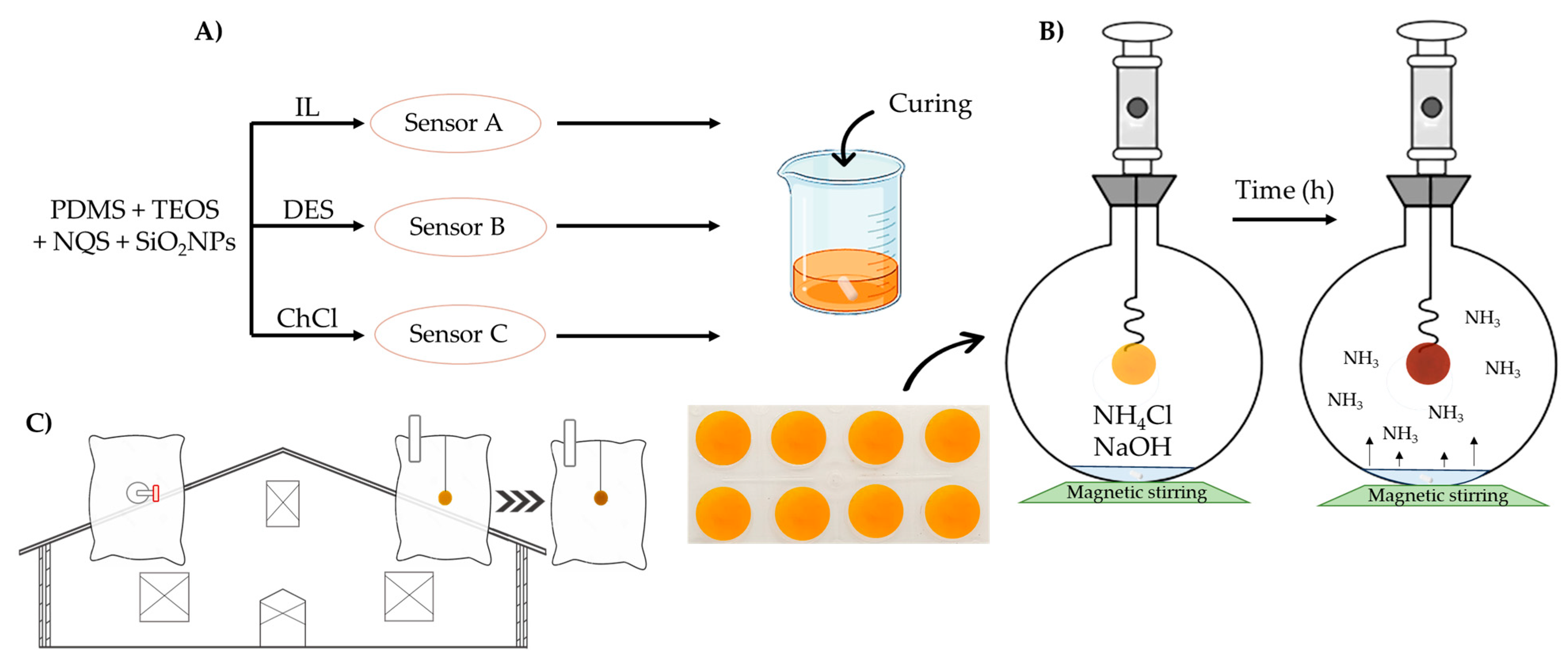
3.1. Characterization of Deep Eutectic Solvents
Different choline chloride-based deep eutectic solvents were synthetized for the development of sensor B, their composition is shown in
Table 1. As indicated in
Section 2.3, two approaches were employed, dilution of the trade mixtures with water and synthesis employing water. All of these DESs and NADESs were characterized by FTIR spectroscopy.
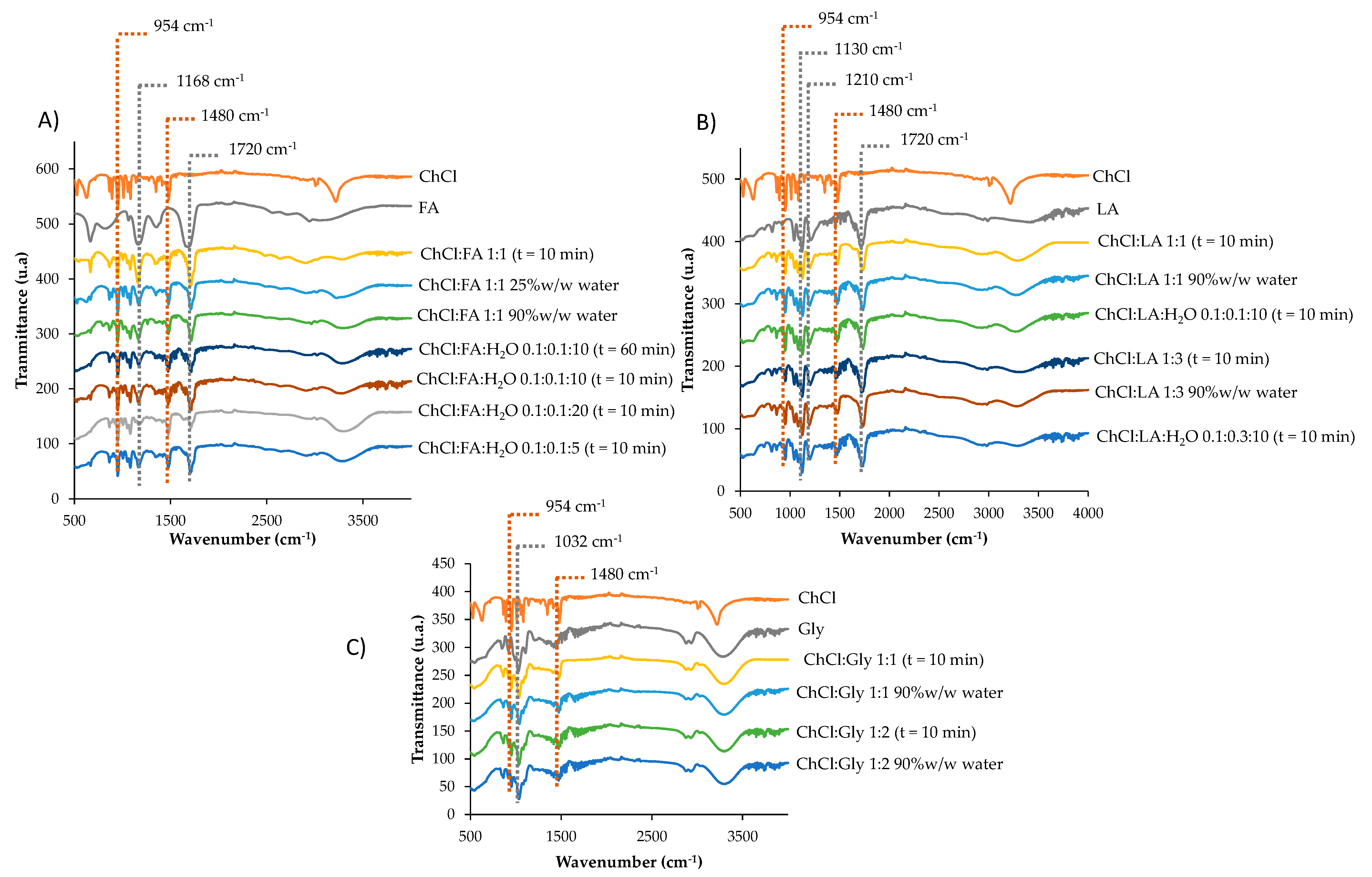
As shown in
Figure 3A, the characteristics peaks of ChCl:FA appeared at 1720 (C=O), 1480 (CH
2), 1168 (C-O),and 954 (C-N) cm
−1 [
16,
34] with 10 min synthesis time. Peaks with similar transmittance appeared in this DES diluted up to 90%
w/
w in water and ChCl:FA:H
2O 0.1:0.1:10 with a synthesis time up to 60 min. Moreover, similar results were observed with ChCl:FA:H
2O 0.1:0.1:10 and ChCl:FA:H
2O 0.1:0.1:5,; nevertheless, a reduction in transmittance peaks in ChCl:FA:H
2O 0.1:0.1:20 was observed. In any case, all of the synthesized types of choline chloride and acid formic deep eutectic solvents showed the characteristic peaks of it.
Figure 3B shows the characteristics peaks of ChCl:LA 1:1, which appeared at 1720, 1480, 1210 (C-O), 1130 (C-O-H), and 954 cm
−1 [
18,
34] with 10 min synthesis time. Peaks with similar transmittance appeared in the NADES diluted up to 90%
w/
w in water and ChCl:LA:H
2O 0.1:0.1:10 with 10 min synthesis. Moreover, similar results were observed with ChCl:LA 1:3 and ChCl:LA:H
2O 0.1:0.3:10. In all synthesized types of choline chloride and acid lactic natural deep eutectic solvents showed the characteristic peaks of it.
The characteristics peaks of ChCl:Gly 1:1 appeared at 1480, 1032 (C-O), and 954 cm
−1 [
18,
34] with 10 min synthesis time. Similar results were observed in ChCl:Gly 1:2 and both NADESs diluted up to 90%
w/
w in water (
Figure 3C).
3.2. Characterizing the Response of the Sensors A, B, and C
The influence of adding IL, DES-NADES, or ChCl into the polymeric matrix for sensor A, B, and C, respectively, was studied. Synthesis of sensor A was optimized in previous studies [
14]; however, synthesis optimization of sensors B and C was carried out in this work.
For sensor B synthesis, the same percentages of PDMS:TEOS and SiO
2NPs were used as in sensor A (see
Table 2). Assays were carried out in static dilution bottles (V = 2 L air) for 6 h with a concentration of 18.8 and 28.3 ppmv NH
3 for optimization of sensor B.
Table 3 shows the influence of the order of addition of several compounds on the sensor gelation. As derived from
Table 3, the synthesis B2 is the best option providing a homogenous membrane.
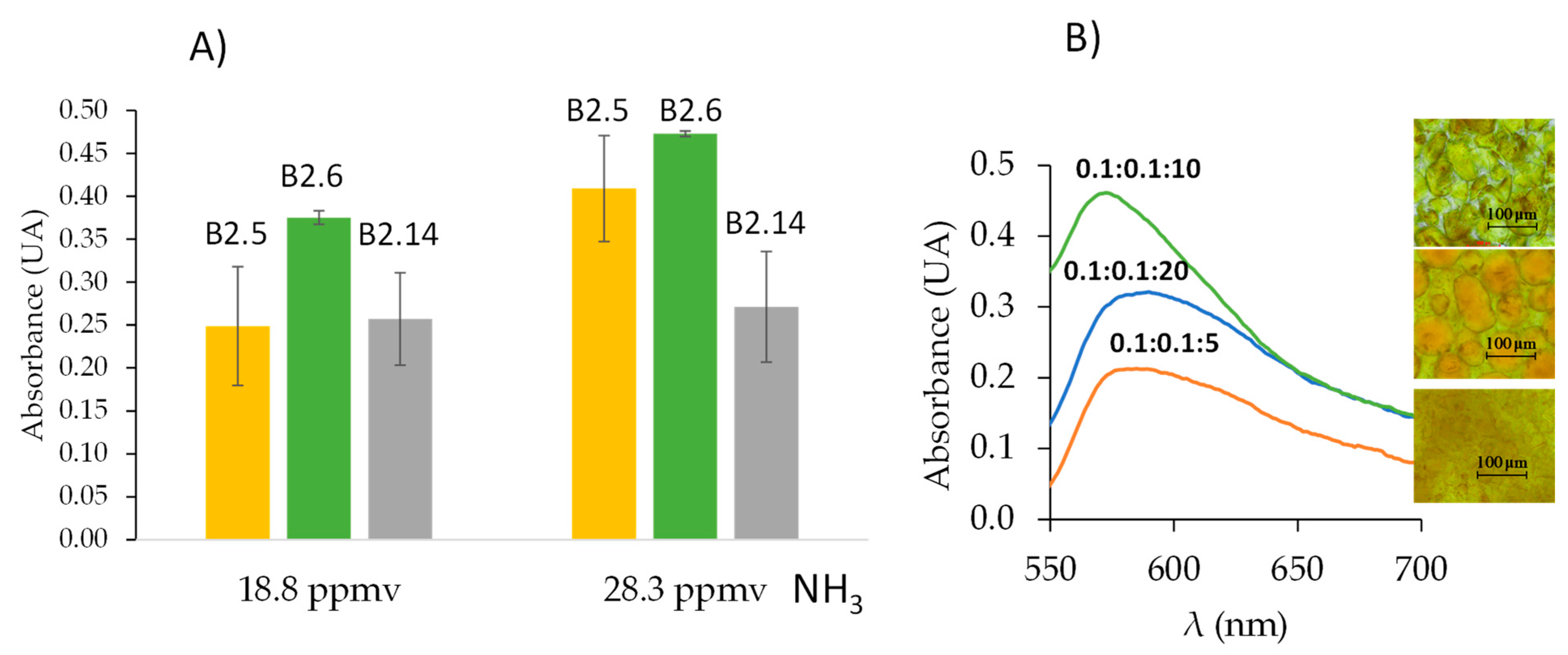
Different types and compositions of deep eutectic solvents such as ChCl:FA, ChCl:LA, and ChCl:Gly as
Table 4 shows, were tested with the objective to obtain comparable responses with those provided by sensor A. It was demonstrated that DESs and NADESs without high water content did not work because the sensor did not gel. Water can cause variations in the interactions of DESs within the sensor, allowing its gelation in the presence of a high percentage of water in the deep eutectic solvent [
35]. Synthesis B2.5, B2.6, and B2.14 provided sensors with response to ammonia, seen in
Table 4 and
Figure 4A. However, synthesis B2.6 provided better precision (see
Figure 4A). The deep eutectic solvent selected was ChCl:FA:H
2O 0.1:0.1:10 with a synthesis time of 10 min. Other molar ratios were tested: 0.1:0.1:20 and 0.1:0.1:5 but the ammonia responses were lower as
Figure 4B shows, the morphology of the membranes were different (see inserts of
Figure 4B).
For the synthesis of sensor C, an additional order of reagents was studied from assays of the sensor in static dilution bottles (V = 2 L air) for one or two h with a concentration of 18.8 ppmv NH
3. These syntheses are described in
Table 5, selecting synthesis C1 for further assays because the membrane obtained was homogenous.
Additionally, PDMS:TEOS percentages were studied, as
Table 6 indicates, 50:50% was selected for the following assays. In addition, the concentration of ChCl was studied up to 5 M, and 1 M was selected for this study. The selected synthesis was C1.4, which provided the highest absorbance values and good precision, as can be seen in
Table 6.
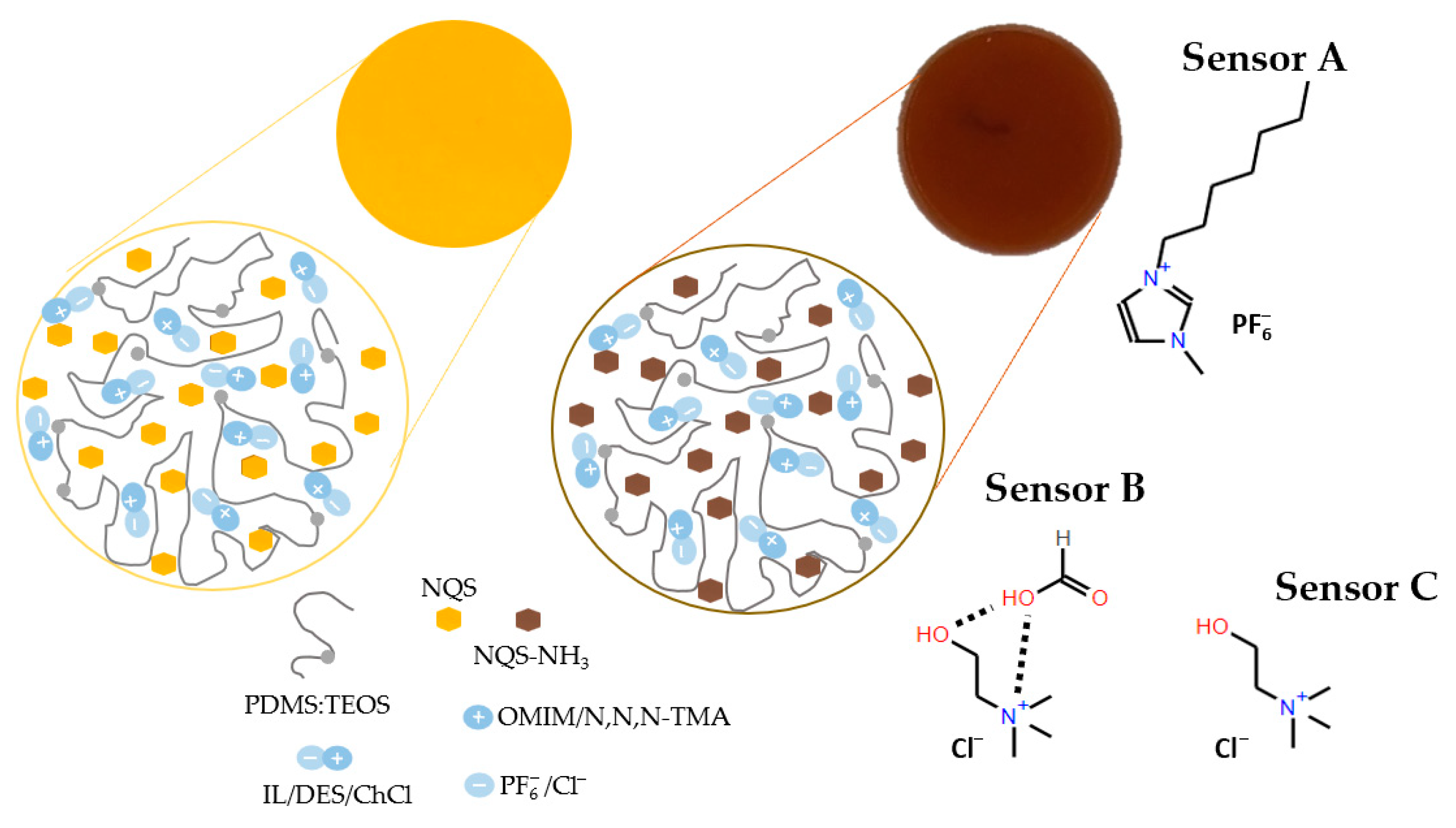
Figure 5 shows a schematic diagram of sensing membrane compositions and their responses for (sensor A) OMMIM PF6, (sensor B) ChCl:FA:H2O 0.1:0.1:10, (sensor C) ChCl. For sensor A, which contained an ionic liquid with an imidazolium cation and a PF
6− anion, simultaneous hydrogen bonds between PF
6, and silica matrix together with imidazolium groups π-π stacking interactions can occur [
14]. For sensor B and C, the interactions are probably due to the Ch
+ cation and Cl
− anion, through a physical interaction between silanol group and Cl
−, which in turn disrupts the electrostatic interaction between Ch
+ and Cl
− [
36].
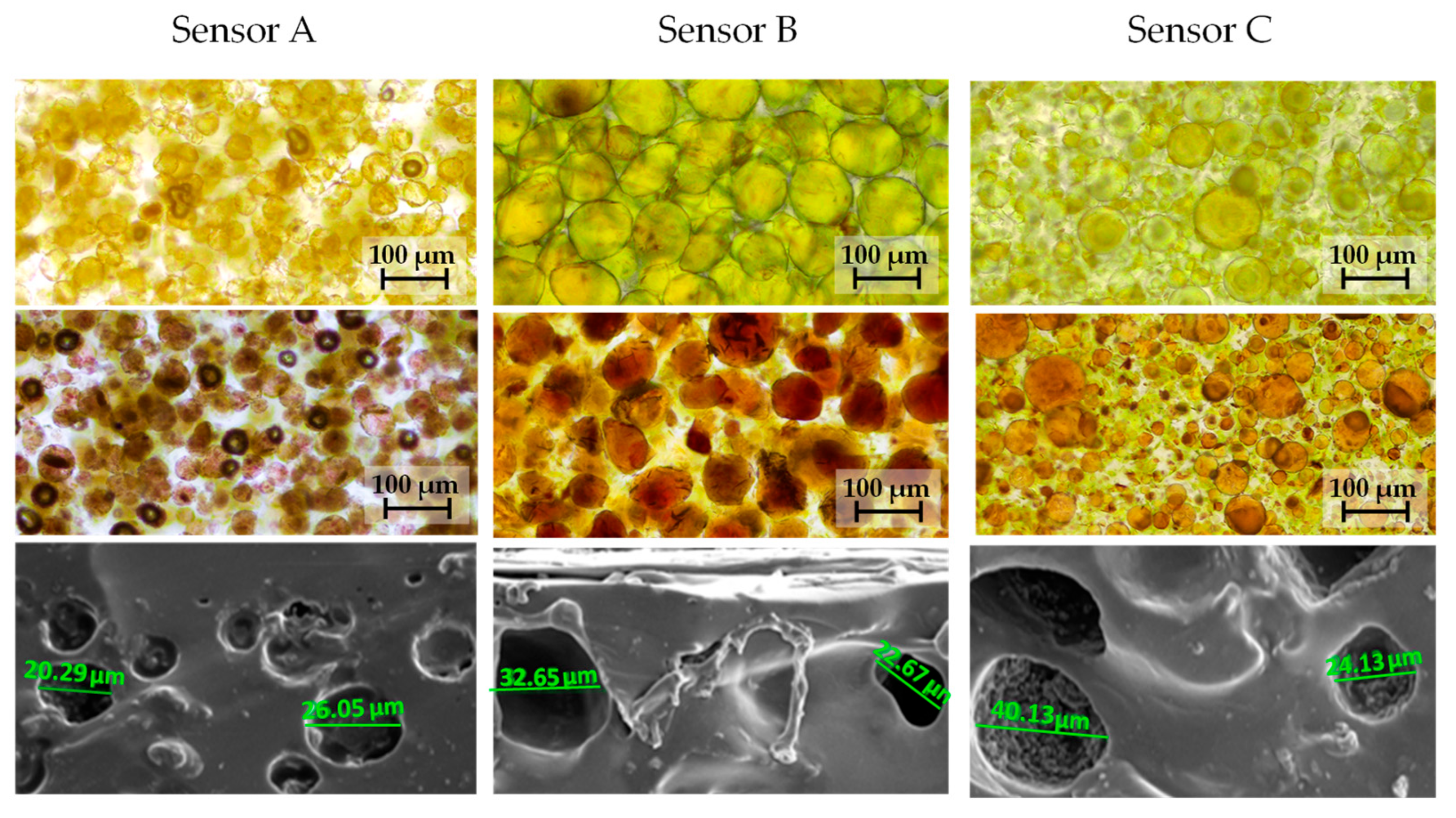
The morphology presented some differences between the three sensors, as can be derived from
Figure 6, which was obtained from bright-field microscopy photographs and SEM micrographs. The porosities are more similar for sensors B and C.
The kinetic reactions between NH
3 and the composites A, B, and C were studied. Kinetic data were collected by measuring the absorbance of the sensors at various exposure times and ammonia concentrations at room temperature, with a two L air sampling volume. The results are presented in
Figure 7. The effect of ammonia concentration on the reaction rate for sensors A and B was evaluated over 24 h at a concentration of 4.72 ppmv NH
3, which followed first-order kinetics. The slopes of the linear regressions of Ln(Abs) versus time (hours) (
n = 6) were used to determine the initial reaction rates, calculated as 0.055 ± 0.012 h
−1 for sensor A and 0.040 ± 0.011 h
−1 for sensor B. For sensor C, the kinetics were studied over five h, yielding a slope of 0.0035 ± 0.0006 h
−1 for the linear regression of Ln(Abs) versus time (hours) (
n = 6). Langmuir adsorption isotherms were examined to characterize the interaction between the sensors and ammonia. A plot of 1/A (where A represents absorbance, proportional to the amount of ammonia adsorbed by the sensor) versus 1/ammonia concentration (ppmv) was generated for sensors A and B at 3, 8, and 24 h, and for sensor C at 1, 3, and 5 h. The slopes of these plots are summarized in
Table 7, with R
2 values exceeding 0.95, demonstrating the reliability of the Langmuir model in describing the adsorption behavior.
In the case of sensor A, the uptake of ammonia exhibited a progressive increase between three and eight h of exposure. In contrast, for sensor B, the caption levels remained similar between three and eight h. Notably, the adsorption observed in sensor B after three h was comparable to that of sensor A after eight h, indicating that sensor B showed enhanced ammonia uptake efficiency over a reduced exposure time. For sensor C, a similar capture behavior was observed between three and five h, suggesting that ammonia uptake reached equilibrium by three h, as no significant variation in the slope was detected at five h. Additionally, the slope values for 24 h assays were similar for both sensors A and B, reinforcing the idea of saturation at longer exposure times.
As shown in
Figure 7, sensor C achieved equilibrium faster than sensor B, while sensor B reached saturation faster than sensor A. These findings indicate that sensors B and C demonstrated more efficient ammonia absorption in less time compared to sensor A, highlighting their suitability for applications requiring rapid response times.
3.3. Stablishing Analytical Parameters from the Response of Sensor A, B and C
The solutions used to generate the ammonia standards were analyzed according to
Section 2.5 for obtaining the ammonia volatility percentage. The volatilization percentages of ammonium in ammonia under the test conditions studied were between 96 and 99% (
n = 20, 98 ± 2). There were no significant differences between the use of the static dilution bottle and the Tedlar bag, so there is the ability to choose any of the two test vessels as required. The values were similar at different test times between one and eight h, thus ensuring volatilization of most of the ammonium present in ammonia to the confined atmosphere. Furthermore, the percentage of volatilization was similar regardless of the ammonia concentration used for the test. These results agreed with previous studies [
14].
Analytical parameters such as sensitivities, limit of quantifications (LOQ), and limit of detections (LOD) at different assay times were obtained. Suitable regression coefficients in all studied cases and relative standard deviation, RSD < 8%, as shown in
Table 8, were achieved.
For sensors A and B, the ionic liquid and the deep eutectic solvent resulted in comparable responses across different exposure times, achieving similar sensitivity and detection limits. This suggests that the deep eutectic solvent can replace the ionic liquid, leading to a more environmentally friendly sensor with lower cost and improved biodegradability, while maintaining equivalent performance under the same conditions. In the case of sensor C, similar sensitivities and better detection limits were achieved within shorter exposure times. As demonstrated previously, sensor C exhibited a faster NH3 uptake capacity compared to sensors A and B. These data indicated the capacity of the doping agents to determine the time-response and the amount of NQS and ammonia retained in the membranes, this versatility can be useful in sampling plan strategies.
3.4. Stability of the Three Synthetized Composites
At the lab, some composites were stored in a freezer at −20 °C, while others were kept at room temperature (25 °C) and exposed to atmospheric conditions. Blank absorption measurements were performed using diffuse reflectance for sensors A, B, and C under both storage conditions over a period of up to 12 months, as illustrated in
Figure 8.
The stability of sensors stored in the freezer was demonstrated, at least, for 12 months, maintaining the analytical signal characteristic of the blank of each sensor during all this time stored at low temperatures. However, for composites exposed to the atmosphere at room temperature, the degradation of the NQS reagent started after one week of exposure.
The preservation of the sensors to maintain their stability for sampling in farms did not present any problems, as they remained stable for at least three months at 4 °C; the absorbance variation for non-exposed sensors (n = 20) was 0.06 ± 0.01 UA. All the sampled farms are equipped with a refrigerator capable of storing them until their use.
3.5. Application of Sensors in Real Poultry Farms
Measurements of air in two poultry and two rabbit farms were carried out by sampling, with a 500 mL syringe, two L of air inside farm ship in hermetically sealed bags, in which the sensor A, B, or C was inside, and Tedlar bags for a UHPLC-QTOF method developed here (see
Section 2.5 and
Section 2.6).
The linear working ranges for ammonia and methylamine obtained by UHPLC-QTOF were as follows: 0.05 (LOQ) − 50 ppmv and 0.05 (LOQ) − 10 ppmv and the calibration graphs: (−1.2 ± 0.1) + (56 ± 2) ppmv; R2 = 0.998 (n = 7) and (−0.3 ± 0.2) + (14.6 ± 0.7) ppmv; R2 = 0.998 (n = 7), respectively. The precision achieved was lower than 7% in all cases.
Sensors A and B were maintained for eight h and sensor C for three h inside bags at room temperature and after, diffuse reflectance as absorbances were measured. Some blanks of each sensor were assayed sampling atmospheric air from outside the farm building and any modification of the signal sensor was obtained.
The NH
3 concentrations measured using UHPLC-QTOF and the sensors evaluated in this study showed comparable results across the 30 atmospheres analyzed as can be seen in
Table 9. Layer, broiler, and rabbit farms with natural ventilation or cross-flow mechanical ventilation were sampled in winter, spring, or summer, as can be seen in
Table 9. In all cases for each farm, the normal manure management was carried out. Some differences can be due to the possible lack of homogeneity of the farm atmosphere. Additionally, methylamine was studied by UHPLC-QTOF, and the presence was detected only in two farm atmosphere samples at concentrations close to the limit of detection, indicating NH
3 was the predominant component in the farms (
Table 9). These results are in accordance with [
37].
The results obtained for samples S7, S8, and S9, which used sensors kept in farm at four °C during 21, 27, and 41 days before sampling corroborated their stability as indicated in the previous section, similar results for ammonia were achieved. NQS provided selectivity in reference to other family compounds because this reagent is a derivatization agent for primary and secondary amino groups. Ammonia in presence of hydrogen sulfide was tested in [
14,
38], and no effect was observed. This statement was also supported by the results obtained from the confirmatory study from air sampling in Tedlar bags and analysis by UHPLC-QTOF because comparable results were obtained (see
Table 9).