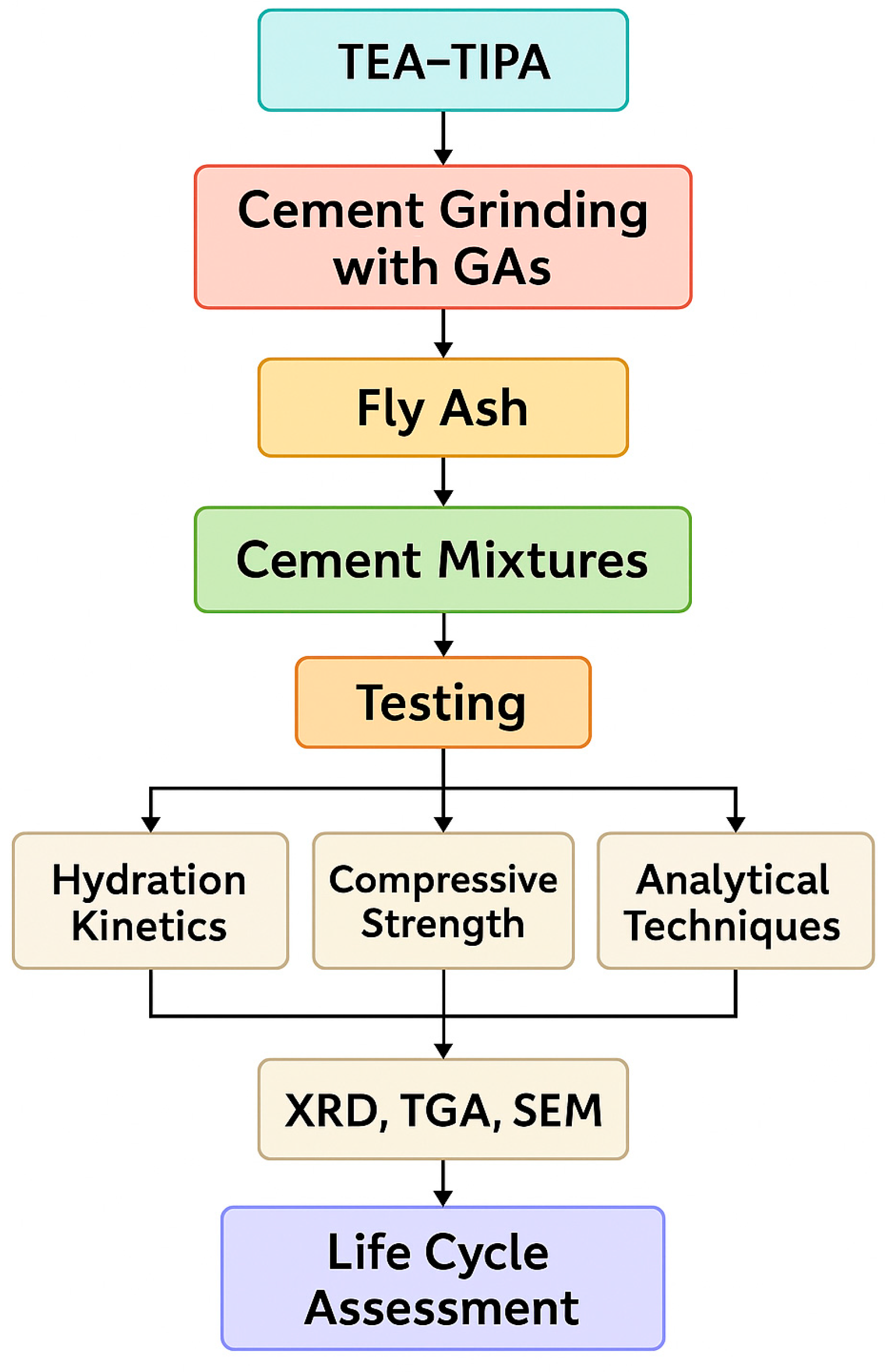
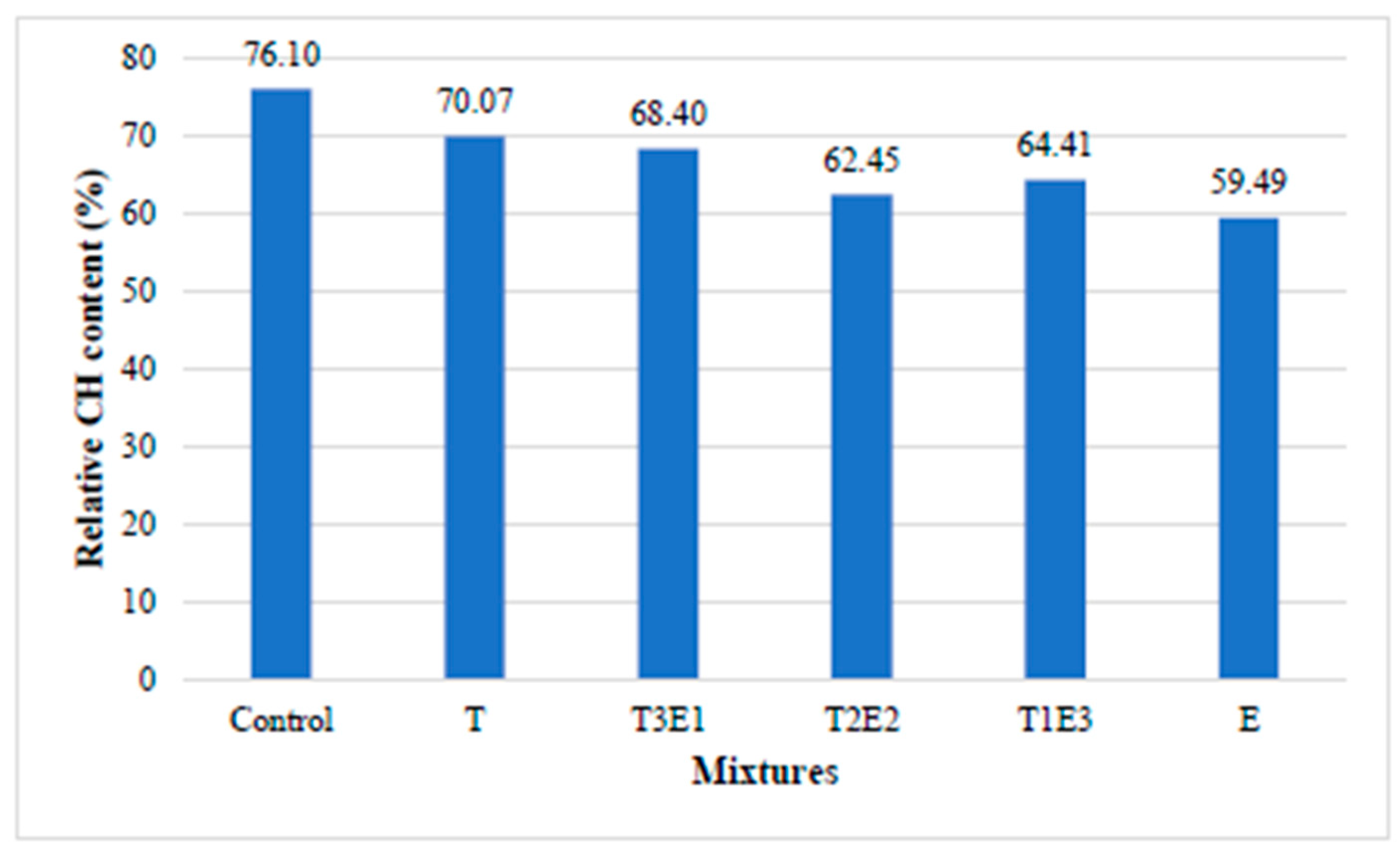
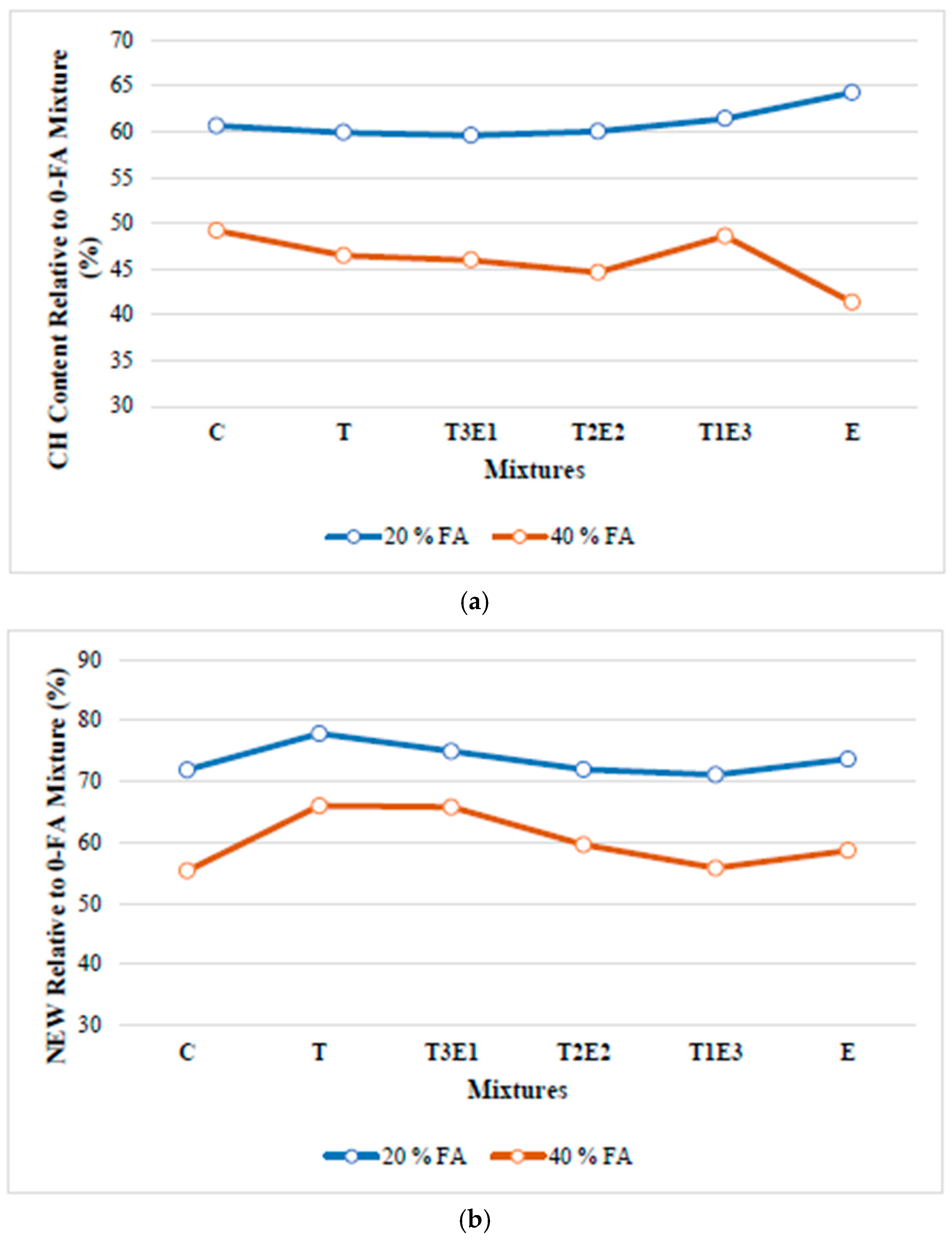
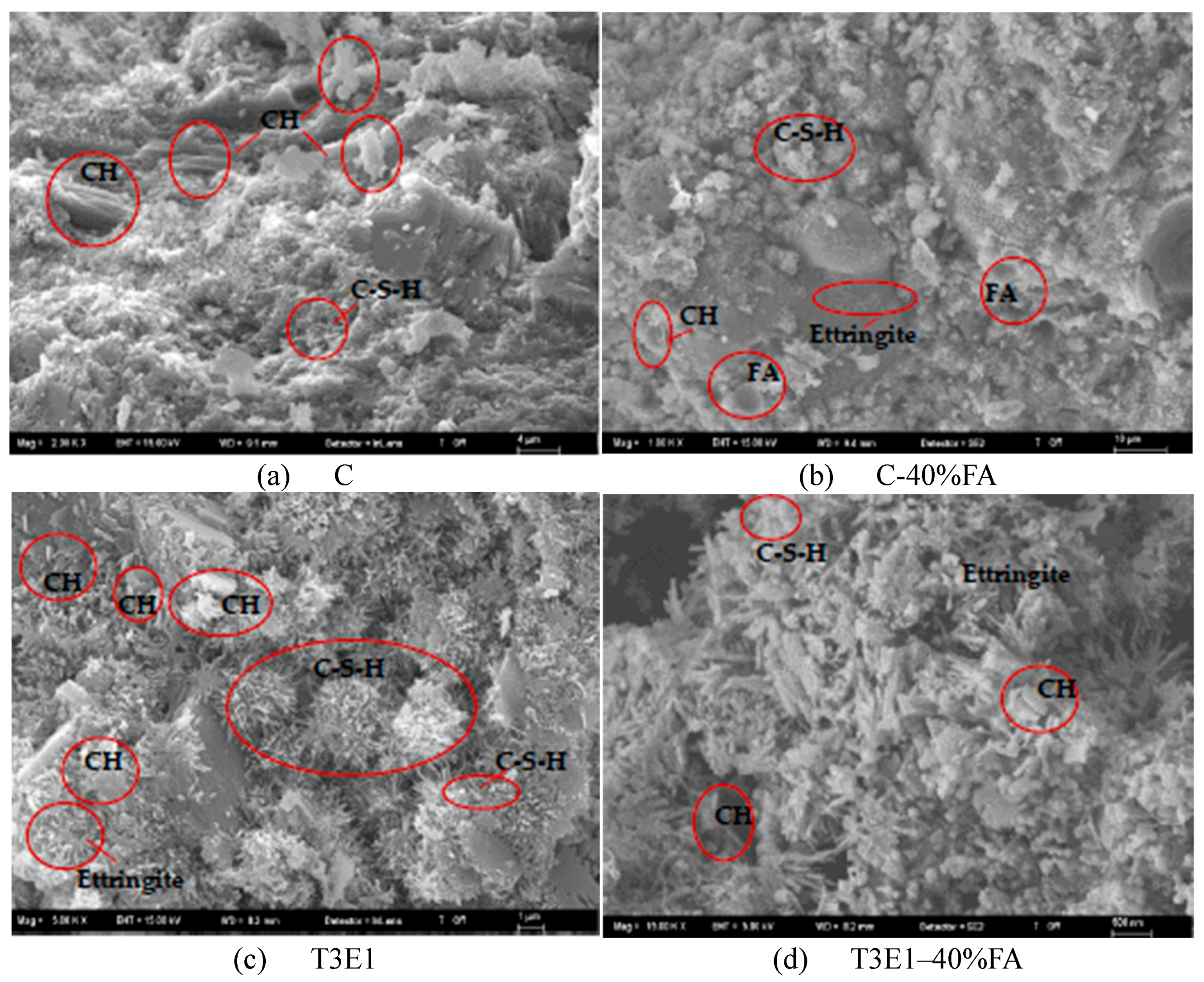
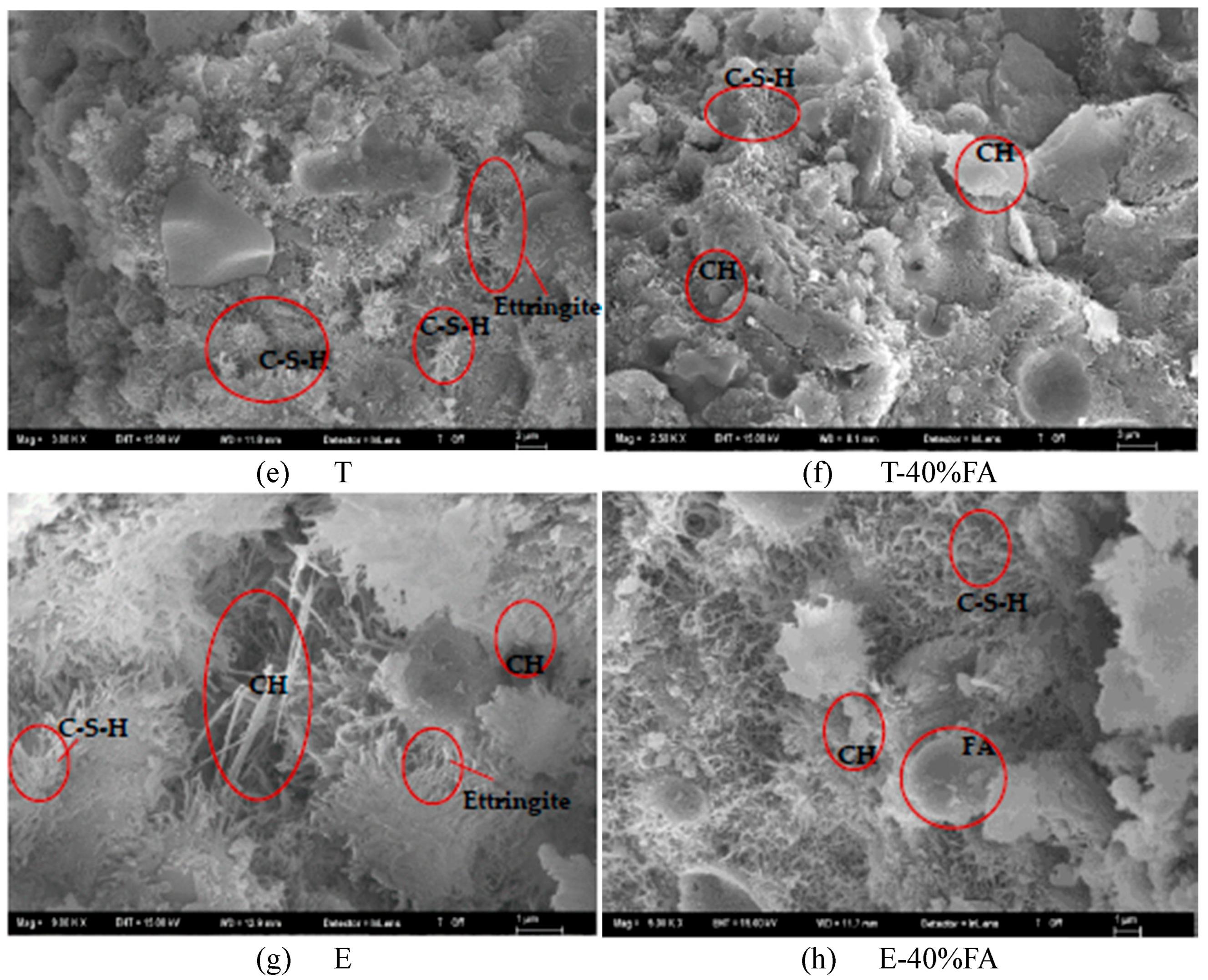
3.2.1. Development of Compressive Strength in Cementitious Systems Without FA
In this section, the effect of the GA used in the study on the development of compressive strength in cementitious mixtures without fly ash is investigated.
As shown in
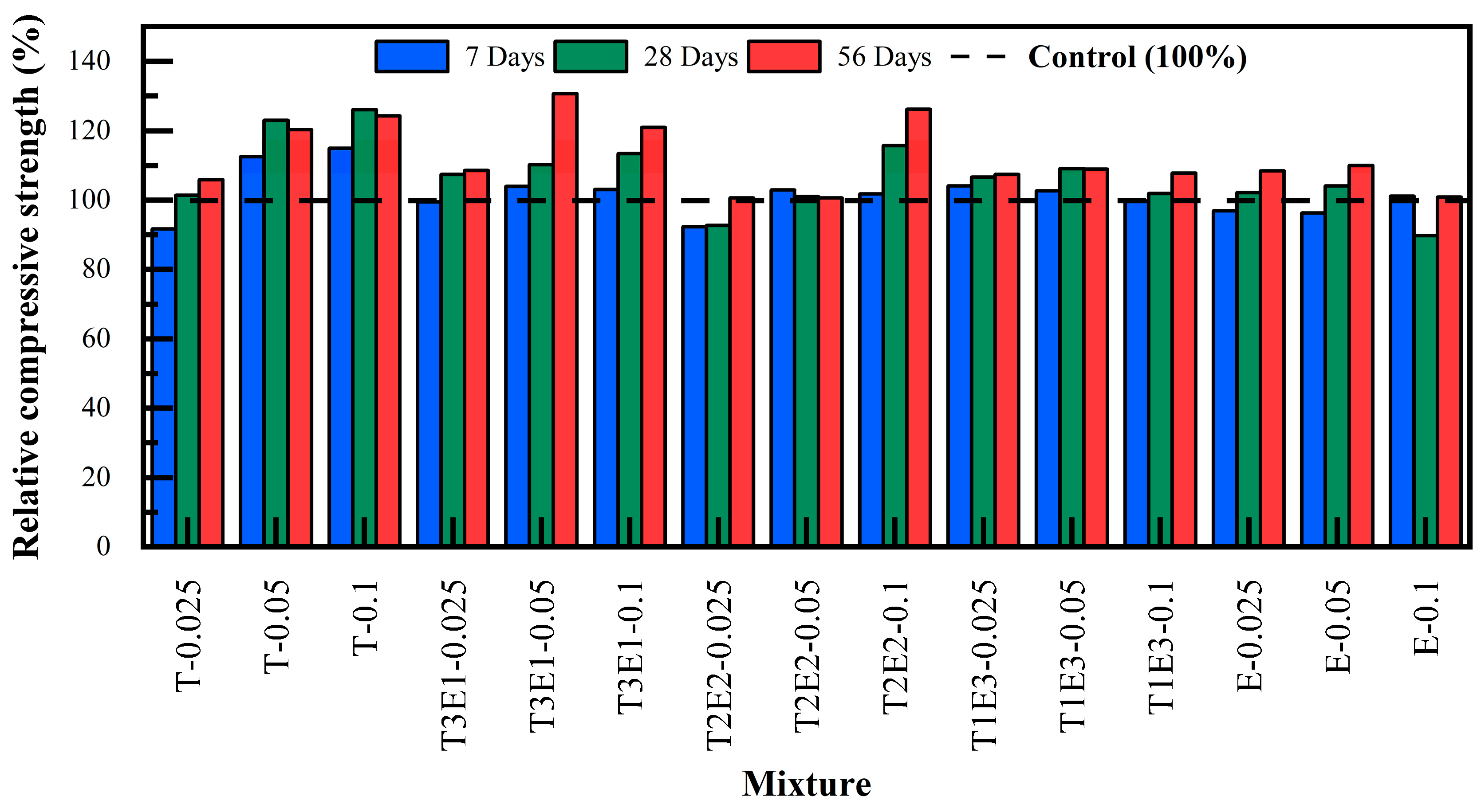
Figure 9, the dosage of GAs had a direct effect on compressive strength performance. In general, increased dosage led to enhanced strength development, particularly evident in TIPA-rich systems. However, beyond a certain threshold (i.e., 0.1%), a plateauing or even slightly diminishing effect was observed in TEA-only systems, likely due to over-retardation of silicate hydration.
The mixtures with TEA (E) generally showed a positive trend in terms of long-term strength. The E-0.025 mixture showed a slight decrease of 3.1% in 7-day strength compared to the control, but no significant change was observed in 28-day strength. At 56 days, an 8.4% increase in strength was detected. Similarly, the E-0.05 mixture showed a 3.7% decrease in 7-day strength compared to the control, but a 4.1% increase at 28 days and a 10.0% increase at 56 days. In the E-0.1 mixture, there was no important distinction in 7-day strength compared to the control, but a 10% decrease in 28-day strength, and at 56 days, the strength was similar to the control.
These results are related to TEA’s acceleration of the hydration of the C
3A phase at early ages but show that when used at high dosages, it can delay the hydration of silicates [
49,
50,
51]. Therefore, the limited development in 28-day strength is followed by a more significant strength gain in the 56-day curing period. TEA, when used at lower dosages, does not significantly accelerate early-age hydration but maintains comparable strength levels. This can be explained by TEA’s regulatory role in the hydration kinetics of the C
3A phase. When used at low dosages, TEA temporarily suppresses the formation of the aluminate phase by forming a stable structure with C
3A, while contributing to the formation of sufficient C-S-H to maintain strength development [
37,
52,
53].
The TIPA addition provided a strength increase at all ages, with particularly higher performance compared to TEA in the long term. The T-0.025 mixture showed an 8% lower performance in 7-day strength compared to the control, and did not show a significant increase in 28-day strength, but showed a 6% increase at 56 days. The T-0.05 mixture showed one of the highest improvements after 56 days with a 13% increase. The T-0.1 mixture showed a 12% increase in 56-day strength compared to the control. TIPA addition accelerates the formation of ettringite and monosulfate phases, which can lead to slower mechanical strength development within the first 7 days [
54,
55,
56].
Looking at long-term strengths, it is believed that the simultaneous promotion of silicate and aluminate phase hydration by TIPA facilitates dissolution, contributing to more dense C-S-H gel formation, and tightening the microstructure, which are the key reasons for this improvement [
57,
58].
Development of Compressive Strength in Cementitious Systems Containing FA
The development of compressive strength in FA-containing systems was evaluated across multiple parameters, including curing age, FA replacement level, and the influence of GA composition, particularly the TEA/TIPA ratio. The discussion integrates both experimental results and comparative insights with the control group to understand the hydration behavior and long-term performance of blended systems.
3.2.4. Effect of GA Type and TEA/TIPA Ratio
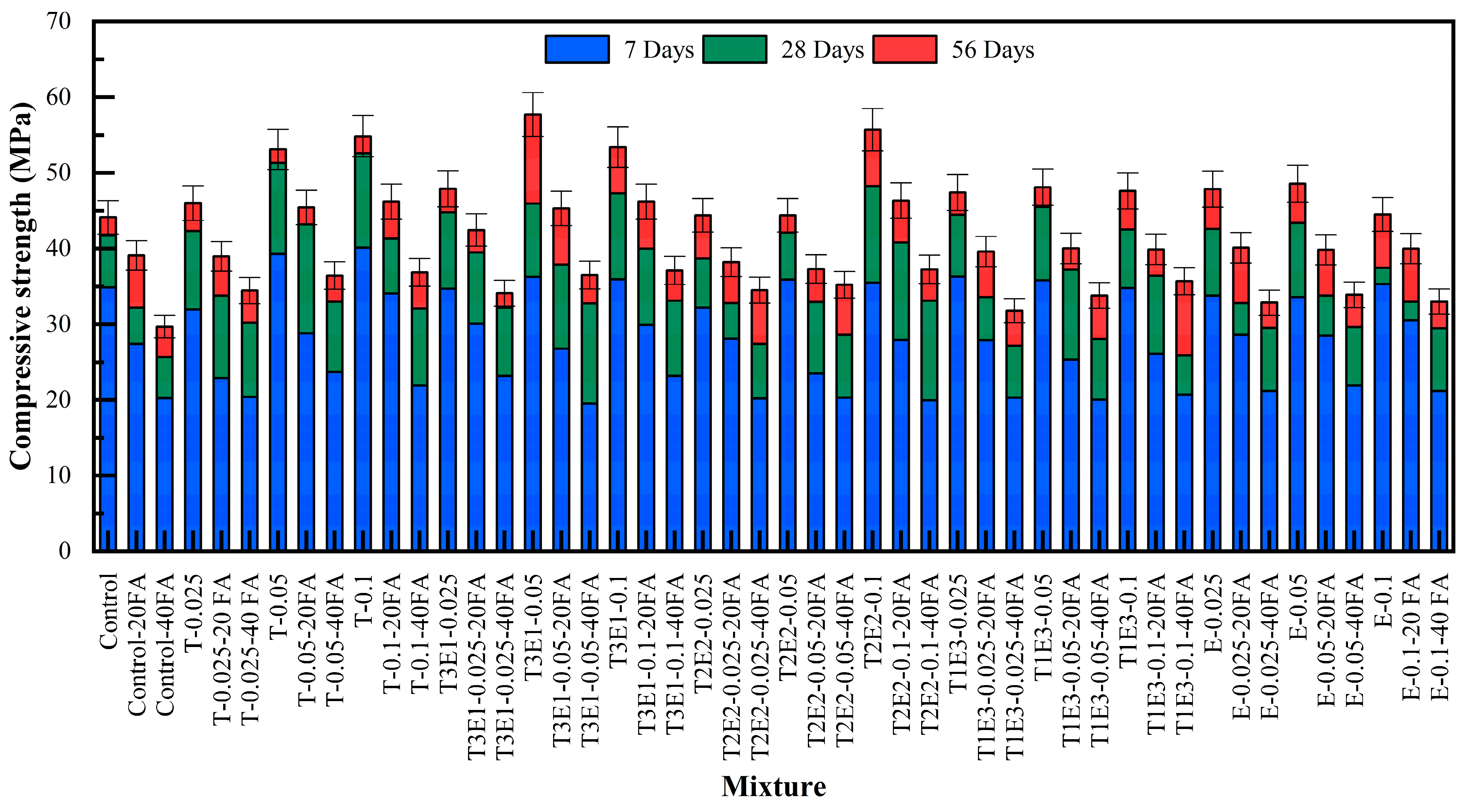
Mixtures containing only TIPA (T-0.025 to T-0.1) achieved the highest strength levels at all ages. T-0.1 recorded 40.10 MPa (7d), 52.60 MPa (28d), and 54.84 MPa (56d), confirming TIPA’s superior impact on both aluminate and silicate phase hydration.
The T3E1 group (75% TIPA/25% TEA) also showed excellent performance, slightly trailing T at early ages but often surpassing it by 56 days. T3E1–0.05: 36.27 MPa (7d), 45.95 MPa (28d), 57.70 MPa (56d)—the highest 56-day strength across all mixtures. This indicates that the balanced combination of TEA’s early-age accelerating effect and TIPA’s later-age pozzolanic activation is effective [
36,
63,
64,
65,
66].
T2E2 mixtures (50% TIPA / 50% TEA) yielded moderate early-age strength but exhibited strong long-term development. For instance, T2E2–0.1 achieved 55.70 MPa at 56 days, suitable for applications emphasizing durability over early strength.
T1E3 mixtures (25% TIPA/75% TEA) reflected TEA’s retarding impact at higher dosages. T1E3–0.1 showed slower development at 28 days but recovered well by 56 days (47.60 MPa), indicating delayed but effective pozzolanic interaction.
Mixtures containing only TEA (E-0.025 to E-0.1) recorded the lowest 28-day strengths but improved notably by 56 days. For instance, E-0.05 reached 48.55 MPa, highlighting TEA’s capacity to activate long-term pozzolanic reactions, albeit with limited early-age benefits.
Notably, hybrid mixtures like T1E3–0.05–40FA displayed a 22.3% strength increase at 56 days relative to the control, validating the benefit of blending TEA and TIPA. Overall, amino alcohol-based GAs such as TIPA and TEA significantly improved compressive strength in FA-containing systems. While TEA primarily enhances later-stage pozzolanic activity, TIPA contributes more strongly to both early and late hydration kinetics. Among all formulations, a TIPA:TEA ratio of 75:25 at a 0.05–0.1% dosage (T3E1) demonstrated the most effective performance, offering a high-strength and sustainable solution for blended cementitious systems.
3.2.5. Life Cycle Assessment
This section examines the mechanical performance and environmental impacts of high levels of FA replacement in mixtures containing TIPA and TEA. The most favorable results were obtained for the mixture containing 0.1% GA (by cement weight) and 75% TIPA with 25% TEA (T3E1–0.1).
This formulation achieved a 56-day compressive strength of 53.4 MPa, showing a 20.9% increase compared to the control mixture (44.13 MPa), despite 46.1% FA replacement. This is the maximum replacement level evaluated in this study. High levels of FA replacement generally lead to strength loss at early ages and, in some cases, over the long term [
58,
59]. However, the strength increase observed here suggests that the selected GAs can enhance the pozzolanic activity of FA. This was also emphasized in a study by He et al. [
67]. The presence of TIPA, which is known to support C
3A hydration and late-age strength development, likely accelerates the formation of additional C–S–H, contributing to the densification of the microstructure and the maintenance of strength as the curing period extends [
60].
This system also significantly impacts environmental advantages. The environmental effects and parameters, such as consumption and savings of the cement-based system mixtures in the study, were evaluated by a life cycle assessment. The consumption and energy requirements considered during the life cycle assessment are calculated according to
Table 8.
When 1.4 tons of raw material is used to produce 1 ton of cement, approximately 680 kg of CO
2 is emitted during the calcination process; when emissions from electricity and transportation are included, the total emissions reach about 950 kg [
70,
71]. Additionally, the production process generates 500–700 kg of industrial waste. Therefore, the use of pozzolans is being investigated as a lower-emission alternative, with CO
2 emissions from these materials reported to be in the range of 70–100 kg per ton [
68,
72].
Beyond its environmental advantages, the combined use of FA and GAs significantly enhances the performance of blended cementitious systems. Grinding aids such as TEA and TIPA not only improve the energy efficiency of grinding but also modify the hydration behavior of fly ash–cement blends. Several studies have demonstrated that GAs can promote early-stage strength development by improving particle dispersion and enhancing the dissolution of active silica and alumina phases within FA, thereby accelerating pozzolanic reactions [
1,
24,
35]. For instance, Chang et al. [
35] reported that TIPA enhances ferrite phase hydration, contributing to Ca(OH)
2 formation, which is then consumed by FA to produce secondary C–S–H gels. Similarly, Liu et al. [
37] observed that GAs improve the microstructural densification in FA-blended cements, leading to improved mechanical performance at later ages. Moreover, the synergistic effect of TEA and TIPA, as confirmed in this study, can regulate both aluminate and silicate phase kinetics, thus compensating for the delayed reactivity typically associated with FA at early curing ages. Consequently, integrating FA with optimized grinding aid formulations offers not only environmental savings but also superior hydration kinetics and long-term mechanical performance compared to FA use alone.
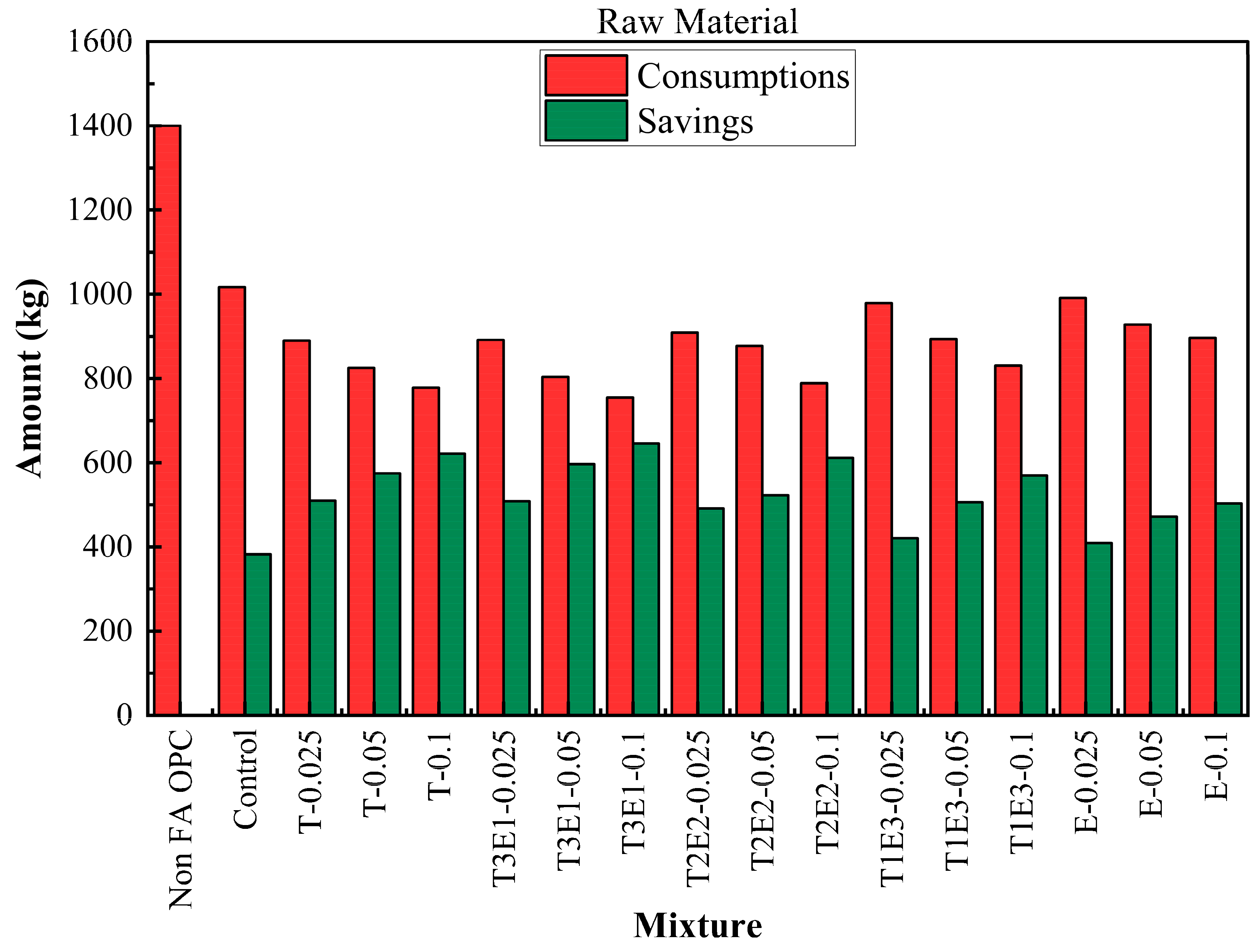
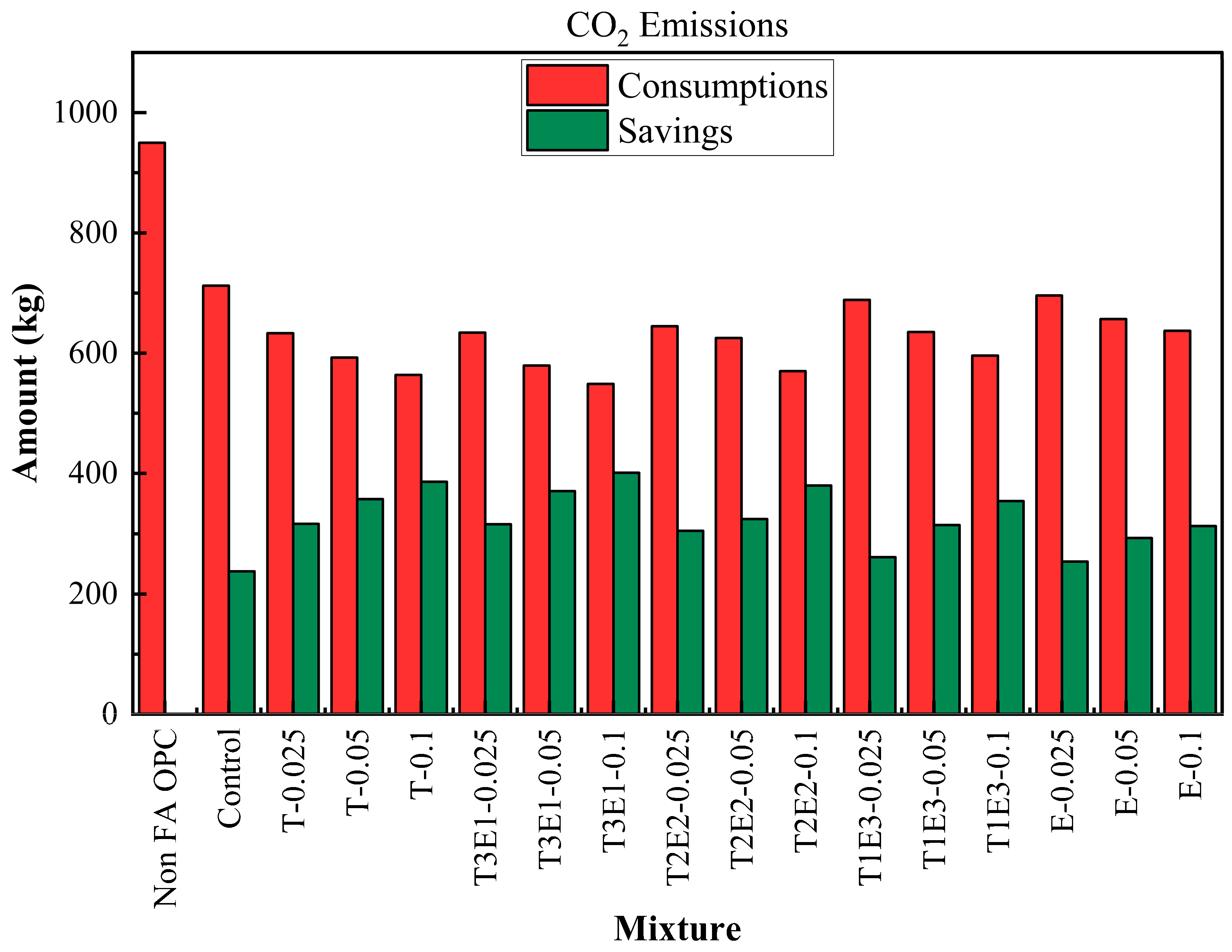
The life cycle process diagram for the life cycle assessment is presented in
Figure 10. The consumption and savings that are determined according to the life cycle analysis, as raw material, CO
2 emissions, and waste generation, are shown in
Figure 11,
Figure 12 and
Figure 13. In addition,
Table 9 shows the maximum FA substitution for target strength values. Here, maximum FA substitution refers to the maximum percentage of fly ash that can be applied to achieve the compressive strengths determined in the study.
Compared to the baseline non-FA OPC mixture, all other mixtures demonstrated substantial raw material savings, primarily due to the replacement of clinker with FA. The control mixture, which includes FA but no GA, already reduced consumption to 1017.45 kg, yielding a 382.55 kg saving. The integration of GAs further enhanced savings. The T3E1–0.1 mixture (75% TIPA + 25% TEA at 0.1% dosage) achieved the lowest raw material usage at 754.50 kg, marking a maximum saving of 645.50 kg. This trend confirms that synergistic use of TEA and TIPA not only promotes hydration but significantly improves material efficiency.
A similar pattern was observed in CO2 emissions. The reference OPC emitted 950 kg CO2 per ton, while the control mixture brought this down to 712.27 kg. Emission reductions became more pronounced with increasing GA effectiveness and FA content. The T3E1–0.1 mixture again recorded the lowest CO2 emissions (548.87 kg), offering a saving of 401.13 kg—over a 42% reduction compared to OPC. This reduction is attributed to two factors: the lower clinker demand (the major CO2 contributor in cement production) and the improved grinding efficiency offered by optimized TEA-TIPA blends.
Waste material savings followed the same trajectory. While the OPC mixture generated 600 kg of waste, the control dropped this to 436.05 kg. As with other metrics, T3E1–0.1 emerged as the most efficient, producing only 323.36 kg of waste and saving 276.64 kg. Other TIPA-rich or balanced TIPA-TEA combinations (e.g., T-0.1, T2E2–0.1) also showed strong reductions. These results reinforce that fly ash replacement and GA optimization not only affect strength and hydration kinetics but significantly reduce industrial waste—supporting circular economy goals.
The data highlight that the combined use of TEA and TIPA, especially in the T3E1 formulation at higher dosages, optimizes environmental performance without compromising strength. The reductions in raw material use, CO2 emissions, and waste generation across T3E1–0.1 reflect a high-efficiency binder system with both mechanical and ecological benefits. This synergy not only enhances pozzolanic activity but also enables deeper clinker substitution, positioning the T3E1–0.1 mixture as the most sustainable and performance-balanced option in this study.
In summary, significant savings have been achieved in the T3E1–0.1 mixture as follows:
A reduction of 645.5 kg in raw material consumption;
A reduction of 401.1 kg in CO2 emissions;
A reduction of 276.6 kg in solid waste generation (per ton of cement).
These gains are believed to be based on two interrelated mechanisms: (1) the partial substitution of clinker, which requires high energy and generates significant amounts of CO
2, with FA, and (2) the increase in grinding efficiency provided by TEA/TIPA additives, reducing the energy demand of the mill and optimizing the particle size distribution [
4,
73]. In particular, this system supports a circular economy approach by utilizing industrial waste (FA) and reducing the environmental footprint of cement production.
Furthermore, the T3E1–0.1 mixture, with FA replacement, has been shown to exceed the strength of the control mixture, demonstrating the potential to lower the overall cement demand in concrete mix design. This has the potential to further enhance sustainability gains through material efficiency.
In conclusion, the findings suggest that the use of amino-based GAs in conjunction with high levels of FA substitution does not result in any loss of mechanical performance. On the contrary, in optimized systems such as T3E1–0.1, this approach offers a high-performance and environmentally sustainable solution.