Surface Properties of Recombinant Pea Vicilin and Cupin-1.2 Solutions in 8M Urea
Abstract
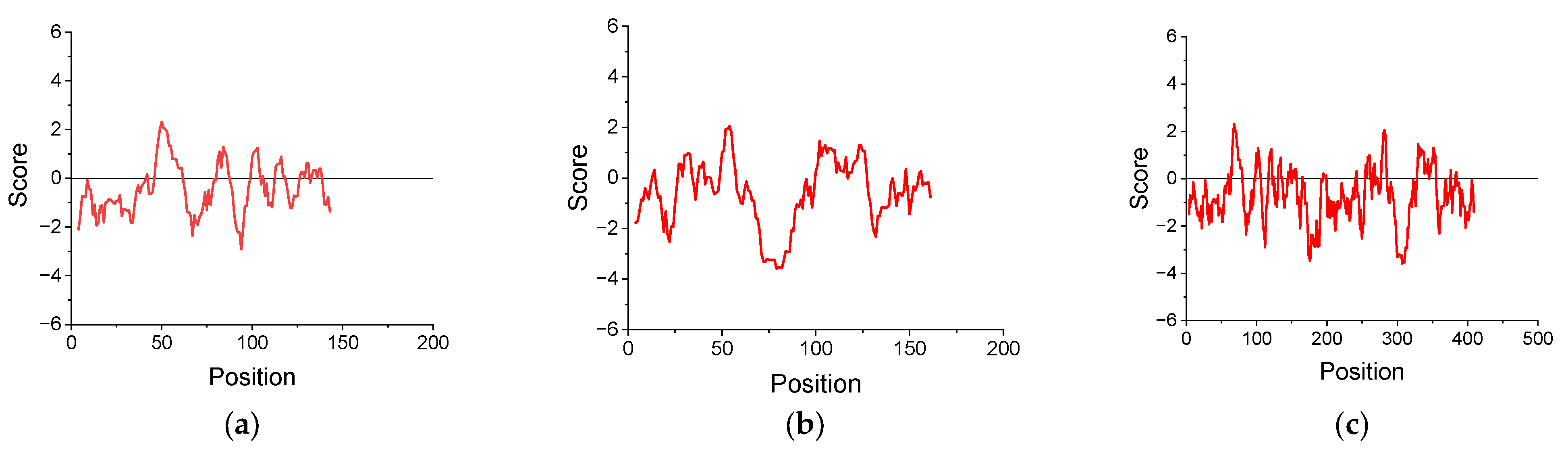
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
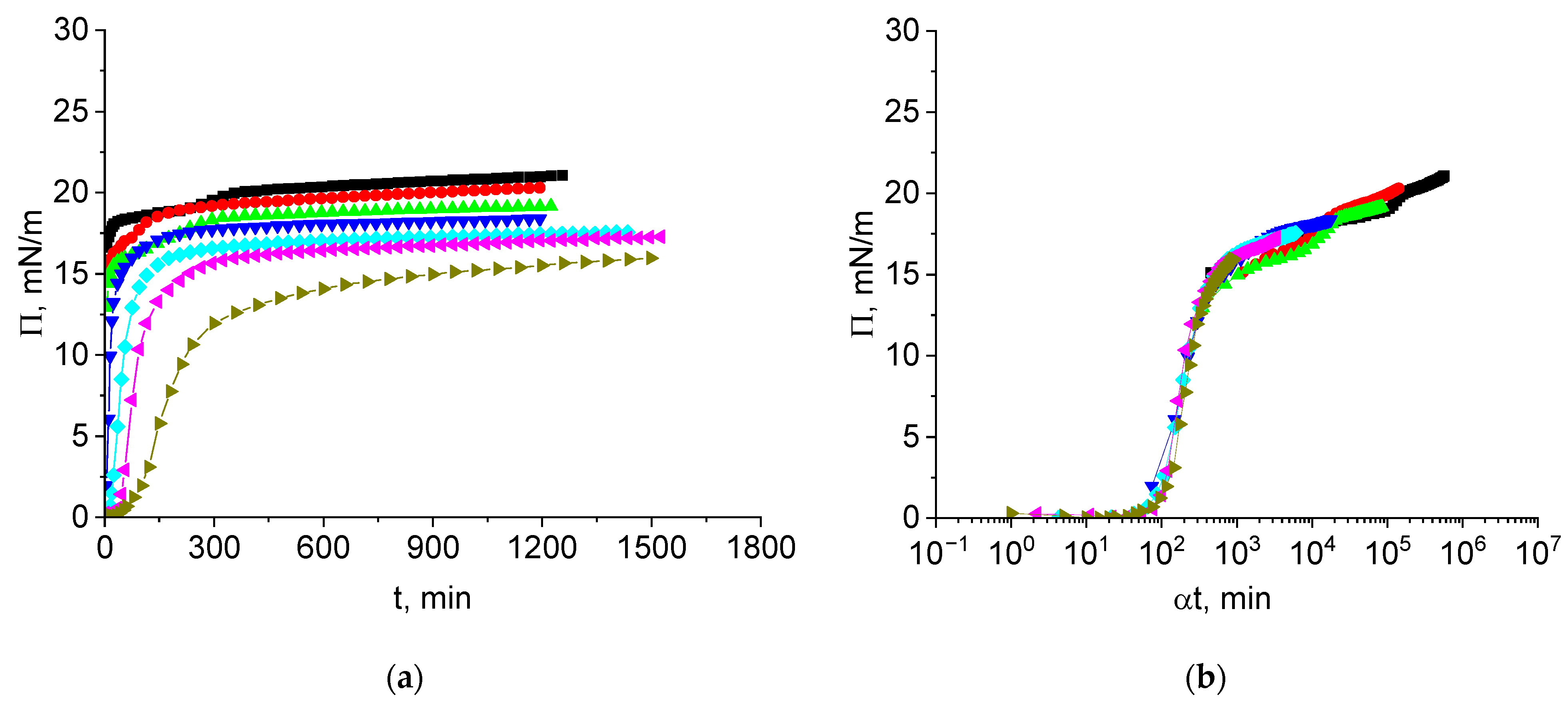
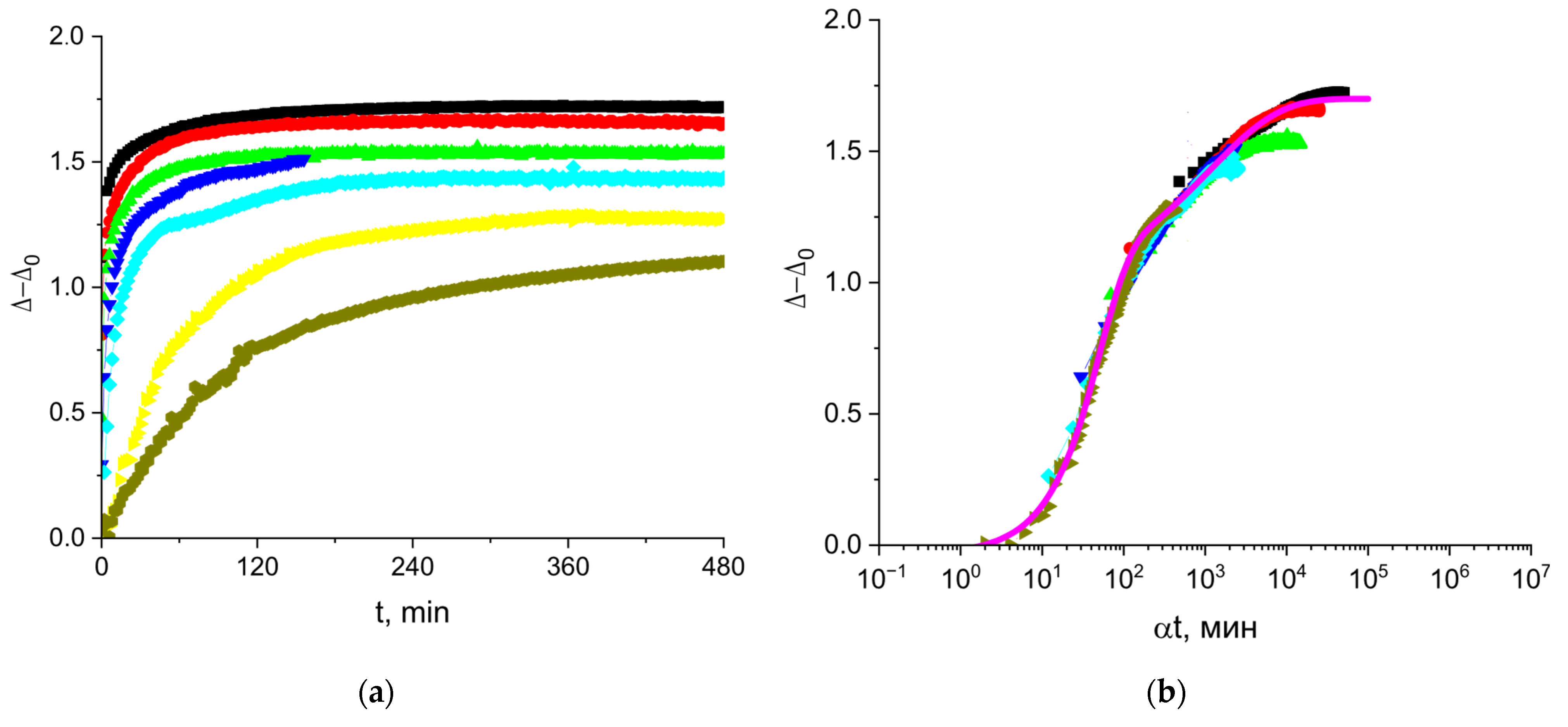
3.1. Cupin-1.2 Adsorption Kinetics
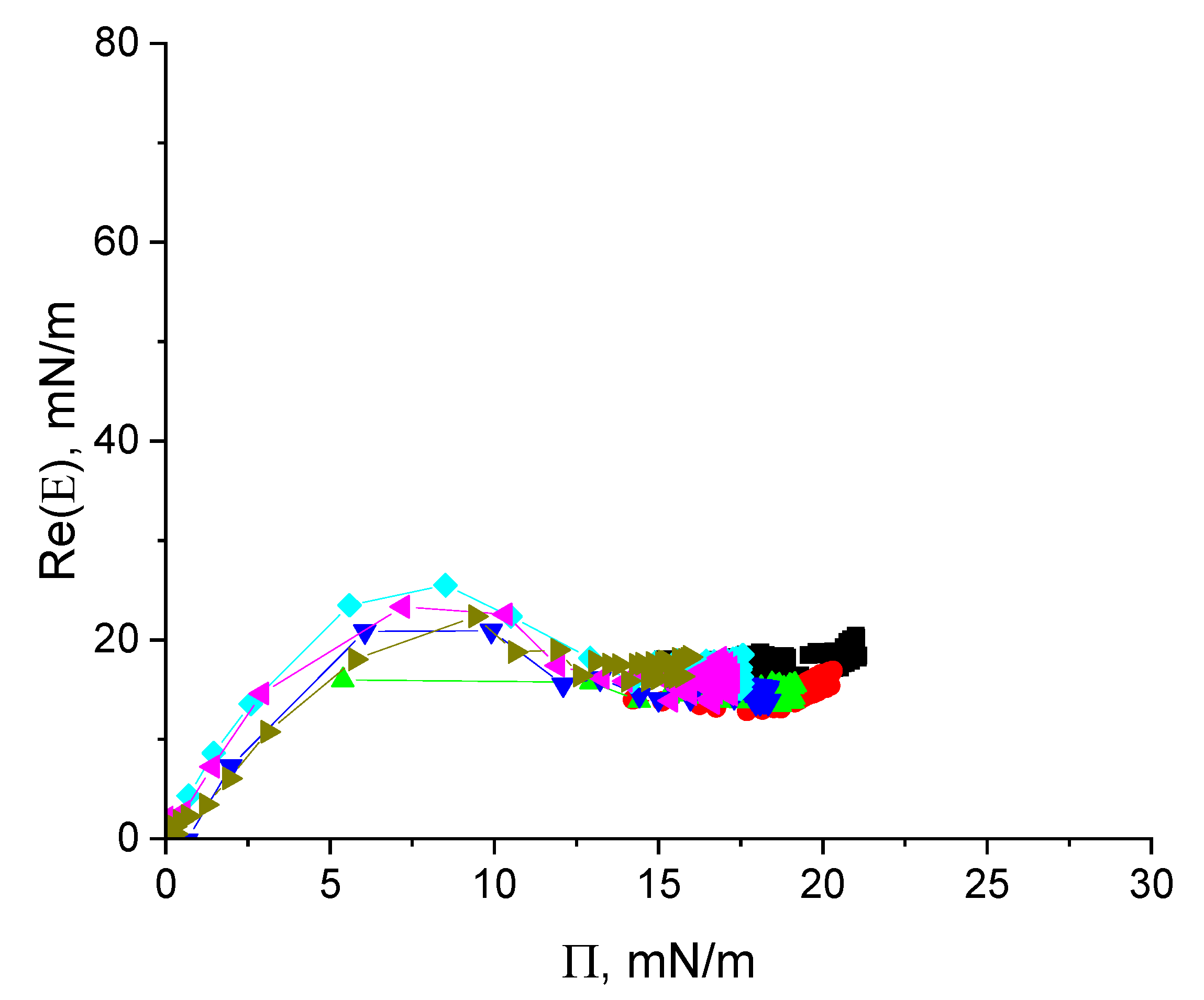
3.2. Dynamic Surface Elasticity of Cupin-1.2 Solutions
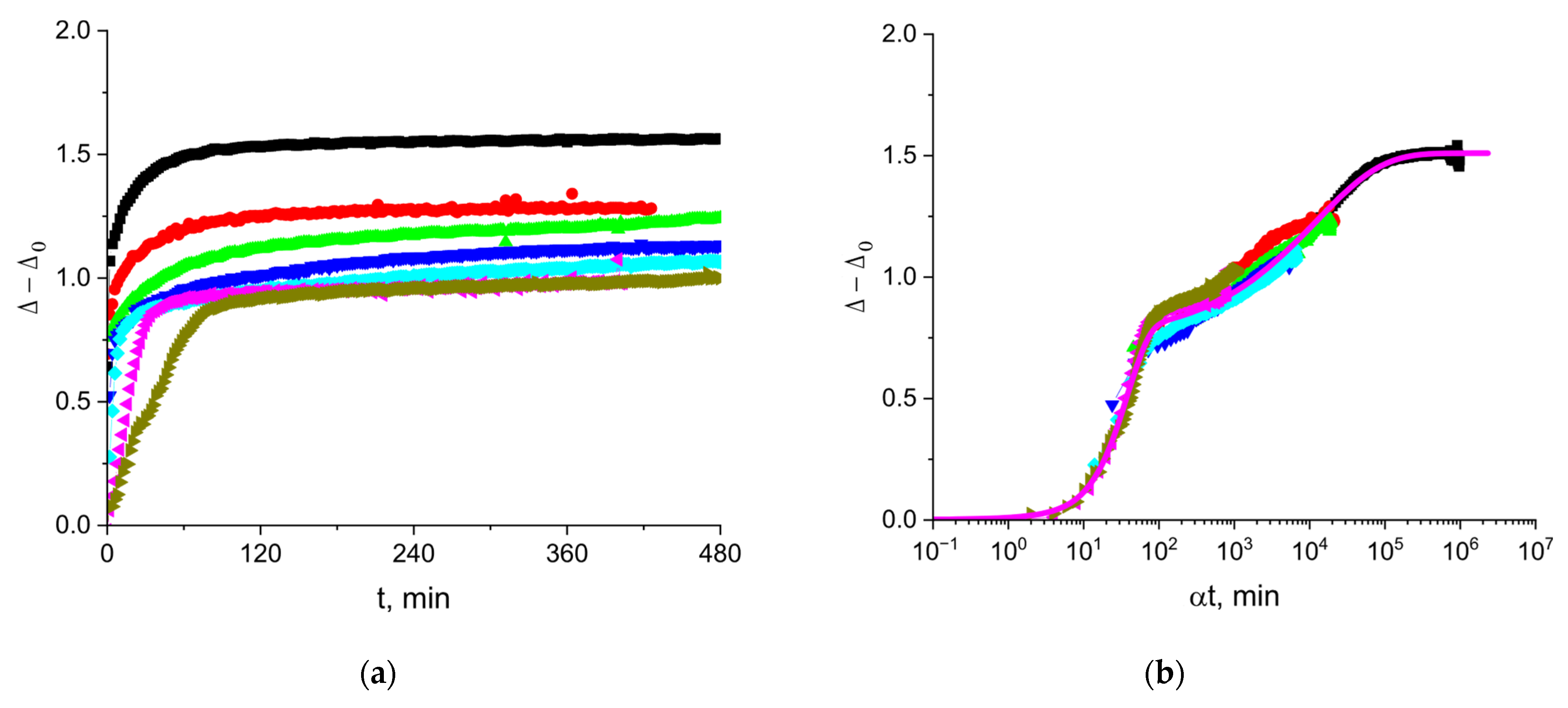
3.3. The Kinetic Dependencies of the Ellipsometric Angle Δ of Cupin-1.2 Solutions
3.4. Vicilin Adsorption Kinetics
3.5. Dynamic Surface Elasticity of Vicilin Solutions
3.6. The Kinetic Dependencies of the Ellipsometric Angle Δ of Vicilin Solutions
3.7. Dynamic Properties and Morphology of Cupin-1.2 and Vicilin Spread Layers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| GRAVY | Grand average of hydropathy |
| AI | Aliphatic index |
References
- Jarpa-Parra, M.; Bamdad, F.; Tian, Z.; Zeng, H.; Temelli, F.; Chen, L. Impact of pH on Molecular Structure and Surface Properties of Lentil Legumin-like Protein and Its Application as Foam Stabilizer. Colloids Surf. B Biointerfaces 2015, 132, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Chen, B.; Rao, J. Synergistic Effect of pH-Shift and Controlled Heating on Improving Foaming Properties of Pea Vicilin and Its Adsorption Behavior at the Air-Water Interface. Food Hydrocoll. 2023, 145, 109022. [Google Scholar] [CrossRef]
- Amagliani, L.; Schmitt, C. Globular Plant Protein Aggregates for Stabilization of Food Foams and Emulsions. Trends Food Sci. Technol. 2017, 67, 248–259. [Google Scholar] [CrossRef]
- Zembyla, M.; Liamas, E.; Andablo-Reyes, E.; Gu, K.; Krop, E.M.; Kew, B.; Sarkar, A. Surface Adsorption and Lubrication Properties of Plant and Dairy Proteins: A Comparative Study. Food Hydrocoll. 2021, 111, 106364. [Google Scholar] [CrossRef]
- Sagis, L.M.; Yang, J. Protein-Stabilized Interfaces in Multiphase Food: Comparing Structure-Function Relations of Plant-Based and Animal-Based Proteins. Curr. Opin. Food Sci. 2022, 43, 53–60. [Google Scholar] [CrossRef]
- Kaur, G.; Ahmadzadeh-Hashemi, S.; Amir, S.; Khan, Z.S.; Gulsunoglu-Konuskan, Z.; Karimidastjerd, A.; Fayaz, S.; Bhat, M.S.; Rustagi, S.; Bekhit, A.E.-D.A.; et al. Exploring Sustainable Novel Millet Protein: A Look at the Future Foods through Innovative Processing. Future Foods 2024, 9, 100367. [Google Scholar] [CrossRef]
- Tang, J.; Yao, D.; Xia, S.; Cheong, L.; Tu, M. Recent Progress in Plant-Based Proteins: From Extraction and Modification Methods to Applications in the Food Industry. Food Chem. X 2024, 23, 101540. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Liu, Z.; Zhi, L.; Jiao, B.; Hu, H.; Ma, X.; Agyei, D.; Shi, A. Plant Protein-Based Emulsifiers: Mechanisms, Techniques for Emulsification Enhancement and Applications. Food Hydrocoll. 2023, 144, 109008. [Google Scholar] [CrossRef]
- Knowles, T.P.J.; Mezzenga, R. Amyloid Fibrils as Building Blocks for Natural and Artificial Functional Materials. Adv. Mater. 2016, 28, 6546–6561. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Food Protein Amyloid Fibrils: Origin, Structure, Formation, Characterization, Applications and Health Implications. Adv. Colloid Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef]
- Zhu, Z.; Pius Bassey, A.; Cao, Y.; Ma, Y.; Huang, M.; Yang, H. Food Protein Aggregation and Its Application. Food Res. Int. 2022, 160, 111725. [Google Scholar] [CrossRef]
- Richards, B.A.; Goncalves, A.G.; Sullivan, M.O.; Chen, W. Engineering Protein Nanoparticles for Drug Delivery. Curr. Opin. Biotechnol. 2024, 86, 103070. [Google Scholar] [CrossRef]
- Wouters, A.G.B.; Schaefer, S.; Joye, I.J.; Delcour, J.A. Relating the Structural, Air-Water Interfacial and Foaming Properties of Wheat (Triticum aestivum L.) Gliadin and Maize (Zea mays L.) Zein Based Nanoparticle Suspensions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 249–259. [Google Scholar] [CrossRef]
- Antonets, K.S.; Belousov, M.V.; Sulatskaya, A.I.; Belousova, M.E.; Kosolapova, A.O.; Sulatsky, M.I.; Andreeva, E.A.; Zykin, P.A.; Malovichko, Y.V.; Shtark, O.Y.; et al. Accumulation of Storage Proteins in Plant Seeds Is Mediated by Amyloid Formation. PLoS Biol. 2020, 18, e3000564. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sagis, L.M.C. Interfacial Behavior of Plant Proteins—Novel Sources and Extraction Methods. Curr. Opin. Colloid Interface Sci. 2021, 56, 101499. [Google Scholar] [CrossRef]
- Poirier, A.; Banc, A.; Stocco, A.; In, M.; Ramos, L. Multistep Building of a Soft Plant Protein Film at the Air-Water Interface. J. Colloid Interface Sci. 2018, 526, 337–346. [Google Scholar] [CrossRef]
- Poirier, A.; Stocco, A.; Kapel, R.; In, M.; Ramos, L.; Banc, A. Sunflower Proteins at Air–Water and Oil–Water Interfaces. Langmuir 2021, 37, 2714–2727. [Google Scholar] [CrossRef]
- Poirier, A.; Banc, A.; Kapel, R.; In, M.; Stocco, A.; Ramos, L. Impact of Structural Flexibility in the Adsorption of Wheat and Sunflower Proteins at an Air/Water Interface. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129317. [Google Scholar] [CrossRef]
- Shen, P.; Yang, J.; Nikiforidis, C.V.; Mocking-Bode, H.C.M.; Sagis, L.M.C. Cruciferin versus Napin—Air-Water Interface and Foam Stabilizing Properties of Rapeseed Storage Proteins. Food Hydrocoll. 2023, 136, 108300. [Google Scholar] [CrossRef]
- Chutinara, C.; Sagis, L.M.C.; Landman, J. Interfacial Rheological Properties of Pepsin-Hydrolyzed Lentil Protein Isolate at Oil-Water Interfaces. Food Hydrocoll. 2024, 155, 110201. [Google Scholar] [CrossRef]
- Peng, D.; Yang, J.; De Groot, A.; Jin, W.; Deng, Q.; Li, B.; Sagis, L.M.C. Soft Gliadin Nanoparticles at Air/Water Interfaces: The Transition from a Particle-Laden Layer to a Thick Protein Film. J. Colloid Interface Sci. 2024, 669, 236–247. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, J.; Waterink, B.; Venema, P.; De Vries, R.; Sagis, L.M.C. Mung Bean Protein Colloid Mixtures and Their Fractions—A Novel and Excellent Foam Stabiliser. Food Hydrocoll. 2024, 155, 110174. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Q.; Waterink, B.; Venema, P.; De Vries, R.; Sagis, L.M.C. Physical, Interfacial and Foaming Properties of Different Mung Bean Protein Fractions. Food Hydrocoll. 2023, 143, 108885. [Google Scholar] [CrossRef]
- Isakov, N.A.; Belousov, M.V.; Nizhnikov, A.A.; Noskov, B.A. Dynamic Properties of the Layers of Cupin-1.1 Aggregates at the Air/Water Interface. Biophys. Chem. 2024, 307, 107166. [Google Scholar] [CrossRef] [PubMed]
- Kontogiorgos, V.; Prakash, S. Adsorption Kinetics and Dilatational Rheology of Plant Protein Concentrates at the Air- and Oil-Water Interfaces. Food Hydrocoll. 2023, 138, 108486. [Google Scholar] [CrossRef]
- Langevin, D. Recent Advances on Emulsion and Foam Stability. Langmuir 2023, 39, 3821–3828. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Habibi, M.; Sagis, L.M.C. Interfacial and Foaming Properties of Soluble Lupin Protein Isolates: Effect of pH. Food Hydrocoll. 2024, 155, 110228. [Google Scholar] [CrossRef]
- Shen, P.; Peng, J.; Sagis, L.M.C.; Landman, J. Molecular, Interfacial and Foaming Properties of Pulse Proteins. Food Hydrocoll. 2024, 156, 110313. [Google Scholar] [CrossRef]
- Antonets, K.; Nizhnikov, A. Predicting Amyloidogenic Proteins in the Proteomes of Plants. Int. J. Mol. Sci. 2017, 18, 2155. [Google Scholar] [CrossRef]
- Isakov, N.A.; Belousov, M.V.; Loglio, G.; Miller, R.; Nizhnikov, A.A.; Panda, A.K.; Noskov, B.A. Cupin-1.1 Adsorption Layers at the Surface of 8 M Urea Solutions. J. Phys. Chem. B 2024, 128, 11992–11998. [Google Scholar] [CrossRef]
- Denkov, N.; Tcholakova, S.; Politova-Brinkova, N. Physicochemical Control of Foam Properties. Curr. Opin. Colloid Interface Sci. 2020, 50, 101376. [Google Scholar] [CrossRef]
- Noskov, B.A. Protein Conformational Transitions at the Liquid–Gas Interface as Studied by Dilational Surface Rheology. Adv. Colloid Interface Sci. 2014, 206, 222–238. [Google Scholar] [CrossRef]
- Noskov, B.A.; Rafikova, A.R.; Milyaeva, O.Y. β-Lactoglobulin Microgel Layers at the Surface of Aqueous Solutions. J. Mol. Liq. 2022, 351, 118658. [Google Scholar] [CrossRef]
- Benjamins, J.-W.; Thuresson, K.; Nylander, T. Ellipsometry Studies of Nonionic Surfactant Adsorption at the Oil−Water Interface. Langmuir 2005, 21, 149–159. [Google Scholar] [CrossRef]
- Kyte, J.; Doolittle, R.F. A Simple Method for Displaying the Hydropathic Character of a Protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef] [PubMed]
- Ikai, A. Thermostability and Aliphatic Index of Globular Proteins. J. Biochem. 1980, 88, 1895–1898. [Google Scholar] [CrossRef]
- Ma, X.; Shen, P.; Habibi, M.; Sagis, L.M.C. Interfacial Properties and Functionality of Lupin Protein-Pectin Complexes at the Air-Water Interface. Food Hydrocoll. 2024, 154, 110050. [Google Scholar] [CrossRef]
- Campbell, R.A.; Tummino, A.; Varga, I.; Milyaeva, O.Y.; Krycki, M.M.; Lin, S.-Y.; Laux, V.; Haertlein, M.; Forsyth, V.T.; Noskov, B.A. Adsorption of Denaturated Lysozyme at the Air–Water Interface: Structure and Morphology. Langmuir 2018, 34, 5020–5029. [Google Scholar] [CrossRef]
- Noskov, B.A.; Mikhailovskaya, A.A.; Lin, S.-Y.; Loglio, G.; Miller, R. Bovine Serum Albumin Unfolding at the Air/Water Interface as Studied by Dilational Surface Rheology. Langmuir 2010, 26, 17225–17231. [Google Scholar] [CrossRef] [PubMed]
- Noskov, B.A.; Akentiev, A.V.; Bilibin, A.Y.; Zorin, I.M.; Miller, R. Dilational Surface Viscoelasticity of Polymer Solutions. Adv. Colloid Interface Sci. 2003, 104, 245–271. [Google Scholar] [CrossRef]
- Douillard, R.; Daoud, M.; Aguié-Béghin, V. Polymer Thermodynamics of Adsorbed Protein Layers. Curr. Opin. Colloid Interface Sci. 2003, 8, 380–386. [Google Scholar] [CrossRef]
- Ward, A.F.H.; Tordai, L. Time-Dependence of Boundary Tensions of Solutions I. The Role of Diffusion in Time-Effects. J. Chem. Phys. 1946, 14, 453–461. [Google Scholar] [CrossRef]
- Noskov, B.A.; Akentiev, A.V.; Miller, R. Dynamic Surface Properties of Poly(Vinylpyrrolidone) Solutions. J. Colloid Interface Sci. 2002, 255, 417–424. [Google Scholar] [CrossRef]
- Noskov, B.A.; Akentiev, A.V.; Bilibin, A.Y.; Grigoriev, D.O.; Loglio, G.; Zorin, I.M.; Miller, R. Dynamic Surface Properties of Poly(N-Isopropylacrylamide) Solutions. Langmuir 2004, 20, 9669–9676. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y.F. Kinetics of Protein Unfolding at Interfaces. J. Phys. Condens. Matter 2012, 24, 503101. [Google Scholar] [CrossRef]
- Noskov, B.A. Dynamic Surface Elasticity of Polymer Solutions. Colloid Polym. Sci. 1995, 273, 263–270. [Google Scholar] [CrossRef]
- Bykov, A.G.; Lin, S.-Y.; Loglio, G.; Miller, R.; Noskov, B.A. Kinetics of Adsorption Layer Formation in Solutions of Polyacid/Surfactant Complexes. J. Phys. Chem. C 2009, 113, 5664–5671. [Google Scholar] [CrossRef]
- Pinaud, F.; Geisel, K.; Massé, P.; Catargi, B.; Isa, L.; Richtering, W.; Ravaine, V.; Schmitt, V. Adsorption of Microgels at an Oil–Water Interface: Correlation between Packing and 2D Elasticity. Soft Matter 2014, 10, 6963–6974. [Google Scholar] [CrossRef] [PubMed]
- Tatry, M.-C.; Laurichesse, E.; Vermant, J.; Ravaine, V.; Schmitt, V. Interfacial Rheology of Model Water–Air Microgels Laden Interfaces: Effect of Cross-Linking. J. Colloid Interface Sci. 2023, 629, 288–299. [Google Scholar] [CrossRef]
- Migliozzi, S.; He, Y.; Angeli, P.; Lan, Y. Enhancing Interfacial Elasticity via Supramolecular Microgel Assembly for Improved Pickering Emulsions Stability. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 699, 134538. [Google Scholar] [CrossRef]
- Rodríguez Patino, J.M.; Molina Ortiz, S.E.; Sánchez, C.C.; Rodríguez Niño, M.R.; Añon, M.C. Behavior of Soy Globulin Films at the Air−Water Interface. Structural and Dilatational Properties of Spread Films. Ind. Eng. Chem. Res. 2003, 42, 5011–5017. [Google Scholar] [CrossRef]
- Rodrı́guez Patino, J.M.; Molina Ortiz, S.E.; Carrera Sánchez, C.; Rodrı́guez Niño, M.R.; Añón, M.C. Dynamic Properties of Soy Globulin Adsorbed Films at the Air–Water Interface. J. Colloid Interface Sci. 2003, 268, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Choe, U.; Chang, L.; Ohm, J.-B.; Chen, B.; Rao, J. Structure Modification, Functionality and Interfacial Properties of Kidney Bean (Phaseolus vulgaris L.) Protein Concentrate as Affected by Post-Extraction Treatments. Food Hydrocoll. 2022, 133, 108000. [Google Scholar] [CrossRef]
- Meng, Y.; Wei, Z.; Xue, C. Correlation among Molecular Structure, Air/Water Interfacial Behavior and Foam Properties of Naringin-Treated Chickpea Protein Isolates. Food Hydrocoll. 2024, 147, 109309. [Google Scholar] [CrossRef]
- Solanilla-Duque, J.F.; Roa-Acosta, D.F.; Bravo-Gómez, J.E. Effect of pH and Concentration on Physicochemical, Adsorption Kinetics and Rheology Properties of Quinoa Protein: Functional Correlations. JCIS Open 2025, 18, 100131. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, L.; Wang, G.; Awais, M.; Tong, L.; Fan, B.; Hu, A.; Wang, F. Comparative Study on the Foam and Air-Water Interface Properties of Ethanol-Soluble and Non-Ethanol Components in Wheat Aqueous Phase Protein. Food Hydrocoll. 2024, 150, 109700. [Google Scholar] [CrossRef]
- Hüsecken, A.K.; Evers, F.; Czeslik, C.; Tolan, M. Effect of Urea and Glycerol on the Adsorption of Ribonuclease A at the Air−Water Interface. Langmuir 2010, 26, 13429–13435. [Google Scholar] [CrossRef]



| Characteristic | Cupin-1.1 | Cupin-1.2 | Vicilin |
|---|---|---|---|
| Aliphatic index | 96.22 | 83.31 | 84.28 |
| GRAVY | −0.495 | −0.640 | −0.744 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isakov, N.; Angel, D.; Belousov, M.; Loglio, G.; Miller, R.; Nizhnikov, A.; Noskov, B. Surface Properties of Recombinant Pea Vicilin and Cupin-1.2 Solutions in 8M Urea. Polymers 2025, 17, 2463. https://doi.org/10.3390/polym17182463
Isakov N, Angel D, Belousov M, Loglio G, Miller R, Nizhnikov A, Noskov B. Surface Properties of Recombinant Pea Vicilin and Cupin-1.2 Solutions in 8M Urea. Polymers. 2025; 17(18):2463. https://doi.org/10.3390/polym17182463
Chicago/Turabian StyleIsakov, Nikolay, Dmitry Angel, Mikhail Belousov, Giuseppe Loglio, Reinhard Miller, Anton Nizhnikov, and Boris Noskov. 2025. "Surface Properties of Recombinant Pea Vicilin and Cupin-1.2 Solutions in 8M Urea" Polymers 17, no. 18: 2463. https://doi.org/10.3390/polym17182463
APA StyleIsakov, N., Angel, D., Belousov, M., Loglio, G., Miller, R., Nizhnikov, A., & Noskov, B. (2025). Surface Properties of Recombinant Pea Vicilin and Cupin-1.2 Solutions in 8M Urea. Polymers, 17(18), 2463. https://doi.org/10.3390/polym17182463











