, U.; Ryszkowska, J. Structure and Properties of Sprayed Polyurethane Bio-Based Foams Produced Under Varying Fabrication Parameters. Polymers 2025, 17, 2522. https://doi.org/10.3390/polym17182522
Węgrzyk G, Grzęda D, Leszczyńska M, Vēvere L, Cābulis U, Ryszkowska J. Structure and Properties of Sprayed Polyurethane Bio-Based Foams Produced Under Varying Fabrication Parameters. Polymers. 2025; 17(18):2522. https://doi.org/10.3390/polym17182522
Chicago/Turabian StyleWęgrzyk, Grzegorz, Dominik Grzęda, Milena Leszczyńska, Laima Vēvere, Uģis Cābulis, and Joanna Ryszkowska. 2025. "Structure and Properties of Sprayed Polyurethane Bio-Based Foams Produced Under Varying Fabrication Parameters" Polymers 17, no. 18: 2522. https://doi.org/10.3390/polym17182522
APA StyleWęgrzyk, G., Grzęda, D., Leszczyńska, M., Vēvere, L., Cābulis, U., & Ryszkowska, J. (2025). Structure and Properties of Sprayed Polyurethane Bio-Based Foams Produced Under Varying Fabrication Parameters. Polymers, 17(18), 2522. https://doi.org/10.3390/polym17182522









