Preparation of Highly Antibacterial MXene Nanofiltration Membranes and Investigation of Their Separation Performance
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Preparation of Nanofiltration Membranes
2.3. Characterization of Nanofiltration Membranes
2.4. Performance Evaluation of Nanofiltration Membranes
3. Results and Discussion
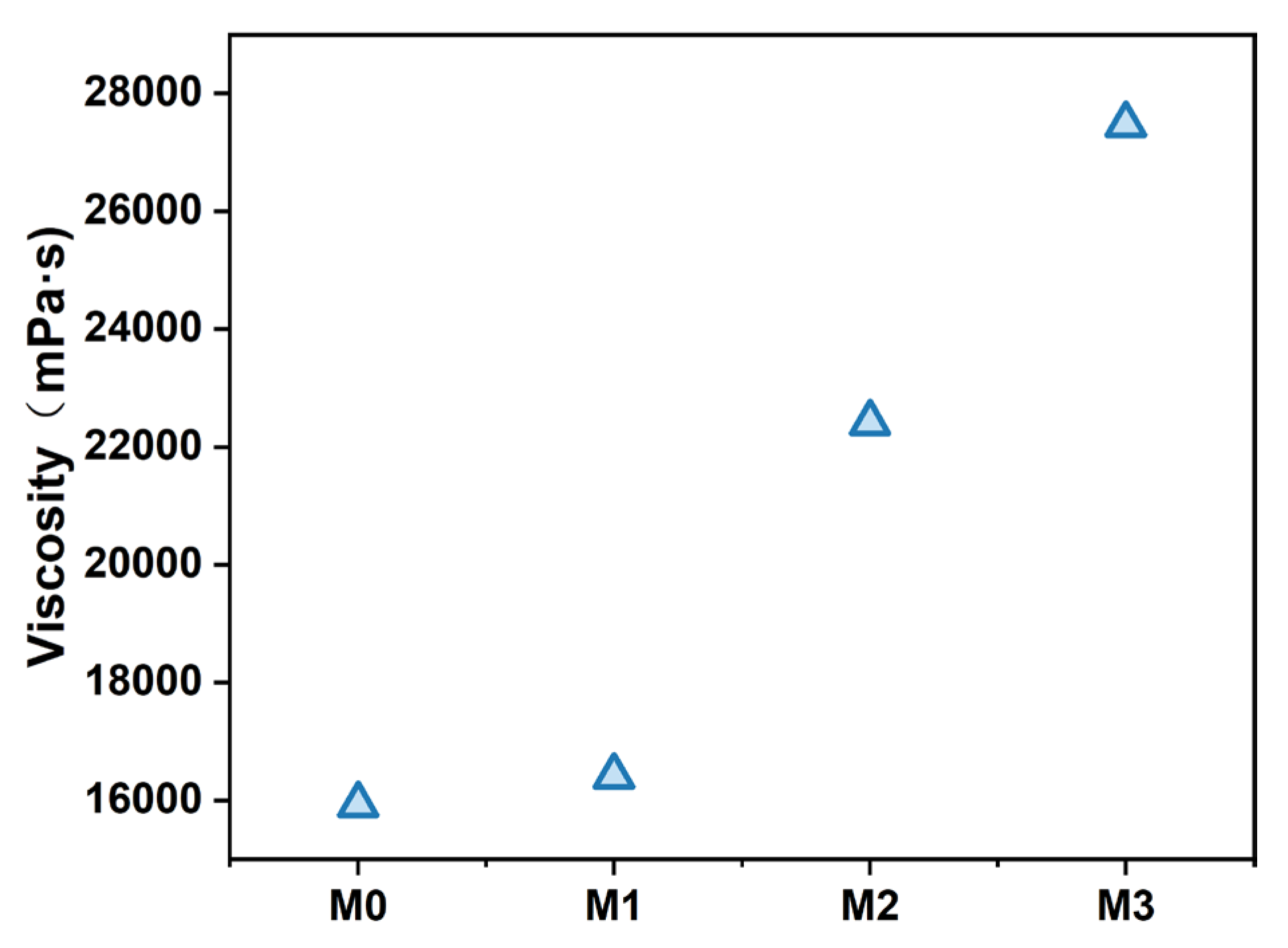
3.1. Viscosity Measurement of Different PES/SPES Membranes
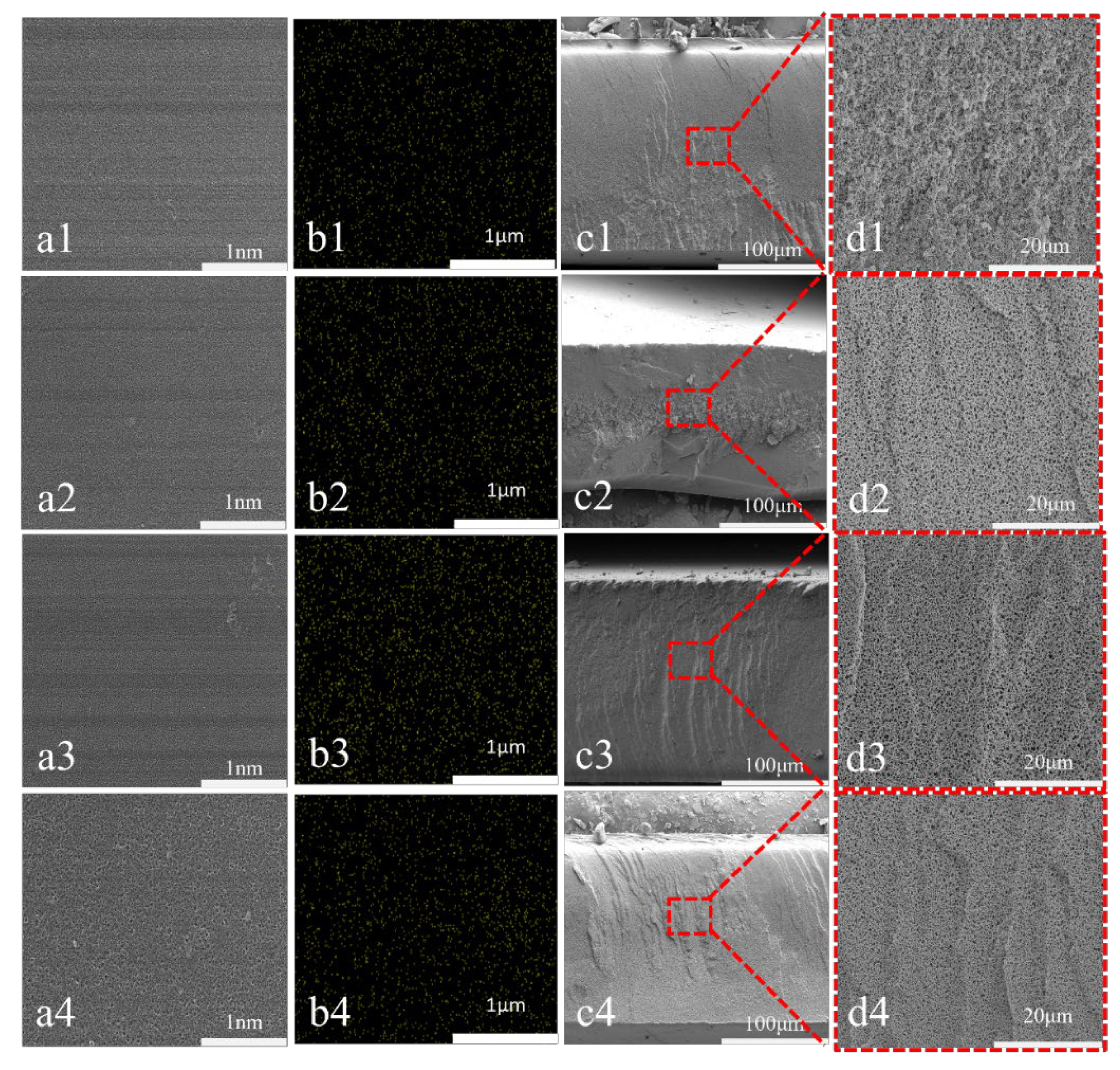
3.2. SEM Characterization of Different Types of PES/SPES Membranes
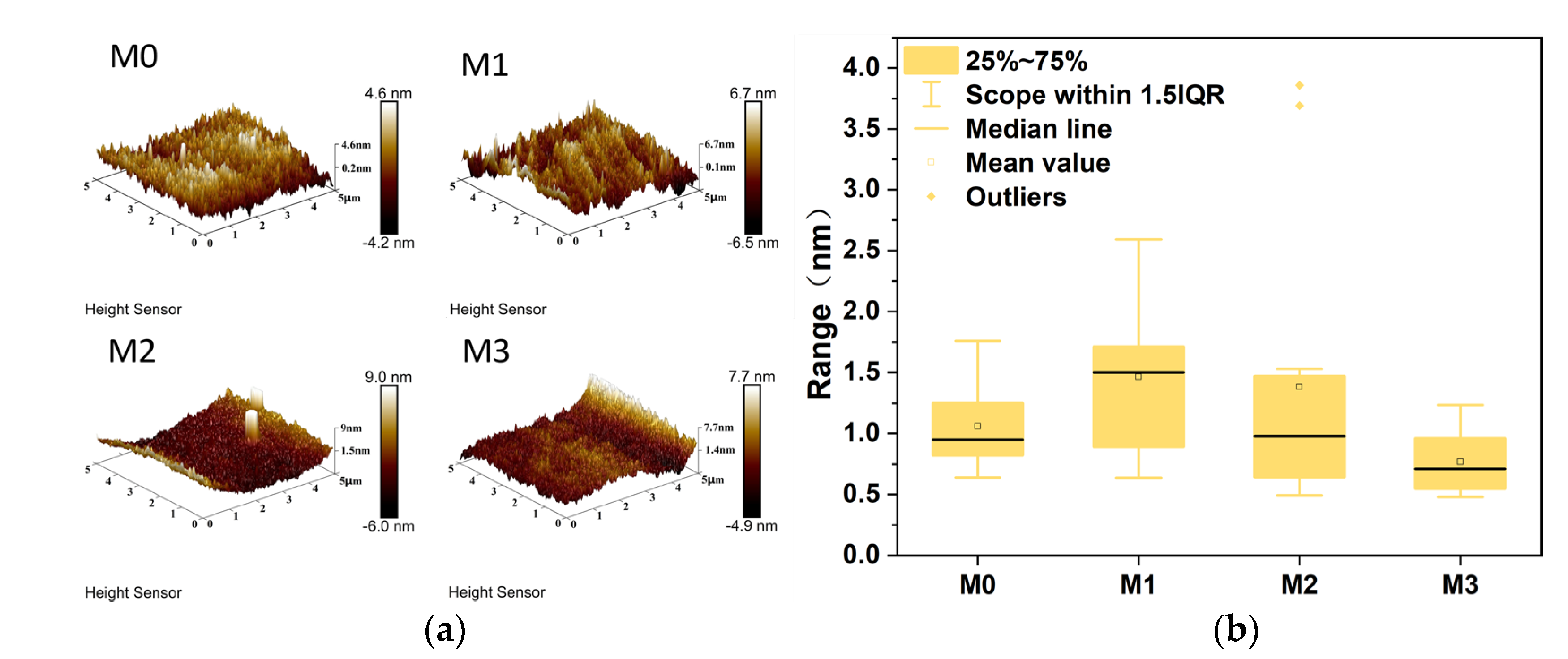
3.3. AFM Characterization of Different PES/SPES Membranes
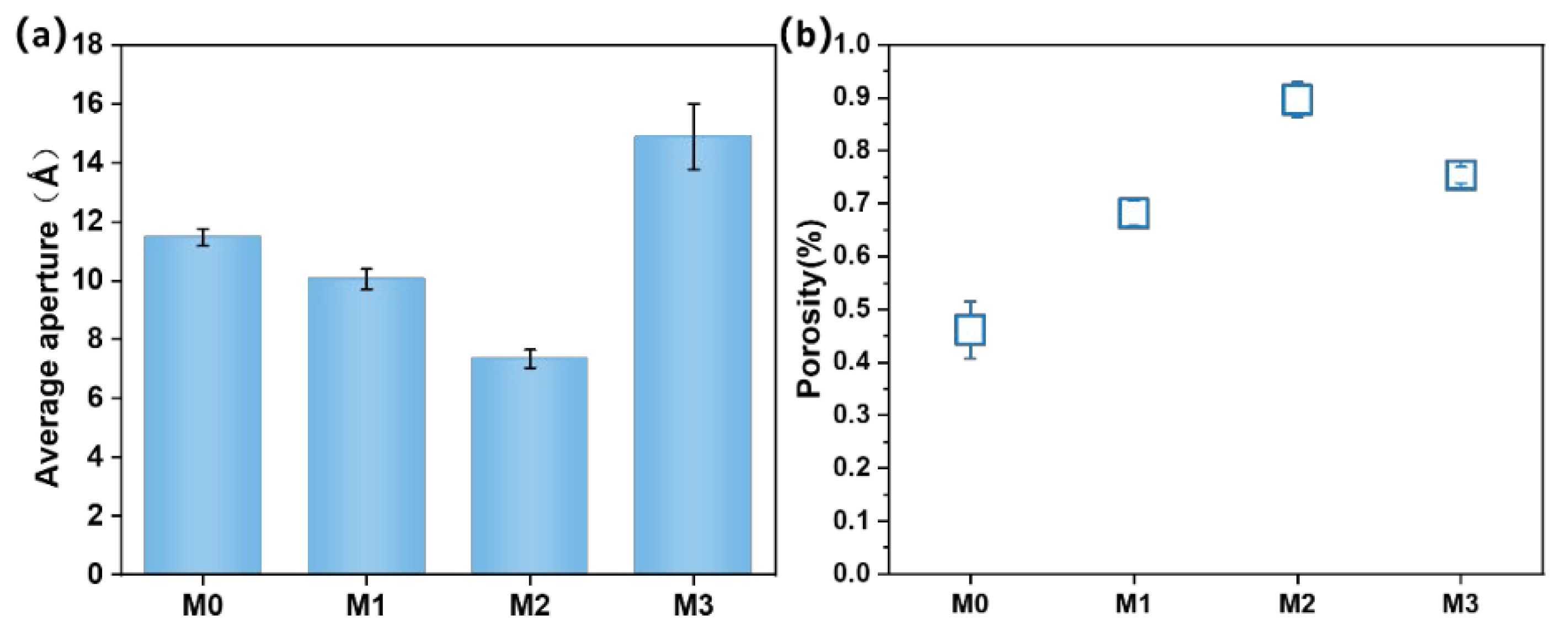
3.4. Average Pore Size and Porosity of PES/SPES Membranes with Different Types
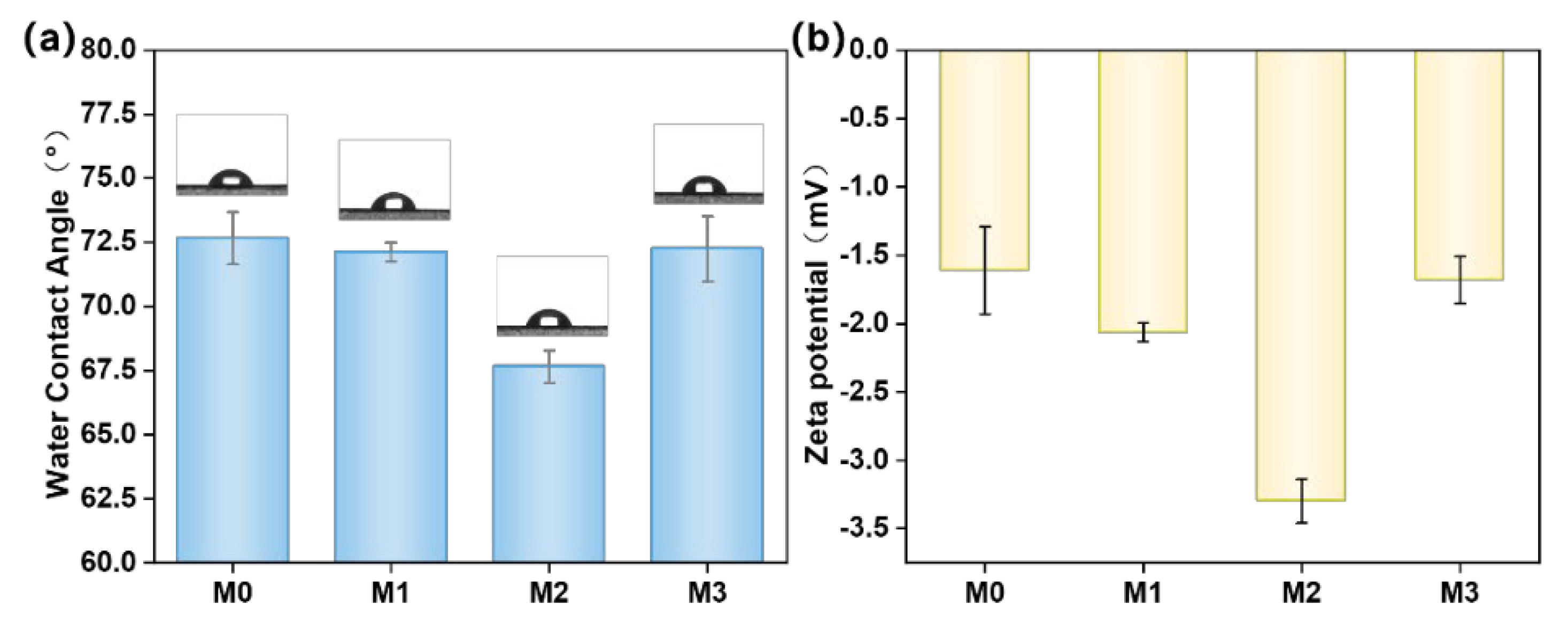
3.5. Water Contact Angle and Zeta Potential of Different PES/SPES Membranes
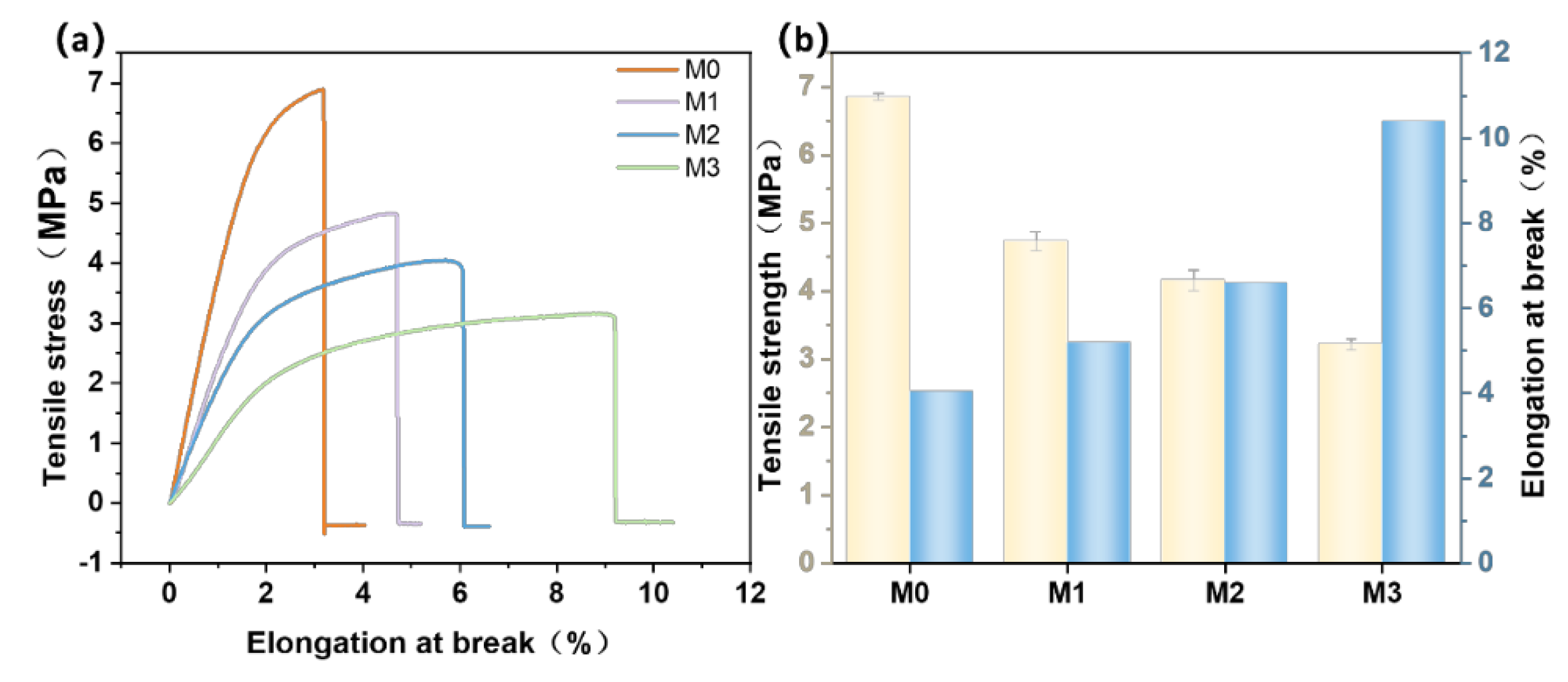
3.6. Tensile Properties of Different Types of PES/SPES Membranes
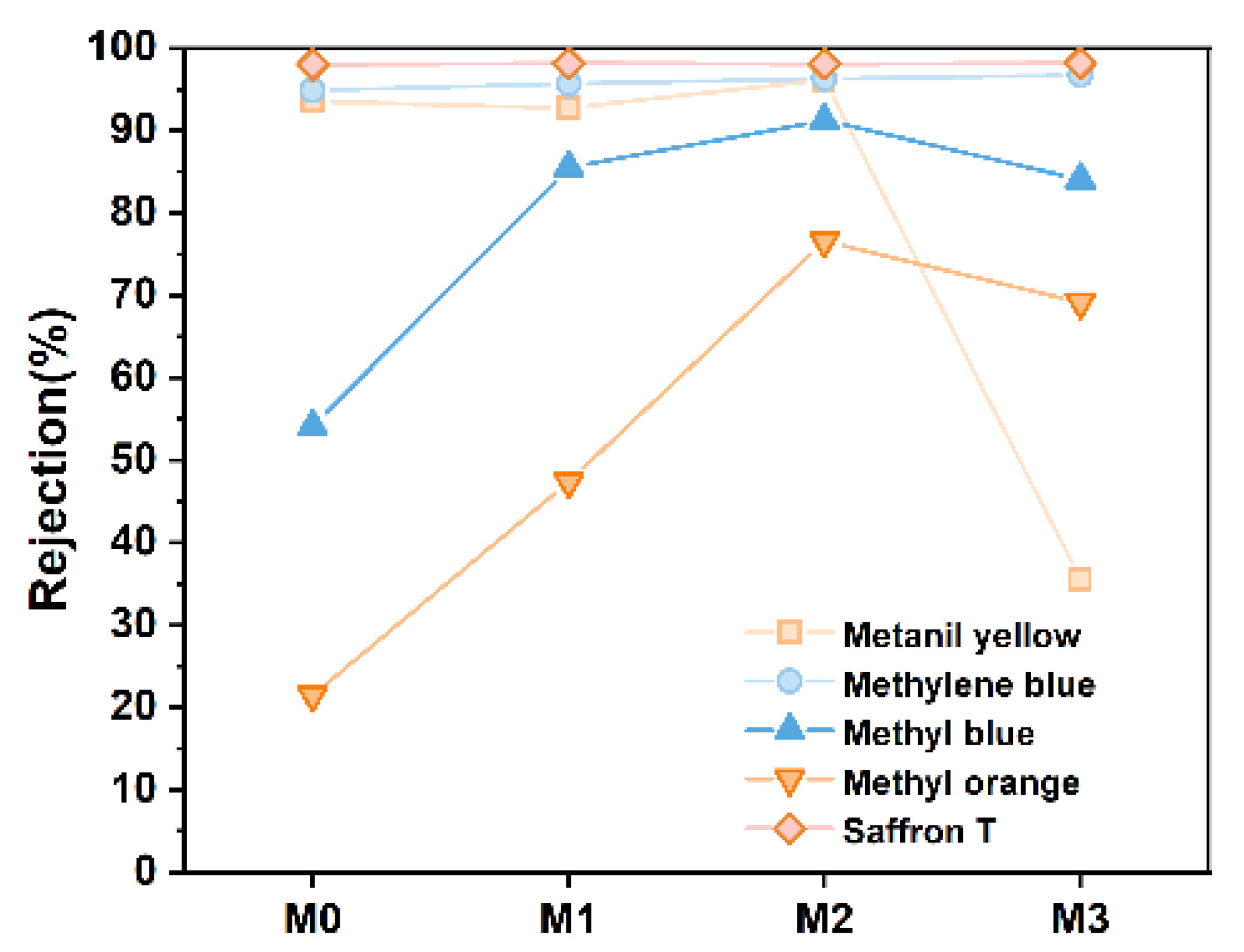
3.7. Dye Retention Test of Different PES/SPES Membranes
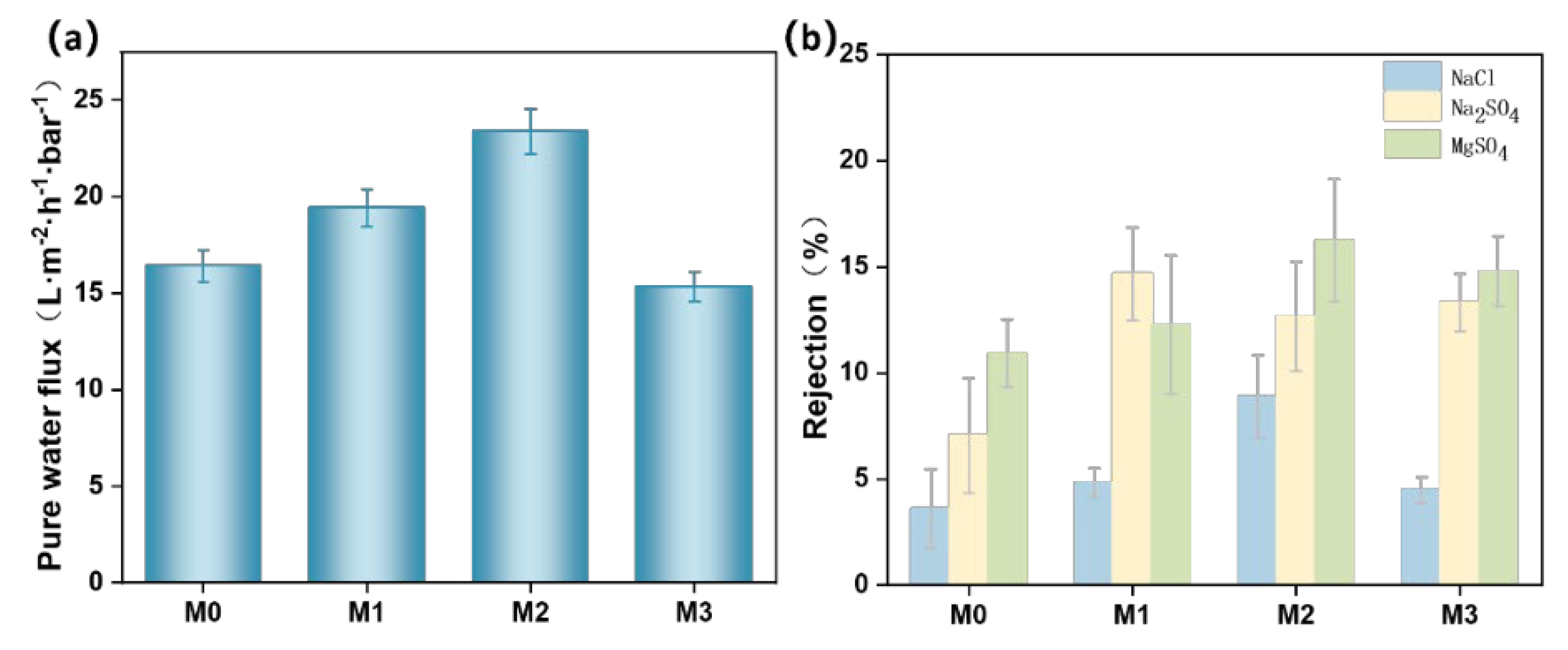
3.8. Measurement of Water Flux and Salt Ion Retention of Different PES/SPES Membranes
3.9. Flux Recovery Test of Different Types of PES/SPES Membranes
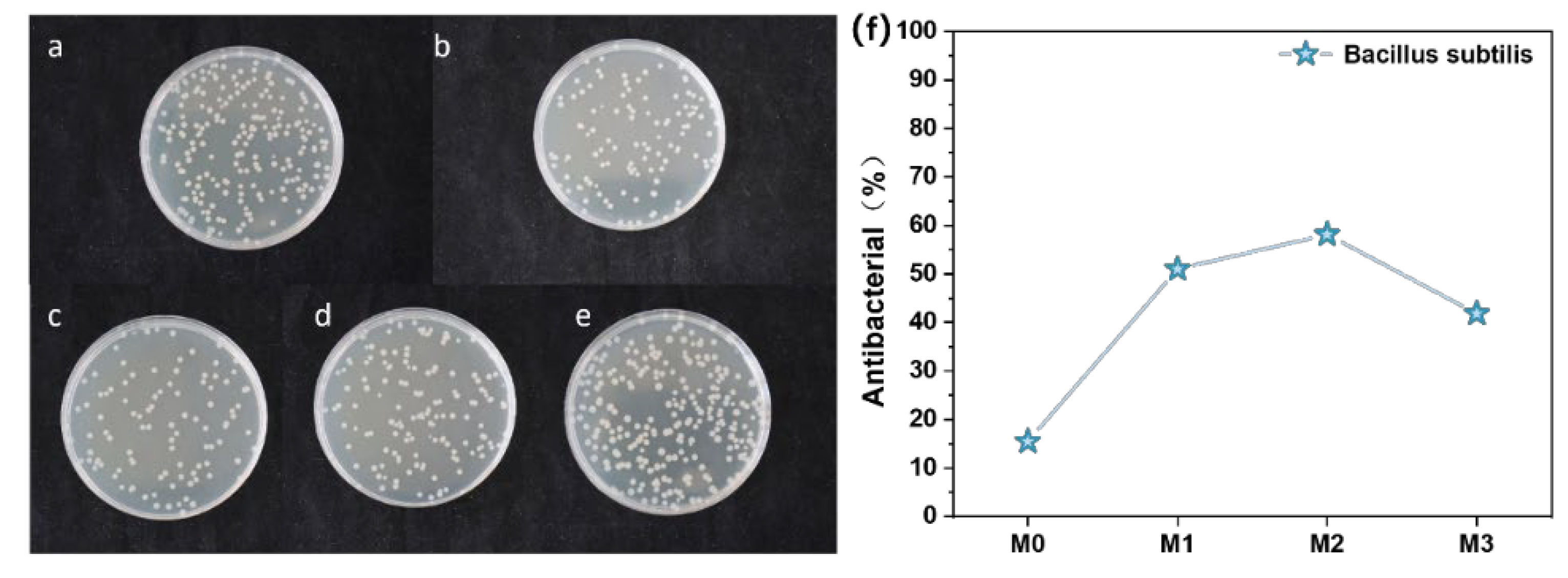
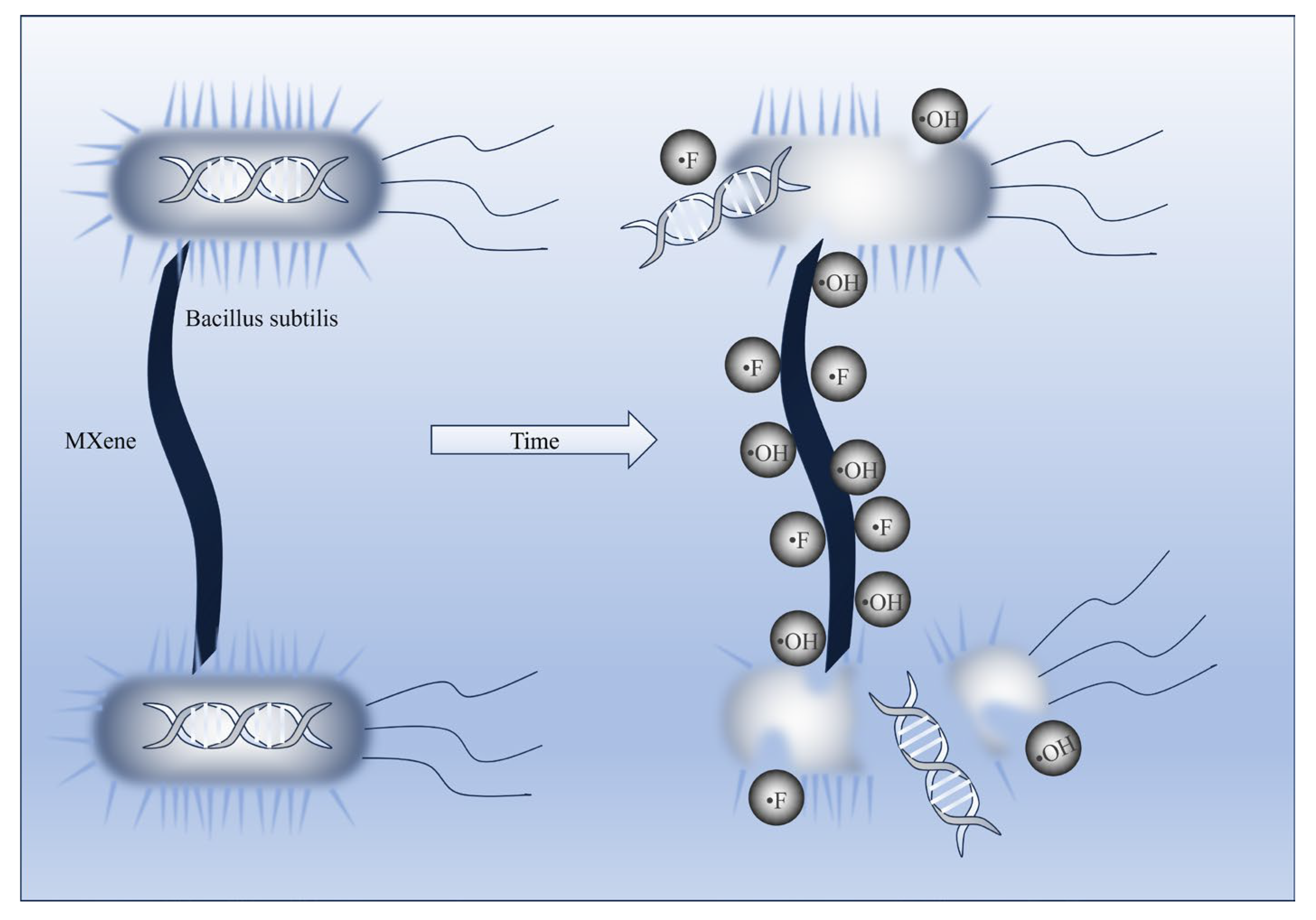
3.10. Antibacterial Test of Different Types of PES/SPES Membranes
3.11. Performance Comparison Between M2 Membrane and the Membrane Reported in the Literature
4. Conclusions
- (1)
- Physicochemical properties: The incorporation of Ti3C2TX significantly influenced the physicochemical properties of the membrane. With the increase of Ti3C2TX content, the water contact angle of the nanofiltration membrane first decreased and then increased. In addition, the changes in phase inversion rate induced by Ti3C2TX content led to corresponding variations in the average pore size and porosity of the membrane.
- (2)
- Separation performance: The addition of Ti3C2TX notably affected membrane performance. As Ti3C2TX content increased, key performance metrics such as water flux, dye rejection rate, flux recovery rate and salt ion rejection rate initially improved and then declined. These findings provide valuable insights for the optimization of membrane technology in salt/dye separation applications.
- (3)
- Antibacterial performance: Ti3C2TX doped membranes exhibited significant antibacterial advantages. The bacteriostatic rate against Bacillus subtilis increased from 15% (the M0 membrane) to 58% (the M2 membrane), which demonstrated enhanced antimicrobial efficacy.
- (4)
- Advantages and disadvantages: The incorporation of a small amount of Ti3C2TX into the hybrid membrane offers several advantages, including an increased flux alongside an enhanced rejection rate, thereby overcoming the trade-off effect. Additionally, the antibacterial performance of the hybrid membrane is significantly improved. However, a disadvantage arises as the amount of Ti3C2TX increases, leading to a continuous decline in the mechanical properties of the membrane, which may affect its service life. Despite the limited usage of Ti3C2TX, the improvement remains evident. Moreover, the one-step phase inversion method enables preparation of the membrane, indicating potential for industrialization.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feng, L.F.; Yan, H.Y.; Liang, C.; Kato, N.; Jeon, S.; Takagi, R.; Matsuyama, H. Effect of Molecular Weight of Sulfonated Poly (ether sulfone) (SPES) on the Mechanical Strength and Antifouling Properties of Poly (ether sulfone)/SPES Blend Membranes. Ind. Eng. Chem. Res. 2017, 56, 11302–11311. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Z.; Kong, X.; Sun, Y.; Zhu, C.; Liu, P.; Pang, J.; Jiang, L.; Wen, L. Engineered PES/SPES nanochannel membrane for salinity gradient power generation. Nano Energy 2019, 59, 354–362. [Google Scholar] [CrossRef]
- Akin, E.; Gharibzahedi, T.M.S.; Qiu, H.; Aliyeva, A.; Altintas, Z. Chitosan-functionalized PVDF and PES membranes integrated by epitope-imprinted polymers for targeted hepatitis A virus capture. J. Membr. Sci. 2024, 709, 123084. [Google Scholar] [CrossRef]
- Athira, V.B.; Mohanty, S.; Nayak, S. Preparation and characterization of porous polyethersulfone (PES) membranes with improved biocompatibility by blending sulfonated polyethersulfone (SPES) and cellulose acetate (CA)–A comparative study. Mater. Today Commun. 2020, 25, 101544. [Google Scholar]
- Almanassra, I.W.; Lubna, J.; Anjaneyulu, C.; Abushawish, A.; Shanableh, A.; Ali Atieh, M. Unveiling the relationship between MOF porosity, particle size, and polyethersulfone membranes properties for efficient decontamination of dye and organic matter. Chem. Eng. J. 2023, 471, 144616. [Google Scholar] [CrossRef]
- Dana, K.; Thanigaivelan, A.; Lina, T.; Hasan, S.W. Enhanced antifouling and separation capabilities of polydopamine@Ce-MOF functionalized PES ultrafiltration membrane. npj Clean Water 2024, 7, 7. [Google Scholar]
- Kashif, R.; Mahmoud, K.A.; Johnson, D.J.; Helal, M.; Berdiyorov, G.R.; Gogotsi, Y. Efficient Antibacterial Membrane based on Two-Dimensional Ti3C2TX (MXene) Nanosheets. Sci. Rep. 2017, 7, 1598. [Google Scholar]
- Tian, Y.L.; Cao, W.; Geng, H.W.; Liu, X.-C.; Chang, R.Y.; Li, N.; Wang, S.W.; Zhang, Y.S.; Li, J.; Geng, H.Z. Modified multi-walled carbon nanotube and silanized MXene electrostatic assembly enables antimicrobial, conductive Mixed Matrix Membranes for efficient electrochemical cleaning. J. Membr. Sci. 2024, 707, 123017. [Google Scholar] [CrossRef]
- Runlin, H.; Xu, M.; Yongli, X.; Teng, D.; Zhang, S. Preparation of a new 2D MXene/PES composite membrane with excellent hydrophilicity and high flux. RSC Adv. 2017, 7, 56204–56210. [Google Scholar]
- Hung, W.L.; Wang, D.M.; Lai, J.Y.; Chou, S.C. On the initiation of macrovoids in polymeric membranes–effect of polymer chain entanglement. J. Membr. Sci. 2016, 505, 70–81. [Google Scholar] [CrossRef]
- Meng, N.; Zhao, W.; Shamsaei, E.; Wang, G.; Zeng, X.; Lin, X.; Xu, T.; Wang, H.; Zhang, X. A low-pressure GO nanofiltration membrane crosslinked via ethylenediamine. J. Membr. Sci. 2018, 548, 363–371. [Google Scholar] [CrossRef]
- Yang, H.; Han, M.; Zhang, W.; Yi, M.; Xia, L.; Meng, F.; Wang, Y.; Zhao, S. High performance mixed-dimensional assembled MXene composite membranes for molecular sieving. J. Membr. Sci. 2024, 698, 122606. [Google Scholar] [CrossRef]
- Banerjee, S.; Das, A.; Das, R.; Bhattacharjee, C. Mesoporous Mg–Al–Ti composite oxide incorporated mixed matrix ultrafiltration membrane with superior multi-heavy metal removal capacity, anti-fouling & anti-microbial property. J. Membr. Sci. 2024, 703, 122836. [Google Scholar]
- Feng, G.; Honglin, L.; Yue, Z.; Liu, D.M.; Xie, Z.H.; Peng, W.X.; Song, Y.C.; Hu, R.; Chen, D.; Kang, J.; et al. Polyamide membrane with nanoscale stripes and internal voids for high-performance nanofiltration. J. Membr. Sci. 2023, 671, 121406. [Google Scholar]
- Enis, H.K.; Kunli, G.; John, C.Z.; Yang, E.; Yıldırım, C.; Chuah, C.Y.; Ahunbay, M.G.; Lee, J.; Tantekin-Ersolmaz, Ş.B.; Chen, Y. MXene Materials for Designing Advanced Separation Membranes. Adv. Mater. 2020, 32, e1906697. [Google Scholar]
- Hua, S.L.; Hao, J.Z.; Chunrui, W.; Zhang, P.; Cao, X.; Sun, J.K. Sub-8 nm networked cage nanofilm with tunable nanofluidic channels for adaptive sieving. Nat. Commun. 2024, 15, 2478. [Google Scholar]
- Anan, Z.; Min, Z.; Yuemei, B.; Lingfeng, Z.; Guanhua, L.; Yanjun, J.; Peng, Z.; Xingzhong, C. Loose nanofiltration membrane constructed via interfacial polymerization using porous organic cage RCC3 for dye/salt separation. J. Membr. Sci. 2022, 664, 121081. [Google Scholar]
- Nan, L.; Lou, T.-J.; Wen, W.; Li, M.; Jing, L.C.; Yang, Z.X.; Chang, R.Y.; Li, J.; Geng, H.Z. MXene-PANI/PES composite ultrafiltration membranes with conductive properties for anti-fouling and dye removal. J. Membr. Sci. 2023, 668, 121271. [Google Scholar]
- Jiawei, H.; Shixuan, G.; Nigel, G.; Yu, W.; Sun, K.; Liu, T. r-HGO/MXene composite membrane with enhanced permeability and rejection performance for water treatment. J. Membr. Sci. 2024, 691, 122216. [Google Scholar]
- Meng, N.; Mi, J.; Chen, X.; Liu, J.; Zhu, H.; Zheng, X. Preparation of Highly Antibacterial Polyethersulfone/Sulfonated Polyethersulfone Blend Composite Membrane and Research on Its Dye Separation Performance. Molecules 2025, 30, 781. [Google Scholar] [CrossRef]
- Meng, N.; Sun, X.; Liu, J.; Mi, J.; Rong, R. Effect of Addition Amount of Ethylenediamine on Interlayer Nanochannels and the Separation Performance of Graphene Oxide Membranes. Polymers 2024, 16, 3123. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.; Sun, X.; Liu, J.; Mi, J.; Chen, X.; Rong, R. Modulation of Interlayer Nanochannels via the Moderate Heat Treatment of Graphene Oxide Membranes. Polymers 2024, 16, 2200. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.H.; Weber, M.; Huang, Y.H.; Lai, J.Y.; Chung, T.S. Investigating the impact of the sulfonation degree in sulfonated polyphenylsulfone (sPPSU) on PES/sPPSU polymer blend membranes. J. Membr. Sci. 2024, 705, 122890. [Google Scholar] [CrossRef]
- Onuk, E.; Gungormus, E.; Cihanoğlu, A.; Altinkaya, S.A. Development of a dopamine-based surface modification technique to enhance protein fouling resistance in commercial ultrafiltration membranes. J. Membr. Sci. 2025, 717, 123554. [Google Scholar] [CrossRef]
- Shaobin, L.; Helen, T.Z.; Mario, H.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar]
- Rostam, B.A.; Peyravi, M.; Ghorbani, M.; Jahanshahi, M. Antibacterial surface modified of novel nanocomposite sulfonated polyethersulfone/polyrhodanine membrane. Appl. Surf. Sci. 2018, 427, 17–28. [Google Scholar] [CrossRef]
- Arash, M.; Hadi, R.; Julker, M.N.; James, L.; Alexandra, T.; Thanh, T.T.; Reza, G.; Stephen, K.; Sarah, V.; Dusan, L. Comparative antibacterial activity of 2D materials coated on porous-titania. J. Mater. Chem. B 2021, 9, 32. [Google Scholar]
- Kai, Y.; Ma, Y.-Q. Computer simulation of the translocation of nanoparticles with different shapes across a lipid bilayer. Nat. Nanotechnol. 2010, 5, 579–583. [Google Scholar]
- Zou, X.; Zhang, L.; Wang, Z.; Yang, L. Mechanisms of the Antimicrobial Activities of Graphene Materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef]
- Jastrzębska, M.A.; Karwowska, E.; Wojciechowski, T.; Ziemkowska, W.; Rozmysłowska, A.; Chlubny, L.; Olszyna, A. The Atomic Structure of Ti2C and Ti3C2 MXenes is Responsible for Their Antibacterial Activity Toward E. coli Bacteria. J. Mater. Eng. Perform. 2019, 28, 1272–1277. [Google Scholar] [CrossRef]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial Activity of Ti3C2TX MXene. Nanotechnol. Wkly. 2016, 10, 3674–3684. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Luo, J.; Wan, Y.; Wang, X.; Chen, X.; Zeng, G. Dual-functional reverse osmosis membranes: A novel Approach to Combat biofouling with enhanced antibacterial and Antiadhesion properties. J. Membr. Sci. 2025, 718, 123699. [Google Scholar] [CrossRef]
- Omid, A.; Elham, G. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar]
- Zhou, S.; Gao, J.; Zhu, J.; Peng, D.; Zhang, Y.; Zhang, Y. Self-cleaning, antibacterial mixed matrix membranes enabled by photocatalyst Ti-MOFs for efficient dye removal. J. Membr. Sci. 2020, 610, 18219. [Google Scholar] [CrossRef]
- Tengfei, T.; Xiaoze, S.; Liang, C.; Luo, Y.; Dong, Z.; Gong, H.; Xu, L.; Zhong, Z.; Peng, R.; Liu, Z. Graphene-based nanocomposite as an effective, multifunctional, and recyclable antibacterial agent. ACS Appl. Mater. Interfaces 2014, 6, 8542–8548. [Google Scholar]
- Beibei, L.; Zhenye, Z.; Biyuan, M.; Wang, W.; Zhu, R.; Zhang, J. 2D MXene Nanomaterials for Versatile Biomedical Applications: Current Trends and Future Prospects. Small 2021, 17, e2100946. [Google Scholar]
- Mahmoud, K.; Rasool, K.; Helal, M.; Ali, A.; Ren, C.; Gogotsi, Y. Antibacterial activity of Ti3C2TX (MXene): Towards new antifouling membranes. Am. Chem. Soc. 2016, 251. [Google Scholar]
- Pandey, R.P.; Rasheed, P.A.; Gomez, T.; Azam, R.S.; Mahmoud, K.A. A fouling-resistant mixed-matrix nanofiltration membrane based on covalently cross-linked Ti3C2TX (MXene)/cellulose acetate. J. Membr. Sci. 2020, 607, 118139. [Google Scholar] [CrossRef]
- Kim, Y.I.; Park, S.; Kim, H.; Park, S.; Ruoff, R.S.; Hwang, S. Strongly-Coupled Freestanding Hybrid Films of Graphene and Layered Titanate Nanosheets: An Effective Way to Tailor the Physicochemical and Antibacterial Properties of Graphene Film. Adv. Funct. Mater. 2014, 24, 2288–2294. [Google Scholar] [CrossRef]
- Jian, L.; Lei, L.; Yilin, X.; Junyong, Z.; Fei, L.; Jiangnan, S.; Zhenyu, W.; Jiuyang, L. MXene nanosheet stacks with tunable nanochannels for efficient molecular separation. Chem. Eng. J. 2022, 427, 132070. [Google Scholar]
- Junyong, Z.; Lijuan, Q.; Adam, U.; Jingwei, H.; Jing, W.; Yatao, Z.; Xin, L.; Shushan, Y.; Jian, L.; Miaomiao, T.; et al. Elevated Performance of Thin Film Nanocomposite Membranes Enabled by Modified Hydrophilic MOFs for Nanofiltration. ACS Appl. Mater. Interfaces 2017, 9, 1975–1986. [Google Scholar]
- Liu, M.; Zhou, C.; Dong, B.; Wu, Z.; Wang, L.; Yu, S.; Gao, C. Enhancing the permselectivity of thin-film composite poly (vinyl alcohol) (PVA) nanofiltration membrane by incorporating poly(sodium-p-styrene-sulfonate) (PSSNa). J. Membr. Sci. 2014, 463, 173–182. [Google Scholar] [CrossRef]
- Lin, J.; Ye, W.; Zeng, H.; Yang, H.; Shen, J.; Darvishmanesh, S.; Luis, P.; Sotto, A.; Bruggen, B.V.d. Fractionation of direct dyes and salts in aqueous solution using loose nanofiltration membranes. J. Membr. Sci. 2015, 477, 183–193. [Google Scholar] [CrossRef]
- Aaron, M.; Rodolfo, C.; Hiroyuki, M.; Ortiz-Medina, J.; Araki, T.; Fukuyo, T.; Tejima, S.; Takeuchi, K.; Hayashi, T.; Terrones, M. Effective NaCl and dye rejection of hybrid graphene oxide/graphene layered membranes. Nat. Nanotechnol. 2017, 12, 1083–1088. [Google Scholar]
- Kang, Y.; Jang, J.; Kim, S.; Lim, J.; Lee, Y.; Kim, I.S. PIP/TMC interfacial polymerization with electrospray: Novel loose nanofiltration membrane for dye wastewater treatment. ACS Appl. Mater. Interfaces 2020, 12, 36148–36158. [Google Scholar] [CrossRef]
- Jin, J.; Du, X.; Yu, J.; Qin, S.; He, M.; Zhang, K.; Chen, G.J. High performance nanofiltration membrane based on SMA-PEI cross-linked coating for dye/salt separation. J. Membr. Sci. 2020, 611, 118307. [Google Scholar] [CrossRef]

| Membrane | Casting Solution Composition (wt%) | Ti3C2TX Mass Ratio (%) | |||
|---|---|---|---|---|---|
| PES | SPES | DMAc | Ti3C2TX | ||
| M0 | 5.4 g | 3.6 g | 21 g | 0 g | 0% |
| M1 | 5.4 g | 3.6 g | 21 g | 0.03 g | 0.1% |
| M2 | 5.4 g | 3.6 g | 21 g | 0.09 g | 0.3% |
| M3 | 5.4 g | 3.6 g | 21 g | 0.15 g | 0.5% |
| Dye Molecule | Electrical Property | Molecular Weight g/mol | Maximum Absorption Wavelength (nm) | Molecular Size (Ǻ) |
|---|---|---|---|---|
 MO | Positive | 327.33 | 463 | 4.8 × 14.9 (Ǻ) [16] |
 MB | Negative | 799.8 | 664 | 5.8 × 14.4 (Ǻ) [16] |
 MEB | Positive | 373.9 | 664 | 15.805 × 7.91 × 4.019 (Ǻ) [17] |
| Membrane | Permeability (LMH bar−1) | Types of Dye | Dye Rejection |
|---|---|---|---|
| M2 | 23.4 | Safranine T Methylene blue | 98 96.3 |
| TFN-mZIF2 [41] | 14.9 | Reactive blue 2 Reactive black 5 | 99.2 99.0 |
| PVA/PSSNa [42] | 8.3 | Congo Red | 99.7 |
| Sepro NF 6 [43] | 10.5 | Congo red Direct red 80 Direct red 23 | 99.9 99.9 99.9 |
| GO/FLG [44] | 6.7 | Rhodamine B Acid Blue 9 | 8.0 96.0 |
| NF90 [45] | 20.2 | Congo red | 99.6 |
| SMA-PEI/PES [46] | 23.0 | Congo red Rhodamine B Methylene blue | 99.4 44.7 7.3 |
| SC-MXene [45] | 9.0 | - | - |
| TFC-5 [40] | 20.9 | Congo red Reactive blue 19 Methyl blue | 99.429% 99.02% 98.84% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, N.; Liu, J.; Mi, J.; Chen, X.; Rong, R.; Hang, J.; Jiang, Z. Preparation of Highly Antibacterial MXene Nanofiltration Membranes and Investigation of Their Separation Performance. Polymers 2025, 17, 1493. https://doi.org/10.3390/polym17111493
Meng N, Liu J, Mi J, Chen X, Rong R, Hang J, Jiang Z. Preparation of Highly Antibacterial MXene Nanofiltration Membranes and Investigation of Their Separation Performance. Polymers. 2025; 17(11):1493. https://doi.org/10.3390/polym17111493
Chicago/Turabian StyleMeng, Na, Jinxin Liu, Jialing Mi, Xuan Chen, Rong Rong, Junjie Hang, and Zihan Jiang. 2025. "Preparation of Highly Antibacterial MXene Nanofiltration Membranes and Investigation of Their Separation Performance" Polymers 17, no. 11: 1493. https://doi.org/10.3390/polym17111493
APA StyleMeng, N., Liu, J., Mi, J., Chen, X., Rong, R., Hang, J., & Jiang, Z. (2025). Preparation of Highly Antibacterial MXene Nanofiltration Membranes and Investigation of Their Separation Performance. Polymers, 17(11), 1493. https://doi.org/10.3390/polym17111493







