Dual Micromechanical Interlocking Through Filler Surface Modification for Enhanced Dental Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
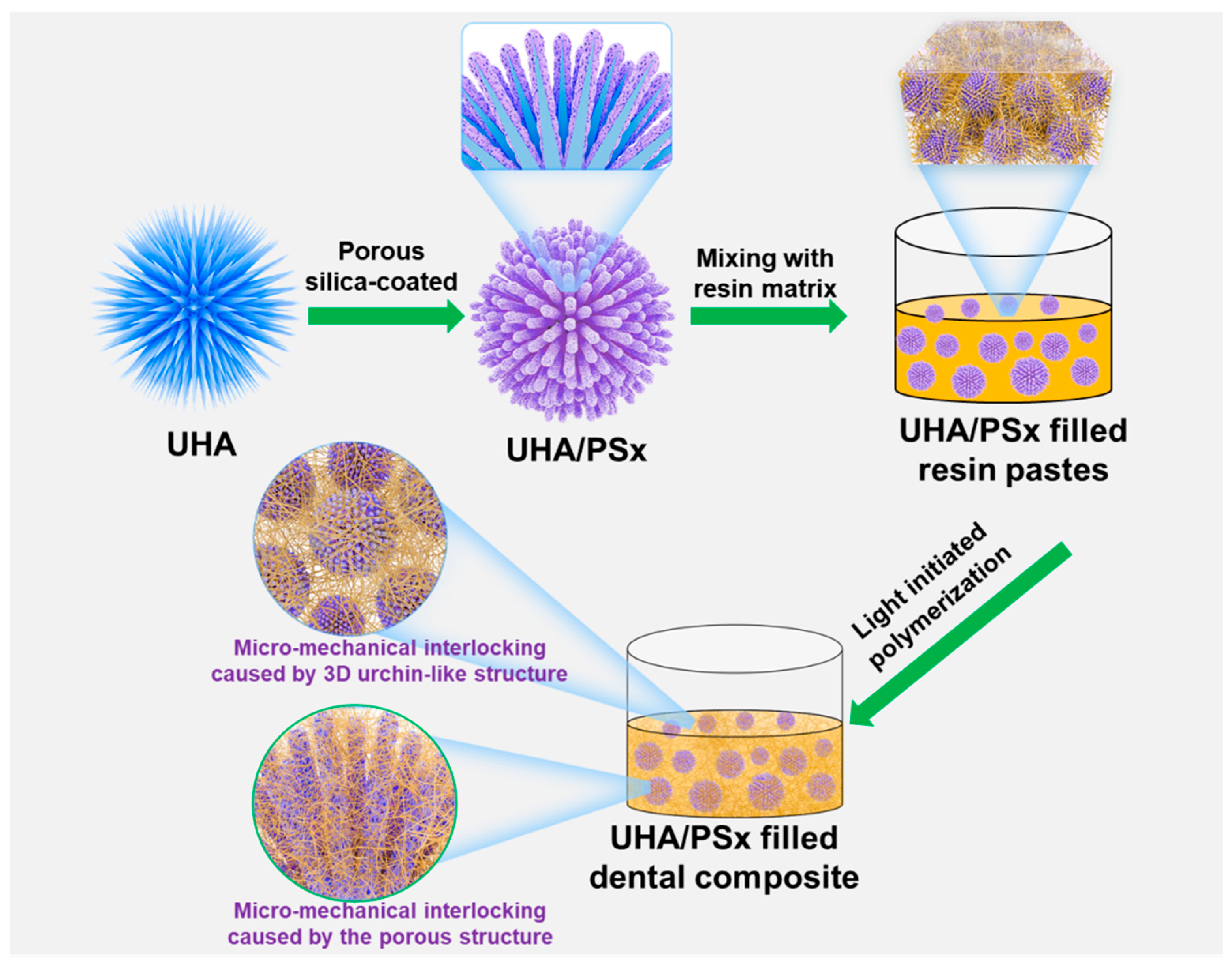
2.2. Synthesis of UHA/PSx Particles
2.3. Preparation of Dental Resin Composites (DRCs)
2.4. Characterization
2.4.1. Morphology and Crystal Structure of UHA/PSx
2.4.2. Mechanical Properties of UHA/PSx-Filled DRCs
2.4.3. Fracture Surface of UHA/PSx-Filled DRCs
2.4.4. Water Sorption and Solubility of UHA/PSx-Filled DRCs
2.4.5. Curing Depth of UHA/PSx-Filled DRCs
2.4.6. Mineralization of DRCs Surfaces
2.4.7. In Vitro Cell Activity of UHA/PSx-Filled DRCs
- Human dental pulp stem cells (hDPSCs) culture
- 2.
- Preparation of DRCs extracts
- 3.
- Cytotoxicity and cell proliferation
- 4.
- Alkaline phosphatase (ALP) activity test
- 5.
- Real-time polymerase chain reaction (RT-PCR) analysis
2.5. Statistical Analysis
3. Results and Discussion
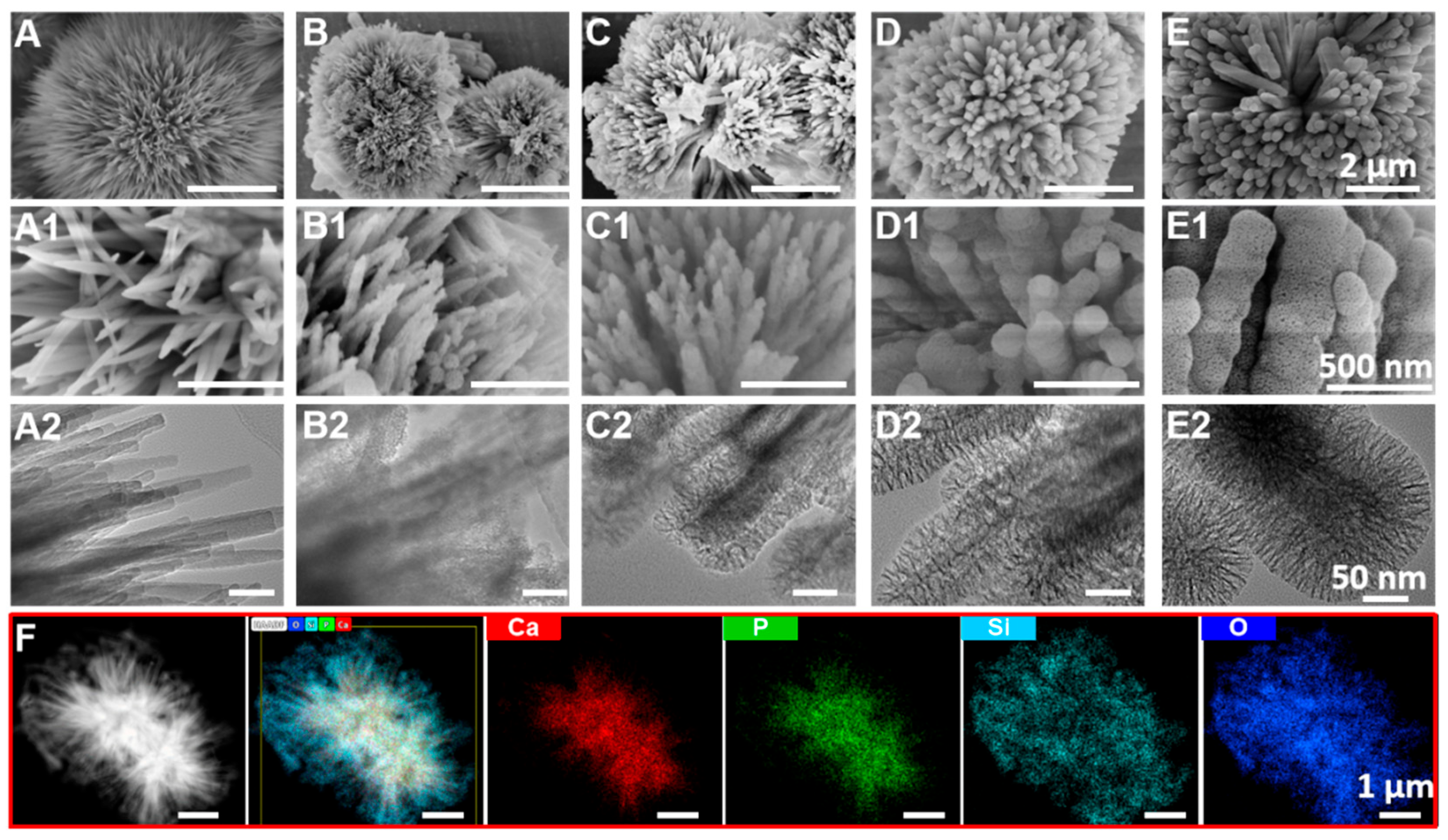
3.1. Morphology of UHA/PSx
3.2. Structure of UHA/PSx
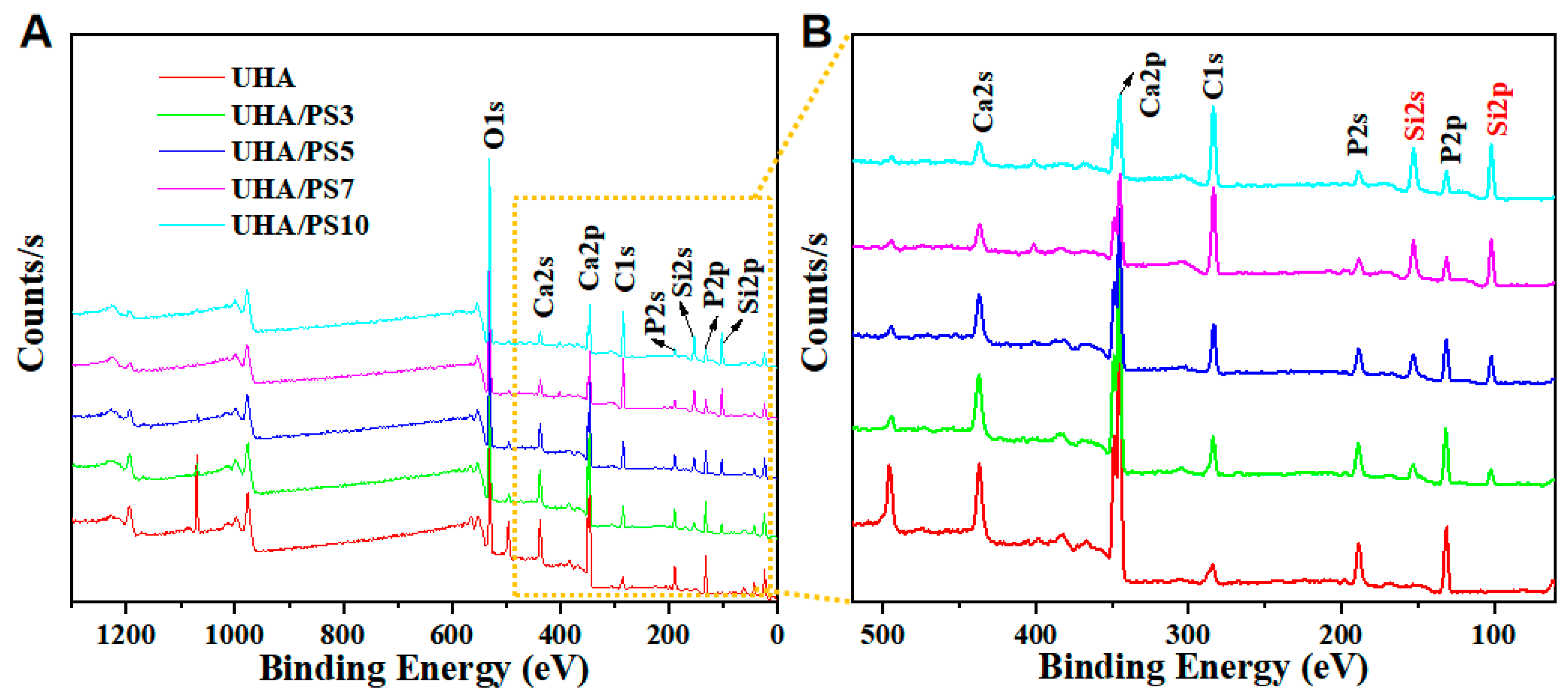
3.3. Compositional Elements of UHA/PSx
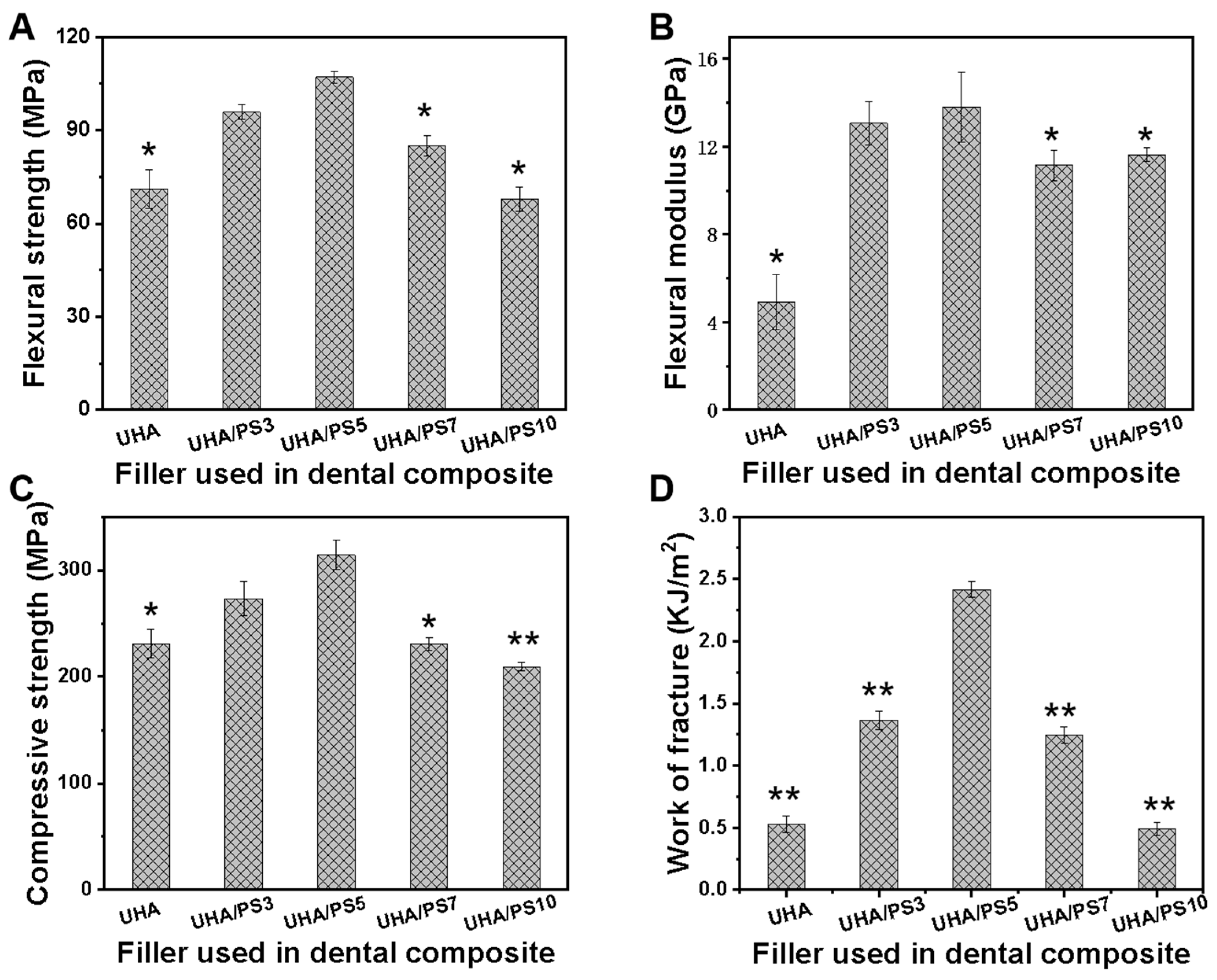
3.4. Mechanical Performance of UHA/PSx-Filled RDCs
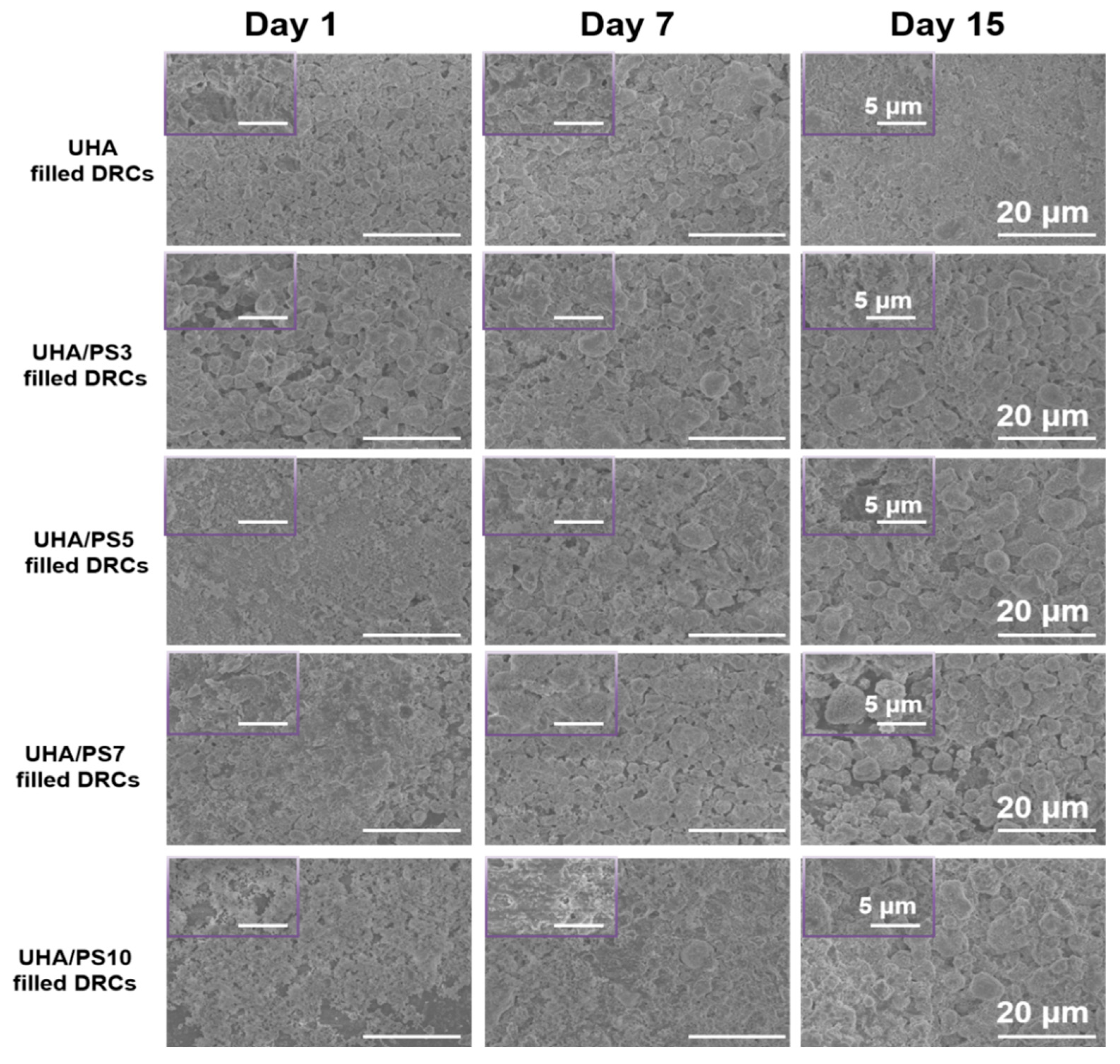
3.5. Fractured Morphology of UHA/PSx-Filled DRCs
3.6. Water Sorption and Solubility of UHA/PSx-Filled DRCs
3.7. Curing Depth of UHA/PSx-Filled DRCs
3.8. Remineralization of UHA/PSx-Filled DRCs
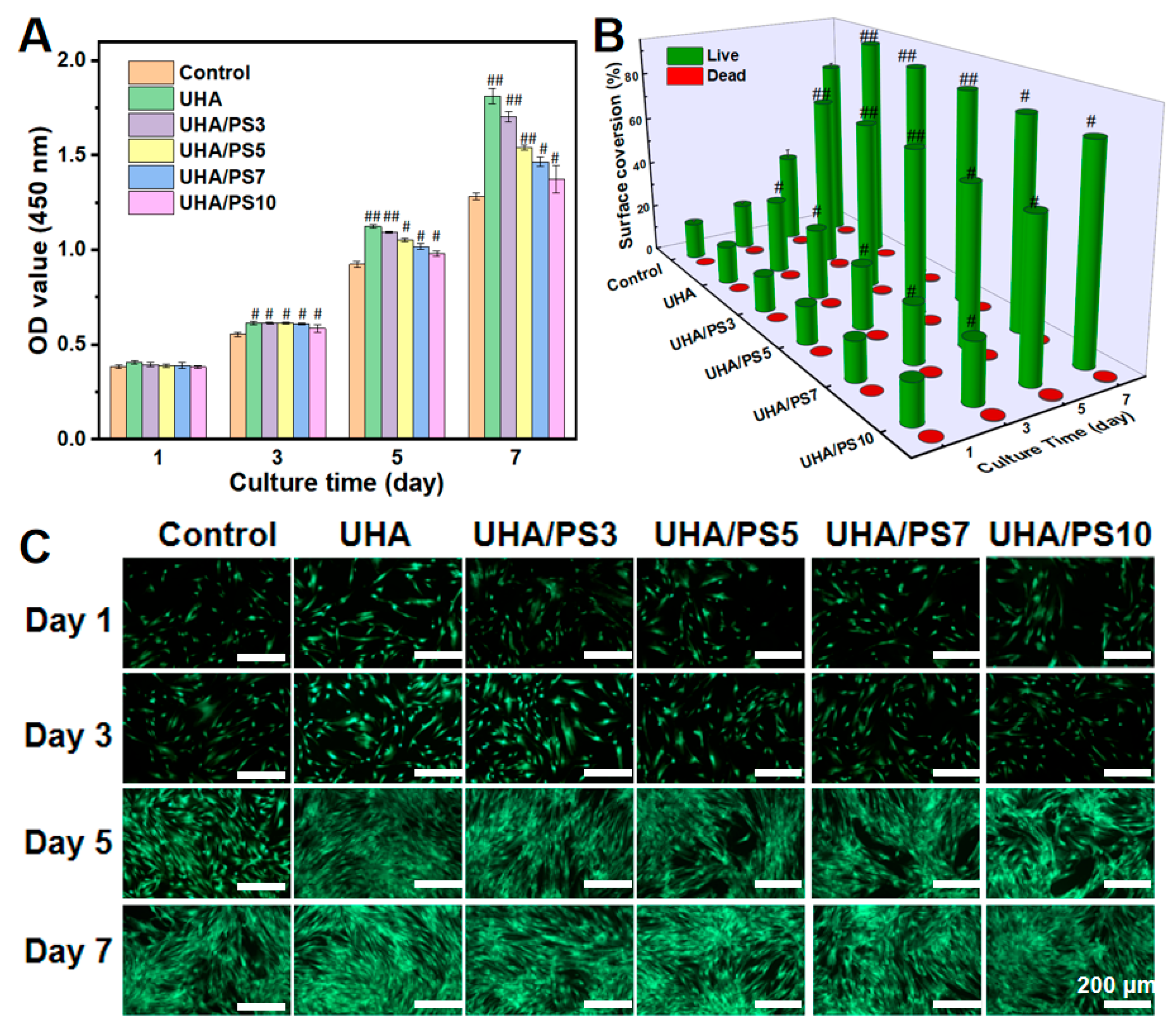
3.9. Cell Viability of UHA/PSx-Filled DRCs
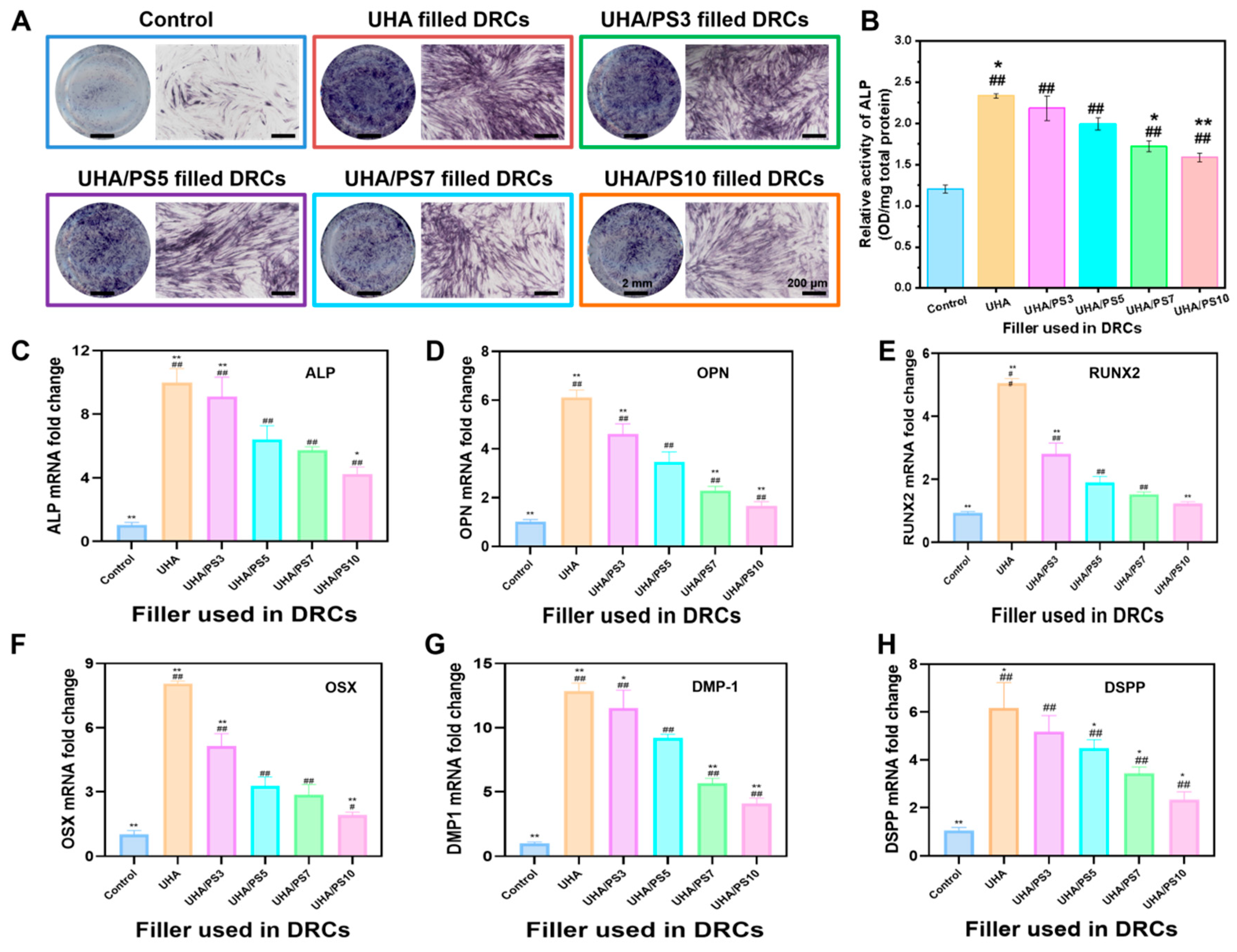
3.10. In Vitro Mineralized Differentiation of hDPSCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cho, K.; Rajan, G.; Farrar, P.; Prentice, L.; Prusty, B.G. Dental resin composites: A review on materials to product realizations. Compos. Part B Eng. 2022, 230, 109495. [Google Scholar] [CrossRef]
- Aminoroaya, A.; Neisiany, R.E.; Khorasani, S.N.; Panahi, P.; Das, O.; Madry, H.; Cucchiarini, M.; Ramakrishna, S. A review of dental composites: Challenges, chemistry aspects, filler influences, and future insights. Compos. B Eng. 2021, 216, 108852. [Google Scholar] [CrossRef]
- Wang, R.L.; Li, Z.H.; Tian, Q.Y.; Ma, Z.Y.; Zhu, M.F. Making graphene oxide (GO)-cladded SiO2 spheres (SiO2@GO) as inorganic fillers for dental restorative resin composites. Dent. Mater. 2023, 39, 1076. [Google Scholar] [CrossRef]
- Duan, C.; Wang, J.J.; Li, M.S.; Wang, R.L. Synergetic effect of ZnO-doped dendritic porous silica and barium glass powder as functional bimodal fillers for dental composite applications. Dent. Mater. 2025, 41, 880. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Wang, R.L.; Qian, L.; Liu, H.M.; Wang, J.X.; Zhu, M.F. Surface modification of urchin-like serried hydroxyapatite with sol-gel method and its application in dental composites. Compos. B Eng. 2020, 182, 107621. [Google Scholar] [CrossRef]
- Ferracane, J.L. Resin composite–state of the art. Dent. Mater. 2011, 27, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Hua, H.F.; Yu, Y.J.; Chen, G.Y.; Zhu, M.F.; Zhu, X.X. Dental resin composites reinforced by rough core-shell SiO2 nanoparticles with a controllable mesoporous structure. ACS Appl. Bio Mater. 2019, 2, 4233. [Google Scholar]
- Xu, X.Y.; He, L.B.; Zhu, B.G.; Li, J.Y.; Li, J.S. Advances1npolymeric materials for dental applications. Polym. Chem. 2017, 8, 807. [Google Scholar] [CrossRef]
- Chen, H.Y.; Wang, R.L.; Qian, L.; Ren, Q.Y.; Jiang, X.Z.; Zhu, M.F. Dental restorative resin composites: Modification technologies for the matrix/filler interface. Macromol. Mater. Eng. 2018, 303, 1800264. [Google Scholar] [CrossRef]
- Chen, H.Y.; Wang, R.L.; Zhang, J.D.; Hua, H.F.; Zhu, M.F. Synthesis of core-shell structured ZnO@m-SiO2, with excellent reinforcing effect and antimicrobial activity for dental resin composites. Dent. Mater. 2018, 34, 1846. [Google Scholar] [CrossRef]
- Chen, H.Y.; Liu, H.M.; Wang, R.L.; Jiang, X.Z.; Zhu, M.F. Size controllable synthesis of dendritic porous silica as reinforcing fillers for dental composites. Dent. Mater. 2021, 37, 961. [Google Scholar] [CrossRef]
- Antonucci, J.M.; Dickens, S.H.; Fowler, B.O.; Xu, H.H.K. Chemistry of silanes: Interfaces in dental polymers and composites. J. Res. Natl. Inst. Stand. Technol. 2005, 110, 541. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.; Habib, E.; Wang, R.; Zhang, Q.; Sun, B.; Zhu, M.F. Surface modification of quartz fibres for dental composites through a sol-gel process. Mater. Sci. Eng. C 2017, 74, 21. [Google Scholar] [CrossRef] [PubMed]
- Klapdohr, S.; Moszner, N. New inorganic components for dental filling composites. Monatsh. Chem. 2005, 136, 21. [Google Scholar] [CrossRef]
- Chen, H.Y.; Wang, J.J.; Yin, S.; Wang, R.L.; Jiang, X.Q.; Zhu, M.F. Micromechanical interlocking-inspired dendritic porous silica-based multimodal resin composites for the tooth restoration. Nano Res. 2024, 17, 9065. [Google Scholar] [CrossRef]
- Matinlinna, J.P.; Lung, C.Y.K.; Tsoi, J.K.H. Silane adhesion mechanism in dental applications and surface treatments: A review. Dent. Mater. 2018, 34, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Drummond, J.L. Degradation, fatigue, and failure of resin dental composite materials. J. Dent. Res. 2008, 87, 710. [Google Scholar] [CrossRef]
- Lei, H.; Ma, Q.; Li, W.F.; Wen, J.; Ma, H.B.; Qn, M.; Wang, W.; Cao, Y. An ester bond underlies the mechanical strength of a pathogen surface protein. Nat. Commun. 2021, 12, 5082. [Google Scholar] [CrossRef]
- Zhang, S.N.; Wang, X.; Yang, J.W.; Chen, H.Y.; Jiang, X.Q. Micromechanical interlocking structure at the filler/resin interface for dental composites: A review. Int. Oral Sci. 2023, 15, 21. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, F.L.; Chen, X.D. Bioinspired mechanically interlocking structures. Small Struct. 2020, 1, 2000045. [Google Scholar] [CrossRef]
- Zandinejad, A.A.; Atai, M.; Pahlevan, A. The effect of ceramic and porous fillers on the mechanical properties of experimental dental composites. Dent. Mater. 2006, 22, 382. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, Y.; Lei, T.; Xiang, Q.; Han, Y.; Huang, B. Effect of porous glass-ceramic fillers on mechanical properties of light-cured dental resin composites. Dent. Mater. 2009, 25, 709. [Google Scholar] [CrossRef]
- Samuel, S.P.; Li, S.; Mukherjee, I.; Guo, Y.; Patel, A.C.; Baran, G.; Wei, Y. Mechanical properties of experimental dental composites containing a combination of mesoporous and nonporous spherical silica as fillers. Dent. Mater. 2009, 25, 296. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.X.; Lin, C.C.; Wang, Y.Y.; Ma, J.; Wang, X.; Yao, X.H.; Tang, B. Preparation of Zn doped mesoporous silica nanoparticles (Zn-MSNs) for the improvement of mechanical and antibacterial properties of dental resin composites. Dent. Mater. 2020, 36, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.N.; Wang, X.; Yin, S.; Wang, J.J.; Chen, H.Y.; Jiang, X.Q. Urchin-like multiscale structured fluorinated hydroxyapatite as versatile filler for caries restoration dental resin composites. Bioact. Mater. 2024, 35, 477. [Google Scholar] [CrossRef]
- Qian, L.; Wang, R.L.; Li, W.; Chen, H.Y.; Jiang, X.Z.; Zhu, M.F. The synthesis of urchin-like serried hydroxyapatite (USHA) and its reinforcing effect for dental resin composites. Macromol. Mater. Eng. 2019, 304, 1800738. [Google Scholar] [CrossRef]
- Dong, Z.; Yang, Q.; Mei, M.; Liu, L.; Sun, J.; Zhao, L.; Zhou, C. Preparation and characterization of fluoride calcium silicate composites with multi-biofunction for clinical application in dentistry. Compos. Part B Eng. 2018, 143, 243. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Quinn, J.B.; Smith, D.T.; Giuseppetti, A.A.; Eichmiller, F.C. Effects of different whiskers on the reinforcement of dental resin composites. Dent. Mater. 2003, 19, 359. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, C.; Wei, J. Microscopic reinforcement for cement based composite materials, Construct. Build. Mater. 2013, 40, 14. [Google Scholar] [CrossRef]
- Liu, F.W.; Sun, B.; Jiang, X.Z.; Aldeyab, S.S.; Zhang, Q.H.; Zhu, M.F. Mechanical properties of dental resin/composite containing urchin-like hydroxyapatite. Dent. Mater. 2014, 30, 1358. [Google Scholar] [CrossRef]
- Huo, F.; Jin, Z.; Han, D.; Zhang, K.; Nishikawa, H. Interface design and the strengthening-ductility behavior of tetra-needle-like ZnO whisker reinforced Sn1.0Ag0.5Cu composite solders prepared with ultrasonic agitation. Mater. Des. 2021, 210, 110038. [Google Scholar] [CrossRef]
- Wang, C.; Huang, Y.; Zhai, H.X. The effect of whisker orientation in SiC whisker-reinforced Si3N4 ceramic matrix composites. J. Eur. Ceram. Soc. 1999, 19, 1903. [Google Scholar]
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and fluorapatite in conservative dentistry and oral implantology—A review. Materials 2019, 12, 2683. [Google Scholar] [CrossRef]
- Chen, H.Y.; Wang, J.J.; Wang, R.L.; Zhu, M.F. Synthesis offluorinated urchin-like serried hydroxyapatite with improved watersorption-solubility and bioactivity for dental composites. Chem. Res. Chin. Univ. 2021, 37, 1092. [Google Scholar] [CrossRef]
- Liu, J.N.; Zhang, H.; Sun, H.J.; Lu, Y.R.; Liu, W.L.; Su, B.; Li, S.B. The development of filler morphology in dental resin composites: A review. Materials 2021, 14, 5612. [Google Scholar] [CrossRef]
- Wang, R.L.; Habib, E.; Zhu, X.X. Synthesis of wrinkled mesoporous silica and its reinforcing effect for dental resin composites. Dent. Mater. 2017, 33, 1139. [Google Scholar] [CrossRef]
- Chen, H.Y.; Luo, J.X.; Yang, J.W.; Zeng, C.; Jiang, X.Q. Synthesis of pore-size-tunable porous silica particles and their effects on dental resin composites. Biomolecules 2023, 13, 1290. [Google Scholar] [CrossRef]
- ISO 4049-2019; Dentistry-Polymer-based Restorative Materials. ISO: Geneva, Switzerland, 2019.
- ISO 10993-12; Biological Evaluation of Medical Devices-Part 12: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2021.
- Schacht, S.; Janicke, M.; Schüth, F. Modeling X-ray patterns and TEM images of MCM-41. Micropor. Mesopor. Mater. 1998, 22, 485. [Google Scholar] [CrossRef]
- Shen, D.K.; Yang, J.P.; Li, X.M.; Zhou, L.; Zhang, R.Y.; Li, W. Biphase stratification approach to three-dimensional dendritic biodegradable mesoporous silica nanospheres. Nano Lett. 2014, 14, 9232. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; He, J.H. Amino-functionalized silica nanoparticles with center-radially hierarchical mesopores as ideal catalyst carriers. Nanoscale 2012, 4, 8529. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Du, X.; Shi, B.Y.; Bi, J.X.; Kleitz, F.; Qiao, S.Z. Tunable stellate mesoporous silica nanoparticles for intracellular drug delivery. J. Mater. Chem. B 2015, 3, 1712. [Google Scholar] [CrossRef]
- Yu, Y.J.; Xing, J.L.; Pang, J.L.; Jiang, S.H.; Lam, K.F.; Yang, T.Q. Facile synthesis of size controllable dendritic mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 22655. [Google Scholar] [CrossRef]
- Zhang, H.; Darvel, B. Mechanical properties of hydroxyapatite whisker reinforced Bis-GMA-based resin composites. Dent. Mater. 2012, 28, 824. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Martin, T.; Antonucci, J.; Eichmiller, F. Ceramic whisker reinforcement of dental resin composites. J. Dent. Res. 1999, 78, 706. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.W.; Jiang, X.Z.; Zhang, Q.H.; Zhu, M.F. Strong and bioactive dental resin composite containing poly (Bis-GMA) grafted hydroxyapatite whiskers and silica nanoparticles. Compo. Sci. Technol. 2014, 101, 86. [Google Scholar] [CrossRef]
- Santos, C.; Clarke, R.L.; Braden, M.; Guitian, F.; Davy, K.W. Water absorption characteristics of dental composites incorporating hydroxyapatite filler. Biomaterials 2002, 23, 1897. [Google Scholar] [CrossRef]
- Flury, S.; Hayoz, S.; Peutzfeldt, A.; Hüsler, J.; Lussi, A. Depth of cure of resin composites: Is the ISO 4049 method suitable for bulk fill materials? Dent. Mater. 2012, 28, 521. [Google Scholar] [CrossRef]
- Dong, Z.; Ni, Y.; Yang, X.; Hu, C.; Sun, J.; Li, L.; Zhou, C.; Fan, H. Characterization and analysis of fluoride calcium silicate composite interface in remineralization of dental enamel. Compos. Part B Eng. 2018, 153, 393. [Google Scholar] [CrossRef]
- Li, X.K.; Wang, J.F.; Joiner, A.; Chang, J. The remineralisation of enamel: A review of the literature. J. Dent. 2014, 42, S12. [Google Scholar] [CrossRef]
- Garcia, I.M.; Balhaddad, A.A.; Lan, Y.; Simionato, A.; Ibrahim, M.S.; Weir, M.D.; Masri, R.; Xu, H.H.; Collares, F.M.; Melo, M.A.S. Magnetic motion of superparamagnetic iron oxide nanoparticles-loaded dental adhesives: Physicochemical/biological properties, and dentin bonding performance studied through the tooth pulpal pressure model. Acta Biomater. 2021, 134, 337. [Google Scholar] [CrossRef]
- Wang, J.J.; Chen, H.Y.; Liu, H.M.; Wang, R.L.; Qin, Z.Y.; Zhu, M.F. Surface modifications of short quartz fibers and their influence on the physicochemical properties and in vitro cell viability of dental composites. Dent. Mater. 2024, 40, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Gurucharan, I.; Saravana, K.B.; Mahalaxmi, S.; Baskar, K.; Rajkumar, G.; Dhivya, V.; Kishen, A.; Sankaranarayanan, S.; Gurucharan, N. Characterization of nano-hydroxyapatite incorporated carboxymethyl chitosan composite on human dental pulp stem cells. Int. Endod. J. 2023, 56, 486. [Google Scholar] [CrossRef] [PubMed]
- Kulan, P.; Karabiyik, O.; Kose, G.T.; Kargul, B. The effect of accelerated mineral trioxide aggregate on odontoblastic differentiation in dental pulp stem cell niches. Int. Endod. J. 2018, 51, 758. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, M.; Zhang, Q.; Zhang, T.; Tian, T.; Ma, Q.; Xue, C.; Lin, S.; Cai, X. Stiffness regulates the proliferation and osteogenic/odontogenic differentiation of human dental pulp stem cells via the WNT signalling pathway. Cell Prolif. 2018, 51, e12435. [Google Scholar] [CrossRef] [PubMed]
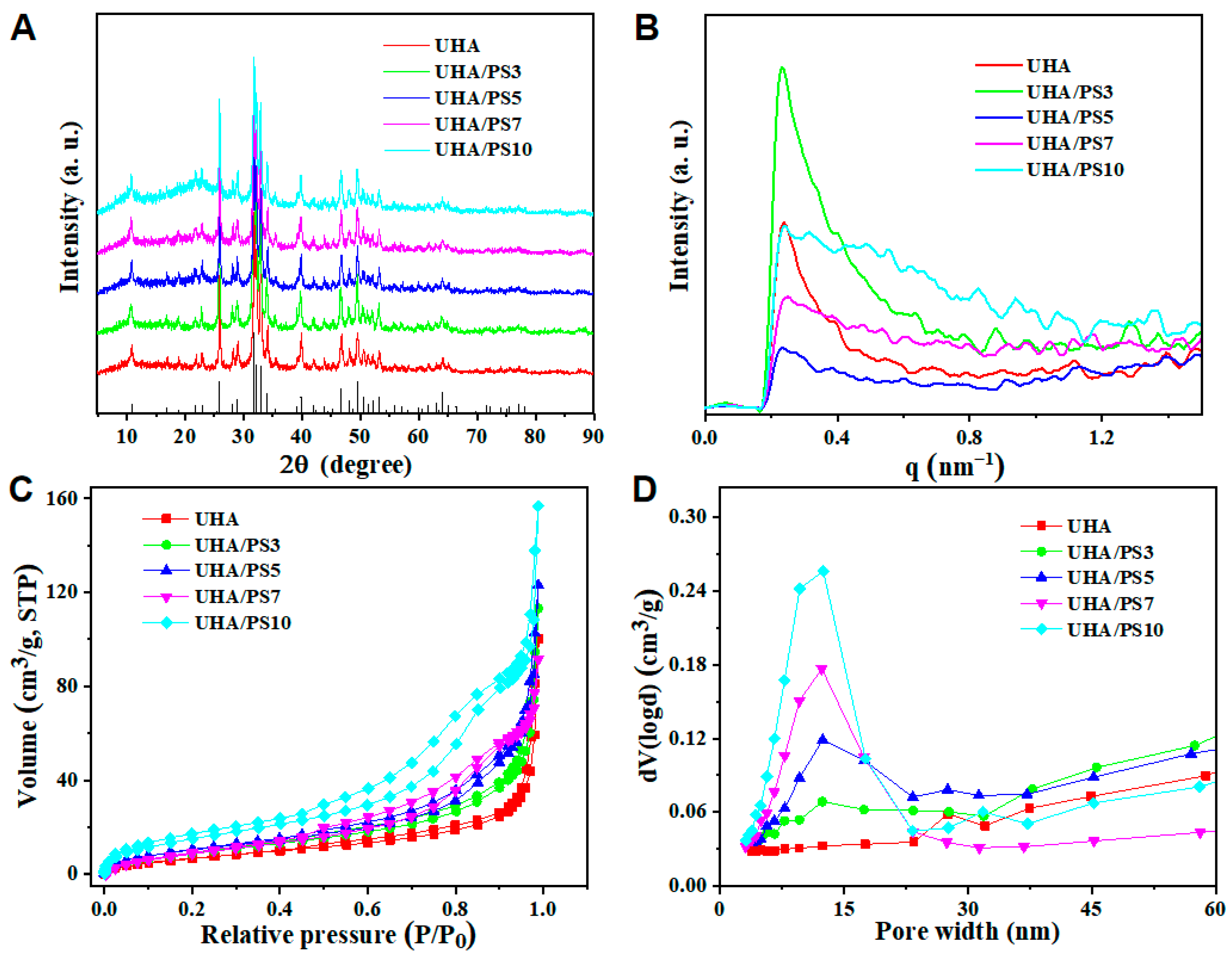


| Particle | Specific Surface Area, SBET (m2/g) a | Cumulative Pore Volume, Vtotal (cm3/g) b |
|---|---|---|
| UHA | 28.27 | 0.15 |
| UHA/PS3 | 35.13 | 0.18 |
| UHA/PS5 | 39.16 | 0.19 |
| UHA/PS7 | 41.37 | 0.22 |
| UHA/PS10 | 59.72 | 0.24 |
| Method | Sample | UHA | UHA/PS3 | UHA/PS5 | UHA/PS7 | UHA/PS10 | |
|---|---|---|---|---|---|---|---|
| Element | |||||||
| XPS (Ar%) | Si | 0.70 | 5.06 | 8.74 | 14.43 | 15.98 | |
| Ca | 17.24 | 14.45 | 11.51 | 6.10 | 5.47 | ||
| O | 57.71 | 54.51 | 52.55 | 46.14 | 48.69 | ||
| P | 13.42 | 11.48 | 9.26 | 4.85 | 4.71 | ||
| C | 10.92 | 14.50 | 18.24 | 28.48 | 25.13 | ||
| ICP-AES (mg/g) | Si a | / | 2.40 | 4.67 | 13.55 | 28.93 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Lyu, J.; Nie, J.; Wang, X.; Yang, N.; Han, S.; Zhou, M. Dual Micromechanical Interlocking Through Filler Surface Modification for Enhanced Dental Composites. Polymers 2025, 17, 2384. https://doi.org/10.3390/polym17172384
Chen H, Lyu J, Nie J, Wang X, Yang N, Han S, Zhou M. Dual Micromechanical Interlocking Through Filler Surface Modification for Enhanced Dental Composites. Polymers. 2025; 17(17):2384. https://doi.org/10.3390/polym17172384
Chicago/Turabian StyleChen, Hongyan, Jiaxuan Lyu, Jia Nie, Xuhui Wang, Na Yang, Sheng Han, and Mingliang Zhou. 2025. "Dual Micromechanical Interlocking Through Filler Surface Modification for Enhanced Dental Composites" Polymers 17, no. 17: 2384. https://doi.org/10.3390/polym17172384
APA StyleChen, H., Lyu, J., Nie, J., Wang, X., Yang, N., Han, S., & Zhou, M. (2025). Dual Micromechanical Interlocking Through Filler Surface Modification for Enhanced Dental Composites. Polymers, 17(17), 2384. https://doi.org/10.3390/polym17172384








