Three-Dimensional Printing and Bioprinting Strategies for Cardiovascular Constructs: From Printing Inks to Vascularization
Abstract
1. Introduction

2. Bioink Development for Cardiovascular Tissue Engineering
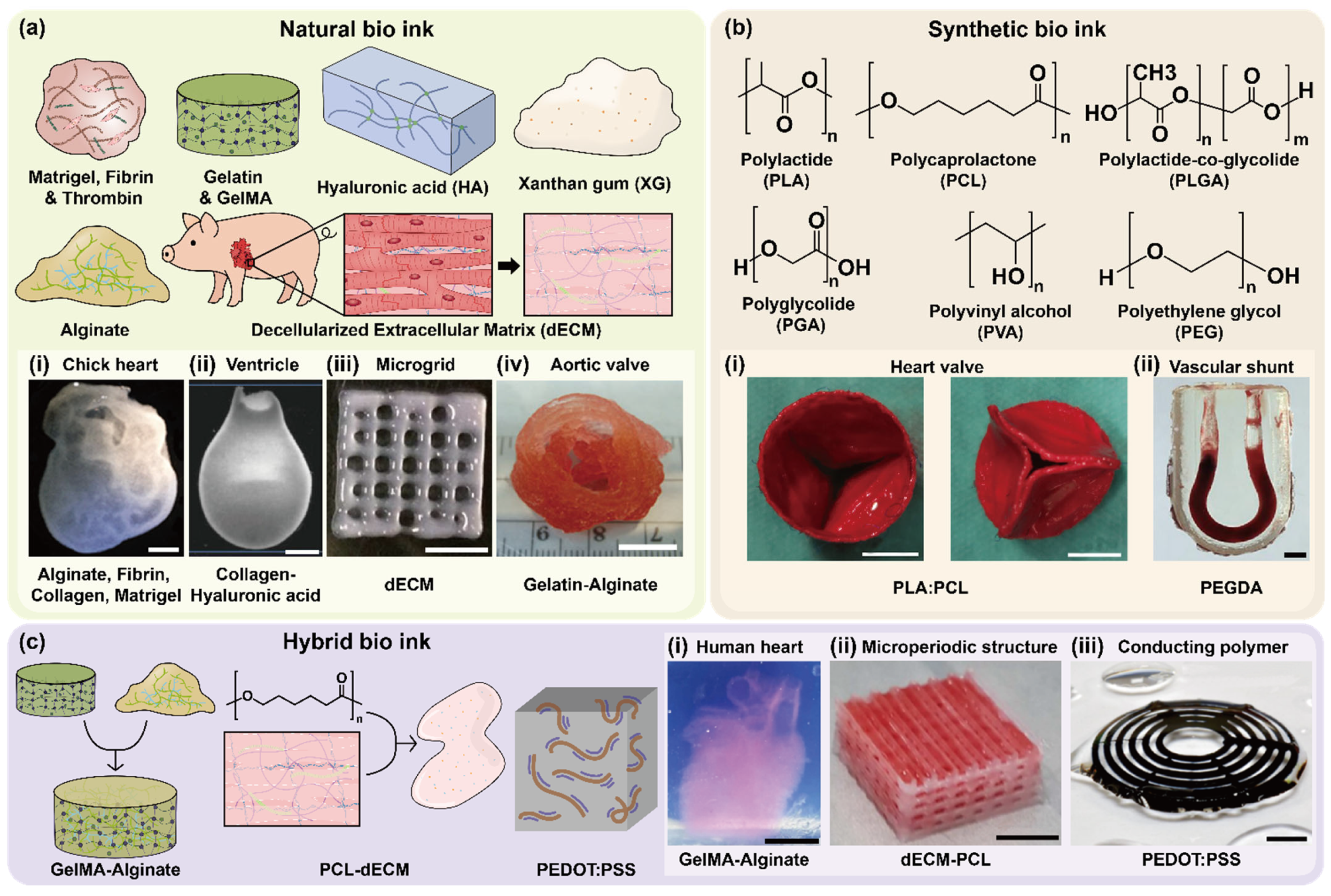
2.1. Natural Bioinks
2.2. Synthetic Bioinks
2.3. Hybrid Bioinks
2.4. Functional Categorization of Bioinks
2.5. Emerging and Stimuli-Responsive Bioinks
2.6. AI-Assisted Bioink Optimization and Predictive Design
| Bioink Composition | Key Properties | Bioprinting Method | Crosslinking | Applications | Cited Models/Cell Type | References |
|---|---|---|---|---|---|---|
| Alginate, Methacrylated collagen, CNT | Electrically conductive, enhanced adhesion | Extrusion | Photo/ionic crosslinking | Cardiac patch development | HCAECs | [61] |
| Gelatin | Contact guidance, enhanced adhesion | Extrusion | Enzymatic | Cardiac patch development, in vitro cardiac modeling | Neonatal rat CMs, hMSCs | [42] |
| GelMa, HAGM, PEGDA | Contact guidance, Respond to inotropic agents at low concentrations | DLP | Photocrosslinking | in vitro cardiac modeling | Neonatal rat CMs | [44] |
| Cardiac dECM, GelMA, Eosin Y | Biochemical specificity, pro-angiogenic | Extrusion | Photocrosslinking | Cardiac patch development | hCPCs, rat CFBs | [40] |
| Gelatin, Fibronectin, HA, Fibrinogen, Thrombin | High cell viability, electrophysiological activity | Extrusion | Photocrosslinking | in vitro cardiac modeling | hiPSC-CMs, hCFBs | [68] |
| GelMA, Alginate | Contact guidance, bioactive, structural stability | Extrusion | Photo/ionic crosslinking | Endothelialized cardiac chips | Neonatal rat CMs, HUVECs | [18] |
| PCL, CNT | Electroconductivity, contact guidance | Extrusion | Thermal | in vitro cardiac modeling | H9c2 | [47] |
| PCL, Human dECM | Regenerative, supports migration | Extrusion | Thermal | Epicardial stem cell patch | hCPCs, hMSCs | [39] |
| Alginate, PEGDA, Fibrinogen, Irgacure 295 | Vascular integration, endothelialization | Extrusion | Photocrosslinking | Vascularized cardiac tissue engineering | iPSC-CMs, HUVECs | [49] |
| Carbopol, Alginate, Gelatin | Various complex structures were fabricated using ionic, photocrosslinkable and thermoresponsive bioinks by adjusting the concentration and pH of the Carbopol support bath | FRESH | Ionic crosslinking | Vascular tissue engineering | NIH 3T3 | [69] |
| Adamantane/β-cyclodextrin-modified MeHA | Supramolecular assembly, guest–host interaction, free-standing structure fabrication | FRESH | Photocrosslinking | Spiral and branching channels | hMSCs, NIH 3T3 | [70] |
| Pluronic F127 diacrylate, Pluronic F127 | Microvascular structuring, tissue engineering, organ modeling | FRESH | Photocrosslinking | Microvascular networks | - | [15] |
| Pluronic F127, Alginate, GelMA | Continuous multimaterial extrusion, digitally tunable printhead, shear-thinning bioinks | FRESH | Photo/ionic crosslinking | Multimaterial tissue fabrication | HDFs, HepG2, hMSCs, HUVECs, MC3T3 | [16] |
| Agarose, GelMA, Matrigel®, Fibronectin, Alginate | CLASS (agarose slurry support), freestanding construct stability, multi-bioink compatibility | FRESH | Photo/ionic crosslinking | Freestanding soft tissue printing, long-term in vitro culture | HEK 293 | [43] |
| Perfluoro-tributylamine support bath, Agarose | Long-term structural stability, Cell viability, and proliferation | FRESH | Thermal | 3D construct mimicking a vascular bifurcation and printed cylinders | hMSC, MG63 | [71] |
| Gellan gum, Laponite, Alginate, Gelatin, PEGDA | Gellan support baths support the printing of functional bioinks that can crosslink with physical, enzymatic, and photocrosslinking mechanisms | FRESH | Ionic, enzymatic, photocrosslinking | Freestanding soft tissue printing | NIH 3T3 | [72] |
| Alginate, Xanthan gum, Collagen, Carboxymethylcellulose | Biocompatibility, multi-cellular printing | FRESH | Thermal | Freestanding soft tissue printing, long-term in vitro culture | NIH 3T3, HUVECs | [73] |
| Collagen, Matrigel®, Gelatin (sacrificial) | High cell density, Perfusable vascular channels | FRESH | Thermal, enzymatic crosslinking | Organ-specific tissues, vascularized cardiac engineering, and pharmaceutical testing | HUVEC, iPSC-derived cardiac/cerebral organ building blocks | [12] |
| Gelatin, Pluronic F127, Gum arabic, Alginate, Collagen, Fibronectin, MeHA, Fibrinogen | Freeform constructs composed of multiple materials and nonplanar features | FRESH | Ionic, enzymatic crosslinking, pH-driven gelation | Freestanding soft tissue printing, vascularized cardiac tissue engineering | hESC-CMs, C2C12, MC3T3 | [9,19,74,75] |
| PCL | Faster pore bridging for the radial pattern | MEW | Thermal | Heart valve tissue engineering, integration with cell-laden hydrogels | hUVSMCs | [76] |
| PCL | Contact guidance | MEW | Thermal | Scaffold design for orthopedic surgery, tubular and gradient scaffold design | MC3T3-E1 | [77] |
| pHMGCL/PCL | Contact guidance | MEW | Thermal | Cardiac tissue engineering | CPCs | [78] |
| PCL | Contact guidance | MEW | Thermal | Anatomical model fabrication, long-term cell-laden structure maintenance | hMSCs | [79] |
| PCL | Elongation along the fibers for a higher laydown angle, while the lamellar shape of cells is on the smaller laydown angle | MEW | Thermal | Optimization of scaffold architecture for controlled cell confinement using machine learning | NHDFs | [80] |
| PCL | Increased cell proliferation and cell–cell interactions on more dense coils | MEW | Thermal | In vivo implantation scaffold, mechanically tunable scaffold platform | hMSCs | [81] |
| PCL | Unidirectional cell alignment for rhombus pores with increased gene expressions. | MEW | Thermal | in vitro renal tubule model, drug screening, regenerative medicine | ciPTECs, HUVECs | [82] |
| GelMA, GM-HA | μCOB bioprinting, prevascularized architecture, multi-cell encapsulation | DLP | Photocrosslinking | Engineering of vascularized tissue constructs with complex microarchitectures | HUVECs, C3H/10T1/2, SCID mice | [83] |
| GelMA, GM-HA | Hexagonal microarchitecture, triculture (hiPSC-HPCs, HUVECs | DLP | Photocrosslinking | 3D triculture liver model | hiPSC-HPCs, ASCs, HUVECs | [84] |
| GelMA, GM-HA | Tri-regional patterning, HA-based, stiffness-tunable | DLP | Photocrosslinking | Tri-regional GBM model | GBM cells, HUVECs | [85] |
| GelMA, GM-HA | HA-rich hydrogel, multicellular co-culture (GSCs, astrocytes | DLP | Photocrosslinking | GBM environment model | GSCs, macrophages, NPCs, astrocytes | [86] |
| GelMA, dECM (Liver) | Tunable stiffness, UV curable | DLP | Photocrosslinking | Liver model of hepatocellular carcinoma | HepG2 | [16] |
| GelMA, nHA | GelMA-nHA Composite supports stromal-cancer cell co-culture, stereolithographic printing | SLA | Photocrosslinking | Breast cancer model | MSCs, human osteoblasts, BrCa cells | [87] |
| OMA-PEGDA | Spatial co-culture, maskless patterning, tunable mechanical and degradation properties | SLA | Photocrosslinking | Co-culture of neurons and muscle myoblasts | Primary hippocampus neurons, skeletal muscle myoblasts | [88] |
| TAZ, DAS | Water-soluble, photocurable, high cell viability | 2PP | Photocrosslinking | 3D cell-laden constructs, stem cell culture, in vitro tissue modeling | ASCs | [89] |
| PEGDA, Irgacure 369 | Photocurable, high-resolution structuring, RGD motif-presenting | 2PP | Photocrosslinking | Bone tissue engineering, microstructured scaffolds | Ovine endothelial cells | [90] |
3. Three-Dimensional Printing and Bioprinting Technologies for Cardiovascular Constructs: Strengths, Limitations, and Translational Perspectives
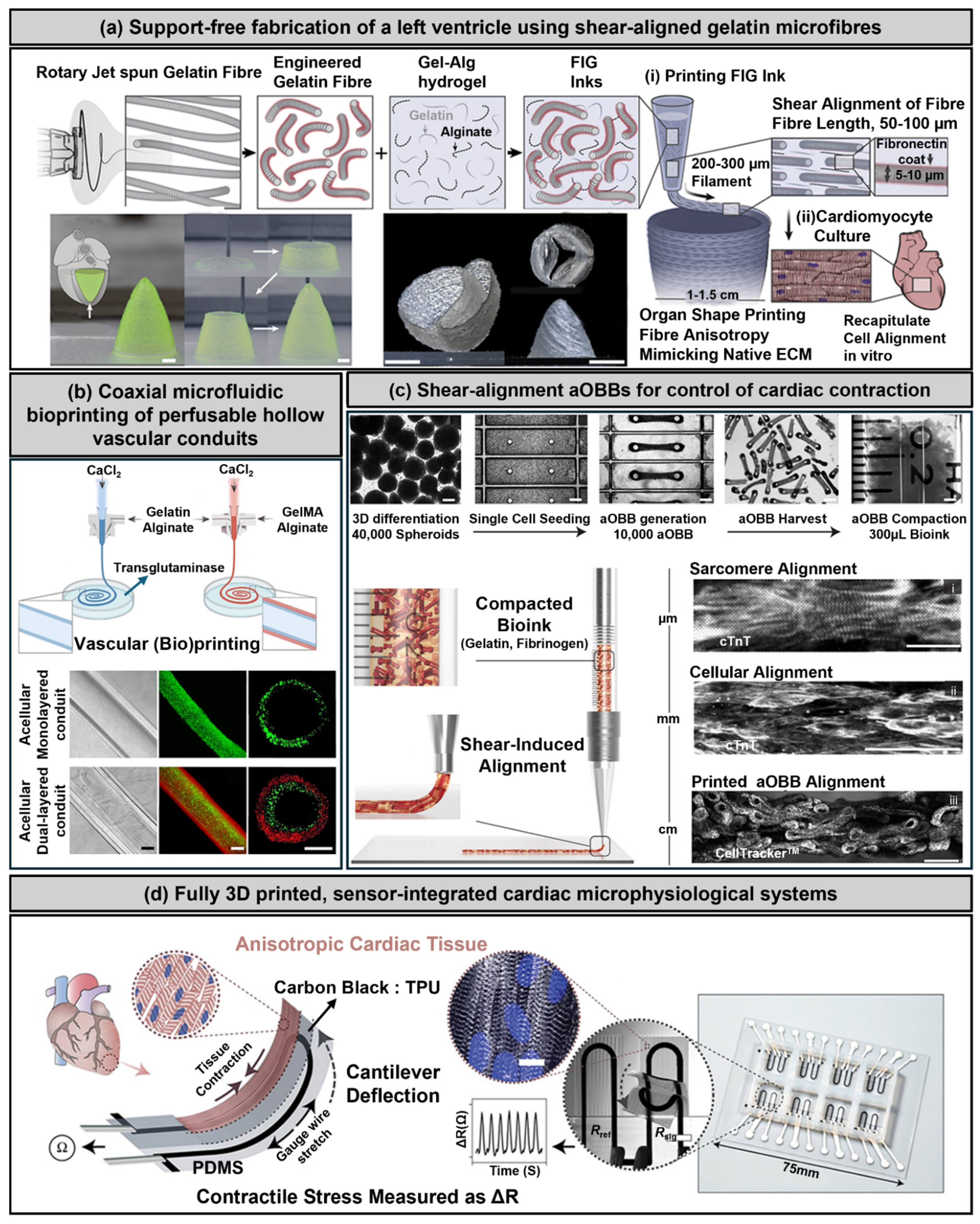
3.1. Extrusion-Based Bioprinting
3.2. Freeform Reversible Embedding of Suspended Hydrogels (FRESH)
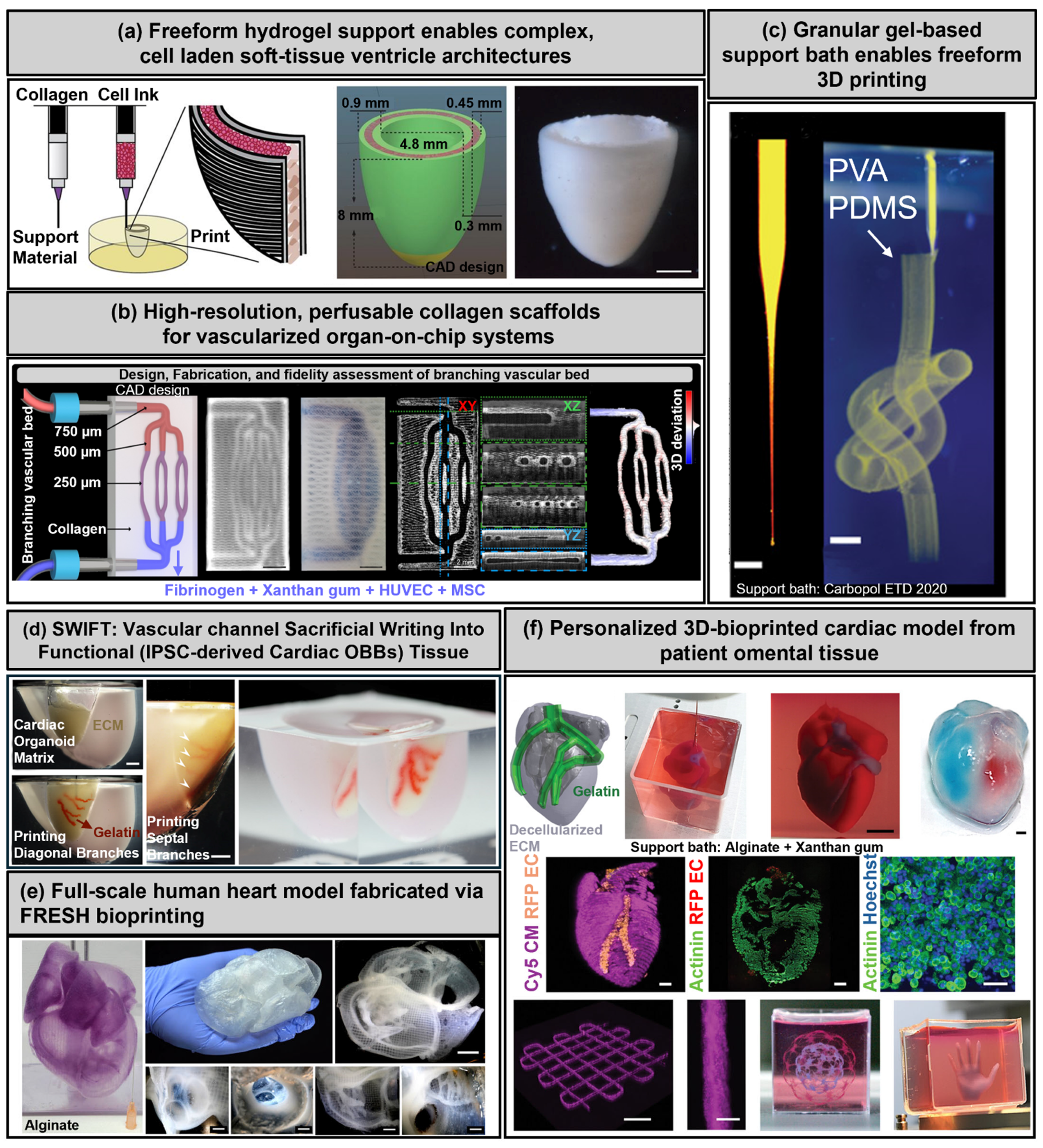
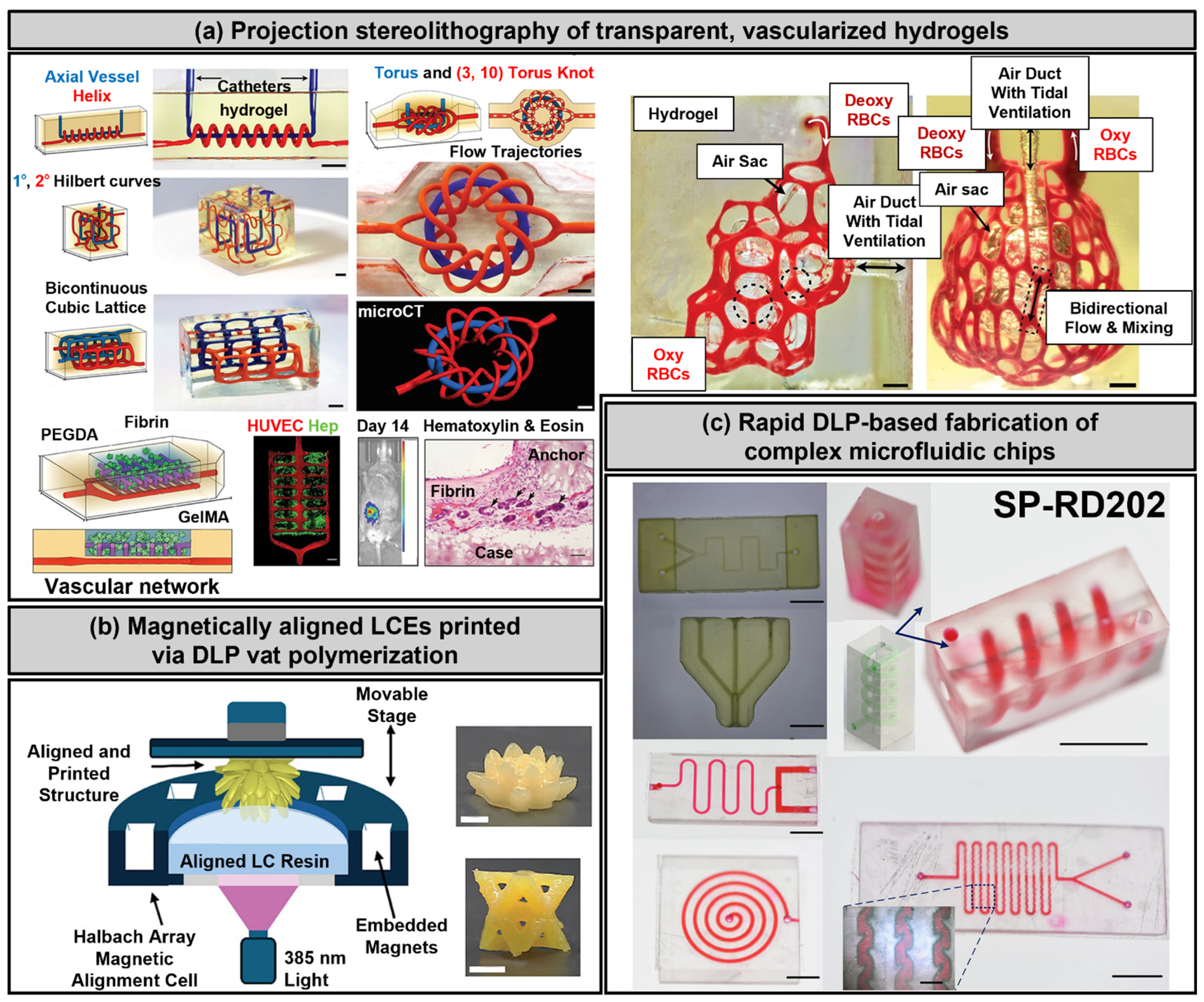
3.3. Stereolithography and Digital Light Processing
3.4. Melt Electrowriting
3.5. Two-Photon Polymerization
3.6. Challenges and Mitigation Strategies
4. Challenges in Cardiovascular Bioprinting
4.1. Achieving Functional Vascularization
4.2. Ensuring Mechanical Stability
4.3. Maintaining Long-Term Functionality
4.4. Promoting Functional Engraftment
5. Applications of 3D-Bioprinted Cardiovascular Tissues
5.1. Clinical and Therapeutic Applications
5.2. Toward Whole-Organ Bioprinting
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CNT | Carbon nanotube |
| HCAECs | Human coronary artery endothelial cells |
| CMs | Cardiomyocytes |
| hMSCs | Human mesenchymal stem cells |
| GelMa | Methacrylated gelatin |
| HAGM | Hyaluronic acid glycidyl methacrylate |
| LAP | Lithium phenyl-2,4,6-trimethylbenzoylphosphinate |
| PEGDA | Poly(ethylene glycol) diacrylate |
| dECM | Decellularized extracellular matrix |
| hCPCs | Human cardiac progenitor cells |
| hiPSC-CMs | Human induced pluripotent stem cell-derived cardiomyocytes |
| hCFBs | Human cardiac fibroblasts |
| HA | Hyaluronic acid |
| HUVECs | Human umbilical vein endothelial cells |
| PCL | Polycaprolactone |
| TOCNF | TEMPO-oxidized cellulose nanofibrils (TOCNF) (where TEMPO: 2,2,6,6-Tetramethylpiperidine-1-oxyl) |
| FRESH | Freeform reversible embedding of suspended hydrogels |
| MeHA | Methacrylated Hyaluronic Acid |
| PCL | Poly ε-caprolactone |
| HUVSMCs | Human umbilical cord vein smooth muscle cells |
| pHMGCL | Poly(hydroxymethylglycolide-co-ε-caprolactone |
| ciPTECs | Conditionally immortalized proximal tubular epithelial cells |
| GM-HA | Glycidal methacrylate–hyaluronic acid |
| SCID | Severe combined immunodeficiency |
| hiPSC-HPCs | hiPSC-derived hepatic progenitor cells |
| GBM | Glioblastoma multiform |
| GSCs | Glioblastoma stem cells |
| NPCs | Neural progenitor cells |
| ASCs | Adipose-derived stem cells |
| nHA | Nanocrystalline hydroxyapatite |
| OMA | Oxidized methacrylic alginate |
| TAZ | 4,4′-(1,2-ethenediyl)bis [3-sulfobenzenediazonium]dichloride |
| DAS | 4,4′-(1,2-ethenediyl)bis [2-(3-sulfophenyl)diazenesulfonate] |
References
- He, L.; Nguyen, N.B.; Ardehali, R.; Zhou, B. Heart Regeneration by Endogenous Stem Cells and Cardiomyocyte Proliferation. Circulation 2020, 142, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef]
- Hwang, D.G.; Choi, Y.-m.; Jang, J. 3D Bioprinting-Based Vascularized Tissue Models Mimicking Tissue-Specific Architecture and Pathophysiology for in vitro Studies. Front. Bioeng. Biotechnol. 2021, 9, 685507. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Dong, C.; Petrovic, M.; Davies, I.J. Applications of 3D printing in medicine: A review. Ann. 3D Print. Med. 2024, 14, 100149. [Google Scholar] [CrossRef]
- Choi, S.; Lee, K.Y.; Kim, S.L.; MacQueen, L.A.; Chang, H.; Zimmerman, J.F.; Jin, Q.; Peters, M.M.; Ardoña, H.A.M.; Liu, X.; et al. Fibre-infused gel scaffolds guide cardiomyocyte alignment in 3D-printed ventricles. Nat. Mater. 2023, 22, 1039–1046. [Google Scholar] [CrossRef]
- Gardin, C.; Ferroni, L.; Latremouille, C.; Chachques, J.C.; Mitrečić, D.; Zavan, B. Recent Applications of Three Dimensional Printing in Cardiovascular Medicine. Cells 2020, 9, 742. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.; Shiwarski, D.; Tashman, J.; Hinton, T.; Yerneni, S.; Bliley, J.; Campbell, P.; Feinberg, A. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Taghizadeh, A.; Yazdi, M.K.; Zarrintaj, P.; Stadler, F.J.; Ramsey, J.D.; Habibzadeh, S.; Hosseini Rad, S.; Naderi, G.; Saeb, M.R.; et al. Chitosan-based inks for 3D printing and bioprinting. Green Chem. 2022, 24, 62–101. [Google Scholar] [CrossRef]
- Panda, S.; Hajra, S.; Mistewicz, K.; Nowacki, B.; In-na, P.; Krushynska, A.; Mishra, Y.K.; Kim, H.J. A focused review on three-dimensional bioprinting technology for artificial organ fabrication. Biomater. Sci. 2022, 10, 5054–5080. [Google Scholar] [CrossRef]
- Skylar-Scott, M.A.; Uzel, S.G.; Nam, L.L.; Ahrens, J.H.; Truby, R.L.; Damaraju, S.; Lewis, J.A. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 2019, 5, eaaw2459. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, S.M.; George, S.C. Nonsteady State Oxygen Transport in Engineered Tissue: Implications for Design. Tissue Eng. Part A 2013, 19, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; DeConinck, A.; Lewis, J.A. Omnidirectional printing of 3D microvascular networks. Adv. Mater. 2011, 23, H178–H183. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.S.; Heinrich, M.A.; De Ferrari, F.; Jang, H.L.; Bakht, S.M.; Alvarez, M.M.; Yang, J.; Li, Y.C.; Trujillo-de Santiago, G. Rapid continuous multimaterial extrusion bioprinting. Adv. Mater. 2017, 29, 1604630. [Google Scholar] [CrossRef]
- O’Connor, C.; Brady, E.; Zheng, Y.; Moore, E.; Stevens, K.R. Engineering the multiscale complexity of vascular networks. Nat. Rev. Mater. 2022, 7, 702–716. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V.; et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016, 110, 45–59. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef]
- He, Y.; Wu, Y.; Fu, J.Z.; Gao, Q.; Qiu, J.J. Developments of 3D printing microfluidics and applications in chemistry and biology: A review. Electroanalysis 2016, 28, 1658–1678. [Google Scholar] [CrossRef]
- Moroni, L.; Burdick, J.A.; Highley, C.; Lee, S.J.; Morimoto, Y.; Takeuchi, S.; Yoo, J.J. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater. 2018, 3, 21–37. [Google Scholar] [CrossRef]
- Burke, J.F.; Yannas, I.V.; Quinby, W.C., Jr.; Bondoc, C.C.; Jung, W.K. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann. Surg. 1981, 194, 413–428. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Cao, Y.; Vacanti, J.P.; Paige, K.T.; Upton, J.; Vacanti, C.A. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast. Reconstr. Surg. 1997, 100, 297–302; discussion 294–303. [Google Scholar] [CrossRef]
- Brassard, J.A.; Nikolaev, M.; Hübscher, T.; Hofer, M.; Lutolf, M.P. Recapitulating macro-scale tissue self-organization through organoid bioprinting. Nat. Mater. 2021, 20, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Davidson, M.D.; Burdick, J.A. 3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels. Nat. Commun. 2021, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Kim, H.; Kim, B.S.; Kong, J.S.; Lee, J.Y.; Park, B.W.; Chae, S.; Kim, J.; Ban, K.; Jang, J.; et al. Tissue-engineering of vascular grafts containing endothelium and smooth-muscle using triple-coaxial cell printing. Appl. Phys. Rev. 2019, 6, 041402. [Google Scholar] [CrossRef]
- Lind, J.U.; Busbee, T.A.; Valentine, A.D.; Pasqualini, F.S.; Yuan, H.; Yadid, M.; Park, S.-J.; Kotikian, A.; Nesmith, A.P.; Campbell, P.H. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater. 2017, 16, 303–308. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Zeng, H.; Liu, D.; Ji, Z.; Xu, X.; Jia, X.; Jiang, P.; Fan, Z.; Wang, X.; et al. Biomechanically Compatible Hydrogel Bioprosthetic Valves. Chem. Mater. 2022, 34, 6129–6141. [Google Scholar] [CrossRef]
- Jungst, T.; Pennings, I.; Schmitz, M.; Rosenberg, A.J.W.P.; Groll, J.; Gawlitta, D. Heterotypic Scaffold Design Orchestrates Primary Cell Organization and Phenotypes in Cocultured Small Diameter Vascular Grafts. Adv. Funct. Mater. 2019, 29, 1905987. [Google Scholar] [CrossRef]
- Michas, C.; Karakan, M.Ç.; Nautiyal, P.; Seidman, J.G.; Seidman, C.E.; Agarwal, A.; Ekinci, K.; Eyckmans, J.; White, A.E.; Chen, C.S. Engineering a living cardiac pump on a chip using high-precision fabrication. Sci. Adv. 2022, 8, eabm3791. [Google Scholar] [CrossRef]
- Shi, H.; Wang, C.; Ma, Z. Stimuli-responsive biomaterials for cardiac tissue engineering and dynamic mechanobiology. APL Bioeng. 2021, 5, 011506. [Google Scholar] [CrossRef] [PubMed]
- Raees, S.; Ullah, F.; Javed, F.; Akil, H.M.; Jadoon Khan, M.; Safdar, M.; Din, I.U.; Alotaibi, M.A.; Alharthi, A.I.; Bakht, M.A.; et al. Classification, processing, and applications of bioink and 3D bioprinting: A detailed review. Int. J. Biol. Macromol. 2023, 232, 123476. [Google Scholar] [CrossRef] [PubMed]
- Ratheesh, G.; Venugopal, J.R.; Chinappan, A.; Ezhilarasu, H.; Sadiq, A.; Ramakrishna, S. 3D Fabrication of Polymeric Scaffolds for Regenerative Therapy. ACS Biomater. Sci. Eng. 2017, 3, 1175–1194. [Google Scholar] [CrossRef] [PubMed]
- Esser, T.U.; Anspach, A.; Muenzebrock, K.A.; Kah, D.; Schrüfer, S.; Schenk, J.; Heinze, K.G.; Schubert, D.W.; Fabry, B.; Engel, F.B. Direct 3D-Bioprinting of hiPSC-Derived Cardiomyocytes to Generate Functional Cardiac Tissues. Adv. Mater. 2023, 35, 2305911. [Google Scholar] [CrossRef]
- Puluca, N.; Lee, S.; Doppler, S.; Münsterer, A.; Dreßen, M.; Krane, M.; Wu, S.M. Bioprinting Approaches to Engineering Vascularized 3D Cardiac Tissues. Curr. Cardiol. Rep. 2019, 21, 90. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.-H.; Won Kim, S.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef]
- Jang, J.; Park, H.J.; Kim, S.W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.; et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Bejleri, D.; Streeter, B.W.; Nachlas, A.L.Y.; Brown, M.E.; Gaetani, R.; Christman, K.L.; Davis, M.E. A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair. Adv. Healthc. Mater. 2018, 7, e1800672. [Google Scholar] [CrossRef]
- Yoon, J.; Jang, J. Decellularized Tissue-Derived Materials as Advanced Bioinks. In Handbook of the Extracellular Matrix: Biologically-Derived Materials; Maia, F.R., Oliveira, J.M., Reis, R.L., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 903–945. [Google Scholar]
- Tijore, A.; Irvine, S.A.; Sarig, U.; Mhaisalkar, P.; Baisane, V.; Venkatraman, S. Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel. Biofabrication 2018, 10, 025003. [Google Scholar] [CrossRef]
- Mirdamadi, E.; Muselimyan, N.; Koti, P.; Asfour, H.; Sarvazyan, N. Agarose slurry as a support medium for bioprinting and culturing freestanding cell-laden hydrogel constructs. 3D Print. Addit. Manuf. 2019, 6, 158–164. [Google Scholar] [CrossRef]
- Liu, J.; Miller, K.; Ma, X.; Dewan, S.; Lawrence, N.; Whang, G.; Chung, P.; McCulloch, A.D.; Chen, S. Direct 3D bioprinting of cardiac micro-tissues mimicking native myocardium. Biomaterials 2020, 256, 120204. [Google Scholar] [CrossRef]
- Kim, J. Characterization of Biocompatibility of Functional Bioinks for 3D Bioprinting. Bioengineering 2023, 10, 457. [Google Scholar] [CrossRef]
- Costantini, M.; Idaszek, J.; Szöke, K.; Jaroszewicz, J.; Dentini, M.; Barbetta, A.; Brinchmann, J.E.; Święszkowski, W. 3D bioprinting of BM-MSCs-loaded ECM biomimetic hydrogels for in vitro neocartilage formation. Biofabrication 2016, 8, 035002. [Google Scholar] [CrossRef]
- Ho, C.M.; Mishra, A.; Lin, P.T.; Ng, S.H.; Yeong, W.Y.; Kim, Y.J.; Yoon, Y.J. 3D Printed Polycaprolactone Carbon Nanotube Composite Scaffolds for Cardiac Tissue Engineering. Macromol. Biosci. 2017, 17, 1600250. [Google Scholar] [CrossRef] [PubMed]
- Motta, S.E.; Peters, M.M.; Chantre, C.O.; Chang, H.; Cera, L.; Liu, Q.; Cordoves, E.M.; Fioretta, E.S.; Zaytseva, P.; Cesarovic, N.; et al. On-demand heart valve manufacturing using focused rotary jet spinning. Matter 2023, 6, 1860–1879. [Google Scholar] [CrossRef]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.; Seliktar, D.; et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018, 8, 13532. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; He, J.; Li, D. Electrohydrodynamic 3D printing of layer-specifically oriented, multiscale conductive scaffolds for cardiac tissue engineering. Nanoscale 2019, 11, 15195–15205. [Google Scholar] [CrossRef]
- Galván, N.T.N.; Paulsen, S.J.; Kinstlinger, I.S.; Marini, J.C.; Didelija, I.C.; Yoeli, D.; Grigoryan, B.; Miller, J.S. Blood Flow Within Bioengineered 3D Printed Vascular Constructs Using the Porcine Model. Front. Cardiovasc. Med. 2021, 8, 629313. [Google Scholar] [CrossRef]
- Xu, C.; Lee, W.; Dai, G.; Hong, Y. Highly Elastic Biodegradable Single-Network Hydrogel for Cell Printing. ACS Appl. Mater. Interfaces 2018, 10, 9969–9979. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Y.; Hu, Z.; Chen, M.; Chen, S.; Yao, Y.; Yao, J.; Shao, X.; Wu, K.; Zhu, Y.; et al. Cation-crosslinked κ-carrageenan sub-microgel medium for high-quality embedded bioprinting. Biofabrication 2024, 16, 025009. [Google Scholar] [CrossRef]
- Zhu, K.; Shin, S.R.; van Kempen, T.; Li, Y.C.; Ponraj, V.; Nasajpour, A.; Mandla, S.; Hu, N.; Liu, X.; Leijten, J.; et al. Gold Nanocomposite Bioink for Printing 3D Cardiac Constructs. Adv. Funct. Mater. 2017, 27, 1605352. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Testa, S.; Mozetic, P.; Barbetta, A.; Fuoco, C.; Fornetti, E.; Tamiro, F.; Bernardini, S.; Jaroszewicz, J.; Święszkowski, W.; et al. Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo. Biomaterials 2017, 131, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Roshanbinfar, K.; Schiffer, M.; Carls, E.; Angeloni, M.; Koleśnik-Gray, M.; Schruefer, S.; Schubert, D.W.; Ferrazzi, F.; Krstić, V.; Fleischmann, B.K.; et al. Electrically Conductive Collagen-PEDOT:PSS Hydrogel Prevents Post-Infarct Cardiac Arrhythmia and Supports hiPSC-Cardiomyocyte Function. Adv. Mater. 2024, 36, 2403642. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D printing of conducting polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef]
- Keriquel, V.; Oliveira, H.; Rémy, M.; Ziane, S.; Delmond, S.; Rousseau, B.; Rey, S.; Catros, S.; Amédée, J.; Guillemot, F.; et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci. Rep. 2017, 7, 1778. [Google Scholar] [CrossRef]
- Zhang, G.; Li, W.; Yu, M.; Huang, H.; Wang, Y.; Han, Z.; Shi, K.; Ma, L.; Yu, Z.; Zhu, X. Electric-field-driven printed 3D highly ordered microstructure with cell feature size promotes the maturation of engineered cardiac tissues. Adv. Sci. 2023, 10, 2206264. [Google Scholar] [CrossRef]
- Zandrini, T.; Florczak, S.; Levato, R.; Ovsianikov, A. Breaking the resolution limits of 3D bioprinting: Future opportunities and present challenges. Trends Biotechnol. 2023, 41, 604–614. [Google Scholar] [CrossRef]
- Izadifar, M.; Chapman, D.; Babyn, P.; Chen, X.; Kelly, M.E. UV-Assisted 3D Bioprinting of Nanoreinforced Hybrid Cardiac Patch for Myocardial Tissue Engineering. Tissue Eng. Part C Methods 2018, 24, 74–88. [Google Scholar] [CrossRef]
- ISO 10993-4; Biological Evaluation of Medical Devices—Part 4: Selection of Tests for Interactions with Blood. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Sarah, R.; Rohauer, R.; Schimmelpfennig, K.; Limon, S.M.; Lewis, C.L.; Habib, A. Data-Driven Optimization of Bioink Formulations for Extrusion-Based Bioprinting: A Predictive Modeling Approach. J. Manuf. Sci. Eng. 2025, 147, 101001. [Google Scholar] [CrossRef]
- Zhang, C.; Elvitigala, K.C.M.L.; Mubarok, W.; Okano, Y.; Sakai, S. Machine learning-based prediction and optimisation framework for as-extruded cell viability in extrusion-based 3D bioprinting. Virtual Phys. Prototyp. 2024, 19, e2400330. [Google Scholar] [CrossRef]
- Allencherry, J.; Pradeep, N.; Shrivastava, R.; Joy, L.; Imbriacco, F.; Özel, T. Investigation of hydrogel and gelatin bath formulations for extrusion-based 3D bioprinting using deep learning. Procedia CIRP 2022, 110, 360–365. [Google Scholar] [CrossRef]
- Yu, J.; Yao, D.; Wang, L.; Xu, M. Machine Learning in Predicting and Optimizing Polymer Printability for 3D Bioprinting. Polymers 2025, 17, 1873. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. Part A 2013, 101A, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Chikae, S.; Kubota, A.; Nakamura, H.; Oda, A.; Yamanaka, A.; Akagi, T.; Akashi, M. Three-dimensional bioprinting human cardiac tissue chips of using a painting needle method. Biotechnol. Bioeng. 2019, 116, 3136–3142. [Google Scholar] [CrossRef]
- Jin, Y.; Compaan, A.; Bhattacharjee, T.; Huang, Y. Granular gel support-enabled extrusion of three-dimensional alginate and cellular structures. Biofabrication 2016, 8, 025016. [Google Scholar] [CrossRef]
- Highley, C.B.; Rodell, C.B.; Burdick, J.A. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv. Mater. 2015, 27, 5075–5079. [Google Scholar] [CrossRef]
- Campos, D.F.D.; Blaeser, A.; Weber, M.; Jäkel, J.; Neuss, S.; Jahnen-Dechent, W.; Fischer, H. Three-dimensional printing of stem cell-laden hydrogels submerged in a hydrophobic high-density fluid. Biofabrication 2012, 5, 015003. [Google Scholar]
- Compaan, A.M.; Song, K.; Huang, Y. Gellan fluid gel as a versatile support bath material for fluid extrusion bioprinting. ACS Appl. Mater. Interfaces 2019, 11, 5714–5726. [Google Scholar] [CrossRef]
- Shapira, A.; Noor, N.; Oved, H.; Dvir, T. Transparent support media for high resolution 3D printing of volumetric cell-containing ECM structures. Biomed. Mater. 2020, 15, 045018. [Google Scholar] [CrossRef] [PubMed]
- Mirdamadi, E.; Tashman, J.W.; Shiwarski, D.J.; Palchesko, R.N.; Feinberg, A.W. FRESH 3D bioprinting a full-size model of the human heart. ACS Biomater. Sci. Eng. 2020, 6, 6453–6459. [Google Scholar] [CrossRef] [PubMed]
- Bliley, J.; Tashman, J.; Stang, M.; Coffin, B.; Shiwarski, D.; Lee, A.; Hinton, T.; Feinberg, A. FRESH 3D bioprinting a contractile heart tube using human stem cell-derived cardiomyocytes. Biofabrication 2022, 14, 024106. [Google Scholar] [CrossRef] [PubMed]
- Saidy, N.T.; Wolf, F.; Bas, O.; Keijdener, H.; Hutmacher, D.W.; Mela, P.; De-Juan-Pardo, E.M. Biologically Inspired Scaffolds for Heart Valve Tissue Engineering via Melt Electrowriting. Small 2019, 15, 1900873. [Google Scholar] [CrossRef]
- Paxton, N.C.; Lanaro, M.; Bo, A.; Crooks, N.; Ross, M.T.; Green, N.; Tetsworth, K.; Allenby, M.C.; Gu, Y.; Wong, C.S.; et al. Design tools for patient specific and highly controlled melt electrowritten scaffolds. J. Mech. Behav. Biomed. Mater. 2020, 105, 103695. [Google Scholar] [CrossRef]
- Castilho, M.; Feyen, D.; Flandes-Iparraguirre, M.; Hochleitner, G.; Groll, J.; Doevendans, P.A.F.; Vermonden, T.; Ito, K.; Sluijter, J.P.G.; Malda, J. Melt Electrospinning Writing of Poly-Hydroxymethylglycolide-co-ε-Caprolactone-Based Scaffolds for Cardiac Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700311. [Google Scholar] [CrossRef]
- Gwiazda, M.; Kumar, S.; Świeszkowski, W.; Ivanovski, S.; Vaquette, C. The effect of melt electrospun writing fiber orientation onto cellular organization and mechanical properties for application in Anterior Cruciate Ligament tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103631. [Google Scholar] [CrossRef]
- Tourlomousis, F.; Jia, C.; Karydis, T.; Mershin, A.; Wang, H.; Kalyon, D.M.; Chang, R.C. Machine learning metrology of cell confinement in melt electrowritten three-dimensional biomaterial substrates. Microsyst. Nanoeng. 2019, 5, 15. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Z.; Wan, Y.; Zhang, Y.; Wang, Z.; Klausen, L.H.; Huang, P.; Dong, M.; Han, X.; Cui, B.; et al. A hierarchically ordered compacted coil scaffold for tissue regeneration. NPG Asia Mater. 2020, 12, 55. [Google Scholar] [CrossRef]
- van Genderen, A.M.; Jansen, K.; Kristen, M.; van Duijn, J.; Li, Y.; Schuurmans, C.C.L.; Malda, J.; Vermonden, T.; Jansen, J.; Masereeuw, R.; et al. Topographic Guidance in Melt-Electrowritten Tubular Scaffolds Enhances Engineered Kidney Tubule Performance. Front. Bioeng. Biotechnol. 2021, 8, 617364. [Google Scholar] [CrossRef]
- Zhu, W.; Qu, X.; Zhu, J.; Ma, X.; Patel, S.; Liu, J.; Wang, P.; Lai, C.S.E.; Gou, M.; Xu, Y. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials 2017, 124, 106–115. [Google Scholar] [CrossRef]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.-S.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.E.; Zanella, F. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Tiwari, S.K.; Agrawal, K.; Tan, M.; Dang, J.; Tam, T.; Tian, J.; Wan, X.; Schimelman, J.; You, S. Rapid 3D bioprinting of glioblastoma model mimicking native biophysical heterogeneity. Small 2021, 17, 2006050. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Xie, Q.; Gimple, R.C.; Zhong, Z.; Tam, T.; Tian, J.; Kidwell, R.L.; Wu, Q.; Prager, B.C.; Qiu, Z. Three-dimensional bioprinted glioblastoma microenvironments model cellular dependencies and immune interactions. Cell Res. 2020, 30, 833–853. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhu, W.; Nowicki, M.; Miao, S.; Cui, H.; Holmes, B.; Glazer, R.I.; Zhang, L.G. 3D bioprinting a cell-laden bone matrix for breast cancer metastasis study. ACS Appl. Mater. Interfaces 2016, 8, 30017–30026. [Google Scholar] [CrossRef]
- Zorlutuna, P.; Jeong, J.H.; Kong, H.; Bashir, R. Stereolithography-based hydrogel microenvironments to examine cellular interactions. Adv. Funct. Mater. 2011, 21, 3642–3651. [Google Scholar] [CrossRef]
- Tromayer, M.; Dobos, A.; Gruber, P.; Ajami, A.; Dedic, R.; Ovsianikov, A.; Liska, R. A biocompatible diazosulfonate initiator for direct encapsulation of human stem cells via two-photon polymerization. Polym. Chem. 2018, 9, 3108–3117. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Gruene, M.; Pflaum, M.; Koch, L.; Maiorana, F.; Wilhelmi, M.; Haverich, A.; Chichkov, B. Laser printing of cells into 3D scaffolds. Biofabrication 2010, 2, 014104. [Google Scholar] [CrossRef]
- Loukelis, K.; Koutsomarkos, N.; Mikos, A.G.; Chatzinikolaidou, M. Advances in 3D bioprinting for regenerative medicine applications. Regen. Biomater. 2024, 11, rbae033. [Google Scholar] [CrossRef]
- Eghosasere, E.; Osasumwen, E.; Emmanuella, O. 3D Bioprinting in Tissue Engineering: Advancements, Challenges, and Pathways to Clinical Translation. JSM Regen. Med. Bioeng. 2025, 7, 1023. [Google Scholar]
- Chen, X.B.; Fazel Anvari-Yazdi, A.; Duan, X.; Zimmerling, A.; Gharraei, R.; Sharma, N.K.; Sweilem, S.; Ning, L. Biomaterials/bioinks and extrusion bioprinting. Bioact. Mater. 2023, 28, 511–536. [Google Scholar] [CrossRef]
- Wang, D.; Maharjan, S.; Kuang, X.; Wang, Z.; Mille, L.S.; Tao, M.; Yu, P.; Cao, X.; Lian, L.; Lv, L.; et al. Microfluidic bioprinting of tough hydrogel-based vascular conduits for functional blood vessels. Sci. Adv. 2022, 8, eabq6900. [Google Scholar] [CrossRef]
- Ahrens, J.H.; Uzel, S.G.; Skylar-Scott, M.; Mata, M.M.; Lu, A.; Kroll, K.T.; Lewis, J.A. Programming cellular alignment in engineered cardiac tissue via bioprinting anisotropic organ building blocks. Adv. Mater. 2022, 34, 2200217. [Google Scholar] [CrossRef]
- Fortunato, G.; Sigismondi, S.; Nicoletta, M.; Condino, S.; Montemurro, N.; Vozzi, G.; Ferrari, V.; De Maria, C. Analysis of the Robotic-Based In Situ Bioprinting Workflow for the Regeneration of Damaged Tissues through a Case Study. Bioengineering 2023, 10, 560. [Google Scholar] [CrossRef]
- Shiwarski, D.J.; Hudson, A.R.; Tashman, J.W.; Bakirci, E.; Moss, S.; Coffin, B.D.; Feinberg, A.W. 3D bioprinting of collagen-based high-resolution internally perfusable scaffolds for engineering fully biologic tissue systems. Sci. Adv. 2025, 11, eadu5905. [Google Scholar] [CrossRef]
- Gong, X.; Wen, Z.; Liang, Z.; Xiao, H.; Lee, S.; Rossello-Martinez, A.; Xing, Q.; Wright, T.; Nguyen, R.Y.; Mak, M. Instant assembly of collagen for tissue engineering and bioprinting. Nat. Mater. 2025, 24, 1307–1318. [Google Scholar] [CrossRef]
- Sexton, Z.A.; Rütsche, D.; Herrmann, J.E.; Hudson, A.R.; Sinha, S.; Du, J.; Shiwarski, D.J.; Masaltseva, A.; Solberg, F.S.; Pham, J.; et al. Rapid model-guided design of organ-scale synthetic vasculature for biomanufacturing. Science 2025, 388, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, T.; Zehnder, S.M.; Rowe, K.G.; Jain, S.; Nixon, R.M.; Sawyer, W.G.; Angelini, T.E. Writing in the granular gel medium. Sci. Adv. 2015, 1, e1500655. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, D.; Alexander, P.G.; Yang, G.; Tan, J.; Cheng, A.W.; Tuan, R.S. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials 2013, 34, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Serpooshan, V.; Mahmoudi, M.; Hu, D.A.; Hu, J.B.; Wu, S.M. Bioengineering cardiac constructs using 3D printing. J. 3D Print. Med. 2017, 1, 123–139. [Google Scholar] [CrossRef]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef]
- Herman, J.A.; Telles, R.; Cook, C.C.; Leguizamon, S.C.; Lewis, J.A.; Kaehr, B.; White, T.J.; Roach, D.J. Digital Light Process 3D Printing of Magnetically Aligned Liquid Crystalline Elastomer Free–forms. Adv. Mater. 2024, 36, 2414209. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Li, J.; Guo, Z.; Zhang, Y.; Nie, B.; Qi, G.; Zhang, X.; Zhang, J.; Wei, R. 3D Printing of Individualized Microfluidic Chips with DLP-Based Printer. Materials 2023, 16, 6984. [Google Scholar] [CrossRef] [PubMed]
- Kade, J.C.; Dalton, P.D. Polymers for melt electrowriting. Adv. Healthc. Mater. 2021, 10, 2001232. [Google Scholar] [CrossRef] [PubMed]
- Ryma, M.; Genç, H.; Nadernezhad, A.; Paulus, I.; Schneidereit, D.; Friedrich, O.; Andelovic, K.; Lyer, S.; Alexiou, C.; Cicha, I. A print-and-fuse strategy for sacrificial filaments enables biomimetically structured perfusable microvascular networks with functional endothelium inside 3D hydrogels. Adv. Mater. 2022, 34, 2200653. [Google Scholar] [CrossRef]
- Castilho, M.; van Mil, A.; Maher, M.; Metz, C.H.G.; Hochleitner, G.; Groll, J.; Doevendans, P.A.; Ito, K.; Sluijter, J.P.G.; Malda, J. Melt Electrowriting Allows Tailored Microstructural and Mechanical Design of Scaffolds to Advance Functional Human Myocardial Tissue Formation. Adv. Funct. Mater. 2018, 28, 1803151. [Google Scholar] [CrossRef]
- Saidy, N.T.; Shabab, T.; Bas, O.; Rojas-González, D.M.; Menne, M.; Henry, T.; Hutmacher, D.W.; Mela, P.; De-Juan-Pardo, E.M. Melt Electrowriting of Complex 3D Anatomically Relevant Scaffolds. Front. Bioeng. Biotechnol. 2020, 8, 793. [Google Scholar] [CrossRef]
- Kim, W.; Lee, H.; Lee, J.; Atala, A.; Yoo, J.J.; Lee, S.J.; Kim, G.H. Efficient myotube formation in 3D bioprinted tissue construct by biochemical and topographical cues. Biomaterials 2020, 230, 119632. [Google Scholar] [CrossRef]
- Sundaram, S.; Lee, J.H.; Bjørge, I.M.; Michas, C.; Kim, S.; Lammers, A.; Mano, J.F.; Eyckmans, J.; White, A.E.; Chen, C.S. Sacrificial capillary pumps to engineer multiscalar biological forms. Nature 2024, 636, 361–367. [Google Scholar] [CrossRef]
- Grebenyuk, S.; Abdel Fattah, A.R.; Kumar, M.; Toprakhisar, B.; Rustandi, G.; Vananroye, A.; Salmon, I.; Verfaillie, C.; Grillo, M.; Ranga, A. Large-scale perfused tissues via synthetic 3D soft microfluidics. Nat. Commun. 2023, 14, 193. [Google Scholar] [CrossRef]
- Sala, F.; Ficorella, C.; Martínez Vázquez, R.; Eichholz, H.M.; Käs, J.A.; Osellame, R. Rapid Prototyping of 3D Biochips for Cell Motility Studies Using Two-Photon Polymerization. Front. Bioeng. Biotechnol. 2021, 9, 664094. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Z.-Q.; Lin, B.; Liu, M.-C.; Zhang, Y.; Liu, S.-J.; Li, Y.; Zhao, Q. Two-photon polymerization-based 3D micro-scaffolds toward biomedical devices. Chem. Eng. J. 2024, 493, 152469. [Google Scholar] [CrossRef]
- Hippler, M.; Lemma, E.D.; Bertels, S.; Blasco, E.; Barner-Kowollik, C.; Wegener, M.; Bastmeyer, M. 3D scaffolds to study basic cell biology. Adv. Mater. 2019, 31, 1808110. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Griffin, M.A.; Sen, S.; Bönnemann, C.G.; Sweeney, H.L.; Discher, D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004, 166, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.S.; Davis, B.N.; Madden, L.; Bursac, N.; Truskey, G.A. Physiology and metabolism of tissue-engineered skeletal muscle. Exp. Biol. Med. 2014, 239, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lei, D.; Huang, S.; Yang, Q.; Song, B.; Guo, Y.; Shen, A.; Yuan, Z.; Li, S.; Qing, F.L.; et al. Elastic 3D-Printed Hybrid Polymeric Scaffold Improves Cardiac Remodeling after Myocardial Infarction. Adv. Healthc. Mater. 2019, 8, e1900065. [Google Scholar] [CrossRef]
- Huang, S.; Lei, D.; Yang, Q.; Yang, Y.; Jiang, C.; Shi, H.; Qian, B.; Long, Q.; Chen, W.; Chen, Y.; et al. A perfusable, multifunctional epicardial device improves cardiac function and tissue repair. Nat. Med. 2021, 27, 480–490. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S. 3D Bioprinting: Challenges in Commercialization and Clinical Translation. J. 3D Print. Med. 2023, 7, 3DP8. [Google Scholar] [CrossRef]
- Kucukgul, C.; Ozler, S.B.; Inci, I.; Karakas, E.; Irmak, S.; Gozuacik, D.; Taralp, A.; Koc, B. 3D bioprinting of biomimetic aortic vascular constructs with self-supporting cells. Biotechnol. Bioeng. 2015, 112, 811–821. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Fu, C.-P.; Li, X.-Y.; Lu, X.-C.; Hu, L.-G.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Three-Dimensional Bioprinting of Decellularized Extracellular Matrix-Based Bioinks for Tissue Engineering. Molecules 2022, 27, 3442. [Google Scholar] [CrossRef]
- Włodarczyk-Biegun, M.K.; Villiou, M.; Koch, M.; Muth, C.; Wang, P.; Ott, J.; Del Campo, A. Melt electrowriting of graded porous scaffolds to mimic the matrix structure of the human trabecular meshwork. ACS Biomater. Sci. Eng. 2022, 8, 3899–3911. [Google Scholar] [CrossRef]
- Saha, S.K.; Wang, D.; Nguyen, V.H.; Chang, Y.; Oakdale, J.S.; Chen, S.-C. Scalable submicrometer additive manufacturing. Science 2019, 366, 105–109. [Google Scholar] [CrossRef]
- Kupfer, M.E.; Lin, W.-H.; Ravikumar, V.; Qiu, K.; Wang, L.; Gao, L.; Bhuiyan, D.B.; Lenz, M.; Ai, J.; Mahutga, R.R. In situ expansion, differentiation, and electromechanical coupling of human cardiac muscle in a 3D bioprinted, chambered organoid. Circ. Res. 2020, 127, 207–224. [Google Scholar] [CrossRef]


| Method | Resolution | Cell Viability | Scalability | Material Compatibility | Cost | Clinical Readiness | Limitations | Reference |
|---|---|---|---|---|---|---|---|---|
| Extrusion | 100–1200 µm | 40–86% (variable due to shear stress), capable of high-cell density printing for thick tissues | Suitable for large construct fabrication (e.g., vascular grafts, heart tissue scaffolds), but slow for high-resolution complex structures | High-viscosity hydrogels (alginate, gelatin, PEG blends), cell-laden bioinks (fibroblasts, cardiomyocytes, stem cells), thermoplastics (PCL, PLA, PLGA). Specific cardiac bioinks like dECM, PEG, Laponite | Low-Medium | Widely used in preclinical studies; early clinical trials for cardiovascular constructs (e.g., valves, vascular grafts) | Shear stress reduces cell viability, low microscale resolution, structural fragility, and limited porosity | [18,39,41,43,45,48,50,62,68] |
| FRESH | Approx. 100 µm | High (due to cell-friendly environment of the support bath, beneficial for sensitive cardiac cells) | High potential for patient-specific, full-scale tissue production (e.g., heart models), though current software limitations exist | Low-viscosity and fragile ECM-based bioinks (collagen I, dECM, fibrin, Matrigel), cell-laden bioinks (fibroblasts, cardiomyocytes, stem cells); highly relevant for cardiac ECM mimicry | Medium | Preclinical demonstrations (e.g., patient-specific heart and valve models); no direct clinical translation yet | Post-processing complexity, support removal challenges for vascular structures, and software limitations for non-planar designs | [9,12,15,16,19,44,69,70,71,72,73,74,75,98,99] |
| DLP/SLA | 10–150 µm | High | Faster than point-by-point methods, but scalability is limited for very large constructs; beneficial for intricate vascular networks | Photocurable hydrogels (PEGDA, GelMA, ECM-methacrylates); used for vascularized constructs | Medium-High | Strong preclinical successes in vascularized tissues (e.g., perfusable liver constructs, HUVEC-based anastomosis); still experimental with no clinical translation | Restricted to photocurable inks, phototoxicity risks for sensitive cells, and variable resolution between DLP and SLA | [16,83,84,85,86,87,88] |
| MEW | Fiber diameter: 2–50 µm | Not applicable | High scalability for structural scaffold fabrication, which can then be vascularized | Thermoplastics (PCL, PLA, PLGA); provides mechanical strength; can be integrated with hydrogels for enhanced bioactivity | Medium | Ongoing in vivo studies; high translation potential for structural applications (e.g., bone regeneration, cardiovascular grafts) | Thermal degradation, limited bioactivity requiring hybrid strategies, and fiber bridging from electrostatic attraction | [76,77,78,79,80,81,82] |
| 2PP | Submicron (up to 100 nm possible, <500 nm for biodegradable materials). | Over 90% possible (improved with recent technological advancements) | Very low; excellent for micro-models and vascular topologies | PEGDA and other photocurable hybrid hydrogels, functionalized photopolymers, nanoparticle-loaded systems; used for vascular models | High (costly femtosecond lasers, low throughput) | Proof-of-concept stage; used for vascular models and complex biomimetic tissues (millimeter-scale) | Very low throughput, high cost, and difficulty in large-scale production of full-scale cardiovascular organs | [89,90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.S.; Choi, Y.; Lee, K.Y. Three-Dimensional Printing and Bioprinting Strategies for Cardiovascular Constructs: From Printing Inks to Vascularization. Polymers 2025, 17, 2337. https://doi.org/10.3390/polym17172337
Kim MS, Choi Y, Lee KY. Three-Dimensional Printing and Bioprinting Strategies for Cardiovascular Constructs: From Printing Inks to Vascularization. Polymers. 2025; 17(17):2337. https://doi.org/10.3390/polym17172337
Chicago/Turabian StyleKim, Min Suk, Yuri Choi, and Keel Yong Lee. 2025. "Three-Dimensional Printing and Bioprinting Strategies for Cardiovascular Constructs: From Printing Inks to Vascularization" Polymers 17, no. 17: 2337. https://doi.org/10.3390/polym17172337
APA StyleKim, M. S., Choi, Y., & Lee, K. Y. (2025). Three-Dimensional Printing and Bioprinting Strategies for Cardiovascular Constructs: From Printing Inks to Vascularization. Polymers, 17(17), 2337. https://doi.org/10.3390/polym17172337







