Determination of Odor Compounds in Lignocellulose-Based Panels Using DHS-GC/MS Combined with Odor Activity Value Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
2.2. Sample Preparation
2.3. Experimental Methods
2.3.1. Methods of Dynamic Headspace
2.3.2. Methods of GC-MS
2.3.3. Data Analysis
2.3.4. Analysis of Odor Substance Characteristics
2.4. Standard Solution Preparation and Volatilization Simulation
2.4.1. Preparation of Mother Liquor
2.4.2. Preparation of Standard Working Solution
2.4.3. Standardization of Filter Paper Load
3. Results and Discussion
3.1. Optimization of Instrument Conditions
3.1.1. Optimization of Sample State
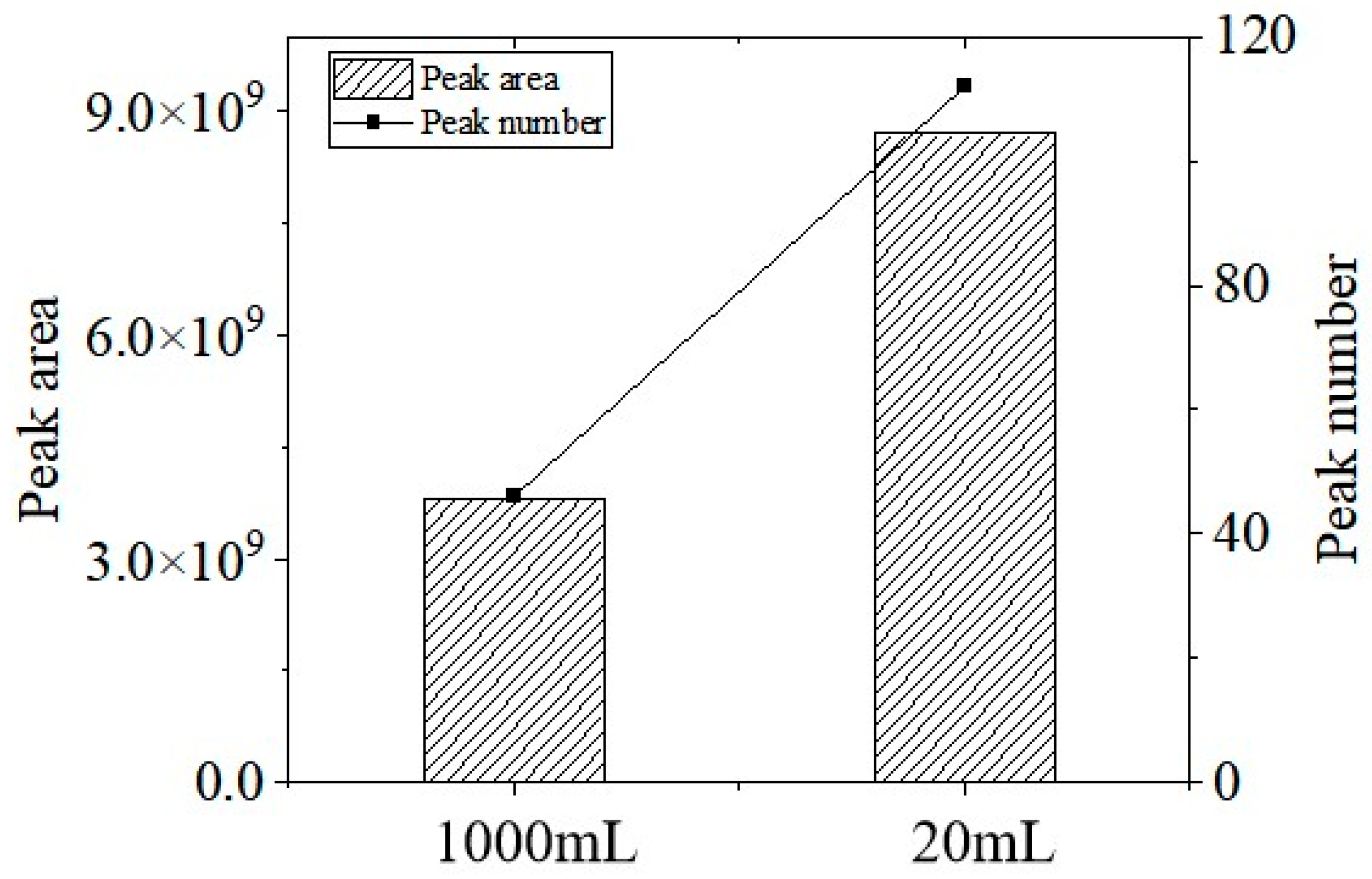
3.1.2. Optimization of Headspace Vial Volume
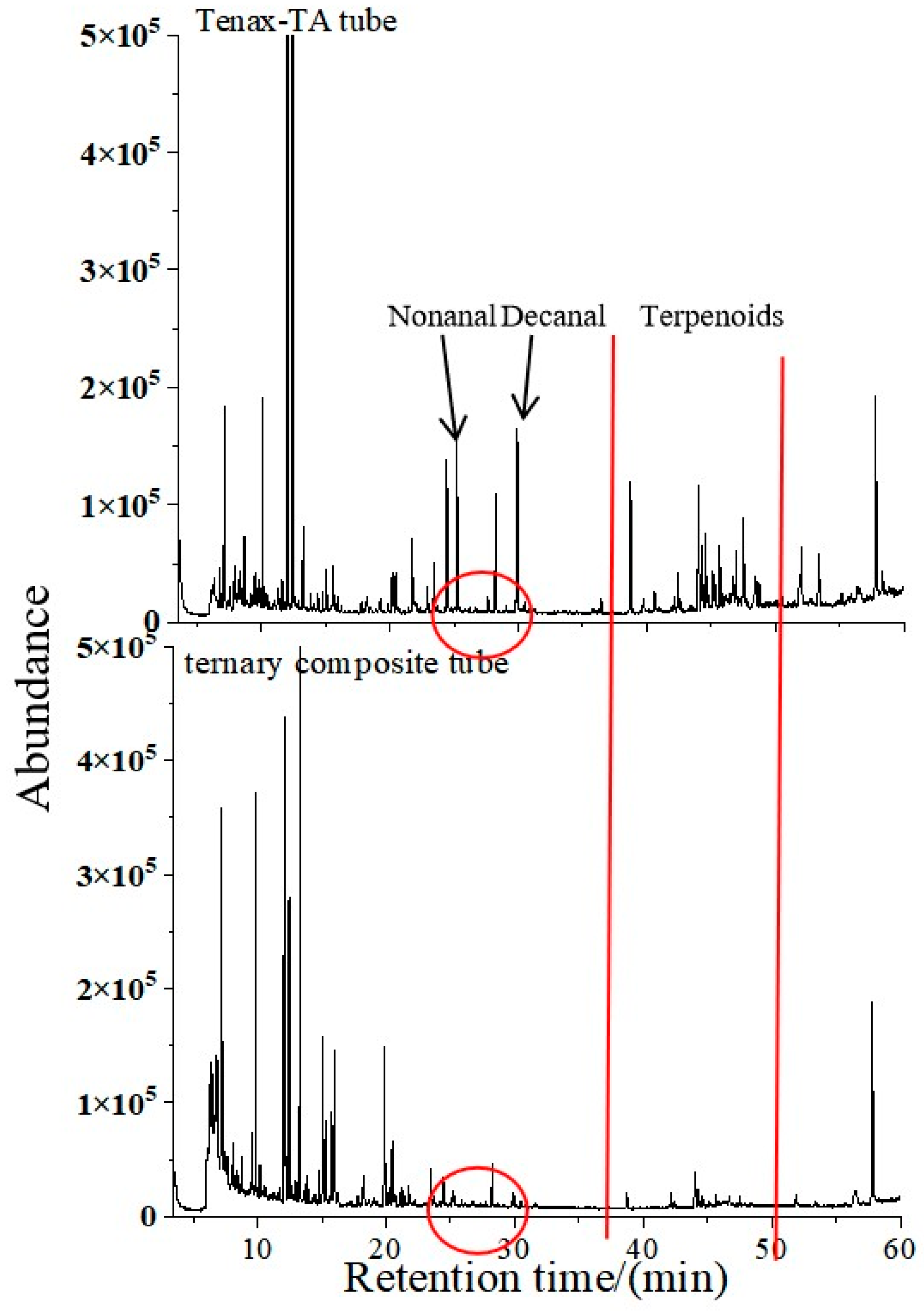
3.1.3. Selection of Packing for Adsorption Tubes
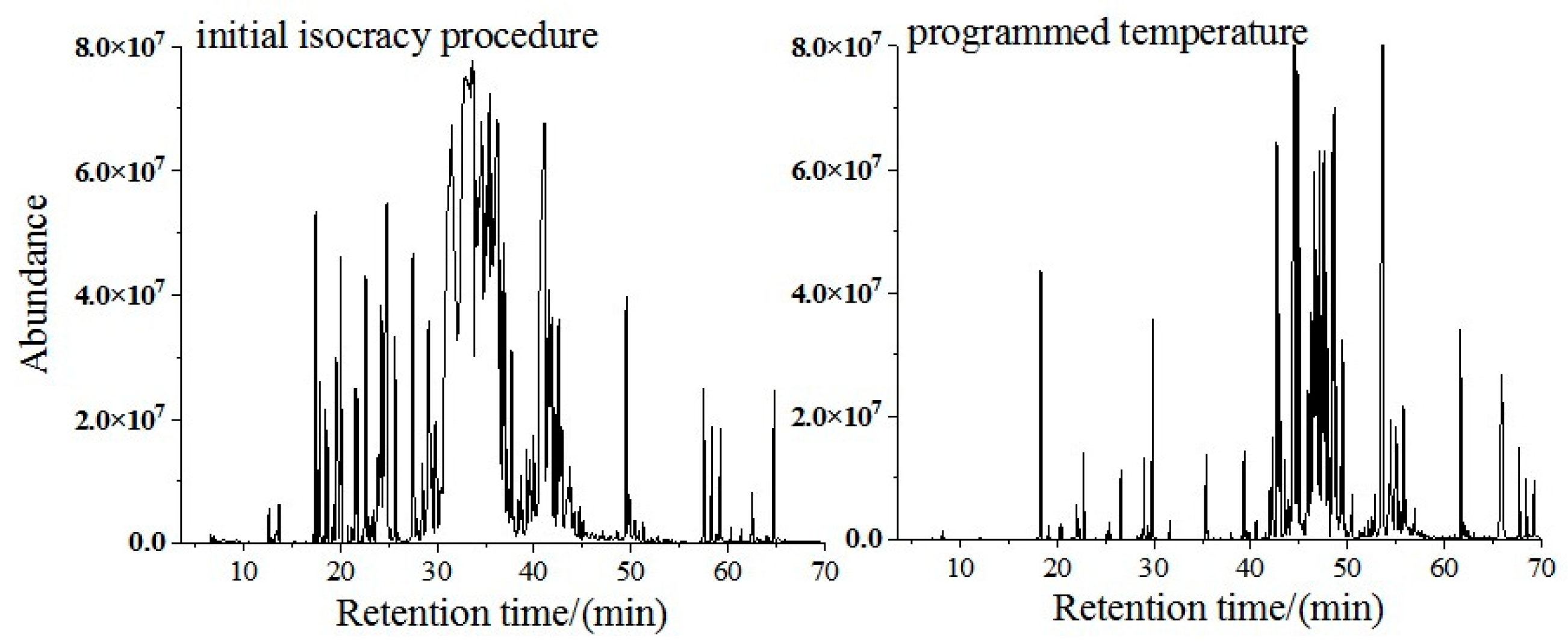
3.1.4. Optimization of the Temperature Rise Procedure of the Column Oven
3.1.5. Optimization of the Split Ratio
3.2. Methodological Validation
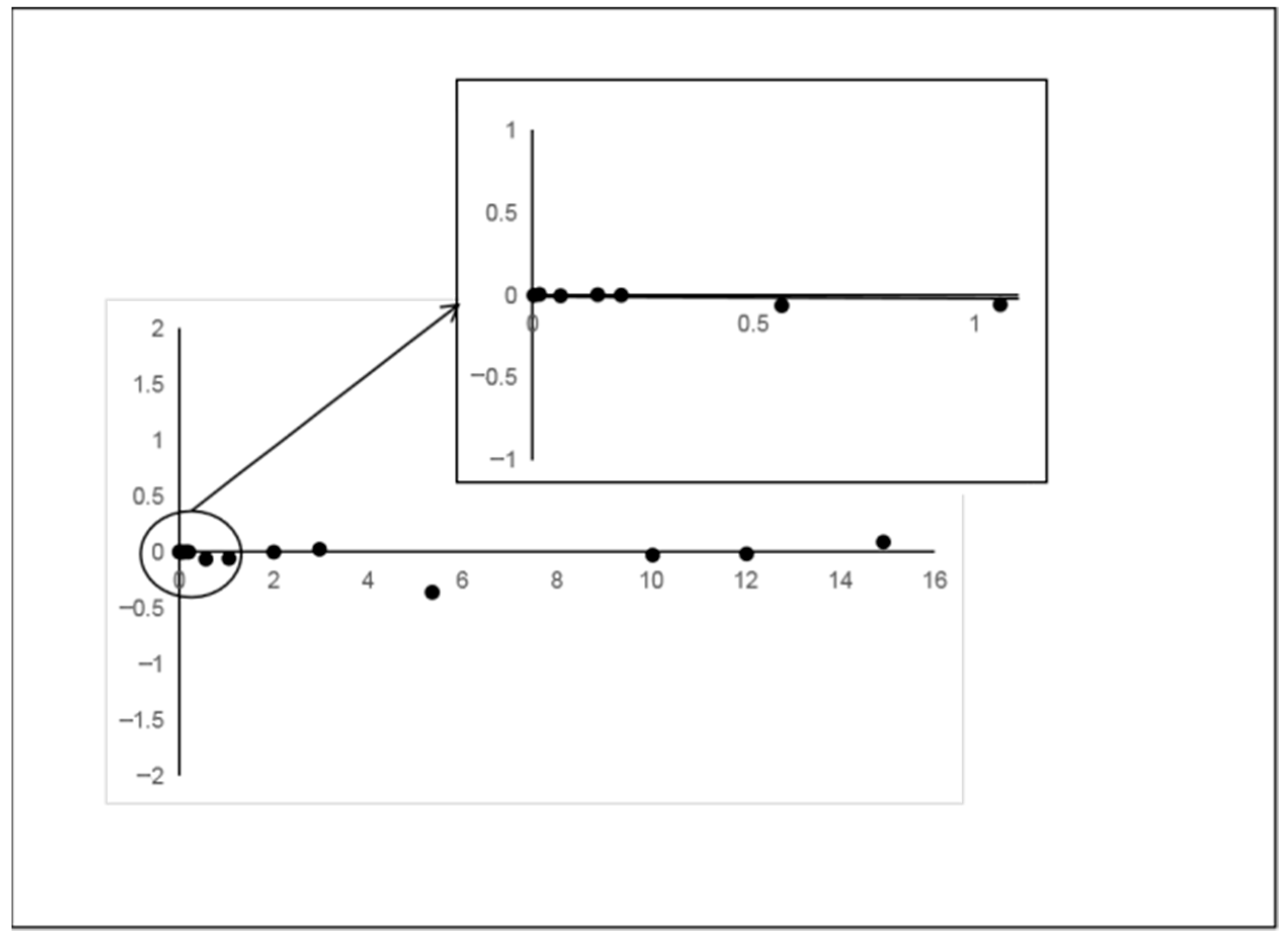
3.2.1. Linear Range and Detection Limit
3.2.2. Recovery Rate and Precision of Spiked Products
3.3. Sample Determination
| Compounds | Retention Time (min) | Characteristic Fragment Ions (m/z) | Odor | Odor Threshold mg/m3 | Particleboard | Fiberboard | Plywood | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration mg/m3 | OAV | Concentration mg/m3 | OAV | Concentration mg/m3 | OAV | ||||||
| Aldehydes | isobutyraldehyde | 8.95 | 72, 57, 73, 55 | A pungent odor * | 0.0010 * | 0.011 | 10.573 | - | - | - | - |
| methacrolein | 9.21 | 70, 69, 71 | Wild hyacinth scent * | 0.0244 * | 0.018 | 0.721 | - | - | - | - | |
| isovaleraldehyde | 11.12 | 58, 57, 71 | Ethereal, chocolate, and peach flavors * | 0.0004 * | 0.032 | 92.122 | - | - | - | - | |
| 2-methylbutyraldehyde | 11.38 | 57, 58, 43, 86 | Moldy smell, fermented baking smell * | - | 0.015 | - | - | - | - | - | |
| pentanal | 12.09 | 58, 57, 71, 86 | Grassy [26] | 0.0014 [27] | 0.029 | 20.954 | - | - | - | - | |
| hexanal | 14.84 | 56, 57, 72 | Stimulating grassy scent and apple aroma [26] | 0.0011 [26] | 0.355 | 322.869 | 0.003 | 2.798 | 0.044 | 40.079 | |
| furfural | 17.44 | 96, 95, 67 | Sweet woody almond-flavored toasted bread mixed with aroma [24] | - | - | - | 0.006 | - | - | ||
| heptanal | 18.26 | 70, 55, 57 | fruity aroma [26] | 0.0008 [26] | 0.033 | 40.762 | 0.003 | 3.867 | - | - | |
| benzaldehyde | 20.97 | 77, 106, 105 | Bitter almond flavor [26] | 0.0260 [26] | 0.080 | 3.085 | - | - | - | - | |
| nonanal | 30.38 | 57, 56, 55, 70 | Strong oily and sweet orange aroma [26] | 0.0020 [26] | 0.543 | 271.419 | - | - | 0.062 | 30.813 | |
| Acids | acetic acid | 5.93 | 45, 60 | Strong, pungent, vinegar-like odor * | 0.0147 * | 1.854 | 125.786 | 0.119 | 8.069 | - | - |
| propanoic acid | 10.24 | 74, 45, 73 | The taste of spicy acidic cheese vinegar * | 0.0173 * | - | - | 0.015 | 0.880 | - | - | |
| butanoic acid | 15.60 | 60, 73, 45 | Yogurt and cheese flavor * | 0.0007* | - | - | 0.006 | 9.326 | - | - | |
| benzoic acid | 31.29 | 105, 122, 77 | Light fragrance * | - | - | - | 0.003 | - | - | ||
| Terpenoids | alpha-terpineol | 29.85 | 59, 93, 122, 136 | Lilac aroma [26] | 0.3855 [26] | - | - | - | - | 0.443 | 1.150 |
| longifolene | 44.08 | 161, 94, 91, 107 | Wood fragrance and iris-like aroma [26] | 0.0024 [26] | - | - | - | - | 0.108 | 45.135 | |
| α-cedrene | 44.31 | 119, 93, 105 | Sweet and gentle cypress characteristic aroma [27] | 0.0113 [26] | - | - | - | - | 0.418 | 36.973 | |
| cis-thujopsene | 45.02 | 119, 123, 105, 121 | A faint woody fragrance [26] | 0.0630 [26] | - | - | - | - | 0.162 | 2.573 | |
| widdrol | 53.12 | 151, 95, 69 | Odorous [28] | - | - | - | - | - | 0.474 | - | |
| cedrol | 53.44 | 95, 150, 151 | Weak wood fragrance with some ointment fragrance [26] | 0.5126 [26] | - | - | - | - | 12.607 | 24.593 | |
| β-acorenol | 54.62 | 119, 121, 161 | Woody [26] | 0.0144 [26] | - | - | - | - | 0.050 | 3.497 | |
| α-bisabolol | 56.87 | 69, 109, 119 | Woody [26] | 0.0013 [26] | - | - | - | - | 0.137 | 105.638 | |
| (-)-camphene | 43.61 | 93, 121, 79 | Fir terpene flavor * | - | 0.053 | - | - | - | - | - | |
| Monoaromatic series | benzene | 11.60 | 78, 77, 519 | Aromatic odor [26] | 8.6130 [26] | 0.010 | 0.001 | - | - | - | - |
| toluene | 14.36 | 91, 92, 65 | Special fragrant aroma [26] | 1.2408 [26] | 0.037 | 0.030 | - | - | - | - | |
| p-xylene | 17.69 | 91, 106, 105 | Stimulating aromatic scent [26] | 0.2517 [26] | 0.026 | 0.103 | - | - | - | - | |
| Alcohol | 1,3-dichloro-2-Propanol | 15.21 | 79, 81, 49 | Ether odor * | - | - | - | - | - | 0.160 | |
| Ester | nerolidyl acetate | 45.70 | 69, 93, 107 | Fresh, sweet, citrus and orchid wood fragrance * | 0.058 | - | - | - | - | - | |
| Ketones | 1-hydroxy-2-propanone | 7.84 | 74, 45 | Caramel aroma * | - | - | - | 0.007 | - | - | - |
| acetone | 7.16 | 58, 59, 57 | Spicy and stimulating [18] | 99.76 [29] | - | - | - | - | 0.447 | 0.005 | |
| Halohydrocarbon | 1,2-dichloro-ethane | 7.68 | 62, 64, 49, 63 | Chloroform-like odor | - | - | - | 0.004 | |||
| Ether | 1-methoxy-2-propanol | 9.87 | 45, 47, 75 | Sweet ether-like odor * | 0.012 [25] | - | - | - | - | 0.060 | 5.000 |
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, Z.K.; Zhang, Y.P.; Wei, W.J. Formaldehyde and VOC emissions at different manufacturing stages of wood-based panels. Build. Environ. 2012, 47, 197–204. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X.D.; Qin, X.; Wang, W.C.; Hu, Y.B.; Yuan, D.L. Identification and characterization of odorous volatile organic compounds emitted from wood-based panels. Environ. Monit. Assess. 2020, 192, 348. [Google Scholar] [CrossRef]
- GB/T 44689-2024; Classification and Evaluation Methods for Odour from Wood-Based Panels and Their Products. Standards Press of China: Beijing, China, 2024.
- Schreiner, L.; Bauer, P.; Buettner, A. Resolving the smell of wood—Identification of odour-active compounds in Scots pine (Pinus sylvestris L.). Sci. Rep. 2018, 8, 8294. [Google Scholar] [CrossRef]
- Wang, Q.F.; Shen, J.; Shen, X.W.; Du, J.H. Volatile Organic Compounds and Odor Emissions from Alkyd Resin Enamel-coated Particleboard. BioResources 2018, 13, 6837–6849. [Google Scholar] [CrossRef]
- Qian, M.C.; Wang, Y.Y. Seasonal Variation of Volatile Composition and Odor Activity Value of ‘Marion’(Rubus spp. hyb) and’Thornless Evergreen’(R. laciniatus L.) Blackberries. J. Food Sci. 2010, 70, C13–C20. [Google Scholar] [CrossRef]
- Vilanova, M.; Campo, E.; Escudero, A.; Grana, M.; Masa, A.; Cacho, J. Volatile composition and sensory properties of Vitis vinifera red cultivars from north west Spain: Correlation between sensory and instrumental analysis. Anal. Chim. Acta 2012, 720, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Xi, Z.M.; Luo, M.J.; Zhang, Z.W. Comparison on aroma compounds in Cabernet Sauvignon and Merlot wines from four wine grape-growing regions in China. Food Res. Int. 2013, 51, 482–489. [Google Scholar] [CrossRef]
- Zhu, J.C.; Cao, X.Y.; Niu, Y.W.; Xiao, Z.B. Investigation of Lactone Chiral Enantiomers and Their Contribution to the Aroma of Longjing Tea by Odor Activity Value and S-Curve. J. Agric. Food Chem. 2023, 71, 6691–6698. [Google Scholar] [CrossRef]
- Rodríguez, A.; Peris, J.E.; Redondo, A.; Shimada, T.; Costell, E.; Carbonell, I.; Rojas, C.; Pena, L. Impact of d -limonene synthase up- or down-regulation on sweet orange fruit and juice odor perception. Food Chem. 2017, 217, 139–150. [Google Scholar] [CrossRef]
- Szudera-Kończal, K.; Myszka, K.; Kubiak, P.; Drabińska, N.; Majcher, M.A. The Combined Effect of Lactic Acid Bacteria and Galactomyces geotrichum Fermentation on the Aroma Composition of Sour Whey. Molecules 2023, 28, 4308. [Google Scholar] [CrossRef]
- GB/T 29899-2024; Determination of the Emission of Volatile Organic Compounds from Wood-Based Panels and Furnishing Products—Small Chamber Method. Standards Press of China: Beijing, China, 2024.
- Radovic, B.S.; Careri, M.; Mangia, A.; Musci, M.; Anklam, E. Contribution of dynamic headspace GC–MS analysis of aroma compounds to authenticity testing of honey. Food Chem. 2001, 72, 511–520. [Google Scholar] [CrossRef]
- Soria, A.C.; Garcia-sarrio, M.J.; Sanz, M.L. Volatile sampling by headspace techniques. TrAC Trends Anal. Chem. 2015, 71, 85–99. [Google Scholar] [CrossRef]
- Morales, M.T.; Aparicio, R.; Rios, J.J. Dynamic headspace gas chromatographic method for determining volatiles in virgin olive oil. J. Chromatogr. A 1994, 668, 455–462. [Google Scholar] [CrossRef]
- Na, J.J.; Liu, C.B.; Ran, J.G. Study on the preparation of chloride ion standard curve using segmented multiple regression method. Sichuan Chem. Ind. 2005, 8, 31–33+37. [Google Scholar]
- Tian, J. A study on Emission Characteristics and Odor Evaluation of Volatile Organic Compounds from Typical Wood-Based Panels in Customized Furniture. Master’s Thesis, South China University of Technology, Guangzhou, China, 2023. [Google Scholar]
- Shen, J. Research on VOCs Release from Particleboar; Science Press: Beijing, China, 2013; pp. 1–228. [Google Scholar]
- Wang, D.W. Control of Very Volatile Organic Compounds Emission from Decorative Medium Fiber Board and Research on Odor Analysis. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2023. [Google Scholar] [CrossRef]
- Qi, L.H.; Zou, X.W.; Lv, B.; Fu, Y.J.; Tang, L.N.; Chen, Q.; Liu, B.; Li, B.T. VOCs Composition and Odor Characteristics of Main Plantation Wood in China. Sci. Silvae Sin. 2023, 11, 103–117. [Google Scholar]
- Lv, S.Y.; Zhang, L.F.; Li, S.M.; Jiang, P.; Peng, L.M. Research Progress in Functional Plywood. China Wood-Based Panels 2021, 28, 1–6. [Google Scholar]
- Jiang, X.F.; Xie, H.; Lu, T.L.; Feng, F.; Chen, H.; Xv, J.G.; He, C.; Mao, C.Q. Study on material basis of odor changes in stir-frying process of Hordei Fructus Germinatus based on Heracles Neo ultra-fast gas phase electronic nose technology. Chin. Tradit. Herb. Drugs 2022, 1, 41–50. [Google Scholar]
- Shu, M.S.; Dan, M.; Ji, X.H.; Wang, Y.; Zhang, Q.; Zhou, F.Y.; Ding, D.; Hang, W. Detection of 2-methylpropanal,2-methyl butanal and 3-methyl butanal in workplace air by portable gas chromatography-mass spectrometry. China Occup. Med. 2019, 3, 367–370. [Google Scholar]
- Huang, C.L. Analysis of Aroma Characteristics of Dianhong Gongfu Tea Based on Molecular Sensory Science. Master’s Thesis, Yunnan Agricultural University, Kunming, China, 2024. [Google Scholar]
- Su, Q.; Lu, X.Y.; Shi, X.Q.; Nie, H.J.; Zhang, P.; Wang, Y.P. Research progress of glue-free fiberboard based on straw. Acta Mater. Compos. Sin. 2024, 4, 1750–1763. [Google Scholar] [CrossRef]
- LY/T 3413-2024; Determination of Odour Compounds in Wood-Based Panels and Finishing Products—Gas Chromatography-Mass Spectrometry-Olfactometry Method. Standards Press of China: Beijing, China, 2024.
- Kwon, H.J.; Hong, Y.K.; Park, C.; Choi, Y.H.; Yun, H.J.; Lee, E.W.; Kim, B.W. Widdrol induces cell cycle arrest, associated with MCM down-regulation, in human colon adenocarcinoma cells. Cancer Lett. 2010, 290, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.Q.; Du, X.M.; Li, Q.; Yu, X.H. Comprehensive Evaluation on the Olfaction Quality of Trademark Paper Base on Volatile Organic Compounds. Pap. Pap. Mak. 2015, 34, 4. [Google Scholar] [CrossRef]
- Li, W.F.; Li, C.J.; Cao, Y.; Zhai, Z.X.; Yan, F.Y.; Shang, X.B. Odor Pollution Conditions and Typical Gaseous Pollutants in Urban Ambient Air in Tianjin City. Res. Environ. Sci. 2014, 27, 8. [Google Scholar] [CrossRef]




| No. | Original (mg/m3) | Added (mg/m3) | Detected (mg/m3) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| 1 | 0.43 | 2.00 | 2.32 | 94.7 | 3.2 |
| 2 | 0.44 | 2.00 | 2.50 | 103.2 | |
| 3 | 0.45 | 2.00 | 2.51 | 103.0 | |
| 4 | 0.43 | 2.00 | 2.50 | 103.4 | |
| 5 | 0.43 | 2.00 | 2.46 | 101.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Chen, Q.; Zhu, L.; Liu, X.; Zou, X.; Fu, Y.; Liu, B. Determination of Odor Compounds in Lignocellulose-Based Panels Using DHS-GC/MS Combined with Odor Activity Value Analysis. Polymers 2025, 17, 2421. https://doi.org/10.3390/polym17172421
Tang L, Chen Q, Zhu L, Liu X, Zou X, Fu Y, Liu B. Determination of Odor Compounds in Lignocellulose-Based Panels Using DHS-GC/MS Combined with Odor Activity Value Analysis. Polymers. 2025; 17(17):2421. https://doi.org/10.3390/polym17172421
Chicago/Turabian StyleTang, Lina, Qian Chen, Liming Zhu, Xiaorui Liu, Xianwu Zou, Yuejin Fu, and Bo Liu. 2025. "Determination of Odor Compounds in Lignocellulose-Based Panels Using DHS-GC/MS Combined with Odor Activity Value Analysis" Polymers 17, no. 17: 2421. https://doi.org/10.3390/polym17172421
APA StyleTang, L., Chen, Q., Zhu, L., Liu, X., Zou, X., Fu, Y., & Liu, B. (2025). Determination of Odor Compounds in Lignocellulose-Based Panels Using DHS-GC/MS Combined with Odor Activity Value Analysis. Polymers, 17(17), 2421. https://doi.org/10.3390/polym17172421





