The Impact of Zinc Oxide Nanoparticles on the Color Stability and Surface Roughness of Heat-Polymerized Maxillofacial Silicone Elastomer Subjected to Artificial Aging: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
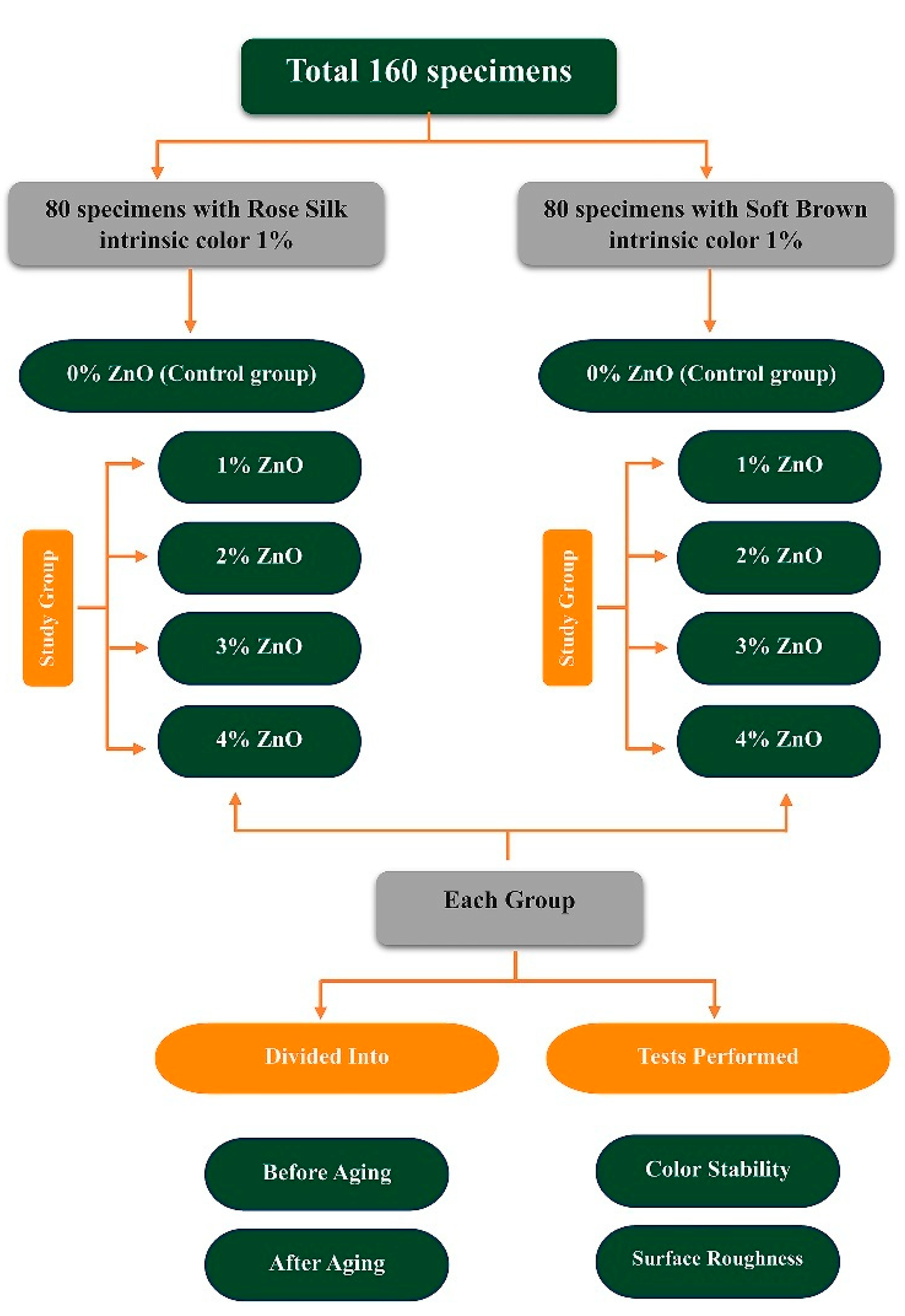
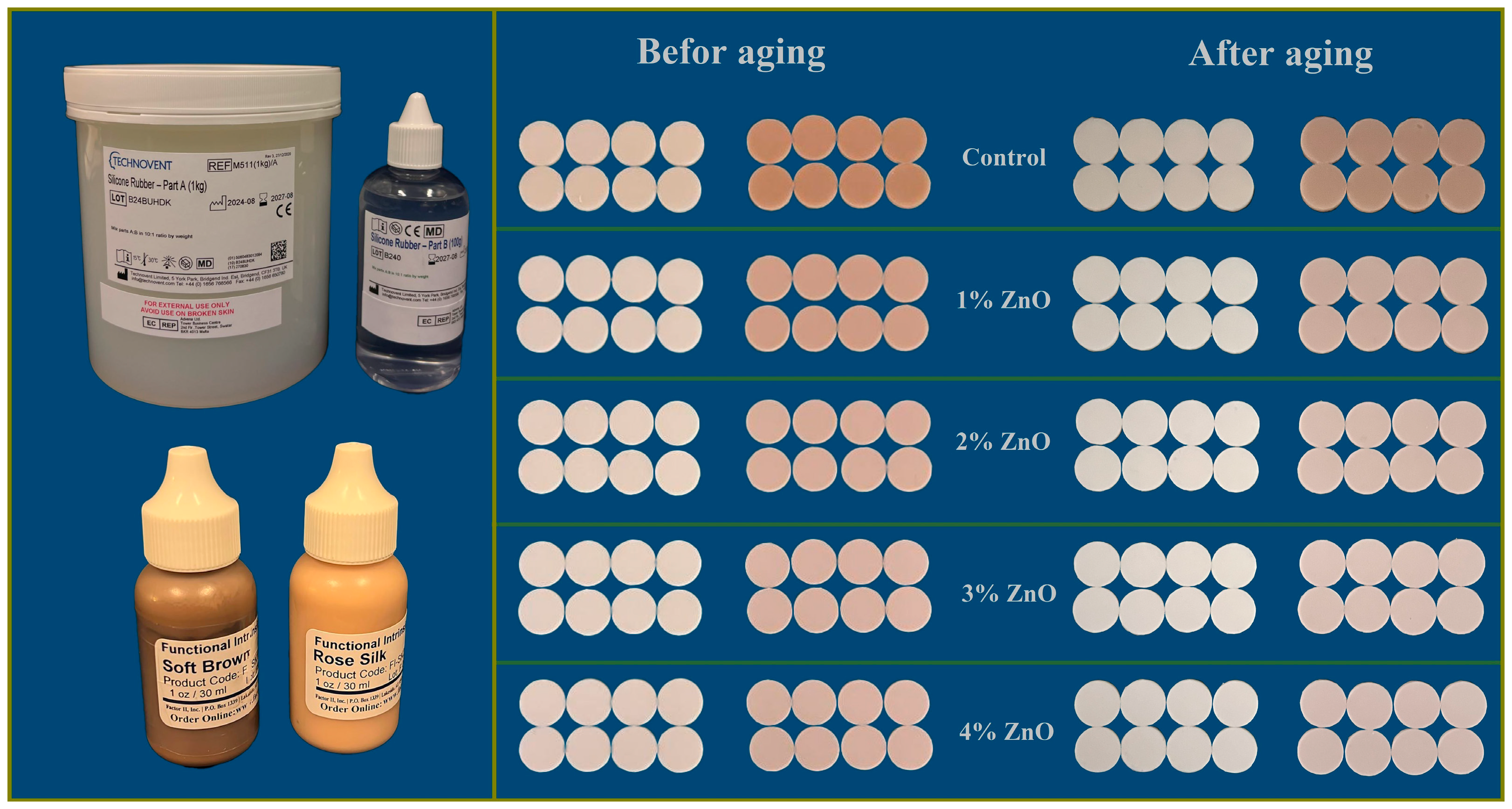
2.2. Study Design and Sample Preparation
2.3. Characterizations
2.4. Statistical Analysis
3. Results
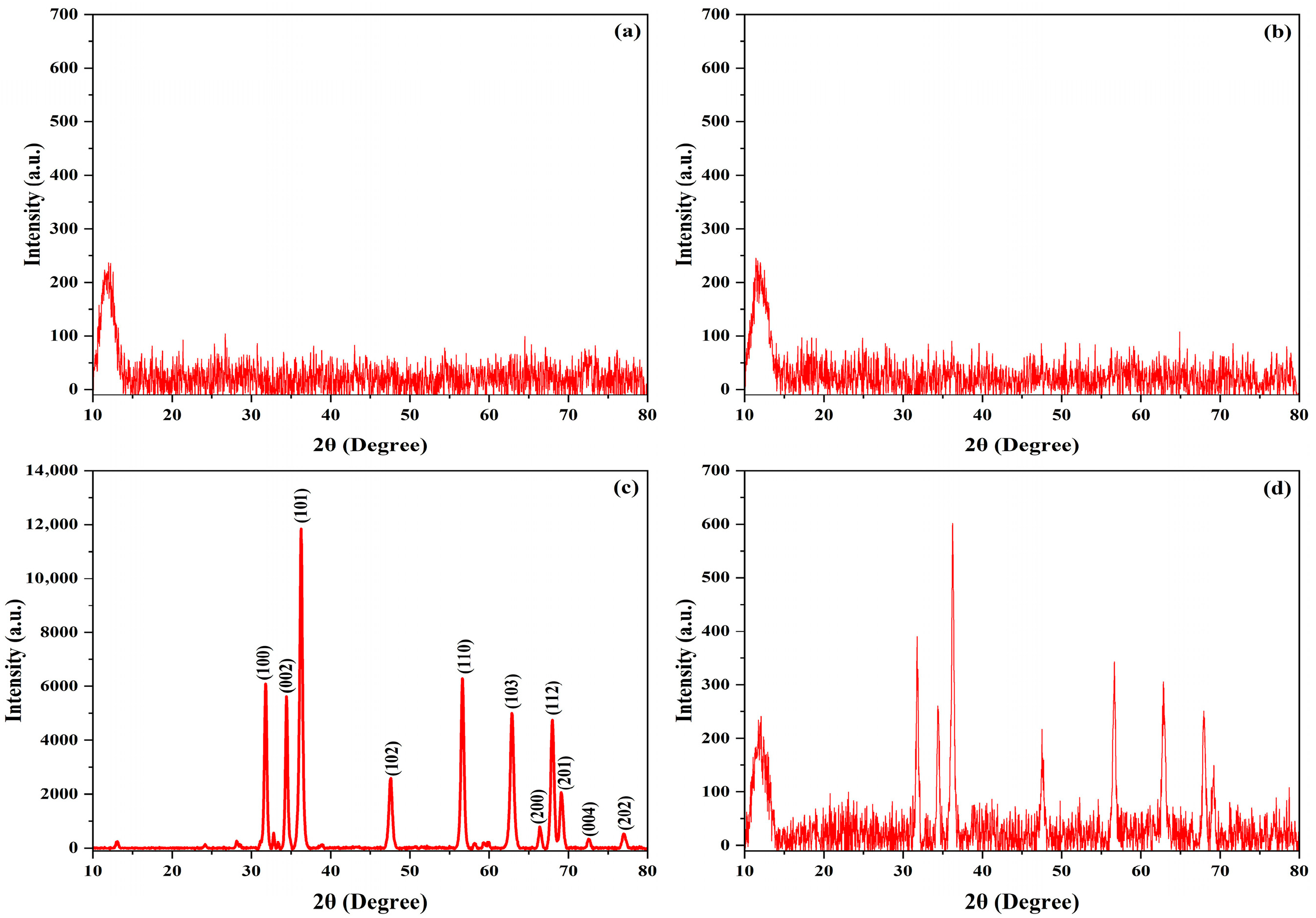
3.1. X-Ray Diffraction
3.2. FTIR-ATR
3.3. FE-SEM
3.4. Atomic Force Microscopy
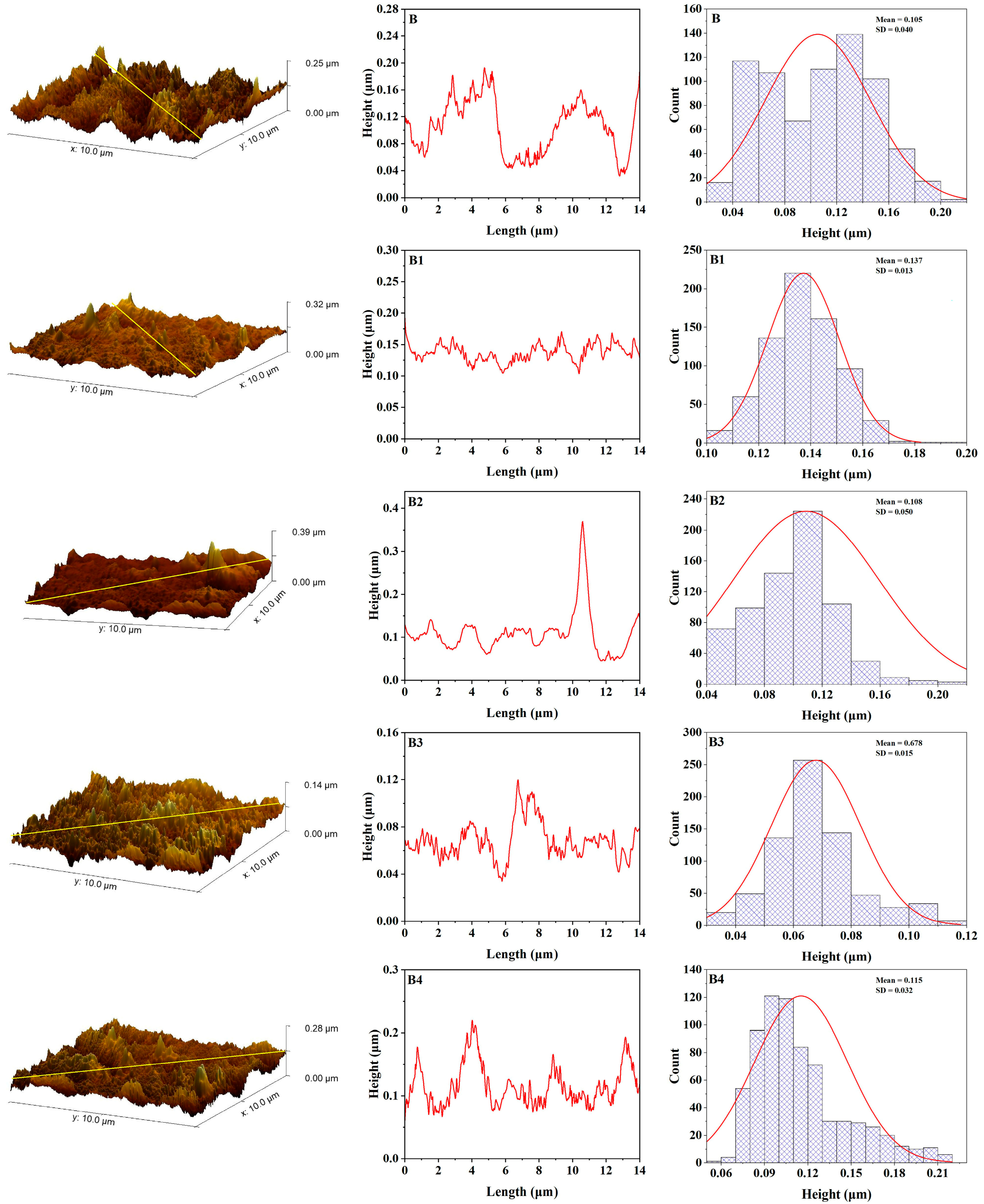
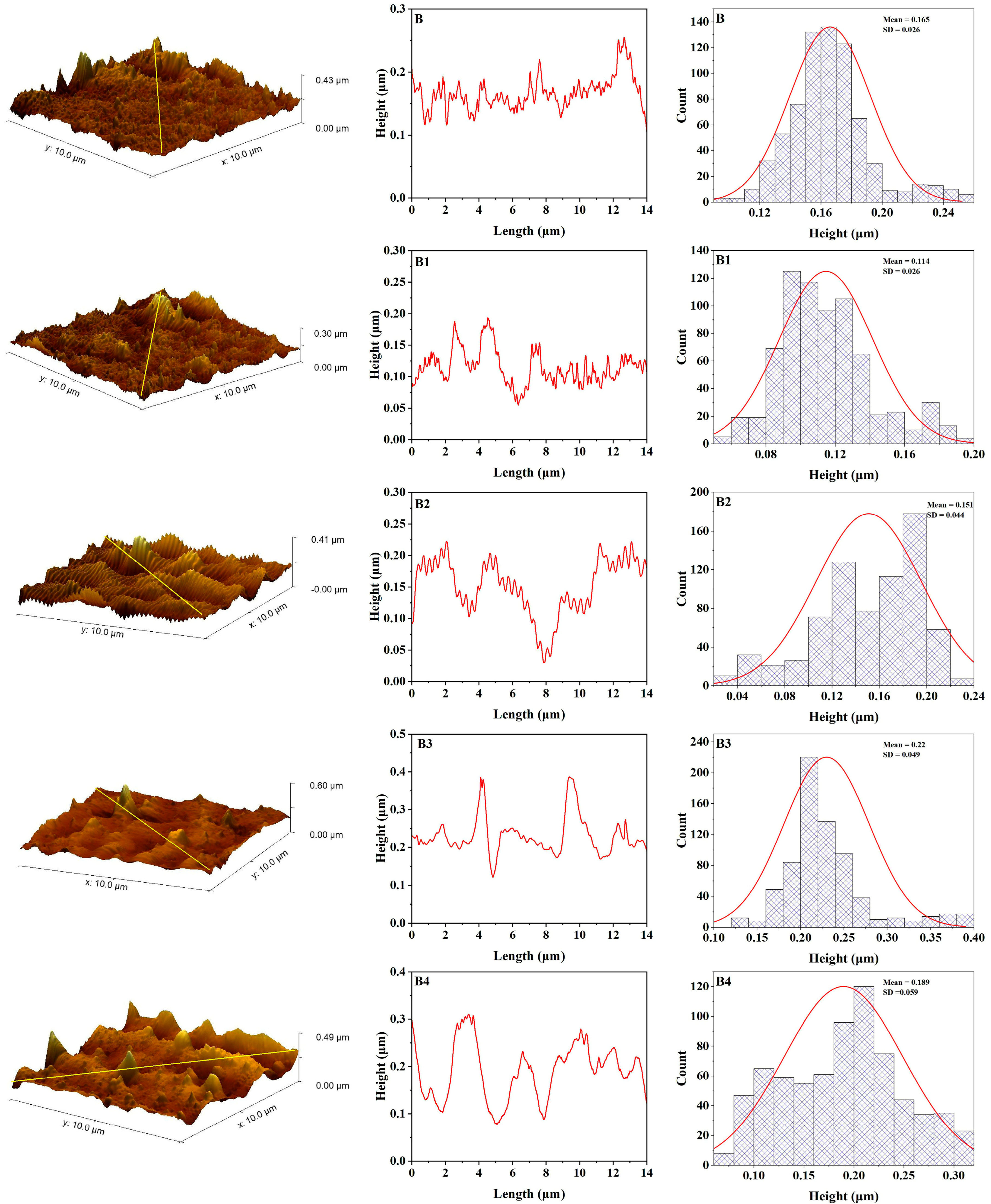
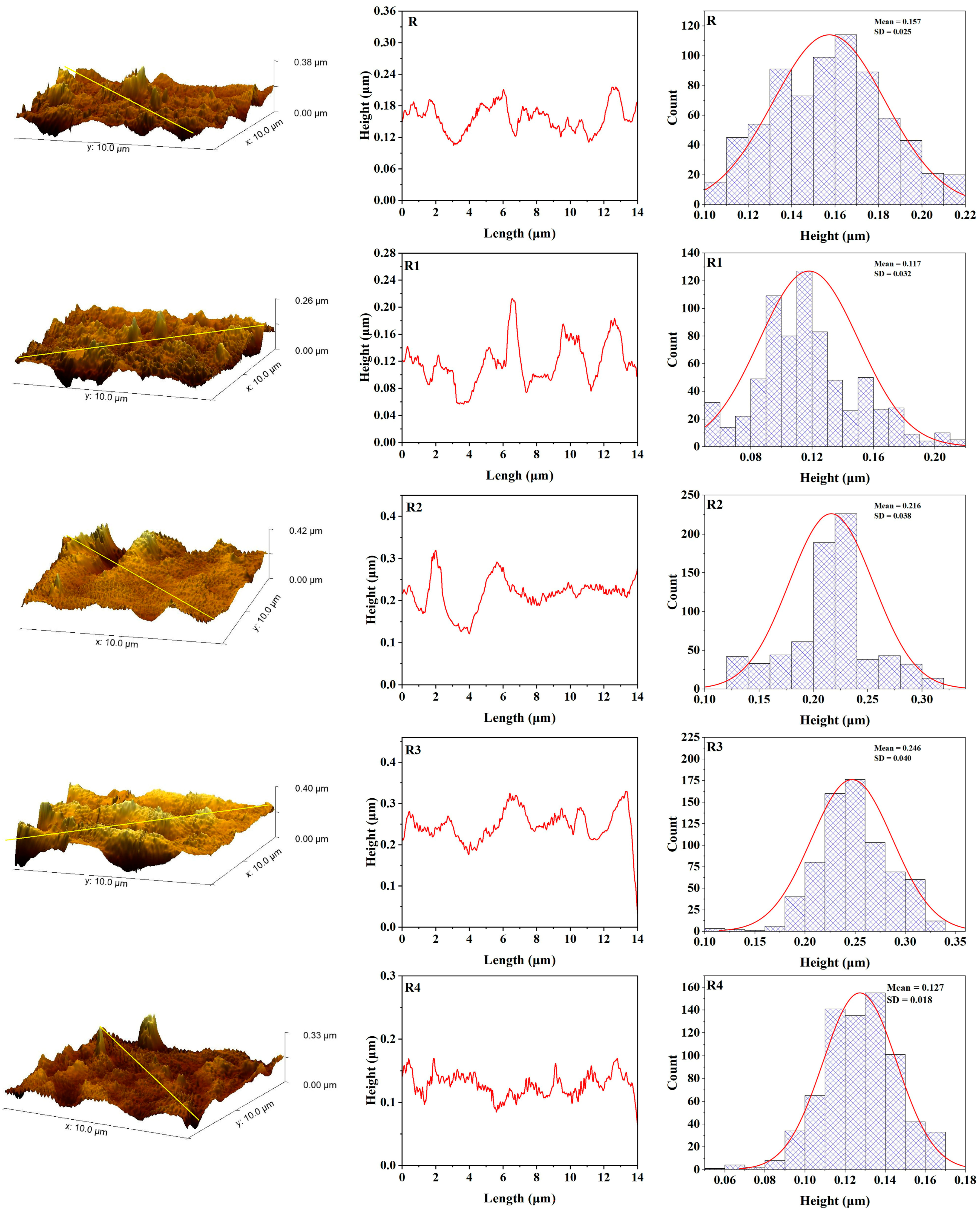
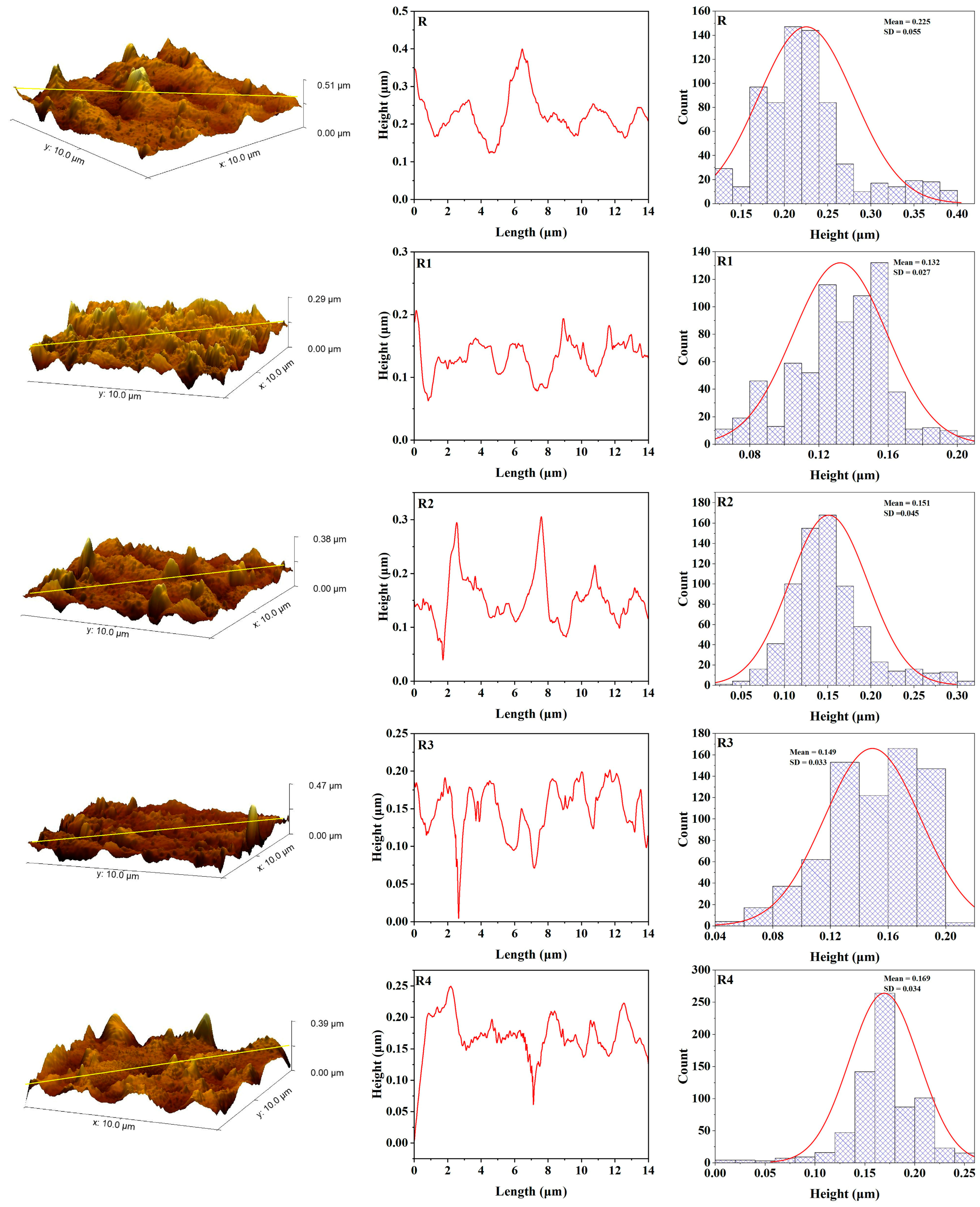
3.5. Color Stability
4. Discussion
4.1. Color Stability
4.2. Surface Roughness
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Zno | Zinc oxide |
| NPs | Nanoparticles |
| HTV | Heat-temperature vulcanized |
| AFM | Atomic force microscopy |
| UV | Ultraviolet |
| CIELAB | Commission internationale de l’Éclairage Lab* color space |
| ΔE | Color Difference (Delta E*) |
| XRD | X-ray diffraction |
| FTIR-ATR | Fourier Transform Infrared Spectroscopy-attenuated total reflectance |
| FE-SEM | Field-Emission Scanning Electron Microscopy |
| SPSS | Statistical Package for the Social Sciences |
| Ra | Average Roughness |
| Rq | Root Mean Square Roughness |
| Rt | Maximum Height of the Profile |
| IQR (Q1–Q3) | Interquartile Range First and Third Quartiles |
| MPa | Megapascal |
| nm | Nanometer |
References
- Mohammed, K.; Zardawi, F.; Azhdar, B. Influence of Silver Nanoparticles on Color Stability of Room-Temperature-Vulcanizing Maxillofacial Silicone Subjected to Accelerated Artificial Aging. Appl. Sci. 2023, 13, 11201. [Google Scholar] [CrossRef]
- Abdalqadir, M.; Mohammed, K.; Azhdar, B. The impact of zirconium dioxide nanoparticles on the color stability of artificially aged heat-polymerized maxillofacial silicone elastomer. Sci. Prog. 2023, 106, 1–11. [Google Scholar] [CrossRef]
- Andres, C.J.; Haug, S.P.; Munoz, C.A.; Bernal, G. Effects of environmental factors on maxillofacial elastomers: Part I—Literature review. J. Prosthet. Dent. 1992, 68, 327–330. [Google Scholar] [CrossRef]
- Haug, S.P.; Andres, C.J.; Moore, B.K. Color stability and colorant effect on maxillofacial elastomers. Part I: Colorant effect on physical properties. J. Prosthet. Dent. 1999, 81, 418–422. [Google Scholar] [CrossRef]
- Sonnahalli, N.K.; Chowdhary, R. Effect of nanoparticles on color stability and mechanical and biological properties of maxillofacial silicone elastomer: A systematic review. J. Indian Prosthodont. Soc. 2020, 20, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Zayed, S.M.; Alshimy, A.M.; Fahmy, A.E. Effect of surface treated silicon dioxide nanoparticles on some mechanical properties of maxillofacial silicone elastomer. Int. J. Biomater. 2014, 2014, 750398. [Google Scholar] [CrossRef]
- Kiat-Amnuay, S.; Mekayarajjananonth, T.; Powers, J.M.; Chambers, M.S.; Lemon, J.C. Interactions of pigments and opacifiers on color stability of MDX4-4210/type A maxillofacial elastomers subjected to artificial aging. J. Prosthet. Dent. 2006, 95, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Charoenkijkajorn, D.; Sanohkan, S. The Effect of Nano Zinc Oxide Particles on Color Stability of MDX4-4210 Silicone Prostheses. Eur. J. Dent. 2020, 14, 525–532. [Google Scholar] [CrossRef]
- Abdalqadir, M.; Saeed, Z.; Azhdar, B. Surface roughness of pigmented and non-pigmented maxillofacial silicone elastomer before and after artificial aging. Mater. Res. Express 2024, 11, 015401. [Google Scholar] [CrossRef]
- Mehta, S.; Nandeeshwar, D. A spectrophotometric analysis of extraoral aging conditions on the color stability of maxillofacial silicone. J. Indian Prosthodont. Soc. 2017, 17, 355–360. [Google Scholar] [CrossRef]
- Watson, R.M.; Coward, T.J.; Forman, G.H. Results of treatment of 20 patients with implant-retained auricular prostheses. Int. J. Oral Maxillofac. Implant. 1995, 10, 445–449. [Google Scholar]
- Beatty, M.W.; Mahanna, G.K.; Dick, K.; Jia, W. Color changes in dry-pigmented maxillofacial elastomer resulting from ultraviolet light exposure. J. Prosthet. Dent. 1995, 74, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Gary, J.J.; Smith, C.T. Pigments and their application in maxillofacial elastomers: A literature review. J. Prosthet. Dent. 1998, 80, 204–208. [Google Scholar] [CrossRef]
- Shakir, D.A.; Abdul-Ameer, F.M. Effect of nano-titanium oxide addition on some mechanical properties of silicone elastomers for maxillofacial prostheses. J. Taibah Univ. Med. Sci. 2018, 13, 281–290. [Google Scholar] [CrossRef]
- Al-Kadi, F.K.; Abdulkareem, J.F.; Azhdar, B.A. Hybrid Chitosan–TiO2 Nanocomposite Impregnated in Type A-2186 Maxillofacial Silicone Subjected to Different Accelerated Aging Conditions: An Evaluation of Color Stability. Nanomaterials 2023, 13, 2379. [Google Scholar] [CrossRef]
- Akash, R.N.; Guttal, S.S. Effect of Incorporation of Nano-Oxides on Color Stability of Maxillofacial Silicone Elastomer Subjected to Outdoor Weathering. J. Prosthodont. 2015, 24, 569–575. [Google Scholar] [CrossRef]
- Han, Y.; Kiat-Amnuay, S.; Powers, J.M.; Zhao, Y. Effect of nano-oxide concentration on the mechanical properties of a maxillofacial silicone elastomer. J. Prosthet. Dent. 2008, 100, 465–473. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, Y.; Xie, C.; Powers, J.M.; Kiat-Amnuay, S. Color stability of pigmented maxillofacial silicone elastomer: Effects of nano-oxides as opacifiers. J. Dent. 2010, 38, e100–e105. [Google Scholar] [CrossRef]
- Rajagopalan, N.; Khanna, A.S. Effect of size and morphology on UV-blocking property of nanoZnO in epoxy coating. Int. J. Sci. Res. Publ. 2013, 3, 1–14. [Google Scholar]
- Chehna, A.B.; Agrawal, N.; Ricard, L.B.; Smith, G.M.; Varley, A.R. AATCC Technical Manual; American Association of Textile Chemists and Colorists: Durham, NC, USA, 2007; p. 82. [Google Scholar]
- Cao, Z.; Zhang, Z.; Wang, F.; Wang, G. Synthesis and UV shielding properties of zinc oxide ultrafine particles modified with silica and trimethyl siloxane. Colloids Surf. A Physicochem. Eng. Asp. 2009, 340, 161–167. [Google Scholar] [CrossRef]
- Bangera, B.S.; Guttal, S.S. Evaluation of varying concentrations of nano-oxides as ultraviolet protective agents when incorporated in maxillofacial silicones: An in vitro study. J. Prosthet. Dent. 2014, 112, 1567–1572. [Google Scholar] [CrossRef]
- Yu, R.; Koran, A., 3rd; Craig, R.G. Physical properties of a pigmented silicone maxillofacial material as a function of accelerated aging. J. Dent. Res. 1980, 59, 1141–1148. [Google Scholar] [CrossRef]
- Chamaria, A.; Aras, M.A.; Chitre, V.; Rajagopal, P. Effect of Chemical Disinfectants on the Color Stability of Maxillofacial Silicones: An In Vitro Study. J. Prosthodont. 2018, 28, e869–e872. [Google Scholar] [CrossRef]
- Goiato, M.C.; Pesqueira, A.A.; dos Santos, D.M.; Zavanelli, A.C.; Ribeiro, P.D.P. Color stability comparison of silicone facial prostheses following disinfection. J. Prosthodont. 2009, 18, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Goiato, M.C.; Haddad, M.F.; Pesqueira, A.A.; Moreno, A.; dos Santos, D.M.; Bannwart, L.C. Effect of chemical disinfection and accelerated aging on color stability of maxillofacial silicone with opacifiers. J. Prosthodont. 2011, 20, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Tukmachi, M.S.; Ali, M.M.M. Effect of Nano Silicon Dioxide Addition on Some Properties of Heat Vulcanized Maxillofacial Silicone Elastomer. IOSR J. Pharm. Biol. Sci. 2017, 12, 37–43. [Google Scholar] [CrossRef]
- Abdalqadir, M.; Faraj, S.; Azhdar, B. An evaluation of a technique to improve the mechanical properties of maxillofacial silicone elastomers with zinc oxide nanoparticles. J. Prosthet. Dent. 2022, 128, 531–538. [Google Scholar] [CrossRef]
- ASTM G154-06; Standard Practice for Operating Fluorescent Light Apparatus for UV Exposure of Nonmetallic Materials. ASTM International: West Conshohocken, PA, USA, 2006.
- Wyszecki, G.; Stiles, W.S. Color Science: Concepts and Methods, Quantitative Data and Formulas, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1982. [Google Scholar]
- Khalaf, S.; Ariffin, Z.; Husein, A.; Reza, F. Surface Coating of Gypsum-Based Molds for Maxillofacial Prosthetic Silicone Elastomeric Material: The Surface Topography. J. Prosthodont. 2015, 24, 419–423. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2011, 10, 181–188. [Google Scholar] [CrossRef]
- Yadhuraj, S.R.; Kumari, U. Measurement of thickness and roughness using gwyddion. In Proceedings of the 2016 3rd International Conference on Advanced Computing and Communication Systems (ICACCS), Coimbatore, India, 22–23 January 2016; pp. 1–5. [Google Scholar]
- Kim, D.; Hossain, I.; Kim, Y.; Choi, O.; Kim, T.-H. PEG/PPG-PDMS-Adamantane-Based Crosslinked Terpolymer Using the ROMP Technique to Prepare a Highly Permeable and CO2-Selective Polymer Membrane. Polymers 2020, 12, 1674. [Google Scholar] [CrossRef]
- Lewis, R.N.A.H.; McElhaney, R.N. Membrane lipid phase transitions and phase organization studied by Fourier transform infrared spectroscopy. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 2347–2358. [Google Scholar] [CrossRef]
- Haiouani, K.; Hegazy, S.; Alsaeedi, H.; Bechelany, M.; Barhoum, A. Green Synthesis of Hexagonal-like ZnO Nanoparticles Modified with Phytochemicals of Clove (Syzygium aromaticum) and Thymus capitatus Extracts: Enhanced Antibacterial, Antifungal, and Antioxidant Activities. Materials 2024, 17, 4340. [Google Scholar] [CrossRef]
- Mvondo, R.R.N.; Meukam, P.; Jeong, J.; Meneses, D.D.S.; Nkeng, E.G. Influence of water content on the mechanical and chemical properties of tropical wood species. Results Phys. 2017, 7, 2096–2103. [Google Scholar] [CrossRef]
- Alshawwa, S.Z.; Mohammed, E.J.; Hashim, N.; Sharaf, M.; Selim, S.; Alhuthali, H.M.; Alzahrani, H.A.; Mekky, A.E.; Elharrif, M.G. In Situ biosynthesis of reduced alpha hematite (α-Fe2O3) nanoparticles by Stevia Rebaudiana L. leaf extract: Insights into antioxidant, antimicrobial, and anticancer properties. J. Antibiot. Res. 2022, 11, 1252. [Google Scholar] [CrossRef]
- Xiong, G.; Pal, U.; Serrano, J.G.; Ucer, K.B.; Williams, R.T. Photoluminesence and FTIR study of ZnO nanoparticles: The impurity and defect perspective. Phys. Status Solidi (c) 2006, 3, 3577–3581. [Google Scholar] [CrossRef]
- Khashayar, G.; Bain, P.A.; Salari, S.; Dozic, A.; Kleverlaan, C.J.; Feilzer, A.J. Perceptibility and acceptability thresholds for colour differences in dentistry. J. Dent. 2014, 42, 637–644. [Google Scholar] [CrossRef]
- Verma, D.; Kumar, R.; Sunil Kumar, M.V.; Kumar, K. Silicone Colour Stability: The TiO2 and ZnO Nanoparticle Showdown. Int. J. Innov. Sci. Res. Technol. 2024, 9, 1295–1304. [Google Scholar]
- Chen, Y.; Liu, L.; Yang, Q.; Wen, S.; Zhang, L.; Zhong, C. Computational study of nanoparticle dispersion and spatial distribution in polymer matrix under oscillatory shear flow. Langmuir 2013, 29, 13932–13942. [Google Scholar] [CrossRef] [PubMed]
- Lanzara, R. Comparative evaluation of tear strength and surface roughness of two maxillofacial silicone materials following disinfection: An invitro study. J. Indian Prosthodont. Soc. 2020, 20. [Google Scholar] [CrossRef]
- Tomer, N.S.; Delor-Jestin, F.; Frezet, L.; Lacoste, J. Oxidation, Chain Scission and Cross-Linking Studies of Polysiloxanes upon Ageings. Open J. Org. Polym. Mater. 2012, 2, 13–22. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, R.; Lu, D. Investigation of Polymer Aging Mechanisms Using Molecular Simulations: A Review. Polymers 2023, 15, 1928. [Google Scholar] [CrossRef] [PubMed]
- Alwan, N.; Abdul, M.; Muhsin, S. Aging Effect on the Surface Roughness and Color Stability of VST-06 Maxillofacial Silicone. JODR 2022, 9, 91–101. [Google Scholar]
- Anjali, A.; Chethan, H. Effects of incorporation of nano particles on the mechanical properties of maxillofacial silicone elastomer subjected to outdoor weathering. Heliyon 2024, 10, e25039. [Google Scholar] [CrossRef] [PubMed]
- Shihab, N.M.; Abdul-Ameer, F.M. Studying some mechanical properties of maxillofacial silicone elastomer before and after incorporation of intrinsic pigments and artificial aging. Future Dent. J. 2018, 4, 244–252. [Google Scholar] [CrossRef]


| Samples | Before Aging | After Aging | ||||||
|---|---|---|---|---|---|---|---|---|
| Ra nm | Rq nm | Rt nm | SE | Ra nm | Rq nm | Rt nm | SE | |
| B | 5.35 | 6.95 | 42.75 | 0.00149 | 7.51 | 9.65 | 58.38 | 0.00097 |
| B1 | 4.26 | 5.39 | 30.9 | 0.00048 | 6.84 | 8.82 | 64.16 | 0.00097 |
| B2 | 4.98 | 6.99 | 61.38 | 0.00186 | 7.57 | 8.71 | 44.1 | 0.00164 |
| B3 | 3.53 | 4.55 | 28.1 | 0.00056 | 4.7 | 6.4 | 58.93 | 0.00182 |
| B4 | 7.58 | 9.27 | 22.53 | 0.00119 | 3.36 | 4.21 | 26.58 | 0.0022 |
| R | 3.45 | 4.39 | 25.76 | 0.00093 | 3.84 | 5.11 | 35.15 | 0.00093 |
| R1 | 3.65 | 4.66 | 32.42 | 0.00119 | 4.19 | 5.64 | 41.01 | 0.00119 |
| R2 | 4.3 | 5.5 | 39.06 | 0.00141 | 5 | 6.44 | 54.39 | 0.00141 |
| R3 | 4.27 | 5.66 | 49.01 | 0.00149 | 5.34 | 7.43 | 78.84 | 0.00149 |
| R4 | 4.72 | 5.96 | 35.36 | 0.00067 | 3.96 | 5.33 | 55.43 | 0.00067 |
| Time (h) | Pigment | Control Median (Q1–Q3) | 1% ZnO Median (Q1–Q3) | 2% ZnO Median (Q1–Q3) | 3% ZnO Median (Q1–Q3) | 4% ZnO Median (Q1–Q3) |
|---|---|---|---|---|---|---|
| 252 | Rose Silk | 0.13 (0.08–0.36) | 0.14 (0.07–0.25) | 0.23 (0.22-0.28) | 0.14 (0.11–0.19) | 0.17 (0.14–0.25) |
| Soft Brown | 0.19 (0.13–0.25) | 0.13 (0.09–0.21) | 0.20 (0.15–0.24) | 0.16 (0.12–0.19) | 0.12 (0.10–0.21) | |
| 504 | Rose Silk | 0.53 (0.45–0.71) | 0.44 (0.42–0.50) | 0.54 (0.41–0.59) | 0.42 (0.38-0.51) ** | 0.48 (0.34–0.51) |
| Soft Brown | 0.45 (0.41–0.52) | 0.30 (0.26–0.39) ** | 0.41 (0.25–0.48) | 0.42 (0.38–0.60) | 0.34 (0.31–0.37) ** | |
| 756 | Rose Silk | 0.29 (0.19–0.47) | 0.14 (0.08–0.18) ** | 0.23 (0.16–0.32) | 0.13 (0.07–0.21) ** | 0.18 (0.13–0.30) |
| Soft Brown | 0.16 (0.13–0.24) | 0.18 (0.13–0.24) | 0.18 (0.12–0.24) | 0.16 (0.13–0.19) | 0.19 (0.17–0.21) | |
| 1008 | Rose Silk | 0.36 (0.26–0.59) | 0.16 (0.12–0.18) ** | 0.24 (0.11–0.40) ** | 0.20 (0.12–0.26) ** | 0.21 (0.15–0.28) ** |
| Soft Brown | 0.21 (0.18–0.24) | 0.14 (0.10–0.16) ** | 0.16 (0.13–0.17) ** | 0.18 (0.13–0.23) | 0.14 (0.12–0.24) ** | |
| 1252 | Rose Silk | 0.40 (0.34–0.60) | 0.18 (0.12–0.20) ** | 0.15 (0.12–0.21) ** | 0.16 (0.13–0.23) ** | 0.20 (0.15–0.23) ** |
| Soft Brown | 0.18 (0.16–0.22) | 0.20 (0.16–0.26) | 0.18 (0.13–0.25) | 0.09 (0.06–0.10) ** | 0.16 (0.13–0.24) | |
| 1504 | Rose Silk | 0.43 (0.31–0.76) | 0.14 (0.07–0.16) ** | 0.16 (0.09–0.30) ** | 0.18 (0.10–0.21) ** | 0.20 (0.14–0.25) ** |
| Soft Brown | 0.21 (0.16–0.25) | 0.16 (0.14–0.24) | 0.15 (0.12–0.17) ** | 0.13 (0.10–0.19) ** | 0.13 (0.12–0.19) ** | |
| 1756 | Rose Silk | 0.52 (0.45–0.99) | 0.24 (0.24–0.27) ** | 0.33 (0.20–0.43) ** | 0.27 (0.20–0.31) ** | 0.31 (0.24–0.44) ** |
| Soft Brown | 0.26 (0.20–0.34) | 0.16 (0.09–0.18) ** | 0.16 (0.14–0.24) ** | 0.19 (0.16–0.24) | 0.14 (0.12–0.20) ** |
| Time (h) | Rose Silk Optimal ZnO% Median (Q1–Q3) | Soft Brown Optimal ZnO% Median (Q1–Q3) |
|---|---|---|
| 252 | 0.14 (0.07–0.25)—1% ZnO | 0.13 (0.09–0.21)—1% ZnO |
| 504 | 0.42 (0.38–0.51)—3% ZnO | 0.30 (0.26–0.39)—1% ZnO |
| 756 | 0.13 (0.07–0.21)—3% ZnO | 0.16 (0.13–0.19)—3% ZnO |
| 1008 | 0.16 (0.12–0.18)—1% ZnO | 0.14 (0.10–0.16)—1% ZnO |
| 1252 | 0.18 (0.12–0.20)—1% ZnO | 0.09 (0.06–0.10)—3% ZnO |
| 1504 | 0.14 (0.07–0.16)—1% ZnO | 0.13 (0.10–0.19)—3% ZnO |
| 1756 | 0.24 (0.24–0.27)—1% ZnO | 0.14 (0.12–0.20)—4% ZnO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, L.; Othman, K.; Azhdar, B. The Impact of Zinc Oxide Nanoparticles on the Color Stability and Surface Roughness of Heat-Polymerized Maxillofacial Silicone Elastomer Subjected to Artificial Aging: An In Vitro Study. Polymers 2025, 17, 2336. https://doi.org/10.3390/polym17172336
Othman L, Othman K, Azhdar B. The Impact of Zinc Oxide Nanoparticles on the Color Stability and Surface Roughness of Heat-Polymerized Maxillofacial Silicone Elastomer Subjected to Artificial Aging: An In Vitro Study. Polymers. 2025; 17(17):2336. https://doi.org/10.3390/polym17172336
Chicago/Turabian StyleOthman, Lozan, Kawan Othman, and Bruska Azhdar. 2025. "The Impact of Zinc Oxide Nanoparticles on the Color Stability and Surface Roughness of Heat-Polymerized Maxillofacial Silicone Elastomer Subjected to Artificial Aging: An In Vitro Study" Polymers 17, no. 17: 2336. https://doi.org/10.3390/polym17172336
APA StyleOthman, L., Othman, K., & Azhdar, B. (2025). The Impact of Zinc Oxide Nanoparticles on the Color Stability and Surface Roughness of Heat-Polymerized Maxillofacial Silicone Elastomer Subjected to Artificial Aging: An In Vitro Study. Polymers, 17(17), 2336. https://doi.org/10.3390/polym17172336






