PP-Based Blends with PVP-I Additive: Mechanical, Thermal, and Barrier Properties for Packaging of Iodophor Pharmaceutical Formulations
Abstract
1. Introduction
2. Materials and Methods
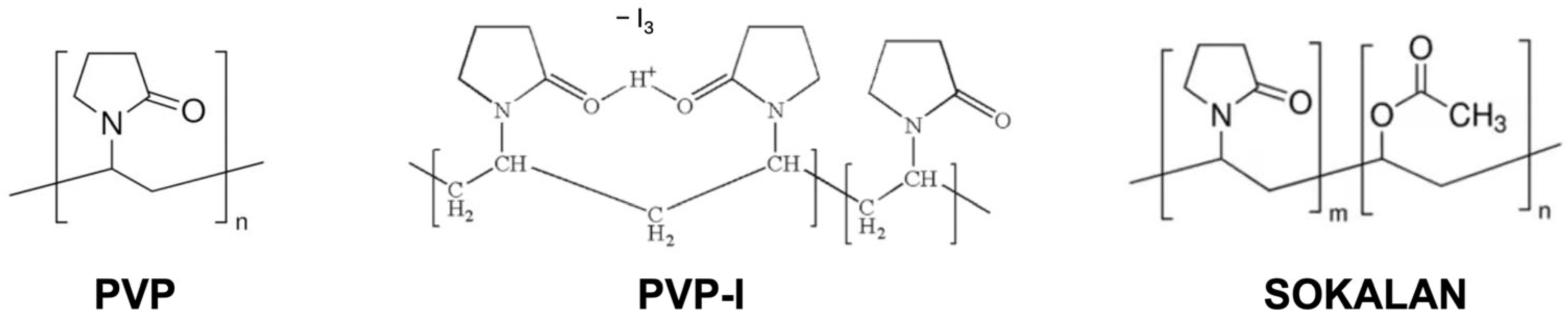
2.1. Materials
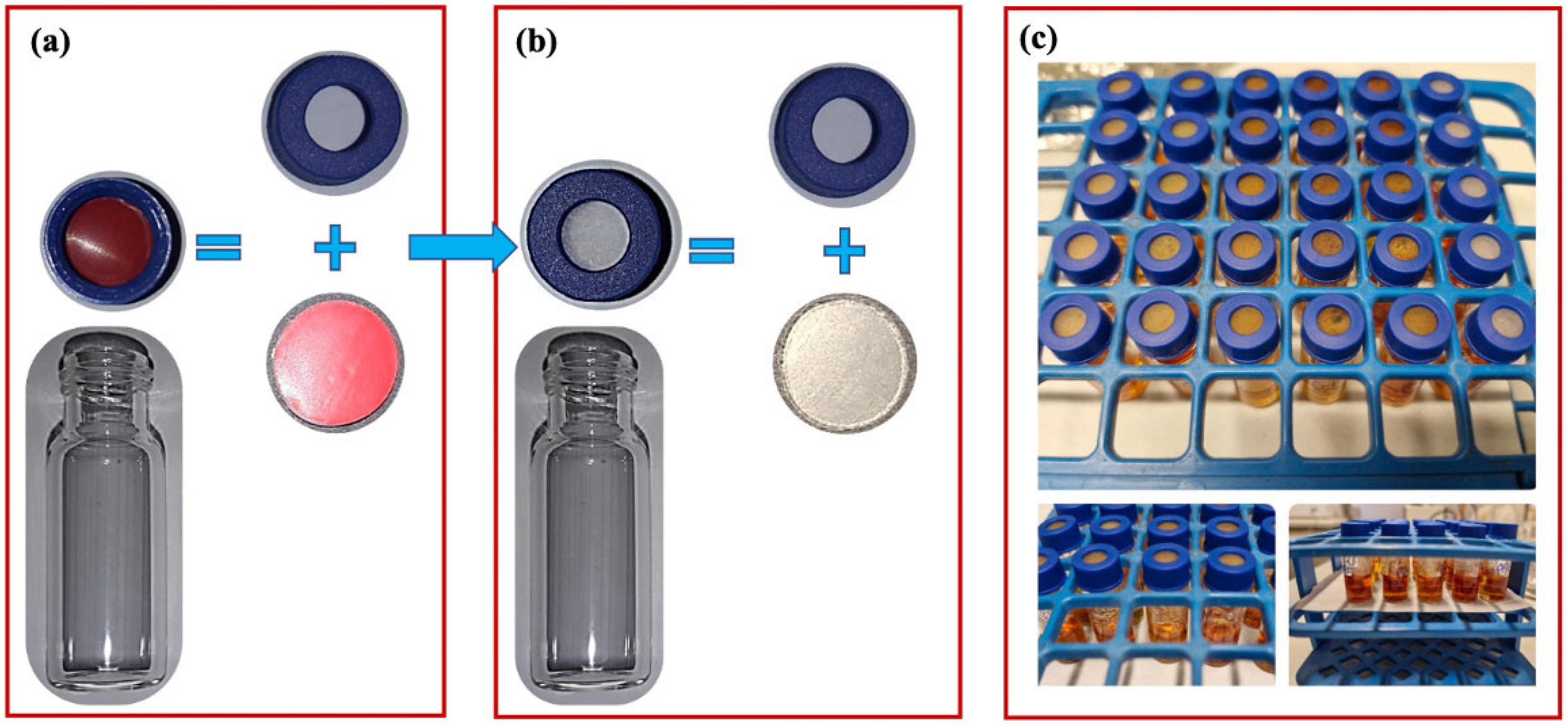
2.2. Sample Preparation
2.3. Characterization Techniques
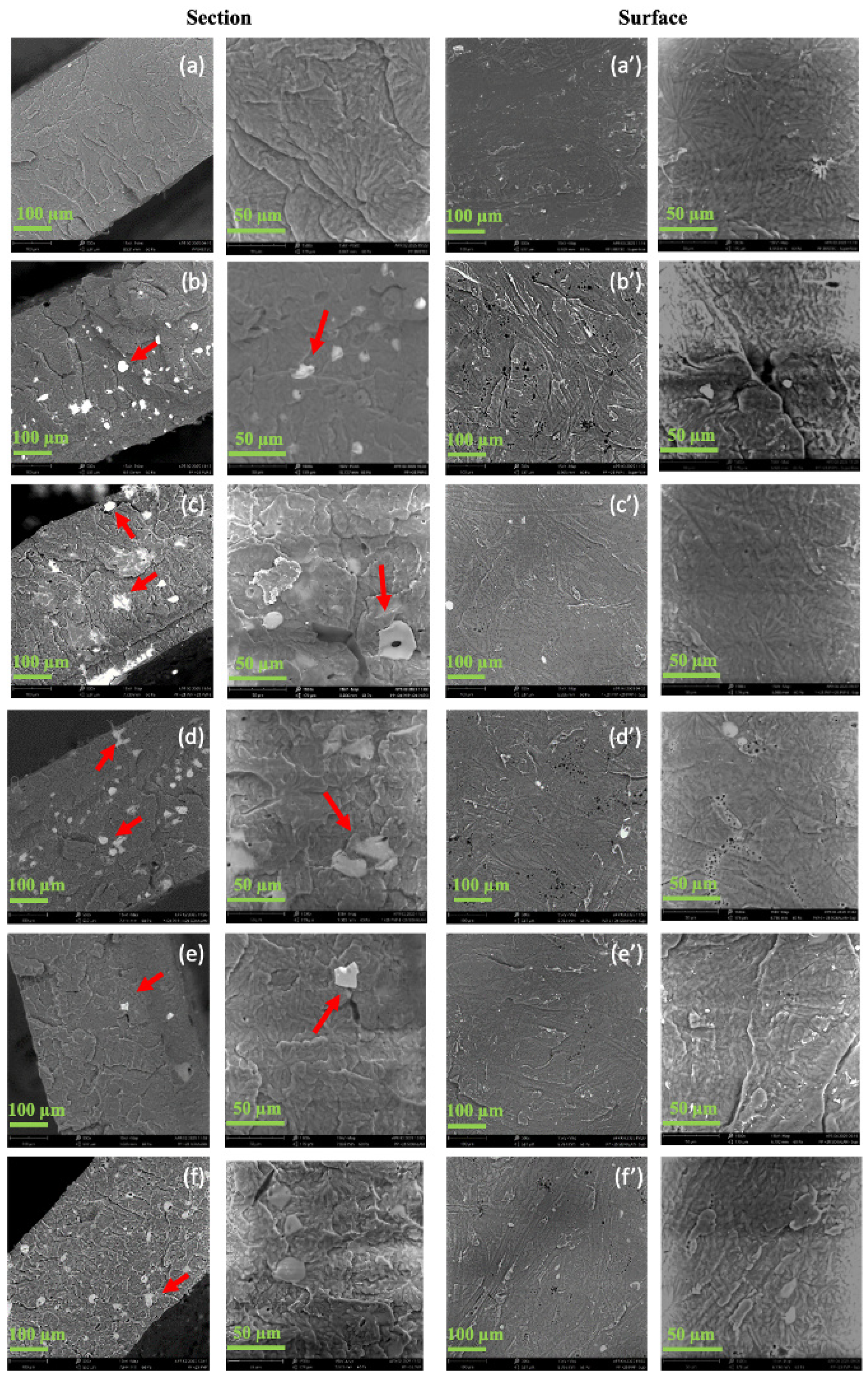
2.3.1. Scanning Electron Microscopy (SEM)
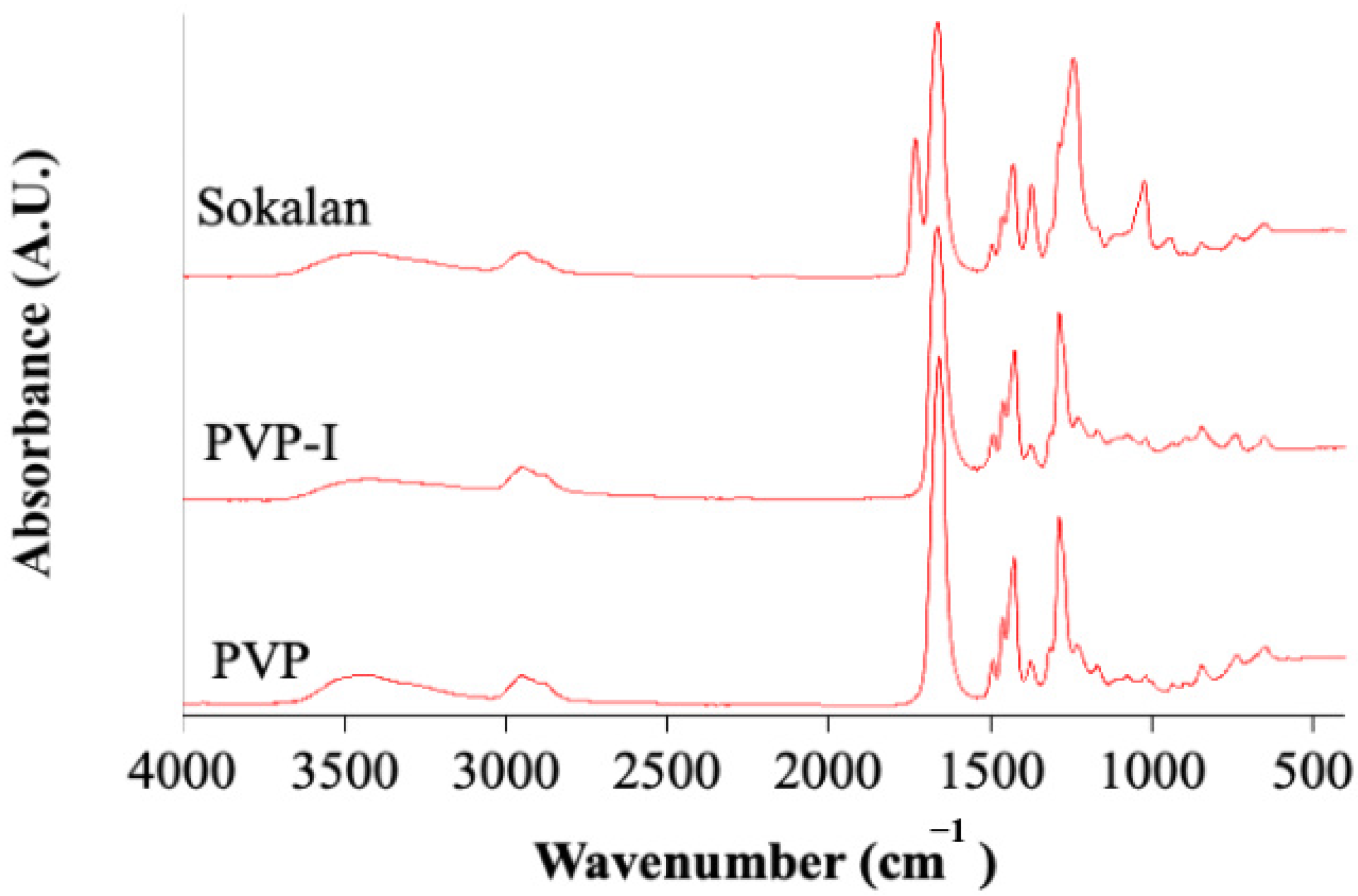
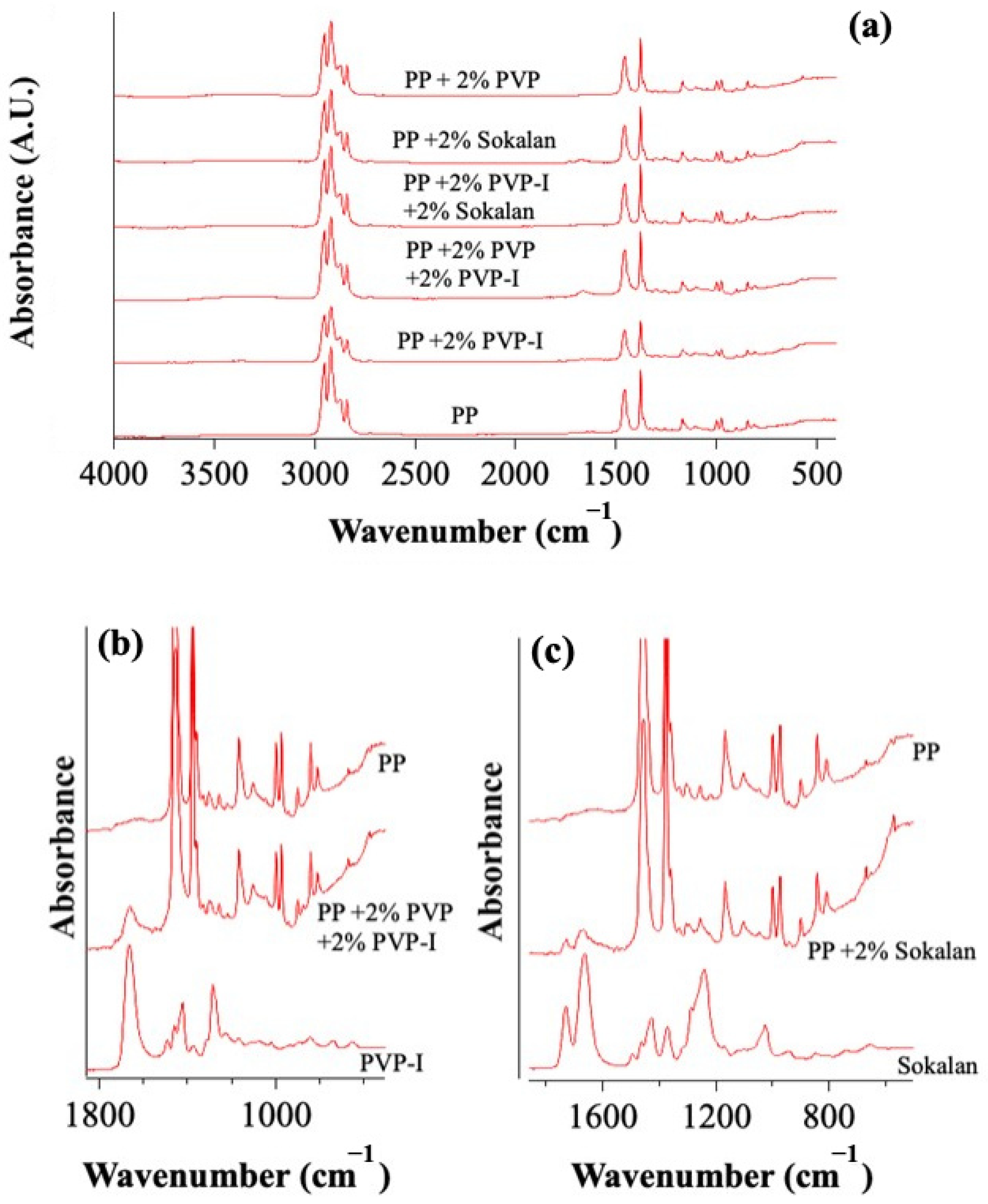
2.3.2. Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) Spectroscopy
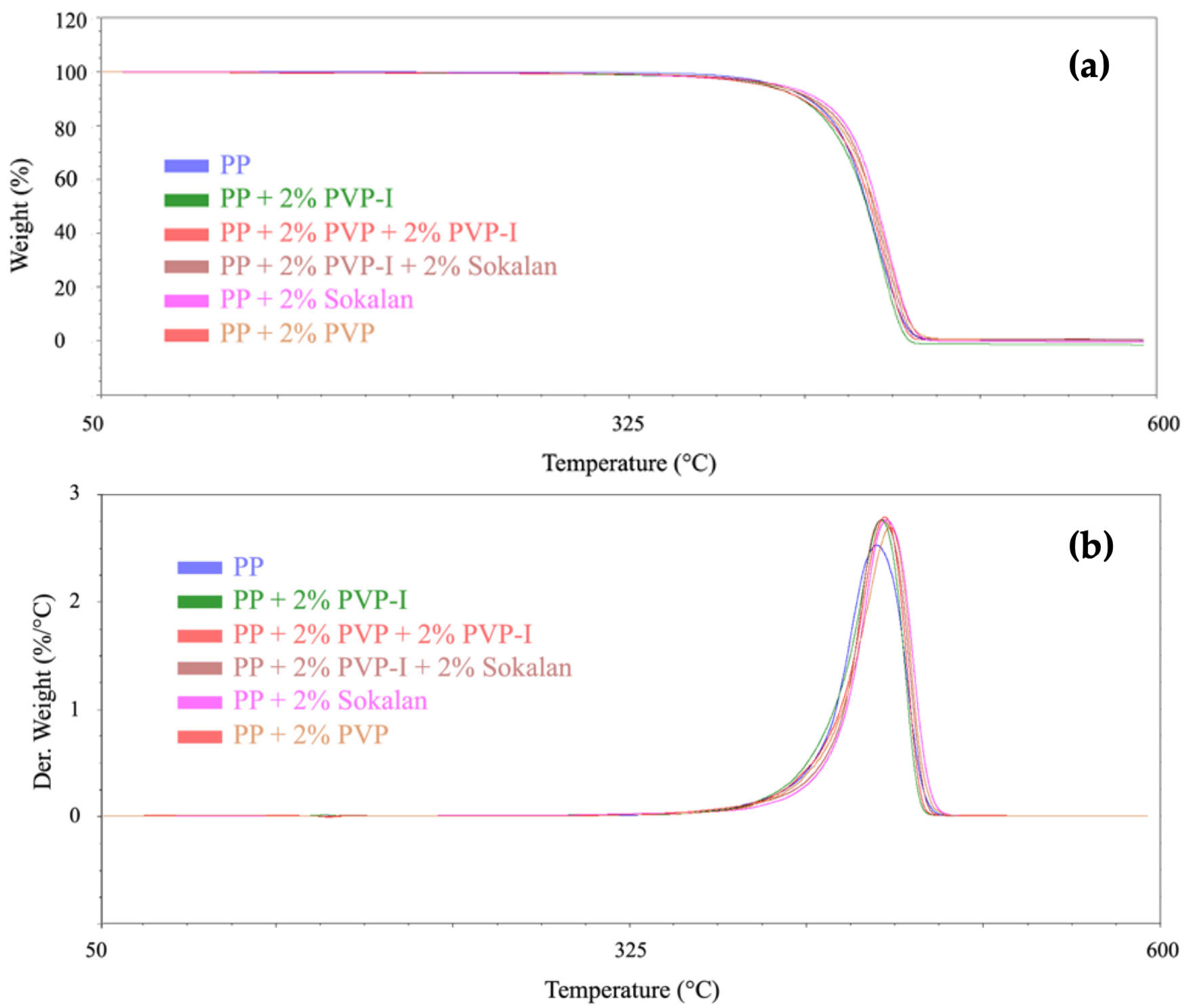
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Mechanical Characterization
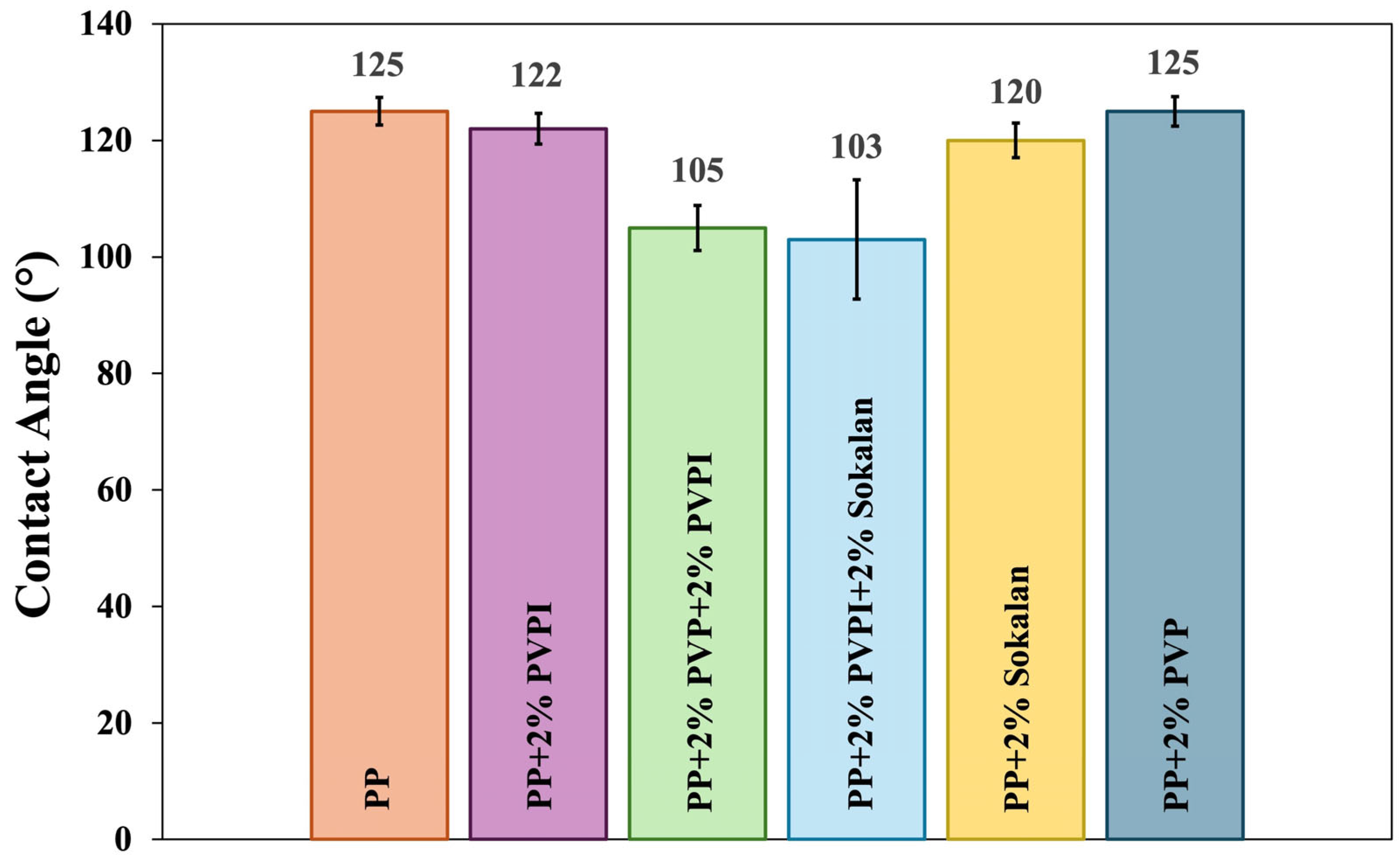
2.3.6. Static Contact Angle (SCA) Measurements
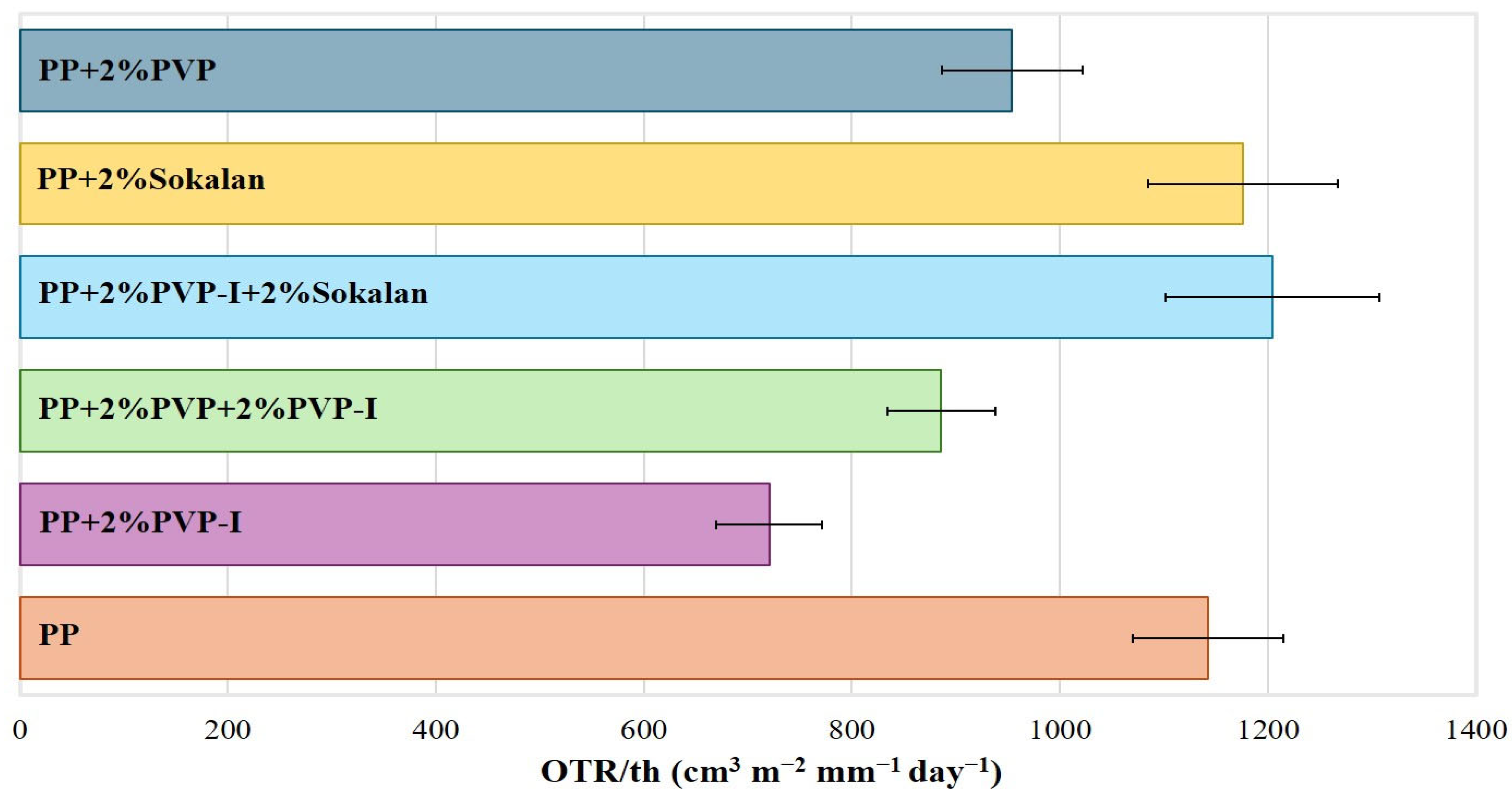
2.3.7. Oxygen Permeability
2.3.8. UV-Vis Spectroscopy
2.3.9. Assessment of Molecular Iodine Leaching
3. Results
3.1. Structural and Morphological Properties
3.2. Thermal Properties
3.3. Mechanical Properties
3.4. Wettability of the Film Surfaces and the SCA
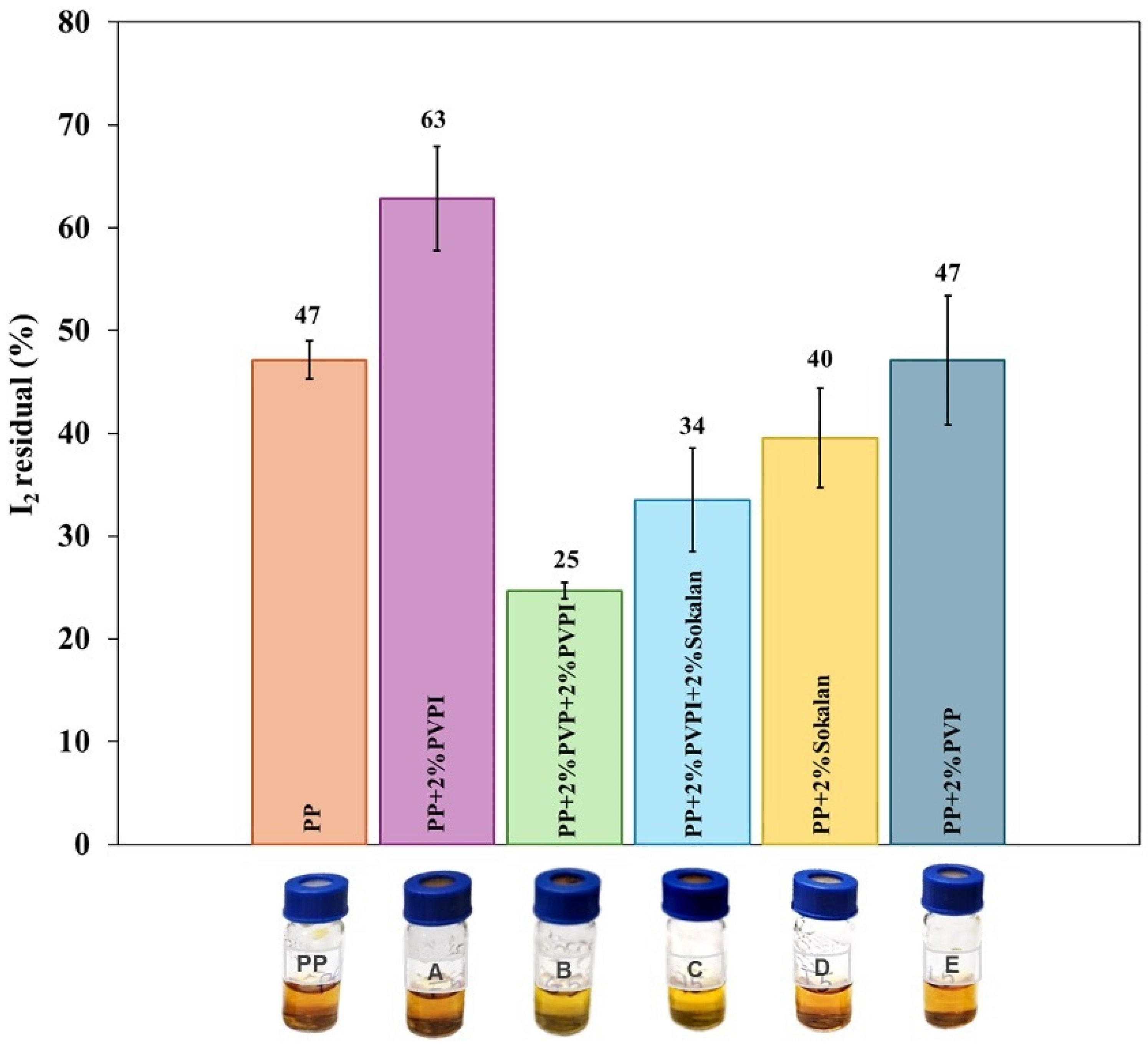
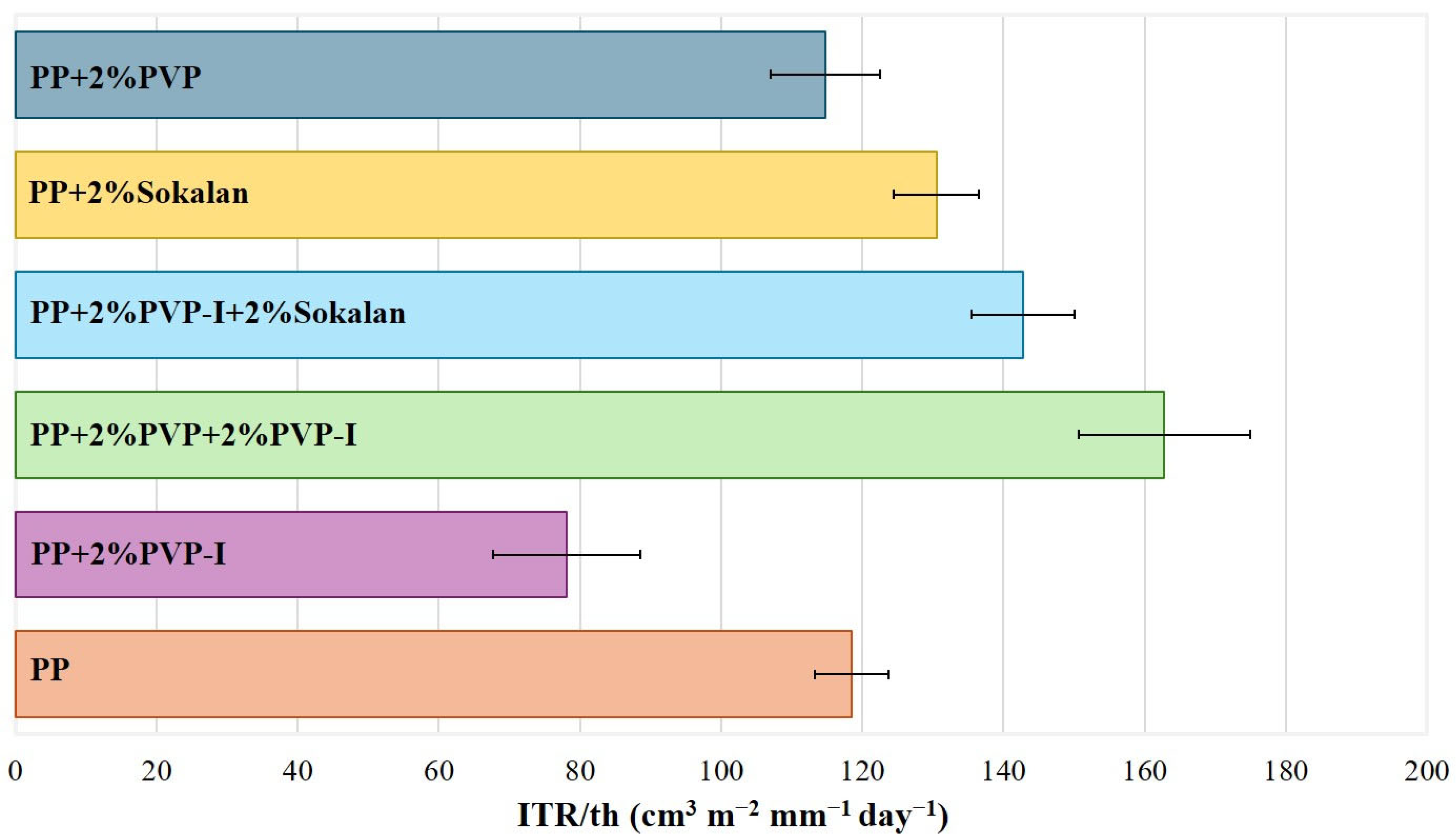
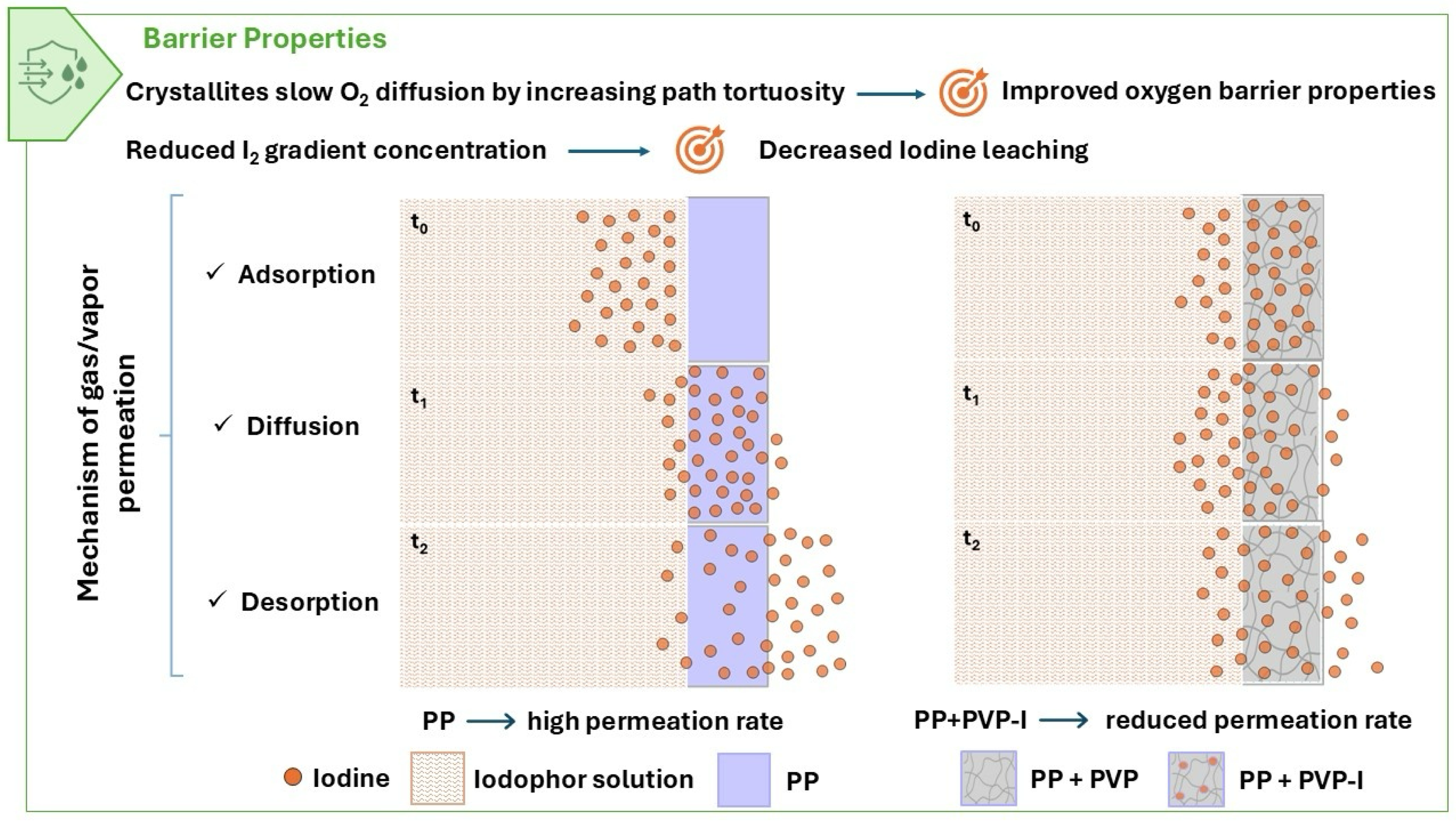
3.5. Barrier Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moulay, S. Molecular iodine/polymer complexes. J. Polym. Eng. 2013, 33, 389–443. [Google Scholar] [CrossRef]
- Makhayeva, D.N.; Irmukhametova, G.S.; Khutoryanskiy, V.V. Polymeric iodophors: Preparation, properties, and biomedical applications. Rev. J. Chem. 2020, 10, 40–57. [Google Scholar] [CrossRef]
- Makhayeva, D.N.; Irmukhametova, G.S.; Khutoryanskiy, V.V. Advances in antimicrobial polymeric iodophors. Eur. Polym. J. 2023, 201, 112573. [Google Scholar] [CrossRef]
- Siggia, S. The chemistry of polyvinylpyrrolidone-iodine. J. Am. Pharm. Assoc. 1957, 46, 201–204. [Google Scholar] [CrossRef]
- Koerner, J.C.; George, M.J.; Meyer, D.R.; Rosco, M.G.; Habib, M.M. Povidone-iodine concentration and dosing in cataract surgery. Surv. Ophthalmol. 2018, 63, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, P.L.; Alsagoff, S.A.L.; El-Kafrawi, H.Y.; Pyon, J.K.; Wa, C.T.C.; Villa, M.A. Povidone iodine in wound healing: A review of current concepts and practices. Int. J. Surg. 2017, 44, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Berkelman, R.L.; Holland, B.W.; Anderson, R.L. Increased bactericidal activity of dilute preparations of povidone-iodine solutions. J. Clin. Microbiol. 1982, 15, 635–639. [Google Scholar] [CrossRef]
- Jenita, J.J.L.; Rathore, S.S.; Manjula, D.; Barnabas, W. Packaging, Container, and Closure of Ophthalmic Products. In Complex Ophthalmic Dosage Forms: Advances in Biomedical Applications and Future Perspectives; Springer: Singapore, 2025; pp. 355–392. [Google Scholar] [CrossRef]
- Bhagwat, D.; Iny, O.; Neck, L.; Pedi, F., Jr. Stabilizing Packaged Iodophor and Minimizing Leaching of Iodine Through Packaging. U.S. Patent 4996048, 26 February 1991. [Google Scholar]
- Bhagwat, D.; Oshlack, B. Stabilized PVP-I Solutions. U.S. Patent 5126127, 30 June 1992. [Google Scholar]
- Liang, B.; Zhang, M. Stable Pharmaceutical Articles Containing Dilute Povidone Iodine Formulations. U.S. Patent 16/962,212, 8 July 2020. [Google Scholar]
- Isenberg, S.J.; Apt, L.; Yoshimori, R.; Pham, C.; Lam, N.K. Efficacy of topical povidone-iodine during the first week after ophthalmic surgery. Am. J. Ophthalmol. 1997, 124, 31–35. [Google Scholar] [CrossRef]
- Ricciardelli, G.; Giannaccare, G.; Di Zazzo, A.; Coassin, M.; Scorcia, V.; Romano, M.R.; Allegrini, D.; Cennamo, M.; Antonini, M.; Bernabei, F.; et al. Efficacy and tolerability of polyvinylpyrrolidone-iodine 0.6% treatment in adenoviral keratoconjunctivitis: A prospective randomized controlled study. Eye 2022, 36, 160–166. [Google Scholar] [CrossRef]
- Amarji, B.; Kulkarni, A.; Deb, P.K.; Maheshwari, R.; Tekade, R.K. Package development of pharmaceutical products: Aspects of packaging materials used for pharmaceutical products. In Dosage Form Design Parameters; Academic Press: Cambridge, MA, USA, 2018; pp. 521–552. [Google Scholar] [CrossRef]
- Ferreri, L.; Rapisarda, M.; Leanza, M.; Munzone, C.; D’Antona, N.; Consoli, G.M.L.; Rizzarelli, P.; Spina, E.T.A. Calix [4]arene derivative for iodine capture and effect on leaching of iodine through packaging. Molecules 2023, 28, 1869. [Google Scholar] [CrossRef]
- Huang, H.-D.; Ren, P.-G.; Zhong, G.-J.; Olah, A.; Li, Z.-M.; Baer, E.; Zhu, L. Promising strategies and new opportunities for high barrier polymer packaging films. Prog. Polym. Sci. 2023, 144, 101722. [Google Scholar] [CrossRef]
- Kilic, A.; Jones, K.; Shim, E.; Pourdeyhimi, B. Surface crystallinity of meltspun isotactic polypropylene filaments. Macromol. Res. 2016, 24, 25–30. [Google Scholar] [CrossRef]
- Lanyi, F.J.; Wenzke, N.; Kaschta, J.; Schubert, D.W. On the determination of the enthalpy of fusion of α-crystalline isotactic polypropylene using differential scanning calorimetry, X-ray diffraction, and Fourier-transform infrared spectroscopy: An old story revisited. Adv. Eng. Mater. 2020, 22, 1900796. [Google Scholar] [CrossRef]
- Virginia, C.; Khasanah, A.; Jauhari, J.; Sriyanti, I. Electrospinning and characterization nanofibers and nanoparticles of polyvinylpyrrolidone. IOP Conf. Ser. Mater. Sci. Eng. 2020, 850, 012039. [Google Scholar] [CrossRef]
- Edikresnha, D.; Sriyanti, I.; Munir, M.M. Synthesis of polyvinylpyrrolidone (PVP)-green tea extract composite nanostructures using electrohydrodynamic spraying technique. IOP Conf. Ser. Mater. Sci. Eng. 2017, 202, 012043. [Google Scholar] [CrossRef]
- Cheng, D.; Xie, R.; Tang, T.; Jia, X.; Cai, Q.; Yang, X. Regulating microstructure and biomineralization of electrospun PVP-based hybridized carbon nanofibers containing bioglass nanoparticles via aging time. RSC Adv. 2016, 6, 3870–3881. [Google Scholar] [CrossRef]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.-J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef]
- Rao, V.; Latha, P.; Ashokan, P.V.; Shridhar, M.H. Thermal degradation of poly(N-vinylpyrrolidone)–poly(vinyl alcohol) blends. Polym. J. 1999, 31, 887–889. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, J.; Zhao, H.; Huang, Z.; Liu, Y.; Fang, M.; Wu, X.; Min, X. Preparation and performance of novel polyvinylpyrrolidone/polyethylene glycol phase change materials composite fibers by centrifugal spinning. Chem. Phys. Lett. 2018, 691, 314–318. [Google Scholar] [CrossRef]
- Wong, A.C.-Y.; Lam, F. Study of selected thermal characteristics of polypropylene/polyethylene binary blends using DSC and TGA. Polym. Test. 2002, 21, 691–696. [Google Scholar] [CrossRef]
- Tariq, A.; Afzal, A.; Rashid, I.A.; Shakir, M.F. Study of thermal, morphological, barrier and viscoelastic properties of PP grafted with maleic anhydride (PP-g-MAH) and PET blends. J. Polym. Res. 2020, 27, 309. [Google Scholar] [CrossRef]
- Simanke, A.G.; de Azeredo, A.P.; de Lemos, C.; Mauler, R.S. Influence of nucleating agent on the crystallization kinetics and morphology of polypropylene. Polímeros 2016, 26, 152–160. [Google Scholar] [CrossRef]
- Lin, J.H.; Pan, Y.J.; Liu, C.F.; Huang, C.L.; Hsieh, C.T.; Chen, C.K.; Lin, Z.I.; Lou, C.W. Preparation and compatibility evaluation of polypropylene/high density polyethylene polyblends. Materials 2015, 8, 8850–8859. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Ceraulo, M.; Giacchi, G.; Mistretta, M.C.; Botta, L. Effect of a compatibilizer on the morphology and properties of polypropylene/polyethylene terephthalate spun fibers. Polymers 2017, 9, 47. [Google Scholar] [CrossRef]
- Salih, S.I.; Jabur, A.R.; Mohammed, T.A. The effect of PVP addition on the mechanical properties of ternary polymer blends. IOP Conf. Ser. Mater. Sci. Eng. 2018, 433, 012071. [Google Scholar] [CrossRef]
- Halimatudahliana, H.; Ismail, M.N. The effect of various compatibilizers on mechanical properties of polystyrene/polypropylene blend. Polym. Test. 2002, 21, 163–170. [Google Scholar] [CrossRef]
- Huhtamäki, T.; Tian, X.; Korhonen, J.T.; Ras, R.H.A. Surface-wetting characterization using contact-angle measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef]
- Mittal, V. Crystallinity, mechanical property and oxygen permeability of polypropylene: Effect of processing conditions, nucleating agent and compatibilizer. J. Thermoplast. Compos. Mater. 2012, 26, 1407–1423. [Google Scholar] [CrossRef]
- Drieskens, M.; Peeters, R.; Mullens, J.; Franco, D.; Lemstra, P.J.; Hristova-Bogaerds, D.G. Structure versus properties relationship of poly(lactic acid). I. Effect of crystallinity on barrier properties. J. Polym. Sci. B Polym. Phys. 2009, 47, 2247–2258. [Google Scholar] [CrossRef]
- Khalil, A.M.; El-Sayed, S.M.; Youssef, A.M. Valorization of polylactic acid bionanocomposites enriched with CuO–TiO2 for packaging applications. Biomass Convers. Biorefin. 2025, 15, 3485–3494. [Google Scholar] [CrossRef]
- Shiva, K.; Soleimani, A.; Morshedian, J.; Farahmandghavi, F.; Shokrolahi, F. Improving the antibacterial properties of polyethylene food packaging films with Ajwain essential oil adsorbed on chitosan particles. Sci. Rep. 2024, 14, 28802. [Google Scholar] [CrossRef]
- Rogers, C.E. Permeation of gases and vapours in polymers. In Polymer Permeability; Comyn, J., Ed.; Springer: Dordrecht, The Netherlands, 1985; pp. 11–73. [Google Scholar] [CrossRef]
- Boydağ, F.S.; Mamedov, S.; Alekperov, V.A.; Özcanli, Y.L. Optical characterization of weakly absorbing PP, PE, and PP/PE films. Opt. Spectrosc. 2003, 95, 225–229. [Google Scholar] [CrossRef]
| Abbreviation | Composition |
|---|---|
| PP | Polypropylene |
| PP + 2% PVP-I | 98% polypropylene + 2% povidone-iodine |
| PP + 2% PVP-I + 2% PVP | 96% polypropylene + 2% povidone-iodine + 2% poly(N-vinylpyrrolidone) |
| PP + 2% PVP-I + 2% Sokalan | 96% polypropylene + 2% povidone-iodine + 2% vinyl pyrrolidone vinyl acetate 55/45 |
| PP + 2% Sokalan | 98% polypropylene + 2% vinyl pyrrolidone vinyl acetate 55/45 |
| PP + 2% PVP | 98% polypropylene + 2% poly(N-vinylpyrrolidone) |
| Sample | WL50°C−250°C (%) | WL250°C−550°C (%) | Tp (°C) * | Tm (°C) * |
|---|---|---|---|---|
| PP | 0.1 | 99.5 | 453 | 170 |
| PP + 2% PVP-I | 0.6 | 100.0 | 455 | 168 |
| PP + 2% PVP + 2% PVP-I | 0.6 | 99.0 | 457 | 167 |
| PP + 2% PVP-I + 2% Sokalan | 0.4 | 99.0 | 456 | 168 |
| PP + 2% Sokalan | 0.4 | 100.0 | 458 | 168 |
| PP + 2% PVP | 0.5 | 99.0 | 460 | 169 |
| First Heating Ramp | |||
|---|---|---|---|
| Sample | Tm (°C) * | ∆Hm (J/g) | Xc (%) |
| PP | 168 | 116 | 56 |
| PP + 2% PVP-I | 166 | 124 | 60 |
| PP + 2% PVP + 2% PVP-I | 165 | 123 | 59 |
| PP + 2% PVP-I + 2% Sokalan | 168 | 122 | 59 |
| PP + 2% Sokalan | 166 | 127 | 61 |
| PP + 2% PVP | 167 | 132 | 64 |
| Second Heating Ramp | |||
| Sample | Tm (°C) * | ∆Hm (J/g) | Xc (%) |
| PP | 167 | 114 | 55 |
| PP + 2% PVP-I | 168 | 119 | 58 |
| PP + 2% PVP + 2% PVP-I | 167 | 120 | 58 |
| PP + 2% PVP-I + 2% Sokalan | 167 | 120 | 58 |
| PP + 2% Sokalan | 166 | 119 | 58 |
| PP + 2% PVP | 166 | 120 | 58 |
| Sample | E [MPa] | TS [MPa] | EB [%] |
|---|---|---|---|
| PP | 950.0 ± 16.0 | 28.6 ± 1.3 | 7.8 ± 0.8 |
| PP + 2% PVP-I | 980.3 ± 46.6 | 25.8 ± 0.8 | 7.2 ± 0.6 |
| PP + 2% PVP + 2% PVP-I | 1008.7 ± 24.5 | 24.8 ± 1.1 | 6.0 ± 0.6 |
| PP + 2% PVP-I + 2% Sokalan | 1050.4 ± 33.7 | 25.7 ± 1.7 | 6.3 ± 0.6 |
| PP + 2% Sokalan | 1042.4 ± 69.3 | 26.0 ± 1.8 | 6.8 ± 0.6 |
| PP + 2% PVP | 988.6 ± 28.5 | 25.4 ± 2.5 | 7.2 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leanza, M.; Carbone, D.C.; Poggi, G.; Rapisarda, M.; Baiamonte, M.; Spina, E.T.A.; Chelazzi, D.; Baglioni, P.; La Mantia, F.P.; Rizzarelli, P. PP-Based Blends with PVP-I Additive: Mechanical, Thermal, and Barrier Properties for Packaging of Iodophor Pharmaceutical Formulations. Polymers 2025, 17, 2442. https://doi.org/10.3390/polym17182442
Leanza M, Carbone DC, Poggi G, Rapisarda M, Baiamonte M, Spina ETA, Chelazzi D, Baglioni P, La Mantia FP, Rizzarelli P. PP-Based Blends with PVP-I Additive: Mechanical, Thermal, and Barrier Properties for Packaging of Iodophor Pharmaceutical Formulations. Polymers. 2025; 17(18):2442. https://doi.org/10.3390/polym17182442
Chicago/Turabian StyleLeanza, Melania, Domenico Carmelo Carbone, Giovanna Poggi, Marco Rapisarda, Marilena Baiamonte, Emanuela Teresa Agata Spina, David Chelazzi, Piero Baglioni, Francesco Paolo La Mantia, and Paola Rizzarelli. 2025. "PP-Based Blends with PVP-I Additive: Mechanical, Thermal, and Barrier Properties for Packaging of Iodophor Pharmaceutical Formulations" Polymers 17, no. 18: 2442. https://doi.org/10.3390/polym17182442
APA StyleLeanza, M., Carbone, D. C., Poggi, G., Rapisarda, M., Baiamonte, M., Spina, E. T. A., Chelazzi, D., Baglioni, P., La Mantia, F. P., & Rizzarelli, P. (2025). PP-Based Blends with PVP-I Additive: Mechanical, Thermal, and Barrier Properties for Packaging of Iodophor Pharmaceutical Formulations. Polymers, 17(18), 2442. https://doi.org/10.3390/polym17182442









