Influence of Material Selection on the Mechanical Properties of 3D-Printed Tracheal Stents for Surgical Applications
Abstract
1. Introduction
2. Materials and Experimental Methodology
2.1. Selection of Materials
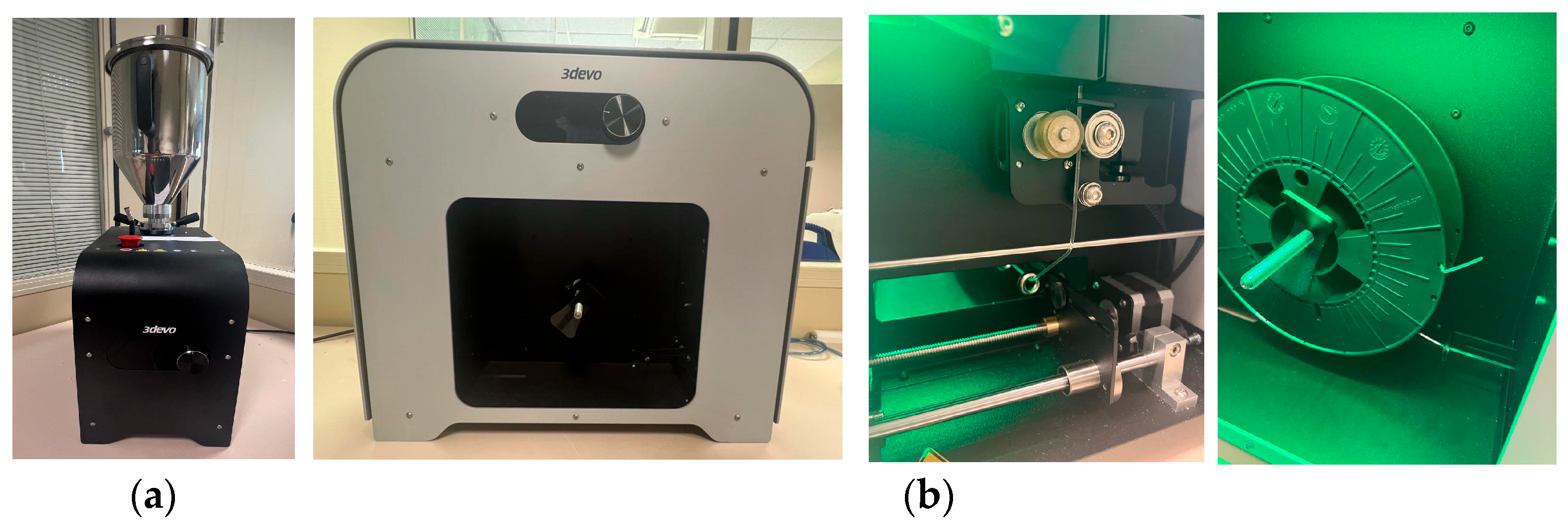
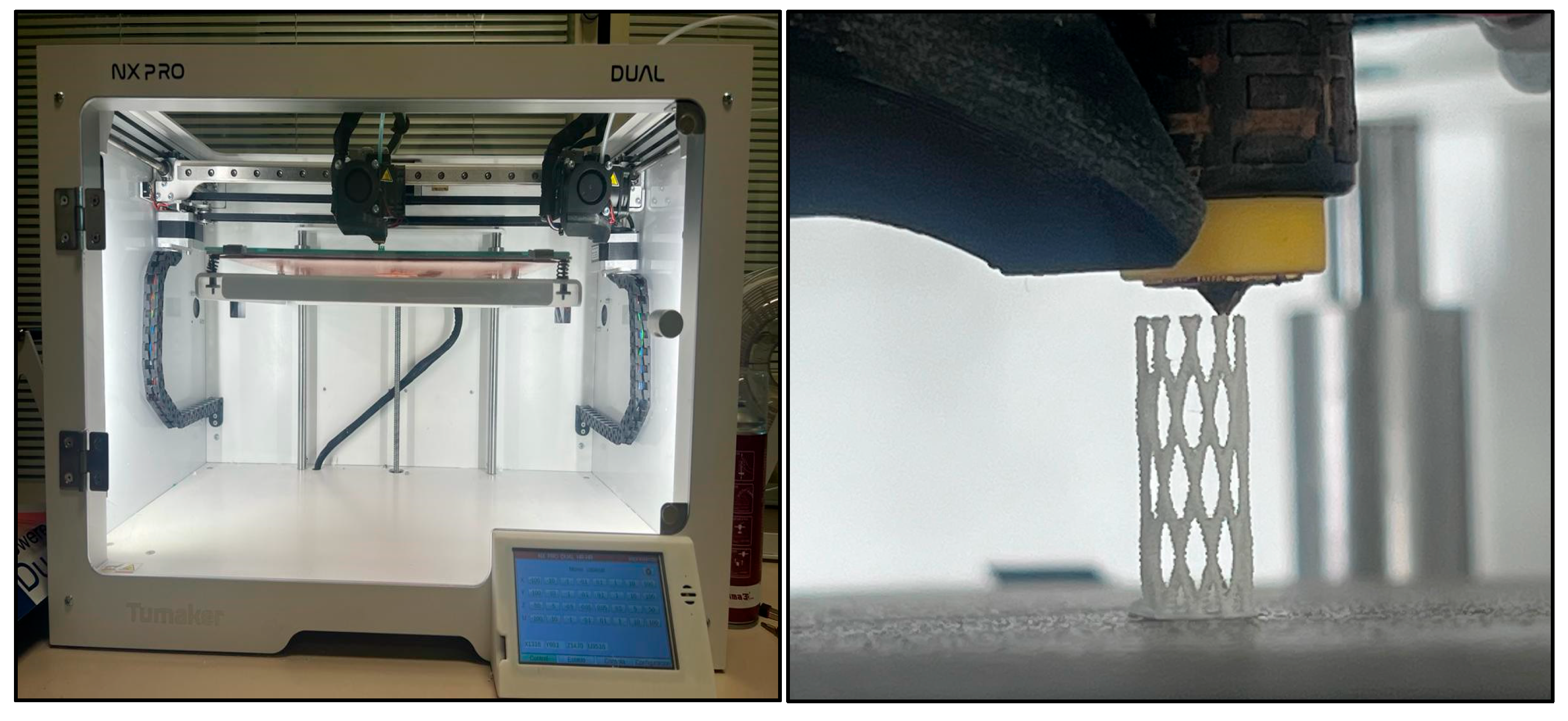
2.2. Manufactured Methods
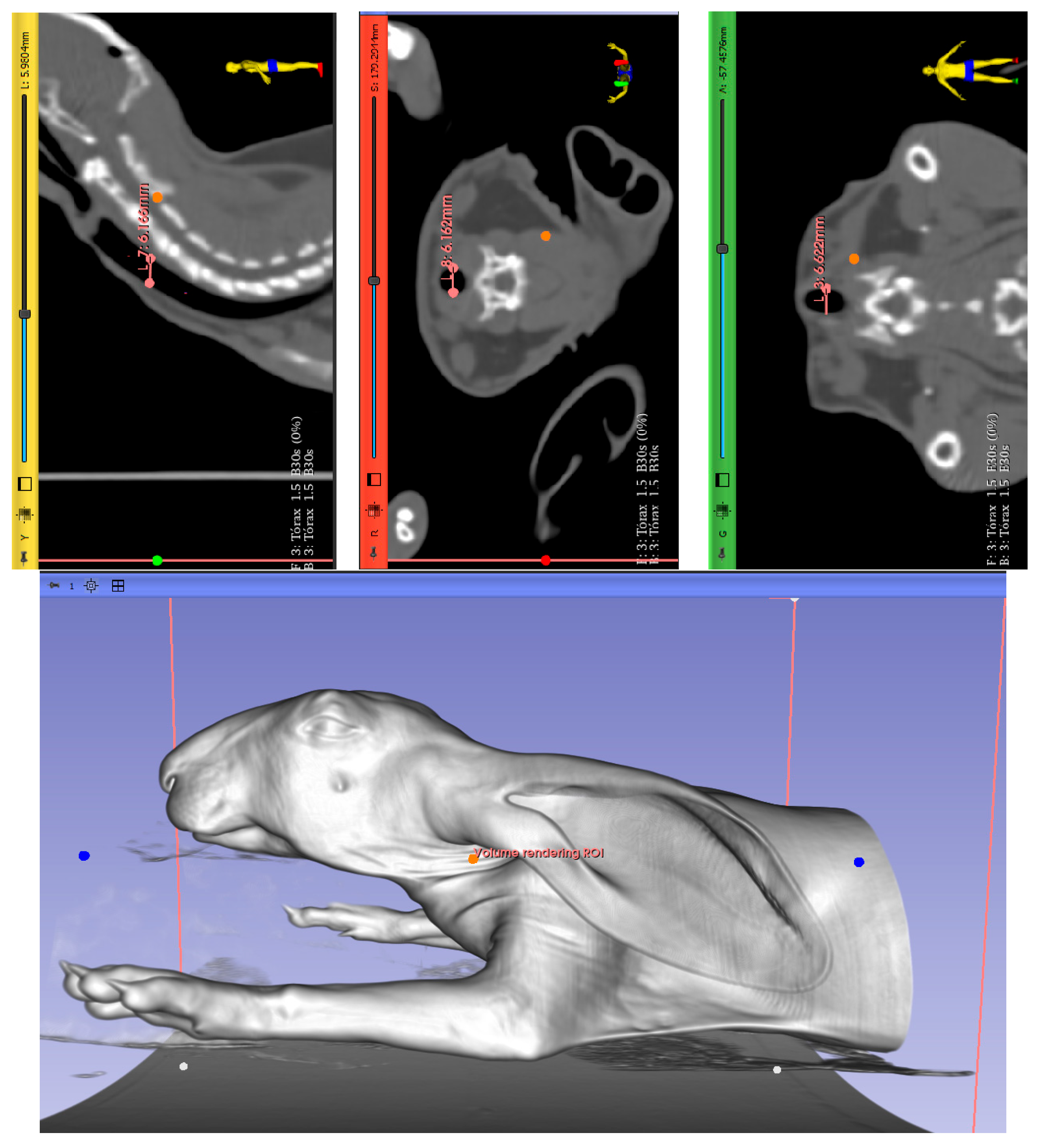
2.3. Determination of Mechanical Properties
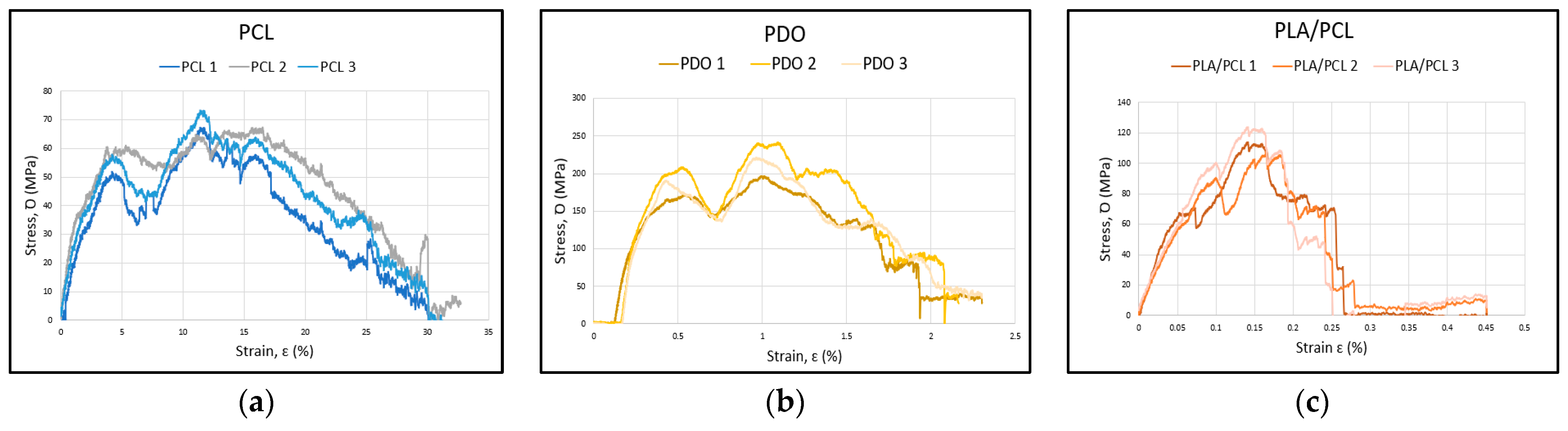
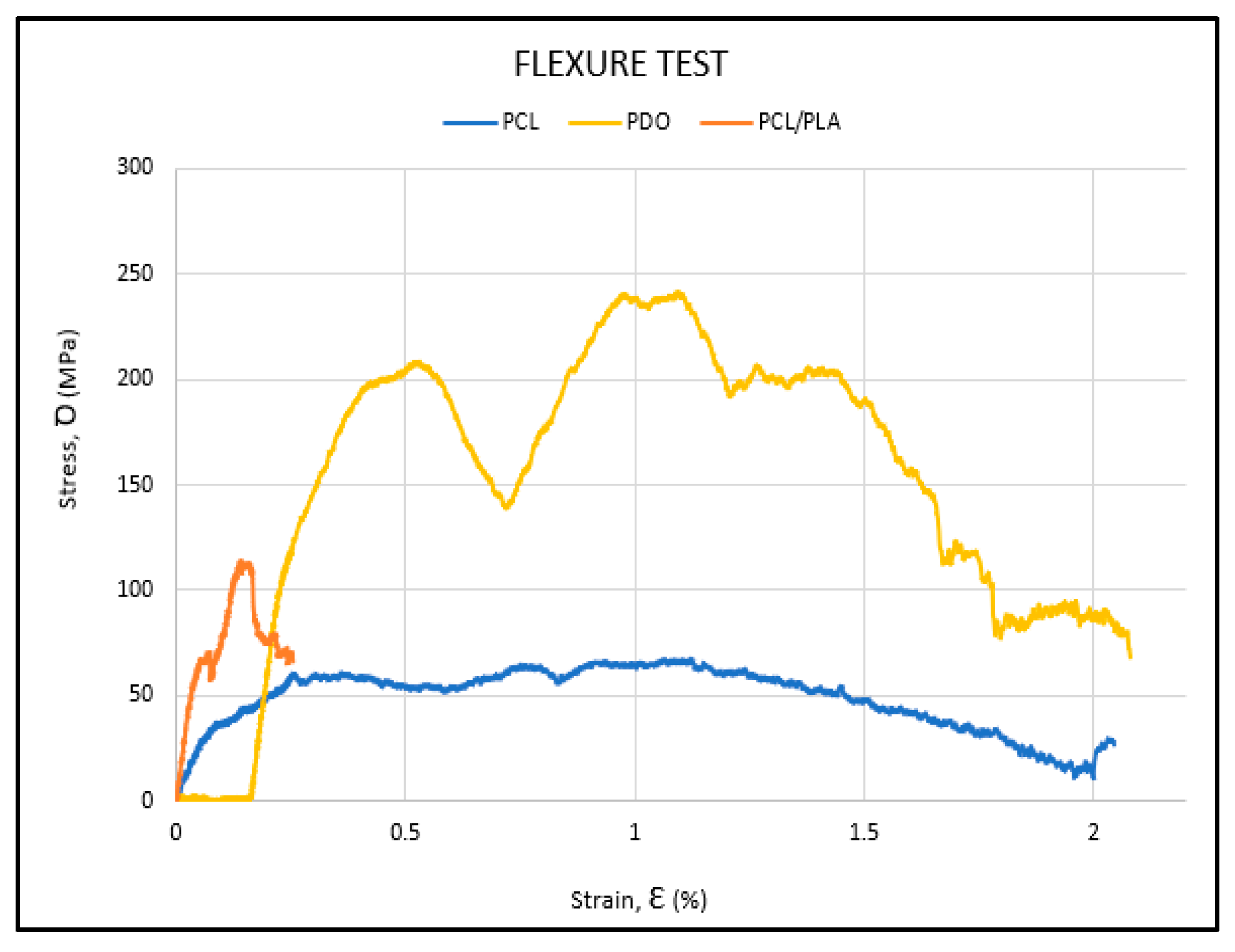
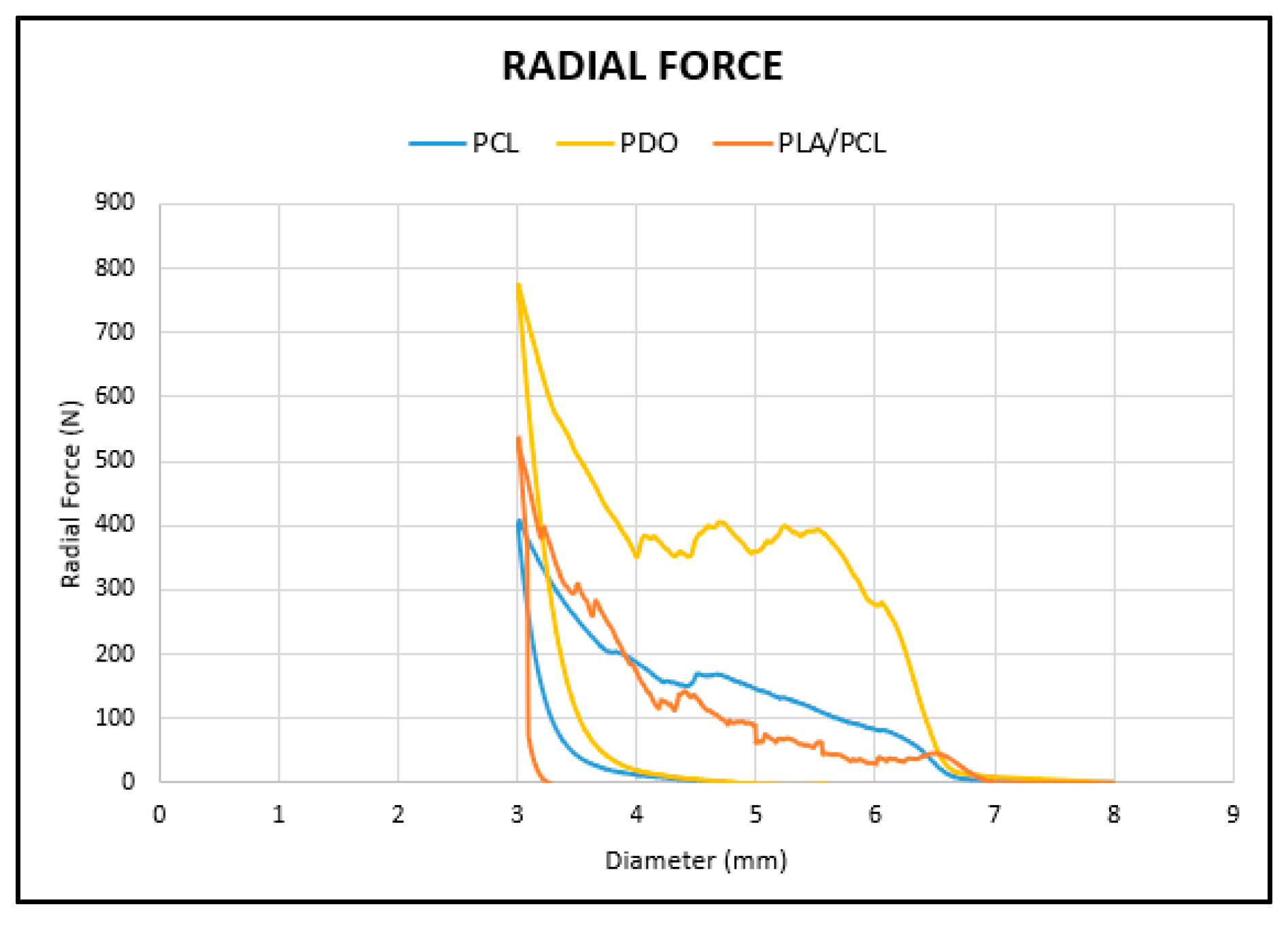
3. Results
3.1. Flexural Test
3.2. Radial Test
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rafanan, A.L.; Mehta, A.C. Stenting of the Tracheobronchial Tree. Radiol. Clin. N. Am. 2000, 38, 395–408. [Google Scholar] [CrossRef]
- Thornton, R.H.; Gordon, R.L.; Kerlan, R.K.; LaBerge, J.M.; Wilson, M.W.; Wolanske, K.A.; Gotway, M.B.; Hastings, G.S.; Golden, J.A. Outcomes of Tracheobronchial Stent Placement for Benign Disease. Radiology 2006, 240, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Delgado Pecellín, I.; González Valencia, J.P.; Machuca Contreras, M.; Pineda Mantecón, M. Clinic, diagnosis and treatment of tracheal stenosis. An. Pediatr. 2009, 70, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Fruchter, O.; Raviv, Y.; Fox, B.D.; Kramer, M.R. Removal of Metallic Tracheobronchial Stents in Lung Transplantation with Flexible Bronchoscopy. J. Cardiothorac. Surg. 2010, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Bolliger, C.T.; Wyser, C.; Wu, X.; Hauser, R.; Studer, W.; Dalquen, P.; Perruchoud, A.P. Evaluation of a New Self-Expandable Silicone Stent in an Experimental Tracheal Stenosis. Chest 1999, 115, 496–501. [Google Scholar] [CrossRef][Green Version]
- Freitag, L.; Gördes, M.; Zarogoulidis, P.; Darwiche, K.; Franzen, D.; Funke, F.; Hohenforst-Schmidt, W.; Dutau, H. Towards Individualized Tracheobronchial Stents: Technical, Practical and Legal Considerations. Respiration 2017, 94, 442–456. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, H.; Koh, W.-J.; Suh, G.Y.; Chung, M.P.; Kwon, O.J. Natural Stent in the Management of Post-Intubation Tracheal Stenosis. Respirology 2009, 14, 583–588. [Google Scholar] [CrossRef]
- Sun, F.; Usón, J.; Ezquerra, J.; Crisóstomo, V.; Luis, L.; Maynar, M. Endotracheal Stenting Therapy in Dogs with Tracheal Collapse. Vet. J. 2008, 175, 186–193. [Google Scholar] [CrossRef]
- Dumon, J.-F. A Dedicated Tracheobronchial Stent. CHEST 1990, 97, 328–332. [Google Scholar] [CrossRef]
- Saad, C.P.; Murthy, S.; Krizmanich, G.; Mehta, A.C. Self-Expandable Metallic Airway Stents and Flexible Bronchoscopy*: Long-Term Outcomes Analysis. CHEST 2003, 124, 1993–1999. [Google Scholar] [CrossRef]
- Chung, F.-T.; Chen, H.-C.; Chou, C.-L.; Yu, C.-T.; Kuo, C.-H.; Kuo, H.-P.; Lin, S.-M. An Outcome Analysis of Self-Expandable Metallic Stents in Central Airway Obstruction: A Cohort Study. J. Cardiothorac. Surg. 2011, 6, 46. [Google Scholar] [CrossRef]
- Husain, S.A.; Finch, D.; Ahmed, M.; Morgan, A.; Hetzel, M.R. Long-Term Follow-Up of Ultraflex Metallic Stents in Benign and Malignant Central Airway Obstruction. Ann. Thorac. Surg. 2007, 83, 1251–1256. [Google Scholar] [CrossRef]
- Ríos, A.E. Intervencionismo pulmonar: Broncoscopia rígida, cirugía endobronquial láser y prótesis traqueobronquiales. NCT Neumol. Cir. Tórax 2006, 65, 26–36. [Google Scholar]
- Saito, Y.; Imamura, H. Airway Stenting. Surg. Today 2005, 35, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Gildea, T.R.; Young, B.P.; Machuzak, M.S. Application of 3D Printing for Patient-Specific Silicone Stents: 1-Year Follow-Up on 2 Patients. Respir. Int. Rev. Thorac. Dis. 2018, 96, 488–494. [Google Scholar] [CrossRef]
- Ghosh, S.; Burks, A.C.; Akulian, J.A. Customizable Airway Stents-Personalized Medicine Reaches the Airways. J. Thorac. Dis. 2019, 11 (Suppl. 9), S1129–S1131. [Google Scholar] [CrossRef]
- Guibert, N.; Saka, H.; Dutau, H. Airway Stenting: Technological Advancements and Its Role in Interventional Pulmonology. Respirology 2020, 25, 953–962. [Google Scholar] [CrossRef]
- She, Y.; Fan, Z.; Wang, L.; Li, Y.; Sun, W.; Tang, H.; Zhang, L.; Wu, L.; Zheng, H.; Chen, C. 3D Printed Biomimetic PCL Scaffold as Framework Interspersed with Collagen for Long Segment Tracheal Replacement. Front. Cell Dev. Biol. 2021, 9, 629796. [Google Scholar] [CrossRef]
- Guerra, A.J.; Cano, P.; Rabionet, M.; Puig, T.; Ciurana, J. 3D-Printed PCL/PLA Composite Stents: Towards a New Solution to Cardiovascular Problems. Materials 2018, 11, 1679. [Google Scholar] [CrossRef]
- Guerra, A.J.; Ciurana, J. 3D-Printed Bioabsordable Polycaprolactone Stent: The Effect of Process Parameters on Its Physical Features. Mater. Des. 2018, 137, 430–437. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, C.; Sun, B.; Wu, Y.; Yu, X.; Cui, J.; Zhang, M.; El-Newehy, M.; El-Hamshary, H.; Barlis, P.; et al. Development of 3D Printed Biodegradable, Entirely X-Ray Visible Stents for Rabbit Carotid Artery Implantation. Adv. Healthc. Mater. 2024, 13, e2304293. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Vates, U.K.; Mishra, S. Proof of Concept Study for Radial Compression Strength & Shape Memory Effect of 3D Printed Double Arrowhead PLA Stent. Emergent Mater. 2024, 7, 2623–2634. [Google Scholar] [CrossRef]
- Sousa, A.M.; Amaro, A.M.; Piedade, A.P. 3D Printing of Polymeric Bioresorbable Stents: A Strategy to Improve Both Cellular Compatibility and Mechanical Properties. Polymers 2022, 14, 1099. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Song, G.; Zhao, Q.; Feng, H.; Wang, Y.; Anderson, J.M.; Zhao, H.; Liu, Q. Development of Three-Dimensionally Printed Vascular Stents of Bioresorbable Poly(l-Lactide-Co-Caprolactone). J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 656–664. [Google Scholar] [CrossRef]
- Joseph, T.M.; Kallingal, A.; Suresh, A.M.; Mahapatra, D.K.; Hasanin, M.S.; Haponiuk, J.; Thomas, S. 3D Printing of Polylactic Acid: Recent Advances and Opportunities. Int. J. Adv. Manuf. Technol. 2023, 125, 1015–1035. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-Based Materials in Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, Production, Recent Developments and Applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Jiang, B.; Jiao, H.; Guo, X.; Chen, G.; Guo, J.; Wu, W.; Jin, Y.; Cao, G.; Liang, Z. Lignin-Based Materials for Additive Manufacturing: Chemistry, Processing, Structures, Properties, and Applications. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2023, 10, e2206055. [Google Scholar] [CrossRef]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-Based Hydrogels for Biomedical Applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Grira, S.; Khalifeh, H.A.; Alkhedher, M.; Ramadan, M. 3D Printing Algae-Based Materials: Pathway towards 4D Bioprinting. Bioprinting 2023, 33, e00291. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, Toxicity, Biocompatibility and Clinical Applications of Polylactic Acid/Polyglycolic Acid Copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Bobel, A.C.; Petisco, S.; Sarasua, J.R.; Wang, W.; McHugh, P.E. Computational Bench Testing to Evaluate the Short-Term Mechanical Performance of a Polymeric Stent. Cardiovasc. Eng. Technol. 2015, 6, 519–532. [Google Scholar] [CrossRef]
- Chen, C.; Xiong, Y.; Li, Z.; Chen, Y. Flexibility of Biodegradable Polymer Stents with Different Strut Geometries. Materials 2020, 13, 3332. [Google Scholar] [CrossRef]
- Ferraro, M.; Auricchio, F.; Boatti, E.; Scalet, G.; Conti, M.; Morganti, S.; Reali, A. An Efficient Finite Element Framework to Assess Flexibility Performances of SMA Self-Expandable Carotid Artery Stents. J. Funct. Biomater. 2015, 6, 585–597. [Google Scholar] [CrossRef] [PubMed]
- McGrath, D.J.; Thiebes, A.L.; Cornelissen, C.G.; O’Brien, B.; Jockenhoevel, S.; Bruzzi, M.; McHugh, P.E. Evaluating the Interaction of a Tracheobronchial Stent in an Ovine In-Vivo Model. Biomech. Model. Mechanobiol. 2018, 17, 499–516. [Google Scholar] [CrossRef] [PubMed]
- McGrath, D.J.; Thiebes, A.L.; Cornelissen, C.G.; O’Shea, M.B.; O’Brien, B.; Jockenhoevel, S.; Bruzzi, M.; McHugh, P.E. An Ovine in Vivo Framework for Tracheobronchial Stent Analysis. Biomech. Model. Mechanobiol. 2017, 16, 1535–1553. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.S.; Tan, L.P.; Joso, J.F.D.; Boey, Y.C.F.; Wang, X. Biodegradable Stents with Elastic Memory. Biomaterials 2006, 27, 1573–1578. [Google Scholar] [CrossRef]
- PDO-Fiche-Technique-EN.Pdf. Available online: https://lattice-services.com/wp-content/uploads/PDO-Fiche-technique-EN.pdf (accessed on 28 May 2025).
- Lattice Medical, PCL-Fiche-Technique-EN.Pdf. Available online: https://lattice-services.com/wp-content/uploads/PCL-Fiche-technique-FR.pdf (accessed on 28 May 2025).
- NatureWorks LLC. Technical Data Sheet 4043D 3D Monofilament, [Online]. Available online: https://www.natureworksllc.com/~/media/Files/NatureWorks/Technical-Documents/Technical-Data-Sheets/TechnicalDataSheet_4043D_3D-monofilament_pdf. (accessed on 28 May 2025).
- Ayechu-Abendaño, A.; Pérez-Jiménez, A.; Sánchez-Matás, C.; López-Villalobos, J.L.; Díaz-Jiménez, C.; Fernández-Parra, R.; Malvè, M. Computational Analysis of Polymeric Biodegradable and Customizable Airway Stent Designs. Polymers 2024, 16, 1691. [Google Scholar] [CrossRef]
- ISO 178; Plastics—Determination of Flexural Properties. International Organization for Standardization: Geneva, Switzerland, 2019.
- Ensayos en Stents. Available online: https://www.zwickroell.com/es/sectores/industria-medica-y-farmaceutica/cateteres-y-stents/ensayos-en-stents/ (accessed on 28 May 2025).
- Noppen, M. Airway Injury and Sequelae: Conservative View. In Surgery for Non-Neoplastic Disorders of the Chest: A Clinical Update; European Respiratory Society: Lausanne, Switzerland, 2025; pp. 234–245. [Google Scholar] [CrossRef]
- Melgoza, E.L.; Serenó, L.; Rosell, A.; Ciurana, J. An Integrated Parameterized Tool for Designing a Customized Tracheal Stent. Comput.-Aided Des. 2012, 44, 1173–1181. [Google Scholar] [CrossRef]
- Xavier, R.G.; Sanches, P.R.S.; de Macedo Neto, A.V.; Kuhl, G.; Vearick, S.B.; Michelon, M.D. Development of a modified Dumon stent for tracheal applications: An experimental study in dogs. J. Bras. Pneumol. 2008, 34, 21–26. [Google Scholar] [CrossRef]
- Zurita-Gabasa, J.; Sánchez-Matás, C.; Díaz-Jiménez, C.; López-Villalobos, J.L.; Malvè, M. A Parametric Tool for Studying a New Tracheobronchial Silicone Stent Prototype: Toward a Customized 3D Printable Prosthesis. Mathematics 2021, 9, 2118. [Google Scholar] [CrossRef]
- Malvè, M.; Barreras, I.; López-Villalobos, J.L.; Ginel, A.; Doblaré, M. Computational Fluid-Dynamics Optimization of a Human Tracheal Endoprosthesis. Int. Commun. Heat Mass Transf. 2012, 39, 575–581. [Google Scholar] [CrossRef]
- Lischke, R.; Pozniak, J.; Vondrys, D.; Elliott, M.J. Novel Biodegradable Stents in the Treatment of Bronchial Stenosis after Lung Transplantation. Eur. J. Cardio-Thorac. Surg. 2011, 40, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Zając, A.; Krysta, M.; Kiszka, A.; Górecki, W. Biodegradable Airway Stents: Novel Treatment of Airway Obstruction in Children. Adv. Clin. Exp. Med. 2019, 28, 961–965. [Google Scholar] [CrossRef]
- Dutau, H.; Reynaud-Gaubert, M.; Thomas, P.A. Endoscopic Management of Post-Lung Transplantation Anastomotic Stenosis: Metallic, Silicone or Biodegradable Stents. Eur. J. Cardio-Thorac. Surg. 2012, 41, 1216–1217. [Google Scholar] [CrossRef]
- McMahon, S.; Bertollo, N.; O’Cearbhaill, E.D.; Salber, J.; Pierucci, L.; Duffy, P.; Dürig, T.; Bi, V.; Wang, W. Bio-Resorbable Polymer Stents: A Review of Material Progress and Prospects. Prog. Polym. Sci. 2024, 83, 79–96. [Google Scholar] [CrossRef]
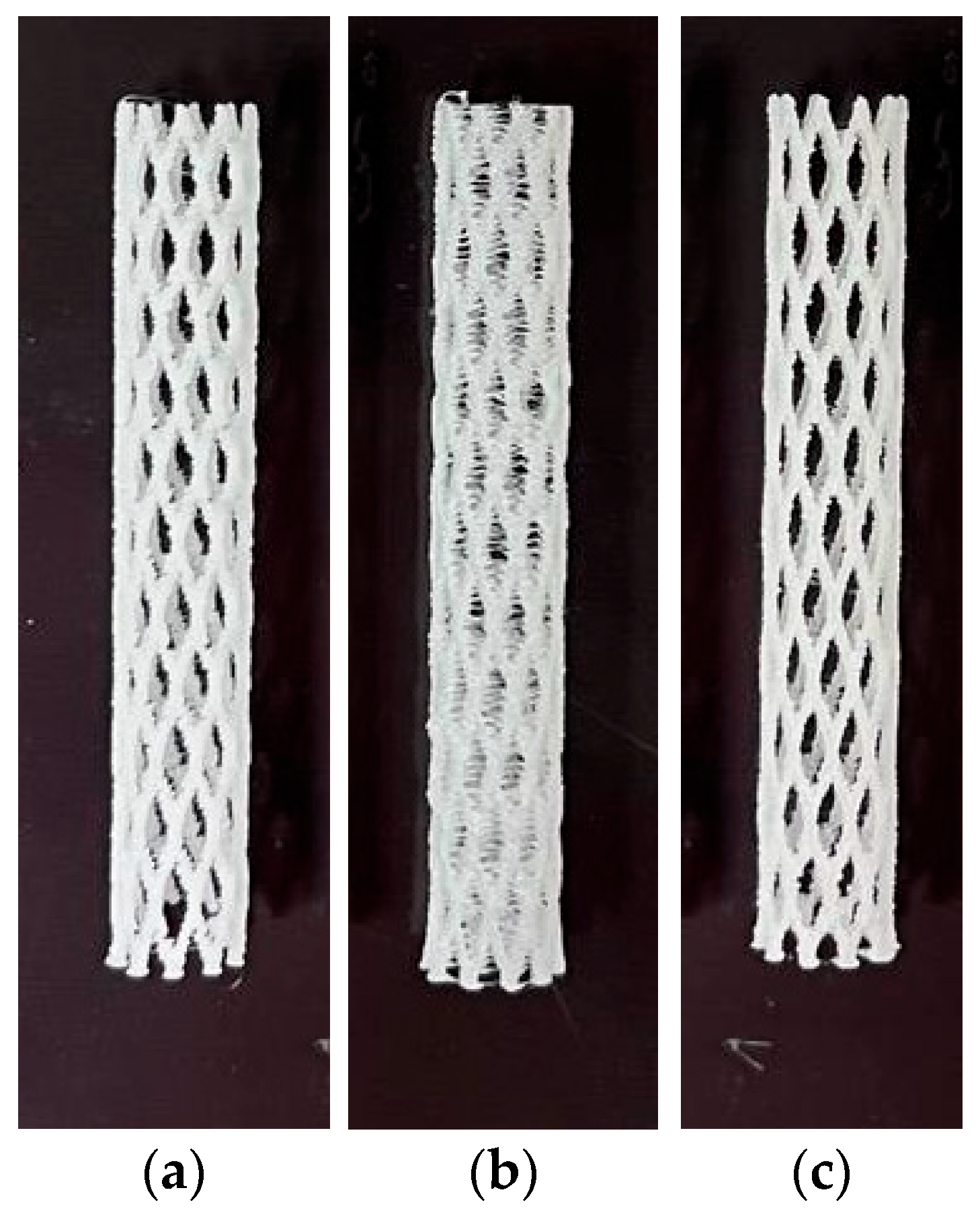




| PCL | PDO | PLA Granules | |
|---|---|---|---|
| Melting range (DSC, 10 °C/min) | 50–70 | 95–110 | 150–180 |
| Glass transition (DSC, 10 °C/min) | <−50 | −21 | 55–60 |
| Degradation temperature (°C) | >250 | >240 | >300 |
| Molar mass (g/mol) | 70,000–90,000 | 140,000–180,000 | 20,000–200,000 |
| Density (g/cm3) | 1.145 | 1.3 | 1.24 |
| Chemical formula | C6H10O2 | C6H8O3 | C3H4O2 |
| PCL | PLA | |
|---|---|---|
| Drying Temperature (°C) | 40 | 60 |
| Drying Time (°C) | 5 | 47 |
| Airflow Rate (%) | 50 | 50 |
| PLA/PCL Blend | ||
|---|---|---|
| Temperature Zones (°C) | Z1 | 160 |
| Z2 | 165 | |
| Z3 | 140 | |
| Z4 | 125 | |
| Screw Speed (rpm) | 12 | |
| Fan Speed (%) | 50 | |
| Filament Diameter (mm) | 1.75 | |
| PCL | PDO | PLA/PCL | |
|---|---|---|---|
| Nozzle Temperature (°C) | 160 | 150 | 190 |
| Bed Temperature (°C) | 45 | 45 | 45 |
| Print Speed (mm/s) | 20 | 20 | 40 |
| Nozzle Diameter (mm) | 0.25 | 0.25 | 0.25 |
| Layer Height (mm) | 0.2 | 0.2 | 0.2 |
| Cooling Fan (%) | 50 | 60 | 100 |
| Retraction Distance (mm) | 0.2 | 0.5 | 1 |
| Retraction Speed (mm/s) | 15 | 10 | 25 |
| Bed Adherence | Texture Surface | Texture Surface | Texture Surface |
| Printing Time (min) | 75 min | 61 min | 32 min |
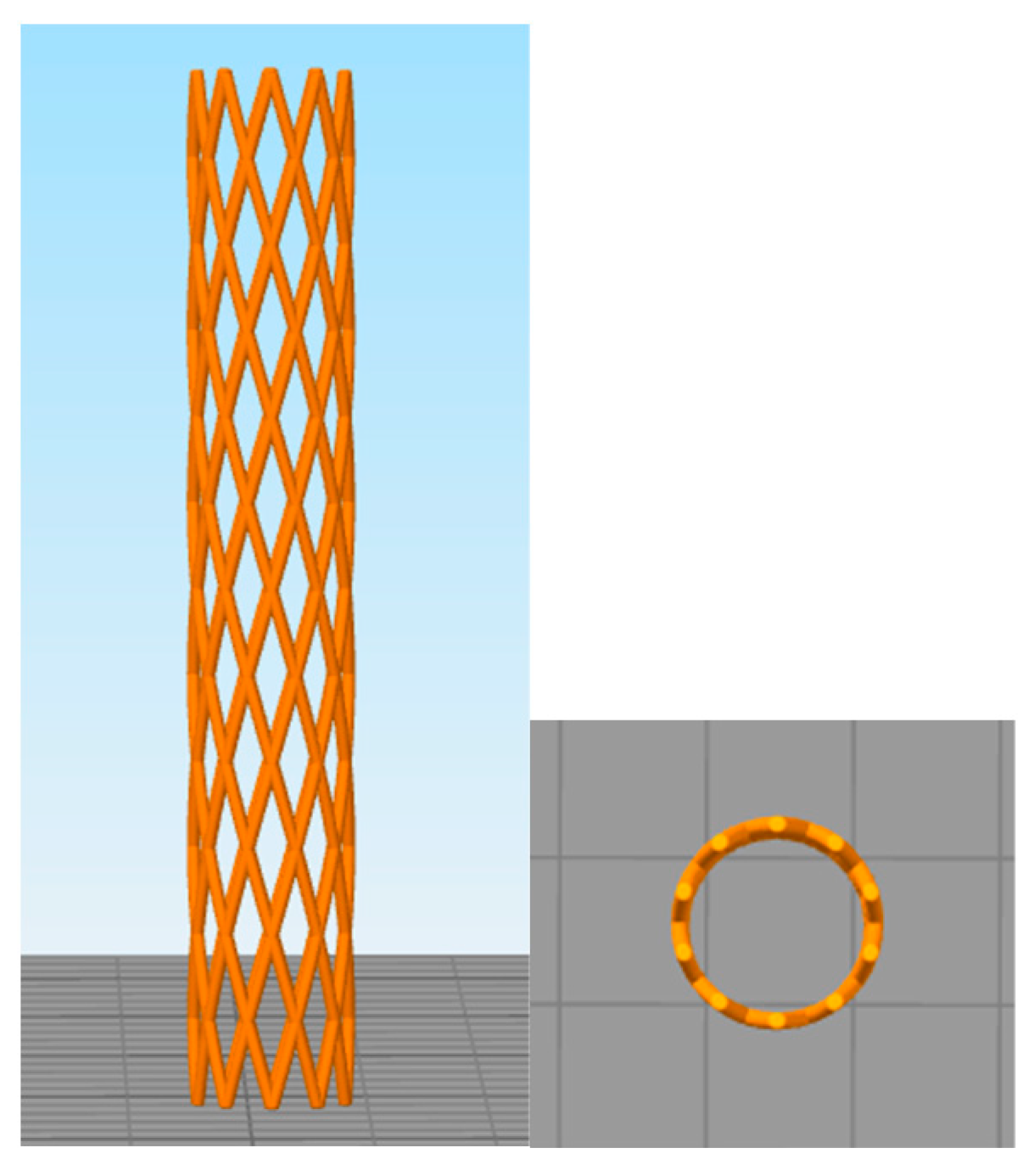
| Parameters | X-Pattern |
|---|---|
| Pitch Angle α (°) | 60 |
| Wire Thickness t (mm) | 0.25 |
| Number of Peaks (or Cell Units) p | 11 |
| Diameter (mm) | 7 |
| Rings | 12 |
| MATERIAL | Ef MPa | ꝹfM MPa | εfM % |
|---|---|---|---|
| PCL 1 | 1460 | 18.2 | 15 |
| PCL 2 | 1530 | 17.6 | 16 |
| PCL 3 | 2020 | 19.1 | 11 |
| PDO 1 | 2010 | 51.3 | 15 |
| PDO 2 | 2900 | 63 | 16 |
| PDO 3 | 3430 | 57.7 | 14 |
| PLA/PCL 1 | 4140 | 29.7 | 2.1 |
| PLA/PCL 2 | 3340 | 28 | 2.4 |
| PLA/PCL 3 | 3138 | 32.5 | 3.5 |
| MATERIAL | RFmax Apply N | RFmax Stand N/mm |
|---|---|---|
| PCL 1 | 404.97 | 9.18 |
| PCL 2 | 396.22 | 9.21 |
| PCL 3 | 377.26 | 8.77 |
| PDO 1 | 773.18 | 17.98 |
| PDO 2 | 760.67 | 17.69 |
| PDO 3 | 744.85 | 17.32 |
| PLA/PCL 1 | 531.10 | 12.35 |
| PLA/PCL 2 | 441.85 | 10.27 |
| PLA/PCL 3 | 463.33 | 10.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez Jiménez, A.; Sánchez González, C.; Pérez Teresí, S.; Landa, N.; Díaz Jiménez, C.; Malvé, M. Influence of Material Selection on the Mechanical Properties of 3D-Printed Tracheal Stents for Surgical Applications. Polymers 2025, 17, 2223. https://doi.org/10.3390/polym17162223
Pérez Jiménez A, Sánchez González C, Pérez Teresí S, Landa N, Díaz Jiménez C, Malvé M. Influence of Material Selection on the Mechanical Properties of 3D-Printed Tracheal Stents for Surgical Applications. Polymers. 2025; 17(16):2223. https://doi.org/10.3390/polym17162223
Chicago/Turabian StylePérez Jiménez, Aurora, Carmen Sánchez González, Sandra Pérez Teresí, Noelia Landa, Cristina Díaz Jiménez, and Mauro Malvé. 2025. "Influence of Material Selection on the Mechanical Properties of 3D-Printed Tracheal Stents for Surgical Applications" Polymers 17, no. 16: 2223. https://doi.org/10.3390/polym17162223
APA StylePérez Jiménez, A., Sánchez González, C., Pérez Teresí, S., Landa, N., Díaz Jiménez, C., & Malvé, M. (2025). Influence of Material Selection on the Mechanical Properties of 3D-Printed Tracheal Stents for Surgical Applications. Polymers, 17(16), 2223. https://doi.org/10.3390/polym17162223






