A Novel Cyclized Polyacrylonitrile Binder Strategy for Efficient Oxygen Evolution Reaction Catalysts
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of NiFe Alloy Catalyst
2.3. Preparation of the Electrodes
2.4. Material Characterization
2.5. Electrochemical Measurements
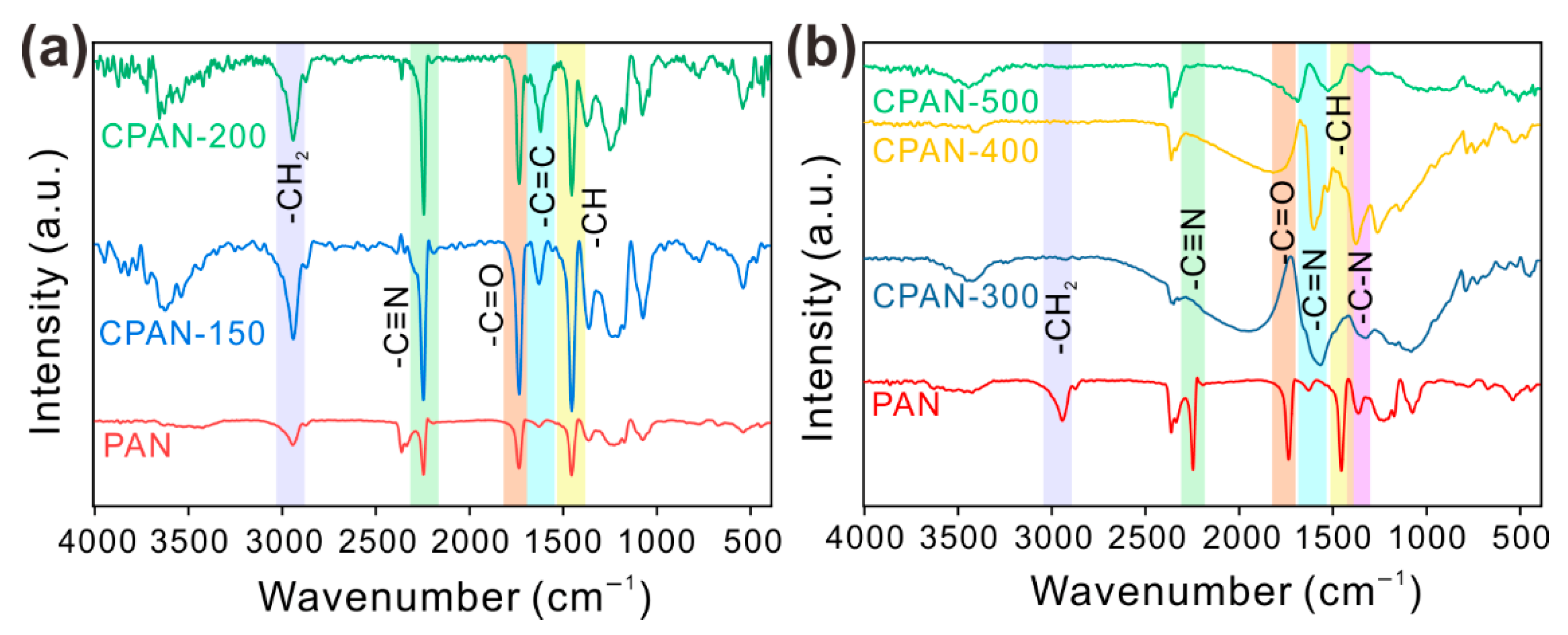
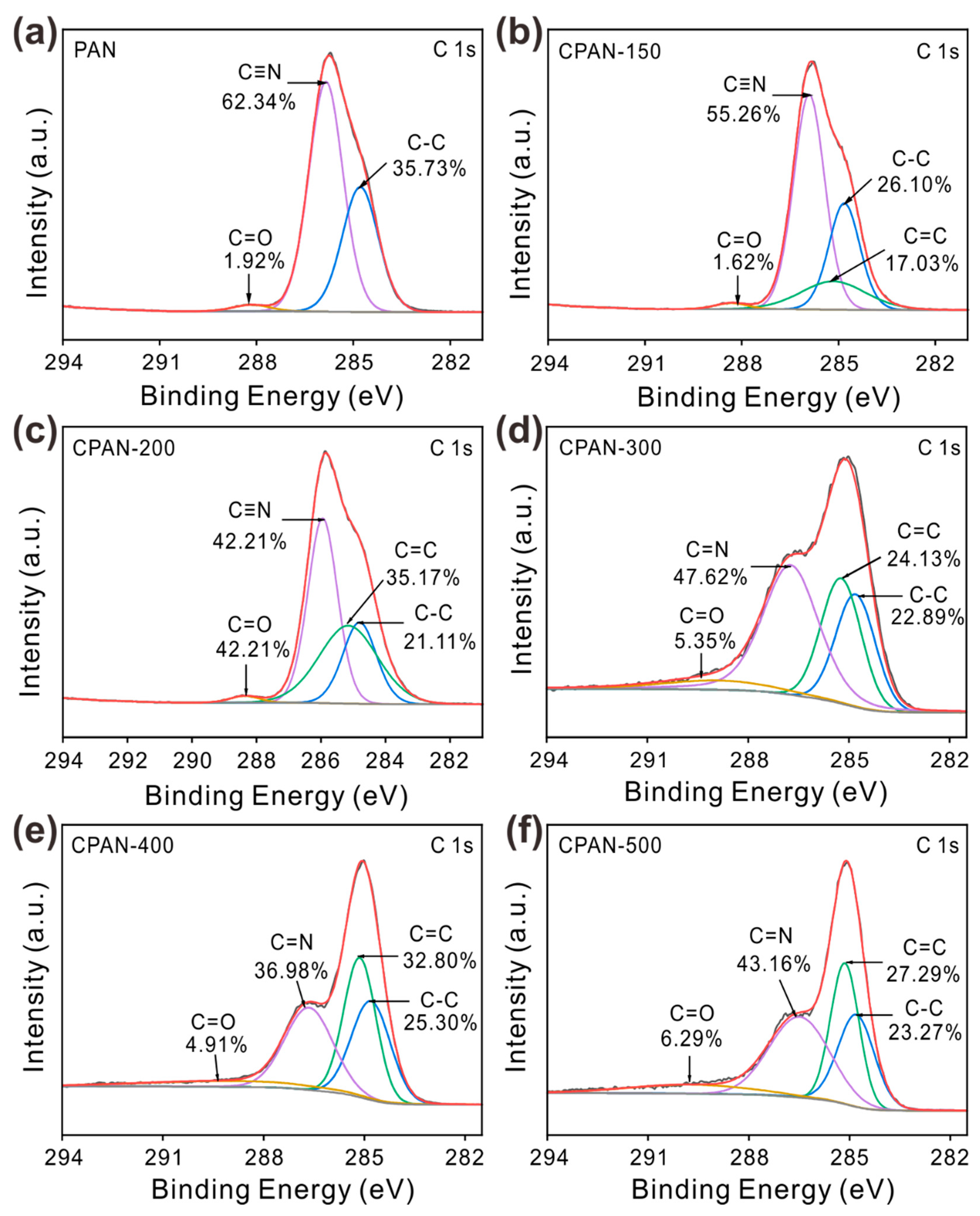
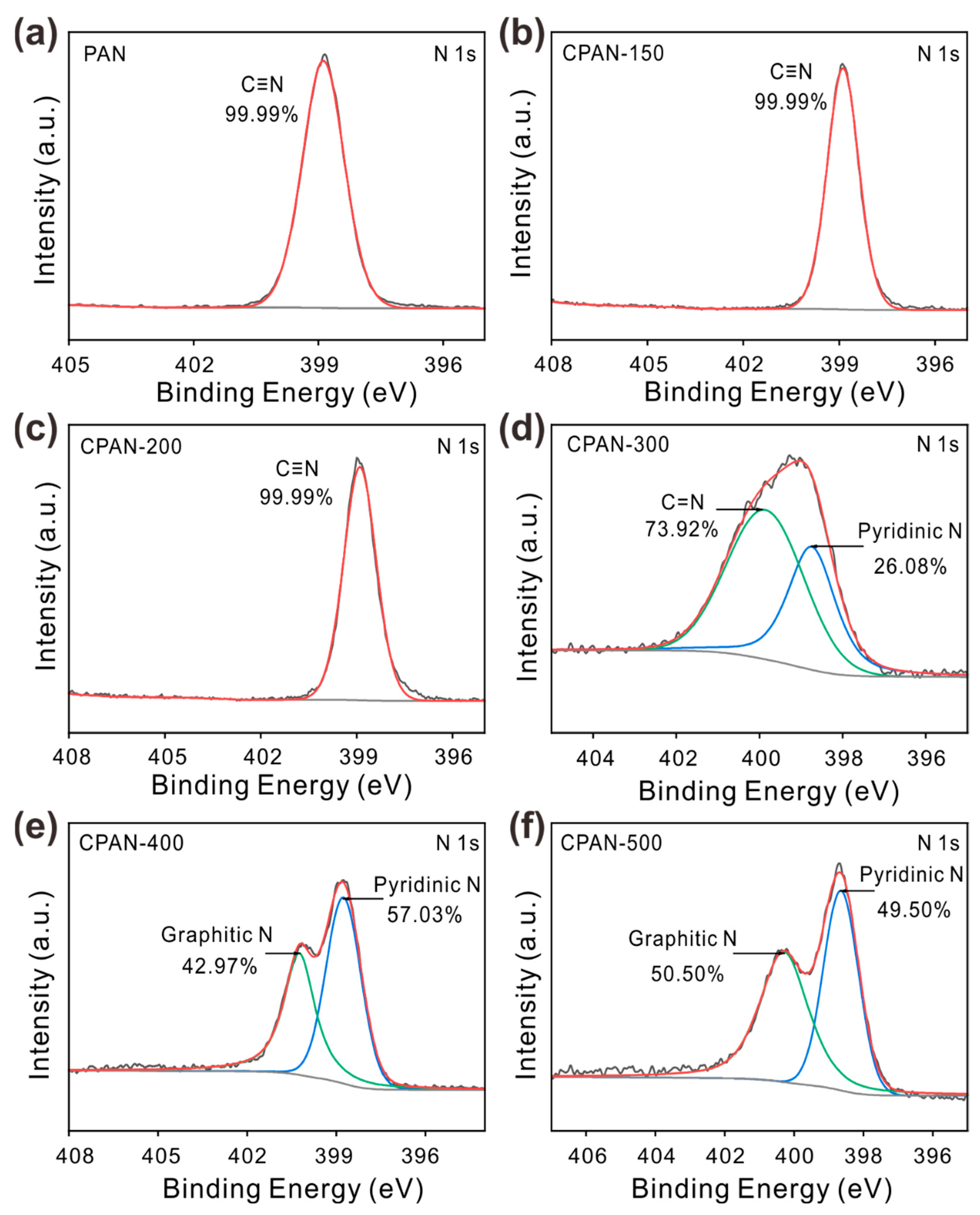
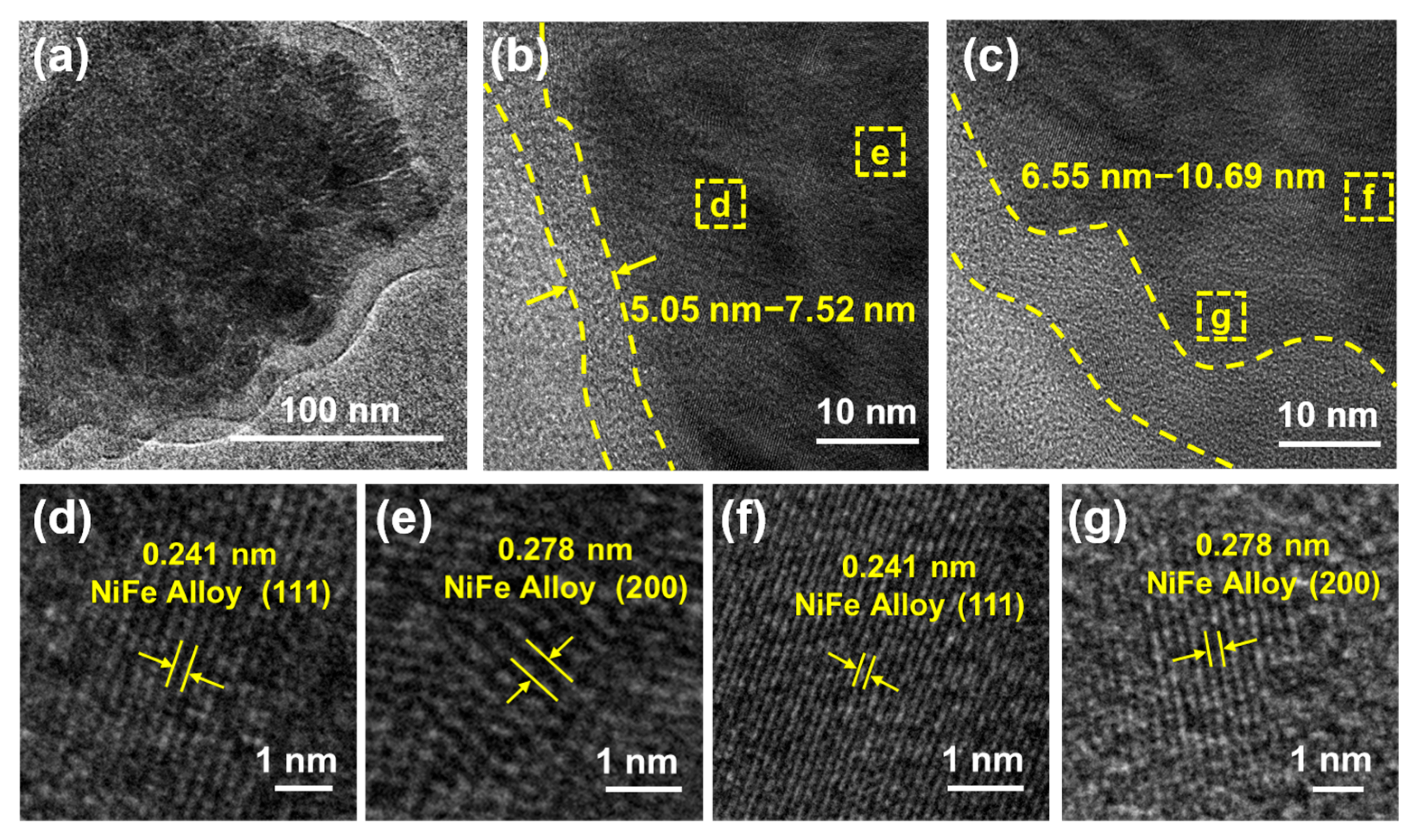
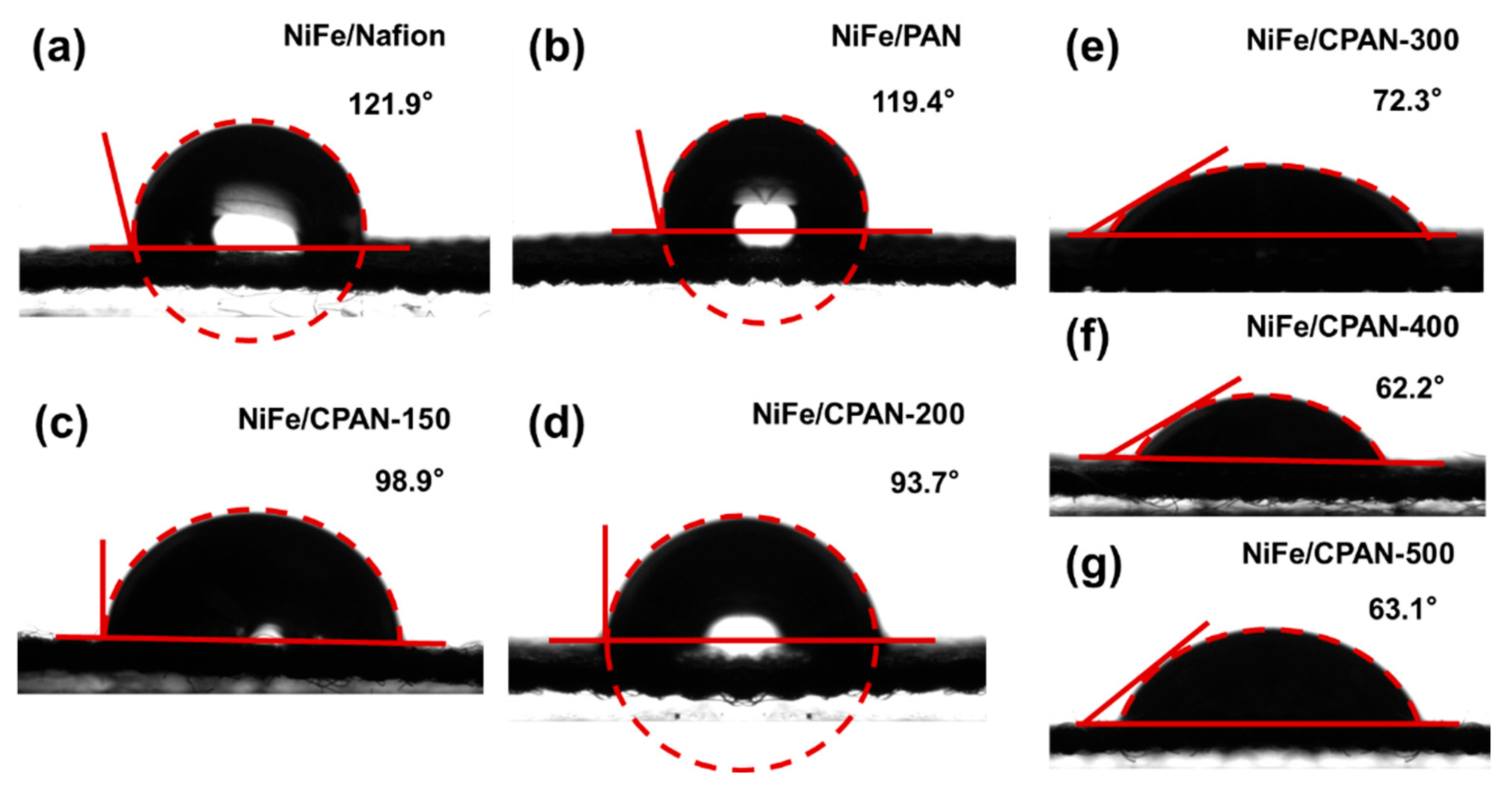
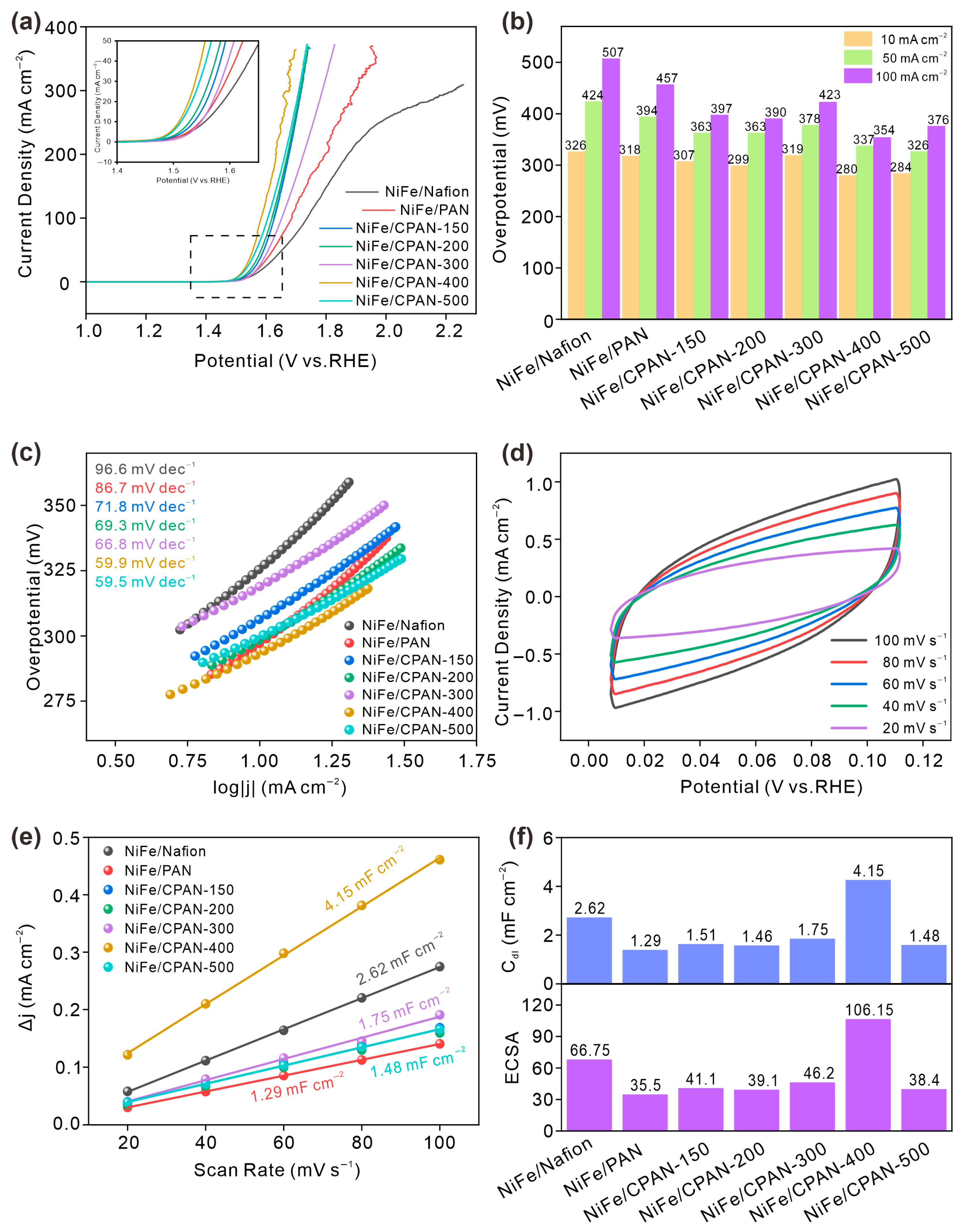
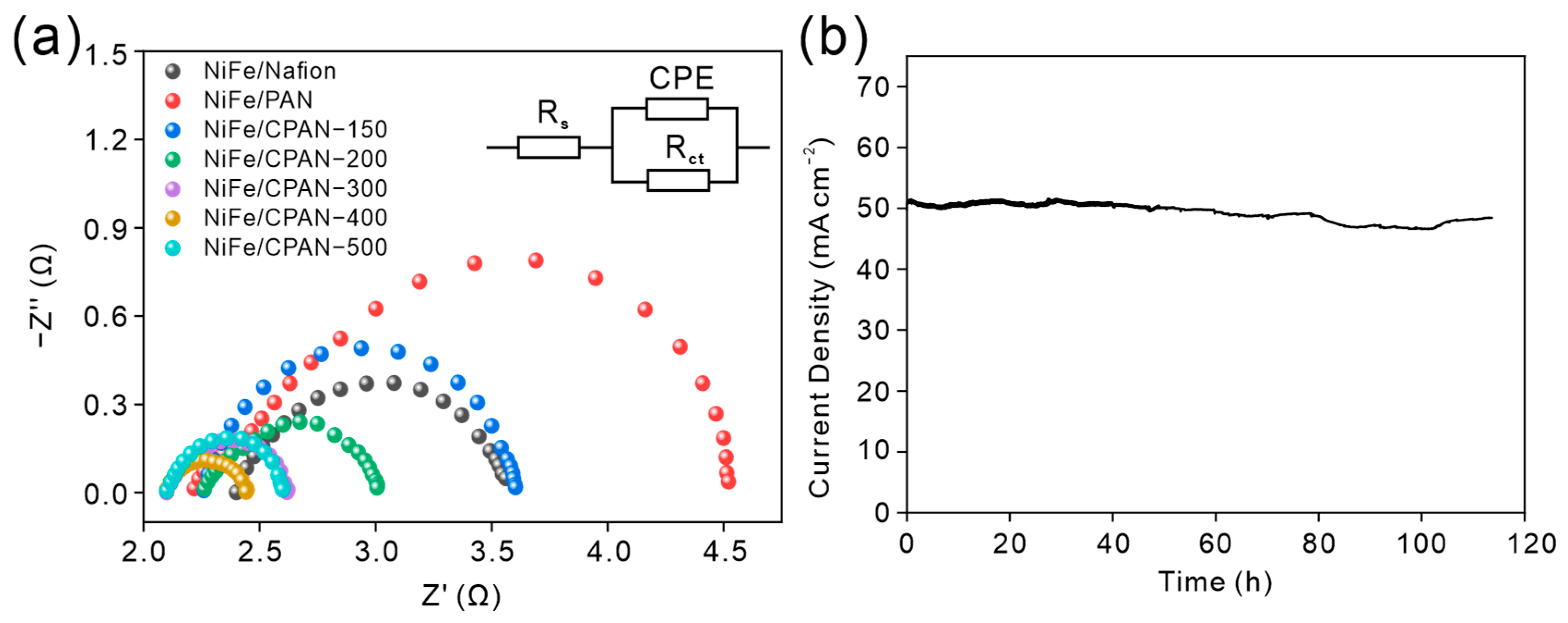
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Wang, M.; Yang, Y.; Kong, D.; Meng, C.; Zhang, D.; Hu, H.; Wu, M. Potential Technology for Seawater Electrolysis: Anion-Exchange Membrane Water Electrolysis. Chem. Catal. 2023, 3, 100643. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Zhang, J.; Wang, J.; Hu, Y.; Jiang, H.; Li, C. Anion Exchange Membrane Water Electrolyzer: Electrode Design, Lab-Scaled Testing System and Performance Evaluation. EnergyChem 2022, 4, 100087. [Google Scholar] [CrossRef]
- Faid, A.Y.; Xie, L.; Barnett, A.O.; Seland, F.; Kirk, D.; Sunde, S. Effect of Anion Exchange Ionomer Content on Electrode Performance in AEM Water Electrolysis. Int. J. Hydrogen Energy 2020, 45, 28272–28284. [Google Scholar] [CrossRef]
- Zhao, J.; Zou, X.; Pan, J.; Wang, B.; Jin, Z.; Xu, G.; He, X.; Sun, Z.; Yan, F. Efficient Transport of Active Species in Triple-Phase Boundary through “Paddle-Effect” of Ionomer for Alkaline Fuel Cells. Chem. Eng. J. 2023, 452, 139498. [Google Scholar] [CrossRef]
- Yang, H.; Driess, M.; Menezes, P.W. Self-Supported Electrocatalysts for Practical Water Electrolysis. Adv. Energy Mater. 2021, 11, 2102074. [Google Scholar] [CrossRef]
- Fang, S.; Liu, G.; Li, M.; Zhang, H.; Yu, J.; Zhang, F.; Pan, M.; Tang, H. Tailoring Ionomer Chemistry for Improved Oxygen Transport in the Cathode Catalyst Layer of Proton Exchange Membrane Fuel Cells. ACS Appl. Energy Mater. 2023, 6, 3590–3598. [Google Scholar] [CrossRef]
- Mardle, P.; Chen, B.; Holdcroft, S. Opportunities of Ionomer Development for Anion-Exchange Membrane Water Electrolysis: Focus Review. ACS Energy Lett. 2023, 8, 3330–3342. [Google Scholar] [CrossRef]
- Lin, N.; Zausch, J. 1D Multiphysics Modelling of PEM Water Electrolysis Anodes with Porous Transport Layers and the Membrane. Chem. Eng. Sci. 2022, 253, 117600. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, Z.; Tian, Y.; Yu, C.; Liu, K.; Jiang, L. Engineering Electrode Wettability to Enhance Mass Transfer in Hydrogen Evolution Reaction. Nano Res. Energy 2023, 2, e9120063. [Google Scholar] [CrossRef]
- Cossar, E.; Murphy, F.; Walia, J.; Weck, A.; Baranova, E.A. Role of Ionomers in Anion Exchange Membrane Water Electrolysis: Is Aemion the Answer for Nickel-Based Anodes? ACS Appl. Energy Mater. 2022, 5, 9938–9951. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shi, K.; Yang, Y.; Yang, Z.; Zhang, W. Polydopamine-Engineered Design of Nafion-Free Powder Electrode with Superhydrophilicity/Superaerophobicity and Superior Adhesive Structure for Efficient Overall Water Splitting. Chem. Eng. Sci. 2023, 281, 119125. [Google Scholar] [CrossRef]
- Li, D.; Park, E.J.; Zhu, W.; Shi, Q.; Zhou, Y.; Tian, H.; Lin, Y.; Serov, A.; Zulevi, B.; Baca, E.D.; et al. Highly Quaternized Polystyrene Ionomers for High Performance Anion Exchange Membrane Water Electrolysers. Nat. Energy 2020, 5, 378–385. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Ma, L.; Tang, R.; Zheng, X.; Zhao, F.; Tang, G.; Wang, Y.; Pang, A.; Li, W.; et al. Polydopamine Blended with Polyacrylic Acid for Silicon Anode Binder with High Electrochemical Performance. Powder Technol. 2021, 388, 393–400. [Google Scholar] [CrossRef]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A Review of Heat Treatment on Polyacrylonitrile Fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef]
- Furushima, Y.; Kumazawa, R.; Yamaguchi, Y.; Hirota, N.; Sawada, K.; Nakada, M.; Murakami, M. Precursory Reaction of Thermal Cyclization for Polyacrylonitrile. Polymer 2021, 226, 123780. [Google Scholar] [CrossRef]
- Nunna, S.; Naebe, M.; Hameed, N.; Fox, B.L.; Creighton, C. Evolution of Radial Heterogeneity in Polyacrylonitrile Fibres during Thermal Stabilization: An Overview. Polym. Degrad. Stab. 2017, 136, 20–30. [Google Scholar] [CrossRef]
- Piper, D.M.; Yersak, T.A.; Son, S.; Kim, S.C.; Kang, C.S.; Oh, K.H.; Ban, C.; Dillon, A.C.; Lee, S. Conformal Coatings of Cyclized-PAN for Mechanically Resilient Si nano-Composite Anodes. Adv. Energy Mater. 2013, 3, 697–702. [Google Scholar] [CrossRef]
- Hong, C.; Tao, R.; Tan, S.; Pressley, L.A.; Bridges, C.A.; Li, H.; Liu, X.; Li, H.; Li, J.; Yuan, H.; et al. In Situ Cyclized Polyacrylonitrile Coating: Key to Stabilizing Porous High-Entropy Oxide Anodes for High-Performance Lithium-Ion Batteries. Adv. Funct. Mater. 2025, 35, 2412177. [Google Scholar] [CrossRef]
- Sun, Y.; Cai, Q.; Wang, Z.; Li, Z.; Zhou, Q.; Li, X.; Zhao, D.; Lu, J.; Tian, S.; Li, Y.; et al. Two-Dimensional SnS Mediates NiFe-LDH-Layered Electrocatalyst toward Boosting OER Activity for Water Splitting. ACS Appl. Mater. Interfaces 2024, 16, 23054–23060. [Google Scholar] [CrossRef]
- Lim, D.; Oh, E.; Lim, C.; Shim, S.E.; Baeck, S.-H. Bimetallic NiFe Alloys as Highly Efficient Electrocatalysts for the Oxygen Evolution Reaction. Catal. Today 2020, 352, 27–33. [Google Scholar] [CrossRef]
- Zheng, J.; Jiang, H.; Xu, X.; Zhao, J.; Ma, X.; Sun, W.; Liu, S.; Xie, W.; Chen, Y.; Xiong, S.; et al. In Situ Partial-Cyclized Polymerized Acrylonitrile-Coated NCM811 Cathode for High-Temperature ≥ 100 °C Stable Solid-State Lithium Metal Batteries. Nano-Micro Lett. 2025, 17, 195. [Google Scholar] [CrossRef]
- Liu, L.; Duan, Y.; Liang, Y.; Kan, A.; Wang, L.; Luo, Q.; Zhang, Y.; Zhang, B.; Li, Z.; Liu, J.; et al. Cyclized Polyacrylonitrile as a Promising Support for Single Atom Metal Catalyst with Synergistic Active Site. Small 2022, 18, 2104142. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Li, Y.; Li, W.; Gao, F.; Chen, X.; Li, T.; Liang, J.; Zhang, H.; Gao, H.; Li, P.; et al. High-Temperature Flexible Electric Piezo/Pyroelectric Bifunctional Sensor with Excellent Output Performance Based on Thermal-Cyclized Electrospun PAN/Zn(Ac)2 Nanofiber Mat. Nano Energy 2024, 124, 109488. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, S.; Ao, Y.; Lan, B.; Sun, Y.; Tian, G.; Yang, T.; Huang, L.; Jin, L.; Tang, L.; et al. High-Temperature-Resistance Flexible Piezoelectric Sensor via Cyclized PAN/BTO Nanofibers. Nano Energy 2025, 138, 110910. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, J.; Liang, J. Correlative Study of Critical Reactions in Polyacrylonitrile Based Carbon Fiber Precursors during Thermal-Oxidative Stabilization. Polym. Degrad. Stab. 2013, 98, 219–229. [Google Scholar] [CrossRef]
- Liu, C.-K.; Feng, Y.; He, H.-J.; Zhang, J.; Sun, R.-J.; Chen, M.-Y. Effect of Carbonization Temperature on Properties of Aligned Electrospun Polyacrylonitrile Carbon Nanofibers. Mater. Des. 2015, 85, 483–486. [Google Scholar] [CrossRef]
- Li, X.; Peng, T.; Zhang, Y.; Wen, Y.; Nan, Z. A New Efficient Visible-Light Photocatalyst Made of SnO 2 and Cyclized Polyacrylonitrile. Mater. Res. Bull. 2018, 97, 517–522. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, X.; Fan, X.; Zhu, H.; Yin, J. Oxygen-Functionalized Polyacrylonitrile Nanofibers with Enhanced Performance for Lithium-Ion Storage. ACS Omega 2021, 6, 2542–2548. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, I.; Wu, G.; Sekiguchi, T.; Baba, Y. Evidence for the Existence of Nitrogen-Substituted Graphite Structure by Polarization Dependence of near-Edge x-Ray-Absorption Fine Structure. Phys. Rev. B 2000, 62, R6053–R6056. [Google Scholar] [CrossRef]
- Gammon, W.J.; Kraft, O.; Reilly, A.C.; Holloway, B.C. Experimental Comparison of N(1s) X-Ray Photoelectron Spectroscopy Binding Energies of Hard and Elastic Amorphous Carbon Nitride Films with Reference Organic Compounds. Carbon 2003, 41, 1917–1923. [Google Scholar] [CrossRef]
- Luo, Q.; Yang, X.; Zhao, X.; Wang, D.; Yin, R.; Li, X.; An, J. Facile Preparation of Well-Dispersed ZnO/Cyclized Polyacrylonitrile Nanocomposites with Highly Enhanced Visible-Light Photocatalytic Activity. Appl. Catal. B Environ. 2017, 204, 304–315. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, X.; Wang, J.; Yuan, Y.; Sun, Q.; Tian, R.; Yang, H.; Lu, J. Restraining the Octahedron Collapse in Lithium and Manganese Rich NCM Cathode toward Suppressing Structure Transformation. Adv. Energy Mater. 2022, 12, 2201323. [Google Scholar] [CrossRef]
- Park, O.-K.; Lee, S.; Joh, H.-I.; Kim, J.K.; Kang, P.-H.; Lee, J.H.; Ku, B.-C. Effect of Functional Groups of Carbon Nanotubes on the Cyclization Mechanism of Polyacrylonitrile (PAN). Polymer 2012, 53, 2168–2174. [Google Scholar] [CrossRef]
- Kang, Q.; Lai, D.; Tang, W.; Lu, Q.; Gao, F. Intrinsic Activity Modulation and Structural Design of NiFe Alloy Catalysts for an Efficient Oxygen Evolution Reaction. Chem. Sci. 2021, 12, 3818–3835. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, M.; Yin, J.; Abou-Hamad, E.; Schwingenschlögl, U.; Costa, P.M.F.J.; Alshareef, H.N. A Cyclized Polyacrylonitrile Anode for Alkali Metal Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 1355–1363. [Google Scholar] [CrossRef]
- Gu, W.; Jiang, K.; Jiao, X.; Gao, C.; Fan, X.; Yang, L.; Song, Y. In-Situ Cyclized Polyacrylonitrile as an Electron Selective Layer for N-i-p Perovskite Solar Cell with Enhanced Efficiency and Stability. Angew. Chem. Int. Ed. 2024, 63, e202403264. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sun, Q.; Zhong, L.; Wang, X.; Chai, L.; Li, Q.; Li, T.-T.; Hu, Y.; Qian, J.; Huang, S. Thermal Conversion of Hollow Nickel-Organic Framework into Bimetallic FeNi3 Alloy Embedded in Carbon Materials as Efficient Oer Electrocatalyst. Electrochim. Acta 2020, 354, 136716. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, X.; Li, X.; Li, D.; Huang, F.; Wei, Q. FeNi Alloy Nanoparticles Embedded in Electrospun Nitrogen-Doped Carbon Fibers for Efficient Oxygen Evolution Reaction. J. Colloid Interface Sci. 2020, 578, 805–813. [Google Scholar] [CrossRef]
- He, F.; Han, Y.; Tong, Y.; Zhong, M.; Wang, Q.; Su, B.; Lei, Z. NiFe Alloys@N-Doped Graphene-Like Carbon Anchored on N-Doped Graphitized Carbon as a Highly Efficient Bifunctional Electrocatalyst for Oxygen and Hydrogen Evolution Reactions. ACS Sustain. Chem. Eng. 2022, 10, 6094–6105. [Google Scholar] [CrossRef]
- Wu, L.; Guan, Z.; Guo, D.; Yang, L.; Chen, X.; Wang, S. High-Efficiency Oxygen Evolution Reaction: Controllable Reconstruction of Surface Interface. Small 2023, 19, 2304007. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Zhao, C. Ultrathin Metal-Organic Framework Array for Efficient Electrocatalytic Water Splitting. Nat. Commun. 2017, 8, 15341. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, X.F.; Wu, Z.; Pei, Z.; Luan, D.; Lou, X.W. (David). Supporting Trimetallic Metal-Organic Frameworks on S/N-Doped Carbon Macroporous Fibers for Highly Efficient Electrocatalytic Oxygen Evolution. Adv. Mater. 2023, 35, 2207888. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, J.; Feng, K.; Xiong, R.; Ma, S.; Wang, R.; Fu, Q.; Rafique, M.; Liu, Z.; Han, J.; et al. Reconstructing the Coordination Environment of Fe/Co Dual-atom Sites towards Efficient Oxygen Electrocatalysis for Zn–Air Batteries. Angew. Chem. Int. Ed. 2025, 64, e202419595. [Google Scholar] [CrossRef]
- Zhai, P.; Wang, C.; Zhao, Y.; Zhang, Y.; Gao, J.; Sun, L.; Hou, J. Regulating Electronic States of Nitride/Hydroxide to Accelerate Kinetics for Oxygen Evolution at Large Current Density. Nat. Commun. 2023, 14, 1873. [Google Scholar] [CrossRef]
- Hashemi, N.; Nandy, S.; Chae, K.H.; Najafpour, M.M. Anodization of a NiFe Foam: An Efficient and Stable Electrode for Oxygen-Evolution Reaction. ACS Appl. Energy Mater. 2022, 5, 11098–11112. [Google Scholar] [CrossRef]
- Jang, E.; Cho, J.; Kim, J.; Kim, J. WC Nanoparticles and NiFe Alloy Co-Encapsulated in N-Doped Carbon Nanocage for Exceptional OER and ORR Bifunctional Electrocatalysis. Appl. Surf. Sci. 2024, 663, 160201. [Google Scholar] [CrossRef]
- Zhang, M.; Deng, C. Temperature-Dependence of Magnetron Sputtered NiFe Films: Structure, Morphology, Optical, Electrical and OER Catalytic Properties. Appl. Surf. Sci. 2024, 653, 159317. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; He, T.; Shi, H.; Yu, H.; Ma, X.; Zhang, Y.; Zhang, C.; Wang, S. Facile Fabrication of Activated NiFe Bimetallic NPs Anchored N-Doped CNTs Arrays as Reliable Self-Standing Electrocatalyst for HER and OER. J. Solid State Chem. 2020, 289, 121498. [Google Scholar] [CrossRef]
- Munonde, T.S.; Zheng, H.; Nomngongo, P.N. Ultrasonic Exfoliation of NiFe LDH/CB Nanosheets for Enhanced Oxygen Evolution Catalysis. Ultrason. Sonochem. 2019, 59, 104716. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Chen, K.; He, X.; Tong, J.; Liu, X.; Tan, P.; Guo, X.; Pan, J. Metal Hydroxide–Organic Framework Mediated Structural Reengineering Enables Efficient NiFe Interaction for Robust Water Oxidation. Nano Lett. 2024, 24, 15436–15443. [Google Scholar] [CrossRef]
- Nairan, A.; Feng, Z.; Zheng, R.; Khan, U.; Gao, J. Engineering Metallic Alloy Electrode for Robust and Active Water Electrocatalysis with Large Current Density Exceeding 2000 mA cm−2. Adv. Mater. 2024, 36, 2401448. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Q.; Lin, Y.; Liu, J.; Pan, J.; Hu, J.; Xu, X. Boosting Oxygen Evolution Reaction by FeNi Hydroxide-Organic Framework Electrocatalyst toward Alkaline Water Electrolyzer. Nat. Commun. 2024, 15, 7278. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Yin, X.; Li, X.; Ding, H.; Zhang, X.; Feng, Y. A Novel Cyclized Polyacrylonitrile Binder Strategy for Efficient Oxygen Evolution Reaction Catalysts. Polymers 2025, 17, 2477. https://doi.org/10.3390/polym17182477
Gu Y, Yin X, Li X, Ding H, Zhang X, Feng Y. A Novel Cyclized Polyacrylonitrile Binder Strategy for Efficient Oxygen Evolution Reaction Catalysts. Polymers. 2025; 17(18):2477. https://doi.org/10.3390/polym17182477
Chicago/Turabian StyleGu, Yifan, Xiaomin Yin, Xinrong Li, Huili Ding, Xiaojie Zhang, and Yi Feng. 2025. "A Novel Cyclized Polyacrylonitrile Binder Strategy for Efficient Oxygen Evolution Reaction Catalysts" Polymers 17, no. 18: 2477. https://doi.org/10.3390/polym17182477
APA StyleGu, Y., Yin, X., Li, X., Ding, H., Zhang, X., & Feng, Y. (2025). A Novel Cyclized Polyacrylonitrile Binder Strategy for Efficient Oxygen Evolution Reaction Catalysts. Polymers, 17(18), 2477. https://doi.org/10.3390/polym17182477





