Impact of Polymer Molecular Weight on Aging of Poly(Ethyleneoxide)/Dextran All-Aqueous Emulsions Stabilized by Oppositely Charged Nanoparticle/Polyelectrolyte Assemblies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Phase Behavior
2.3. Emulsion Preparation
2.4. Fluorescence Imaging
2.5. Rheological Measurements
2.6. Electrophoretic Mobility Measurements
2.7. Interfacial Tension Measurements
3. Results and Discussion
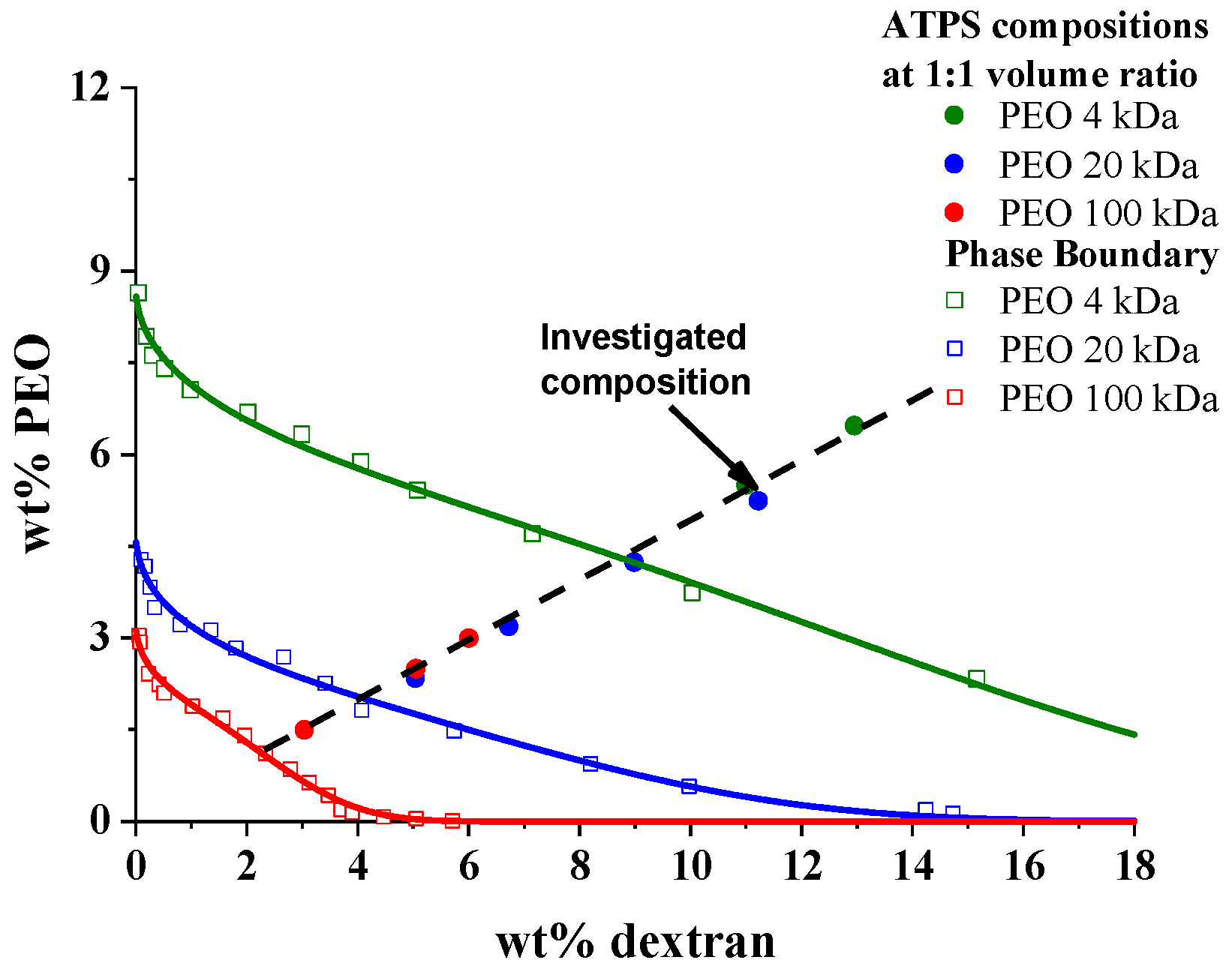
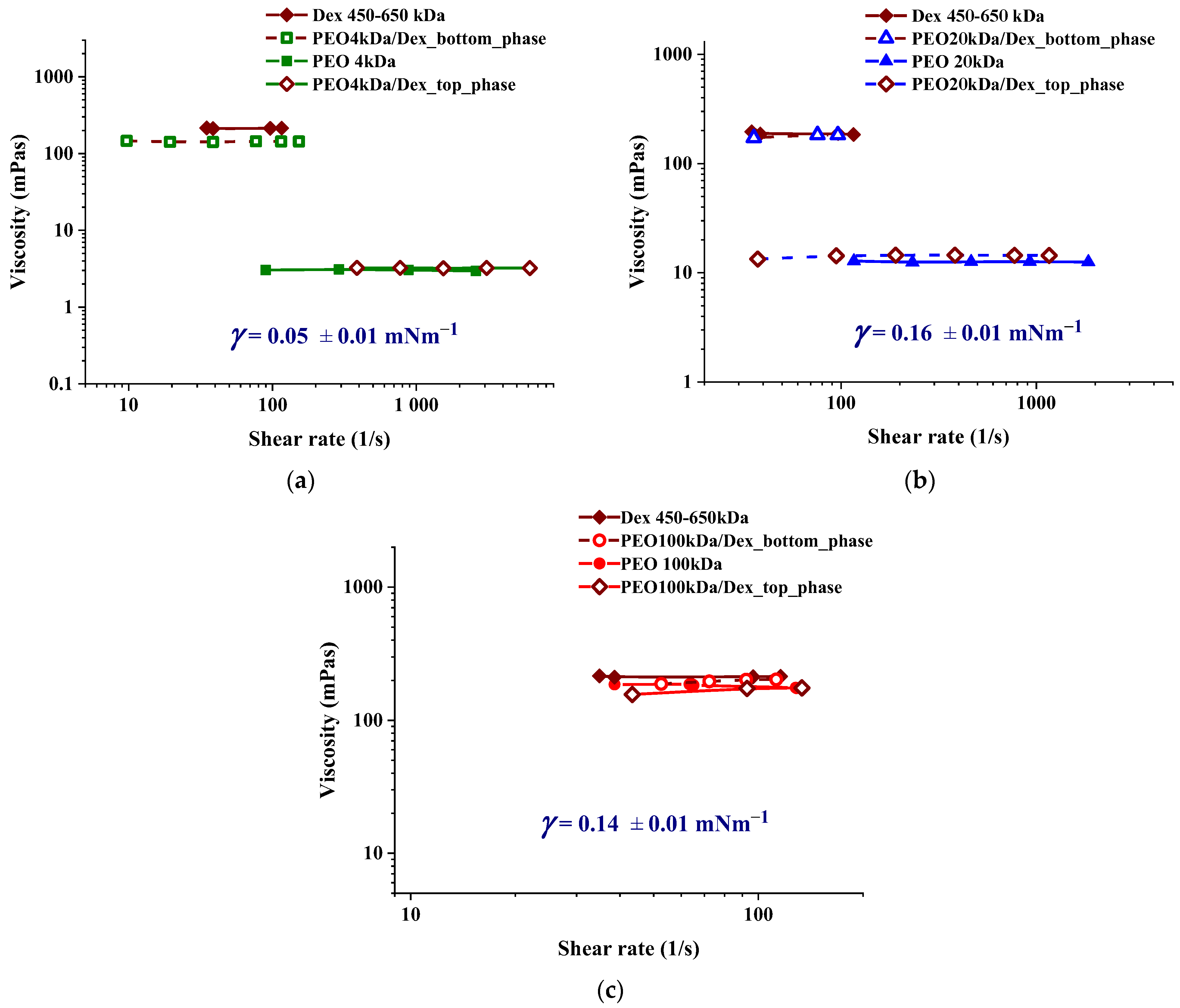
3.1. Characterization of PEO/Dextran ATPS Systems
3.2. Silica Particle/PDADMAC Association
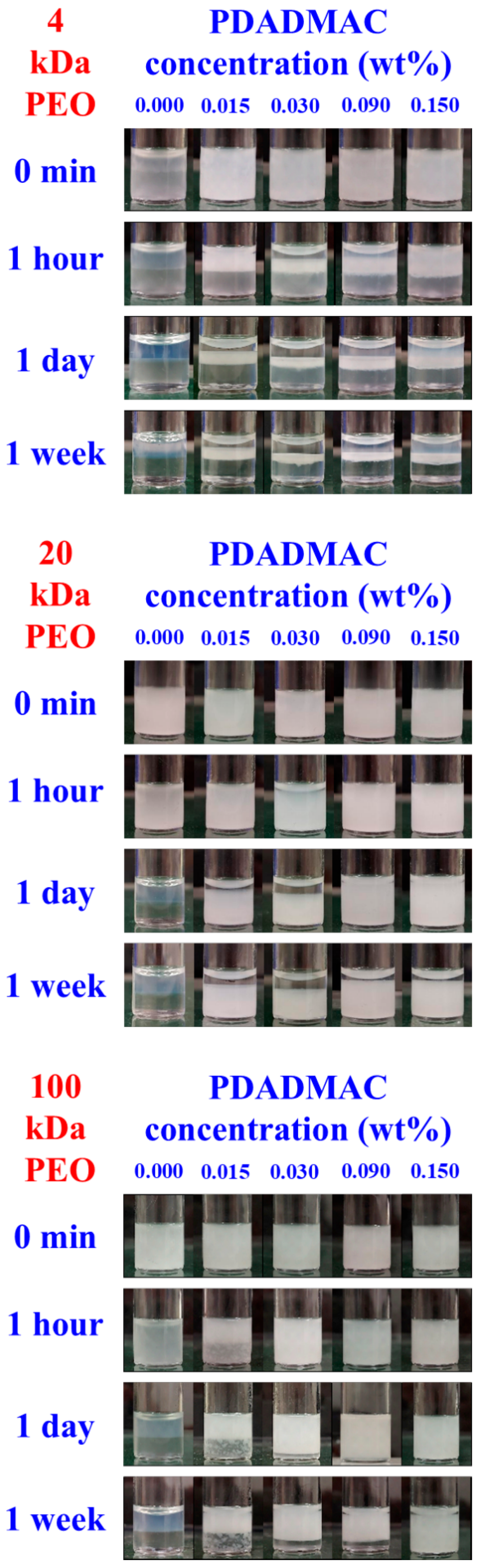
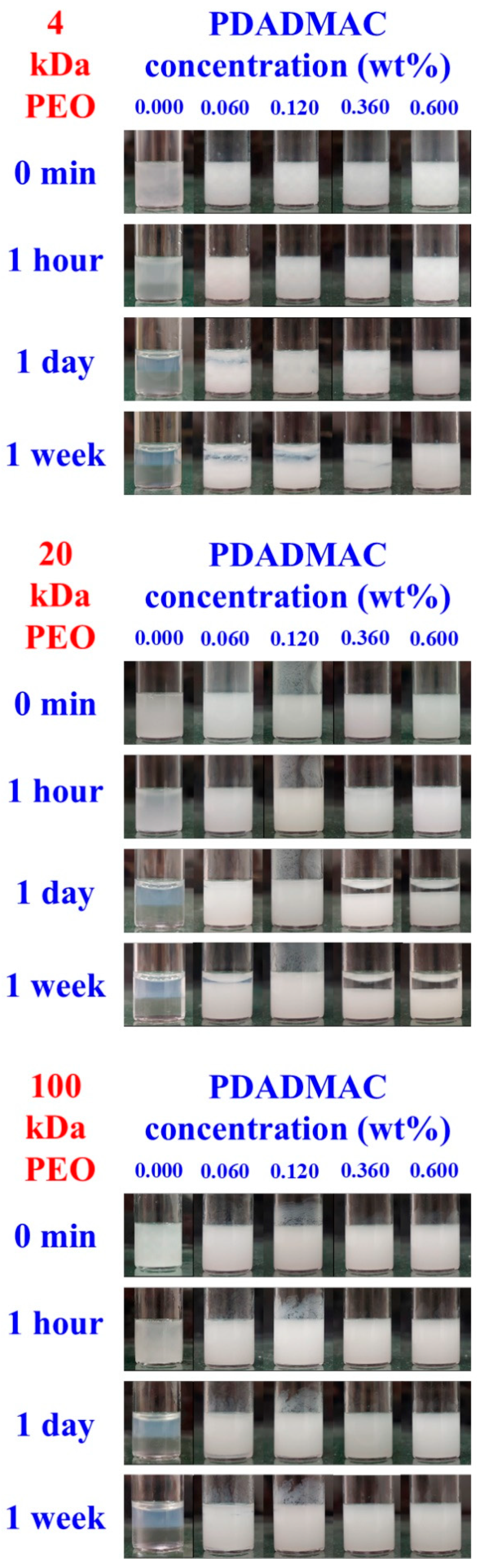
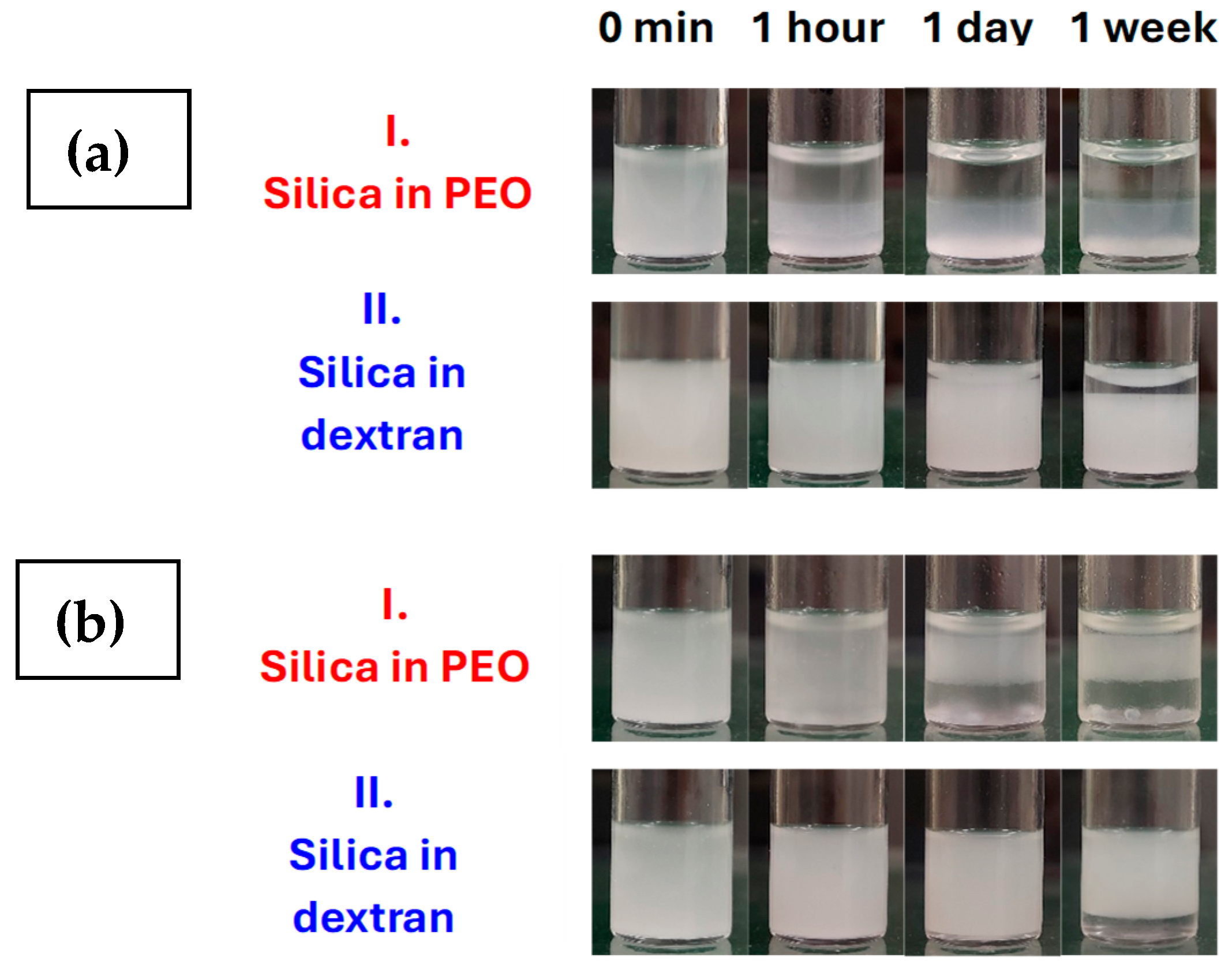
3.3. Aging Processes in Emulsions with 1 and 4 wt% Silica Concentrations
3.4. Dependence of Emulsion Stability on PEO Molecular Weight
3.5. The Effect of Starting Media
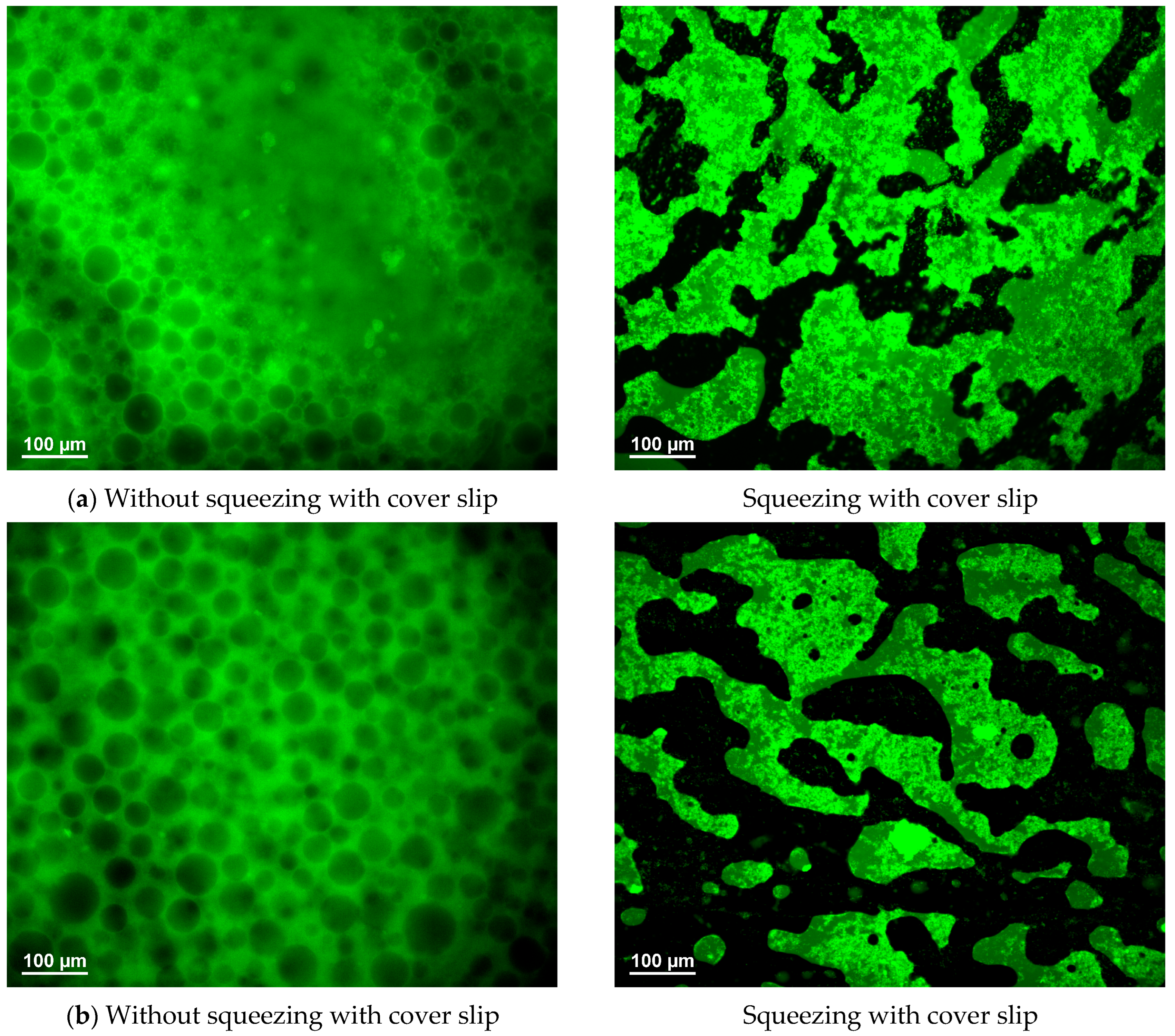
3.6. Formation of Bicontinuous Structures Under Confinement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, T.F.; Borchardt, H.; Wanderley, W.F.; Vasconcelos, U.; Itamara, F.; Leite, I.L. Pequi Pulp (Caryocar brasiliense) Oil-Loaded Emulsions as Cosmetic Products for Topical Use. Polymers 2025, 17, 226. [Google Scholar] [CrossRef]
- Wang, L.; Lin, L.; Li, J.; Liao, J.; Li, B.; Jiao, W.; Guo, S. In Vitro Gastrointestinal Digestion of Corn-Oil-in-Water Pickering Emulsions: Influence of Lignin-Containing Cellulose Nanofibrils Loading. Polymers 2024, 16, 2648. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Yin, N.; Xu, D.; Ge, L.; Guo, R. Dynamically Reconfigurable Complex Emulsion Droplets as Intelligent Microreactors. ACS Sustain. Chem. Eng. 2024, 12, 5129–5138. [Google Scholar] [CrossRef]
- Gao, X.; Hou, L.; Yang, W.; Dong, L.; Ge, X. Lignin-Based Nanoparticles Stabilized Pickering Emulsion for Enhanced Catalytic Hydrogenation. Langmuir 2025, 41, 1937–1947. [Google Scholar] [CrossRef]
- Johnson, E.; Koh, A. Recent Advances in Smart Emulsion Materials: From Synthesis to Applications. Adv. Eng. Mater. 2024, 26, 2400995. [Google Scholar] [CrossRef]
- Cheng, Y.; Xie, J.; Lu, Y.; Tian, W.; Wu, T.; Chen, F.; Gao, W.; Jin, Y.; Yuan, L.; Wang, B. Interfacial Pickering Emulsion Polycondensation for Degradable Nanocomposites. ACS Macro. Lett. 2024, 13, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, X.; Zhang, J.; Liu, Y.; Liu, C.; Huang, B.; Yang, Y. Development and Application of High-Internal-Phase Water-in-Oil Emulsions Using Amphiphilic Nanoparticle- Based Emulsifiers. Polymers 2024, 16, 3148. [Google Scholar] [CrossRef]
- Liang, C.; Qi, N.; Zhao, L.; Li, X.; Li, Z. Development of a Water-Sensitive Self-Thickening Emulsion Temporary Plugging Diverting Agent for High-Temperature and High-Salinity Reservoirs. Polymers 2025, 17, 1543. [Google Scholar] [CrossRef]
- Di Vitantonio, G.; Wang, T.; Stebe, K.J.; Lee, D. Fabrication and Application of Bicontinuous Interfacially Jammed Emulsions Gels. Appl. Phys. Rev. 2021, 8, 021323. [Google Scholar] [CrossRef]
- Ghaffarkhah, A.; Hashemi, S.A.; Isari, A.A.; Panahi-Sarmad, M.; Jiang, F.; Russell, T.P.; Rojas, O.J.; Arjmand, M. Chemistry, Applications, and Future Prospects of Structured Liquids. Chem. Soc. Rev. 2024, 53, 9652–9717. [Google Scholar] [CrossRef]
- Thorson, T.J.; Gurlin, R.E.; Botvinick, E.L.; Mohraz, A. Bijel-Templated Implantable Biomaterials for Enhancing Tissue Integration and Vascularization. Acta Biomater. 2019, 94, 173–182. [Google Scholar] [CrossRef]
- Kharal, S.P.; Haase, M.F. Centrifugal Assembly of Helical Bijel Fibers for PH Responsive Composite Hydrogels. Small 2022, 18, 2106826. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Richter, F.H.; Thijssen, J.H.J.; Bruce, P.G.; Clegg, P.S. Direct Transformation of Bijels into Bicontinuous Composite Electrolytes Using a Pre-Mix Containing Lithium Salt. Mater. Horiz. 2018, 5, 499–505. [Google Scholar] [CrossRef]
- Ching, H.; Thorson, T.J.; Paul, B.; Mohraz, A. Rapid Production of Bicontinuous Macroporous Materials Using Intrinsically Polymerizable Bijels. Mater. Adv. 2021, 2, 5067–5075. [Google Scholar] [CrossRef]
- Neves, B.S.; Gonçalves, R.C.; Mano, J.F.; Oliveira, M.B. Controlling the Diffusion of Small Molecules from Matrices Processed by All-Aqueous Methodologies: Towards the Development of Green Pharmaceutical Products. Green Chem. 2024, 26, 4417–4431. [Google Scholar] [CrossRef]
- Gonçalves, R.C.; Oliveira, M.B.; Mano, J.F. Exploring the Potential of All-Aqueous Immiscible Systems for Preparing Complex Biomaterials and Cellular Constructs. Mater. Horiz. 2024, 11, 4573–4599. [Google Scholar] [CrossRef]
- Esquena, J. Recent Advances on Water-in-Water Emulsions in Segregative Systems of Two Water-Soluble Polymers. Curr. Opin. Food Sci. 2023, 51, 101010. [Google Scholar] [CrossRef]
- Singla, M.; Sit, N. Theoretical Aspects and Applications of Aqueous Two-Phase Systems. ChemBioEng Rev. 2023, 10, 65–80. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Y.; Liu, C.; Cao, H.; Li, Y.; Li, B.; Zhang, Y.; Liu, S. Water-in-Water Pickering Emulsion: A Fascinating Microculture Apparatus for Embedding and Cultivation of Lactobacillus Helveticus. Food Hydrocoll. 2024, 147, 109398. [Google Scholar] [CrossRef]
- Wei, Y.; Cai, Z.; Liu, Z.; Liu, C.; Kong, T.; Li, Z.; Song, Y. All-Aqueous Synthesis of Alginate Complexed with Fibrillated Protein Microcapsules for Membrane-Bounded Culture of Tumor Spheroids. Carbohydr. Polym. 2024, 345, 122580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Karadas, Ö.; Rosenholm, J.M.; Xu, C.; Näreoja, T.; Wang, X. Bioprinting Macroporous Hydrogel with Aqueous Two-Phase Emulsion-Based Bioink: In Vitro Mineralization and Differentiation Empowered by Phosphorylated Cellulose Nanofibrils. Adv. Funct. Mater. 2024, 34, 2400431. [Google Scholar] [CrossRef]
- Fick, C.; Khan, Z.; Srivastava, S. Interfacial Stabilization of Aqueous Two-Phase Systems: A Review. Mater. Adv. 2023, 4, 4665–4678. [Google Scholar] [CrossRef]
- Waldmann, L.; Nguyen, D.-N.-T.; Arbault, S.; Nicolai, T.; Benyahia, L.; Ravaine, V. Tuning the Bis-Hydrophilic Balance of Microgels: A Tool to Control the Stability of Water-in-Water Emulsions. J. Colloid Interface Sci. 2024, 653, 581–593. [Google Scholar] [CrossRef]
- Zhou, C.; Xie, Y.; Li, Y.; Li, B.; Zhang, Y.; Liu, S. Water-in-Water Emulsion Stabilized by Cellulose Nanocrystals and Their High Enrichment Effect on Probiotic Bacteria. J. Colloid Interface Sci. 2023, 633, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Buzza, A.; Fletcher, P.D.I.; Georgiou, T.K.; Ghasdian, N. Water-in-Water Emulsions Based on Incompatible Polymers and Stabilized by Triblock Copolymers-Templated Polymersomes. Langmuir 2013, 29, 14804–14814. [Google Scholar] [CrossRef]
- Cui, W.; Xia, C.; Xu, S.; Ye, X.; Wu, Y.; Cheng, S.; Zhang, R.; Zhang, C.; Miao, Z. Water-in-Water Emulsions Stabilized by Self-Assembled Chitosan Colloidal Particles. Carbohydr. Polym. 2023, 303, 120466. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, C.; Zhang, J.; Li, Y.; Li, B.; Liu, S. Crosslinking Alginate at Water-in-Water Pickering Emulsions Interface to Control the Interface Structure and Enhance the Stress Resistance of the Encapsulated Probiotics. J. Colloid Interface Sci. 2024, 655, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.S.; Phisarnchananan, N. The Effect of Nanoparticles on the Phase Separation of Waxy Corn Starch+locust Bean Gum or Guar Gum. Food Hydrocoll. 2014, 42, 92–99. [Google Scholar] [CrossRef]
- Ma, Q.; Song, Y.; Kim, J.W.; Choi, H.S.; Shum, H.C. Affinity Partitioning-Induced Self-Assembly in Aqueous Two-Phase Systems: Templating for Polyelectrolyte Microcapsules. ACS Macro Lett. 2016, 5, 666–670. [Google Scholar] [CrossRef]
- Shekhar, C.; Kiran, A.; Mehandia, V.; Dugyala, V.R.; Sabapathy, M. Droplet–Bijel–Droplet Transition in Aqueous Two-Phase Systems Stabilized by Oppositely Charged Nanoparticles: A Simple Pathway to Fabricate Bijels. Langmuir 2021, 37, 7055–7066. [Google Scholar] [CrossRef]
- Zhu, Y.; Beaumont, M.; Solin, K.; Spiliopoulos, P.; Zhao, B.; Tao, H.; Kontturi, E.; Bai, L.; Rojas, O.J. Interfacial Membranization of Regenerated Cellulose Nanoparticles and a Protein Renders Stable Water-in-Water Emulsion. Small 2024, 20, 2400952. [Google Scholar] [CrossRef]
- Hann, S.D.; Stebe, K.J.; Lee, D. AWE-Somes: All Water Emulsion Bodies with Permeable Shells and Selective Compartments. ACS Appl. Mater. Interfaces 2017, 9, 25023–25028. [Google Scholar] [CrossRef]
- Mistry, S.L.; Kaul, A.; Merchuk, J.C.; Asenjo, J.A. Mathematical Modelling and Computer Simulation of Aqueous Two-Phase Continuous Protein Extraction. J. Chromatogr. A 1996, 741, 151–163. [Google Scholar] [CrossRef]
- Atefi, E.; Fyffe, D.; Kaylan, K.B.; Tavana, H. Characterization of Aqueous Two-Phase Systems from Volume and Density Measurements. J. Chem. Eng. Data 2016, 61, 1531–1539. [Google Scholar] [CrossRef]
- Stratford, K.; Adhikari, R.; Pagonabarraga, I.; Desplat, J.-C.; Cates, M.E. Colloidal Jamming at Interfaces: A Route to Fluid-Bicontinuous Gels. Science 2005, 309, 2198–2201. [Google Scholar] [CrossRef] [PubMed]
- Merchuk, J.C.; Andrews, B.A.; Asenjo, J.A. Aqueous Two-Phase Systems for Protein Separation: Studies on Phase Inversion. J. Chromatogr. B 1998, 711, 285–293. [Google Scholar] [CrossRef]
- Ryden, J.; Albertsson, P.-A. Interfacial Tension of Dextran-Polyethylene Glycol-Water Two-Phase Systems. J. Colloid Interface Sci. 1971, 37, 219–222. [Google Scholar] [CrossRef]
- Tirtaatmadja, V.; Dunstan, D.E.; Boger, D.V. Rheology of dextran solutions. J. Non-Newton. Fluid Mech. 2001, 97, 295–301. [Google Scholar] [CrossRef]
- Ebagninin, K.W.; Benchabane, A.; Bekkour, K. Rheological characterization of poly(ethylene oxide) solutions of different molecular weights. J. Colloid Interface Sci. 2009, 336, 360–367. [Google Scholar] [CrossRef]
- Gonzalez-Tello, P.; Camacho, F.; Blazquez, G. Density and Viscosity of Concentrated Aqueous Solutions of Polyethylene Glycol. J. Chem. Eng. Data 1994, 39, 611–614. [Google Scholar] [CrossRef]
- Hierrezuelo, J.; Sadeghpour, A.; Szilágyi, I.; Vaccaro, A.; Borkovec, M. Electrostatic Stabilization of Charged Colloidal Particles with Adsorbed Polyelectrolytes of Opposite Charge. Langmuir 2010, 26, 15109–15111. [Google Scholar] [CrossRef]
- Voigt, U.; Jaeger, W.; Findenegg, G.H.; von Klitzing, R. Charge Effects on the Formation of Multilayers Containing Strong Polyelectrolytes. J. Phys. Chem. B 2003, 107, 5273–5280. [Google Scholar] [CrossRef]
- Binks, B.P. Colloidal Particles at a Range of Fluid–Fluid Interfaces. Langmuir 2017, 33, 6947–6963. [Google Scholar] [CrossRef]
- Ben Ayed, E.; Cochereau, R.; Dechancé, C.; Capron, I.; Nicolai, T.; Benyahia, L. Water-In-Water Emulsion Gels Stabilized by Cellulose Nanocrystals. Langmuir 2018, 34, 6887–6893. [Google Scholar] [CrossRef]
- Ramos, D.M.; Sadtler, V.; Marchal, P.; Lemaitre, C.; Niepceron, F.; Benyahia, L.; Roques-Carmes, T. Particles’ Organization in Direct Oil-in-Water and Reverse Water-in-Oil Pickering Emulsions. Nanomaterials 2023, 13, 371. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P.; Lumsdon, S.O. Effects of Oil Type and Aqueous Phase Composition on Oil–Water Mixtures Containing Particles of Intermediate Hydrophobicity. Phys. Chem. Chem. Phys. 2000, 2, 2959–2967. [Google Scholar] [CrossRef]
- Guzmán, E.; Maestro, A.; Ortega, F.; Rubio, R.G. Association of Oppositely Charged Polyelectrolyte And Surfactant In Solution: Equilibrium and Nonequilibrium Features. J. Phys. Condens. Matter 2023, 35, 323001. [Google Scholar] [CrossRef] [PubMed]
- Bali, K.; Varga, Z.; Kardos, A.; Mészáros, R. Impact of Local Inhomogeneities on the Complexation between Poly(Diallyldimethylammoniumchloride) and Sodium Dodecyl Sulfate. Colloids Surf. A 2019, 574, 21–28. [Google Scholar] [CrossRef]
- Pojják, K.; Fegyver, E.; Mészáros, R. Effect of Linear Nonionic Polymer Additives on the Kinetic Stability of Dispersions of Poly(Diallyldimethylammonium Chloride)/Sodium Dodecylsulfate Nanoparticles. Langmuir 2013, 29, 10077–10086. [Google Scholar] [CrossRef]
- Mathur, S.; Moudgil, B.M. Adsorption Mechanism(s) of Poly (Ethylene Oxide) on Oxide Surfaces. J. Colloid Interface Sci. 1997, 196, 92–98. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, Y.; Song, A.; Hao, J. Particle–Polymer Union with Changeable Wettability for Constructing Bijels Using a Simple Mixing Method. Langmuir 2023, 39, 16513–16521. [Google Scholar] [CrossRef]
- Macmillan, K.A.; Royer, J.R.; Morozov, A.; Joshi, Y.M.; Cloitre, M.; Clegg, P.S. Rheological Behavior and in Situ Confocal Imaging of Bijels Made by Mixing. Langmuir 2019, 35, 10927–10936. [Google Scholar] [CrossRef]
- Jiao, J.; Rhodes, D.G.; Burgess, D.J. Multiple Emulsion Stability: Pressure Balance and Interfacial Film Strength. J. Colloid Interface Sci. 2002, 250, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Clegg, P.S.; Herzig, E.M.; Schofield, A.B.; Egelhaaf, S.U.; Horozov, T.S.; Binks, B.P.; Cates, M.E.; Poon, W.C.K. Emulsification of Partially Miscible Liquids Using Colloidal Particles: Nonspherical and Extended Domain Structures. Langmuir 2007, 23, 5984–5994. [Google Scholar] [CrossRef] [PubMed]
- Gam, S.; Corlu, A.; Chung, H.-J.; Ohno, K.; Hore, M.J.A.; Composto, R.J. A Jamming Morphology Map of Polymer Blend Nanocomposite Films. Soft Matter 2011, 7, 7262. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kardos, A.; Bak, M.; Kovács, E.; Juhász, G.; Cserepes, M.; Tóvári, J.; Mészáros, R. Impact of Polymer Molecular Weight on Aging of Poly(Ethyleneoxide)/Dextran All-Aqueous Emulsions Stabilized by Oppositely Charged Nanoparticle/Polyelectrolyte Assemblies. Polymers 2025, 17, 2305. https://doi.org/10.3390/polym17172305
Kardos A, Bak M, Kovács E, Juhász G, Cserepes M, Tóvári J, Mészáros R. Impact of Polymer Molecular Weight on Aging of Poly(Ethyleneoxide)/Dextran All-Aqueous Emulsions Stabilized by Oppositely Charged Nanoparticle/Polyelectrolyte Assemblies. Polymers. 2025; 17(17):2305. https://doi.org/10.3390/polym17172305
Chicago/Turabian StyleKardos, Attila, Mónika Bak, Emese Kovács, György Juhász, Mihály Cserepes, József Tóvári, and Róbert Mészáros. 2025. "Impact of Polymer Molecular Weight on Aging of Poly(Ethyleneoxide)/Dextran All-Aqueous Emulsions Stabilized by Oppositely Charged Nanoparticle/Polyelectrolyte Assemblies" Polymers 17, no. 17: 2305. https://doi.org/10.3390/polym17172305
APA StyleKardos, A., Bak, M., Kovács, E., Juhász, G., Cserepes, M., Tóvári, J., & Mészáros, R. (2025). Impact of Polymer Molecular Weight on Aging of Poly(Ethyleneoxide)/Dextran All-Aqueous Emulsions Stabilized by Oppositely Charged Nanoparticle/Polyelectrolyte Assemblies. Polymers, 17(17), 2305. https://doi.org/10.3390/polym17172305






