Advanced Electrospun Chitosan-(Polylactic Acid)-(Silver Nanoparticle)-Based Scaffolds for Facilitated Healing of Purulent Wounds: A Preclinical Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospun Patches Structural Assessment
2.3. Experimental Animals
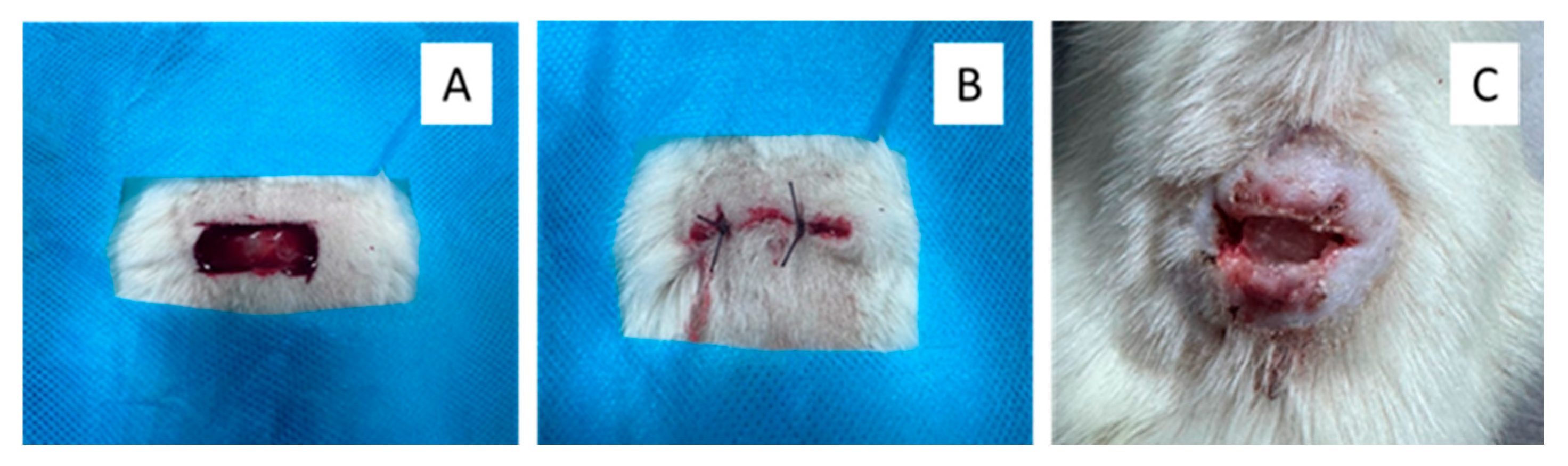
2.4. Rat Skin Defect Model and Treatment Protocol
2.5. Wound Size and Microbiology Profile Monitoring
2.6. Histology and Immunohistochemistry
3. Results
3.1. Patches Structure
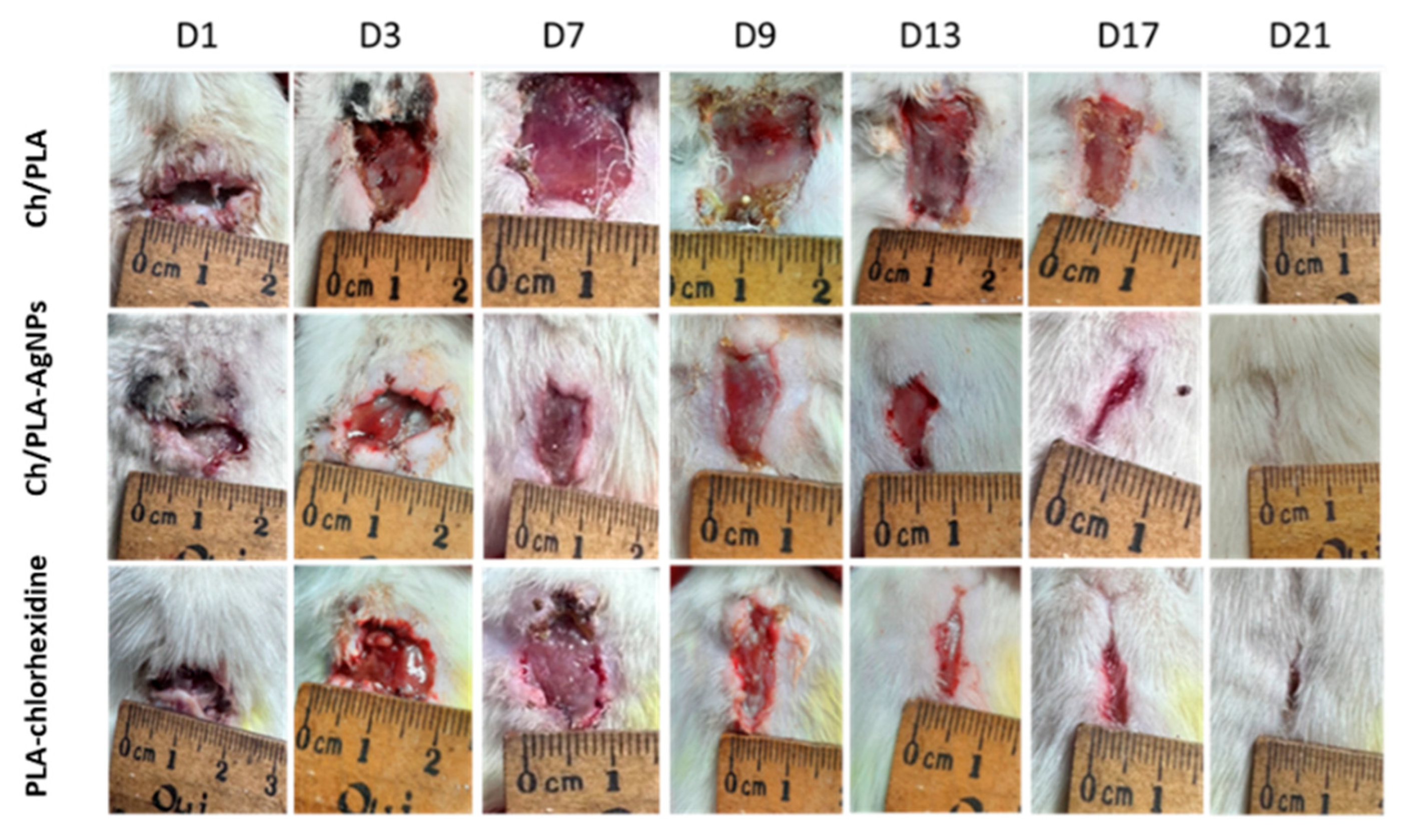
3.2. Wound Healing Rate
3.3. Wound Microbiology
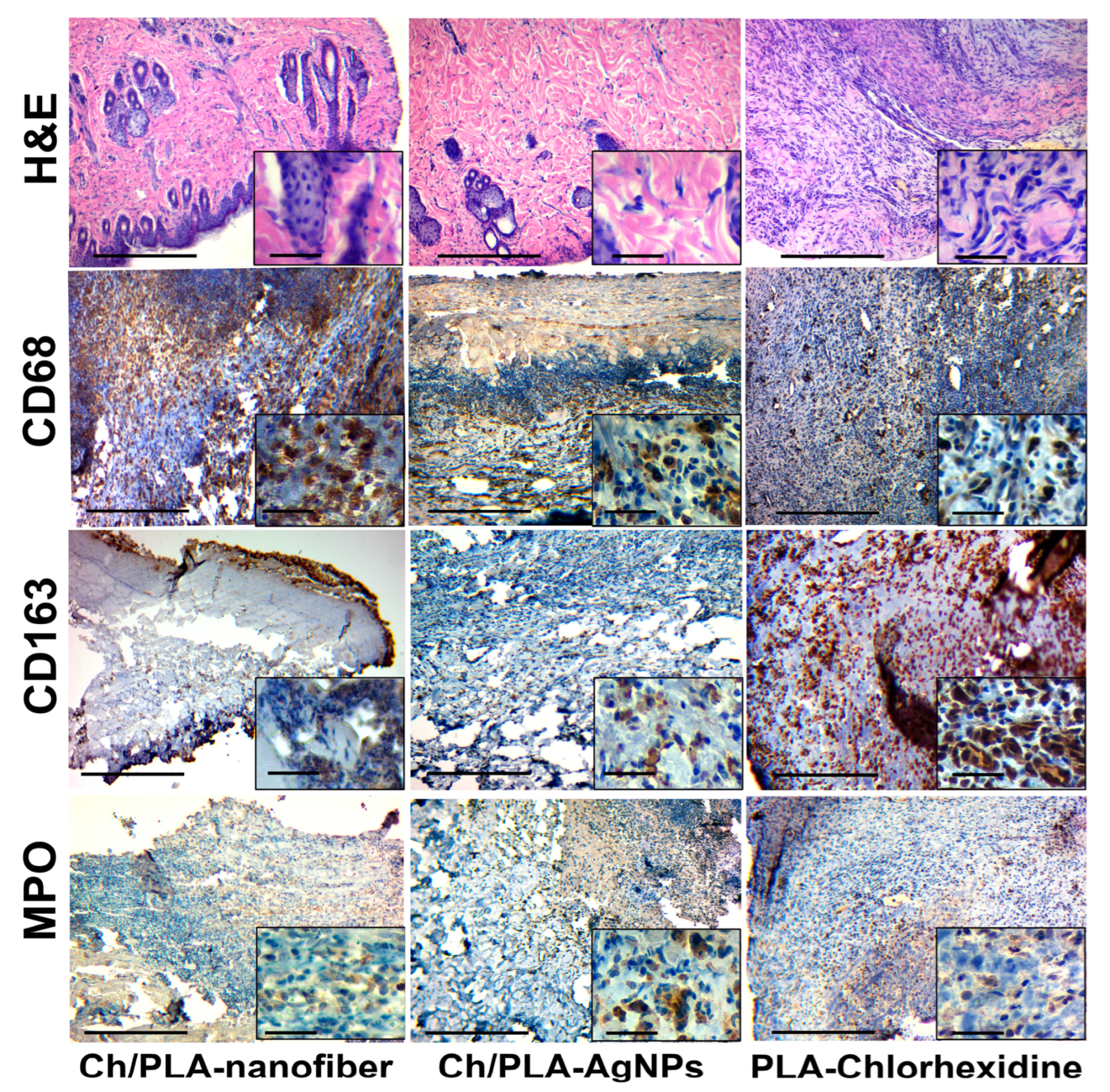
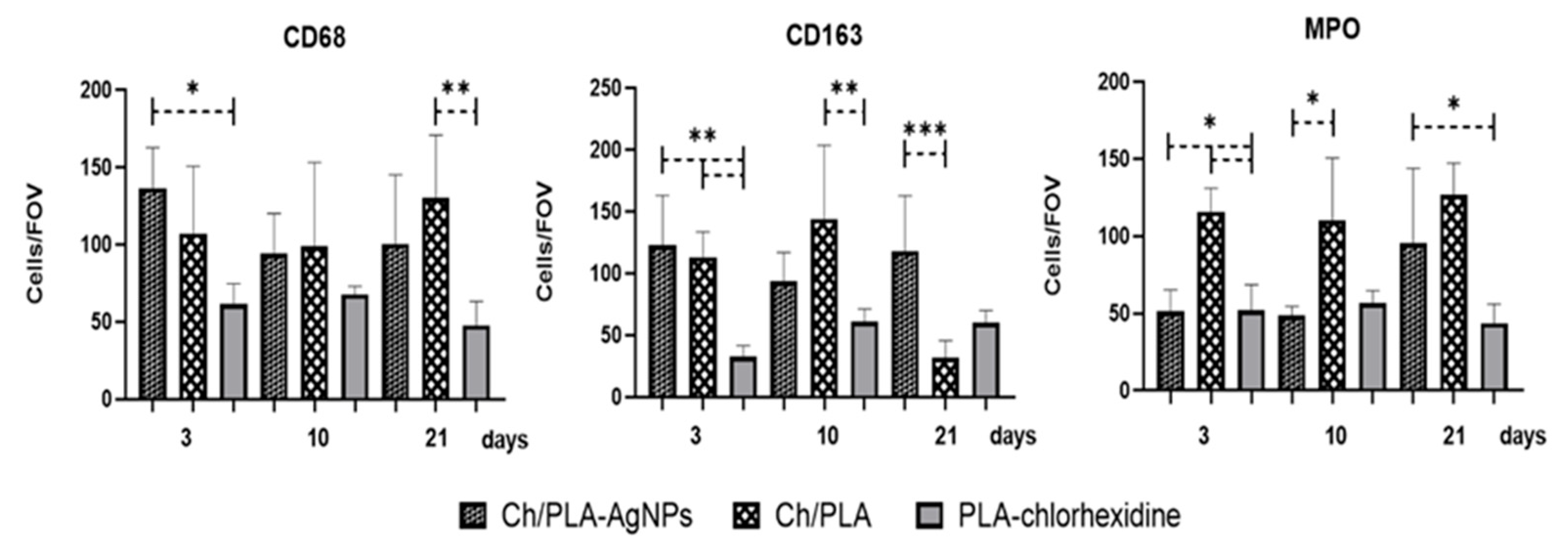
3.4. Histological and Immunohistochemical Assessment
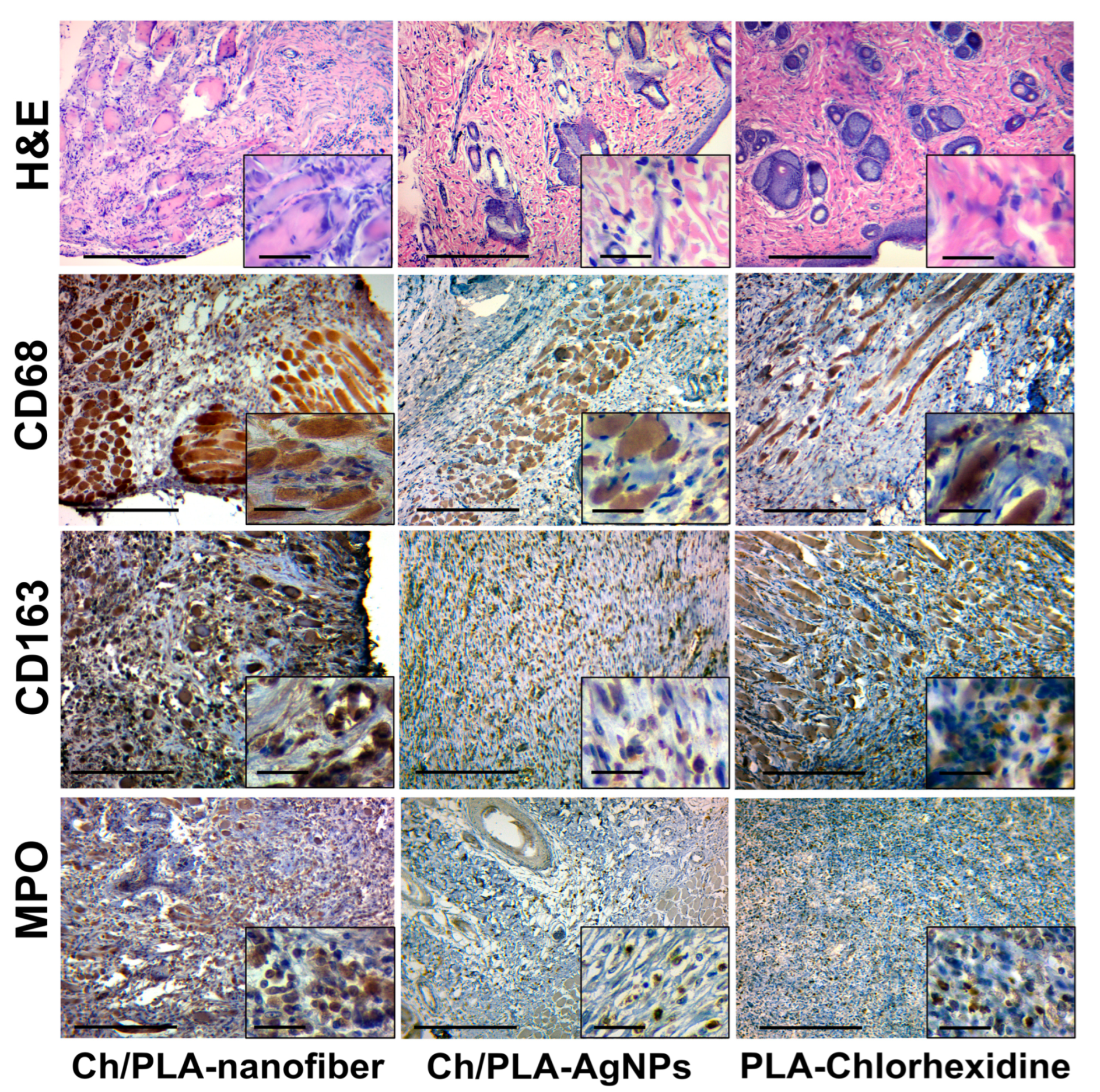
3.5. Systemic Reaction for Biomaterials Application
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Maawi, S.; Orlowska, A.; Sader, R.; James Kirkpatrick, C.; Ghanaati, S. In vivo cellular reactions to different biomaterials-Physiological and pathological aspects and their consequences. Semin. Immunol. 2017, 29, 49–61. [Google Scholar] [CrossRef]
- Freedman, B.R.; Hwang, C.; Talbot, S.; Hibler, B.; Matoori, S.; Mooney, D.J. Breakthrough treatments for accelerated wound healing. Sci. Adv. 2023, 9, eade7007. [Google Scholar] [CrossRef]
- Naomi, R.; Bahari, H.; Ridzuan, P.M.; Othman, F. Natural-Based Biomaterial for Skin Wound Healing (Gelatin vs. Collagen): Expert Review. Polymers 2021, 13, 2319. [Google Scholar] [CrossRef] [PubMed]
- Otte, A.; Damen, F.; Goergen, C.; Park, K. Coupling the in vivo performance to the in vitro characterization of PLGA microparticles. Int. J. Pharm. 2021, 604, 120738. [Google Scholar] [CrossRef] [PubMed]
- Hatlen, T.J.; Miller, L.G. Staphylococcal Skin and Soft Tissue Infections. Infect. Dis. Clin. N. Am. 2021, 35, 81–105. [Google Scholar] [CrossRef]
- Hurlow, J.; Bowler, P.G. Acute and chronic wound infections: Microbiological, immunological, clinical and therapeutic distinctions. J. Wound Care 2022, 31, 436–445. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, C.; Wang, B.; Yin, J.; Yan, S. Progress in Hydrogels for Skin Wound Repair. Macromol. Biosci. 2022, 22, e2100475. [Google Scholar] [CrossRef]
- Sabarees, G.; Velmurugan, V.; Tamilarasi, G.P.; Alagarsamy, V.; Raja Solomon, V. Recent Advances in Silver Nanoparticles Containing Nanofibers for Chronic Wound Management. Polymers 2022, 14, 3994. [Google Scholar] [CrossRef]
- Kaiser, P.; Wächter, J.; Windbergs, M. Therapy of infected wounds: Overcoming clinical challenges by advanced drug delivery systems. Drug Deliv. Transl. Res. 2021, 11, 1545–1567. [Google Scholar] [CrossRef]
- Fuglestad, M.A.; Tracey, E.L.; Leinicke, J.A. Evidence-based Prevention of Surgical Site Infection. Surg. Clin. N. Am. 2021, 101, 951–966. [Google Scholar] [CrossRef]
- Daeschlein, G. Antimicrobial and antiseptic strategies in wound management. Int. Wound J. 2013, 10 (Suppl. S1), 9–14. [Google Scholar] [CrossRef]
- Rowan, M.P.; Cancio, L.C.; Elster, E.A.; Burmeister, D.M.; Rose, L.F.; Natesan, S.; Chan, R.K.; Christy, R.J.; Chung, K.K. Burn wound healing and treatment: Review and advancements. Crit. Care 2015, 19, 243. [Google Scholar] [CrossRef]
- Haldar, J. Confronting the Rising Threat of Antimicrobial Resistance: A Global Health Imperative. ACS Infect. Dis. 2024, 10, 1–2. [Google Scholar] [CrossRef]
- MacLeod, A.S.; Mansbridge, J.N. The Innate Immune System in Acute and Chronic Wounds. Adv. Wound Care 2016, 5, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Holl, J.; Kowalewski, C.; Zimek, Z.; Fiedor, P.; Kaminski, A.; Oldak, T.; Moniuszko, M.; Eljaszewicz, A. Chronic Diabetic Wounds and Their Treatment with Skin Substitutes. Cells 2021, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair. Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef]
- Mihai, M.M.; Preda, M.; Lungu, I.; Gestal, M.C.; Popa, M.I.; Holban, A.M. Nanocoatings for Chronic Wound Repair-Modulation of Microbial Colonization and Biofilm Formation. Int. J. Mol. Sci. 2018, 19, 1179. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Rao, P.; Hm, S.; Gs, S.; Rai, R.V. Biofilm-Associated Infections in Chronic Wounds and Their Management. Adv. Exp. Med. Biol. 2023, 1370, 55–75. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert. Rev. Anti Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Cavallo, I.; Sivori, F.; Mastrofrancesco, A.; Abril, E.; Pontone, M.; Di Domenico, E.G.; Pimpinelli, F. Bacterial Biofilm in Chronic Wounds and Possible Therapeutic Approaches. Biology 2024, 13, 109. [Google Scholar] [CrossRef]
- Miron, A.; Giurcaneanu, C.; Mihai, M.M.; Beiu, C.; Voiculescu, V.M.; Popescu, M.N.; Soare, E.; Popa, L.G. Antimicrobial Biomaterials for Chronic Wound Care. Pharmaceutics 2023, 15, 1606. [Google Scholar] [CrossRef] [PubMed]
- Haalboom, M. Chronic Wounds: Innovations in Diagnostics and Therapeutics. Curr. Med. Chem. 2018, 25, 5772–5781. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and biocompatibility: An historical overview. J. Biomed. Mater. Res. A 2020, 108, 1617–1633. [Google Scholar] [CrossRef] [PubMed]
- Bravi Costantino, M.L.; Belluzo, M.S.; Oberti, T.G.; Cortizo, A.M.; Cortizo, M.S. Terpolymer-chitosan membranes as biomaterial. J. Biomed. Mater. Res. A 2022, 110, 383–393. [Google Scholar] [CrossRef]
- Polak, R.; Pitombo, R.N. Care during freeze-drying of bovine pericardium tissue to be used as a biomaterial: A comparative study. Cryobiology 2011, 63, 61–66. [Google Scholar] [CrossRef] [PubMed]
- De France, K.J.; Xu, F.; Hoare, T. Structured Macroporous Hydrogels: Progress, Challenges, and Opportunities. Adv. Healthc. Mater. 2018, 7, 1700927. [Google Scholar] [CrossRef]
- Mishra, A.; Srivastava, V. Biomaterials and 3D printing techniques used in the medical field. J. Med. Eng. Technol. 2021, 45, 290–302. [Google Scholar] [CrossRef]
- John, J.V.; McCarthy, A.; Wang, H.; Luo, Z.; Li, H.; Wang, Z.; Cheng, F.; Zhang, Y.S.; Xie, J. Freeze-Casting with 3D-Printed Templates Creates Anisotropic Microchannels and Patterned Macrochannels within Biomimetic Nanofiber Aerogels for Rapid Cellular Infiltration. Adv. Healthc. Mater. 2021, 10, 2100238. [Google Scholar] [CrossRef]
- Zamani, M.; Shakhssalim, N.; Ramakrishna, S.; Naji, M. Electrospinning: Application and Prospects for Urologic Tissue Engineering. Front. Bioeng. Biotechnol. 2020, 8, 579925. [Google Scholar] [CrossRef]
- Korniienko, V.; Varava, Y.; Banasiuk, R.; Korniienko, V.; Diedkova, K.; Petricenko, O.; Arora, D.; Denysenko, A.; Moskalenko, R.; Pogorielov, M. Layer-by-Layer Chitosan/PCL Electrospun Membrane Loaded with Copper Nanoparticles as Antibacterial Wound Healing Dressing. In Nanocomposite and Nanocrystalline Materials and Coatings: Microstructure, Properties and Applications; Pogrebnjak, A.D., Bing, Y., Sahul, M., Eds.; Springer Nature: Singapore, 2024; pp. 149–162. [Google Scholar]
- Rodrigues MÁ, V.; Bertolo, M.R.V.; Horn, M.M.; Lugão, A.B.; Mattoso, L.H.C.; de Guzzi Plepis, A.M. Comparing solution blow spinning and electrospinning methods to produce collagen and gelatin ultrathin fibers: A review. Int. J. Biol. Macromol. 2024, 283, 137806. [Google Scholar] [CrossRef] [PubMed]
- Mirek, A.; Grzeczkowicz, M.; Belaid, H.; Bartkowiak, A.; Barranger, F.; Abid, M.; Wasyłeczko, M.; Pogorielov, M.; Bechelany, M.; Lewińska, D. Electrospun UV-cross-linked polyvinylpyrrolidone fibers modified with polycaprolactone/polyethersulfone microspheres for drug delivery. Biomater. Adv. 2023, 147, 213330. [Google Scholar] [CrossRef] [PubMed]
- Zahra, F.T.; Quick, Q.; Mu, R. Electrospun PVA Fibers for Drug Delivery: A Review. Polymers 2023, 15, 3837. [Google Scholar] [CrossRef] [PubMed]
- Karan, A.; Sharma, N.S.; Darder, M.; Su, Y.; Andrabi, S.M.; Shahriar, S.M.S.; John, J.V.; Luo, Z.; Decoster, M.A.; Zhang, Y.S.; et al. Copper–cystine Biohybrid-embedded Nanofiber Aerogels Show Antibacterial and Angiogenic Properties. ACS Omega 2024, 9, 9765–9781. [Google Scholar] [CrossRef]
- Purushothaman, A.E.; Abhinandan, R.; Adithya, S.P.; Sidharthan, D.S.; Balagangadharan, K.; Selvamurugan, N. Bioactive Molecule-incorporated Polymeric Electrospun Fibers for Bone Tissue Engineering. Curr. Stem Cell Res. Ther. 2023, 18, 470–486. [Google Scholar] [CrossRef]
- Mousavi, S.-M.; Nejad, Z.M.; Hashemi, S.A.; Salari, M.; Gholami, A.; Ramakrishna, S.; Chiang, W.-H.; Lai, C.W. Bioactive Agent-Loaded Electrospun Nanofiber Membranes for Accelerating Healing Process: A Review. Membranes 2021, 11, 702. [Google Scholar] [CrossRef]
- Memic, A.; Abudula, T.; Mohammed, H.S.; Joshi Navare, K.; Colombani, T.; Bencherif, S.A. Latest Progress in Electrospun Nanofibers for Wound Healing Applications. ACS Appl. Bio Mater. 2019, 2, 952–969. [Google Scholar] [CrossRef]
- Nezamoleslami, S.; Fattahi, A.; Nemati, H.; Bagrezaie, F.; Pourmanouchehri, Z.; Kiaie, S.H. Electrospun sandwich-structured of polycaprolactone/gelatin-based nanofibers with controlled release of ceftazidime for wound dressing. Int. J. Biol. Macromol. 2023, 236, 123819. [Google Scholar] [CrossRef]
- Kheradvar Kolour, A.; Ghoraishizadeh, S.; Zaman, M.S.; Alemzade, A.; Banavand, M.; Esmaeili, J.; Shahrousvand, M. Janus Films Wound Dressing Comprising Electrospun Gelatin/PCL Nanofibers and Gelatin/Honey/Curcumin Thawed Layer. ACS Appl. Bio Mater. 2024, 7, 8642–8655. [Google Scholar] [CrossRef]
- Miranda, C.C.; Gomes, M.R.; Moço, M.; Cabral, J.M.S.; Ferreira, F.C.; Sanjuan-Alberte, P. A Concise Review on Electrospun Scaffolds for Kidney Tissue Engineering. Bioengineering 2022, 9, 554. [Google Scholar] [CrossRef]
- Hoveizi, E.; Nabiuni, M.; Parivar, K.; Rajabi-Zeleti, S.; Tavakol, S. Functionalisation and surface modification of electrospun polylactic acid scaffold for tissue engineering. Cell Biol. Int. 2014, 38, 41–49. [Google Scholar] [CrossRef]
- Chanda, A.; Adhikari, J.; Ghosh, A.; Chowdhury, S.R.; Thomas, S.; Datta, P.; Saha, P. Electrospun chitosan/polycaprolactone-hyaluronic acid bilayered scaffold for potential wound healing applications. Int. J. Biol. Macromol. 2018, 116, 774–785. [Google Scholar] [CrossRef]
- Jiang, A.; Patel, R.; Padhan, B.; Palimkar, S.; Galgali, P.; Adhikari, A.; Varga, I.; Patel, M. Chitosan Based Biodegradable Composite for Antibacterial Food Packaging Application. Polymers 2023, 15, 2235. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Lu, W.; Hu, Y. Review on chitosan-based antibacterial hydrogels: Preparation, mechanisms, and applications. Int. J. Biol. Macromol. 2024, 255, 128080. [Google Scholar] [CrossRef] [PubMed]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- Khatoon, U.T.; Rao, G.V.S.N.; Mohan, M.K.; Ramanaviciene, A.; Ramanavicius, A. Comparative study of Antifungal Activity of Silver and Gold Nanoparticles Synthesized by Facile Chemical Approach. J. Environ. Chem. Eng. 2018, 6, 5837–5844. [Google Scholar] [CrossRef]
- Korniienko, V.; Husak, Y.; Diedkova, K.; Varava, Y.; Grebnevs, V.; Pogorielova, O.; Bērtiņš, M.; Korniienko, V.; Zandersone, B.; Ramanaviciene, A.; et al. Antibacterial Potential and Biocompatibility of Chitosan/Polycaprolactone Nanofibrous Membranes Incorporated with Silver Nanoparticles. Polymers 2024, 16, 1729. [Google Scholar] [CrossRef]
- Samokhin, Y.; Varava, Y.; Diedkova, K.; Yanko, I.; Husak, Y.; Radwan-Pragłowska, J.; Pogorielova, O.; Janus, Ł.; Pogorielov, M.; Korniienko, V. Fabrication and Characterization of Electrospun Chitosan/Polylactic Acid (CH/PLA) Nanofiber Scaffolds for Biomedical Application. J. Funct. Biomater. 2023, 14, 414. [Google Scholar] [CrossRef]
- Myronov, P.; Sulaieva, O.; Korniienko, V.; Banasiuk, R.; Vielikov, M.; Husak, Y.; Pernakov, M.; Deineka, V.; Yusupova, A.; Hristova, M.T.; et al. Combination of Chlorhexidine and Silver Nanoparticles: An Efficient Wound Infection and Healing Control System. BioNanoScience 2021, 11, 256–268. [Google Scholar] [CrossRef]
- Butsyk, A.; Varava, Y.; Moskalenko, R.; Husak, Y.; Piddubnyi, A.; Denysenko, A.; Korniienko, V.; Ramanaviciute, A.; Banasiuk, R.; Pogorielov, M.; et al. Copper Nanoparticle Loaded Electrospun Patches for Infected Wound Treatment: From Development to In-Vivo Application. Polymers 2024, 16, 2733. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 macrophages and their overlaps-myth or reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef] [PubMed]
- Saylor, J.; Ma, Z.; Goodridge, H.S.; Huang, F.; Cress, A.E.; Pandol, S.J.; Shiao, S.L.; Vidal, A.C.; Wu, L.; Nickols, N.G.; et al. Spatial Mapping of Myeloid Cells and Macrophages by Multiplexed Tissue Staining. Front. Immunol. 2018, 9, 2925. [Google Scholar] [CrossRef] [PubMed]
- Sharifiaghdam, M.; Shaabani, E.; Faridi-Majidi, R.; De Smedt, S.C.; Braeckmans, K.; Fraire, J.C. Macrophages as a therapeutic target to promote diabetic wound healing. Mol. Ther. 2022, 30, 2891–2908. [Google Scholar] [CrossRef]
- Kolomiiets, O.; Moskalenko, R. Immunohistochemical Study of M1 and M2 Macrophages in Breast Cancer With Microcalcifications. East. Ukr. Med. J. 2023, 11, 155–163. [Google Scholar] [CrossRef]
- Gauchotte, G.; Bochnakian, A.; Campoli, P.; Lardenois, E.; Brix, M.; Simon, E.; Colomb, S.; Martrille, L.; Peyron, P.A. Myeloperoxydase and CD15 with Glycophorin C Double Staining in the Evaluation of Skin Wound Vitality in Forensic Practice. Front. Med. 2022, 9, 910093. [Google Scholar] [CrossRef]
- de Melo, D.F.; Guedes, G.G.; de Carvalho Moreira, L.M.C.; Oshiro-Júnior, J.A.; de Lima Damasceno, B.P.G. Physicochemical Characterization of Silver Sulfadiazine in Polymeric Wound Dressings. Curr. Pharm. Des. 2023, 29, 865–882. [Google Scholar] [CrossRef]
- Hadrup, N.; Sharma, A.K.; Loeschner, K. Toxicity of silver ions, metallic silver, and silver nanoparticle materials after in vivo dermal and mucosal surface exposure: A review. Regul. Toxicol. Pharmacol. 2018, 98, 257–267. [Google Scholar] [CrossRef]
- Vishwanath, N.; Whitaker, C.; Allu, S.; Clippert, D.; Jouffroy, E.; Hong, J.; Stone, B.; Connolly, W.; Barrett, C.C.; Antoci, V.; et al. Silver as an Antibiotic-Independent Antimicrobial: Review of Current Formulations and Clinical Relevance. Surg. Infect. 2022, 23, 769–780. [Google Scholar] [CrossRef]
- Ingavle, G.C.; Leach, J.K. Advancements in electrospinning of polymeric nanofibrous scaffolds for tissue engineering. Tissue Eng. Part B Rev. 2014, 20, 277–293. [Google Scholar] [CrossRef]
- Purssell, E.; Gallagher, R.; Gould, D. Aseptic versus clean technique during wound management? Systematic review with meta-analysis. Int. J. Environ. Health Res. 2024, 34, 1580–1591. [Google Scholar] [CrossRef]
- Wang, X.; Song, R.; Johnson, M.; A, S.; Shen, P.; Zhang, N.; Lara-Sáez, I.; Xu, Q.; Wang, W. Chitosan-Based Hydrogels for Infected Wound Treatment. Macromol. Biosci. 2023, 23, e2300094. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Khatoon, U.T.; Rao, N.G.V.S.; Mohan, K.M.; Ramanaviciene, A.; Ramanavicius, A. Antibacterial and antifungal activity of silver nanospheres synthesized by tri-sodium citrate assisted chemical approach. Vacuum 2017, 146, 259–265. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Maróstica, H.V.; Ames-Sibin, A.P.; Pateis, V.d.O.; de Souza, G.H.; Silva, B.P.; Bracht, L.; Comar, J.F.; Peralta, R.M.; Bracht, A.; Sá-Nakanishi, A.B. Harmful effects of chlorhexidine on hepatic metabolism. Environ. Toxicol. Pharmacol. 2023, 102, 104217. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samokhin, Y.; Varava, Y.; Butsyk, A.; Moskalenko, R.; Husak, Y.; Dryhval, B.; Korniienko, V.; Zhyvotovskyi, I.; Kukurika, V.; Shmatkov, A.; et al. Advanced Electrospun Chitosan-(Polylactic Acid)-(Silver Nanoparticle)-Based Scaffolds for Facilitated Healing of Purulent Wounds: A Preclinical Investigation. Polymers 2025, 17, 2225. https://doi.org/10.3390/polym17162225
Samokhin Y, Varava Y, Butsyk A, Moskalenko R, Husak Y, Dryhval B, Korniienko V, Zhyvotovskyi I, Kukurika V, Shmatkov A, et al. Advanced Electrospun Chitosan-(Polylactic Acid)-(Silver Nanoparticle)-Based Scaffolds for Facilitated Healing of Purulent Wounds: A Preclinical Investigation. Polymers. 2025; 17(16):2225. https://doi.org/10.3390/polym17162225
Chicago/Turabian StyleSamokhin, Yevhen, Yuliia Varava, Anna Butsyk, Roman Moskalenko, Yevheniia Husak, Bohdan Dryhval, Valeriia Korniienko, Ihor Zhyvotovskyi, Vyacheslav Kukurika, Artem Shmatkov, and et al. 2025. "Advanced Electrospun Chitosan-(Polylactic Acid)-(Silver Nanoparticle)-Based Scaffolds for Facilitated Healing of Purulent Wounds: A Preclinical Investigation" Polymers 17, no. 16: 2225. https://doi.org/10.3390/polym17162225
APA StyleSamokhin, Y., Varava, Y., Butsyk, A., Moskalenko, R., Husak, Y., Dryhval, B., Korniienko, V., Zhyvotovskyi, I., Kukurika, V., Shmatkov, A., Ramanaviciute, A., Banasiuk, R., Pogorielov, M., Ramanavicius, A., & Korniienko, V. (2025). Advanced Electrospun Chitosan-(Polylactic Acid)-(Silver Nanoparticle)-Based Scaffolds for Facilitated Healing of Purulent Wounds: A Preclinical Investigation. Polymers, 17(16), 2225. https://doi.org/10.3390/polym17162225






