Influence of Drug Properties, Formulation Composition, and Processing Parameters on the Stability and Dissolution Performance of Amorphous Solid Dispersions-Based Tablets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
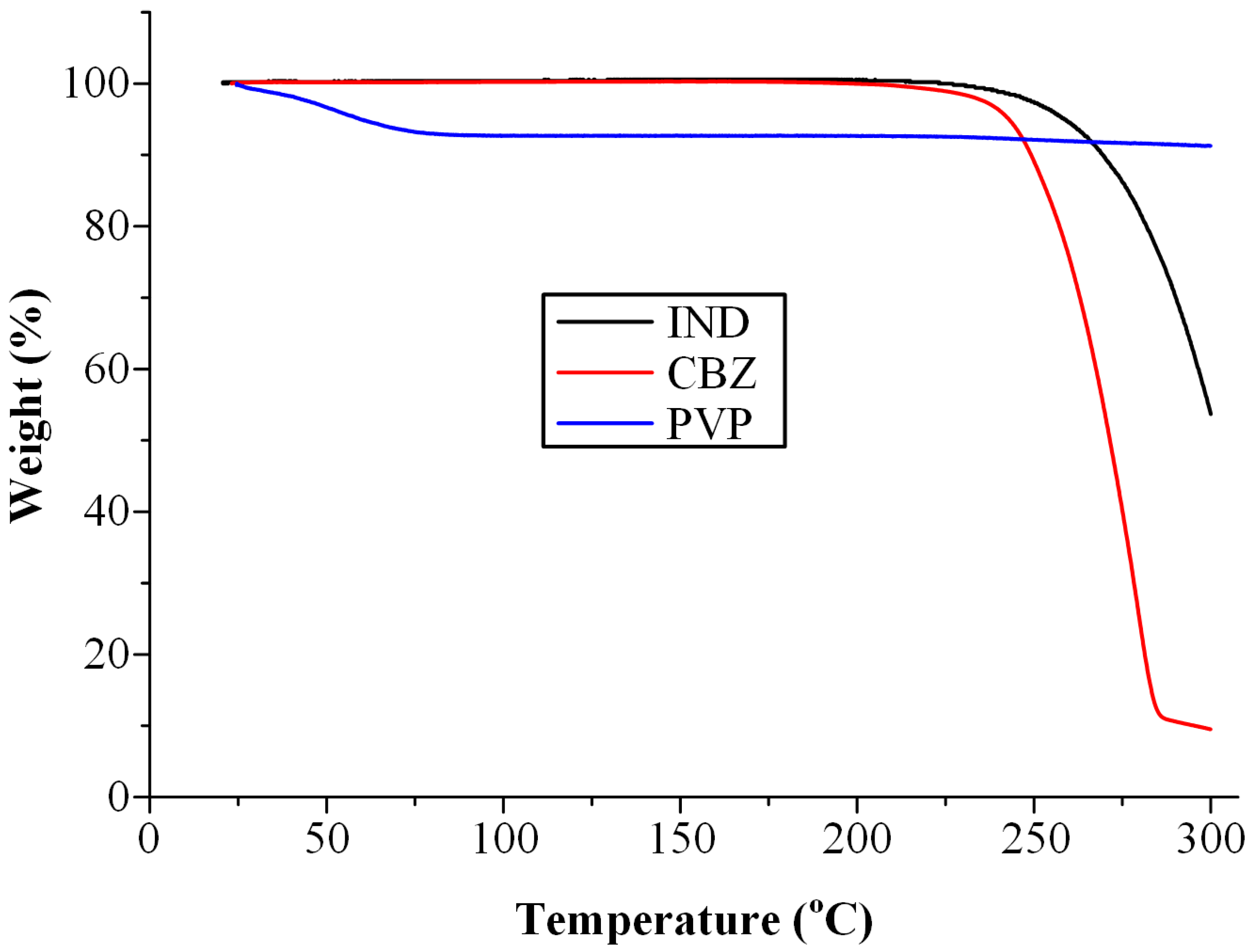
2.2. Thermal Stability of Pure Materials
2.3. Preparation of the ASDs
2.4. Physicochemical and Thermophysical Characterization of the Prepared ASDs
2.4.1. Differential Scanning Calorimetry (DSC)
2.4.2. Powder X-Ray Diffractometry (pXRD)
2.4.3. Attenuated Total Reflectance-FTIR Spectroscopy (ATR-FTIR)
2.5. Tablet Manufacturing
2.6. Characterization of Prepared Tablets
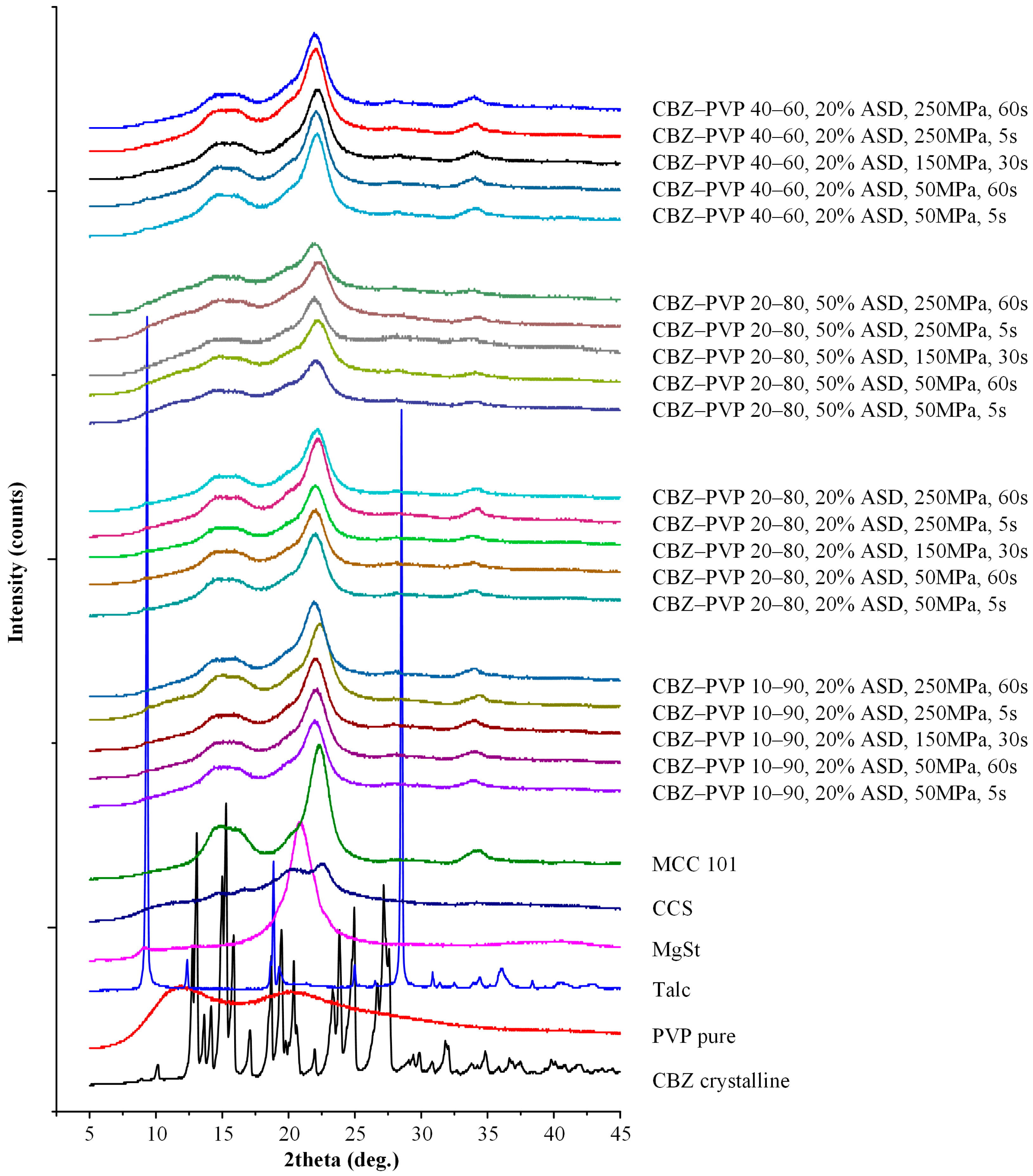
2.6.1. pXRD Analysis of the Tablets
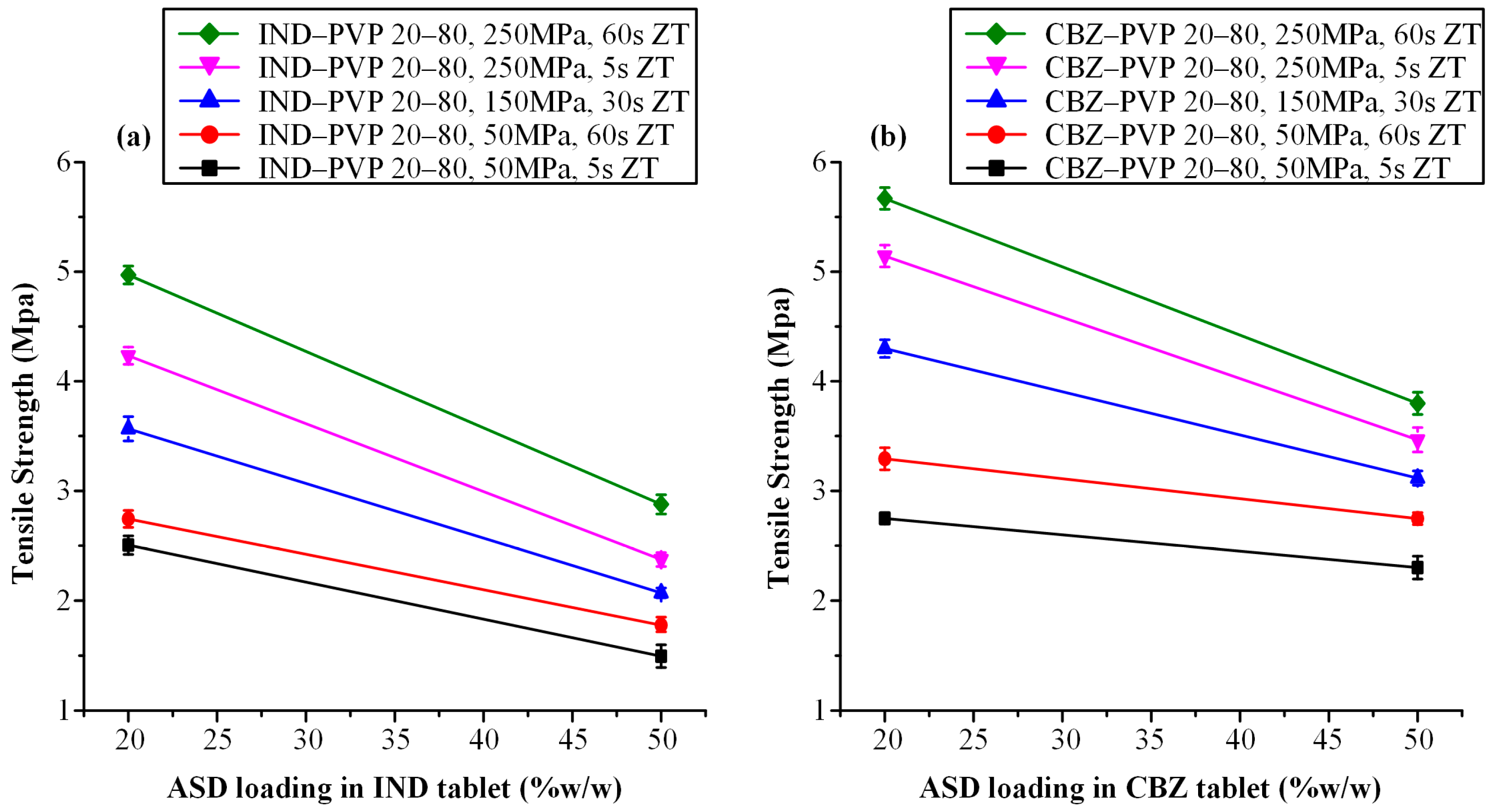
2.6.2. Evaluation of Tensile Strength
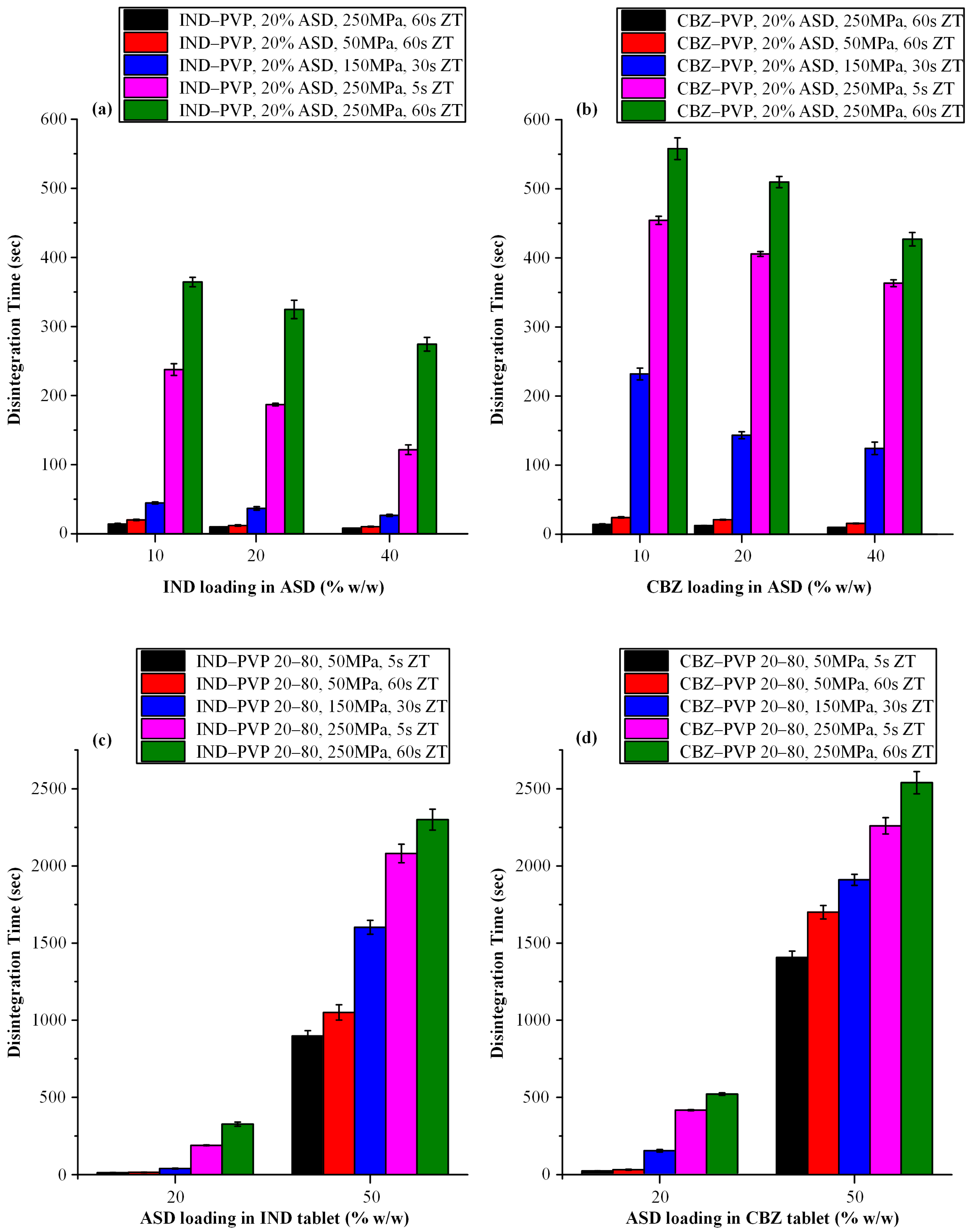
2.6.3. Disintegration Time Assessment
2.7. IND/CBZ Solubility and Dissolution Studies
2.7.1. API’s Crystalline Saturation Solubility Determination
2.7.2. In-Vitro Dissolution Studies Under Non-Sink Conditions
2.8. Stability Studies
2.9. Statistical Analysis
3. Results and Discussion
3.1. Thermal Stability Profile of Raw Materials
3.2. Evaluation of ASDs
3.2.1. DSC Results
3.2.2. Evaluation of Molecular Interactions via ATR-FTIR
3.3. Characterization of the Prepared Tablets Immediately After Preparation
3.3.1. Physical State Evaluation
3.3.2. Tensile Strength Evaluation
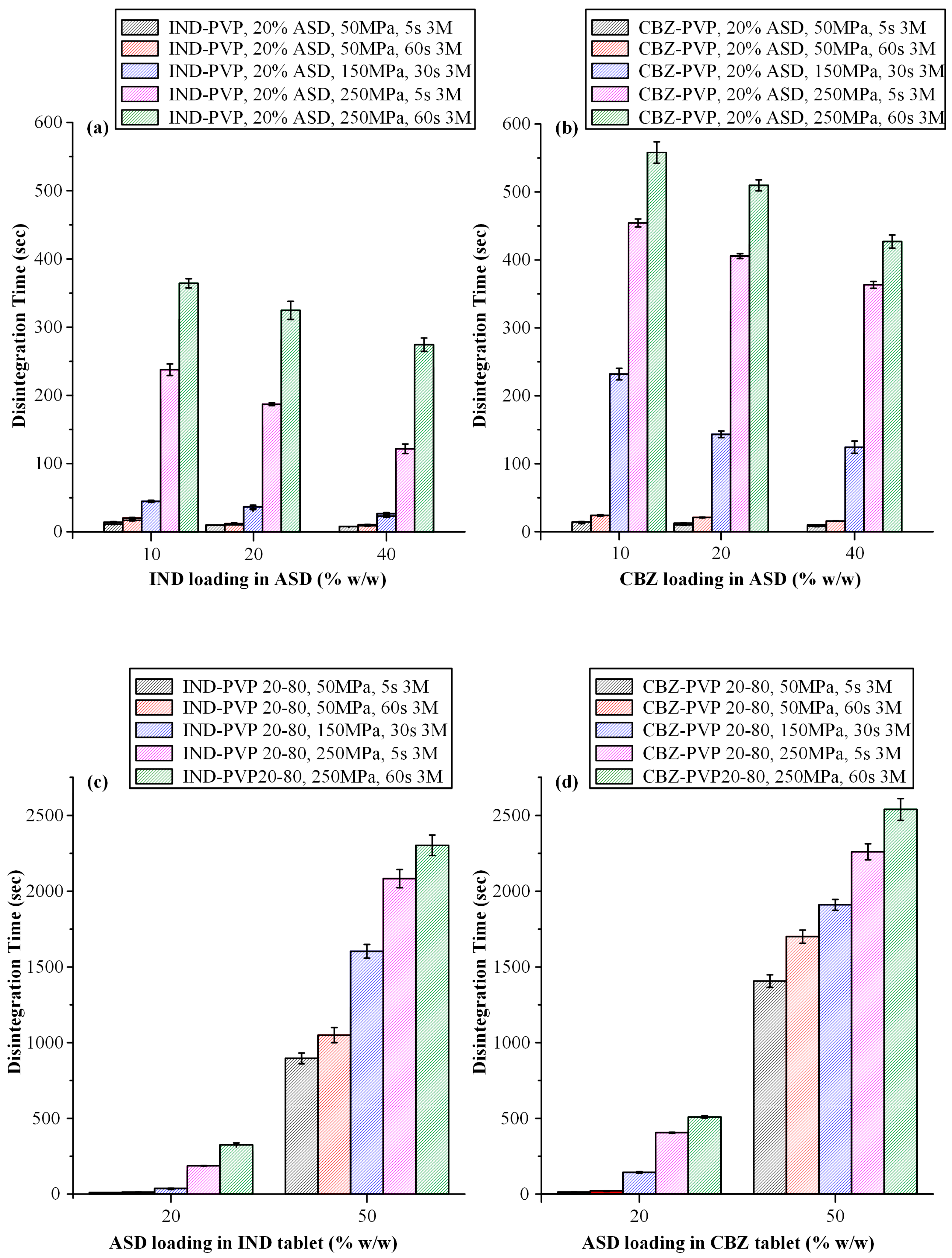
3.3.3. Disintegration Time Evaluation
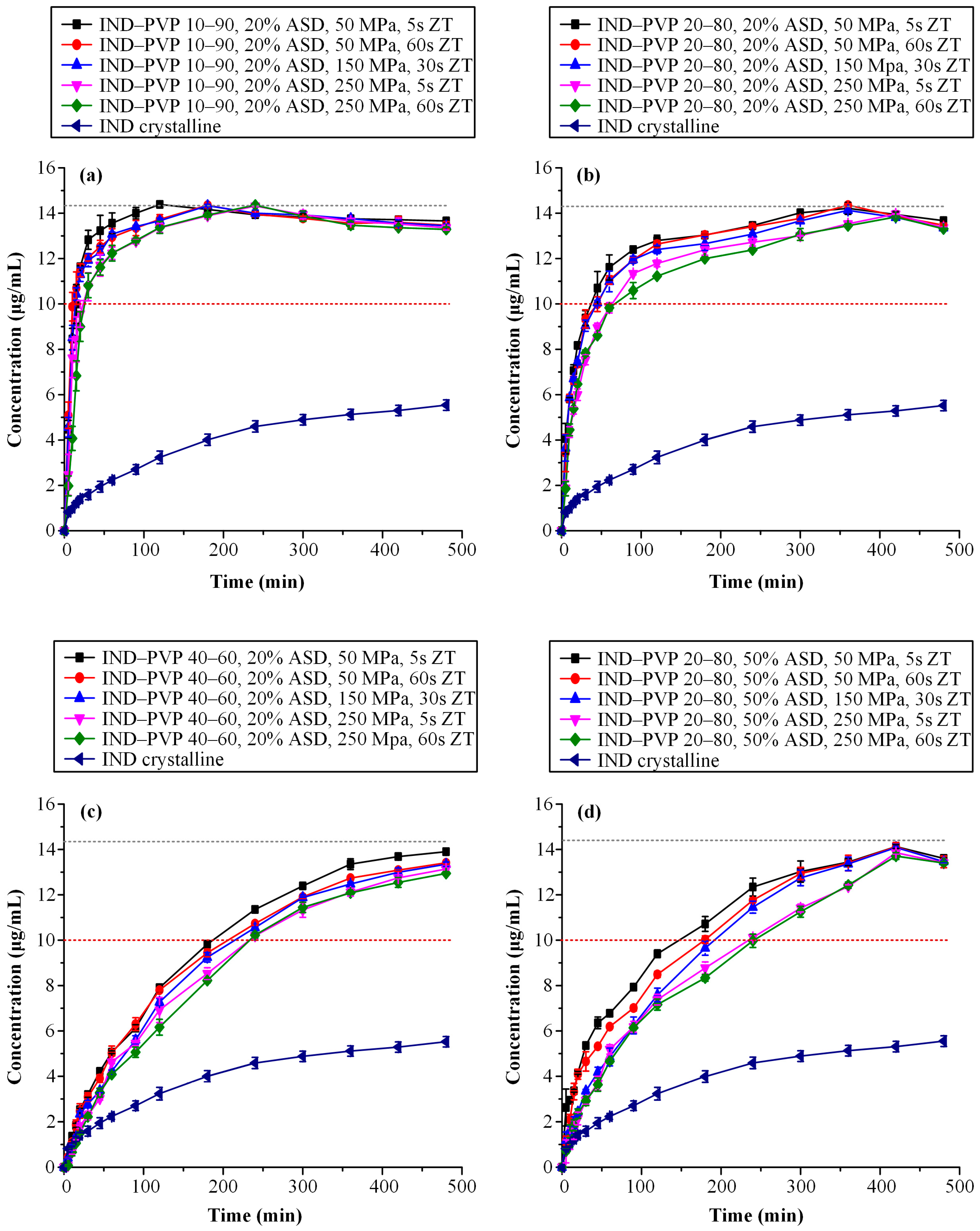
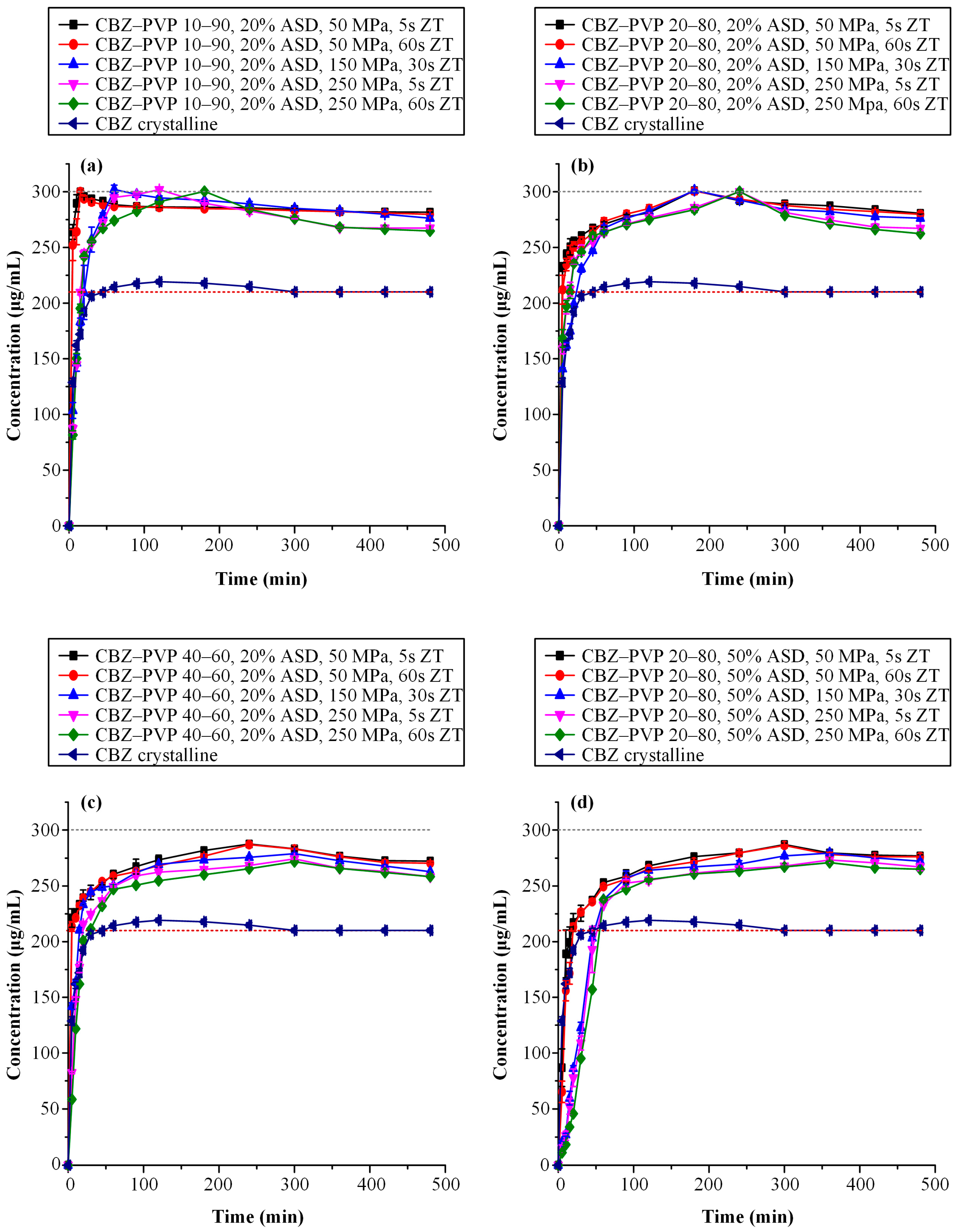
3.3.4. Supersaturated Dissolution Studies
3.4. Stability Studies Results
3.4.1. Physical Stability
3.4.2. Tensile Strength Evaluation After Storage
3.4.3. Disintegration Time Evaluation After Storage
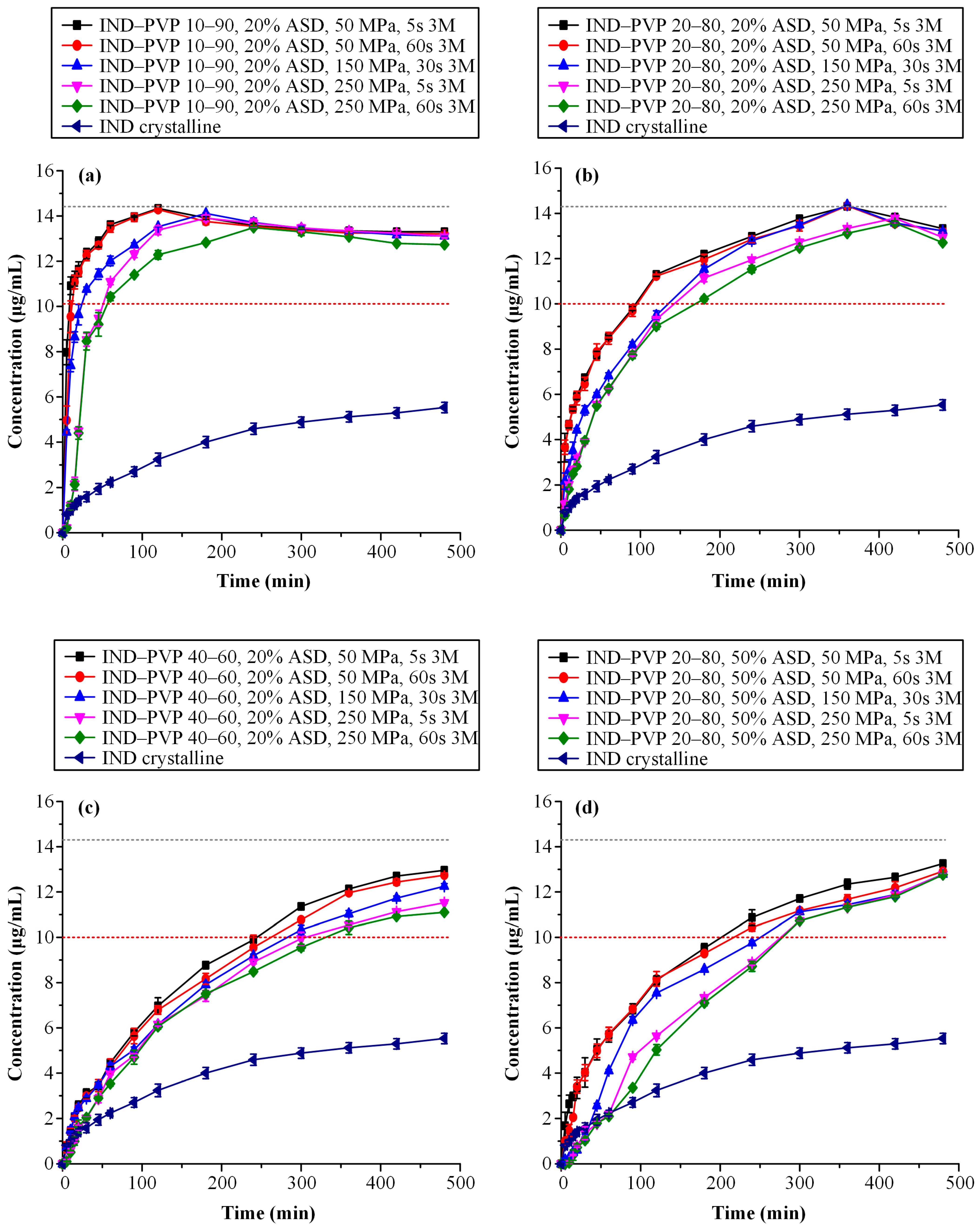
3.4.4. Dissolution Studies After Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, P.; Thakur, A.S.; Deshmukh, K.; Jha, A.K.; Verma, S. Routes of drug administration. Int. J. Pharm. Stud. Res. 2010, 1, 54–59. [Google Scholar]
- Rang, H.P.; Dale, M.M.; Ritter, J.M.; Flower, R.J. Rang & Dale’s Pharmacology, 9th ed.; Elsevier: London, UK, 2020. [Google Scholar]
- Xie, B.; Liu, Y.; Li, X.; Yang, P.; He, W. Solubilization techniques used for poorly water-soluble drugs. Acta Pharm. Sin. B 2024, 14, 4683–4716. [Google Scholar] [CrossRef]
- Nyamba, I.; Sombié, C.B.; Yabré, M.; Zimé-Diawara, H.; Yaméogo, J.; Ouédraogo, S.; Lechanteur, A.; Semdé, R.; Evrard, B. Pharmaceutical approaches for enhancing solubility and oral bioavailability of poorly soluble drugs. Eur. J. Pharm. Biopharm. 2024, 204, 114513. [Google Scholar] [CrossRef] [PubMed]
- Vimalson, C.; Sundararajan, P.; Jeganathan, N.; Anbazhagan, S. Techniques to enhance solubility of hydrophobic drugs: An overview. Asian J. Pharm. 2016, 10, S67–S75. [Google Scholar]
- Narmada, I. Contemporary Review on Solubility Enhancement Techniques. J. Drug Deliv. Ther. 2023, 13, 110–120. [Google Scholar] [CrossRef]
- Han, J.; Tang, M.; Yang, Y.; Sun, W.; Yue, Z.; Zhang, Y.; Zhu, Y.; Liu, X.; Wang, J. Amorphous solid dispersions: Stability mechanism, design strategy and key production technique of hot melt extrusion. Int. J. Pharm. 2023, 646, 123490. [Google Scholar] [CrossRef] [PubMed]
- Ricarte, R.; Zee, N.; Li, Z.; Johnson, L.; Lodge, T.; Hillmyer, M. Recent Advances in Understanding the Micro- and Nanoscale Phenomena of Amorphous Solid Dispersions. Mol. Pharm. 2019, 16, 4089–4103. [Google Scholar] [CrossRef]
- Teng, M.; Li, J.; Li, Z.; Zhang, G.; Zhao, P.; Fu, Q. Recrystallization Mediates the Gelation of Amorphous Drugs: The Case of Acemetacin. Pharmaceutics 2023, 15, 219. [Google Scholar] [CrossRef]
- Fung, M.H.; DeVault, M.; Kuwata, K.T.; Suryanarayanan, R. Drug-Excipient Interactions: Effect on Molecular Mobility and Physical Stability of Ketoconazole–Organic Acid Coamorphous Systems. Mol. Pharm. 2018, 15, 1052–1061. [Google Scholar] [CrossRef]
- Wu, J.; Mooter, G.V.d. The influence of hydrogen bonding between different crystallization tendency drugs and PVPVA on the stability of amorphous solid dispersions. Int. J. Pharm. 2023, 646, 123440. [Google Scholar] [CrossRef]
- Kapourani, A.; Eleftheriadou, K.; Kontogiannopoulos, K.N.; Barmpalexis, P. Evaluation of rivaroxaban amorphous solid dispersions physical stability via molecular mobility studies and molecular simulations. Eur. J. Pharm. Sci. 2021, 157, 105642. [Google Scholar] [CrossRef] [PubMed]
- Wolbert, F.; Fahrig, I.-K.; Gottschalk, T.; Luebbert, C.; Thommes, M.; Sadowski, G. Factors Influencing the Crystallization-Onset Time of Metastable ASDs. Pharmaceutics 2022, 14, 269. [Google Scholar] [CrossRef]
- Luo, C.; Li, R.; Tang, M.; Gao, Y.; Zhang, J.; Qian, S.; Wei, Y.; Shen, P. Amorphous solid dispersion to facilitate the delivery of poorly water-soluble drugs: Recent advances on novel preparation processes and technology coupling. Expert Opin. Drug Deliv. 2024, 21, 1807–1822. [Google Scholar] [CrossRef]
- Bhujbal, S.V.; Mitra, B.; Jain, U.; Gong, Y.; Agrawal, A.; Karki, S.; Taylor, L.S.; Kumar, S.; Zhou, Q. Pharmaceutical amorphous solid dispersion: A review of manufacturing strategies. Acta Pharm. Sin. B 2021, 11, 2505–2536. [Google Scholar] [CrossRef]
- Davis, M.T.; Potter, C.B.; Walker, G.M. Downstream processing of a ternary amorphous solid dispersion: The impacts of spray drying and hot melt extrusion on powder flow, compression and dissolution. Int. J. Pharm. 2018, 544, 242–253. [Google Scholar] [CrossRef]
- Sakai, T.; Hirai, D.; Kimura, S.-i.; Iwao, Y.; Itai, S. Effects of tablet formulation and subsequent film coating on the supersaturated dissolution behavior of amorphous solid dispersions. Int. J. Pharm. 2018, 540, 171–177. [Google Scholar] [CrossRef]
- DiNunzio, J.C.; Schilling, S.U.; Coney, A.W.; Hughey, J.R.; Kaneko, N.; McGinity, J.W. Use of highly compressible Ceolus™ microcrystalline cellulose for improved dosage form properties containing a hydrophilic solid dispersion. Drug Dev. Ind. Pharm. 2012, 38, 180–189. [Google Scholar] [CrossRef]
- Leane, M.; Sinclair, W.; Qian, F.; Haddadin, R.; Brown, A.; Tobyn, M.; Dennis, A. Formulation and process design for a solid dosage form containing a spray-dried amorphous dispersion of ibipinabant. Pharm. Dev. Technol. 2012, 18, 359–366. [Google Scholar] [CrossRef]
- Yu, D.; Hoag, S.W. The impact of diluents on the compaction, dissolution, and physical stability of amorphous solid dispersion tablets. Int. J. Pharm. 2024, 654, 123924. [Google Scholar] [CrossRef] [PubMed]
- Démuth, B.; Farkas, A.; Balogh, A.; Bartosiewicz, K.; Kállai-Szabó, B.; Bertels, J.; Vigh, T.; Mensch, J.; Verreck, G.; Assche, I.; et al. Lubricant-Induced Crystallization of Itraconazole From Tablets Made of Electrospun Amorphous Solid Dispersion. J. Pharm. Sci. 2016, 105, 2982–2988. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Dantuluri, A.; Puri, V. Barrier Coated Drug Layered Particles for Formulation Design of an Amorphous Solid Dispersion. In Proceedings of the 1st Electronic Conference on Pharmaceutical Sciences, Basel, Switzerland, 28 February 2011; p. 518. [Google Scholar]
- Kokott, M.; Klinken, S.; Breitkreutz, J.; Wiedey, R. Downstream processing of amorphous solid dispersions into orodispersible tablets. Int. J. Pharm. 2023, 631, 122493. [Google Scholar] [CrossRef]
- Zhang, W.; Sluga, K.; Yost, E.; Phan, J.; Nagapudi, K.; Hou, H. Impact of Drug Loading on the Compaction Properties of Itraconazole-PVPVA Amorphous Solid Dispersions. Int. J. Pharm. 2022, 629, 122366. [Google Scholar] [CrossRef]
- Zhang, W.; Noland, R.; Chin, S.; Petkovic, M.; Zuniga, R.; Santarra, B.; Conklin, B.; Hou, H.; Nagapudi, K.; Gruenhagen, J.; et al. Impact of polymer type, ASD loading and polymer-drug ratio on ASD tablet disintegration and drug release. Int. J. Pharm. 2020, 592, 120087. [Google Scholar] [CrossRef]
- Patel, S.; Kou, X.; Hou, H.; Huang, Y.; Strong, J.; Zhang, G.; Sun, C. Mechanical Properties and Tableting Behavior of Amorphous Solid Dispersions. J. Pharm. Sci. 2016, 106, 217–223. [Google Scholar] [CrossRef]
- Dohrn, S.; Kyeremateng, S.O.; Bochmann, E.; Sobich, E.; Wahl, A.; Liepold, B.; Sadowski, G.; Degenhardt, M. Thermodynamic Modeling of the Amorphous Solid Dispersion-Water Interfacial Layer and Its Impact on the Release Mechanism. Pharmaceutics 2023, 15, 1539. [Google Scholar] [CrossRef]
- Bērziņš, K.; Fraser-Miller, S.J.; Walker, G.F.; Rades, T.; Gordon, K.C. Investigation on Formulation Strategies to Mitigate Compression-Induced Destabilization in Supersaturated Celecoxib Amorphous Solid Dispersions. Mol. Pharm. 2021, 18, 3882–3893. [Google Scholar] [CrossRef]
- Bērziņš, K.; Suryanarayanan, R. Compression-Induced Crystallization in Sucrose-Polyvinylpyrrolidone Amorphous Solid Dispersions. Cryst. Growth Des. 2017, 18, 839–848. [Google Scholar] [CrossRef]
- Bērziņš, K.; Fraser-Miller, S.J.; Di, R.; Liu, J.; Peltonen, L.; Strachan, C.J.; Rades, T.; Gordon, K.C. Combined Effect of the Preparation Method and Compression on the Physical Stability and Dissolution Behavior of Melt-Quenched Amorphous Celecoxib. Mol. Pharm. 2021, 18, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.A.; Van Eerdenbrugh, B.; Taylor, L.S. A Classification System to Assess the Crystallization Tendency of Organic Molecules from Undercooled Melts. J. Pharm. Sci. 2010, 99, 3787–3806. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.D.; Wen, H.; Taylor, L.S. Non-Sink Dissolution Conditions for Predicting Product Quality and In Vivo Performance of Supersaturating Drug Delivery Systems. J. Pharm. Sci. 2016, 105, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Kapourani, A.; Vardaka, E.; Katopodis, K.; Kachrimanis, K.; Barmpalexis, P. Crystallization tendency of APIs possessing different thermal and glass related properties in amorphous solid dispersions. Int. J. Pharm. 2020, 579, 119149. [Google Scholar] [CrossRef]
- Ditzinger, F.; Price, D.J.; Nair, A.; Becker-Baldus, J.; Glaubitz, C.; Dressman, J.B.; Saal, C.; Kuentz, M. Opportunities for Successful Stabilization of Poor Glass-Forming Drugs: A Stability-Based Comparison of Mesoporous Silica Versus Hot Melt Extrusion Technologies. Pharmaceutics 2019, 11, 577. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, M.; Luo, M.; Cai, T. Advances in the development of amorphous solid dispersions: The role of polymeric carriers. Asian J. Pharm. Sci. 2023, 18, 100834. [Google Scholar] [CrossRef] [PubMed]
- Lehmkemper, K.; Kyeremateng, S.O.; Heinzerling, O.; Degenhardt, M.; Sadowski, G. Impact of Polymer Type and Relative Humidity on the Long-Term Physical Stability of Amorphous Solid Dispersions. Mol. Pharm. 2017, 14, 4374–4386. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Jacobs, E.; Jones, D.S.; McCoy, C.P.; Wu, H.; Andrews, G.P. The design and development of high drug loading amorphous solid dispersion for hot-melt extrusion platform. Int. J. Pharm. 2020, 586, 119545. [Google Scholar] [CrossRef] [PubMed]
- Bērziņš, K.; Fraser-Miller, S.J.; Rades, T.; Gordon, K.C. Low-Frequency Raman Spectroscopic Study on Compression-Induced Destabilization in Melt-Quenched Amorphous Celecoxib. Mol. Pharm. 2019, 16, 3678–3686. [Google Scholar] [CrossRef]
- Thakral, N.K.; Thakral, S.; Stephenson, G.A.; Sedlock, R.; Suryanarayanan, R. Compression-Induced Polymorphic Transformation in Tablets: Role of Shear Stress and Development of Mitigation Strategies. J. Pharm. Sci. 2019, 108, 476–484. [Google Scholar] [CrossRef]
- Punia, A.; Biyyala, V.; Faassen, F.; Ash, J.; Lamm, M.S. Detrimental Effect of the Film Coat Chemistry and Thickness on the Physical Stability of Amorphous Solid Dispersions in Tablet Formulations. J. Pharm. Sci. 2023, 112, 708–717. [Google Scholar] [CrossRef]
- Yohannes, B.; Abebe, A. Determination of tensile strength of shaped tablets. Powder Technol. 2021, 383, 11–18. [Google Scholar] [CrossRef]
- Fell, J.T.; Newton, J.M. Determination of Tablet Strength by the Diametral-Compression Test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef]
- Keramatnia, F.; Shayanfar, A.; Jouyban, A. Thermodynamic Solubility Profile of Carbamazepine–Cinnamic Acid Cocrystal at Different pH. J. Pharm. Sci. 2015, 104, 2559–2565. [Google Scholar] [CrossRef] [PubMed]
- Alhalaweh, A.; Roy, L.; Rodríguez-Hornedo, N.; Velaga, S.P. pH-Dependent Solubility of Indomethacin–Saccharin and Carbamazepine–Saccharin Cocrystals in Aqueous Media. Mol. Pharm. 2012, 9, 2605–2612. [Google Scholar] [CrossRef]
- Pantazos, I.; Kapourani, A.; Chortis, A.; Cholevas, C.; Verykokou, S.; Ioannidis, C.; Katakalos, K.; Barmpalexis, P. Personalized drug-loaded 3D-printed scaffolds for periodontal bone repair: Structural, mechanical, and controlled release properties. J. Pharm. Sci. 2025, 114, 103807. [Google Scholar] [CrossRef]
- Qi, Z.-L.; Zhang, D.-F.; Chen, F.-X.; Miao, J.-Y.; Ren, B.-Z. Thermal decomposition and non-isothermal decomposition kinetics of carbamazepine. Russ. J. Phys. Chem. A 2014, 88, 2308–2313. [Google Scholar] [CrossRef]
- de Alvarenga, B.R., Jr.; Moseson, D.E.; Carneiro, R.L.; Taylor, L.S. Impact of Polymer Type on Thermal Degradation of Amorphous Solid Dispersions Containing Ritonavir. Mol. Pharm. 2022, 19, 332–344. [Google Scholar] [CrossRef]
- Newman, A.; Zografi, G. Considerations in the Development of Physically Stable High Drug Load API- Polymer Amorphous Solid Dispersions in the Glassy State. J. Pharm. Sci. 2023, 112, 8–18. [Google Scholar] [CrossRef]
- Chen, Y.; Pui, Y.; Chen, H.; Wang, S.; Serno, P.; Tonnis, W.; Chen, L.; Qian, F. Polymer-Mediated Drug Supersaturation Controlled by Drug–Polymer Interactions Persisting in an Aqueous Environment. Mol. Pharm. 2019, 16, 205–213. [Google Scholar] [CrossRef]
- Li, J.; Fan, N.; Wang, X.; Li, C.; Sun, M.; Wang, J.; Fu, Q.; He, Z. Interfacial interaction track of amorphous solid dispersions established by water-soluble polymer and indometacin. Eur. J. Pharm. Sci. 2017, 106, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, A.L.; Lang, M.; Kim, K.; Matzger, A.J. Comparison of the Four Anhydrous Polymorphs of Carbamazepine and the Crystal Structure of Form I. J. Pharm. Sci. 2003, 92, 2260–2271. [Google Scholar] [CrossRef] [PubMed]
- Benmore, C.; Edwards, A.; Alderman, O.; Cherry, B.; Smith, P.; Smith, D.; Byrn, S.; Weber, J.K.R.; Yarger, J. The Structure of Liquid and Glassy Carbamazepine. Quantum Beam Sci. 2022, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.; Knipp, G.; Zografi, G. Assessing the performance of amorphous solid dispersions. J. Pharm. Sci. 2012, 101, 1355–1377. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Huang, J.; Hussain, M.A. Drug-Polymer Solubility and Miscibility: Stability Consideration and Practical Challenges in Amorphous Solid Dispersion Development. J. Pharm. Sci. 2010, 99, 2941–2947. [Google Scholar] [CrossRef] [PubMed]
- Abedinoghli, D.; Charkhpour, M.; Osouli-Bostanabad, K.; Selselehjonban, S.; Emami, S.; Barzegar-Jalali, M.; Adibkia, K. Electrosprayed Nanosystems of Carbamazepine-PVP K30 for Enhancing Its Pharmacologic Effects. Iran. J. Pharm. Res. IJPR 2018, 17, 1431–1443. [Google Scholar]
- Hsu, C.-C.; Hung, C.-T.; Lin, Y.-H.; Tsai, H.-J.; Hu, P.-C.; Lin, Y.-P.; Chen, J.-C.; Hsu, S.-F.; Hsieh, H.-J. Preparation of Indomethacin Co-Crystals; Comparison of XRD, THz, and FT-IR Spectral Analyses; and Enhancement of Solubility. J. Pharm. BioTech Ind. 2024, 1, 2–17. [Google Scholar] [CrossRef]
- Deng, J.; Staufenbiel, S.; Bodmeier, R. Evaluation of a biphasic in vitro dissolution test for estimating the bioavailability of carbamazepine polymorphic forms. Eur. J. Pharm. Sci. 2017, 105, 64–70. [Google Scholar] [CrossRef]
- Al-khattawi, A.; Alyami, H.; Townsend, B.; Ma, X.; Mohammed, A. Evidence-Based Nanoscopic and Molecular Framework for Excipient Functionality in Compressed Orally Disintegrating Tablets. PLoS ONE 2014, 9, e101369. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Zheng, G.; Jiang, X. Optimization of the removal of reactive golden yellow SNE dye by cross-linked cationic starch and its adsorption properties. J. Eng. Fibers Fabr. 2019, 14, 155892501986526. [Google Scholar] [CrossRef]
- Pivsa-Art, W.; Pivsa-Art, S. Effect of Talc on Mechanical Characteristics and Fracture Toughness of Poly(lactic acid)/Poly(butylene succinate) Blend. J. Polym. Environ. 2019, 27, 1821–1827. [Google Scholar] [CrossRef]
- Wang, T.; Potts, A.R.; Hoag, S.W. Elucidating the Variability of Magnesium Stearate and the Correlations With Its Spectroscopic Features. J. Pharm. Sci. 2019, 108, 1569–1580. [Google Scholar] [CrossRef]
- Flügel, K.; Schmidt, K.; Mareczek, L.; Gäbe, M.; Hennig, R.; Thommes, M. Impact of incorporated drugs on material properties of amorphous solid dispersions. Eur. J. Pharm. Biopharm. 2021, 159, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ritters, L.; Tian, Y.; Reichl, S. Spray-Dried Paracetamol/Polyvinylpyrrolidone Amorphous Solid Dispersions: Part I—Stability of Powders and Tablets. Pharmaceutics 2021, 13, 1938. [Google Scholar] [CrossRef]
- Xu, X.; Vallabh, C.K.P.; Hoag, S.W.; Dave, V.S.; Cetinkaya, C. Early detection of capping risk in pharmaceutical compacts. Int. J. Pharm. 2018, 553, 338–348. [Google Scholar] [CrossRef]
- Sun, C.C.; Kleinebudde, P. Mini review: Mechanisms to the loss of tabletability by dry granulation. Eur. J. Pharm. Biopharm. 2016, 106, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Wang, C.; Sun, C.C. Tabletability Flip–Role of Bonding Area and Bonding Strength Interplay. J. Pharm. Sci. 2020, 109, 3569–3573. [Google Scholar] [CrossRef]
- Ayenew, Z.; Paudel, A.; Van den Mooter, G. Can compression induce demixing in amorphous solid dispersions? A case study of naproxen–PVP K25. Eur. J. Pharm. Biopharm. 2012, 81, 207–213. [Google Scholar] [CrossRef]
- Sauer, A.; Warashina, S.; Mishra, S.M.; Lesser, I.; Kirchhöfer, K. Downstream processing of spray-dried ASD with hypromellose acetate succinate–Roller compaction and subsequent compression into high ASD load tablets. Int. J. Pharm. X 2021, 3, 100099. [Google Scholar] [CrossRef]
- Xi, H.; Ren, J.; Novak, J.M.; Kemp, E.; Johnson, G.; Klinzing, G.; Johnson, M.A.; Xu, W. The Effect of Inorganic Salt on Disintegration of Tablets with High Loading of Amorphous Solid Dispersion Containing Copovidone. Pharm. Res. 2020, 37, 70. [Google Scholar] [CrossRef] [PubMed]
- Kapourani, A.; Chatzitheodoridou, M.; Valkanioti, V.; Manioudaki, A.-E.; Bikiaris, N.D.; Barmpalexis, P. Evaluating the effect of kosmotropic inorganic salts in the in vitro dissolution behavior of tablets containing amorphous indomethacin-polyvinylpyrrolidone solid dispersions. J. Drug Deliv. Sci. Technol. 2022, 72, 103421. [Google Scholar] [CrossRef]
- Mishra, S.M.; Richter, M.; Mejia, L.; Sauer, A. Downstream Processing of Itraconazole:HPMCAS Amorphous Solid Dispersion: From Hot-Melt Extrudate to Tablet Using a Quality by Design Approach. Pharmaceutics 2022, 14, 1429. [Google Scholar] [CrossRef]
- The Metabolomics Innovation Center. Carbamazepine-Toxin and Toxin-Target Database (T3DB). Available online: https://www.t3db.ca/toxins/T3D2826 (accessed on 25 June 2025).
- Almeida e Sousa, L.; Reutzel-Edens, S.M.; Stephenson, G.A.; Taylor, L.S. Assessment of the Amorphous “Solubility” of a Group of Diverse Drugs Using New Experimental and Theoretical Approaches. Mol. Pharm. 2015, 12, 484–495. [Google Scholar] [CrossRef]
- Alonzo, D.E.; Gao, Y.; Zhou, D.; Mo, H.; Zhang, G.G.Z.; Taylor, L.S. Dissolution and precipitation behavior of amorphous solid dispersions. J. Pharm. Sci. 2011, 100, 3316–3331. [Google Scholar] [CrossRef]
- Sun, D.D.; Lee, P.I. Haste Makes Waste: The Interplay Between Dissolution and Precipitation of Supersaturating Formulations. AAPS J. 2015, 17, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Zhang, X.; Xu, D.; Zhang, H.; Baak, J.P.; Luo, L.; Xia, Y.; Wang, J.; Ke, X.; Sun, P. Evaluating supersaturation in vitro and predicting its performance in vivo with Biphasic gastrointestinal Simulator: A case study of a BCS IIB drug. Int. J. Pharm. 2020, 578, 119043. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, J.; Brewster, M.E.; Augustijns, P. Supersaturating Drug Delivery Systems: The Answer to Solubility-Limited Oral Bioavailability? J. Pharm. Sci. 2009, 98, 2549–2572. [Google Scholar] [CrossRef] [PubMed]
- Schver, G.C.R.M.; Lee, P.I. On the usefulness of sink index in characterizing the degree of nonsinkness in dissolution studies. Int. J. Pharm. 2021, 605, 120845. [Google Scholar] [CrossRef]
- Borrmann, D.; Friedrich, P.; Smuda, J.; Sadowski, G. Counteracting the loss of release for indomethacin-copovidone ASDs. J. Pharm. Sci. 2025, 114, 449–457. [Google Scholar] [CrossRef]
- Saboo, S.; Moseson, D.E.; Kestur, U.S.; Taylor, L.S. Patterns of drug release as a function of drug loading from amorphous solid dispersions: A comparison of five different polymers. Eur. J. Pharm. Sci. 2020, 155, 105514. [Google Scholar] [CrossRef]
- Löffler, J.F. Metallic Glasses, Bulk. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2003; pp. 1–12. [Google Scholar]
- Blaabjerg, L.I.; Lindenberg, E.; Löbmann, K.; Grohganz, H.; Rades, T. Is there a correlation between the glass forming ability of a drug and its supersaturation propensity? Int. J. Pharm. 2018, 538, 243–249. [Google Scholar] [CrossRef]




| Formulation Type I | Formulation Type II | |
|---|---|---|
| Component | % w/w | % w/w |
| ASD * | 20 | 50 |
| MCC 101 | 74 | 44 |
| SCC | 4 | 4 |
| MgSt | 1 | 1 |
| Talc | 1 | 1 |
| Sample ID | AUC(0→t) (Mean ± SD) [μg/(mL·min) × 102] | AUC(0→t) Ratio (Mean) |
|---|---|---|
| IND–PVP 10–90, 20% ASD, 50 MPa, 5 s | 65.17 ± 0.31 | 3.34 |
| IND–PVP 10–90, 20% ASD, 50 MPa, 60 s | 64.37 ± 0.20 | 3.30 |
| IND–PVP 10–90, 20% ASD, 150 MPa, 30 s | 64.04 ± 0.20 | 3.28 |
| IND–PVP 10–90, 20% ASD, 250 MPa, 5 s | 62.92 ± 0.21 | 3.22 |
| IND–PVP 10–90, 20% ASD, 250 MPa, 60 s | 62.39 ± 0.24 | 3.20 |
| IND–PVP 20–80, 20% ASD, 50 MPa, 5 s | 61.77 ± 0.29 | 3.17 |
| IND–PVP 20–80, 20% ASD, 50 MPa, 60 s | 60.93 ± 0.08 | 3.12 |
| IND–PVP 20–80, 20% ASD, 150 MPa, 30 s | 60.07 ± 0.18 | 3.08 |
| IND–PVP 20–80, 20% ASD, 250 MPa, 5 s | 57.60 ± 0.30 | 2.95 |
| IND–PVP 20–80, 20% ASD, 250 MPa, 60 s | 56.61 ± 0.20 | 2.90 |
| IND–PVP 40–60, 20% ASD, 50 MPa, 5 s | 48.46 ± 0.22 | 2.48 |
| IND–PVP 40–60, 20% ASD, 50 MPa, 60 s | 46.61 ± 0.38 | 2.39 |
| IND–PVP 40–60, 20% ASD, 150 MPa, 30 s | 45.35 ± 0.26 | 2.33 |
| IND–PVP 40–60, 20% ASD, 250 MPa, 5 s | 43.65 ± 0.22 | 2.24 |
| IND–PVP 40–60, 20% ASD, 250 MPa, 60 s | 42.78 ± 0.20 | 2.19 |
| IND–PVP 20–80, 50% ASD, 50 MPa, 5 s | 52.69 ± 0.32 | 2.70 |
| IND–PVP 20–80, 50% ASD, 50 MPa, 60 s | 50.72 ± 0.31 | 2.60 |
| IND–PVP 20–80, 50% ASD, 150 MPa, 30 s | 48.66 ± 0.27 | 2.49 |
| IND–PVP 20–80, 50% ASD, 250 MPa, 5 s | 45.54 ± 0.24 | 2.33 |
| IND–PVP 20–80, 50% ASD, 250 MPa, 60 s | 44.83 ± 0.20 | 2.30 |
| IND crystalline | 19.51 ± 0.05 | 1.00 |
| Sample ID | AUC(0→t) (Mean ± SD) [μg/(mL·min) × 102] | AUC(0→t) Ratio (Mean) |
|---|---|---|
| CBZ–PVP 10–90, 20% ASD, 50 MPa, 5 s | 1361 ± 1.72 | 1.35 |
| CBZ–PVP 10–90, 20% ASD, 50 MPa, 60 s | 1355 ± 2.09 | 1.34 |
| CBZ–PVP 10–90, 20% ASD, 150 MPa, 30 s | 1342 ± 1.74 | 1.33 |
| CBZ–PVP 10–90, 20% ASD, 250 MPa, 5 s | 1315 ± 0.95 | 1.31 |
| CBZ–PVP 10–90, 20% ASD, 250 MPa, 60 s | 1305 ± 3.04 | 1.30 |
| CBZ–PVP 20–80, 20% ASD, 50 MPa, 5 s | 1355 ± 1.25 | 1.34 |
| CBZ–PVP 20–80, 20% ASD, 50 MPa, 60 s | 1351 ± 1.03 | 1.34 |
| CBZ–PVP 20–80, 20% ASD, 150 MPa, 30 s | 1321 ± 1.91 | 1.32 |
| CBZ–PVP 20–80, 20% ASD, 250 MPa, 5 s | 1306 ± 2.46 | 1.30 |
| CBZ–PVP 20–80, 20% ASD, 250 MPa, 60 s | 1300 ± 1.50 | 1.29 |
| CBZ–PVP 40–60, 20% ASD, 50 MPa, 5 s | 1305 ± 3.10 | 1.30 |
| CBZ–PVP 40–60, 20% ASD, 50 MPa, 60 s | 1296 ± 0.71 | 1.29 |
| CBZ–PVP 40–60, 20% ASD, 150 MPa, 30 s | 1267 ± 1.47 | 1.26 |
| CBZ–PVP 40–60, 20% ASD, 250 MPa, 5 s | 1231 ± 1.23 | 1.22 |
| CBZ–PVP 40–60, 20% ASD, 250 MPa, 60 s | 1211 ± 4.26 | 1.20 |
| CBZ–PVP 20–80, 50% ASD, 50 MPa, 5 s | 1282 ± 0.80 | 1.27 |
| CBZ–PVP 20–80, 50% ASD, 50 MPa, 60 s | 1270 ± 1.55 | 1.26 |
| CBZ–PVP 20–80, 50% ASD, 150 MPa, 30 s | 1209 ± 2.48 | 1.20 |
| CBZ–PVP 20–80, 50% ASD, 250 MPa, 5 s | 1179 ± 4.23 | 1.17 |
| CBZ–PVP 20–80, 50% ASD, 250 MPa, 60 s | 1161 ± 1.08 | 1.15 |
| CBZ crystalline | 1008 ± 1.0 | 1.00 |
| Sample ID | AUC(0→t) (Mean ± SD) [μg/(mL·min) × 102] 3 Μ | AUC(0→t) Ratio (Mean) |
|---|---|---|
| IND–PVP 10–90, 20% ASD, 50 MPa, 5 s | 64.05 ± 0.21 | 3.28 |
| IND–PVP 10–90, 20% ASD, 50 MPa, 60 s | 63.44 ± 0.23 | 3.25 |
| IND–PVP 10–90, 20% ASD, 150 MPa, 30 s | 62.00 ± 0.23 | 3.18 |
| IND–PVP 10–90, 20% ASD, 250 MPa, 5 s | 59.72 ± 0.19 | 3.07 |
| IND–PVP 10–90, 20% ASD, 250 MPa, 60 s | 57.26 ± 0.29 | 2.93 |
| IND–PVP 20–80, 20% ASD, 50 MPa, 5 s | 57.30 ± 0.22 | 2.94 |
| IND–PVP 20–80, 20% ASD, 50 MPa, 60 s | 56.63 ± 0.39 | 2.90 |
| IND–PVP 20–80, 20% ASD, 150 MPa, 30 s | 54.00 ± 0.18 | 2.77 |
| IND–PVP 20–80, 20% ASD, 250 MPa, 5 s | 51.09 ± 0.15 | 2.62 |
| IND–PVP 20–80, 20% ASD, 250 MPa, 60 s | 49.92 ± 0.10 | 2.56 |
| IND–PVP 40–60, 20% ASD, 50 MPa, 5 s | 43.99 ± 0.31 | 2.26 |
| IND–PVP 40–60, 20% ASD, 50 MPa, 60 s | 42.55 ± 0.15 | 2.18 |
| IND–PVP 40–60, 20% ASD, 150 MPa, 30 s | 40.31 ± 0.09 | 2.07 |
| IND–PVP 40–60, 20% ASD, 250 MPa, 5 s | 38.21 ± 0.17 | 1.96 |
| IND–PVP 40–60, 20% ASD, 250 MPa, 60 s | 37.28 ± 0.15 | 1.91 |
| IND–PVP 20–80, 50% ASD, 50 MPa, 5 s | 47.06 ± 0.16 | 2.41 |
| IND–PVP 20–80, 50% ASD, 50 MPa, 60 s | 45.36 ± 0.21 | 2.33 |
| IND–PVP 20–80, 50% ASD, 150 MPa, 30 s | 42.36 ± 0.02 | 2.17 |
| IND–PVP 20–80, 50% ASD, 250 MPa, 5 s | 38.89 ± 0.09 | 1.99 |
| IND–PVP 20–80, 50% ASD, 250 MPa, 60 s | 38.01 ± 0.20 | 1.95 |
| IND crystalline | 19.51 ± 0.05 | 1.00 |
| Sample ID | AUC(0→t) (Mean ± SD) [μg/(mL·min) × 102] 3 M | AUC(0→t) Ratio (Mean) |
|---|---|---|
| CBZ–PVP 10–90, 20% ASD, 50 MPa, 5 s | 1314 ± 3.63 | 1.30 |
| CBZ–PVP 10–90, 20% ASD, 50 MPa, 60 s | 1308 ± 1.93 | 1.30 |
| CBZ–PVP 10–90, 20% ASD, 150 MPa, 30 s | 1297 ± 2.97 | 1.29 |
| CBZ–PVP 10–90, 20% ASD, 250 MPa, 5 s | 1242 ± 5.68 | 1.23 |
| CBZ–PVP 10–90, 20% ASD, 250 MPa, 60 s | 1202 ± 3.30 | 1.19 |
| CBZ–PVP 20–80, 20% ASD, 50 MPa, 5 s | 1290 ± 3.12 | 1.28 |
| CBZ–PVP 20–80, 20% ASD, 50 MPa, 60 s | 1285 ± 2.52 | 1.28 |
| CBZ–PVP 20–80, 20% ASD, 150 MPa, 30 s | 1256 ± 4.94 | 1.25 |
| CBZ–PVP 20–80, 20% ASD, 250 MPa, 5 s | 1206 ± 2.00 | 1.20 |
| CBZ–PVP 20–80, 20% ASD, 250 MPa, 60 s | 1173 ± 2.74 | 1.16 |
| CBZ–PVP 40–60, 20% ASD, 50 MPa, 5 s | 1239 ± 2.91 | 1.22 |
| CBZ–PVP 40–60, 20% ASD, 50 MPa, 60 s | 1229 ± 4.76 | 1.22 |
| CBZ–PVP 40–60, 20% ASD, 150 MPa, 30 s | 1202 ± 0.88 | 1.19 |
| CBZ–PVP 40–60, 20% ASD, 250 MPa, 5 s | 1138 ± 1.52 | 1.13 |
| CBZ–PVP 40–60, 20% ASD, 250 MPa, 60 s | 1095 ± 3.09 | 1.09 |
| CBZ–PVP 20–80, 50% ASD, 50 MPa, 5 s | 1204 ± 2.00 | 1.19 |
| CBZ–PVP 20–80, 50% ASD, 50 MPa, 60 s | 1193 ± 3.25 | 1.18 |
| CBZ–PVP 20–80, 50% ASD, 150 MPa, 30 s | 1128 ± 3.03 | 1.12 |
| CBZ–PVP 20–80, 50% ASD, 250 MPa, 5 s | 1071 ± 3.65 | 1.06 |
| CBZ–PVP 20–80, 50% ASD, 250 MPa, 60 s | 1038 ± 1.70 | 1.03 |
| CBZ crystalline | 1008 ± 1.0 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantazos, I.; Poimenidou, M.; Kouskouridas, D.; Tzaferas, E.; Karava, V.; Cholevas, C.; Kapourani, A.; Barmpalexis, P. Influence of Drug Properties, Formulation Composition, and Processing Parameters on the Stability and Dissolution Performance of Amorphous Solid Dispersions-Based Tablets. Polymers 2025, 17, 2484. https://doi.org/10.3390/polym17182484
Pantazos I, Poimenidou M, Kouskouridas D, Tzaferas E, Karava V, Cholevas C, Kapourani A, Barmpalexis P. Influence of Drug Properties, Formulation Composition, and Processing Parameters on the Stability and Dissolution Performance of Amorphous Solid Dispersions-Based Tablets. Polymers. 2025; 17(18):2484. https://doi.org/10.3390/polym17182484
Chicago/Turabian StylePantazos, Ioannis, Maria Poimenidou, Dimitrios Kouskouridas, Evangelos Tzaferas, Vasiliki Karava, Christos Cholevas, Afroditi Kapourani, and Panagiotis Barmpalexis. 2025. "Influence of Drug Properties, Formulation Composition, and Processing Parameters on the Stability and Dissolution Performance of Amorphous Solid Dispersions-Based Tablets" Polymers 17, no. 18: 2484. https://doi.org/10.3390/polym17182484
APA StylePantazos, I., Poimenidou, M., Kouskouridas, D., Tzaferas, E., Karava, V., Cholevas, C., Kapourani, A., & Barmpalexis, P. (2025). Influence of Drug Properties, Formulation Composition, and Processing Parameters on the Stability and Dissolution Performance of Amorphous Solid Dispersions-Based Tablets. Polymers, 17(18), 2484. https://doi.org/10.3390/polym17182484








