Production and Characterisation of an Exopolysaccharide by Vreelandella titanicae Zn11_249 Isolated from Salar de Uyuni (Bolivia)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Bacterial Strain Sample
2.3. Analysis of Bacterial Growth, Colony Forming Units (CFU)/mL, pH, and EPS Production
2.4. Scanning Electron Microscopy (SEM) in Three Different Media
2.5. Isolation, Purification, and Molecular Weight of EPS in Three Different Media
2.6. Molecular Weight, Compositional Analysis, and Characterisation of the EPSs in Three Different Media
2.6.1. Molecular Weight
2.6.2. Compositional Analysis
2.6.3. Attenuated Total Reflectance/FT-Infrared Spectroscopy (ATR-FTIR)
2.6.4. Differential Scanning Calorimetry (DSC) Analysis
2.7. PCR Amplification and GENOMIC Analysis of EPSs Synthesis.
2.8. Antioxidant Activities of the EPSs in Three Different Media
2.8.1. DPPH Free Radical Scavenging Activity
2.8.2. Hydroxyl Radical Scavenging Activity
2.8.3. Superoxide Anion Scavenging Activity
2.9. Biocompatibility Studies in Three Different Media
2.9.1. Culture of Cells
2.9.2. Cytotoxicity Assay
2.10. Determination of the Antioxidant Ability at the Cellular Level in Three Different Media
Establishment of Injury Model
2.11. Statistical Analysis
3. Results and Discussion
3.1. Strain Identification
3.2. Bacterial Growth, EPS Optimization in Three Different Media, and Scanning Electron Microscopy (SEM)
3.3. Compositional Analysis and Characterisation of EPSs Exopolymer
3.3.1. Molecular Weight Determination of EPSs (EPSU1, EPSU2, and EPSU3) Obtained in Three Different Media
3.3.2. GC-MS Analysis of EPSs (EPSU1, EPSU2, and EPSU3) in Three Different Media
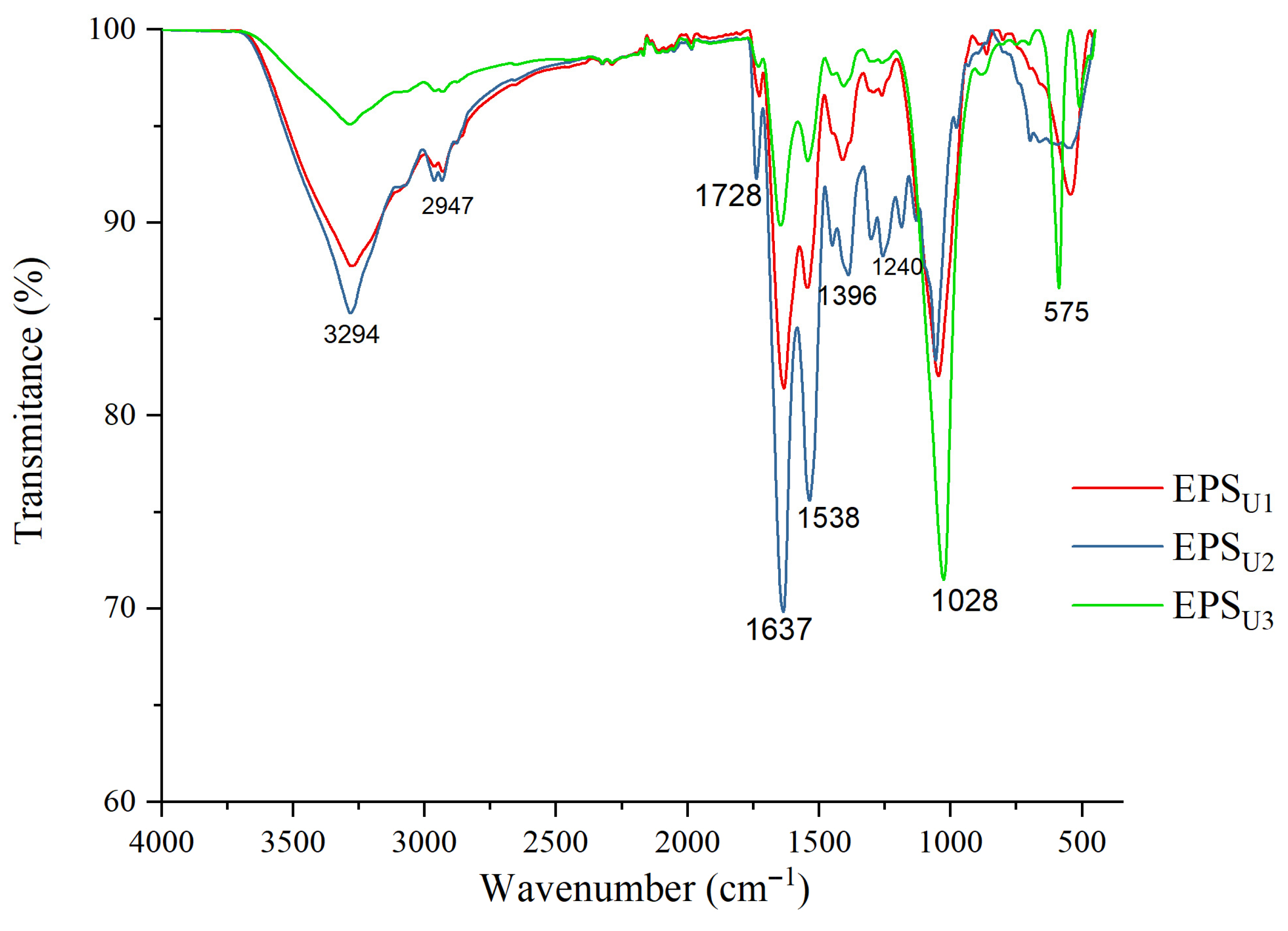
3.3.3. ATR–FTIR Analysis of EPSs
3.3.4. Characterisation of the Thermal Properties of EPSs
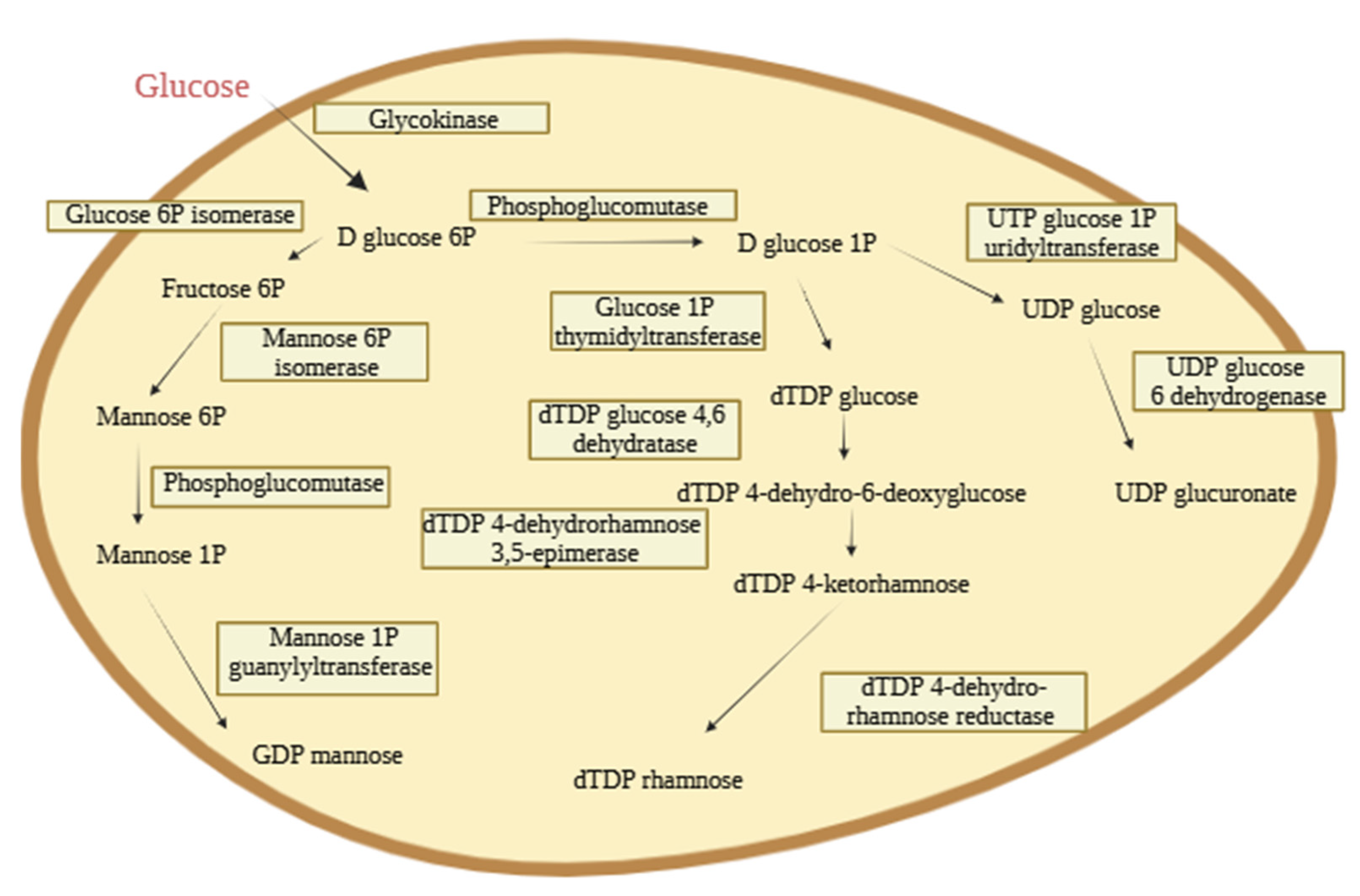
3.3.5. Genomic Analysis of EPS Pathways by Vreelandella titanicae Zn11_249
3.4. Biotechnological Applications
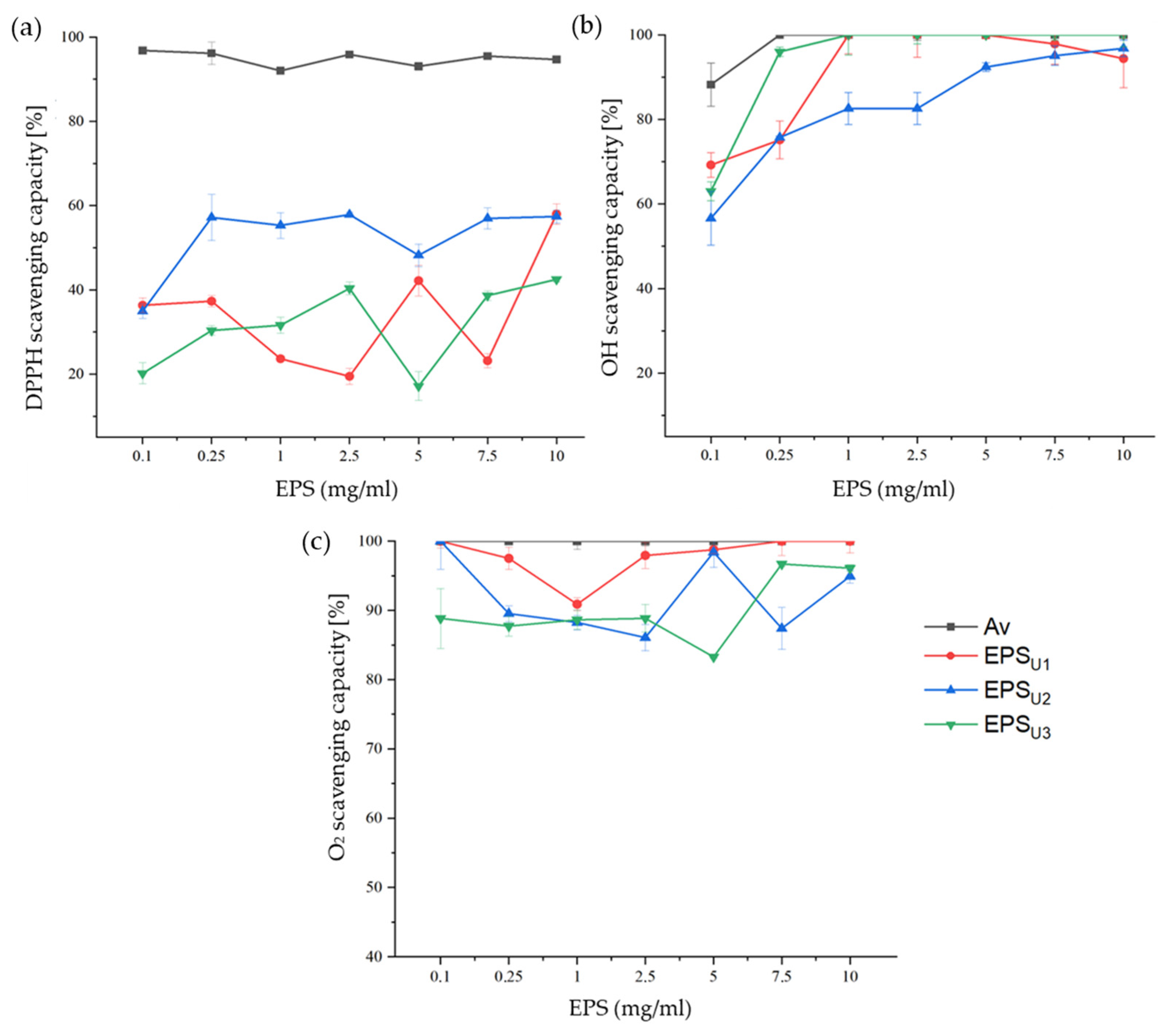
3.4.1. Antioxidant Activity Tests of EPSs
3.4.2. Biocompatibility Studies and Antioxidant Ability at Cellular Level of EPSs (EPSU1, EPSU2, and EPSU3) in Three Different Media
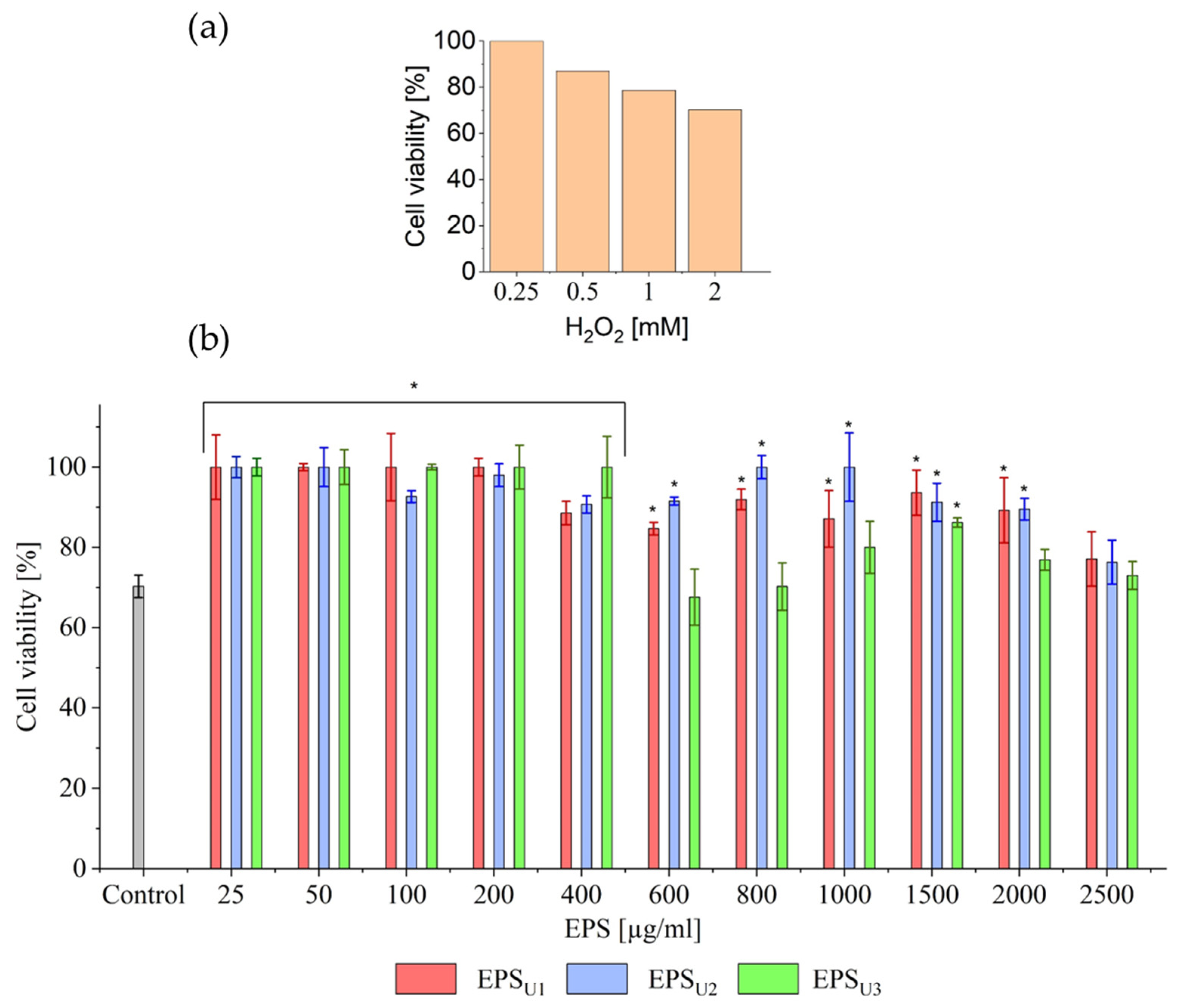
3.4.3. H2O2-Induced Assay and Effects of EPSs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Zeng, S.; Hao, L.; Yao, S.; Wang, D.; Yang, H.; Wu, C. Structural characterization and bioactivity of novel exopolysaccharides produced by Tetragenococcus halophilus. Food Res. Int. 2022, 155, 111083. [Google Scholar] [CrossRef]
- Upadhyaya, C.; Patel, H.; Patel, I.; Upadhyaya, T. Extremophilic Exopolysaccharides: Bioprocess and Novel Applications in 21st Century. Fermentation 2025, 11, 16. [Google Scholar] [CrossRef]
- Ruijgrok, G.; Wu, D.; Overkleeft, H.S.; Codée, J.D.C. Synthesis and application of bacterial exopolysaccharides. Curr. Opin. Chem. Biol. 2024, 78, 102418. [Google Scholar] [CrossRef]
- Thakur, R.; Yadav, S. Biofilm forming, exopolysaccharide producing and halotolerant, bacterial consortium mitigates salinity stress in Triticum aestivum. Int. J. Biol. Macromol. 2024, 262, 130049. [Google Scholar] [CrossRef]
- Ventosa, A.; de la Haba, R.R.; Sánchez-Porro, C.; Papke, R.T. Microbial diversity of hypersaline environments: A metagenomic approach. Curr. Opin. Microbiol. 2015, 25, 87. [Google Scholar] [CrossRef]
- Gan, L.; Huang, X.; He, Z.; He, T. Exopolysaccharide production by salt-tolerant bacteria: Recent advances, current challenges, and future prospects. Int. J. Biol. Macromol. 2024, 264, 130731. [Google Scholar] [CrossRef] [PubMed]
- Llamas, I.; Amjres, H.; Mata, J.A.; Quesada, E.; Béjar, V. The Potential Biotechnological Applications of the Exopolysaccharide Produced by the Halophilic Bacterium Halomonas almeriensis. Molecules 2012, 17, 7103–7120. [Google Scholar] [CrossRef] [PubMed]
- de la Haba, R.R.; Arahal, D.R.; Sánchez-Porro, C.; Chuvochina, M.; Wittouck, S.; Hugenholtz, P.; Ventosa, A. A long-awaited taxogenomic investigation of the family Halomonadaceae. Front. Microbiol. 2023, 14, 1293707. [Google Scholar] [CrossRef]
- Chikkanna, A.; Ghosh, D.; Kishore, A. Expression and characterization of a potential exopolysaccharide from a newly isolated halophilic thermotolerant bacteria Halomonas nitroreducens strain WB1. PeerJ 2018, 6, e4684. [Google Scholar] [CrossRef]
- Amjres, H.; Béjar, V.; Quesada, E.; Carranza, D.; Abrini, J.; Sinquin, C.; Ratiskol, J.; Colliec-Jouault, S.; Llamas, I. Characterization of haloglycan, an exopolysaccharide produced by Halomonas stenophila HK30. Int. J. Biol. Macromol. 2015, 72, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Joulak, I.; Azabou, S.; Finore, I.; Poli, A.; Nicolaus, B.; Donato, P.D.I.; Bkhairia, I.; Dumas, E.; Gharsallaoui, A.; Immirzi, B.; et al. Structural characterization and functional properties of novel exopolysaccharide from the extremely halotolerant Halomonas elongata S6. Int. J. Biol. Macromol. 2020, 164, 95–104. [Google Scholar] [CrossRef]
- Joulak, I.; Finore, I.; Poli, A.; Abid, Y.; Bkhairia, I.; Nicolaus, B.; Di Donato, P.; Dal Poggetto, G.; Gharsallaoui, A.; Attia, H.; et al. Hetero-exopolysaccharide from the extremely halophilic Halomonas smyrnensis K2: Production, characterization and functional properties in vitro. 3 Biotech. 2020, 10, 395. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, C.; Srivastava, G.K.; Carranza, D.; Mata, J.A.; Llamas, I.; Santamaría, M.; Quesada, E.; Molina, I.J. An exopolysaccharide produced by the novel halophilic bacterium Halomonas stenophila strain B100 selectively induces apoptosis in human T leukaemia cells. Appl. Microbiol. Biotechnol. 2011, 89, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Kazak Sarilmiser, H.; Toksoy Oner, E. Investigation of anti-cancer activity of linear and aldehyde-activated levan from Halomonas smyrnensis AAD6T. Biochem. Eng. J. 2014, 92, 28–34. [Google Scholar] [CrossRef]
- Sanchez-Lopez, M. From a White Desert to the Largest World Deposit of Lithium: Symbolic Meanings and Materialities of the Uyuni Salt Flat in Bolivia. Antipode 2019, 51, 1318–1339. [Google Scholar] [CrossRef]
- Hallsworth, J.E.; Prior, B.A.; Nomura, Y.; Iwahara, M.; Timmis, K.N. Compatible Solutes Protect against Chaotrope (Ethanol)-Induced, Nonosmotic Water Stress. Appl. Environ. Microbiol. 2003, 69, 7032–7034. [Google Scholar] [CrossRef]
- Duda, V.I.; Danilevich, V.N.; Suzina, N.E.; Shorokhova, A.P.; Dmitriev, V.V.; Mokhova, O.N.; Akimov, V.N. Changes in the Fine Structure of Microbial Cells Induced by Chaotropic Salts. Microbiology 2004, 73, 341–349. [Google Scholar] [CrossRef]
- Martínez, J.M.; Escudero, C.; Rodríguez, N.; Rubin, S.; Amils, R. Subsurface and surface halophile communities of the chaotropic Salar de Uyuni. Environ. Microbiol. 2021, 23, 3987–4001. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Porro, C.; Kaur, B.; Mann, H.; Ventosa, A. Halomonas titanicae sp. nov., a halophilic bacterium isolated from the RMS Titanic. Int. J. Syst. Evol. Microbiol. 2010, 60, 2768–2774. [Google Scholar] [CrossRef]
- Abrusci, C.; Pablos, J.L.; Marín, I.; Espí, E.; Corrales, T.; Catalina, F. Comparative effect of metal stearates as pro-oxidant additives on bacterial biodegradation of thermal- and photo-degraded low density polyethylene mulching films. Int. Biodeterior. Biodegrad. 2013, 83, 25–32. [Google Scholar] [CrossRef]
- Gómez, F.; Cavalazzi, B.; Rodríguez, N.; Amils, R.; Ori, G.G.; Olsson-Francis, K.; Escudero, C.; Martínez, J.M.; Miruts, H. Ultra-small microorganisms in the polyextreme conditions of the Dallol volcano, Northern Afar, Ethiopia. Sci. Rep. 2019, 9, 7907. [Google Scholar] [CrossRef]
- Sánchez-León, E.; Bello-Morales, R.; López-Guerrero, J.A.; Poveda, A.; Jiménez-Barbero, J.; Gironès, N.; Abrusci, C. Isolation and characterization of an exopolymer produced by Bacillus licheniformis: In vitro antiviral activity against enveloped viruses. Carbohydr. Polym. 2020, 248, 116737. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Aullybux, A.A.; Puchooa, D.; Bahorun, T.; Jeewon, R.; Wen, X.; Matin, P. Antioxidant and Cytotoxic Activities of Exopolysaccharides from Alcaligenes faecalis Species Isolated from the Marine Environment of Mauritius. J. Polym. Environ. 2022, 30, 1462–1477. [Google Scholar] [CrossRef]
- García, R.; Martínez José, M.; Leandro, T.; Amils, R. Draft Genome Sequence of Rhizobium sp. Strain T2.30D-1.1, Isolated from 538.5 Meters Deep on the Subsurface of the Iberian Pyrite Belt. Microbiol. Resour. Announc. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid Annotations using Subsystems Technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef] [PubMed]
- Niknezhad, S.V.; Najafpour-Darzi, G.; Morowvat, M.H.; Ghasemi, Y. Eexopolysaccharide production of Pantoea sp. BCCS 001 GH: Physical characterizations, emulsification, and antioxidant activities. Int. J. Biol. Macromol. 2018, 118, 1103–1111. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, F.; Shi, M.; Zhang, X.; Zhou, B.; Zhang, Y.; Chen, X. Characterization and Biotechnological Potential Analysis of a New Exopolysaccharide from the Arctic Marine Bacterium Polaribacter sp. SM1127. Scietific Rep. 2015, 5, 18435. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Prasad, B.; Rai, A.K.; Velappan, S.P.; Subbanna, M.N.; Narayan, B. In vitro antioxidant and antibacterial properties of hydrolysed proteins of delimed tannery fleshings: Comparison of acid hydrolysis and fermentation methods. Biodegradation 2011, 22, 287–295. [Google Scholar] [CrossRef]
- Huang-Lin, E.; Sánchez-León, E.; Amils, R.; Abrusci, C. Potential Applications of an Exopolysaccharide Produced by Bacillus xiamenensis RT6 Isolated from an Acidic Environment. Polymers 2022, 14, 3918. [Google Scholar] [CrossRef]
- Pérez-Blanco, C.; Huang-Lin, E.; Abrusci, C. Characterization, biodegradation and cytotoxicity of thermoplastic starch and ethylene-vinyl alcohol copolymer blends. Carbohydr. Polym. 2022, 298, 120085. [Google Scholar] [CrossRef] [PubMed]
- Morro, A.; Abrusci, C.; Pablos, J.L.; Marín, I.; García, F.C.; García, J.M. Inherent antibacterial activity and in vitro biocompatibility of hydrophilic polymer film containing chemically anchored sulfadiazine moieties. Eur. Polym. J. 2017, 91, 274–282. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for in vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Lu, S.; Dou, W.; Gu, T.; Chen, S.; Cheng, X.; Hou, R.; Wang, Y.; Zhang, Y.; Liu, G. Extracellular electron transfer corrosion mechanism of two marine structural steels caused by nitrate reducing Halomonas titanicae. Corros. Sci. 2023, 217, 111125. [Google Scholar] [CrossRef]
- Lebre, P.H.; De Maayer, P.; Cowan, D.A. Xerotolerant bacteria: Surviving through a dry spell. Nat. Rev. Microbiol. 2017, 15, 285–296. [Google Scholar] [CrossRef]
- Li, R.; Hou, Y.; Li, H.; Han, Y.; Zhang, D.; Song, Y.; Huang, C.; Guo, J.; Liu, Z.; Wei, W.; et al. Salinity responsive mechanisms of sulfur-based mixotrophic denitrification and ectoine induced tolerance enhancement. Chem. Eng. J. 2024, 496, 154266. [Google Scholar] [CrossRef]
- Park, Y.; Choi, T.; Han, Y.; Song, H.; Park, J.; Bhatia, S.K.; Gurav, R.; Choi, K.; Kim, Y.; Yang, Y. Effects of osmolytes on salt resistance of Halomonas socia CKY01 and identification of osmolytes-related genes by genome sequencing. J. Biotechnol. 2020, 322, 21–28. [Google Scholar] [CrossRef]
- Joulak, I.; Finore, I.; Nicolaus, B.; Leone, L.; Moriello, A.S.; Attia, H.; Poli, A.; Azabou, S. Evaluation of the production of exopolysaccharides by newly isolated Halomonas strains from Tunisian hypersaline environments. Int. J. Biol. Macromol. 2019, 138, 658–666. [Google Scholar] [CrossRef]
- Mata, J.A.; Béjar, V.; Llamas, I.; Arias, S.; Bressollier, P.; Tallon, R.; Urdaci, M.C.; Quesada, E. Exopolysaccharides produced by the recently described halophilic bacteria Halomonas ventosae and Halomonas anticariensis. Res. Microbiol. 2006, 157, 827–835. [Google Scholar] [CrossRef]
- Martínez, F.L.; Rajal, V.B.; Irazusta, V. Removal of lithium from aqueous solutions using halotolerant bacteria from El Salar del Hombre Muerto. J. Environ. Chem. Eng. 2021, 9, 105099. [Google Scholar] [CrossRef]
- Fagliarone, C.; Fernandez, B.G.; Di Stefano, G.; Mosca, C.; Billi, D. Insights into the chaotropic tolerance of the desert cyanobacterium Chroococcidiopsis sp. 029 (Chroococcidiopsales, Cyanobacteria). J. Phycol. 2024, 60, 185–194. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; Hugenholtz, J.; Zoon, P. An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int. Dairy. J. 2002, 12, 163–171. [Google Scholar] [CrossRef]
- Gan, L.; Li, X.; Zhang, H.; Zhang, R.; Wang, H.; Xu, Z.; Peng, B.; Tian, Y. Preparation, characterization and functional properties of a novel exopolysaccharide produced by the halophilic strain Halomonas saliphila LCB169T. Int. J. Biol. Macromol. 2020, 156, 372–380. [Google Scholar] [CrossRef]
- Kim, B.; Yang, A.; Joe, H.; Kim, K.H.; Choe, H.; Joe, S.; Jun, M.O.; Shin, N. Genomic attributes and characterization of novel exopolysaccharide-producing bacterium Halomonas piscis sp. nov. isolated from jeotgal. Front. Microbiol. 2023, 14, 1303039. [Google Scholar] [CrossRef]
- Athmika; Ghate, S.D.; Arun, A.B.; Rao, S.S.; Kumar, S.T.A.; Kandiyil, M.K.; Saptami, K.; Rekha, P.D. Genome analysis of a halophilic bacterium Halomonas malpeensis YU-PRIM-29T reveals its exopolysaccharide and pigment producing capabilities. Sci. Rep. 2021, 11, 1749. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, Y.; Guo, H.; Gan, R.; Peng, L.; Zhao, G.; Zou, L. Physicochemical and Biological Properties of Polysaccharides from Dictyophora indusiata Prepared by Different Extraction Techniques. Polymers 2021, 13, 2357. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ma, X.; Peng, X.; Yao, Z.; Yang, F.; Dai, M. Comparative investigation between co-pyrolysis characteristics of protein and carbohydrate by TG-FTIR and Py-GC/MS. J. Anal. Appl. Pyrolysis 2018, 135, 209–218. [Google Scholar] [CrossRef]
- Bremer, P.J.; Geesey, G.G. An evaluation of biofilm development utilizing non-destructive attenuated total reflectance Fourier transform infrared spectroscopy. Biofouling 1991, 3, 89–100. [Google Scholar] [CrossRef]
- Sánchez-León, E.; Huang-Lin, E.; Amils, R.; Abrusci, C. Production and Characterisation of an Exopolysaccharide by Bacillus amyloliquefaciens: Biotechnological Applications. Polymers 2023, 15, 1550. [Google Scholar] [CrossRef]
- Shang, N.; Xu, R.; Li, P. Structure characterization of an exopolysaccharide produced by Bifidobacterium animalis RH. Carbohydr. Polym. 2013, 91, 128–134. [Google Scholar] [CrossRef]
- Choi, I.S.; Ko, S.H.; Lee, M.E.; Kim, H.M.; Yang, J.E.; Jeong, S.; Lee, K.H.; Chang, J.Y.; Kim, J.; Park, H.W. Production, Characterization, and Antioxidant Activities of an Exopolysaccharide Extracted from Spent Media Wastewater after Leuconostoc mesenteroides WiKim32 Fermentation. ACS Omega 2021, 6, 8171–8178. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, K.; Yin, S.; Liu, S.; Zhu, Y.; Yang, Y.; Wang, C. Purification and characterization of an exopolysaccharide produced by Lactobacillus plantarum HY isolated from home-made Sichuan Pickle. Int. J. Biol. Macromol. 2019, 134, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Karuppiah, P.; Venkatasamy, V.; Viswaprakash, N.; Ramasamy, T. A statistical approach on optimization of exopolymeric substance production by Halomonas sp. S19 and its emulsification activity. Bioresour. Bioprocess. 2015, 2, 48. [Google Scholar] [CrossRef]
- Pandey, P.; Mishra, H.N. Co-microencapsulation of γ-aminobutyric acid (GABA) and probiotic bacteria in thermostable and biocompatible exopolysaccharides matrix. LWT 2021, 136, 110293. [Google Scholar] [CrossRef]
- Chouchane, H.; Boutiti, S.; Ouertani, A.; Hassen, W.; Guesmi, S.; Neifar, M.; Jelassi, H.; Sghaier, H.; Masmoudi, A.S.; Cherif, A. Effect of Gamma Irradiation on Enhanced Biological Activities of Exopolysaccharide from Halomonas desertis G11: Biochemical and Genomic Insights. Polymers 2021, 13, 3798. [Google Scholar] [CrossRef]
- Rahnama Vosough, P.; Habibi Najafi, M.B.; Edalatian Dovom, M.R.; Javadmanesh, A.; Mayo, B. Evaluation of antioxidant, antibacterial and cytotoxicity activities of exopolysaccharide from Enterococcus strains isolated from traditional Iranian Kishk. J. Food Meas. Charact. 2021, 15, 5221–5230. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, G.; Wang, F.; Zhao, H.; Wei, Y.; Liu, L.; Zhang, H. Extraction, characterization, antioxidant activity and rheological behavior of a polysaccharide produced by the extremely salt tolerant Bacillus subtilis LR1. LWT 2022, 162, 113413. [Google Scholar] [CrossRef]
- Arun, J.; Selvakumar, S.; Sathishkumar, R.; Moovendhan, M.; Ananthan, G.; Maruthiah, T.; Palavesam, A. In vitro antioxidant activities of an exopolysaccharide from a salt pan bacterium Halolactibacillus miurensis. Carbohydr. Polym. 2017, 155, 400–406. [Google Scholar] [CrossRef]
- Xiong, Y.; Ju, X.; Li, X.; Gong, Y.; Xu, M.; Zhang, C.; Yuan, B.; Lv, Z.; Qin, S. Fermentation conditions optimization, purification, and antioxidant activity of exopolysaccharides obtained from the plant growth-promoting endophytic actinobacterium Glutamicibacter halophytocola KLBMP 5180. Int. J. Biol. Macromol. 2020, 153, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Liu, F.; Wang, J.; Wang, H.; Zhang, M. Antioxidant activities of an exopolysaccharide isolated and purified from marine Pseudomonas PF-6. Carbohydr. Polym. 2012, 87, 764–770. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Li, D.; Tian, J.; Xiao, L.; Kwok, L.; Li, W.; Sun, Z. Structure characterization, antioxidant capacity, rheological characteristics and expression of biosynthetic genes of exopolysaccharides produced by Lactococcus lactis subsp. lactis IMAU11823. Food Chem. 2022, 384, 132566. [Google Scholar] [CrossRef]
- Ning, Y.; Cao, H.; Zhao, S.; Gao, D.; Zhao, D. Structure and Properties of Exopolysaccharide Produced by Gluconobacter frateurii and Its Potential Applications. Polymers 2024, 16, 1004. [Google Scholar] [CrossRef]
- Sharma, A.; Liu, N.; Ma, Q.; Zheng, H.; Kawazoe, N.; Chen, G.; Yang, Y. PEG assisted P/Ag/Ag2O/Ag3PO4/TiO2 photocatalyst with enhanced elimination of emerging organic pollutants in salinity condition under solar light illumination. Chem. Eng. J. 2020, 385, 123765. [Google Scholar] [CrossRef]
- Dong, J.; Chi, Z.; Lu, S.; Xie, X.; Gong, P.; Li, H.; Liu, W. Bacterial exopolysaccharides: Characteristics and antioxidant mechanism. Int. J. Biol. Macromol. 2025, 289, 138849. [Google Scholar] [CrossRef] [PubMed]
- Usuldin, S.R.; Ilham, Z.; Jamaludin, A.A.; Ahmad, R.; Wan-Mohtar, W. Enhancing Biomass-Exopolysaccharides Production of Lignosus rhinocerus in a High-Scale Stirred-Tank Bioreactor and Its Potential Lipid as Bioenergy. Energies 2023, 16, 2330. [Google Scholar] [CrossRef]
- Wang, J.; Salem, D.R.; Sani, R.K. Two new exopolysaccharides from a thermophilic bacterium Geobacillus sp. WSUCF1: Characterization and bioactivities. New Biotechnol. 2021, 61, 29–39. [Google Scholar] [CrossRef]


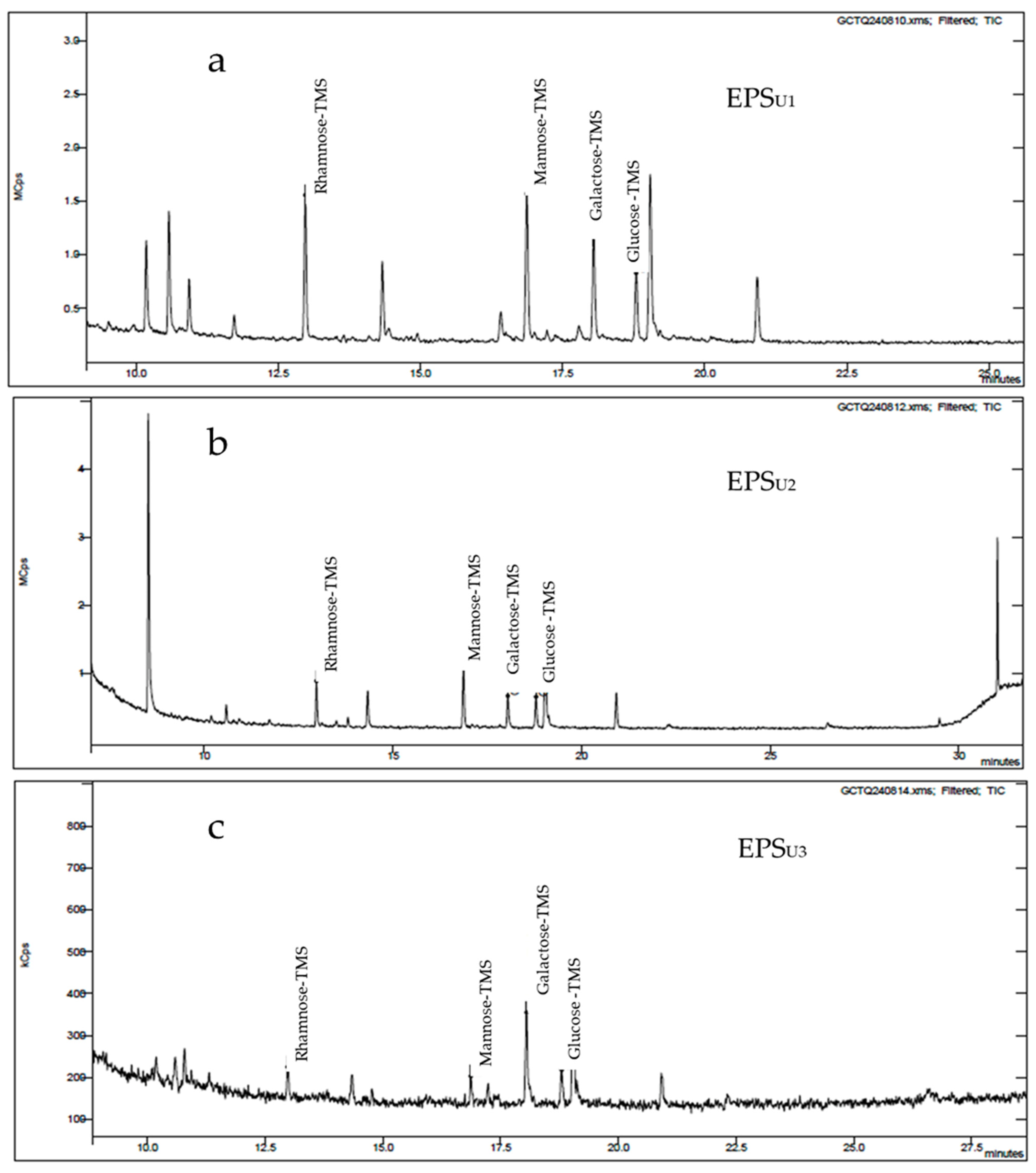


| EPS | Glucose (Molar Ratio [%]) | Mannose (Molar Ratio [%]) | Galactose (Molar Ratio [%]) | Rhamnose (Molar Ratio [%]) |
|---|---|---|---|---|
| EPSU1 | 10 | 40 | 25 | 25 |
| EPSU2 | 30 | 10 | 30 | 30 |
| EPSU3 | 25 | 25 | 25 | 25 |
| EPS | Initial Weight Loss 20–250 °C (%) | Major Weight Loss 250–500 °C (%) | DSC Tm1 (°C) | DSC Tm2 (°C) |
|---|---|---|---|---|
| EPSU1 | 14.99 | 45.31 | 88.76 | 272.09 |
| EPSU2 | 10.90 | 66.30 | 88.76 | 270.00 |
| EPSU3 | 4.30 | 25.51 | 63.83 | 253.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabroso, E.; Martínez, J.M.; Sánchez-León, E.; Rodríguez, N.; Amils, R.; Abrusci, C. Production and Characterisation of an Exopolysaccharide by Vreelandella titanicae Zn11_249 Isolated from Salar de Uyuni (Bolivia). Polymers 2025, 17, 2362. https://doi.org/10.3390/polym17172362
Sabroso E, Martínez JM, Sánchez-León E, Rodríguez N, Amils R, Abrusci C. Production and Characterisation of an Exopolysaccharide by Vreelandella titanicae Zn11_249 Isolated from Salar de Uyuni (Bolivia). Polymers. 2025; 17(17):2362. https://doi.org/10.3390/polym17172362
Chicago/Turabian StyleSabroso, Esteban, José M. Martínez, Enrique Sánchez-León, Nuria Rodríguez, Ricardo Amils, and Concepción Abrusci. 2025. "Production and Characterisation of an Exopolysaccharide by Vreelandella titanicae Zn11_249 Isolated from Salar de Uyuni (Bolivia)" Polymers 17, no. 17: 2362. https://doi.org/10.3390/polym17172362
APA StyleSabroso, E., Martínez, J. M., Sánchez-León, E., Rodríguez, N., Amils, R., & Abrusci, C. (2025). Production and Characterisation of an Exopolysaccharide by Vreelandella titanicae Zn11_249 Isolated from Salar de Uyuni (Bolivia). Polymers, 17(17), 2362. https://doi.org/10.3390/polym17172362









