Advanced Cellulose Triacetate-Based Mixed Matrix Membranes Enhanced by Bimetallic Ni-Cu-BTC MOFs for CO2/CH4 Separation
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
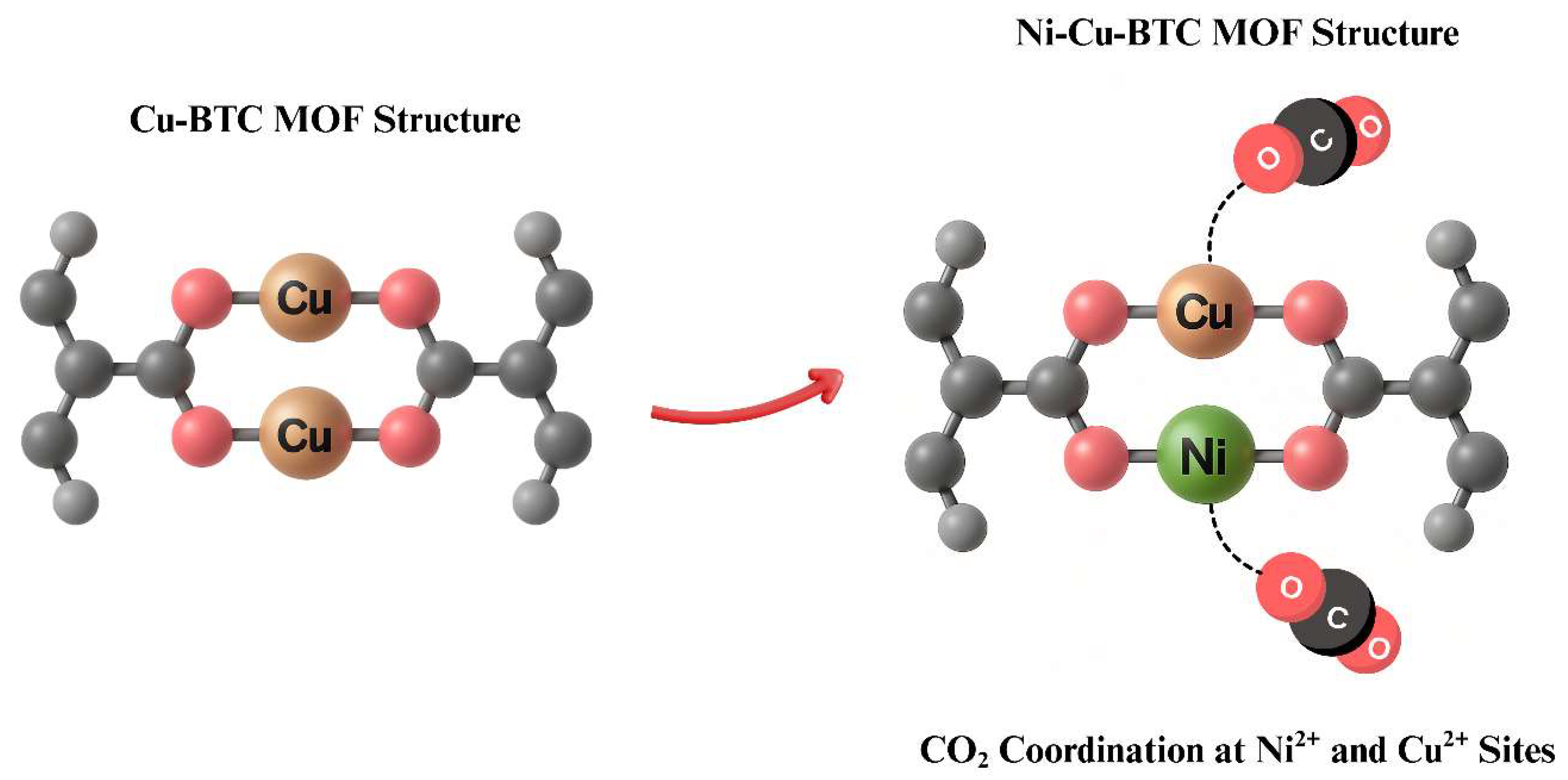
2.2. Synthesis Procedures for Cu-BTC and Ni-Cu-BTC MOFs
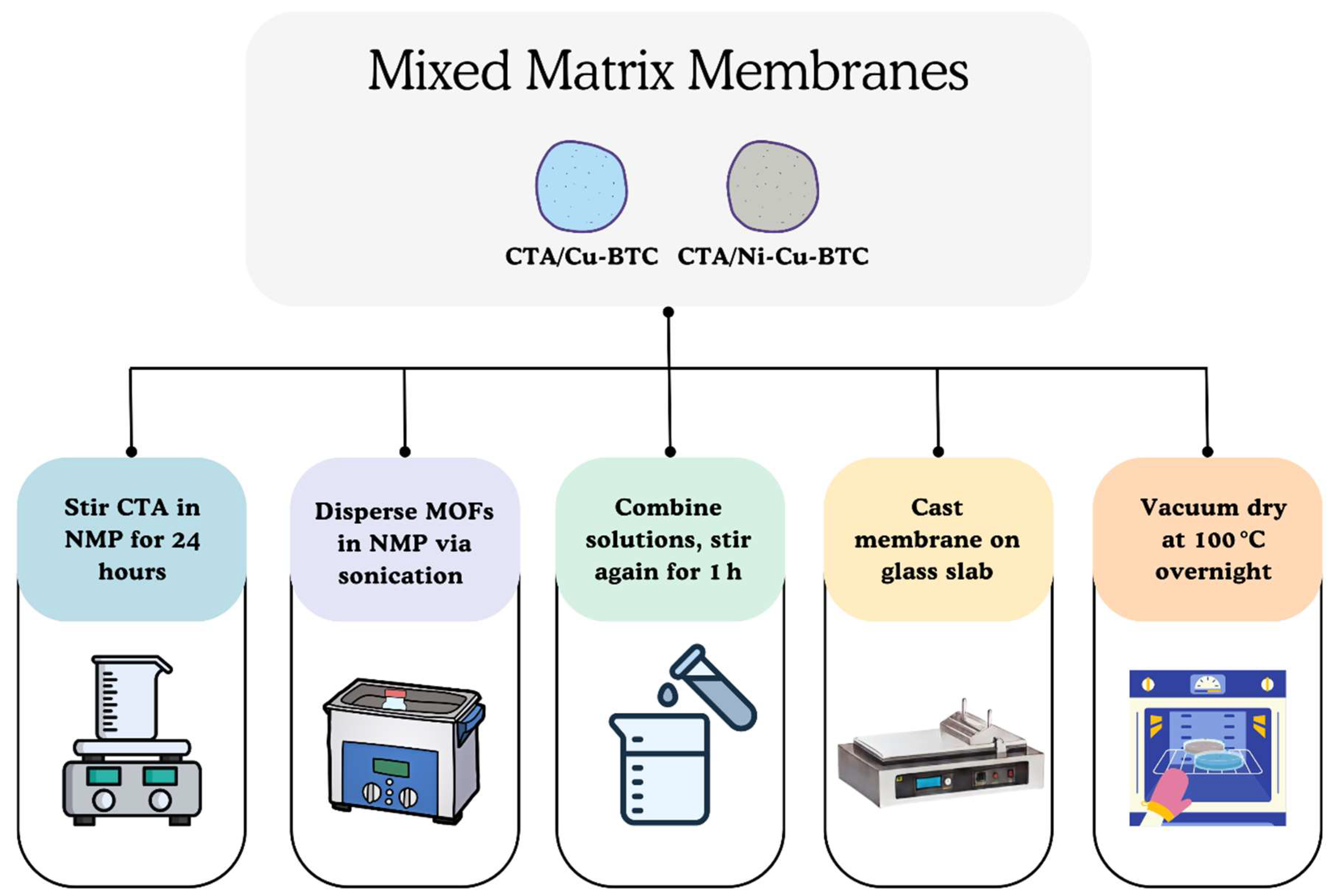
2.3. Membrane Fabrication
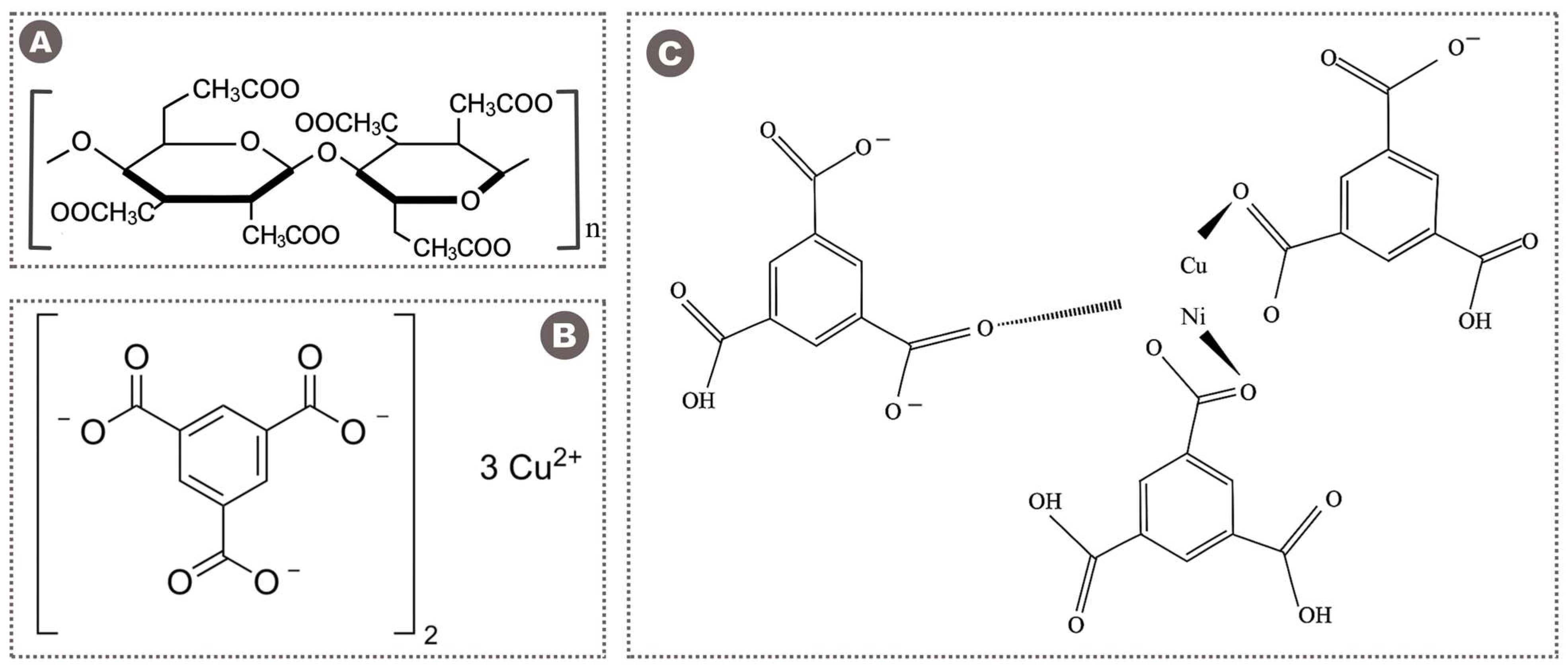
2.3.1. Pristine Cellulose Triacetate (CTA) Membrane
2.3.2. Mixed Matrix Membranes (MMMs)
2.4. Membrane Characterizations
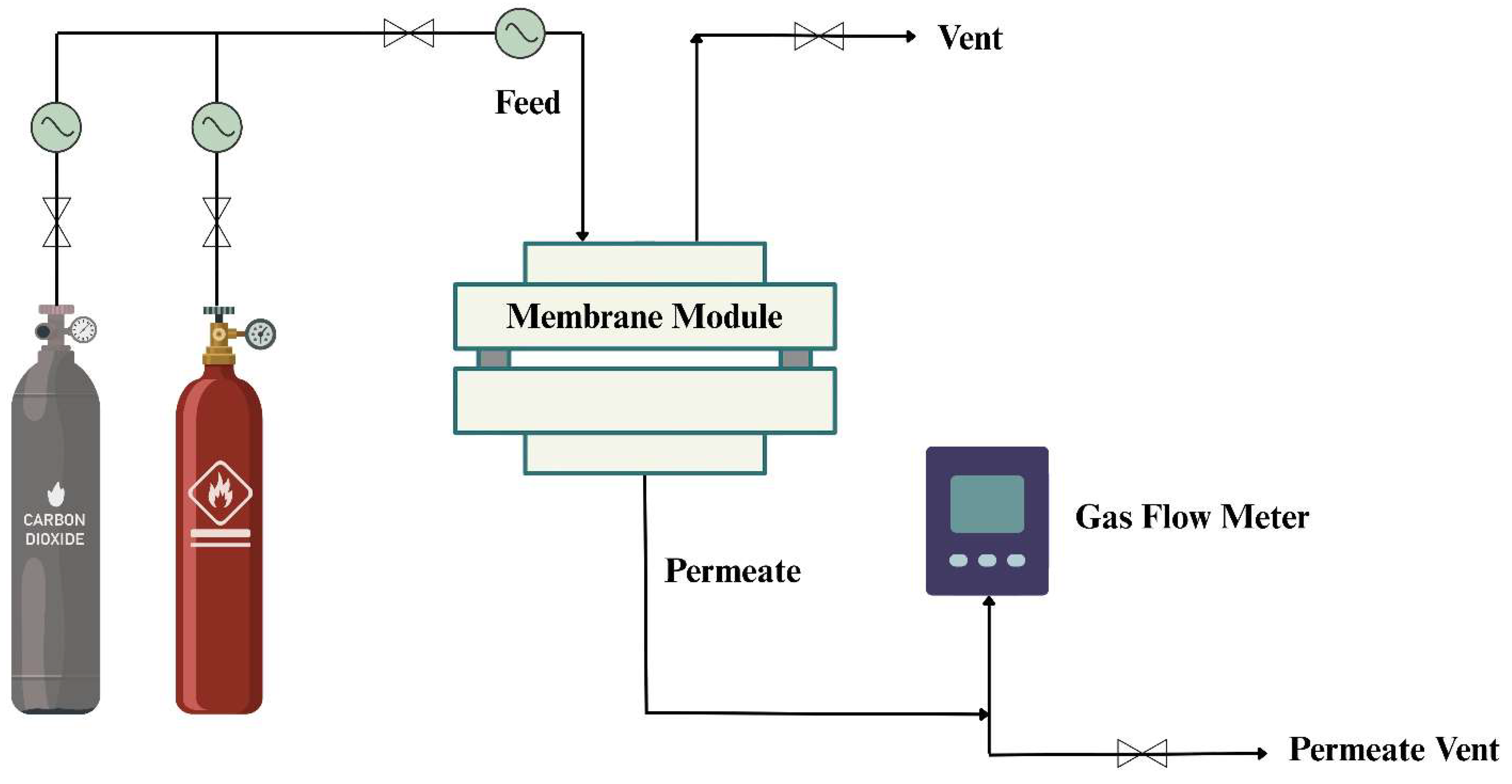
2.5. Gas Permeation Measurements
3. Results and Discussion
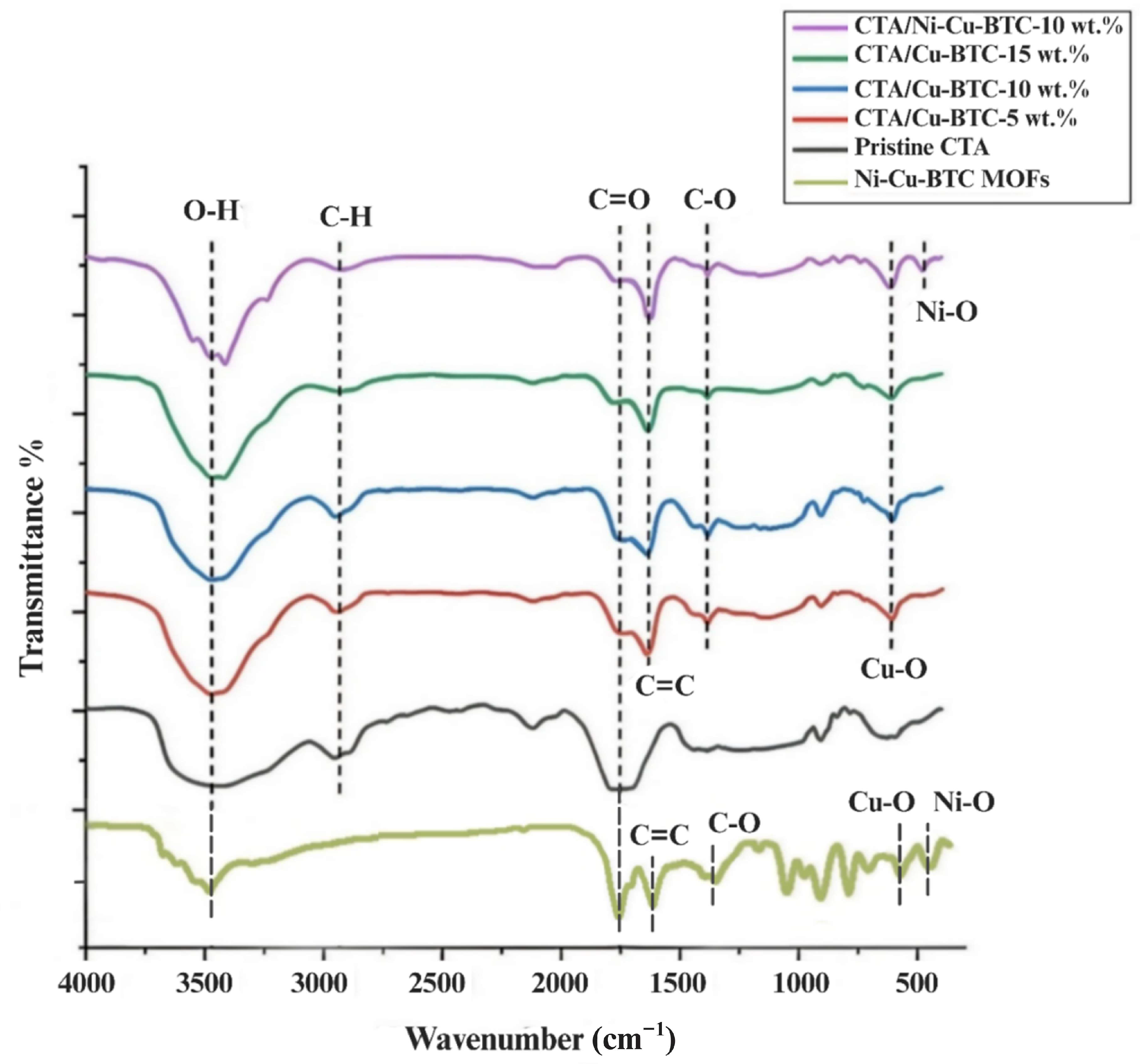
3.1. Fourier Transform Infrared (FTIR) Analysis
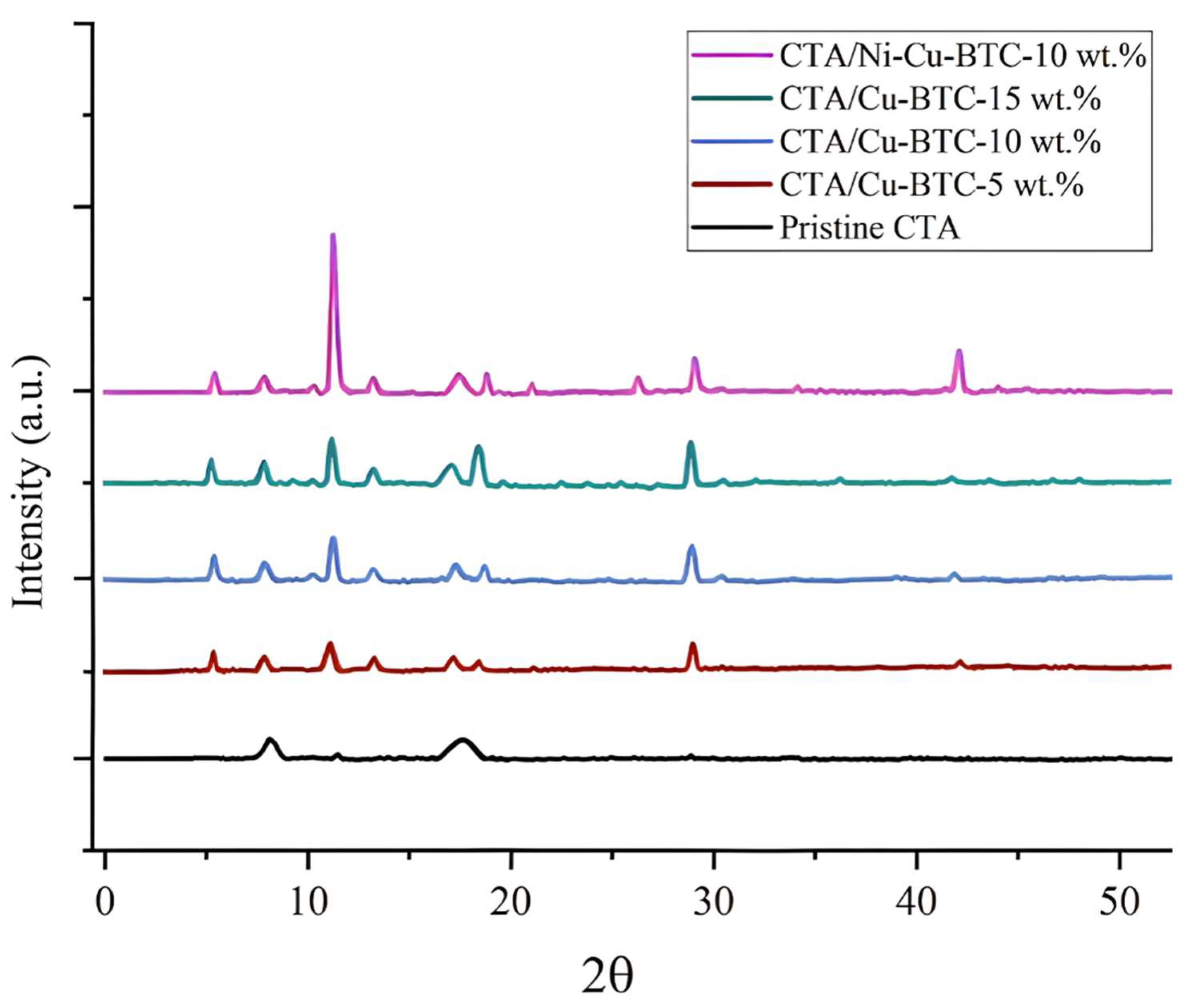
3.2. X-Ray Diffraction (XRD) Analysis
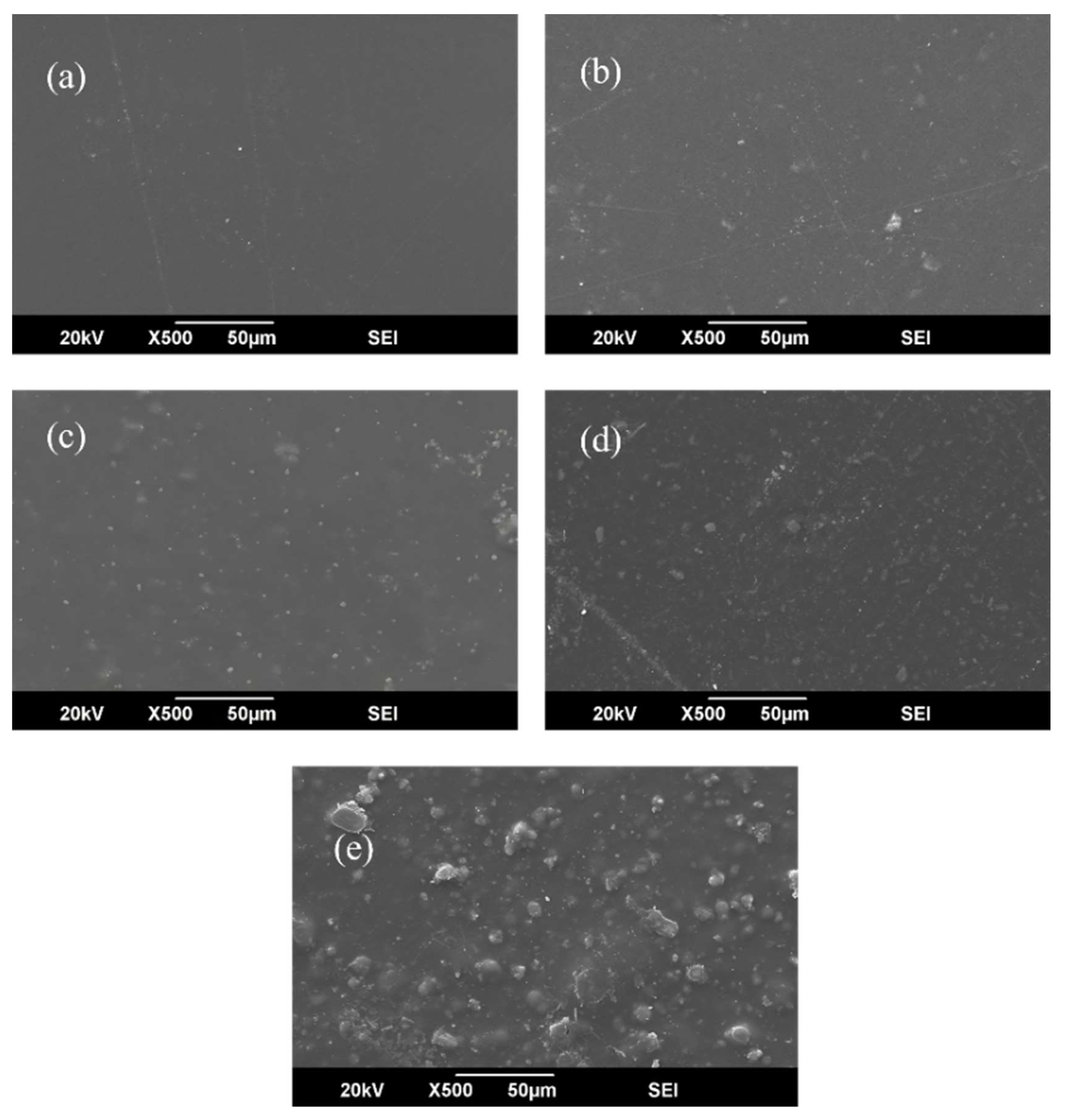
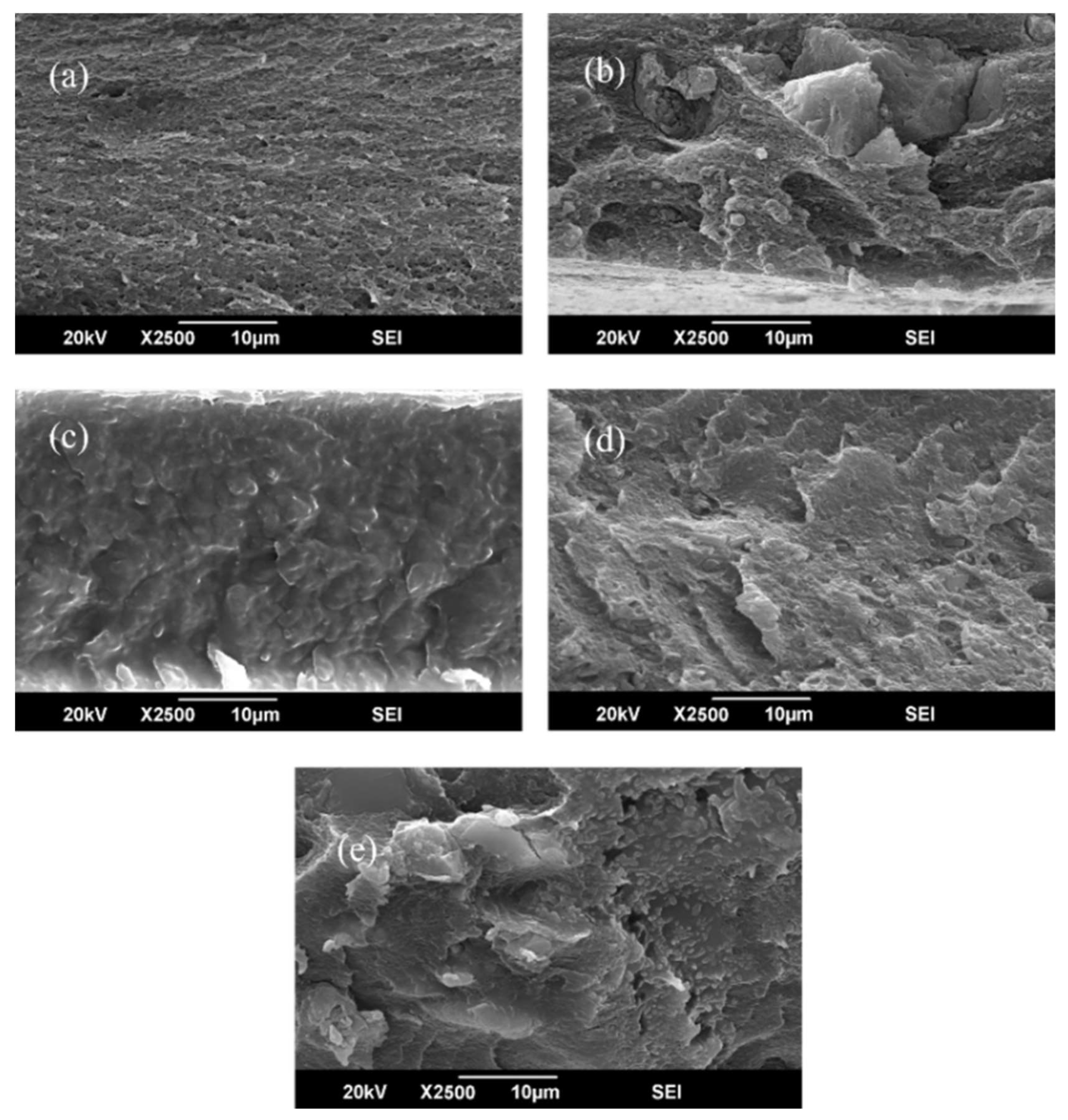
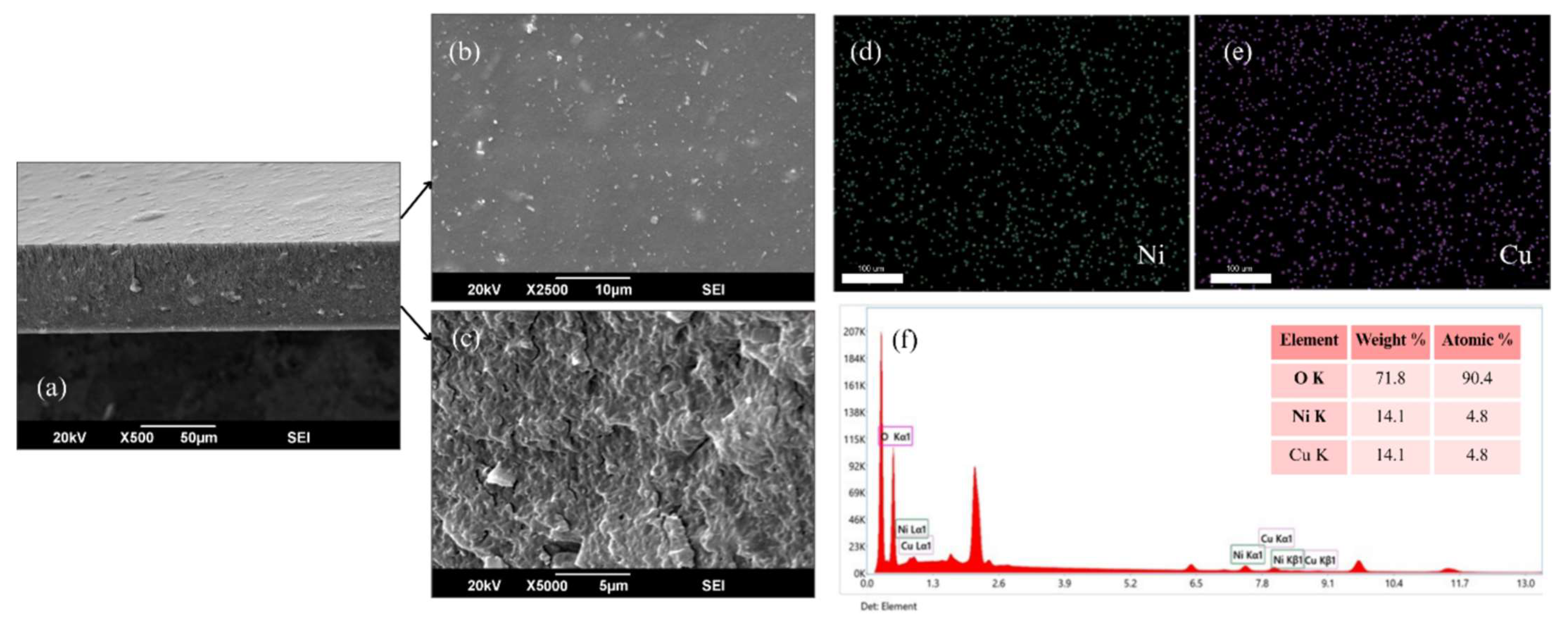
3.3. Scanning Electron Microscopy (SEM) and EDX Analysis
3.4. Fractional Free Volume (FFV) Analysis
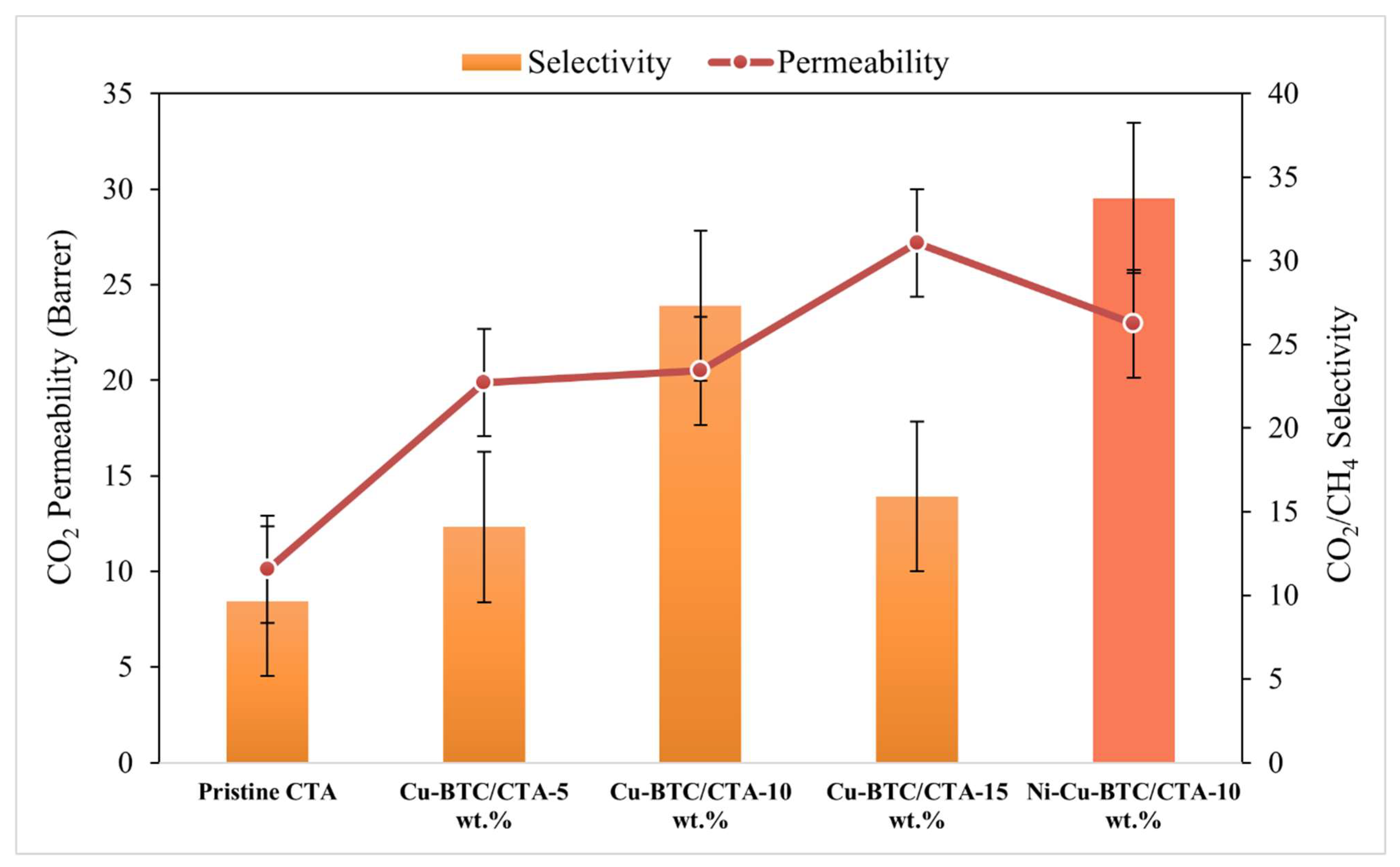
3.5. Gas Permeation Analysis
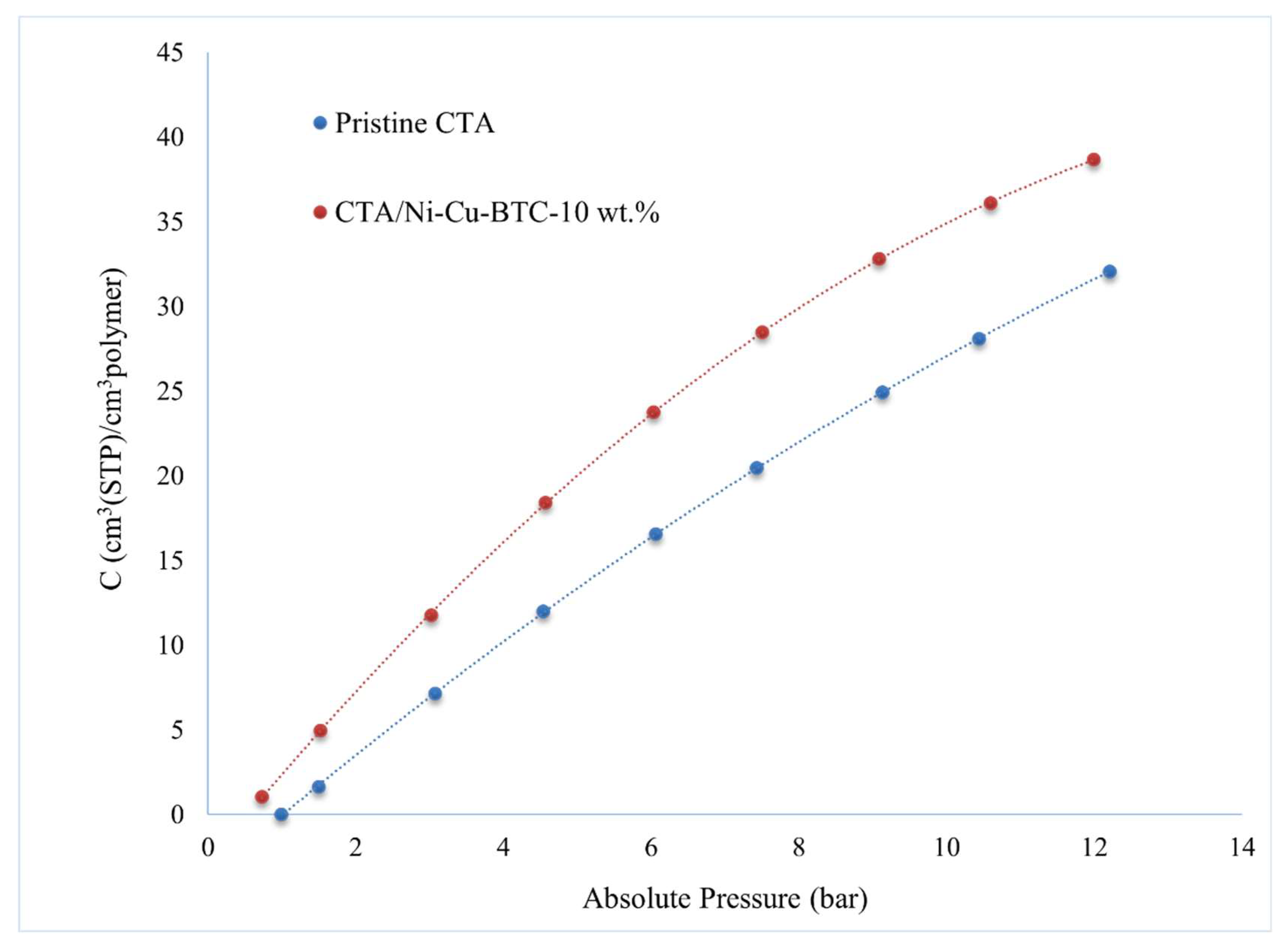
3.6. Sorption Analysis
3.7. Benchmark with the Literature
4. Conclusions
- (a)
- The increase in CO2 permeability observed at a filler concentration of 10 wt.% loading can be primarily attributed to two key factors: (1) the strong interactions between quadrupolar CO2 molecules and the Lewis acidic Cu2+ centers present in the Cu-BTC structure, and (2) the enhancement in fractional free volume resulting from the disruption of the polymer matrix structure.
- (b)
- However, CO2/CH4 selectivity exhibited a fluctuating trend. This trend could result from particle agglomeration at higher loadings and excessive fractional free volume (FFV), which may create non-selective pathways. Therefore, optimized loading is required to fabricate higher-performance CTA/Cu-BTC MMMs.
- (c)
- Pure gas permeation analysis revealed that the optimized bimetallic CTA/Ni-Cu-BTC mixed matrix membrane containing 10 wt.% filler showed a 126.7% increase in CO2 permeability along with a 252.1% enhancement in CO2/CH4 selectivity relative to the pristine CTA membrane. Further validation through mixed-gas separation using a 50:50 CO2/CH4 mixture confirmed these enhancements, with CO2 permeability rising by 91.7% and selectivity improving by 154.8%. This improved performance is primarily due to the synergistic effects introduced by the Ni-Cu-BTC framework, which contributes additional unsaturated metal sites, a higher surface area, and a well-structured pore network—factors that collectively enhance CO2 interaction and diffusion.
- (d)
- The enhancement in gas separation performance of CTA/Ni-Cu-BTC-10 wt.% MMM is primarily the result of a 49.30% increase in the solubility coefficient and a 51.94% rise in the diffusion coefficient compared with the pristine membrane. This improvement likely arises from the excellent molecular sieving capability and high surface area of the dual-metal framework. Additionally, the inclusion of Ni-Cu-BTC MOF improved the Langmuir capacity constant () by 15.04%, Henry’s coefficient () by 14.81%, and the hole affinity constant () by 65%, highlighting significant enhancements in gas sorption and interaction properties.
- (e)
- The FFV analysis supports the permeation and sorption trends, with CTA/Ni-Cu-BTC-10 wt.% showing a notable increase in FFV (0.229) relative to the pristine CTA membrane (0.151).
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mubashir, M.; Fong, Y.Y.; Leng, C.T.; Keong, L.K.; Jusoh, N. Study on the effect of process parameters on CO2/CH4 binary gas separation performance over NH2-MIL-53(Al)/cellulose acetate hollow fiber mixed matrix membrane. Polym. Test. 2020, 81, 106223. [Google Scholar] [CrossRef]
- Doman, L.E.; Smith, K.A.; Mayne, L.D.; Yucel, E.M.; Barden, J.L.; Fawzi, A.M.; Martin, P.D.; Zaretskaya, V.V.; Mellish, M.L.; Kearney, D.R.; et al. International Energy Outlook; USA Energy Information Administration (EIA): Washington, DC, USA, 2010. [Google Scholar]
- Iulianelli, A.; Russo, F.; Galiano, F.; Manisco, M.; Figoli, A. Novel bio-polymer based membranes for CO2/CH4 separation. Int. J. Greenh. Gas Control 2022, 117, 103657. [Google Scholar] [CrossRef]
- Zeeshan, M.H.; Yeong, Y.F.; Chew, T.L. Current Progress on Dual-Layer Hollow Fiber Mixed-Matrix Membrane in CO2 Capture. ChemBioEng Rev. 2024, 11, 513–542. [Google Scholar] [CrossRef]
- Alcheikhhamdon, Y.; Hoorfar, M. Natural gas purification from acid gases using membranes: A review of the history, features, techno-commercial challenges, and process intensification of commercial membranes. Chem. Eng. Process.-Process Intensif. 2017, 120, 105–113. [Google Scholar] [CrossRef]
- Bashir, Z.; Lock, S.S.M.; e Hira, N.; Ilyas, S.U.; Lim, L.G.; Lock, I.S.M.; Yiin, C.L.; Darban, M.A. A review on recent advances of cellulose acetate membranes for gas separation. RSC Adv. 2024, 14, 19560–19580. [Google Scholar] [CrossRef] [PubMed]
- Patil, T.; Dharaskar, S.A.; Reddy, B.R. Innovations in cryogenic carbon capture. Emerging Carbon Capture Technologies: Towards a Sustainable Future; Elsevier: Amsterdam, The Netherlands, 2022; pp. 239–256. [Google Scholar] [CrossRef]
- Patil, T.; Patel, S.; Sinha, M.K.; Dharaskar, S.; Pandya, J.; Shinde, S.; Sillanpaa, M.; Yoo, C.; Khalid, M. Tailoring CO2/CH4 Separation Efficiency with [THTDP][Cl]/Pebax-1657 Supported Ionic Liquid Membranes: Design, Characterization, and Theoretical Insights. Top. Catal. 2024, 68, 290–306. [Google Scholar] [CrossRef]
- Lee, W.; Xu, R.; Kim, S.; Park, J.H.; Kang, Y.T. Nanofluid and nanoemulsion absorbents for the enhancement of CO2 absorption performance. J. Clean. Prod. 2021, 291, 125848. [Google Scholar] [CrossRef]
- Liu, X.; Lim, G.J.H.; Wang, Y.; Zhang, L.; Mullangi, D.; Wu, Y.; Zhao, D.; Ding, J.; Cheetham, A.K.; Wang, J. Binder-free 3D printing of covalent organic framework (COF) monoliths for CO2 adsorption. Chem. Eng. J. 2021, 403, 126333. [Google Scholar] [CrossRef]
- Chen, S.J.; Yu, B.Y. Rigorous simulation and techno-economic evaluation on the hybrid membrane/cryogenic distillation processes for air separation. J. Taiwan Inst. Chem. Eng. 2021, 127, 56–68. [Google Scholar] [CrossRef]
- Vega, F.; Sanna, A.; Navarrete, B.; Maroto-Valer, M.M.; Cortés, V.J. Degradation of amine-based solvents in CO2 capture process by chemical absorption. Greenh. Gases Sci. Technol. 2014, 4, 707–733. [Google Scholar] [CrossRef]
- Altaf, F.; Ahmed, S.; Ali, S.; Mansha, M.; Kouser, T.; Khan, S.A. Transforming Natural Resources into Advanced Solutions: The Contribution of Clay-Based Adsorbents to Carbon Dioxide (CO2) Adsorption. Trans. Tianjin Univ. 2025, 31, 74–130. [Google Scholar] [CrossRef]
- Okoro, J. Microbial Strategies for Carbon Capture and Environmental Sustainability. Archives of Industrial Biotechnology n.d.;8. Available online: https://www.alliedacademies.org/articles/microbial-strategies-for-carbon-capture-and-environmental-sustainability-31056.html (accessed on 17 August 2025).
- Xie, K.; Fu, Q.; Qiao, G.G.; Webley, P.A. Recent progress on fabrication methods of polymeric thin film gas separation membranes for CO2 capture. J. Membr. Sci. 2019, 572, 38–60. [Google Scholar] [CrossRef]
- Rajpure, M.M.; Mujmule, R.B.; Kim, U.; Kim, H. Fabrication of MgO nanorods blended cellulose acetate-based mixed matrix membranes for selective gas separation of H2/CH4, CO2/CH4 and H2/CO2: Effect of loading and pressure. Int. J. Hydrogen Energy 2024, 50, 615–628. [Google Scholar] [CrossRef]
- Abdulabbas, A.A.; Mohammed, T.J.; Al-Hattab, T.A. Parameters estimation of fabricated polysulfone membrane for CO2/CH4 separation. Results Eng. 2024, 21, 101929. [Google Scholar] [CrossRef]
- Raza, A.; Farrukh, S.; Hussain, A.; Khan, I.; Othman, M.H.D.; Ahsan, M. Performance Analysis of Blended Membranes of Cellulose Acetate with Variable Degree of Acetylation for CO2/CH4 Separation. Membranes 2021, 11, 245. [Google Scholar] [CrossRef]
- Puleo, A.C.; Paul, D.R.; Kelley, S.S. The effect of degree of acetylation on gas sorption and transport behavior in cellulose acetate. J. Membr. Sci. 1989, 47, 301–332. [Google Scholar] [CrossRef]
- Roafi, H.; Farrukh, S.; Salahuddin, Z.; Raza, A.; Karim, S.S.; Waheed, H. Fabrication and Permeation Analysis of Polysulfone (PSf) Modified Cellulose Triacetate (CTA) Blend Membranes for CO2 Separation from Methane (CH4). J. Polym. Environ. 2023, 32, 2414–2430. [Google Scholar] [CrossRef]
- Kamide, K.; Saito, M. Cellulose and Cellulose Derivatives: Recent Advances in Physical Chemistry. In Biopolymers; Springer: Berlin/Heidelberg, Germany, 1987; pp. 1–56. [Google Scholar] [CrossRef]
- Boháčová, M.; Zetková, K.; Knotek, P.; Bouša, D.; Friess, K.; Číhal, P.; Lanč, M.; Hrdlička, Z.; Sofer, Z. Mildly oxidized SWCNT as new potential support membrane material for effective H2/CO2 separation. Appl. Mater. Today 2019, 15, 335–342. [Google Scholar] [CrossRef]
- Regmi, C.; Ashtiani, S.; Průša, F.; Friess, K. Synergistic effect of hybridized TNT@GO fillers in CTA-based mixed matrix membranes for selective CO2/CH4 separation. Sep. Purif. Technol. 2022, 282, 120128. [Google Scholar] [CrossRef]
- Cheng, Y.; Ying, Y.; Zhai, L.; Liu, G.; Dong, J.; Wang, Y.; Christopher, M.P.; Long, S.; Wang, Y.; Zhao, D. Mixed matrix membranes containing MOF@COF hybrid fillers for efficient CO2/CH4 separation. J. Membr. Sci. 2019, 573, 97–106. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Sanip, S.M.; Ng, B.C.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, N.; Wu, L.; Kang, S.; Zhang, Z.; Huo, G.; Dai, Z.; Li, N. Carbon molecular sieve gas separation membranes from crosslinkable bromomethylated 6FDA-DAM polyimide. J. Membr. Sci. 2022, 659, 120781. [Google Scholar] [CrossRef]
- Shao, L.; Chung, T.S.; Goh, S.H.; Pramoda, K.P. Transport properties of cross-linked polyimide membranes induced by different generations of diaminobutane (DAB) dendrimers. J. Membr. Sci. 2004, 238, 153–163. [Google Scholar] [CrossRef]
- Thür, R.; Lemmens, V.; Van Havere, D.; van Essen, M.; Nijmeijer, K.; Vankelecom, I.F.J. Tuning 6FDA-DABA membrane performance for CO2 removal by physical densification and decarboxylation cross-linking during simple thermal treatment. J. Membr. Sci. 2020, 610, 118195. [Google Scholar] [CrossRef]
- Ward, J.K.; Koros, W.J. Crosslinkable mixed matrix membranes with surface modified molecular sieves for natural gas purification: I. Preparation and experimental results. J. Membr. Sci. 2011, 377, 75–81. [Google Scholar] [CrossRef]
- Alkadhem, A.M.; Elgzoly, M.A.A.; Onaizi, S.A. Novel Amine-Functionalized Magnesium Oxide Adsorbents for CO2 Capture at Ambient Conditions. J. Environ. Chem. Eng. 2020, 8, 103968. [Google Scholar] [CrossRef]
- Barooah, M.; Kundu, S.; Kumar, S.; Katare, A.; Borgohain, R.; Uppaluri, R.V.S.; Kundu, L.M.; Mandal, B. New generation mixed matrix membrane for CO2 separation: Transition from binary to quaternary mixed matrix membrane. Chemosphere 2024, 354, 141653. [Google Scholar] [CrossRef]
- Tanvidkar, P.; Jonnalagedda, A.; Kuncharam, B.V.R. Investigation of cellulose acetate and ZIF-8 mixed matrix membrane for CO2 separation from model biogas. Environ. Technol. 2024, 45, 2867–2878. [Google Scholar] [CrossRef]
- Lim, S.Y.; Choi, J.; Kim, H.Y.; Kim, Y.; Kim, S.J.; Kang, Y.S.; Won, J. New CO2 separation membranes containing gas-selective Cu-MOFs. J. Membr. Sci. 2014, 467, 67–72. [Google Scholar] [CrossRef]
- Abid, H.R.; Hanif, A.; Keshavarz, A.; Shang, J.; Iglauer, S. CO2, CH4, and H2 Adsorption Performance of the Metal-Organic Framework HKUST-1 by Modified Synthesis Strategies. Energy Fuels 2023, 37, 7260–7267. [Google Scholar] [CrossRef]
- Dewi, K.K.; Septiani, N.L.W.; Iqbal, M.; Irzaman Nugroho, W.S.; Rusydi, F.; Nugraha; Yuliarto, B. Triethylamine assisted HKUST-1 formation and its electrochemical properties. In Proceedings of the Engineering Physics International Conference 2021–Epic 2021, Yogyakarta, Indonesia, 24–25 August 2021; AIP Publishing LLC: Melville, NY, USA, 2023; Volume 2580. [Google Scholar] [CrossRef]
- Fakoori, M.; Fakoori, M.; Azdarpour, A.; Honarvar, B. Investigation of Thin Film Nanocomposite (TFN) Membrane with NH2-CuBTC for CO2/N2 Separation. Chem. Afr. 2024, 7, 865–876. [Google Scholar] [CrossRef]
- Abraham, D.S.; Athul, K.V.; Shamna, I.; Bhagiyalakshmi, M.; Jeong, S.K. Rationalizing the Efficiency of HKUST-1 for Capture and Biomimetic Sequestration of CO2. Korean J. Chem. Eng. 2024, 41, 1467–1478. [Google Scholar] [CrossRef]
- Nik, O.G.; Chen, X.Y.; Kaliaguine, S. Functionalized metal organic framework-polyimide mixed matrix membranes for CO2/CH4 separation. J. Membr. Sci. 2012, 413–414, 48–61. [Google Scholar] [CrossRef]
- Zulhairun, A.K.; Fachrurrazi, Z.G.; Nur Izwanne, M.; Ismail, A.F. Asymmetric hollow fiber membrane coated with polydimethylsiloxane–metal organic framework hybrid layer for gas separation. Sep. Purif. Technol. 2015, 146, 85–93. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y.; Lester, E.; Wu, T. Optimized synthesis of nano-scale high quality HKUST-1 under mild conditions and its application in CO2 capture. Microporous Mesoporous Mater. 2018, 270, 249–257. [Google Scholar] [CrossRef]
- Peedikakkal, A.M.P.; Aljundi, I.H. Mixed-Metal Cu-BTC Metal-Organic Frameworks as a Strong Adsorbent for Molecular Hydrogen at Low Temperatures. ACS Omega 2020, 5, 28493–28499. [Google Scholar] [CrossRef]
- Sabouni, R.; Kazemian, H.; Rohani, S. Carbon dioxide capturing technologies: A review focusing on metal organic framework materials (MOFs). Environ. Sci. Pollut. Res. 2014, 21, 5427–5449. [Google Scholar] [CrossRef]
- Kim, C.; Talapaneni, S.N.; Dai, L. Porous carbon materials for CO2 capture, storage and electrochemical conversion. Mater. Rep. Energy 2023, 3, 100199. [Google Scholar] [CrossRef]
- Singh, S.; Varghese, A.M.; Karanikolos, G.N. Mixed Matrix CO2 Separation Membranes based on Polysulfone with Cu-BTC Metal-Organic Framework and Poly(ethylene glycol)-grafted-Carbon Nanotubes. In Proceedings of the 15th Greenhouse Gas Control Technologies Conference 2021, GHGT 2021, Abu Dhabi, United Arab Emirates, 15–18 March 2021. [Google Scholar] [CrossRef]
- Rehman, A.; Jahan, Z.; Khan Niazi, M.B.; Noor, T.; Javed, F.; Othman, S.I.; Abukhadra, M.R.; Nawaz, A. Graphene-grafted bimetallic MOF membranes for hazardous & toxic contaminants treatment. Chemosphere 2023, 340, 139721. [Google Scholar] [CrossRef]
- Yang, X.; Xu, Q. Bimetallic Metal-Organic Frameworks for Gas Storage and Separation. Cryst. Growth Des. 2017, 17, 1450–1455. [Google Scholar] [CrossRef]
- He, X.; Chen, D.R.; Wang, W.N. Bimetallic metal-organic frameworks (MOFs) synthesized using the spray method for tunable CO2 adsorption. Chem. Eng. J. 2020, 382, 122825. [Google Scholar] [CrossRef]
- Villajos, J.A.; Orcajo, G.; Martos, C.; Botas, J.Á.; Villacañas, J.; Calleja, G. Co/Ni mixed-metal sited MOF-74 material as hydrogen adsorbent. Int. J. Hydrogen Energy 2015, 40, 5346–5352. [Google Scholar] [CrossRef]
- Botas, J.A.; Calleja, G.; Sánchez-Sánchez, M.; Orcajo, M.G. Effect of Zn/Co ratio in MOF-74 type materials containing exposed metal sites on their hydrogen adsorption behaviour and on their band gap energy. Int. J. Hydrogen Energy 2011, 36, 10834–10844. [Google Scholar] [CrossRef]
- Chen, C.; Kosari, M.; Jing, M.; He, C. Microwave-assisted synthesis of bimetallic NiCo-MOF-74 with enhanced open metal site for efficient CO2 capture. Environ. Funct. Mater. 2022, 1, 253–266. [Google Scholar] [CrossRef]
- Zhou, Z.; Mei, L.; Ma, C.; Xu, F.; Xiao, J.; Xia, Q.; Li, Z. A novel bimetallic MIL-101(Cr, Mg) with high CO2 adsorption capacity and CO2/N2 selectivity. Chem. Eng. Sci. 2016, 147, 109–117. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Liu, J.; Zhang, J.; Ren, Y.; Zhao, J. Construction of Zn–Cu bimetallic metal–organic frameworks for carbon dioxide capture. RSC Adv. 2024, 14, 20780–20785. [Google Scholar] [CrossRef] [PubMed]
- Hon Lau, C.; Babarao, R.; Hill, M.R. A route to drastic increase of CO2 uptake in Zr metal organic framework UiO-66. Chem. Commun. 2013, 49, 3634–3636. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, R.; Li, T.; Sun, Y.; Sharif, S.; He, G.; Ma, C. Amino-modified bimetallic ZIF (Zn/Co-NH2)/Polyimide-based mixed matrix membranes for enhanced hydrogen separation. Sep. Purif. Technol. 2025, 377, 134322. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Han, M.; Xu, S. Efficient N2/CH4 separation by mixed matrix membrane with bimetallic metal–organic framework. Inorg. Chem. Commun. 2025, 172, 113757. [Google Scholar] [CrossRef]
- Khan, A.U.; Samuel, O.; Othman, M.H.D.; Younas, M.; Kamaludin, R.; Khan, Z.I.; Al-Ogaili, M.F.A.; Yoshida, N.; Kurniawan, T.A.; Puteh, M.H.; et al. Modifying polysulfone dual-layer hollow fiber membrane with amine-functionalized bimetallic MOF (PEI@HKUST-1(Cu, Mg)) for improved mechanical stability and CO2/CH4 separation performance. J. Environ. Chem. Eng. 2025, 13, 114913. [Google Scholar] [CrossRef]
- Baldanza, A.; Mallamace, D.; Mensitieri, G.; Brondi, C.; Musto, P.; Scherillo, G. Survey on Adsorption of Low Molecular Weight Compounds in Cu-BTC Metal–Organic Framework: Experimental Results and Thermodynamic Modeling. Int. J. Mol. Sci. 2022, 23, 9406. [Google Scholar] [CrossRef]
- Momin, S.; Mahmood, T.; Ullah, A.; Ali, R. Novel bimetallic organic frameworks of Cu/Ni-BTC for enhanced remediation of methylene blue from aqueous medium. J. Indian Chem. Soc. 2025, 102, 101905. [Google Scholar] [CrossRef]
- Cheng, S.; Chung, T.; Wang, R.; Vora, R. Gas-Sorption Properties of 6FDA-Durene/1,4-Phenylenediamine (pPDA) and 6FDA-Durene/1,3-Phenylenediamine (mPDA) Copolyimides. J. Appl. Polym. Sci. 2003, 90, 2187–2193. [Google Scholar] [CrossRef]
- Suhaimi, N.H.; Yeong, Y.F.; Ch’ng, C.W.M.; Jusoh, N. Tailoring CO2/CH4 Separation Performance of Mixed Matrix Membranes by Using ZIF-8 Particles Functionalized with Different Amine Groups. Polymers 2019, 11, 2042. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A. Van der waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Stern, S.A.; De Meringo, A.H. Solubility of carbon dioxide in cellulose acetate at elevated pressures. J. Polym. Sci. Polym. Phys. Ed. 1978, 16, 735–751. [Google Scholar] [CrossRef]
- Vieth, W.R.; Howell, J.M.; Hsieh, J.H. Dual sorption theory. J. Membr. Sci. 1976, 1, 177–220. [Google Scholar] [CrossRef]
- Vieth, W.R.; Tam, P.M.; Michaels, A.S. Dual sorption mechanisms in glassy polystyrene. J. Colloid. Interface Sci. 1966, 22, 360–370. [Google Scholar] [CrossRef]
- Ricci, E.; De Angelis, M.G. Modelling Mixed-Gas Sorption in Glassy Polymers for CO2 Removal: A Sensitivity Analysis of the Dual Mode Sorption Model. Membranes 2019, 9, 8. [Google Scholar] [CrossRef]
- Douna, I.; Farrukh, S.; Hussain, A.; Salahuddin, Z.; Noor, T.; Pervaiz, E.; Younas, M.; Fan, X.F. Experimental investigation of polysulfone modified cellulose acetate membrane for CO2/H2 gas separation. Korean J. Chem. Eng. 2022, 39, 189–197. [Google Scholar] [CrossRef]
- Hayat, H.; Noor, T.; Iqbal, N.; Ahmed, R.; Zaman, N.; Huang, Y. Electrocatalytic activity of Cu MOF and its g-C3N4-based composites for oxygen reduction and evolution reaction in metal-air batteries. J. Environ. Chem. Eng. 2023, 11, 109627. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, J.H. Copper-based metal-organic framework for highly efficient adsorption of lead ions from aqueous solution. Mater. Res. Express 2022, 9, 095505. [Google Scholar] [CrossRef]
- Abbasi, M.; Noor, T.; Iqbal, N.; Zaman, N. Electrocatalytic study of cu/Ni MOF and its g-C3N4 composites for methanol oxidation reaction. Int. J. Energy Res. 2022, 46, 13915–13930. [Google Scholar] [CrossRef]
- Jaksani, B.; Abburi, J.; Islam, H.; Gonuguntla, S.; Baidya, T.; Pal, U. In situ decorated Ni and Co in a CuBTC MOF for synergistic photocatalytic hydrogen generation. Mater. Adv. 2025, 6, 4211–4219. [Google Scholar] [CrossRef]
- Suleman, M.S.; Lau, K.K.; Yeong, Y.F. Characterization and Performance Evaluation of PDMS/PSF Membrane for CO2/CH4 Separation under the Effect of Swelling. Procedia Eng. 2016, 148, 176–183. [Google Scholar] [CrossRef]
- Nivetha, R.; Sajeev, A.; Mary Paul, A.; Gothandapani, K.; Gnanasekar, S.; Jacob, G.; Sellappan, R.; Raghavan, V.; N, K.C.; Pitchaimuthu, S.; et al. Cu based Metal Organic Framework (Cu-MOF) for electrocatalytic hydrogen evolution reaction. Mater. Res. Express 2020, 7, 114001. [Google Scholar] [CrossRef]
- Ghafuri, H.; Ganjali, F.; Hanifehnejad, P. Cu.BTC MOF as a Novel and Efficient Catalyst for the Synthesis of 1,8-Dioxo-octa-hydro Xanthene 2021. Chem. Proc. 2021, 3, 2. [Google Scholar] [CrossRef]
- Meng, Y.; Shu, L.; Liu, L.; Wu, Y.; Xie, L.; Zhao, M.; Li, J. A high-flux mixed matrix nanofiltration membrane with highly water-dispersible MOF crystallites as filler. J. Membr. Sci. 2019, 591, 117360. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Yuan, S.; Zhou, J.; Wang, B. Challenges and recent advances in MOF-polymer composite membranes for gas separation. Inorg. Chem. Front. 2016, 3, 896–909. [Google Scholar] [CrossRef]
- Tajuddin, M.H.A.; Jaafar, J.; Hasbullah, H.; Awang, N.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Yusof, N.; Aziz, F.; Salleh, W.N.W. Metal Organic Framework in Membrane Separation for Wastewater Treatment: Potential and Way Forward. Arab. J. Sci. Eng. 2021, 46, 6109–6130. [Google Scholar] [CrossRef]
- Chen, G.Q.; Kanehashi, S.; Doherty, C.M.; Hill, A.J.; Kentish, S.E. Water vapor permeation through cellulose acetate membranes and its impact upon membrane separation performance for natural gas purification. J. Membr. Sci. 2015, 487, 249–255. [Google Scholar] [CrossRef]
- Jusoh, N.; Yeong, Y.F.; Lau, K.K.; Shariff, A.M. Fabrication of silanated zeolite T/6FDA-durene composite membranes for CO2/CH4 separation. J. Clean. Prod. 2017, 166, 1043–1058. [Google Scholar] [CrossRef]
- Akbari, A.; Karimi-Sabet, J.; Ghoreishi, S.M. Intensification of helium separation from CH4 and N2 by size-reduced Cu-BTC particles in Matrimid matrix. Sep. Purif. Technol. 2020, 251, 117317. [Google Scholar] [CrossRef]
- Pradanos, P.; Soto, C.; Carmona, F.J.; Lozano, Á.E.; Hernández, A.; Palacio, L. Morphological Study before and after Thermal Treatment of Polymer-Polymer Mixed-Matrix Membranes for Gas Separations. Polymers 2024, 16, 1397. [Google Scholar] [CrossRef]
- Takeuchi, H.; Roe, R.J.; Mark, J.E. Molecular dynamics simulation of diffusion of small molecules in polymers. II. Effect of free volume distribution. J. Chem. Phys. 1990, 93, 9042–9048. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Wang, N.; Cao, Y.; Wang, T. The preparation and gas separation properties of zeolite/carbon hybrid membranes. J. Mater. Sci. 2015, 50, 2561–2570. [Google Scholar] [CrossRef]
- Yang, M.; Han, N.; Shi, L.; Gao, H.; Liu, X.; Mi, Y.; Zeng, X.; Bai, J.; Xiong, D. Effect of nickel doping on the structure, morphology and oxygen evolution reaction performance of Cu-BTC derived CuCoO2. Dalton Trans. 2022, 51, 8757–8765. [Google Scholar] [CrossRef]
- Sirijaraensre, J. Structures and mechanisms of CO2 cycloaddition with styrene oxide on bimetallic M–Cu–BTC MOFs (M = Mg, Ca, Al, and Ga): A DFT study. New J. Chem. 2021, 45, 4729–4737. [Google Scholar] [CrossRef]
- Nguyen, H.; Wang, M.; Hsiao, M.Y.; Nagai, K.; Ding, Y.; Lin, H. Suppression of crystallization in thin films of cellulose diacetate and its effect on CO2/CH4 separation properties. J. Membr. Sci. 2019, 586, 7–14. [Google Scholar] [CrossRef]
- Raza, A.; Japip, S.; Liang, C.Z.; Farrukh, S.; Hussain, A.; Chung, T.S. Novel Cellulose Triacetate (CTA)/Cellulose Diacetate (CDA) Blend Membranes Enhanced by Amine Functionalized ZIF-8 for CO2 Separation. Polymers 2021, 13, 2946. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yavari, M. Upper bound of polymeric membranes for mixed-gas CO2/CH4 separations. J. Membr. Sci. 2015, 475, 101–109. [Google Scholar] [CrossRef]
- Bhide, B.D.; Stern, S.A. Membrane processes for the removal of acid gases from natural gas. II. Effects of operating conditions, economic parameters, and membrane properties. J. Membr. Sci. 1993, 81, 239–252. [Google Scholar] [CrossRef]
- Wind, J.D.; Staudt-Bickel, C.; Paul, D.R.; Koros, W.J. Solid-state covalent cross-linking of polyimide membranes for carbon dioxide plasticization reduction. Macromolecules 2003, 36, 1882–1888. [Google Scholar] [CrossRef]
- Jiang, X.; Li, S.; Shao, L. Pushing CO2-philic membrane performance to the limit by designing semi-interpenetrating networks (SIPN) for sustainable CO2 separations. Energy Environ. Sci. 2017, 10, 1339–1344. [Google Scholar] [CrossRef]
- Ma, X.; Ghanem, B.; Salines, O.; Litwiller, E.; Pinnau, I. Synthesis and effect of physical aging on gas transport properties of a microporous polyimide derived from a novel spirobifluorene-based dianhydride. ACS Macro Lett. 2015, 4, 231–235. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Nasernejad, B.; Kargari, A. Cellulose acetate/nano-porous zeolite mixed matrix membrane for CO2 separation. Greenh. Gases Sci. Technol. 2015, 5, 291–304. [Google Scholar] [CrossRef]
- Khan, A.L.; Li, X.; Vankelecom, I.F.J. Mixed-gas CO2/CH4 and CO2/N2 separation with sulfonated PEEK membranes. J. Membr. Sci. 2011, 372, 87–96. [Google Scholar] [CrossRef]
- Houde, A.Y.; Krishnakumar, B.; Charati, S.G.; Stern, S.A.; Wiley, J. Permeability of Dense (Homogeneous) Cellulose Acetate Membranes to Methane, Carbon Dioxide, and Their Mixtures at Elevated Pressures. J. Appl. Polym. Sci. 1996, 62, 2181–2192. [Google Scholar] [CrossRef]
- Tsujita, Y. Gas sorption and permeation of glassy polymers with microvoids. Prog. Polym. Sci. 2003, 28, 1377–1401. [Google Scholar] [CrossRef]
- Suhaimi, N.H.; Yeong, Y.F.; Jusoh, N.; Chew, T.L.; Bustam, M.A.; Mubashir, M. Altering sorption and diffusion coefficients of gases in 6FDA-based membrane via addition of functionalized Ti-based fillers. Polym. Compos. 2022, 43, 440–453. [Google Scholar] [CrossRef]
- Rehman, A.; Jahan, Z.; Sher, F.; Noor, T.; Khan Niazi, M.B.; Akram, M.A.; Sher, E.K. Cellulose acetate based sustainable nanostructured membranes for environmental remediation. Chemosphere 2022, 307, 135736. [Google Scholar] [CrossRef]
- Nguyen, H.; Hsiao, M.Y.; Nagai, K.; Lin, H. Suppressed crystallization and enhanced gas permeability in thin films of cellulose acetate blends. Polymer 2020, 205, 122790. [Google Scholar] [CrossRef]
- Lam, B.; Wei, M.; Zhu, L.; Luo, S.; Guo, R.; Morisato, A.; Alexandridis, P.; Lin, H. Cellulose triacetate doped with ionic liquids for membrane gas separation. Polymer 2016, 89, 1–11. [Google Scholar] [CrossRef]
- Regmi, C.; Ashtiani, S.; Sofer, Z.; Hrdlička, Z.; Průša, F.; Vopička, O.; Friess, K. CeO2-blended cellulose triacetate mixed-matrix membranes for selective CO2 separation. Membranes 2021, 11, 632. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.H.; Yeong, Y.F.; Chew, T.L. Mixed matrix membrane encompassing of cellulose triacetate and ZIF-90 for CO2 separation from CH4 and N2. Polym. Eng. Sci. 2025, 65, 3334–3345. [Google Scholar] [CrossRef]
- Mehmood, O.; Farrukh, S.; Hussain, A.; Younas, M.; Salahuddin, Z.; Pervaiz, E.; Ayoub, M. Investigation of cellulose acetate/gamma-cyclodextrin MOF based mixed matrix membranes for CO2/CH4 gas separation. Greenh. Gases Sci. Technol. 2021, 11, 313–330. [Google Scholar] [CrossRef]
- Číhal, P.; Vopička, O.; Lanč, M.; Kludský, M.; Velas, J.; Hrdlička, Z.; Michalcová, A.; Dendisová, M.; Friess, K. Poly(butylene succinate)-cellulose triacetate blends: Permeation, pervaporation, sorption and physical structure. Polym. Test. 2018, 65, 468–479. [Google Scholar] [CrossRef]
- Asadi, H.; Alizadehdakhel, A.; Ramazani, A.; Dorosti, F. The effect of Cu-BTC metal–organic framework (MOF) in mixed matrix membranes on permeability and separation of carbon dioxide and methane. Polym. Bull. 2021, 78, 6953–6968. [Google Scholar] [CrossRef]

| Membranes | Density (g/cm3) | FFV |
|---|---|---|
| Pristine CTA | 1.186 ± 0.10 | 0.151 ± 0.12 |
| CTA/Cu-BTC-5 wt.% | 1.143 ± 0.13 | 0.181 ± 0.13 |
| CTA/Cu-BTC-10 wt.% | 1.096 ± 0.11 | 0.215 ± 0.10 |
| CTA/Cu-BTC-15 wt.% | 1.011 ± 0.12 | 0.276 ± 0.12 |
| CTA/Ni-Cu-BTC-10 wt.% | 1.076 ± 0.14 | 0.229 ± 0.13 |
| Membranes | CO2 Permeability (Barrer) | CO2/CH4 Selectivity |
|---|---|---|
| Pristine CTA | 9.6 ± 0.11 | 15.7 |
| CTA/Ni-Cu-BTC-10 wt.% | 18.4 ± 0.12 | 40.0 |
| Membranes | CO2 Permeability (Barrer) | Solubility Coefficient (10−2 cm3(STP)/cm3 cmHg) | Diffusivity Coefficient (10−8 cm2/s) | Standard Error of Estimate | |||
|---|---|---|---|---|---|---|---|
| Pristine CTA | 10.1 ± 0.13 | 3.57 | 2.83 | 1.62 | 16.02 | 0.20 | 1.816 |
| CTA/Ni-Cu-BTC-10 wt.% | 22.9 ± 0.14 | 5.33 | 4.30 | 1.86 | 18.43 | 0.33 | 1.808 |
| Membranes | Pressure (Bar) | Temp. (°C) | CO2 Permeability (Barrer) | CO2/CH4 Selectivity | Ref. |
|---|---|---|---|---|---|
| CDA-CTA | 11 | 35 | 14 | 32 | [98] |
| CTA-GO | 1.5 | 25 | 11.29 | 34.22 | [23] |
| CTA/PSf | 4 | 25 | 11.20 | 30.70 | [20] |
| CTA/[emim][BF4] | 4 | 35 | 12 | 20 | [99] |
| CTA-CeO2-0.32 wt.% | 1 (torr) | 25 | 6.89 | 24.61 | [100] |
| ZIF-90-(1.5)-CTA | 7 | 25 | 46.5 | 10.1 | [101] |
| ZIF-8/CA-10 wt.% | 2 | 25 | 9.5 | 15.3 | [32] |
| CA-γ-CD-MOF-0.8 wt.% | 5 | 25 | 13 | 35.5 | [102] |
| PBS-CTA-10 wt.% | 1.5 | 25 | 3.5 | 35 | [103] |
| PSF-PES-Cu-BTC (52%-28%-20%) | 3 | 25 | 3.90 | 20.52 | [104] |
| CTA/Ni-Cu-BTC-10 wt.% | 5 | 25 | 22.9 | 33.8 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asad, E.; Raza, A.; Safdar, A.; Khan, M.N.A.; Roafi, H. Advanced Cellulose Triacetate-Based Mixed Matrix Membranes Enhanced by Bimetallic Ni-Cu-BTC MOFs for CO2/CH4 Separation. Polymers 2025, 17, 2258. https://doi.org/10.3390/polym17162258
Asad E, Raza A, Safdar A, Khan MNA, Roafi H. Advanced Cellulose Triacetate-Based Mixed Matrix Membranes Enhanced by Bimetallic Ni-Cu-BTC MOFs for CO2/CH4 Separation. Polymers. 2025; 17(16):2258. https://doi.org/10.3390/polym17162258
Chicago/Turabian StyleAsad, Esha, Ayesha Raza, Amna Safdar, Muhammad Nouman Aslam Khan, and Humais Roafi. 2025. "Advanced Cellulose Triacetate-Based Mixed Matrix Membranes Enhanced by Bimetallic Ni-Cu-BTC MOFs for CO2/CH4 Separation" Polymers 17, no. 16: 2258. https://doi.org/10.3390/polym17162258
APA StyleAsad, E., Raza, A., Safdar, A., Khan, M. N. A., & Roafi, H. (2025). Advanced Cellulose Triacetate-Based Mixed Matrix Membranes Enhanced by Bimetallic Ni-Cu-BTC MOFs for CO2/CH4 Separation. Polymers, 17(16), 2258. https://doi.org/10.3390/polym17162258








