Crown Ether-Functionalized Polyethersulfone Membranes with Potential Applications in Hemodialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Surface Functionalization of the Membranes via Plasma Treatment
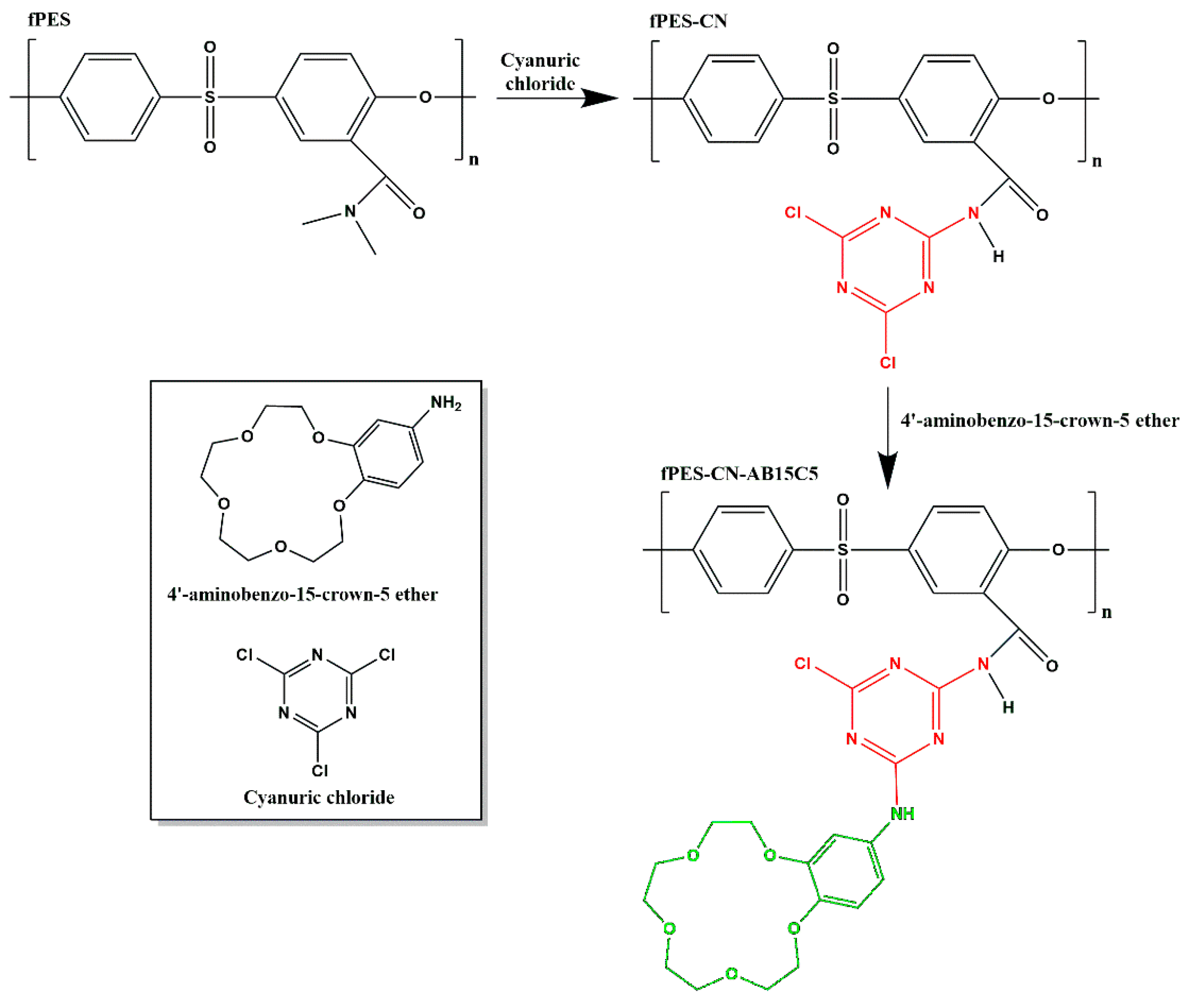
2.3. Chemical Modification of the Membrane Surface with 4′-Aminobenzo-15-Crown-5 Ether
2.4. Characterization Methods
3. Results
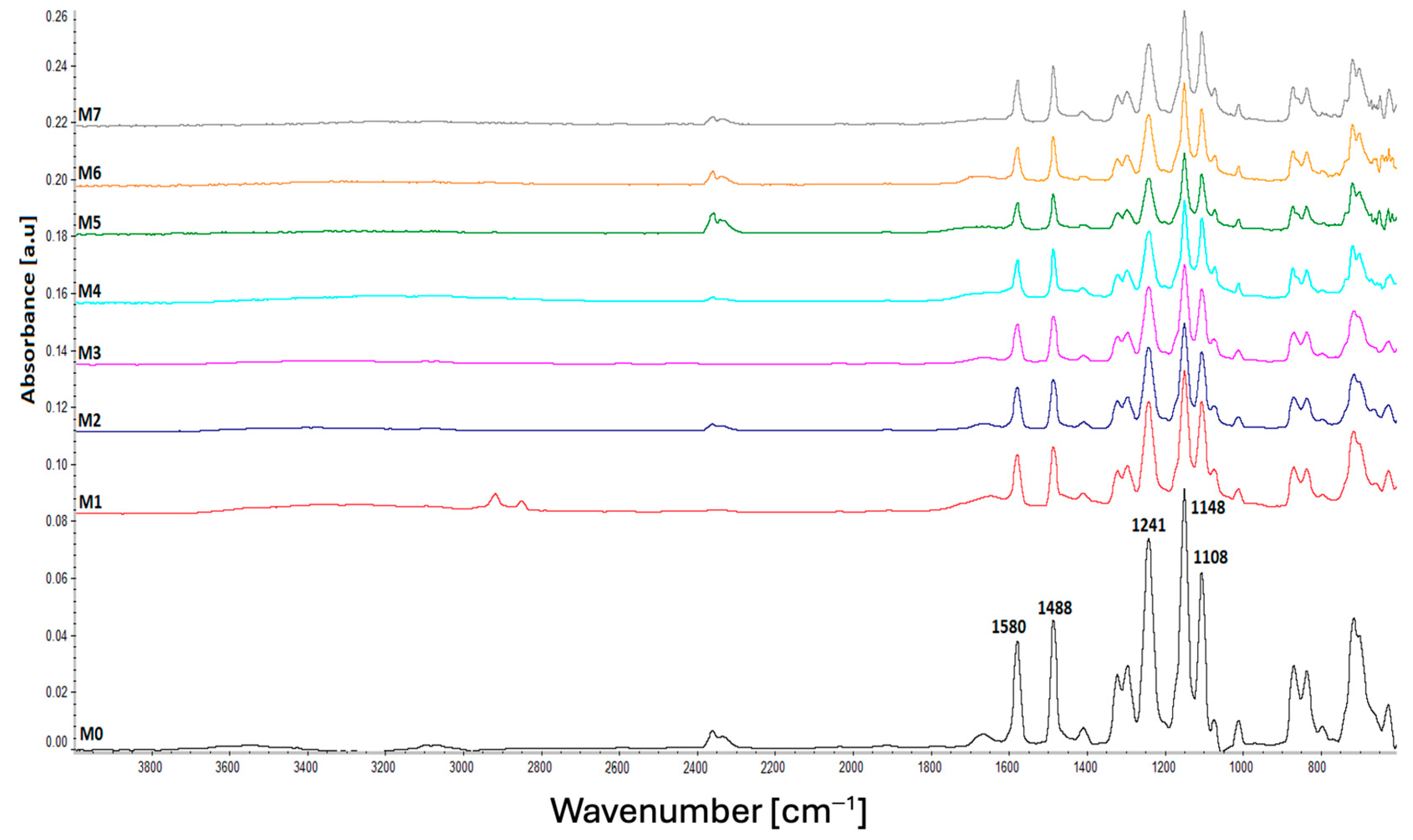
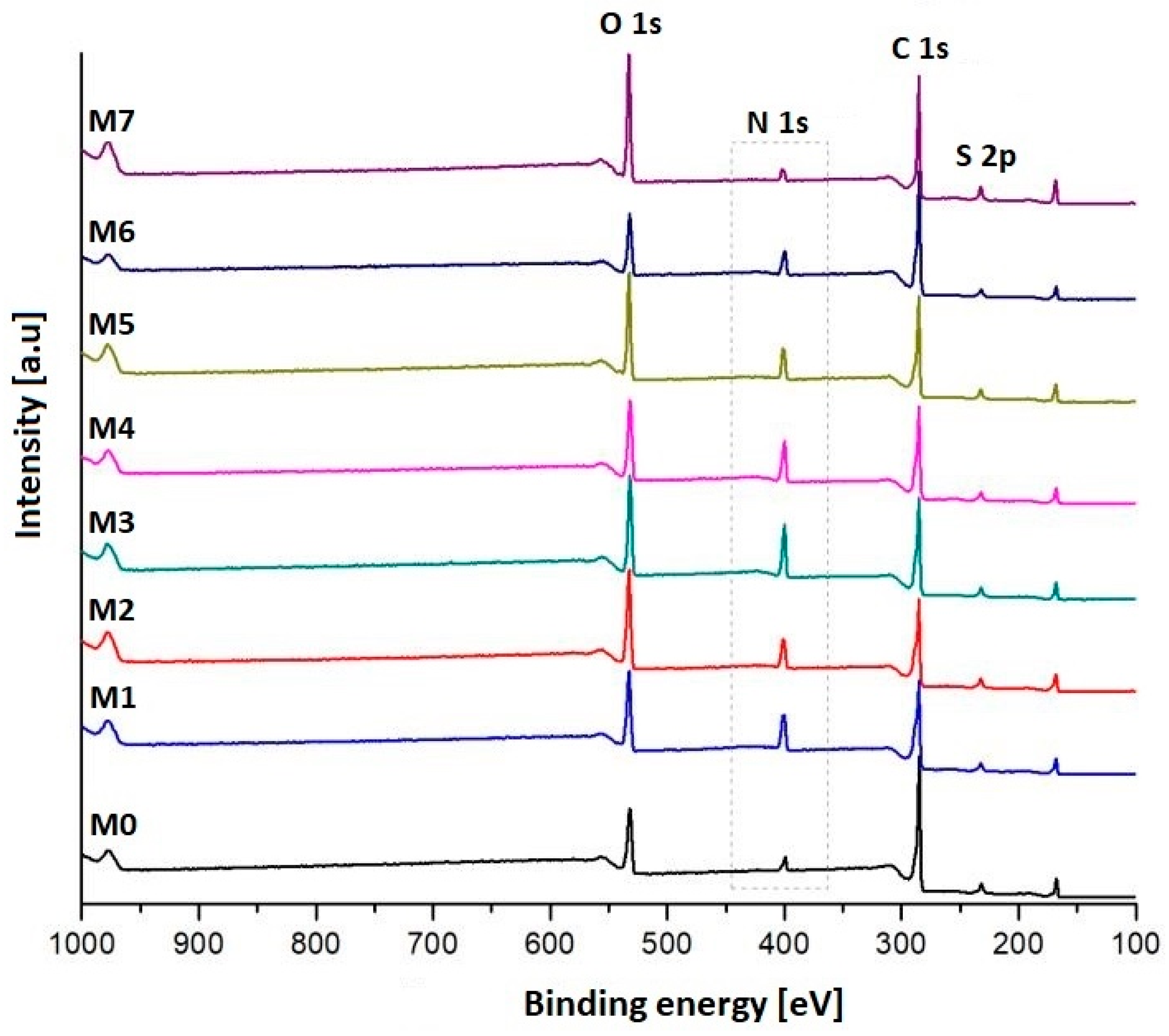
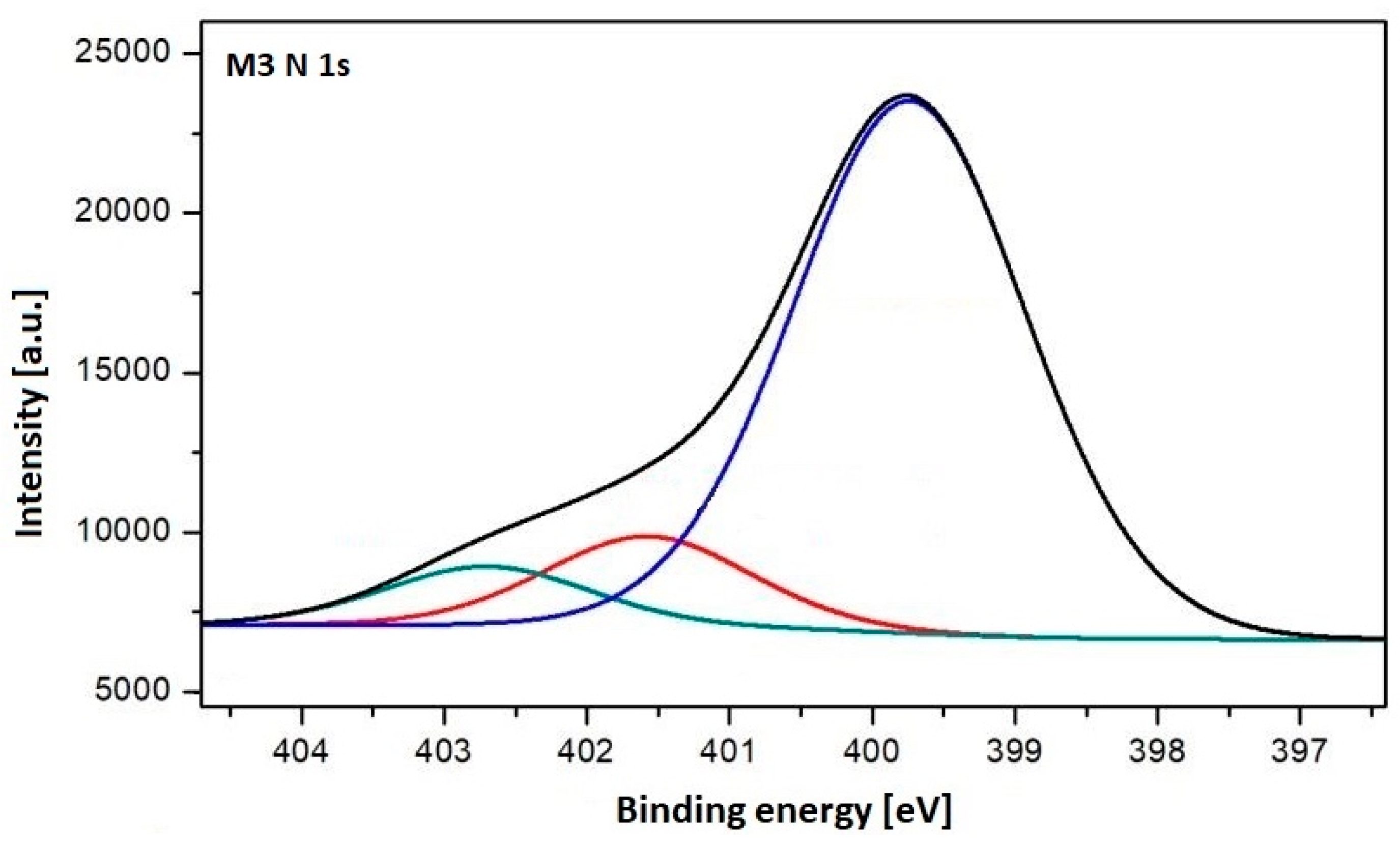
3.1. Characterization of the Plasma-Treated PES Membranes
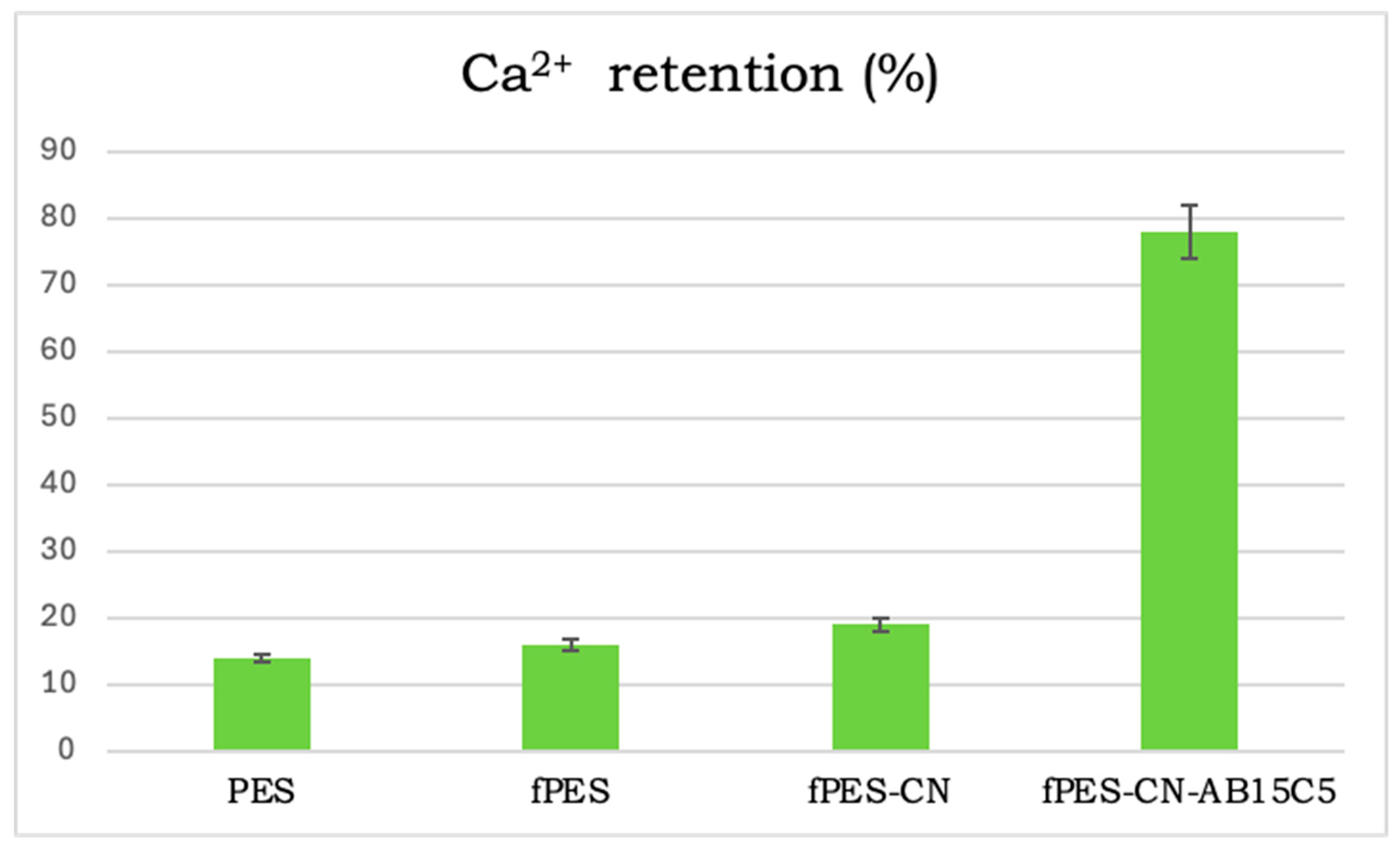
3.2. Characterization of the AB15C5-Functionalized PES Membrane
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mollahosseini, A.; Abdelrasoul, A.; Shoker, A. A critical review of recent advances in hemodialysis membranes hemocompatibility and guidelines for future development. Mater. Chem. Phys. 2020, 248, 122911. [Google Scholar] [CrossRef]
- Sakai, K. Dialysis membranes for blood purification. Front. Med. Biol. Eng. Int. J. Jpn. Soc. Med. Electron. Biol. Eng. 2000, 10, 117–129. [Google Scholar] [CrossRef]
- Ismail, A.; Zainol Abidin, M.N.; Mansur, S.; Zailani, M.; Said, N.; Raharjo, Y.; Rosid, S.; Othman, M.H.; Goh, P.; Hasbullah, H. Hemodialysis Membrane for Blood Purification Process. In Handbooks in Separation Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 283–314. [Google Scholar]
- Hothi, D.K.; Geary, D.F. CHAPTER 57—Pediatric Hemodialysis Prescription, Efficacy, and Outcome. In Comprehensive Pediatric Nephrology; Geary, D.F., Schaefer, F., Eds.; Mosby: Philadelphia, PA, USA, 2008; pp. 867–893. [Google Scholar]
- Wenten, I.G.; Aryanti, P.T.P.; Khoiruddin, K.; Hakim, A.N.; Himma, N.F. Advances in Polysulfone-Based Membranes for Hemodialysis. J. Membr. Sci. Res. 2016, 2, 78–89. [Google Scholar] [CrossRef]
- Marcut, L.; Mohan, A.G.; Corneschi, I.; Grosu, E.; Paltanea, G.; Avram, I.; Badaluta, A.V.; Vasilievici, G.; Nicolae, C.-A.; Ditu, L.M. Improving the Hydrophobicity of Plasticized Polyvinyl Chloride for Use in an Endotracheal Tube. Materials 2023, 16, 7089. [Google Scholar] [CrossRef]
- Bowry, S.K.; Gatti, E.; Vienken, J. Contribution of polysulfone membranes to the success of convective dialysis therapies. Contrib. Nephrol. 2011, 173, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Voicu, S.I.; Thakur, V.K. Green polymers-based membranes for water reuse in a circular economy context. Curr. Opin. Green Sustain. Chem. 2023, 43, 100852. [Google Scholar] [CrossRef]
- Irfan, M.; Irfan, M.; Shah, S.M.; Baig, N.; Saleh, T.A.; Ahmed, M.; Naz, G.; Akhtar, N.; Muhammad, N.; Idris, A. Hemodialysis performance and anticoagulant activities of PVP-k25 and carboxylic-multiwall nanotube composite blended Polyethersulfone membrane. Mater. Sci. Eng. C 2019, 103, 109769. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Cellulose acetate-based membranes for the removal of heavy metals from water in the context of circular economy. Ind. Crops Prod. 2023, 206, 117716. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Cellulose Acetate-Based Materials for Water Treatment in the Context of Circular Economy. Water 2023, 15, 1860. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Qiu, M.; He, C. Heparin-mimicking semi-interpenetrating composite membrane with multiple excellent performances for promising hemodialysis. J. Membr. Sci. 2021, 618, 118740. [Google Scholar] [CrossRef]
- Xu, R.; Feng, Q.; He, Y.; Yan, F.; Chen, L.; Zhao, Y. Dual functionalized poly (vinylidene fluoride) membrane with acryloylmorpholine and argatroban to improve antifouling and hemocompatibility. J. Biomed. Mater. Res. 2017, 105, 178–188. [Google Scholar] [CrossRef]
- Alenazi, N.A.; Hussein, M.A.; Alamry, K.A.; Asiri, A.M. Modified polyether-sulfone membrane: A mini review. Des. Monomers Polym. 2017, 20, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Pandele, A.; Serbanescu, O.S.; Voicu, S.I. Polysulfone Composite Membranes with Carbonaceous Structure. Synthesis and Applications. Coatings 2020, 10, 609. [Google Scholar] [CrossRef]
- Rafieian, F.; Mousavi, M.; Dufresne, A.; Yu, Q. Polyethersulfone membrane embedded with amine functionalized microcrystalline cellulose. Int. J. Biol. Macromol. 2020, 164, 4444–4454. [Google Scholar] [CrossRef]
- Oprea, M.; Pandele, A.M.; Nechifor, A.C.; Nicoara, A.I.; Antoniac, I.V.; Semenescu, A.; Voicu, S.I.; Enachescu, C.I.; Fratila, A.M. Improved Biomineralization Using Cellulose Acetate/Magnetic Nanoparticles Composite Membranes. Polymers 2025, 17, 209. [Google Scholar] [CrossRef]
- Abidin, M.N.Z.; Goh, P.S.; Said, N.; Ismail, A.F.; Othman, M.H.D.; Abdullah, M.S.; Ng, B.C.; Hasbullah, H.; Sheikh Abdul Kadir, S.H.; Kamal, F.; et al. Polysulfone/amino-silanized poly(methyl methacrylate) dual layer hollow fiber membrane for uremic toxin separation. Sep. Purif. Technol. 2020, 236, 116216. [Google Scholar] [CrossRef]
- Ionita, M.; Vasile, E.; Crica, L.E.; Voicu, S.I.; Pandele, A.M.; Dinescu, S.; Predoiu, L.; Galateanu, B.; Hermenean, A.; Costache, M. Synthesis, characterization and in vitro studies of polysulfone/graphene oxide composite membranes. Compos. Part B Eng. 2015, 72, 108–115. [Google Scholar] [CrossRef]
- Satulu, V.; Mitu, B.; Pandele, A.; Voicu, S.; Kravets, L.; Dinescu, G. Composite polyethylene terephthalate track membranes with thin teflon-like layers: Preparation and surface properties. Appl. Surf. Sci. 2019, 476, 452–459. [Google Scholar] [CrossRef]
- Muhulet, A.; Tuncel, C.; Miculescu, F.; Pandele, A.; Bobirica, C.; Orbeci, C.; Bobirica, L.; Palla-Papavlu, A.; Voicu, S.I. Synthesis and characterization of polysulfone–TiO 2 decorated MWCNT composite membranes by sonochemical method. Appl. Phys. A 2020, 126, 233. [Google Scholar] [CrossRef]
- Fu, C.-C.; Hsiao, Y.-S.; Ke, J.-W.; Syu, W.-L.; Liu, T.-Y.; Liu, S.-H.; Juang, R.-S. Adsorptive removal of p-cresol and creatinine from simulated serum using porous polyethersulfone mixed-matrix membranes. Sep. Purif. Technol. 2020, 245, 116884. [Google Scholar] [CrossRef]
- Antoniac, I.; Valeanu, N.; Niculescu, M.; Antoniac, A.; Robu, A.; Popescu, L.; Manescu Paltanea, V.; Anusca, D.; Enachescu, C.I. Outcomes of Birmingham Hip Resurfacing Based on Clinical Aspects and Retrieval Analysis of Failed Prosthesis. Materials 2024, 17, 3965. [Google Scholar] [CrossRef]
- Oprea, M.; Pandele, A.M.; Nicoara, A.I.; Nicolescu, A.; Deleanu, C.; Voicu, S.I. Crown ether-functionalized cellulose acetate membranes with potential applications in osseointegration. Int. J. Biol. Macromol. 2023, 230, 123162. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Voicu, S.I.; Thakur, V.K. Polysulfone functionalized membranes: Properties and challenges. Mater. Today Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]
- ter Beek, O.; Pavlenko, D.; Suck, M.; Helfrich, S.; Bolhuis-Versteeg, L.; Snisarenko, D.; Causserand, C.; Bacchin, P.; Aimar, P.; van Oerle, R.; et al. New membranes based on polyethersulfone—SlipSkin™ polymer blends with low fouling and high blood compatibility. Sep. Purif. Technol. 2019, 225, 60–73. [Google Scholar] [CrossRef]
- ter Beek, O.E.M.; Pavlenko, D.; Stamatialis, D. Hollow fiber membranes for long-term hemodialysis based on polyethersulfone-SlipSkin™ polymer blends. J. Membr. Sci. 2020, 604, 118068. [Google Scholar] [CrossRef]
- Zhong, D.; Wang, Z.; Zhou, J.; Wang, Y. Additive-free preparation of hemodialysis membranes from block copolymers of polysulfone and polyethylene glycol. J. Membr. Sci. 2021, 618, 118690. [Google Scholar] [CrossRef]
- Lusiana, R.A.; Sangkota, V.D.A.; Sasongko, N.A.; Gunawan, G.; Wijaya, A.R.; Santosa, S.J.; Siswanta, D.; Mudasir, M.; Abidin, M.N.Z.; Mansur, S.; et al. Permeability improvement of polyethersulfone-polietylene glycol (PEG-PES) flat sheet type membranes by tripolyphosphate-crosslinked chitosan (TPP-CS) coating. Int. J. Biol. Macromol. 2020, 152, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Saadati, S.; Westphalen, H.; Eduok, U.; Abdelrasoul, A.; Shoker, A.; Choi, P.; Doan, H.; Ein-Mozaffari, F.; Zhu, N. Biocompatibility enhancement of hemodialysis membranes using a novel zwitterionic copolymer: Experimental, in situ synchrotron imaging, molecular docking, and clinical inflammatory biomarkers investigations. Mater. Sci. Eng. C 2020, 117, 111301. [Google Scholar] [CrossRef]
- Streza, A.; Antoniac, A.; Manescu, V.; Paltanea, G.; Robu, A.; Dura, H.; Verestiuc, L.; Stanica, E.; Voicu, S.I.; Antoniac, I.; et al. Effect of Filler Types on Cellulose-Acetate-Based Composite Used as Coatings for Biodegradable Magnesium Implants for Trauma. Materials 2023, 16, 554. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S. Modification of polyethersulfone membranes—A review of methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, K.H.; Cho, K.; Park, C.E. Surface modification of polysulfone ultrafiltration membrane by oxygen plasma treatment. J. Membr. Sci. 2002, 199, 135–145. [Google Scholar] [CrossRef]
- Li, R.; Liang, Y.; Li, Q.; Niu, H.; Zhang, Y.; Li, Q. Studies on surface properties of polyethersulfone membrane by remote argon plasma. Vacuum 2020, 175, 109276. [Google Scholar] [CrossRef]
- Korchowiec, B.; Trojan, S.; Joly, J.-P.; Korchowiec, J.; Beley, M.; Rogalska, E. The interaction of an amphiphile crown ether with divalent metal ions. An electrochemical, Langmuir film, and molecular modeling study. Thin Solid Film. 2019, 683, 49–56. [Google Scholar] [CrossRef]
- Potopnyk, M.A.; Jarosz, S. Chapter 3—Nitrogen-Containing Macrocycles Having a Carbohydrate Scaffold. In Advances in Carbohydrate Chemistry and Biochemistry; Horton, D., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 227–295. [Google Scholar]
- Davis, F.; Higson, S. Chapter 3 Crown Ethers, Cryptands and Other Compounds. In Macrocycles: Construction, Chemistry and Nanptechnology Applications; Wiley: Oxford, UK, 2011; pp. 34–76. [Google Scholar]
- Barboiu, M.; Guizard, C.; Luca, C.; Hovnanian, N.; Palmeri, J.; Cot, L. Facilitated transport of organics of biological interest: II. Selective transport of organic acids by macrocyclic fixed site complexant membranes. J. Membr. Sci. 2000, 174, 277–286. [Google Scholar] [CrossRef]
- Pei, H.; Yan, F.; Wang, Z.; Liu, C.; Hou, S.; Ma, X.; Li, J.; Cui, Z.; He, B.; Wickramsinghe, S.R. Polysulfone-graft-4′- aminobenzo-15-crown-5-ether based tandem membrane chromatography for efficient adsorptive separation of lithium isotopes. J. Chromatogr. A 2019, 1602, 206–216. [Google Scholar] [CrossRef]
- Pei, H.; Yan, F.; Ma, X.; Li, X.; Liu, C.; Li, J.; Cui, Z.; He, B. In situ one-pot formation of crown ether functionalized polysulfone membranes for highly efficient lithium isotope adsorptive separation. Eur. Polym. J. 2018, 109, 288–296. [Google Scholar] [CrossRef]
- Song, L.; Huo, J.; Wang, X.; Yang, F.; He, J.; Li, C. Phosphate adsorption by a Cu(II)-loaded polyethersulfone-type metal affinity membrane with the presence of coexistent ions. Chem. Eng. J. 2016, 284, 182–193. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Luo, F.; Peng, H.-Y.; Zhang, S.-G.; Xie, R.; Ju, X.-J.; Wang, W.; Faraj, Y.; Chu, L.-Y. A novel smart membrane with ion-recognizable nanogels as gates on interconnected pores for simple and rapid detection of trace lead(II) ions in water. J. Membr. Sci. 2019, 575, 28–37. [Google Scholar] [CrossRef]
- Jin, C.; Liu, G.; Wu, G.; Huo, S.; Liu, Z.; Kong, Z. Facile fabrication of crown ether functionalized lignin-based biosorbent for the selective removal of Pb(II). Ind. Crops Prod. 2020, 155, 112829. [Google Scholar] [CrossRef]
- Kazemzadeh, H.; Karimi-Sabet, J.; Towfighi Darian, J.; Adhami, A. Evaluation of polymer inclusion membrane efficiency in selective separation of lithium ion from aqueous solution. Sep. Purif. Technol. 2020, 251, 117298. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, Y.; Pan, Y.; Li, X.; Hu, J.; Lü, J. Single-molecule nanomechanical spectroscopy shows calcium ions contribute to chain association and structural flexibility of blood clotting factor VIII. Biochem. Biophys. Res. Commun. 2019, 513, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Varga-Szabo, D.; Braun, A.; Nieswandt, B. Calcium signaling in platelets. J. Thromb. Haemost. JTH 2009, 7, 1057–1066. [Google Scholar] [CrossRef]
- Singh, S.; Dodt, J.; Volkers, P.; Hethershaw, E.; Philippou, H.; Ivaskevicius, V.; Imhof, D.; Oldenburg, J.; Biswas, A. Structure functional insights into calcium binding during the activation of coagulation factor XIII A. Sci. Rep. 2019, 9, 11324. [Google Scholar] [CrossRef]
- Schmitz, T.; Bäuml, C.A.; Imhof, D. Inhibitors of blood coagulation factor XIII. Anal. Biochem. 2020, 605, 113708. [Google Scholar] [CrossRef]
- Gancarz, I.; Poźniak, G.; Bryjak, M. Modification of polysulfone membranes. Eur. Polym. 2000, 36, 1563–1569. [Google Scholar] [CrossRef]
- Ayazi, Z.; Safarpour, M.; Ahmadi, F. Monolithic polyethersulfone membrane modified with PVA and PVP as a novel extracting media for thin film microextraction of bisphenol A from aquatic samples. Microchem. J. 2022, 175, 107143. [Google Scholar] [CrossRef]
- Singh, K.; Devi, S.; Bajaj, H.; Ingole, P.; Chaudhari, J.; Harshad, B. Optical Resolution of Racemic Mixtures of Amino Acids through Nanofiltration Membrane Process. Sep. Sci. Technol. 2014, 49, 2630–2641. [Google Scholar] [CrossRef]
- Belfer, S.; Fainchtain, R.; Purinson, Y.; Kedem, O. Surface characterization by FTIR-ATR spectroscopy of polyethersulfone membranes-unmodified, modified and protein fouled. J. Membr. Sci. 2000, 172, 113–124. [Google Scholar] [CrossRef]
- Bryjak, M.; Gancarz, I.; Poźniak, G.; Tylus, W. Modification of polysulfone membranes 4. Ammonia plasma treatment. Eur. Polym. J. 2002, 38, 717–726. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Hierro-Oliva, M.; Gallardo-Moreno, A.M.; González-Martín, M.L. Effect of plasma treatment on the surface properties of polylactic acid films. Polym. Test. 2021, 96, 107097. [Google Scholar] [CrossRef]
- Espinosa-Solis, V.; García-Tejeda, Y.V.; Leal-Castañeda, E.J.; Barrera-Figueroa, V. Effect of the Degree of Substitution on the Hydrophobicity, Crystallinity, and Thermal Properties of Lauroylated Amaranth Starch. Polymers 2020, 12, 2548. [Google Scholar] [CrossRef] [PubMed]
- Oprea, M.; Voicu, S.I. Recent Advances in Applications of Cellulose Derivatives-Based Composite Membranes with Hydroxyapatite. Materials 2020, 13, 2481. [Google Scholar] [CrossRef] [PubMed]
- Nechifor, A.; Stoian, M.; Voicu, Ş.I.; Nechifor, G. Modified Fe3O4 colloidal dispersed magnetic particles as carrier in liquid membranes. Optoelectron. Adv. Mater. Rapid Commun. 2010, 4, 1118–1123. [Google Scholar]
- Ji, M.; Luo, J.; Wei, J.; Woodley, J.; Daugaard, A.E.; Pinelo, M. Commercial polysulfone membranes pretreated with ethanol and NaOH: Effects on permeability, selectivity and antifouling properties. Sep. Purif. Technol. 2019, 219, 82–89. [Google Scholar] [CrossRef]
- Xiang, T.; Xie, Y.; Wang, R.; Wu, M.-B.; Sun, S.-D.; Zhao, C.-S. Facile chemical modification of polysulfone membrane with improved hydrophilicity and blood compatibility. Mater. Lett. 2014, 137, 192–195. [Google Scholar] [CrossRef]
- Tang, X.-Z.; Li, W.; Yu, Z.-Z.; Rafiee, M.A.; Rafiee, J.; Yavari, F.; Koratkar, N. Enhanced thermal stability in graphene oxide covalently functionalized with 2-amino-4,6-didodecylamino-1,3,5-triazine. Carbon 2011, 49, 1258–1265. [Google Scholar] [CrossRef]
- Olsen, G.; Ulstrup, J.; Chi, Q. Crown-ether derived graphene hybrid composite for membrane-free potentiometric sensing of alkali metal ions. ACS Appl. Mater. Interfaces 2016, 8, 37–41. [Google Scholar] [CrossRef]
- Chen, L.; Yang, B.; Zhou, P.; Xu, T.; He, C.; Xu, Y.; Zhao, W.; Zhao, C. A polyethersulfone composite ultrafiltration membrane with the in-situ generation of CdS nanoparticles for the effective removal of organic pollutants and photocatalytic self-cleaning. J. Membr. Sci. 2021, 638, 119715. [Google Scholar] [CrossRef]
- Pandele, A.M.; Oprea, M.; Dutu, A.A.; Miculescu, F.; Voicu, S.I. A Novel Generation of Polysulfone/Crown Ether-Functionalized Reduced Graphene Oxide Membranes with Potential Applications in Hemodialysis. Polymers 2022, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Villa-Etchegoyen, C.; Lombarte, M.; Matamoros, N.; Belizán, J.M.; Cormick, G. Mechanisms Involved in the Relationship between Low Calcium Intake and High Blood Pressure. Nutrients 2019, 11, 1112. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Baino, F.; Yamaguchi, S. The Use of Simulated Body Fluid (SBF) for Assessing Materials Bioactivity in the Context of Tissue Engineering: Review and Challenges. Biomimetics 2020, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Olăreț, E.; Voicu, Ș.I.; Oprea, R.; Miculescu, F.; Butac, L.; Stancu, I.-C.; Serafim, A. Nanostructured Polyacrylamide Hydrogels with Improved Mechanical Properties and Antimicrobial Behavior. Polymers 2022, 14, 2320. [Google Scholar] [CrossRef] [PubMed]
- Radu, E.R.; Voicu, S.I. Functionalized Hemodialysis Polysulfone Membranes with Improved Hemocompatibility. Polymers 2022, 14, 1130. [Google Scholar] [CrossRef] [PubMed]
- Radu, E.R.; Voicu, S.I.; Thakur, V.K. Polymeric Membranes for Biomedical Applications. Polymers 2023, 15, 619. [Google Scholar] [CrossRef]
- Ji, H.; Li, Y.; Su, B.; Zhao, W.; Kizhakkedathu, J.N.; Zhao, C. Advances in Enhancing Hemocompatibility of Hemodialysis Hollow-Fiber Membranes. Adv. Fiber Mater. 2023, 5, 1198–1240. [Google Scholar] [CrossRef] [PubMed]
- Oprea, M.; Ionita, M. Antisense oligonucleotides-based approaches for the treatment of multiple myeloma. Int. J. Biol. Macromol. 2025, 291, 139186. [Google Scholar] [CrossRef]
- Mikaelsson, M.E. The Role of Calcium in Coagulation and Anticoagulation. In Coagulation and Blood Transfusion: Proceedings of the Fifteenth Annual Symposium on Blood Transfusion, Groningen 1990, Organized by the Red Cross Blood Bank Groningen-Drenthe; Sibinga, C.T.S., Das, P.C., Mannucci, P.M., Eds.; Springer: Boston, MA, USA, 1991; pp. 29–37. [Google Scholar]
- Liu, Y.; Li, G.; Han, Q.; Lin, H.; Li, Q.; Hua, J.; Liu, F. Anticoagulant dialyzer with enhanced Ca2+ chelation and hydrophilicity for heparin free hemodialysis. J. Membr. Sci. 2020, 604, 118082. [Google Scholar] [CrossRef]
- Xiao, T.; Wang, Y.; Wang, H.; Zhou, J.; Zhao, Q.; Zhang, Y. Preparation of antioxidant hemodialysis membranes by in situ growth of citric acid-terminated Prussian blue nanoenzymes. J. Membr. Sci. 2025, 722, 123847. [Google Scholar] [CrossRef]
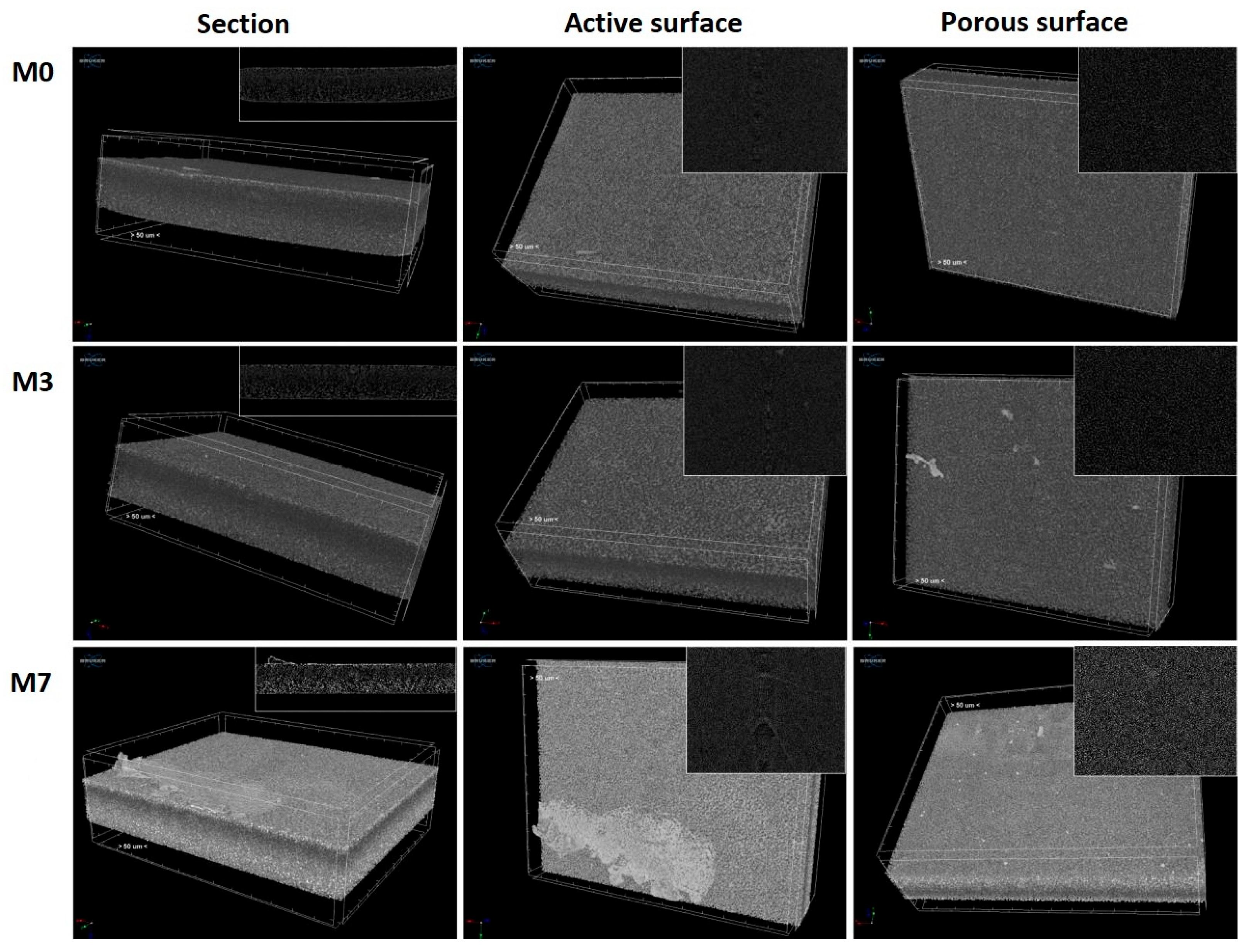
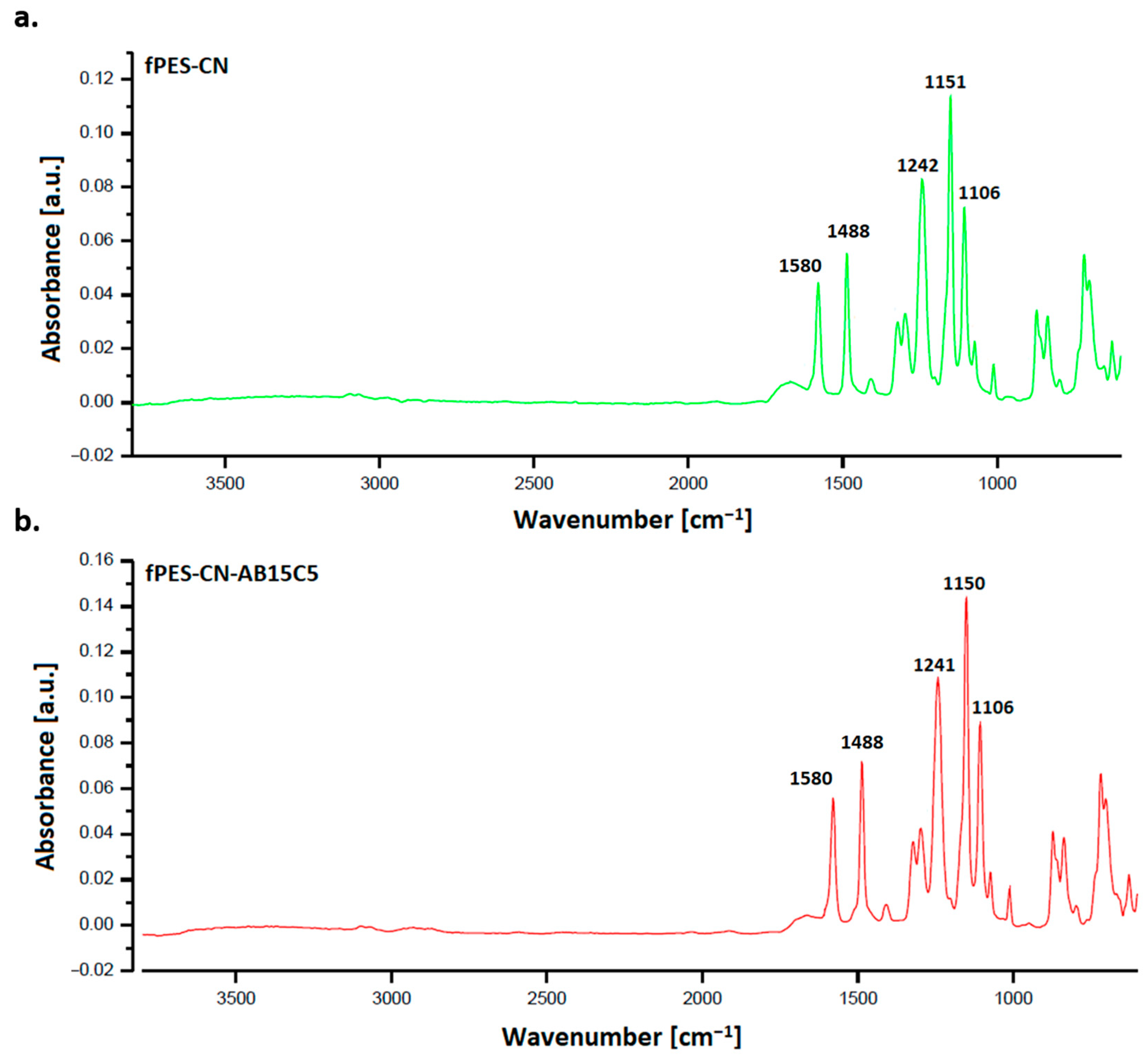
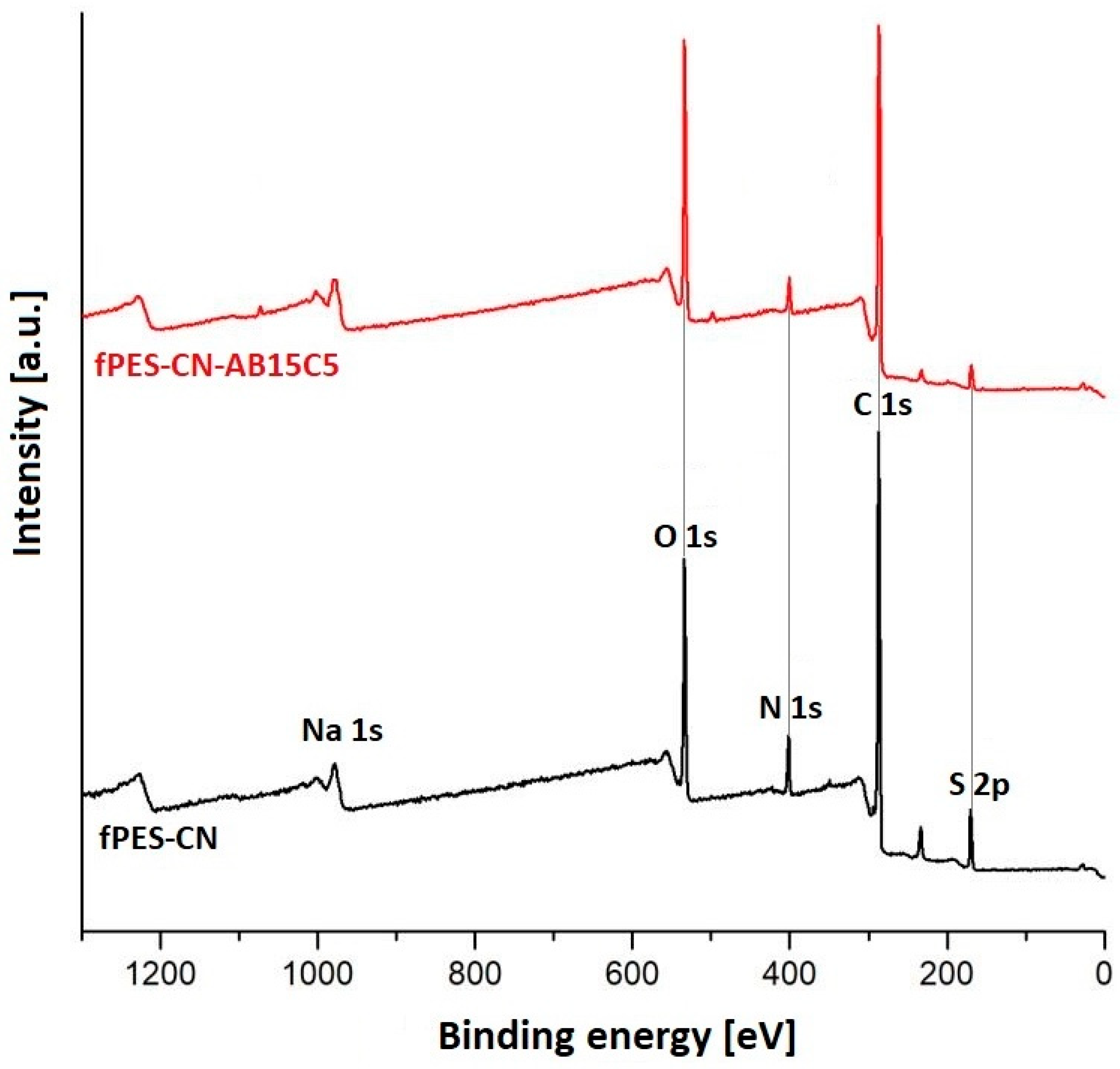
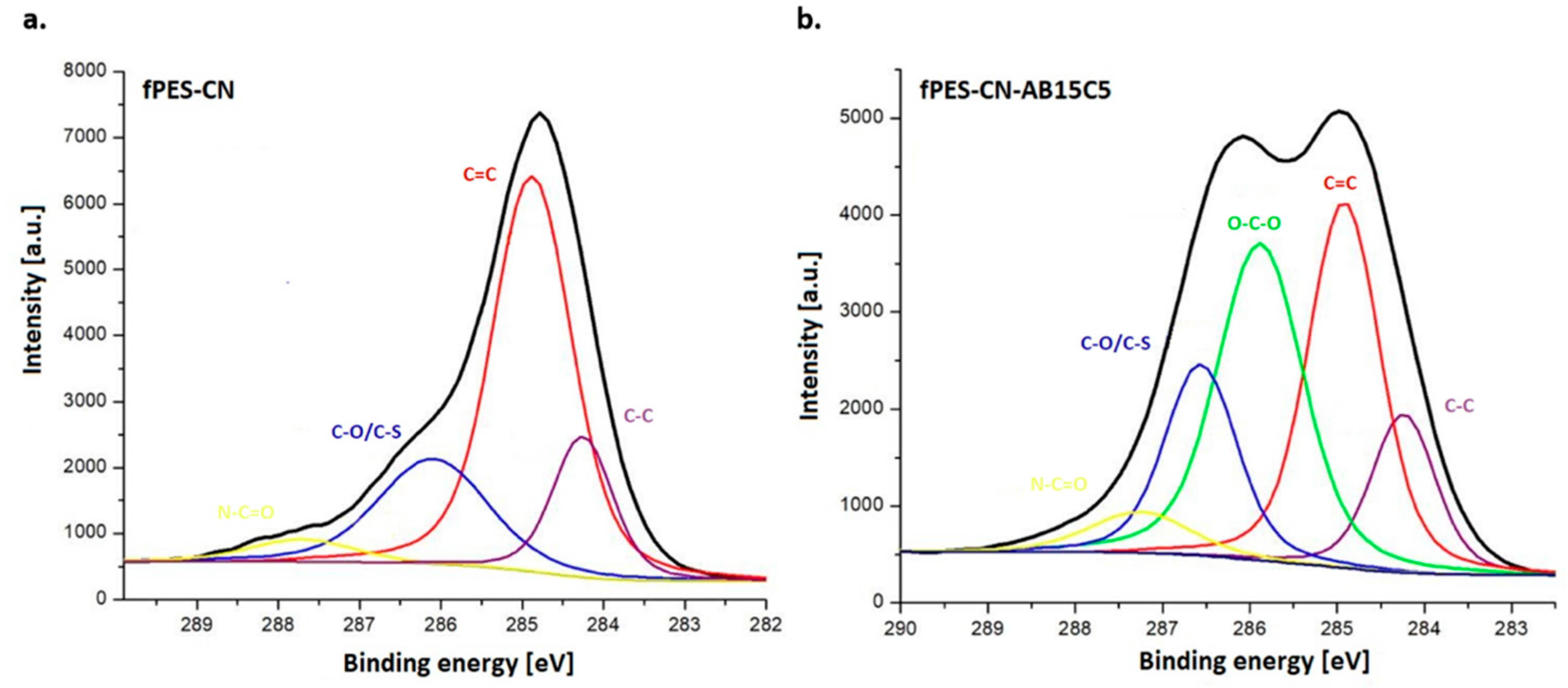


| Sample | Discharge Gas | Flow [cm3/min] | RF Power [W] | Pressure [Pa] | Time [min] |
|---|---|---|---|---|---|
| M1 | Ar/N2 | 10/10 | 20 | 4 × 102 | 10 |
| M2 | Ar/N2 | 10/25 | 20 | 6 × 102 | 10 |
| M3 | Ar/NH3 | 10/10 | 20 | 5.5 × 102 | 10 |
| M4 | Ar/NH3 | 10/25 | 20 | 7.5 × 102 | 10 |
| M5 | Ar/H2 + Ar/N2 | 10/25 + 10/25 | 20 | 8 × 102 | 5 + 5 |
| M6 | Ar/(H2 + N2) | 10/(25 + 25) | 20 | 103 | 10 |
| M7 | Ar | 10 | 20 | 2.4 × 102 | 10 |
| Sample | Atomic Percentage [%] | |||
|---|---|---|---|---|
| C 1s | O 1s | S 2p | N 1s | |
| M0 | 73.05 | 17.48 | 4.79 | 4.68 |
| M1 | 57.17 | 25.70 | 5.07 | 12.06 |
| M2 | 59.70 | 20.69 | 4.27 | 15.34 |
| M3 | 55.91 | 22.69 | 4.40 | 16.99 |
| M4 | 58.17 | 21.64 | 4.52 | 15.67 |
| M5 | 59.49 | 24.52 | 4.83 | 11.15 |
| M6 | 70.97 | 15.48 | 3.81 | 9.74 |
| M7 | 59.45 | 29.28 | 6.47 | 4.79 |
| N 1s Peak | Assignment | Chemical Structure * |
|---|---|---|
| 399.74 | Amine | 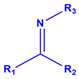 |
| 401.58 | Amide | 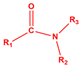 |
| 402.70 | Imine | 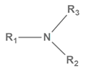 |
| Sample | Atomic Percentage [%] | ||||
|---|---|---|---|---|---|
| C1s | O1s | S2p | N1s | Na1s | |
| fPES | 55.91 | 22.69 | 4.40 | 16.99 | |
| fPES-CN | 70.26 | 17.89 | 5.78 | 6.07 | 0.49 |
| fPES-CN-AB15C5 | 70.52 | 21.06 | 2.71 | 4.72 | - |
| Sample | Tmax [°C] | WL [%] | R800 [%] |
|---|---|---|---|
| PES | 520 | 72 | 28 |
| fPES-CN | 565 | 63 | 37 |
| fPES-CN-AB15C5 | 550 | 65 | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oprea, M.; Pandele, A.M.; Enachescu, C.I.; Antoniac, I.V.; Voicu, S.I.; Fratila, A.M. Crown Ether-Functionalized Polyethersulfone Membranes with Potential Applications in Hemodialysis. Polymers 2025, 17, 2184. https://doi.org/10.3390/polym17162184
Oprea M, Pandele AM, Enachescu CI, Antoniac IV, Voicu SI, Fratila AM. Crown Ether-Functionalized Polyethersulfone Membranes with Potential Applications in Hemodialysis. Polymers. 2025; 17(16):2184. https://doi.org/10.3390/polym17162184
Chicago/Turabian StyleOprea, Madalina, Andreea Madalina Pandele, Catalin Ionel Enachescu, Iulian Vasile Antoniac, Stefan Ioan Voicu, and Anca Maria Fratila. 2025. "Crown Ether-Functionalized Polyethersulfone Membranes with Potential Applications in Hemodialysis" Polymers 17, no. 16: 2184. https://doi.org/10.3390/polym17162184
APA StyleOprea, M., Pandele, A. M., Enachescu, C. I., Antoniac, I. V., Voicu, S. I., & Fratila, A. M. (2025). Crown Ether-Functionalized Polyethersulfone Membranes with Potential Applications in Hemodialysis. Polymers, 17(16), 2184. https://doi.org/10.3390/polym17162184










