Methylcellulose-Encapsulated Magnesium-Substituted Biphasic Calcium Phosphate Granules for Local Drug Delivery in Bone Tissue Engineering: Modification for Prolonged Release and Antibacterial Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Magnesium-Substituted Non-Stoichiometric Hydroxyapatite
2.3. Formation of BCP Granules and Loading of Model Antimicrobial Agent
2.4. Physical and Chemical Characterization
2.5. Antimicrobial Tests
2.6. Statistical Analysis
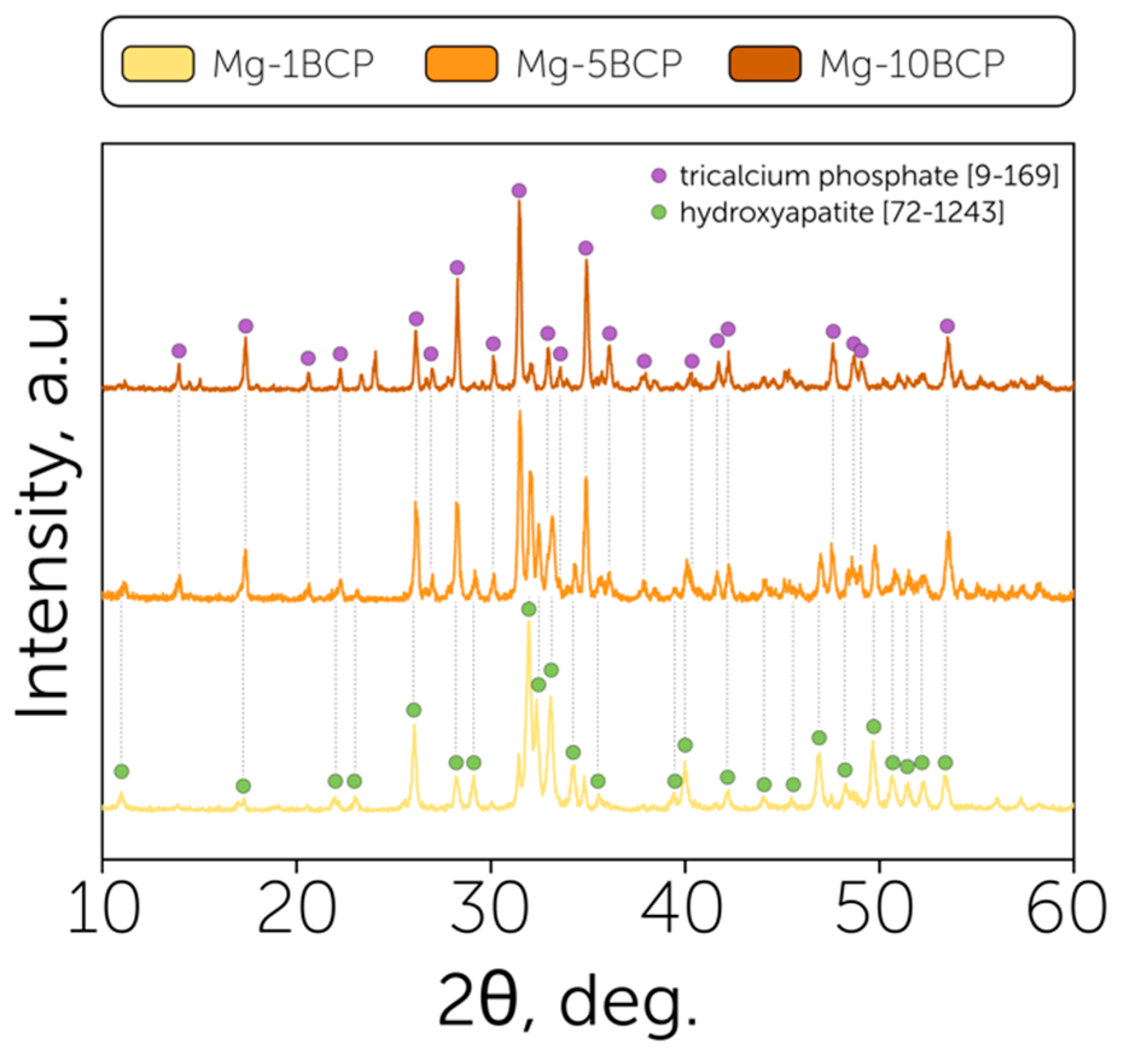
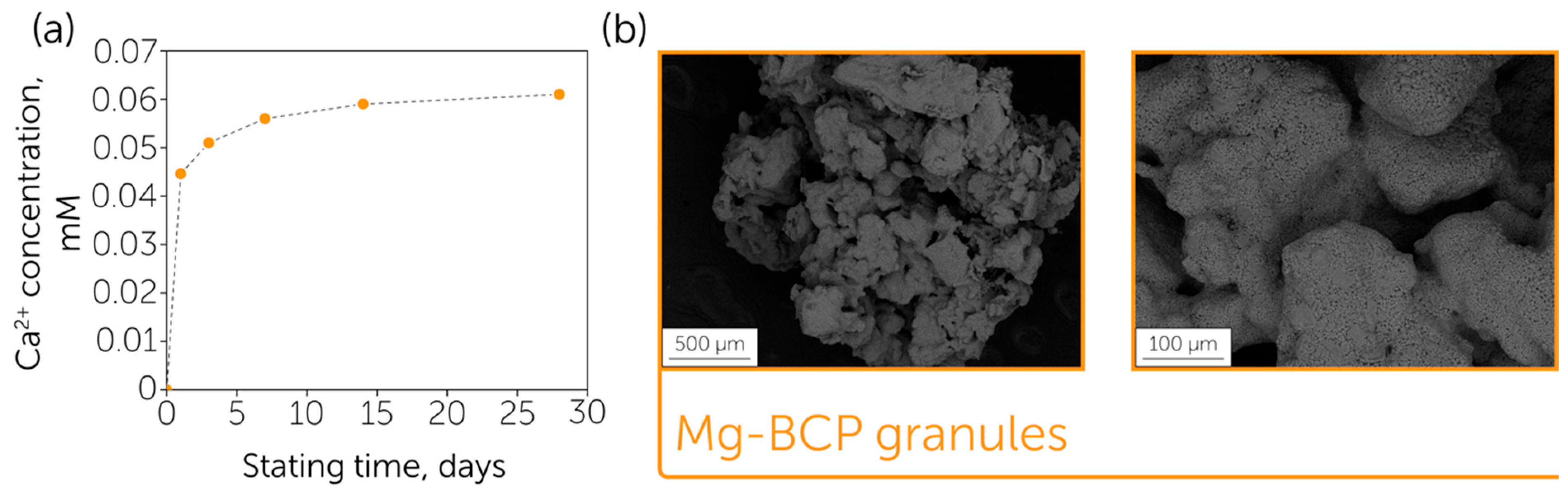
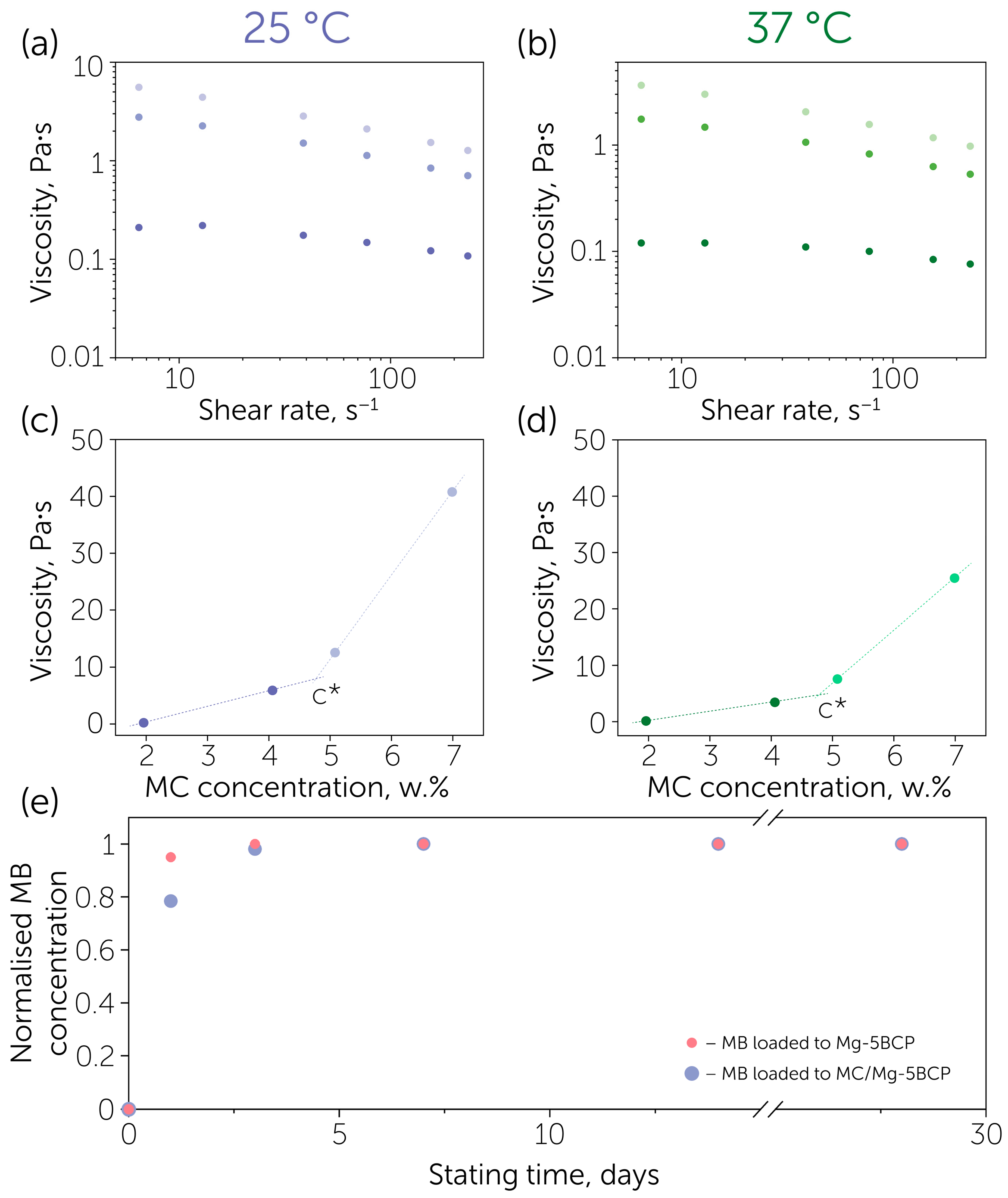
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Mg-BCP | Magnesium-substituted biphasic calcium phosphate |
| HAp | Hydroxyapatite |
| β-TCP | β-tricalcium phosphate |
| BCP | Biphasic calcium phosphate |
| MgHAp | Magnesium-substituted non-stoichiometric hydroxyapatite |
| SA | Sodium alginate |
| XRD | X-ray diffraction |
| MB | Methylene blue |
| MC | Methylcellulose |
References
- Awad, K.; Ahuja, N.; Yacoub, A.S.; Brotto, L.; Young, S.; Mikos, A.; Aswath, P.; Varanasi, V. Revolutionizing Bone Regeneration: Advanced Biomaterials for Healing Compromised Bone Defects. Front. Aging 2023, 4, 1217054. [Google Scholar] [CrossRef] [PubMed]
- Keating, J.F.; Simpson, A.H.R.W.; Robinson, C.M. The Management of Fractures with Bone Loss. J. Bone Joint Surg. Br. 2005, 87-B, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-Y.; Bi, Q.; Zhao, C.; Chen, J.-Y.; Cai, M.-H.; Chen, X.-Y. Recent Advances in Biomaterials for the Treatment of Bone Defects. Organogenesis 2020, 16, 113–125. [Google Scholar] [CrossRef]
- Darghiasi, S.F.; Farazin, A.; Ghazali, H.S. Design of Bone Scaffolds with Calcium Phosphate and Its Derivatives by 3D Printing: A Review. J. Mech. Behav. Biomed. Mater. 2024, 151, 106391. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Li, C. Advances in 3D Printing Technology for Preparing Bone Tissue Engineering Scaffolds from Biodegradable Materials. Front. Bioeng. Biotechnol. 2024, 12, 1483547. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Q.; Liu, S.; Liu, L.; He, Z.; Liu, Y.; Sun, Y.; Liu, X.; Luo, E. The Role of Integrin Avβ3 in Biphasic Calcium Phosphate Ceramics Mediated M2 Macrophage Polarization and the Resultant Osteoinduction. Biomaterials 2024, 304, 122406. [Google Scholar] [CrossRef]
- Sugai, Y.; Hamai, R.; Shiwaku, Y.; Anada, T.; Tsuchiya, K.; Kimura, T.; Tadano, M.; Yamauchi, K.; Takahashi, T.; Egusa, H.; et al. Effect of Octacalcium Phosphate on Osteogenic Differentiation of Induced Pluripotent Stem Cells in a 3D Hybrid Spheroid Culture. Biomimetics 2025, 10, 205. [Google Scholar] [CrossRef]
- Jolic, M.; Åberg, I.; Omar, O.; Engqvist, H.; Engstrand, T.; Palmquist, A.; Thomsen, P.; Shah, F.A. Pyrophosphate-Containing Calcium Phosphates Negatively Impact Heterotopic Bone Quality. Adv. Healthc. Mater. 2025, 14, e2405171. [Google Scholar] [CrossRef]
- Tran, D.L.; Ta, Q.T.H.; Tran, M.H.; Nguyen, T.M.H.; Le, N.T.T.; Nguyen Hong, A.P.; Park, H.-J.; Park, K.D.; Nguyen, D.H. Optimized Synthesis of Biphasic Calcium Phosphate: Enhancing Bone Regeneration with a Tailored β-Tricalcium Phosphate/Hydroxyapatite Ratio. Biomater. Sci. 2025, 13, 969–979. [Google Scholar] [CrossRef]
- Kazakova, G.; Safronova, T.; Golubchikov, D.; Shevtsova, O.; Rau, J.V. Resorbable Mg2+-Containing Phosphates for Bone Tissue Repair. Materials 2021, 14, 4857. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Shin, K.-K.; Jung, J.S.; Chun, H.H.; Park, S.S.; Lee, J.K.; Park, H.-C.; Yoon, S.-Y. The Role of Magnesium Ion Substituted Biphasic Calcium Phosphate Spherical Micro-Scaffolds in Osteogenic Differentiation of Human Adipose Tissue-Derived Mesenchymal Stem Cells. J. Nanosci. Nanotechnol. 2015, 15, 5520–5523. [Google Scholar] [CrossRef]
- Wang, Y.; Geng, Z.; Huang, Y.; Jia, Z.; Cui, Z.; Li, Z.; Wu, S.; Liang, Y.; Zhu, S.; Yang, X.; et al. Unraveling the Osteogenesis of Magnesium by the Activity of Osteoblasts in Vitro. J. Mater. Chem. B 2018, 6, 6615–6621. [Google Scholar] [CrossRef]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. Applications of Metals for Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi Birgani, Z.; Fennema, E.; Gijbels, M.J.; de Boer, J.; van Blitterswijk, C.A.; Habibovic, P. Stimulatory Effect of Cobalt Ions Incorporated into Calcium Phosphate Coatings on Neovascularization in an in Vivo Intramuscular Model in Goats. Acta Biomater. 2016, 36, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of Orthopedic Implants with Emphasis on Bacterial Adhesion Process and Techniques Used in Studying Bacterial-Material Interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef]
- Niazvand, F.; Sharifianjazi, F.; Esmaeilkhanian, A.; Ahmadi, E.; Moradigharibvand, N.; Rabiee, N.; Seifalian, A.; Ghiasvand, A.; Hojjati, M. Sol-Gel Derived Bioactive Glasses Containing Boron and Strontium: Bioactivity, Biocompatibility, and Antibacterial Properties. J. Non. Cryst. Solids 2024, 631, 122909. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Lebedev, V.N.; Barbaro, K.; Titkov, V.V.; Lazoryak, B.I.; Fadeeva, I.V.; Gosteva, A.N.; Udyanskaya, I.L.; Aksenov, S.M.; Rau, J.V. Antimicrobial and Cell-Friendly Properties of Cobalt and Nickel-Doped Tricalcium Phosphate Ceramics. Biomimetics 2023, 9, 14. [Google Scholar] [CrossRef]
- Nicácio, T.C.N.; Castro, M.A.M.; Melo, M.C.N.; Silva, T.A.; Teodoro, M.D.; Bomio, M.R.D.; Motta, F.V. Zn and Ni Doped Hydroxyapatite: Study of the Influence of the Type of Energy Source on the Photocatalytic Activity and Antimicrobial Properties. Ceram. Int. 2024, 50, 27540–27552. [Google Scholar] [CrossRef]
- Golubchikov, D.O.; Fadeeva, I.V.; Knot’ko, A.V.; Kostykov, I.A.; Slonskaya, T.K.; Barbaro, K.; Zepparoni, A.; Fosca, M.; Antoniac, I.V.; Rau, J.V. Mechanochemically-Activated Solid-State Synthesis of Borate-Substituted Tricalcium Phosphate: Evaluation of Biocompatibility and Antimicrobial Performance. Molecules 2025, 30, 1575. [Google Scholar] [CrossRef]
- Pinchuk, N.D.; Piecuch, A.; Charczuk, N.; Sobierajska, P.; Targonska, S.; Bezkrovnyi, O.; Ogórek, R.; Wang, Y.; Wiglusz, R.J. Effect of Silver Ion and Silicate Group on the Antibacterial and Antifungal Properties of Nanosized Hydroxyapatite. Sci. Rep. 2024, 14, 29339. [Google Scholar] [CrossRef]
- Baradaran, S.; Moghaddam, E.; Nasiri-Tabrizi, B.; Basirun, W.J.; Mehrali, M.; Sookhakian, M.; Hamdi, M.; Alias, Y. Characterization of Nickel-Doped Biphasic Calcium Phosphate/Graphene Nanoplatelet Composites for Biomedical Application. Mater. Sci. Eng. C 2015, 49, 656–668. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolanthai, E.; Nivethaa, E.A.K.; Pandian, M.S.; Ramasamy, P.; Catalani, L.H.; Kalkura, S.N. Enhanced in Vitro Inhibition of MCF-7 and Magnetic Properties of Cobalt Incorporated Calcium Phosphate (HAp and β-TCP) Nanoparticles. Ceram. Int. 2023, 49, 855–861. [Google Scholar] [CrossRef]
- Fu, L.-H.; Hu, Y.-R.; Qi, C.; He, T.; Jiang, S.; Jiang, C.; He, J.; Qu, J.; Lin, J.; Huang, P. Biodegradable Manganese-Doped Calcium Phosphate Nanotheranostics for Traceable Cascade Reaction-Enhanced Anti-Tumor Therapy. ACS Nano. 2019, 13, 13985–13994. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, Z.; Liu, C.; Chen, X.; Cao, X. High Performance Injectable Mg Doped Bioactive Glass Bone Cement for the Regulation of Osteogenic Immune Microenvironment. Biomater. Adv. 2024, 160, 213864. [Google Scholar] [CrossRef]
- Kolmas, J.; Romaniuk, P.; Predoi, D.; Drobniewska, A.; Burdan, K.; Kołodziejska, B. Magnesium Ion Substitution in Various Calcium Phosphates: A Way towards Bone Regeneration. Ceram. Int. 2025, 51, 1153–1160. [Google Scholar] [CrossRef]
- Uskoković, V.; Batarni, S.S.; Schweicher, J.; King, A.; Desai, T.A. Effect of Calcium Phosphate Particle Shape and Size on Their Antibacterial and Osteogenic Activity in the Delivery of Antibiotics in Vitro. ACS Appl. Mater. Interfaces 2013, 5, 2422–2431. [Google Scholar] [CrossRef]
- Zhao, X.; Guan, J.; Zhang, Y.; Huang, Q.; Guo, Y.; Yi, S. In Situ Magnesium-Doped Calcium Phosphate Microtube-Reinforced Magnesium-Doped Hydroxyapatite Composites with Tunable Mechanical Properties and Biodegradability. J. Eur. Ceram. Soc. 2024, 44, 7383–7397. [Google Scholar] [CrossRef]
- Ma, J.; Wang, C.Z.; Huang, B.X.; Zhao, X.C.; Chen, C.Z.; Yu, H.J. In Vitro Degradation and Apatite Formation of Magnesium and Zinc Incorporated Calcium Silicate Prepared by Sol-Gel Method. Mater. Technol. 2021, 36, 420–429. [Google Scholar] [CrossRef]
- Griesiute, D.; Garskaite, E.; Antuzevics, A.; Klimavicius, V.; Balevicius, V.; Zarkov, A.; Katelnikovas, A.; Sandberg, D.; Kareiva, A. Synthesis, Structural and Luminescent Properties of Mn-Doped Calcium Pyrophosphate (Ca2P2O7) Polymorphs. Sci. Rep. 2022, 12, 7116. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.K.; Hossain, M.S.; Akash, M.H.; Bashar, M.S.; Bahadur, N.M.; Ahmed, S. Synthesis of Pure and Doped Nano-Calcium Phosphates Using Different Conventional Methods for Biomedical Applications: A Review. J. Mater. Chem. B 2024, 12, 3376–3391. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-C.; Zhang, M.-L.; Lee, J.-S.; Jung, U.-W.; Choi, S.-H. Effect of Different Hydroxyapatite:β-Tricalcium Phosphate Ratios on the Osteoconductivity of Biphasic Calcium Phosphate in the Rabbit Sinus Model. Int. J. Oral Maxillofac. Implant. 2015, 30, 65–72. [Google Scholar] [CrossRef]
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic Calcium Phosphate Ceramics for Bone Reconstruction: A Review of Biological Response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef]
- Champion, E. Sintering of Calcium Phosphate Bioceramics. Acta Biomater. 2013, 9, 5855–5875. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Trofimchuk, E.S. Method for Producing Porous Granules from Calcium Phosphates. Patent RU 2833401 C1, 21 January 2025. [Google Scholar]
- Zhang, L.; Lu, T.; He, F.; Zhang, W.; Yuan, X.; Wang, X.; Ye, J. Physicochemical and Cytological Properties of Poorly Crystalline Calcium-Deficient Hydroxyapatite with Different Ca/P Ratios. Ceram. Int. 2022, 48, 24765–24776. [Google Scholar] [CrossRef]
- Zuev, D.M.; Golubchikov, D.O.; Evdokimov, P.V.; Putlyaev, V.I. Synthesis of Amorphous Calcium Phosphate Powders for Production of Bioceramics and Composites by 3D Printing. Russ. J. Inorg. Chem. 2022, 67, 940–951. [Google Scholar] [CrossRef]
- Rey, C.; Marsan, O.; Combes, C.; Drouet, C.; Grossin, D.; Sarda, S. Characterization of Calcium Phosphates Using Vibrational Spectroscopies. In Advances in Calcium Phosphate Biomaterials; Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2014; pp. 229–266. [Google Scholar]
- Lucio, M.D.S.; Oh, E.-J.; Ha, J.-H.; Lee, J.; Lee, H.-J.; Jung, S.H.; Shin, J.Y.; Song, I.-H. Manufacturing and Properties Evaluation of Al2O3/ZrO2 Granules Derived from Sodium Alginate Gelation. J. Korean Ceram. Soc. 2024, 61, 673–692. [Google Scholar] [CrossRef]
- Bodvik, R.; Dedinaite, A.; Karlson, L.; Bergström, M.; Bäverbäck, P.; Pedersen, J.S.; Edwards, K.; Karlsson, G.; Varga, I.; Claesson, P.M. Aggregation and Network Formation of Aqueous Methylcellulose and Hydroxypropylmethylcellulose Solutions. Colloids Surf. A Physicochem. Eng. Asp. 2010, 354, 162–171. [Google Scholar] [CrossRef]
- Kol, R.; Nachtergaele, P.; De Somer, T.; D’hooge, D.R.; Achilias, D.S.; De Meester, S. Toward More Universal Prediction of Polymer Solution Viscosity for Solvent-Based Recycling. Ind. Eng. Chem. Res. 2022, 61, 10999–11011. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Guo, H.; Lan, T.; Chen, Y.; Chen, X.; Feng, Y.; Luo, Y.; Yao, Y.; Li, Y.; Pan, X.; et al. Co-Delivery of Antibiotic and Baicalein by Using Different Polymeric Nanoparticle Cargos with Enhanced Synergistic Antibacterial Activity. Int. J. Pharm. 2021, 599, 120419. [Google Scholar] [CrossRef] [PubMed]
- Lukina, Y.; Kotov, S.; Bionyshev-Abramov, L.; Serejnikova, N.; Chelmodeev, R.; Fadeev, R.; Toshev, O.; Tavtorkin, A.; Ryndyk, M.; Smolentsev, D.; et al. Low-Temperature Magnesium Calcium Phosphate Ceramics with Adjustable Resorption Rate. Ceramics 2023, 6, 168–194. [Google Scholar] [CrossRef]
- Nabiyouni, M.; Ren, Y.; Bhaduri, S.B. Magnesium Substitution in the Structure of Orthopedic Nanoparticles: A Comparison between Amorphous Magnesium Phosphates, Calcium Magnesium Phosphates, and Hydroxyapatites. Mater. Sci. Eng. C 2015, 52, 11–17. [Google Scholar] [CrossRef]
- Sinulingga, K.; Sirait, M.; Siregar, N.; Abdullah, H. Synthesis and Characterizations of Natural Limestone-Derived Nano-Hydroxyapatite (HAp): A Comparison Study of Different Metals Doped HAps on Antibacterial Activity. RSC Adv. 2021, 11, 15896–15904. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Stan, G.E.; Buton, N. Synthesis, Characterization, and Antimicrobial Activity of Magnesium-Doped Hydroxyapatite Suspensions. Nanomaterials 2019, 9, 1295. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.C.; Araújo, R.; Quadros, P.A.; Sousa, S.R.; Monteiro, F.J. Antibacterial Bone Substitute of Hydroxyapatite and Magnesium Oxide to Prevent Dental and Orthopaedic Infections. Mater. Sci. Eng. C 2019, 97, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Megha, M.; Joy, A.; Unnikrishnan, G.; Haris, M.; Thomas, J.; Deepti, A.; Chakrapani, P.S.B.; Kolanthai, E.; Muthuswamy, S. Structural and Biological Properties of Novel Vanadium and Strontium Co-Doped HAp for Tissue Engineering Applications. Ceram. Int. 2023, 49, 30156–30169. [Google Scholar] [CrossRef]
- Veljovic, D.; Matic, T.; Stamenic, T.; Kojic, V.; Dimitrijevic-Brankovic, S.; Lukic, M.J.; Jevtic, S.; Radovanovic, Z.; Petrovic, R.; Janackovic, D. Mg/Cu Co-Substituted Hydroxyapatite—Biocompatibility, Mechanical Properties and Antimicrobial Activity. Ceram. Int. 2019, 45, 22029–22039. [Google Scholar] [CrossRef]
- Lu, T.; Miao, Y.; Yuan, X.; Zhang, Y.; Ye, J. Adjusting Physicochemical and Cytological Properties of Biphasic Calcium Phosphate by Magnesium Substitution: An in Vitro Study. Ceram. Int. 2023, 49, 15588–15598. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubchikov, D.O.; Fadeeva, I.V.; Trofimchuk, E.S.; Barbaro, K.; Yankova, V.G.; Antoniac, I.V.; Putlayev, V.I.; Rau, J.V.; Saceleanu, V. Methylcellulose-Encapsulated Magnesium-Substituted Biphasic Calcium Phosphate Granules for Local Drug Delivery in Bone Tissue Engineering: Modification for Prolonged Release and Antibacterial Behavior. Polymers 2025, 17, 2422. https://doi.org/10.3390/polym17172422
Golubchikov DO, Fadeeva IV, Trofimchuk ES, Barbaro K, Yankova VG, Antoniac IV, Putlayev VI, Rau JV, Saceleanu V. Methylcellulose-Encapsulated Magnesium-Substituted Biphasic Calcium Phosphate Granules for Local Drug Delivery in Bone Tissue Engineering: Modification for Prolonged Release and Antibacterial Behavior. Polymers. 2025; 17(17):2422. https://doi.org/10.3390/polym17172422
Chicago/Turabian StyleGolubchikov, Daniil O., Inna V. Fadeeva, Elena S. Trofimchuk, Katia Barbaro, Viktoriya G. Yankova, Iulian V. Antoniac, Valery I. Putlayev, Julietta V. Rau, and Vicentiu Saceleanu. 2025. "Methylcellulose-Encapsulated Magnesium-Substituted Biphasic Calcium Phosphate Granules for Local Drug Delivery in Bone Tissue Engineering: Modification for Prolonged Release and Antibacterial Behavior" Polymers 17, no. 17: 2422. https://doi.org/10.3390/polym17172422
APA StyleGolubchikov, D. O., Fadeeva, I. V., Trofimchuk, E. S., Barbaro, K., Yankova, V. G., Antoniac, I. V., Putlayev, V. I., Rau, J. V., & Saceleanu, V. (2025). Methylcellulose-Encapsulated Magnesium-Substituted Biphasic Calcium Phosphate Granules for Local Drug Delivery in Bone Tissue Engineering: Modification for Prolonged Release and Antibacterial Behavior. Polymers, 17(17), 2422. https://doi.org/10.3390/polym17172422









